Abstract
Objective
Understanding how best to measure renal function in HIV-infected patients is critical because estimated glomerular filtration rate (eGFR) in HIV-infected patients can be affected by ethnicity and body composition. We validated the available eGFR equations and compared them to the plasma 99mTc-diethylenetriaminepentaacetic acid (99mTc-DTPA) clearance in HIV-infected patients.
Design
Test of diagnostic accuracy.
Methods
196 HIV-infected patients underwent measuring 99mTc-DTPA plasma clearance, 5 creatinine-based eGFR equations, cystatin-C GFR and 24-hour urine creatinine clearance (CrCl).
Results
Mean (SD) 99mTc-DTPA GFR was 117.7±29.2 mL/min per 1.73 m2. The re-expressed MDRD, CKD-EPI, re-expressed MDRD formula with Thai racial correction factor, Thai eGFR equation, Cockcroft & Gault, cystatin-C GFR, and 24 hr urine CrCl, underestimated the reference GFR. The bias estimated by the mean of differences the limits of agreement for the re-expressed MDRD equation, CKD-EPI, re-expressed MDRD formula with Thai racial correction factor, Thai eGFR, Cockcroft & Gault, cystatin C, and 24 hr urine CrCl can be expressed as 18.9±27.3, 11.1±25.5, 6.2±28.8, 13.5±27.0, 30.4±28.0, 3.2±36.1, and 5.0±12.1 mL/min per 1.73 m2 respectively.
Conclusion
The available eGFR equations underestimated GFR in HIV-infected adults. However, the eGFR by cystacin C GFR was the most precise and accurate. Among Cr-based eGFR equations, re-expressed MDRD formula with Thai racial correction factor was the most precise and accurate. The racial factor for each ethnicity is important and the existing eGFR equation should be validated before using it in the HIV population.
Keywords: 99mTc-DTPA GFR, eGFR equation, glomerular filtration rate, HIV-infected patients, race
Introduction
Antiretroviral therapy (ART) has significantly reduced mortality and progression to AIDS. Instead, complications of long-standing HIV infection and treatment, including renal disease, have become increasingly important. Aging, concomitant metabolic diseases, and use of potentially nephrotoxic ART can lead to higher risks for developing renal diseases in HIV-infected people; therefore, it is critical that physicians have the best tool to measure renal function in HIV-infected patients. However, there is no clear guidance which tool is the most appropriate for measuring the renal function in patients with HIV.
Physicians who treat HIV-infected patients are concerned whether the calculated eGFRs derived from non-HIV population are precise and accurate in assessing HI-associated chronic kidney disease (CKD) because the body compositions of HIV and non-HIV patients vary. None of the methods used to date have been well validated in HIV-infected Asian patients. Since all of the GFR measuring tools were validated in HIV-negative caucasians and blacks (African Americans) [1–4], therefore its use in other ethnic groups casts doubt to its appropriateness. Even though 99mTc DTPA plasma clearance is highly accurate and is the gold standard for GFR assessment, it is impractical to scale up in resource-limited settings. The serum creatinine is the simplest method but its inability to detect early decline of the renal function is its major pitfall [5]. Serum cystatin C is more expensive than serum creatinine and the effect of HIV replication may limit its use in this population [6]. The calculated methods for GFR may be the best tool for use in resource-limited settings because it does not require the use of sophisticated equipments nor has any other additional expenses aside from serum creatinine tests. Each of the equations has its own pitfall but the most important issue is that it has not been well validated in HIV-infected patients, especially Asians. The Cockcroft-Gault (CG) method [7] is the most commonly used because it is easy to calculate. Lately, there is increasing evidence suggesting that the Modification of Diet in Renal Disease (MDRD) [1,3] and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [4] may be more accurate in assessing GFR in caucasians and African Americans yet in Asians, the MDRD tends to overestimate the prevalence of renal disease [8]. As a result of this, Praditpornsilpa K et al. [9] proposed a racial factor for Thais that appears to give a more accurate GFR measurement, at least in HIV-negative patients.
