Abstract
Assimilating new information into existing knowledge is a fundamental part of consolidating new memories and allowing them to guide behavior optimally and is vital for conceptual knowledge (semantic memory), which is accrued over many years. Sleep is important for memory consolidation, but its impact upon assimilation of new information into existing semantic knowledge has received minimal examination. Here, we examined the integration process by training human participants on novel words with meanings that fell into densely or sparsely populated areas of semantic memory in two separate sessions. Overnight sleep was polysomnographically monitored after each training session and recall was tested immediately after training, after a night of sleep, and 1 week later. Results showed that participants learned equal numbers of both word types, thus equating amount and difficulty of learning across the conditions. Measures of word recognition speed showed a disadvantage for novel words in dense semantic neighborhoods, presumably due to interference from many semantically related concepts, suggesting that the novel words had been successfully integrated into semantic memory. Most critically, semantic neighborhood density influenced sleep architecture, with participants exhibiting more sleep spindles and slow-wave activity after learning the sparse compared with the dense neighborhood words. These findings provide the first evidence that spindles and slow-wave activity mediate integration of new information into existing semantic networks.
Introduction
Sleep facilitates memory consolidation and has been implicated in various aspects of the process whereby new memories become part of existing knowledge structures (Diekelmann and Born, 2010). Specifically, classic consolidation theory suggests that new memories are initially mediated by synaptic changes within the medial temporal lobe, supporting immediate influence of new learning on activation of representations in the neocortex (McClelland et al., 1995; McClelland and Goddard, 1996). According to this framework, these memories gradually gain neocortical representation via reactivation during sleep. Tamminen et al. (2010) showed that time and sleep after initial study increase interference of new phonological representations with processing of existing words at the behavioral level and that this increase in interference is predicted by sleep spindle activity, consistent with the view that neocortical representations begin to change very soon after initial encoding. No meanings were assigned to the new words learned in that study, however, and little is known about how new semantic information interacts with existing semantic memories or which aspects of sleep architecture mediate such interactions.
Semantic neighborhood density (the number of semantically associated concepts) has been shown to affect memory and learning, typically through better memory for items with few semantic neighbors. If participants are given a list of words to recall or names to learn, performance is superior for words with few neighbors (Nelson and Zhang, 2000; Storkel and Adlof, 2009). These studies suggest that newly learned materials suffer from interference from preexisting neighbors and that the number of neighbors affects the degree of interference. We used a learning paradigm to teach adults new words with meanings that were novel semantic concepts that fit in either dense or sparse semantic neighborhoods and used the emergence of neighborhood interference as a marker of semantic integration. We hypothesized that novel words that fell in sparse semantic neighborhoods would be associated with a more rapidly accessible memory trace and thus faster behavioral reaction times (RTs) because of less interference from competing semantic neighbors.
Our second prediction was that if sleep plays an active role in integrating new memories with existing semantic knowledge, then semantic neighborhood density should affect sleep architecture after learning. Previous research has demonstrated a critical role for two categories of neural activity occurring mostly during slow-wave sleep: slow-wave activity (SWA; <4 Hz) and sleep spindles. SWA is increased after learning and at the slower range of SWA oscillations (<1 Hz) synchronize neocortical activity and hippocampal ripples, with the latter likely reflecting hippocampal reactivation of new memories (Diekelmann and Born, 2010). Sleep spindles (11–15 Hz oscillations lasting up to 3 s) align temporally with hippocampal ripples (Siapas and Wilson, 1998), implicating them as part of hippocampal-neocortical dialog and, like SWA, spindles are associated with priming cortical plasticity (Rosanova and Ulrich, 2005). We predicted that these neural measures mediate integration of new memories into preexisting semantic knowledge and expected to observe more SWA and increased spindle activity associated with learning sparse neighborhood words.
Materials and Methods
Participants and design.
Twenty-four normal sleepers (12 females, 12 males) with a mean age of 21 (SEM = 0.8) maintained a regular sleep schedule for 3 d before participating in the study.
