Abstract
Rationale
Early-life events can cause long-term neurobiological and behavioural changes with a resultant effect upon reward and addiction processes that enhance risk to develop alcohol use disorders. Maternal separation (MS) is used to study the mediating mechanisms of early-life influences in rodents. In MS studies, the pups are exposed to maternal absence during the first postnatal weeks. The outcome of MS experiments exhibits considerable variation and questions have been raised about the validity of MS models.
Objectives
This short review aims to provide information about experimental conditions that are important to consider when assessing the impact of early-life environment on voluntary ethanol consumption.
Results
The results from currently used MS protocols are not uniform. However, studies consistently show that longer separations of intact litters predispose for higher ethanol consumption and/or preference in adult male rats as compared to shorter periods of MS. Studies using individual pup MS paradigms, other controls, low ethanol concentrations, adult females or examining adolescent consumption reported no differences or inconsistent results.
Conclusions
There is no “a rodent MS model”, there are several models and they generate different results. The compiled literature shows that MS is a model of choice for analysis of early-life effects on voluntary ethanol consumption but there are examples of MS paradigms that are not suitable. These studies emphasize the importance to carefully designed MS experiments to supply the optimal conditions to definitely test the research hypothesis and to be particulate in the interpretation of the outcome.
Keywords: Neonatal handling, Early handling, Maternal deprivation, Early-life stress, Rearing condition, Intermittent access, Continuous access, Alcohol, Home-cage drinking
Introduction
There are pronounced differences in the liability to develop alcohol use disorders (AUD) and many factors interact to determine the individual vulnerability or resilience to AUD. Numerous studies have reported associations between genetic make-up and AUD and show that AUD clearly is a polygenetic disorder (Kalsi et al. 2009; Mayfield et al. 2008). AUD is also a multifactorial disorder and environmental influences, particularly early in life, have profound impact on the individual risk for AUD (De Bellis 2002; Langeland et al. 2004; Schwandt et al. 2013). Multiple environmental factors throughout the prenatal period, childhood and adolescence interact with genetic factors through epigenetic and transcriptional mechanisms and shape the brain (Andersen and Teicher 2009; Cirulli et al. 2003; Crews 2008; McCrory et al. 2011). Interference with these vital processes, for example by emotional and social stress or risk consumption of drugs, can cause long-term neurobiological and behavioural changes, affect alcohol-induced reward and addiction processes and thereby result in enhanced vulnerability for AUD (Crews 2008; de Kloet et al. 2005; Holmes et al. 2005; Sinha 2008). It is therefore of interest to further study how early-life factors can alter the sensitivity to challenges later in life such as stress and drug exposure and how they contribute to the individual vulnerability or resilience to AUD.
Although the relationship between early-life events and prevalence of AUD is undisputed, the mechanisms and mediators of the early environmental impact are poorly understood. In clinical research, studies of causal relationships between early environmental factors and later vulnerability/resilience to AUD are restricted. It is for example difficult to distinguish the relationships between the influence of innate factors, early-life adversity and early drug consumption in AUD patients. In preclinical research, attempts are made to find valid animal models in which the early environment is controlled so the causality between genetic factors, early-life events and challenges later in life can be examined. With experimental models, the mechanisms and mediators of early-life impact can be studied further. For example, studies on non-human primates have provided valuable information about the influence of early experiences on later alcohol consumption. An early study reported that rhesus monkeys that were reared without the presence of adults (peer-rearing) during the first months of life voluntarily consumed more ethanol in adulthood than mother-reared control animals (Higley et al. 1991). Later studies have provided evidence for specific genetic variants, such as genes encoding monoamine oxidase (MAO) A, the serotonin transporter (5HTT) and the mu-opioid receptor, and gene–environment interactions that underlie the increased ethanol preference in animals subjected to peer-rearing conditions (Barr 2013). Furthermore, rodent models are commonly used to examine how exposure to various early-life rearing conditions affects ethanol intake behaviour later in life (Moffett et al. 2007; Roman and Nylander 2005). Recent reviews present excellent summaries and discussions of the compiled previous data from studies investigating stress on drug abuse-related behaviours (Neisewander et al. 2012) and on ethanol intake, including early-life stress (Becker et al. 2011). The present review aims to provide more detailed information about rodent models used in studies of early-life impact on ethanol consumption, with emphasis on the rat due to the more comprehensive literature relative to the mouse. Moreover, the experimental conditions that are important to consider when assessing the effects of different early-life environmental conditions on ethanol intake are discussed. Focus is on maternal separation (MS) paradigms used to induce early-life beneficial or risk environmental conditions and on voluntary ethanol consumption paradigms used to assess the consequences. It is evident that the effects on ethanol consumption are dependent on the experimental conditions, and although several studies are concordant, there are studies that report no MS-induced effects on ethanol intake or preference. A systematic analysis of the short- and long-term effects of being separated from the mother is therefore needed to determine whether MS is a model of choice in assessment of the effects on voluntary ethanol consumption.