Aside from that, this adjusted eGFR measurement can be used to monitor the deterioration rate of the renal function so physicians can change the dose of ART to prevent CKD/ESRD. Therefore, this study validated all of the available methods used to assess renal function (reexpressed MDRD formula, CKD-EPI equation, reexpressed MDRD formula with Thai racial factor correction, Thai eGFR equation, Cockcroft & Gault, and cystacin C GFR) in ARV drug experienced HIV-infected patients and compared the results to the gold standard of 99mTc DTPA plasma clearance.
Materials and methods
Patients
The study was approved by the Ethical Committee for Research, Chulalongkorn University, Bangkok, Thailand. All patients have provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki. Stable HIV-infected adults >18 years old, followed up by HIV-NAT (The Netherlands Australia and Thailand Collaboration in HIV Research), Bangkok, Thailand, were recruited into the study. The study was conducted in an ambulatory setting and began at 08:00–09:00 AM to avoid the diurnal variations in the renal function. Patients with acute deterioration of the renal function, amputation, malnutrition (BMI<18 kg/ m2), in a bed ridden state, with infection, in an edematous state, gastrointestinal bleeding, heart failure, or were hospitalized were excluded. Women of childbearing age without a reliable contraceptive method, patients on renal replacement therapy, patients taking methyldopa, levodopa, ascorbic acid, cimetidine, trimethoprim, antibiotics, steroids, or flucytosine were also excluded.
Clinical data and body composition assessment
Body composition was assessed by bioimpedance analysis (BIA) using Body Composition Analyzer (In Body S20, Biospace, Korea). Skeletal muscle mass, body fat mass, and total body water were analyzed. Body weight (BW), height, blood pressure were recorded.
Reference GFR measurement
The reference GFR was determined by plasma collected at 10 different time points by using the 99mTc-DTPA plasma clearance method which was performed at the Department of Radiology, Chulalongkorn University. 99mTc-DTPA was purchased from the Office of Atoms for Peace, Bangkok, Thailand, with a radiopurity of >95 % and 99mTc-DTPA bound to plasma protein of <5 %. The same protocol was applied to all patients. In brief, heparin lock was inserted in the arm to obtain blood samples to determine the radioactivity background and for the serum creatinine assay. A single intravenous bolus of 99mTc-DTPA was injected into each patient. Blood specimens were drawn to assess plasma radioactivity at 5, 10, 20, 30, 60, 90, 120, 180, and 240 minutes post 99mTc-DTPA injection. Plasma radioactive activities were then plotted as a function of time to create a time-activity curve to calculate for GFR. The GFR equation was determined by using bi-exponential fitting method [10]:
D is the dosage of injected 99mTc-DTPA. The result was normalized by the body surface area which was calculated according to Dubois and Dubois [11]. Reference GFR by 99mTc-DTPA plasma clearance were read by a radiologist who was blinded to the clinical status and laboratory results of the patients.
Calibration for the serum creatinine assay
Fasting serum creatinine was measured by using a Roche Diagnostics (Indianapolis, IN, US) CREA plus (11775642) enzymatic assay (CrEnz), on a COBAS, INTRGRA 400 plus analyzer. The measured CrEnz values were adjusted by using traceable high-level IDMS reference serum creatinine, as recommended by the National Kidney Disease Education Program. The IDMS reference serum creatinine (SRM 967) was purchased from the National Institute of Standards and Technology. The certified concentration values for serum creatinine were 0.847 ± 0.018 mg/dL for level 1 and 3.877±0.082 mg/dL for level 2. The coefficient of variation for the serum creatinine assay was 1.21%.