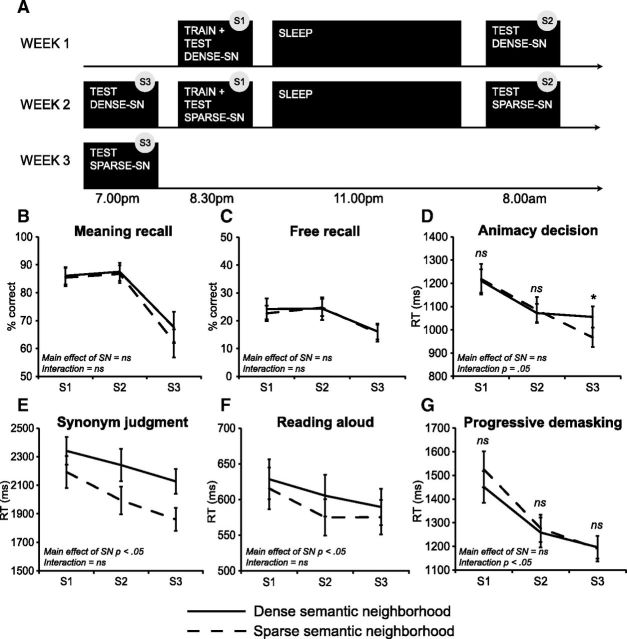
The first session (S1) took place in the evening (Week 1; Fig. 1A). Participants were wired for polysomnography (PSG) and completed training and an immediate test session of the first set of novel words (dense semantic neighborhood, henceforth dense-SN, or sparse semantic neighborhood, henceforth sparse-SN). They then slept in the laboratory and were awakened at 8:00 A.M. The second test session (S2) began after breakfast (30 min from awakening). One week after S1 (mean = 7.0 d, range = 6–7 d), participants returned in the evening (Week 2) and completed a third test session (S3) in which memory for the first set of words was tested. This was followed by wiring for PSG, a training session on a second set of novel words, and an immediate test of this set. Participants slept overnight in the laboratory and were tested in the morning. The third test of the second word set took place 1 week later (mean = 7.4 d, range = 6–11 d).
Figure 1.
A, Timing of the sessions. Dense-SN/sparse-SN order was counterbalanced and Session 3 in Week 3 could occur earlier than 7:00 P.M. Test performance is shown in B–G. B, C, Percentage recalled in meaning recall (B) and free recall (C) tasks. D–G, Response times in the animacy decision (D), synonym judgment (E), reading aloud (F), and progressive demasking (G) tasks. Error bars indicate SEM. *p < 0.05; ns, not significant.
Materials.
Sixty-four novel semantic concepts were generated by taking an existing concept and adding a new semantic feature to it, making it a novel variant of an existing concept (e.g., “bee whose sting feels pleasant”). We manipulated semantic neighborhood density of the new concepts by making variants of existing concepts with either many semantic associates (dense-SN) or few associates (sparse-SN). The number of associates was derived from the Florida Free Association Norms (Nelson et al., 1998). These were compiled by asking >6000 participants to produce the best associate for each concept in a list. The number of different associates produced across the participants constitutes the number of associates for a given concept. We selected 32 concepts that had many associates (mean = 22.3, e.g., “chicken”) and 32 that had few associates (mean = 5.5, e.g., “saddle”), matched in frequency of occurrence and imageability.
Participants were trained on novel fictitious words, each associated with one of the novel concepts as its meaning. Sixty-four pronounceable nonwords (e.g., “feckton”) acted as the novel words. These were divided into two sets of 32 to be associated with the dense-SN and sparse-SN concepts at random. The sets were rotated through the neighborhood conditions.
Procedure.
Participants completed four training tasks. In the meaning matching task (six exposures to each word), a novel word was presented with a possible meaning (one of the novel concepts) and participants indicated whether the pairing was correct (having to guess at the first exposure). It was incorrect half of the time but the correct meaning was always displayed after a response. In the repetition task (two exposures), a novel word was played through headphones and displayed on screen and participants repeated it aloud. In the sentence-generation task (two exposures), a novel word and its meaning were displayed on screen and participants generated a sentence using the word. In the cued recall task (three exposures), a novel word was presented and participants had to recall and produce the meaning of the word. The correct meaning was presented after a response. Across all tasks each novel word was encountered 13 times. Participants were aware that memory would be tested later.