Maternal separation paradigms
Methodological aspects
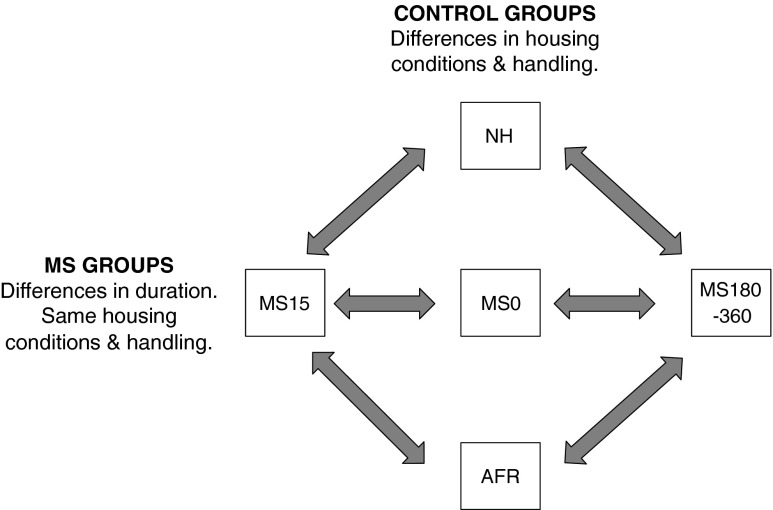
In rodent models, repeated separations of the pups from the mother (or care-giving female) during the first two to three postnatal weeks are frequently used to examine the consequences of different early-life experiences. These models are commonly denominated maternal separation (MS). However, MS is not one defined model but comprises a variety of experimental paradigms, see Table 1 and Fig. 1.
Table 1.
A summary of experimental conditions that commonly are used in maternal separation studies. Note that there are some inconsistencies in the literature regarding the nomenclature of the procedures (for details, see the text). In the table, examples of the most common denominations are listed
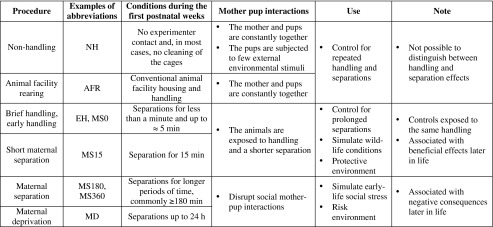
Fig. 1.
Common experimental groups in rodent maternal separation (MS) paradigms. Animals in the horizontal groups are subjected to the same handling by the experimenter and exposed to the same housing conditions. The duration of MS differs and, consequently, the effect of being separated from the dam different length of time can be assessed. The vertical groups represent control groups commonly used to examine the outcome of short or prolonged periods of MS. These groups differ in a number of aspects and care must be taken in choosing the proper control depending on the question asked. Modified from (Nylander and Roman 2012). NH, non-handling; AFR, animal facility rearing; MS0, brief handling of the pup with less than 1 min loss of contact with the dam; MS15 and MS180-360, maternal separation for 15 min or 180–360 min, common protocols utilize 180, 240 or 360 min separations
Early studies showed that handling, i.e. gently holding rat pups for 10 min every day for 3 weeks post-weaning, resulted in positive physiological and behavioural effects in adulthood (Weininger 1954). That early handling was related to long-term effects in the offspring when comparing with non-handled (NH) rats was further supported in studies where it was shown that separating mother and pups daily for only 3 min during the first 3 weeks of life reduced the physiological responses to stress (Levine 1957; Levine and Lewis 1959). Over the years, MS paradigms have been developed and used in a number of studies to investigate the long-term effects of early-life events on the offspring. These studies also include prolonged MS and they have not only provided valuable knowledge about neurobiological and behavioural consequences of early-life experiences but also about experimental factors that influence these effects (e.g. reviews by Kuhn and Schanberg 1998; Lehmann and Feldon 2000; Levine 2002; Macri and Würbel 2006; Nylander and Roman 2012; Pryce and Feldon 2003).