Cystatin C
Serum for cystatin C levels was collected at the time of serum creatinine collection. Cystatin C was measured by a particle Enhanced Turbidimetric ImmunoAssay (PETIA) (ARCHITECT c Systems and AEROSET Cystatin C Reagent, Abbott Diagnostics). The coefficient of variation for the serum cystatin C assay was 1.17%.
eGFR calculation for the Thai HIV population
The eGFR values were calculated by using the reexpressed MDRD equation, CKD-EPI equation, reexpressed MDRD equation with Thai racial factor correction, Thai eGFR equation, Cockcroft & Gault formula, and cystatin C GFR [12] (Table 1).
Table 1.
eGFR equations.
| eGFR Methods | Gender | Serum Cr | Equations |
|---|---|---|---|
| Re-expressed MDRD equation[3] | - | CrEnz | 175 × (CrEnz)−1.154 × (Age)−0.203 (× 0.742 if female) |
| CKD-EPI equation[4] | Female | CrEnz ≤ 0.7 mg/dL | 144 × (CrEnz/0.7)−0.329 × (0.993)Age |
| Female | CrEnz > 0.7 mg/dL | 144 × (CrEnz/0.7)−1.209 × (0.993)Age | |
| Male: | CrEnz ≤ 0.9 mg/dL | 141 × (CrEnz/0.9)−0.411 × (0.993)Age | |
| Male: | CrEnz > 0.9 mg/dL | 141 × (CrEnz/0.9)−1.209 × (0.993)Age | |
| Re-expressed MDRD equation with Thai racial factor[9] | - | CrEnz | 175×CrEnz(−1.154)×Age(−0.203)×0.742 (if female) ×1.129) if Thai |
| Thai eGFR equation[9] | - | CrEnz | 375.5× (CrEnz)−0.848)×(Age)−0.364 ×0.712 (if female) |
| Cockcroft & Gault equation[7] | - | CrEnz | [(140-age)×BW/ CrEnz ×72]/BSA×0.85 if female |
| Cystatin C GFR [12] | - | - | 86.7/cystatin C −4.2 |
Urine 24-hour collection
Urine was collected over a 24-hr period which included the morning when 99mTc DTPA plasma clearance GFR was measured. Verbal and written instructions on appropriate collection technique were provided to the patients beforehand. Container for urine collection was provided to each patient. Urine collection was performed at home 1 day prior the day of radioisotope GFR. Creatinine clearance (CrCl) was calculated by using this equation: CrCl=(Urine creatinine/Serum creatinine) × (urine volume / time of the actual collection). CrCl estimations were adjusted for BSA.
Statistical analysis
Bland-Altman plots were used to assess the agreement between eGFR and reference GFR [13]. The regression of the average and the difference between the reference GFR and eGFR (reference GFR minus eGFR) were analyzed. Statistical analysis was performed by using MedCalc Software version 10 (Mariakerke, Belgium) and SAS/STAT software version 9.3 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the patients
A total of 208 HIV-infected cases were studied; 12 cases were excluded because of the leakage of radioisotope during the plasma isotope clearance study. 196 cases [10 anti-retrovirus (ARV) naıive and 186 well suppressed HIV; 43% were female] were included in this analysis (Table 2). The average exposure to ARV was 8.6±3.5 years. The mean (SD) viral load was 2,647.9±18,590.2 copies/ml, and only 10 subjects (ARV naive) had viral load > 50 copies/mL. The mean (SD) CD4 count was 610.3±241.5 cells/µL; none of the patients had CD4 < 200 cells/µL. The averages of the body mass index (BMI) and body surface area (BSA) were 22.3±3.2 kg/m2 and 1.63±0.18m2, respectively. Only 15% of the patients had a BMI in the overweight (>25 kg/m2) range. Fifty-five percent, 44% and 59% of them were classified as having low skeletal muscle mass, high body fat mass and high body fat percent, respectively. The mean CrEnz was 0.91±0.29 mg/dL (95% confidential interval (95% CI) of 0.86 to 0.95 mg/dL). The mean reference GFR (99mTcDTPA) was 117.7±29.2 mL/min/1.73m2 (95% CI of 113.6 to 121.8 mL/min /1.73 m2). One hundred and sixty-seven patients (85%) had an isotope GFR of > 90 mL/min/1.732 and only 2% had low isotope GFR of < 60 mL/min/1.73m2. Diabetes mellitus and hypertension were found in a minority of the patients (7% and 15%, respectively). None of them were on ganciclovir, adefovir, and cidofovir 6 months prior to this study.
