Abstract
Purpose
Recent reports suggest the association of human papilloma virus (HPV) with retinoblastoma. This study was performed to elucidate whether HPV infection is related to retinoblastoma among Koreans.
Methods
A total of 54 cases diagnosed with retinoblastoma were enrolled from Seoul National University Children's Hospital and Seoul Metropolitan Government-Seoul National University Boramae Medical Center. Presence of human papilloma viral DNA was detected by in situ hybridization in formalin-fixed paraffin-embedded retinoblastoma tissues using both probes against high- and low risk HPV types.
Results
The mean age at diagnosis was 22.0 months (range, 1.1 to 98.0 months), and the mean age at enucleation was 27.8 months (range, 1.5 to 112.7 months) among the 54 patients with retinoblastoma. HPV was not detected in any of the retinoblastoma samples using either high risk or low risk HPV probes.
Conclusions
Our study, being the first study in the Korean population, proposes that HPV infection may have no causal relationship with retinoblastoma in Koreans.
Keywords: DNA, Human papillomavirus, In situ hybridization, Retinoblastoma
Infectious sources have been repeatedly reported as the pathogenesis in various cancers. The relationship between the human papilloma virus (HPV) and cervical cancer is a well-proven example. Especially among the various types of HPV, certain types appear to be more oncogenic and are therefore named high-risk HPV [1]. Helicobacter pylori and gastric cancer is another widely-known example of infective agent-associated cancer [2]. Based on such findings, microorganism or viral eradication has been suggested as a treatment for such cancers.
In the same vein, interest has arisen in the association of ocular tumors and infections. The association between ocular adnexal mucosa-associated lymphoid tissue lymphoma and Chlamydia psittaci infection has been well documented [3].
Among ocular tumors, retinoblastoma (RBL) is the most common primary ocular malignancy in children, accounting for approximately 3% of the tumors occurring in people under 15 years of age and leading to 9,000 deaths annually worldwide [4]. RBL is known to be more prevalent in areas of low hygiene and socioeconomic status, such as South America, Africa and Southeast Asia, raising the possibility of infection as a factor in its development.
Several studies have recently reported the association of HPV with RBL. Anand et al. [5] demonstrated the presence of HPV in a subset of patients with RBL. Shetty et al. [6] also suggested that infection of HPV-16/-18 may interfere with the cell regulatory process and lead to development of RBL in the Indian population. However, contradictory results were reported by Gillison et al. [7] that revealed no relationship between HPV and RBL in North American populations. These discrepant results may suggest geographic differences in the prevalence and etiology of retinoblastoma and HPV infection. To date, no reports are available with respect to the East Asian population.
In this study, we analyzed 54 retinoblastoma patients who underwent enucleation to demonstrate the association with HPV infection in the Korean population.
Materials and Methods
Samples
Formalin-fixed paraffin-embedded tissues from 54 enucleated eyeballs from patients diagnosed with retinoblastoma from 2000 to 2005 from Seoul National University Children's Hospital and Seoul Metropolitan Government-Seoul National University Boramae Medical Center were recruited for the study. We retrospectively reviewed the medical records of 54 patients with retinoblastoma. Reviewed data included demographic information and histopathological diagnosis. The hematoxylin and eosin-stained slides were independently reviewed by one of the authors (JEK) in each case to confirm the original diagnosis. This study was approved by the institutional review board of Seoul Metropolitan Government Seoul National University Boramae Medical Center.
Tissue microarray
Tissue microarray (TMA) recipient blocks containing paraffin-embedded retinoblastoma enucleated tissues from the archived patient specimens, previously fixed in 10% formaldehyde, were constructed according to the established methods. From every archival paraffin block, 1 cylinder of 2.0-mm-diameter tissue was taken from representative areas and transferred to the recipient paraffin block that contained 60 tissue cores. The recipient block of retinoblastoma consisted of 54 tumor cores. Duplicates of each TMA were used for the HPV study.
In situ hybridization of human papilloma virus
The in situ hybridization (ISH) for HPV was performed using the automated staining system (Ventana, Tucson, AZ, USA) according to the manufacturer's instructions. Inform HPV III probe sets (Ventana) can identify 12 types of high-risk HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66) and two types of low-risk HPV (6 and 11). Appropriate positive controls using tissue sections of HPV-related squamous cell carcinoma of the uterine cervix was also applied, and bluish black nuclear signals were interpreted as positive by microscopic examination.
Results
Demographic data and clinical features
The 54 patients who underwent medical record review consisted of 26 males and 28 females. The 54 enucleated eyeballs consisted of 29 right eyeballs and 25 left eyeballs. The mean age at diagnosis was 22.0 months (range, 1.1 to 98.0 months), and the mean age at enucleation was 27.8 months (range, 1.5 to 112.7 months).
Human papilloma virus in situ hybridization
HPV was not detected in any of the retinoblastoma samples. The possibilities of false-negative results remain low due to the fact that positive controls-cervical cancer specimen-consistently expressed HPV, which confirms the reliability of the analysis technique (Fig. 1).