In MS studies, the duration of the separation is used to simulate different environmental settings. When the aim is to simulate a beneficial environment, i.e. conditions that relate to positive behavioural effects and protection against negative influences, the pups are subjected to shorter, that is minutes rather than hours, separations from the dam. For example, repeated short separations for 15 min (MS15) per day are used to simulate naturalistic conditions based on knowledge from the wildlife rearing conditions where the lactating dam leaves the nest regularly, often around 10 min and not longer than 1 h depending on the age of the offspring (Grota and Ader 1969). When the aim is to simulate a risk environment, i.e. adverse conditions relating to risk for negative consequences such as excessive ethanol intake or symptoms reminiscent of pathological conditions, longer periods of maternal absence are used. The newborn rat is dependent on the mother for a normal development and repeated separations for longer periods of time, commonly 180–360 min per day, are used to disrupt the early social mother–pup interactions that are vital for normal neuronal and behavioural development. Many studies support the notion that prolonged MS is regarded a risk environment that is associated with early-life stress and negative consequences later in life (Holmes et al. 2005; Ladd et al. 2000; Levine 2002; Pryce and Feldon 2003). Maternal deprivation (MD) is also seen in the literature but is, however, not used consistently; MD can refer to occasional 24 h MS but also be used interchangeably with MS to describe 180–360 min separations from the dam (Hall et al. 1999; Ogawa et al. 1994; Vazquez et al. 2005; Viveros et al. 2009). Furthermore, early deprivation has been used to describe separation of the pup from the dam and littermates (see below, individual MS) to distinguish from separation of the litter from the dam (Ruedi-Bettschen et al. 2005). In the various MS protocols, the separations are done repeatedly, either daily or occasionally.
The separation conditions differ between MS studies. During the separation from the dam, the rat pups can be either placed individually or kept together in litters, either in the maternity cage or in a separation cage. In the litter-wise paradigm, the pups experience loss of contact with the dam, i.e. they cannot see, communicate with, smell or feel her presence, whereas the contact with the littermates is undisturbed. In individual MS paradigms, the rat pups are individually placed during the separations. They are, for example, placed separately in small compartments in a cage so that they can communicate with and smell the other pups but they are deprived the tactile contact with the littermates. In all MS paradigms, the ambient temperature should be controlled in order to avoid hypothermia (Ruedi-Bettschen et al. 2004; Zimmerberg and Shartrand 1992) and it is of course of particular importance to keep control over temperature when the rat pups are isolated from both dam and littermates. Another factor that can differ is the use of cross-fostering procedures versus the use of biological littermates. Often, pups are cross-fostered on the day of birth and arranged in litters to contain an equal number of male and female pups. However, there is no standardized procedure for cross-fostering and how this is done varies between studies.
Another experimental design used in the literature to investigate the effects of disturbed social interactions is social isolation. The animals are single housed, usually for longer periods of time, and the effects of deprivation of social contacts at different ages can be assessed. Recent comprehensive reviews discuss the behavioural effects seen after isolation rearing immediately after weaning, including effects on ethanol intake (Becker et al. 2011; Neisewander et al. 2012). The compiled studies show that social stress post-weaning facilitates drug abuse-related behaviours and that animal models can be used to further study the consequences of social isolation during adolescence. However, in these social isolation studies, the isolation occurs after weaning and thus does not include disturbance of the early-life environment with the dam. Thus, these conditions are different from the MS conditions and the models should be considered to be different models since they study different aspects of social stress. Hall et al. discuss the different and the common effects, respectively, elicited by either isolation in weanling rats or MS and suggest a common role for mesolimbic dopamine in consequences seen after social stress, but still, they could observe differences in how dopamine function was affected by these two procedures (Hall et al. 1999). Taken together, since social behaviour and social interactions differ before and after weaning and since the social stress induced by the isolation occurs at different developmental time windows, it is important to distinguish between effects elicited by MS from those seen after social isolation later in life.
The choice of control groups in the experimental design of a MS study is of vital importance in the evaluation of results from a MS experiment. As mentioned above, short periods of MS can be used to examine the basis for environmental protective factors per se, but they can also be used as a control condition in the comparison to effects induced by the prolonged separations, i.e. the risk environment. Common experimental groups that are used as controls include MS15 and MS0 or brief (early) handling that usually refer to shorter separations for up to 5 min per day (Jaworski et al. 2005; Lehmann and Feldon 2000; Pryce and Feldon 2003; Roman and Nylander 2005). Comparisons between short/brief and prolonged separations enable analysis of the effects of the duration of maternal absence with all other experimental conditions, such as handling by the experimenter and general housing conditions, kept the same (Fig. 1). Other controls are listed in Table 1 and they include the non-handling (NH) paradigm and animal facility rearing (AFR). NH relates to an unnatural, understimulated environment that in itself may induce effects in the offspring (Macri and Würbel 2006; Pryce and Feldon 2003), which indicates that it is not a suitable control. AFR refers to conventional animal facility housing and conditions with experimenter contact only when the cages are changed. However, although AFR is similar between laboratories, there are still several possible confounding factors due to different laboratory environments and handling procedures (Chesler et al. 2002; Crabbe et al. 1999; Wahlsten et al. 2003).