Table 2.
Characteristics of patients enrolled in the study.
| Mean ± SD | Median | |
|---|---|---|
| • Age (years) | 43.6±7.8 | 43.2 |
| • Body weight (kg) | 59.0 ±10.9 | 56.6 |
| • Height (meter) | 1.63 ±0.82 | 1.62 |
| • ARV vintage (years) | 8.6 ±3.5 | 8.7 |
| • HIV Viral load (copies/ml) | 2,647.9 ± 18,590.2 | 50.0 |
| • CD4 count (cells/uL) | 610.3 ± 241.5 | 585.5 |
| • CD4 (%) | 28.0 ±7.8 | 24.0 |
| • BMI (kg/m2) | 22.3 ±3.2 | 21.9 |
| • BSA (m2) | 1.63 ±0.18 | 1.60 |
| • Skeletal muscle mass (kg) | 24.6 ±5.6 | 23.9 |
| • Body fat mass (kg) | 13.7 ±6.5 | 13.1 |
| • Fat free mass (kg) | 45.0 ±9.2 | 44.2 |
| • Soft lean mass (kg) | 42.5 ±8.7 | 41.5 |
| • MAP (mmHg) | 91.0 ±11.5 | 90 |
| • BUN (mg/dL) | 12.41 ± 4.96 | 12.00 |
| • Serum creatinine (mg/dL) | 0.91 ±0.29 | 0.90 |
| • Urine protein (mg/day) | 439.6±1095.7 | 220.0 |
| • Total cholesterol (mg/dL) | 204.1 ±45.9 | 196 |
| • Serum triglyceride (mg/dL) | 152.0±96 | 119 |
| • HDL (mg/dL) | 50.9 ±16.6 | 49 |
| • Plasma glucose (mg/dL) | 92.1 ±25.1 | 87.0 |
| • rum phosphate (mg/dL) | 3.52 ±0.53 | 3.50 |
Assessing the agreement between eGFR values from different equations and reference GFR
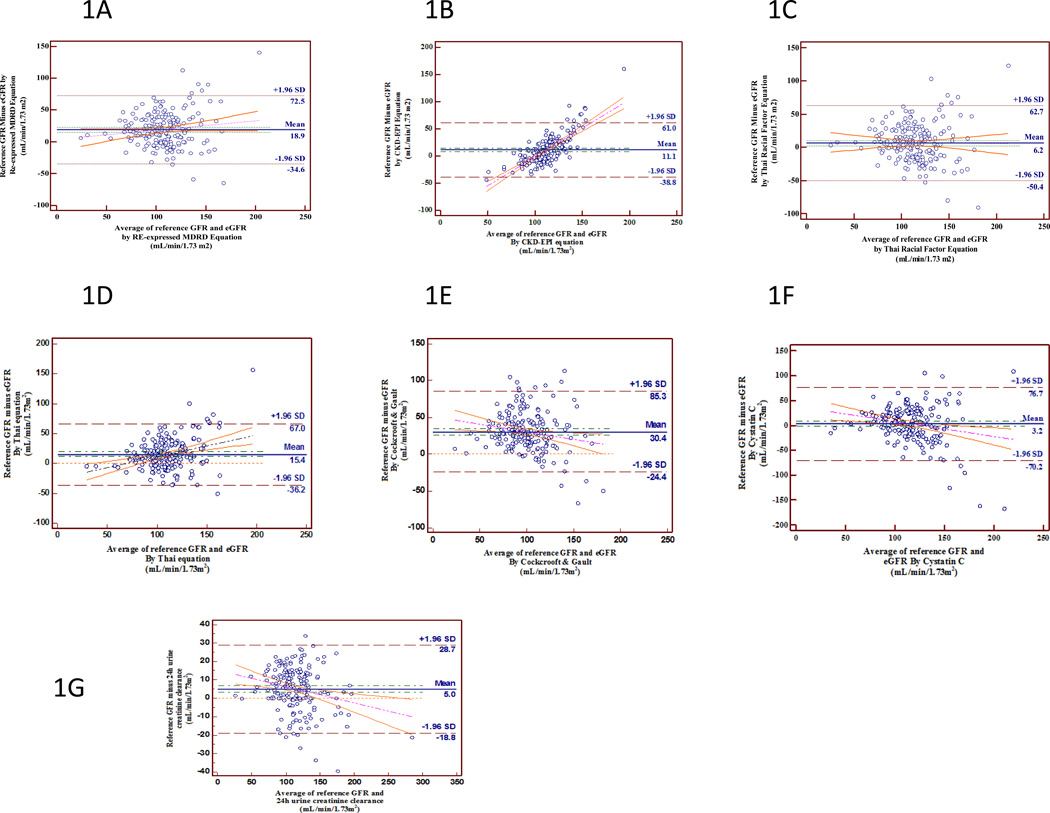