The test phase included six subtests. The first two evaluated explicit memory of the novel words and concepts to ensure that the number of stimuli encoded was equal across the neighborhood conditions. In free recall, participants had 3 min to recall as many novel words as possible. In meaning recall, the list of novel words was presented and participants were asked to recall the meaning of each word. The final four tasks measured speeded access to the novel words and concepts by measuring RTs. In the animacy decision task, a trained novel word appeared on the screen and participants indicated whether it referred to an animate or inanimate concept. In the synonym judgment task, a trained novel word appeared on the screen with three familiar words. One was semantically associated with the meaning of the novel word and participants indicated which one. In the reading aloud task, participants read aloud a trained novel word on the screen as soon as possible. In the progressive demasking task a trained novel word was presented interspersed with a masking stimulus (##########). Initially, the duration of the word was very short and the duration of the mask very long, but through progressively increasing the word duration and decreasing the mask duration, the word became identifiable. Participants indicated when they identified the word. Previous research into familiar word recognition has shown that all of these RT tasks (apart from synonym judgment, which has not been tested on this variable before) are influenced by semantic neighborhood size (Dunabeitia et al., 2008). The order of the tasks was counterbalanced.
EEG recording and analysis.
An Embla N7000 system recorded EEG (200 Hz sampling rate). Six scalp electrodes were positioned using the international 10–20 system (F3, F4, C3, C4, O1, O2) with contralateral mastoid references. Two electrooculographic channels monitored eye movements and two chin electromyographic channel monitored muscle tone. Thirty second epochs of sleep data were categorized into sleep stages (Rechtschaffen and Kales, 1968). Spindle analysis involved artifact-rejected, non-rapid-eye movement sleep (stage 2 and slow-wave sleep). Raw EEG data were band-pass filtered (11–15 Hz) using a linear finite impulse response filter. Automated detection (Ferrarelli et al., 2007) derived the number of discrete spindle events: for each channel, amplitude fluctuations in the filtered time series exceeding a predetermined threshold counted as spindles. Thresholds were calculated relative to the mean channel amplitude (eight times the average amplitude). This algorithm is similar to others and has been widely used.
Power spectral density in non-rapid-eye-movement sleep was analyzed with Welch's method using 4 s Hamming window length with 50% overlap. Inspected frequency bands included slow oscillation (0.5–1 Hz), δ (1–4 Hz), θ (4–7 Hz), α (8–12 Hz), σ (13–15 Hz), and β (17–25 Hz) bands.
Behavioral data were analyzed using ANOVA (Greenhouse-Geisser corrected where necessary) with semantic neighborhood size (dense vs sparse) and test session (S1, S2, S3) as within-subjects variables. In addition to the standard analysis, where data are averaged over stimuli for each participant (reported below), we averaged data over participants for each stimulus (i.e., newly learned concept). This is standard practice in research using language materials. Statistics that are reported significant below also reached significance in this second analysis (not reported). Data in percentages were arcsine transformed and RT data log transformed to better meet the assumption of normality (Fig. 1 shows data retransformed back to raw units).
Results
Behavioral measures
Figure 1 shows behavioral performance. All tasks showed a main effect of session (Table 1). Planned comparisons revealed that free recall and meaning recall showed significant decline from S2 to S3 (t(23) = 4.91, p < 0.001 and t(23) = 11.82, p < 0.001, respectively), but no difference between the neighborhood conditions. In the RT tasks of synonym judgment and reading aloud, performance speeded from S1 to S2 (t(23) = 3.46, p = 0.002 and t(23) = 4.52, p < 0.001, respectively), and in synonym judgment, it further speeded from S2 to S3 (t(23) = 2.64, p = 0.02).
Table 1.
Statistical analyses (ANOVA) of behavioral tasks
| Task | Main effect of session | Main effect of neighborhood | Session × neighborhood interaction |
|---|---|---|---|
| Free recall | F(2,46) = 17.08, p < 0.001 | NS | NS |
| Meaning recall | F(2,46) = 124.46, p < 0.001 | NS | NS |
| Animacy decision | F(2,46) = 47.76, p < 0.001 | NS | F(2,46) = 3.23, p = 0.05* |
| Synonym judgment | F(2,46) = 18.70, p < 0.001 | F(1,23) = 15.49, p = 0.001 | NS |
| Reading aloud | F(2,46) = 10.82, p < 0.001 | F(1,23) = 5.52, p = 0.03 | NS |
| Progressive demasking | F(2,46) = 101.59, p < 0.001 | NS | F(2,46) = 3.86, p = 0.03 |
NS, not significant.