Fig. 1.
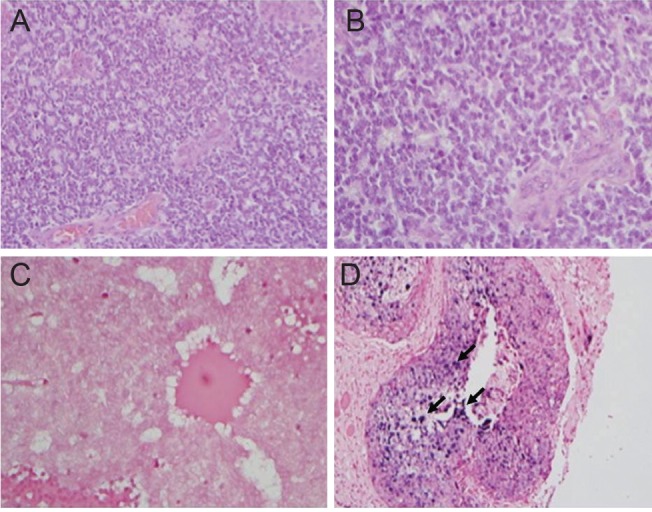
(A,B) Hematoxylin and eosin staining confirm the presence of retinoblastoma cells, ×200, ×400, respectively. (C) In situ hybridization for human papilloma virus (HPV) in retinoblastoma tumor cells is negative for HPV-DNA. (D) Squamous cell carcinoma of the uterine cervix stain positive for HPV-DNA. Dark purple staining (depicted in black arrows) indicates integrated or episomal HPV-DNA.
Discussion
The association between various cancers and infection as their etiology has emerged as a significant issue within the past decade. HPV-related pathogenesis of cervical carcinoma is a well-established example; such pathogenesis occurs when the infection is persistent and oncogenic portions of the high-risk HPV genome can interact with host cells in ways that lead to genetic and cell regulatory changes, which in turn leads to carcinogenesis. After scientific establishment of such a causal relationship, investigations of HPV in other tumors are following.
Several papers have been published regarding the presence or absence of HPV in retinoblastoma [4-10]. Disparity exists between such reports, ranging from greater than 70% HPV detection in RBL to no HPV found in retinoblastoma specimens from certain populations. The highest prevalence of HPV DNA in RBL was reported from the Asian Indian population. Fifty-three of 76 (65.7%) specimens tested positive for HPV DNA, especially high-risk HPV (HPV-16 / HPV-18) [6]. Another study conducted in Southern India revealed 48% positivity of HPV-DNA-all HPV-16-in 44 unilateral RBL cases [8]. However, relevance was as low as 4.5% with respect to HPV-positive samples in 154 RBL tumor tissues from the Brazilian population, being even lower than that of normal retina samples, which strongly implies no relevance of HPV with cancer etiology [9]. No relationship with HPV was also proposed in a study from North America, which analyzed 40 RBL tumor tissues for the presence of 37 types of HPV, all of which were negative [7].
Our study is the first in the Korean population as well as the East-Asian population. Retinoblastoma tissues from 54 enucleated eyeballs all revealed no HPV-DNA. Geographic variation of HPV infection rates may play a role in the low prevalence of HPV-DNA in our study. In a large population study involving 60,775 Korean women aged between 18 and 79 years, based on cervical cytology, HPV-DNA was detected in 34.2% of samples; 17.5 % were infected with high-risk HPV genotypes and 16.7% with low-risk HPV genotypes [11]. On the contrary, higher incidence of HPV infection was reported from India, which also reports higher rates of HPV-DNA detection in RBL patients. The overall prevalence of high-risk HPV reported from India is 37.6% to 54.9% [12,13]. Therefore, when speculating the mode of HPV transmission in RBL patients as maternal exposure to viral infection, which could cause mutations in utero, the lower prevalence of overall HPV infection may explain the low prevalence of HPV-DNA detection in RBL patients in Korea.
High risk-HPV prevalence rates may also vary by detection method [14]. In this study, ISH for HPV was conducted using an Inform HPV III kit (Ventana), a detection system that has high specificity due to its increased signal strength [15]. Using this system, HPV detection rates in oropharyngeal squamous cell carcinoma have been reported to be 59% to 88% in sensitivity and 71% to 94.7% in specificity [14,16]. Although ISH is advantageous in preserving the morphological context of the specimen and direct visualization of signaling, limitations may lie in its detection rate. Therefore, an alternative suggestion to HPV ISH may be determination of p16 protein expression by immunohistochemistry. In HPV-associated cancers, p16 is frequently overexpressed because of inactivation of pRb by the HR-HPV E7 oncoprotein and the consequent release of pRb-mediated negative regulation of p16 [17]. However, although p16 is a more sensitive marker of HPV infection in uterine cervix carcinoma and head and neck carcinoma, one cannot apply the same rule in RBL [18]. In RBL, p16 is almost always highly expressed because of deterioration of the Rb gene [19]. Therefore, ISH is a more proper method of detection in retinoblastoma. Despite the application of ISH, no HPV-DNA was detected in the present study.