The bias estimated by the mean of differences W the limits of agreement for the reexpressed IDMS traceable MDRD equation was 18.9±27.3, 11.1±25.5 for CKD-EPI, 6.2±28.8 for re-expressed MDRD formula with Thai racial correction factor, 15.4±27.0 for Thai eGFR, 30.4±28.0 for Cockcroft & Gault, 3.2±36.1 for cystacin CGFR and 5.0±12.1mL/min per 1.73 m2 for CrCL by 24 hr urine(Fig. 1). The slope of the bias regression line between the reference GFR and the eGFR by re-expressed IDMS traceable MDRD equation was 0.15, 1.06 for CKD-EPI, 0.01 for re-expressed MDRD formula with Thai racial correction factor, 0.29 for Thai eGFR, 0.36 for Cockcroft & Gault, 0.17 for cystatin C GFR, and 0.09 for CrCL by 24 hr urine (Table 3). Reexpressed MDRD equation with Thai racial correction factor had the least slope (0.01) which can be interpreted that at each GFR, the bias was the most evenly distributed and was almost constant at 6.2 mL/min/1.73m2 (Fig. 1c).
Figure 1.
Bland-Altman plots of eGFR values calculated by different equations and the reference GFR which was used to show the disagreement between the equation and the reference. The mean bias and ± 1.96 SD are represented by the horizontal lines. The disagreement of different equations [A; re-expressed MDRD equation, B; CKD-EPI equation, C; re-expressed MDRD equation with Thai racial factor correction, D; Thai eGFR equation, E; Cockcroft & Gault, F; cystacin C GFR, and G; CrCL by 24 hr urine] are shown by the regression trend of the difference and the mean bias of the eGFRs towards the reference GFR.
Table 3.