*Greenhouse-Geisser corrected; p = 0.06.
A main effect of neighborhood was found in synonym judgment and reading aloud, reflecting faster RTs to sparse-SN words (Table 1). Animacy decision and progressive demasking showed a significant interaction between session and neighborhood (Table 1). In animacy decision, there was no difference between the neighborhood conditions in S1 and S2, whereas a significant difference emerged in S3 (t(23) = 2.45, p = 0.02). RTs became faster from S1 to S2 (t(23) = 3.88, p = 0.001) and from S2 to S3 (t(23) = 5.07, p < 0.001) in the sparse-SN items, but this change was observed only from S1 to S2 (t(23) = 5.75, p < 0.001) in the dense-SN items. Progressive demasking showed no significant difference between the neighborhood conditions in any session, so the interaction appears to reflect that the initial numerical dense-SN advantage in the first two sessions disappeared in S3. In the sparse-SN items, RTs became faster from S1 to S2 (t(23) = 8.64, p = 0.001) and from S2 to S3 (t(23) = 4.52, p < 0.001); dense-SN items showed the same trend (S1 to S2: t(23) = 9.98, p < 0.001, S2 to S3: t(23) = 2.80, p = 0.01). These data suggest that the neighborhood size manipulation had an impact on speed of access to novel word meanings: all RT tasks apart from progressive demasking showed faster semantic access to novel words in sparse-SN at one or multiple time points. However, in synonym judgment, participants made more errors on dense-SN words in S1 (p < 0.05), so greater difficulty in judging the semantic relatedness of dense-SN words may have contributed to the behavioral neighborhood effect in this task in this session.
Sleep stage analysis
PSG data from two males and two females were lost due to technical problems or low sleep efficiency (percentage of time in sleep during time in bed <65%) on one of the two nights, making comparison of the two nights unreliable. There were no effects of semantic neighborhood size on global sleep architecture (Table 2). We compared the duration of each sleep stage (percentage of sleep time) between the neighborhood learning sessions using paired-samples t tests. No sleep stage showed a significant difference. This result was unchanged using time in minutes. We next tested for associations between time spent in different sleep stages and overnight change in performance in the test tasks. No correlations reached significance when corrected for multiple comparisons.
Table 2.
Percentage of time spent in sleep stages (± SEM)
| Sleep stage | Sparse-SN | Dense-SN |
|---|---|---|
| Stage 1 | 6.10 ± 1.01% | 4.77 ± 0.55% |
| Stage 2 | 50.94 ± 2.04% | 52.00 ± 1.53% |
| SWS | 18.93 ± 1.65% | 20.17 ± 1.47% |
| REM | 24.02 ± 1.35% | 23.06 ± 0.90% |
| Sleep efficiency | 90.91 ± 1.56% | 91.59 ± 1.38% |
| Total sleep time | 466.30 ± 9.86 min | 470.80 ± 9.56 min |
Sleep spindle analysis
Three further male participants' datasets were lost from the spindle and spectral analyses due to excessive noise in the EEG on one or both nights. An ANOVA with neighborhood size and hemisphere as within-subjects factors was calculated on spindle density (mean number of spindles per 30 s, log-transformed data). Hemisphere was included because semantic neighborhood effects are found dominantly in left hemisphere cortical areas (Pexman et al., 2007) and spindle activity is maximally increased as a result of learning in spatially specific manner (Johnson et al., 2012), leading us to expect that neighborhood differences would be strongest in the left hemisphere. To determine whether the order in which the sparse and dense-SN stimuli were learned affected the results, we initially included order as a between-subjects factor. Because this showed no significant main effect or interaction with neighborhood, it was dropped. A main effect of neighborhood confirmed that learning sparse-SN words resulted in increased spindle density (F(1,16) = 14.31, p = 0.002). There was an interaction between hemisphere and neighborhood (F(1,16) = 4.68, p = 0.046): the effect was significant in the left-side electrodes (t(16) = 3.55, p = 0.003), but not on the right (Fig. 2A).
Figure 2.