Tumors that have previously shown a relationship with HPV, i.e., cervical cancer, certain oropharyngeal cancers, arise on areas that have mucosal exposure, allowing direct transmission of the viral DNA via an external source. Unlike these tumors, the mode of transmission is questionable in RBL, initially an intraocular tumor. To our knowledge, no studies have investigated the relationship of central nervous system tumors and HPV, which would have been a better comparison. However, such studies may be restricted due to difficult obtainability of such specimen, prohibiting the determination of the actual causal relationship.
The exact cause of retinoblastoma tumors lacking genetic alterations or pRb-inactivating tumor viruses remains unknown. Further studies with these retinoblastoma specimens are needed to validate involvement of other components of the pRb pathway, such as p16, a surrogate marker of viral infection and carcinogenesis, as suggested above, and may provide better understanding of infection-tumor relationships and etiology.
In conclusion, we have shown that HPV infection may have no causal relationship with RBL in the Korean population. Further studies to reveal the etiology underlying RBL are encouraged.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000;92:690–698. doi: 10.1093/jnci/92.9.690. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choung HK, Kim YA, Lee MJ, et al. Multigene methylation analysis of ocular adnexal MALT lymphoma and their relationship to Chlamydophila psittaci infection and clinical characteristics in South Korea. Invest Ophthalmol Vis Sci. 2012;53:1928–1935. doi: 10.1167/iovs.11-7668. [DOI] [PubMed] [Google Scholar]
- 4.Dimaras H, Kimani K, Dimba EA, et al. Retinoblastoma. Lancet. 2012;379:1436–1446. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 5.Anand B, Ramesh C, Appaji L, et al. Prevalence of high-risk human papillomavirus genotypes in retinoblastoma. Br J Ophthalmol. 2011;95:1014–1018. doi: 10.1136/bjo.2010.199802. [DOI] [PubMed] [Google Scholar]
- 6.Shetty OA, Naresh KN, Banavali SD, et al. Evidence for the presence of high risk human papillomavirus in retinoblastoma tissue from nonfamilial retinoblastoma in developing countries. Pediatr Blood Cancer. 2012;58:185–190. doi: 10.1002/pbc.23346. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Chen R, Goshu E, et al. Human retinoblastoma is not caused by known pRb-inactivating human DNA tumor viruses. Int J Cancer. 2007;120:1482–1490. doi: 10.1002/ijc.22516. [DOI] [PubMed] [Google Scholar]
- 8.Mohan A, Venkatesan N, Kandalam M, et al. Detection of human papillomavirus DNA in retinoblastoma samples: a preliminary study. J Pediatr Hematol Oncol. 2009;31:8–13. doi: 10.1097/MPH.0b013e31818b373b. [DOI] [PubMed] [Google Scholar]
- 9.Antoneli CB, Ribeiro KB, Sredni ST, et al. Low prevalence of HPV in Brazilian children with retinoblastoma. J Med Virol. 2011;83:115–118. doi: 10.1002/jmv.21925. [DOI] [PubMed] [Google Scholar]
- 10.Palazzi MA, Yunes JA, Cardinalli IA, et al. Detection of oncogenic human papillomavirus in sporadic retinoblastoma. Acta Ophthalmol Scand. 2003;81:396–398. doi: 10.1034/j.1600-0420.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee EH, Um TH, Chi HS, et al. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012;27:1091–1097. doi: 10.3346/jkms.2012.27.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deodhar K, Gheit T, Vaccarella S, et al. Prevalence of human papillomavirus types in cervical lesions from women in rural Western India. J Med Virol. 2012;84:1054–1060. doi: 10.1002/jmv.23310. [DOI] [PubMed] [Google Scholar]
- 13.Vinodhini K, Shanmughapriya S, Sanmugham S, et al. Prevalence of high-risk HPV and associated risk factors in cases of cervical carcinoma in Tamil Nadu, India. Int J Gynaecol Obstet. 2012;119:253–256. doi: 10.1016/j.ijgo.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol. 2011;24:1295–1305. doi: 10.1038/modpathol.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung ES, Shin JH, Lee KJ, et al. HPV in situ hybridization and immunohistochemical expression of L1 capsid protein in cervical intraepithelial neoplasia. Korean J Obstet Gynecol. 2009;52:1279–1286. [Google Scholar]
- 16.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zur Hausen H. Immortalization of human cells and their malignant conversion by high risk human papillomavirus genotypes. Semin Cancer Biol. 1999;9:405–411. doi: 10.1006/scbi.1999.0144. [DOI] [PubMed] [Google Scholar]
- 18.Venuti A, Paolini F. HPV detection methods in head and neck cancer. Head Neck Pathol. 2012;6 Suppl 1:S63–S74. doi: 10.1007/s12105-012-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]