The means of reference GFR and eGFR calculated by the different eGFR equations, the bias between mean and reference GFR and the range of the bias are shown
| Mean GFR± SD (mL/min/1.73m2) |
Mean bias± SD (mL/min/1.73m2) |
Lower limit (mL/min/1.73m2) |
Upper limit (mL/min/1.73m2) |
Slope of bias line by Bland-Altman plot |
|
|---|---|---|---|---|---|
| Reference GFR | 117.7± 29.2 | NA | NA | NA | NA |
| eGFR by reexpressed MDRD | 98.7± 26.1 | 18.9±27.3 | −34.8 | 72.5 | 0.15 |
| eGFR by CKD-EPI equation | 106.6± 11.9 | 11.1±25.5 | −38.8 | 61.0 | 1.06 |
| eGFR by Thai racial factor | 111.5 ± 29.5 | 6.2±28.8 | −50.4 | 62.7 | 0.01 |
| eGFR by Thai equation | 104.1 ± 23.4 | 13.5±27.0 | −39.4 | 66.5 | 0.29 |
| Cockcroft & Gault | 86.4 ± 29.2 | 30.4±28.0 | −24.4 | 85.3 | 0.36 |
| eGFR by Cystatin C | 114.0 ±36.1 | 3.6±36.1 | −70.2 | 76.7 | 0.17 |
| CrCl by24 hr urine | 112.3 ± 31.4 | 5.0±12.1 | −18.8 | 28.7 | 0.09 |
The correlation of each equation varied by 0.81 for the re-expressed MDRD equation, 0.85 for the CKD-EPI equation, 0.92 for the re-expressed MDRD equation with Thai racial correction factor, 0.86 for the Thai eGFR equation, 0.73 for the Cockcroft &Gault, 0.89 for the cystatin C GFR, and 0.96 for the CrCl by the 24-hr urine.
The number of eGFR estimates that fell within 30% of measured GFR was 74% by re-expressed MDRD equation, 80% by CKD-EPI equation, 84% by reexpressed MDRD equation with Thai racial correction factor, 84% by Thai eGFR equation, 53% by Cockcroft &Gault, 84% by cystatin C GFR, and 100% by CrCl by the 24-hr urine.
Discussion
HIV infection remains incurable and indefinite ART is often limited by drug toxicity. Renal toxicity is a major cause for morbidity and mortality in HIV-infected patients. HIV infection is also a risk factor for CKD. Since kidney disease tends to be silent during the initial stages, therefore an accurate and reliable tool for measuring GFR in HIV-infected patients is urgently needed globally to properly monitor and manage HIV and ART-related renal diseases. A recent study showed high prevalence of CKD in HIV population [14]. The exposure to ARV is a unique risk factor for HIV population. Certain ARVs have been shown to be nephrotoxic and can cause renal stone disease as well as chronic tubulointerstitial disease. The expansion of HIV population and the success of HIV treatment can extend the lives of the patients that over time, some of them may develop CKD and progress to ESRD which will ultimately impact all health care services. It is important to find a reliable tool to calculate/measure GFR so physicians can detect patients at risk for developing CKD. In addition, if this tool can accurately monitor the deterioration rate of the renal function so that doses of ART can be reduced, this will significantly help prevent the disease from occurring.
The re-expressed MDRD eGFR equation has been developed primarily for Caucasians and African-Americans with CKD [1–4]. Recent studies have shown that the calculation of eGFR derived from a race without prior validation will result in inaccurate estimations of GFR unless a racial factor is added to the equation to provide a more precise estimation [5–7]. Even though various eGFR equations have been studied in different races, the validation data have not been well studied in a large HIV population, especially in Asians. Our study has a large sample size (N=196) and is one of the first of its kind to compare various equations of estimated GFR against the radioisotope plasma clearance GFR in HIV-infected patients from Asia. The majority (95%) of the patients from the study’s cohort are on ART and their HIV RNA are well suppressed (VL<50 copies/mL). Some of the patients are overweight but many have abnormal body compositions due to ART-related lipodystrophy, resulting in low skeletal mass and high body fat mass.
We demonstrated that the expressed MDRD, CKD-EPI, re-expressed MDRD formula with Thai racial correction factor, Thai eGFR equation, cystatin C GFR, and 24-hr urine CrCl underestimated the reference GFR. The application of re-expressed MDRD and CKD-EPI equations derived from non HIV-CKD population had a bias of 18.9 mL/min/1.73m2 and 11.1 mL/min/ 1.73m2 respectively. The spread of the bias between the reference GFR and the eGFR by CKD-EPI was not evenly distributed (Fig. 1b). When GFR was less than 110 mL/min/1.73m2 or more than 110 mL/min/ 1.73m2, the eGFR from the CKD-EPI overestimated or underestimated the reference GFR, respectively. From all of the serum creatinine based eGFR equations, the reexpressed MDRD equation with Thai racial factor correction was the only equation that had the least bias of 6.2 mL/min/1.73m2 and an evenly distributed spread of bias. Therefore the re-expressed MDRD equation with Thai racial factor correction is more applicable to Thai HIV-CKD population. Our data agrees with Barraclough et al.’s [15] data which showed that the MDRD formula was the most precise method for Caucasians infected with HIV.