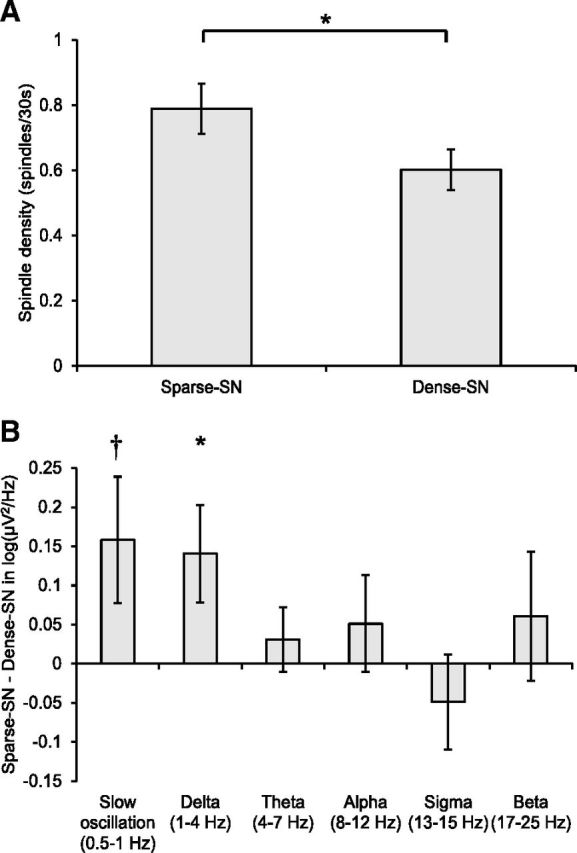
A, Spindle (11–15 Hz) density averaged across left hemisphere electrodes. B, Difference in spectral power between neighborhood conditions averaged across left hemisphere electrodes. Error bars indicate SEM. *p < 0.05; †p < 0.10.
Fast and slow spindles were analyzed separately using the same ANOVAs as above by counting spindles on data filtered to 11–13 Hz (slow) and 13–15 Hz (fast). Learning order was dropped. In slow spindles, the main effect of neighborhood reached significance (F(1,16) = 13.62, p = 0.002) and interacted marginally with hemisphere (F(1,16) = 4.30, p = 0.055). The neighborhood effect was significant in the left electrodes only (t(16)=-3.57, p = 0.003). In fast spindles, we found a main effect of neighborhood (F(1,16) = 10.60, p = 0.005) and an interaction with hemisphere (F(1,16) = 4.52, p = 0.049), reflecting a significant neighborhood effect in the left hemisphere only (t(16) = −3.35, p = 0.004).
Spectral analysis
Statistical analysis of spectral data were performed on log-transformed absolute power (μV2/Hz) using an ANOVA with neighborhood density and hemisphere as within-subject factors and learning order as a between-subject factor. Learning order showed no effects and was dropped. The slow oscillation band revealed a trend-level effect of neighborhood density (F(1,16) = 2.73, p = 0.12), indicating that sparse-SN words may have resulted in increased slow oscillation power. The main effect of hemisphere was significant (F(1,16) = 8.78, p = 0.009), showing higher power in the right-side electrodes. Although the interaction with hemisphere failed to reach significance, we analyzed hemispheres separately because there was a hemisphere-specific neighborhood effect in the spindle data. Consistent with the spindle result, we found a trend-level effect of neighborhood in the left-side electrodes (t(16) = −1.97, p = 0.067; Fig. 2B), but not on the right. The δ band showed no main effect of neighborhood (F(1,16) = 1.81, p = 0.20), but trend-level effects of hemisphere (F(1,16) = 3.63, p = 0.08) and a trend-level interaction between the two (F(1,16) = 3.03, p = 0.10). The neighborhood effect was significant in the left-side electrodes (t(16) = −2.27, p = 0.038; Fig. 2B), but not on the right. Other frequency bands showed no effects of neighborhood.
Discussion
Our behavioral data showed a strong influence of semantic neighborhood density. Three of the four RT tasks showed a dense-SN disadvantage, reflecting greater interference from the related neighbors. This suggests that these novel words were already interacting with previously stored information in semantic memory. In animacy decision, the effect emerged after a lengthier period, whereas in the other tasks, the effect was apparent by the end of training. Critically, neighborhood density influenced sleep architecture after learning: learning sparse-SN words led to higher sleep spindle activity. Spectral analysis revealed higher activity in the δ band and a trend for higher slow oscillation activity (both in the left hemisphere) after learning sparse-SN words. These effects did not stem from the amount or difficulty of learning: two explicit recall measures showed that participants encoded an equal number of words in both neighborhood conditions; therefore, these effects seem to reflect processes directly related to the influence of existing networks of semantic memory upon the processing of new semantic information.