The racial factor for each ethnicity is important. Recently, our group did a study in 350 HIV-uninfected patients with various CKD stages [16]. We found that differences in ethnicity significantly affected the results of the MDRD-based eGFR equation and the racial factor for Thais was 1.129. When we used the adjusted MDRD equation with Thai racial factor on our HIV-infected population with an abnormal body composition but well-preserved kidney function compared to the uninfected population, GFR estimation was precise and accurate. This study showed that re-expressed MRDR equation with Thai racial factor can precisely and accurately be used in Thais with or without HIV infection.
The performance of the MDRD with Thai racial factor suggests that this equation is suitable for GFR estimation in our HIV-infected population. However, this formulation may not be applicable for all HIV-infected Asians because other studies conducted in Chinese [17,18] and Japanese [19–21] non-HIV-infected population have different racial factors of 1.23 and 0.88, respectively. This discrepancy within the Asian population makes it difficult to adopt a universal eGFR equation/racial corrected factor. It is unknown whether the body composition or the differences in determining the reference GFR method affected this disparity in eGFR equation and racial corrected factor for the MDRD-based GFR among Asians. The reference GFR from the Japanese study was obtained from using renal clearance of inulin whereas for the Chinese study, 99mTc DTPA was used. The techniques used in the Chinese study is similar to our group but we incorporated 10 time points within the 4 hours period instead of using only 2 time points as in the Chinese study. Furthermore, we performed all isotopic measurements at the same time during the day for all patients to avoid diurnal GFR variation.
Our data supports Bonjoch et al. [22] who reported that cystatin C had the least bias compared to serum creatinine based eGFR equations in estimating isotopic GFR when used in 15 HIV-infected patients. The drawback of using cystatin C is that it is not standardized even though its use as a biomarker for renal function is increasing. It has been shown that there are systematic shifts in cystatin C levels [23] and standardization is necessary before it can be systematically and routinely utilized in the clinical setting. Following cystatin C GFR, the second less biased technique is the use of CrCl by 24-hr urine collection. Unfortunately, 24-hr urine collection is the most impractical and difficult method to be used routinely in clinical practice; its precise collection of the urine has made this method unattractive.
The strength of this study is its large sample size as well as intensive measurements of isotopic GFR (10 time points). This data can also be applied to females as 43% of the patients in the study were females. Aside from that, the present study is representive for both HIV with and without lipodystrophy/abnormal body composition. The primary limitation of this study is that the results may not be generalizable to non-Thais and very few participants with impaired kidney function were included, therefore the comparison at lower levels of kidney function may be less reliable. In addition, most of the patients had high CD4 and undetectable VL or well-controlled HIV suppression so this data may not be applicable to patients with a more profound immunodeficiency or AIDS-related wasting.
In conclusion, we have proved that there is a need for the racial correction factor for the creatinine based eGFR equation for both non-HIV CKD and HIV population. Therefore, it is highly and strongly recommended that the existing creatinine based eGFR equations should be validated before using it in both non-HIV CKD and HIV population in epidemiologic studies and in the clinical setting.
Acknowledgements
This study was funded by the Thai Research Fund: Grant # RMU538004, Chulalongkorn University (Grant # H-19–79-53) and funding support provided through a grant to amfAR, The Foundation for AIDS Research, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), by the U.S. National Institutes of Health (NIH): National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD) and National Cancer Institute (NCI). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
We would like to thank Chantip Klaowmee, Thidarat Jumpimai, Napassanant Laopraynak and Pirapon June Ohata for their input and assistance on this manuscript.