The involvement of spindles and SWA in semantic learning is consistent with recent findings. Spindles are thought to trigger neural plasticity, as shown by spindle-like firing patterns generating short- and long-term potentiation in rat neocortical pyramidal cells (Rosanova and Ulrich, 2005). Spindles are also closely correlated temporally with hippocampal ripples that reflect hippocampal reactivation of new memories (Girardeau et al., 2009). Specifically, ripple troughs occur within milliseconds of spindle troughs (Sirota et al., 2003), potentially implicating spindles in a hippocampal-neocortical dialog. Furthermore, functional connectivity between the hippocampus and neocortex is increased during spindles (Andrade et al., 2011). Consistent with these findings, spindle activity is influenced by learning: learning word pairs induces more spindle activity compared with a nonlearning condition (Gais et al., 2002) and increasing difficulty of materials to be memorized leads to increased spindle activity (Schmidt et al., 2006).
SWA has also been implicated in consolidation. Learning a motor task increases SWA (0.5–4.5 Hz) over motor cortex (Huber et al., 2004), whereas prevention of motor encoding decreases SWA over the same area (Huber et al., 2006). Inducing SWA through 0.75 Hz electrical stimulation at frontolateral locations during sleep improves recall of word pairs learned before sleep (Marshall and Born, 2007). SWA may enhance cortical plasticity in two ways. First, SWA may be involved in downscaling synaptic potentiation induced by learning, thus returning cortical areas to a state of high plasticity (Tononi and Cirelli, 2006). Second, there is a temporal relationship between slow oscillations and spindles. Neocortically generated slow oscillations synchronize neural activity into up-states of neuronal activity and down-states of neuronal silence, with spindles increasing during the up-state. By exerting top-down control over spindle and ripple events, slow oscillations may allow hippocampally replayed information to reach the neocortex during the up-state, which is associated with increased long-term potentiation and therefore an optimal state for synaptic changes (Mölle et al., 2011). These phenomena may of course not only strengthen new memories in the neocortex, but also within the hippocampus.
Although these studies showed that learning triggers an increase in SWA and spindles, our data provide a significant advance by demonstrating for the first time that spindles and SWA are influenced by the properties of the semantic environment into which new memories are integrated. Spindles have been implicated in integrating new memories with existing memories in learning meaningless word forms (Tamminen et al., 2010), but the present study is the first to target semantic memory specifically.
When newly encoded information is consistent with preexisting knowledge (e.g., a schema), consolidation is accelerated (Tse et al., 2007) and coupling between the hippocampus and cortical areas is reduced (van Kesteren et al., 2012). This fits with insights from computational models of memory consolidation—a two-component (hippocampus and neocortical) consolidation system allows for both rapid encoding of transient episodes and prior-knowledge-dependent consolidation into preexisting knowledge (McClelland, 2013). If consolidation of information that is inconsistent with preexisting knowledge is too rapid, then preexisting knowledge is damaged. In contrast, consolidation of consistent information can be allowed to occur faster. The elements of sleep architecture identified in our study might reflect a neural marker for the appropriate consolidation rate for each new item. Integration into a sparse neighborhood can proceed more rapidly because it is less likely that the neighborhood includes inconsistent knowledge (hence greater spindle and SWA). In contrast, a densely packed representational structure is more likely to include inconsistent knowledge and requires slower consolidation (lower spindle and SWA).
The neighborhood effects observed in our behavioral data do not necessarily indicate that newly learned words are neocortically represented. They could also be accommodated by bidirectional connections between neocortical semantic memory and new hippocampal representations. This possibility is supported by the discovery of bidirectional couplings between hippocampus and neocortex during deeper stages of sleep (Wagner et al., 2010). Furthermore, the hippocampus is involved in representing memories that can be several years old (Squire et al., 1976), suggesting that consolidation may occur over long periods and that our data catch the first moments of the process when the role of the neocortex may still be limited. Nonetheless, our data show that this process begins through neural mechanisms involved in priming cortical plasticity and potentially facilitating hippocampal-neocortical interactions during the first night after learning. How long these mechanisms need to operate on new semantic memories before they become fully independent of the hippocampus remains unknown.