Footnotes
Conflicts of interest
All authors declare that there are no conflict of interest.
Author contributions:
Conception and study design, obtain funding, and interpretation of the data were done by K.P. and A.A.
99mTc-DTPA GFR was done by T.C.
Acquisition of data was done by P.C., J.W., and S.U.,
Bioimpedance analysis was done by A.C.
Review of manuscript was done by Y.Y., K.R., K.T., S.E., and P.P.
References
- 1.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 6.Mauss S, Berger F, Kuschak D, Henke J, Hegener P, Wolf E, et al. Cystatin C as a marker of renal function is affected by HIV replication leading to an underestimation of kidney function in HIV patients. Antivir Ther. 2008;13:1091–1095. [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Cass A, Patel AA, Suriyawongpaisal P, Barzi F, Chadban S, et al. High prevalence of chronic kidney disease in Thailand. Kidney Int. 2008;73:473–479. doi: 10.1038/sj.ki.5002701. [DOI] [PubMed] [Google Scholar]
- 9.Praditpornsilpa KT, Chawatanarat T, Wathanavaha A, Pansin P, Tiranathanagul K, Katawatin P, et al. Differences in the Racial Factor for MDRD-Based Glomerular Filtration Rate Estimation in Different CKD Populations. World Congress of Nephrology 2009 Milan; May 22–26, 2009; Italy. [Google Scholar]
- 10.Sainsbury EJ, Ashley JJ. Curve-fitting in pharmacokinetics–a comparison between gamma- and biexponential fits. Eur J Clin Pharmacol. 1986;30:243–244. doi: 10.1007/BF00614312. [DOI] [PubMed] [Google Scholar]
- 11.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311. discussion 312–303. [PubMed] [Google Scholar]
- 12.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia. 2006;49:1686–1689. doi: 10.1007/s00125-006-0275-7. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 14.Menezes AM, Torelly J, Jr, Real L, Bay M, Poeta J, Sprinz E. Prevalence and risk factors associated to chronic kidney disease in HIV-infected patients on HAART and undetectable viral load in Brazil. PLoS One. 2011;6:e26042. doi: 10.1371/journal.pone.0026042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barraclough K, Er L, Ng F, Harris M, Montaner J, Levin A. A comparison of the predictive performance of different methods of kidney function estimation in a well-characterized HIV infected population. Nephron Clin Pract. 2009;111:c39–c48. doi: 10.1159/000178978. [DOI] [PubMed] [Google Scholar]
- 16.Praditpornsilpa K, Townamchai N, Chawatanarat T, Tiranathanagul K, Katawatin P, Susantitapong P, et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfq815. [DOI] [PubMed] [Google Scholar]
- 17.Ma YC, Zuo L, Zhang CL, Wang M, Wang RF, Wang HY. Comparison of 99mTc-DTPA renal dynamic imaging with modified MDRD equation for glomerular filtration rate estimation in Chinese patients in different stages of chronic kidney disease. Nephrol Dial Transplant. 2007;22:417–423. doi: 10.1093/ndt/gfl603. [DOI] [PubMed] [Google Scholar]
- 18.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 19.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, et al. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927–937. doi: 10.1053/j.ajkd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, et al. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41–50. doi: 10.1007/s10157-006-0453-4. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Bonjoch A, Bayes B, Riba J, Puig J, Estany C, Perez-Alvarez N, et al. Validation of estimated renal function measurements compared with the isotopic glomerular filtration rate in an HIV-infected cohort. Antiviral Res. 2010;88:347–354. doi: 10.1016/j.antiviral.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Maahs DM, Jalal D, McFann K, Rewers M, Snell-Bergeon JK. Systematic shifts in cystatin C between 2006 and 2010. Clin J Am Soc Nephrol. 2011;6:1952–1955. doi: 10.2215/CJN.11271210. [DOI] [PMC free article] [PubMed] [Google Scholar]