Linking newly acquired information with existing knowledge is a crucial process because it allows new information to be used to predict future events and to interpret the current environment. This is vital for semantic memory, which is acquired over a lifetime and is capable of generating generalizations from our broad, multimodal array of experience to generate appropriate behavior (Lambon Ralph et al., 2010). We used the diverse landscape of semantic memory to show that the varying amounts of information stored in different areas of semantic space have a clear influence on learning and memory. We suggest that newly learned information becomes linked to existing semantic memory very rapidly and that this process persists during subsequent sleep. Although the mechanisms mediating this process during wake remain unknown, our data suggest that during sleep spindles and SWA facilitate linking new with old semantic knowledge.
Notes
Supplemental material for this article is available at http://www.psy3.man.ac.uk/projects/penny/tamminen_supplement.pdf. This material has not been peer reviewed. The supplement includes a list of the stimuli, analysis of training data, analysis of error data in the test tasks, analysis of behavioral data calculated over stimuli, and correlations between test performance and PSG measures.
Footnotes
This work was supported by the Economic and Social Research Council (Postdoctoral Fellowship Grant #PTA-026-27-2540 and Grant #RES-062-23-2268 to J.T.) and by the Biotechnology and Biological Sciences Research Council (New Investigator Award #BB/F003048/1 to P.A.L.). We thank Fabio Ferrarelli and Giulio Tononi for the spindle detection algorithm and Nora Hennies for assistance in performing the study.
References
- Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Sämann PG, Czisch M. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011;31:10331–10339. doi: 10.1523/JNEUROSCI.5660-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Duñabeitia JA, Avilés A, Carreiras M. NoA's ark: influence of the number of associates in visual word recognition. Psychon Bull Rev. 2008;15:1072–1077. doi: 10.3758/PBR.15.6.1072. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/appi.ajp.164.3.483. [DOI] [PubMed] [Google Scholar]
- Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci U S A. 2012;109:18583–18588. doi: 10.1073/pnas.1207532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci U S A. 2010;107:2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- McClelland JL. Incorporating rapid neocortical learning of new schema-consistent information into complementary learning systems theory. J Exp Psychol Gen. 2013 doi: 10.1037/a0033812. doi: 10.1037/a0033812. Advance online publication. Retrieved August 27, 2013. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–1421. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Zhang N. The ties that bind what is known to the recall of what is new. Psychon Bull Rev. 2000;7:604–617. doi: 10.3758/BF03212998. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998. http://w3.usf.edu/FreeAssociation/ [DOI] [PubMed]
- Pexman PM, Hargreaves IS, Edwards JD, Henry LC, Goodyear BG. The neural consequences of semantic richness: when more comes to mind, less activation is observed. Psychol Science. 2007;18:401–406. doi: 10.1111/j.1467-9280.2007.01913.x. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stage scoring of human subjects. Bethesda, MD: U.S. Department of Health, Education, and Welfare; 1968. [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25:9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Münch M, de Quervain DJ, Wirz-Justice A, Cajochen C. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–8982. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/S0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LB, Chace PM, Slater PC. Retrograde amnesia following electroconvulsive therapy. Nature. 1976;260:775–777. doi: 10.1038/260775a0. [DOI] [PubMed] [Google Scholar]
- Storkel HL, Adlof SM. The effect of semantic set size on word learning by preschool children. J Speech Lang Hear Res. 2009;52:306–320. doi: 10.1044/1092-4388(2009/07-0175). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J Neurosci. 2010;30:14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- van Kesteren MTR, Ruiter DJ, Fernández G, Henson RN. How schema and novelty augment memory formation. Trends Neurosci. 2012;35:211–219. doi: 10.1016/j.tins.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Wagner T, Axmacher N, Lehnertz K, Elger CE, Fell J. Sleep-dependent directional coupling between human neocortex and hippocampus. Cortex. 2010;46:256–263. doi: 10.1016/j.cortex.2009.05.012. [DOI] [PubMed] [Google Scholar]