Behavioural consequences of maternal separation
Environmental conditions during the first postnatal weeks are critical for developmental processes in rodents. MS during the first postnatal weeks can therefore disturb the social interactions, interfere with critical developmental processes and result in persistent changes in brain function and behaviour. Signs of altered development that can be noted during the MS period are, for example, altered ultrasonic vocalization trajectory after daily 360 min MS compared to MS15 (Ploj et al. 2003b) and delayed eye opening after daily 360 min MS compared to AFR and MS15 (Ploj et al. 2002). The long-term endocrine and behavioural consequences of disturbance or deprivation of early-life social contacts in rodent models have been excellently reviewed by other authors (Claessens et al. 2011; de Kloet et al. 2005; Faturi et al. 2010; Holmes et al. 2005; Ladd et al. 2000; Levine 2002; Neisewander et al. 2012). Here, the focus is MS-induced effects on ethanol consumption (see separate section) and we just give a brief outline of other relevant endpoints within the context of ethanol consumption and propensity for AUD.
The fact that early-life environmental factors can interfere with development of hypothalamus–pituitary–adrenal (HPA) axis function is of importance with regard to the use of MS as an experimental model to evaluate consequences of early-life impact on vulnerability to AUD and on addictive behaviour. There is a close interrelationship between stress and ethanol consumption (Becker et al. 2011; Clarke et al. 2008; Miczek et al. 2008; Pautassi et al. 2010; Prendergast and Little 2007) but the association is not completely understood and needs to be examined further. With regard to effects of MS on HPA axis function, many studies confirm that briefly handled rats displayed a low stress response when compared to non-handled rats (Ader and Grota 1969; Anisman et al. 1998; Meaney 2001; Pryce and Feldon 2003). Prolonged separations resulted in a hyperresponsive HPA axis as compared to early handling, but not non-handling (Ladd et al. 2000; Lehmann and Feldon 2000; Meaney 2001; Nemeroff 2004; Pryce and Feldon 2003), whereas other studies have shown a lower or blunted corticosterone response compared to animals subjected to AFR or short separations (Greisen et al. 2005; Kim et al. 2005; Ogawa et al. 1994; Roman et al. 2006). Thus, the results are not conclusive and the outcome seems to be highly dependent on experimental factors such as control group (see Macri and Würbel 2006; Pryce and Feldon 2003). A recent report addressed the question to what extent the HPA axis is activated by repeated MS (Daskalakis et al. 2011). The study showed that all rats displayed desensitization to the 8-h separation itself but, interestingly, “home-separated rats” (pups remained in their maternity cage during 8 h of MS) were more responsive to subsequent stress than “novelty-separated rats” (placed individually in a novel cage during MS) (Daskalakis et al. 2011). These results not only illustrate the complexity of the MS-induced effects on the HPA axis but also further strengthen the notion that MS modulates stress responses even though the basal activity is unaffected.
Besides disturbed HPA axis function after MS, there are a number of reports that provide evidence for MS-induced interference with brain transmitter function (Miczek et al. 2008; Moffett et al. 2007; Nylander and Roman 2012). Of relevance for ethanol consumption behaviour is for example effects on mesolimbic dopamine (Brake et al. 2004; Hall et al. 1999; Matthews et al. 2001; Matthews et al. 1996; Meaney et al. 2002; Oreland et al. 2011), endocannabinoids (Romano-Lopez et al. 2012), serotonin (Arborelius et al. 2004; Huot et al. 2001; Matthews et al. 2001; Oreland et al. 2009; Oreland et al. 2011; Vicentic et al. 2006), GABA (Giachino et al. 2007; Jaworski et al. 2005; Leussis et al. 2012; Martisova et al. 2012), glutamate (Martisova et al. 2012; Pickering et al. 2006) and endogenous opioids (Nylander and Roman 2012; Ploj et al. 2003b; Vazquez et al. 2005). These neurobiological changes as a consequence of early-life experiences can all contribute to altered drug responsiveness and vulnerability to AUD later in life.
Other behavioural effects that are observed after being subjected to longer periods of MS and that can relate to an AUD prone phenotype include altered balance between the appetitive and aversive motivational effects of ethanol in preweanling rats subjected to MS relative to AFR (Pautassi et al. 2012), alterations in risk-assessment and risk-taking behaviour in adult rats (Roman et al. 2006) and depression-like behaviour (Schmidt et al. 2011).
Ethanol consumption paradigms
Methodological aspects
A number of experimental paradigms can be used to assess effects on ethanol consumption in rodents (Becker 2013; Crabbe et al. 2011; Sanchis-Segura and Spanagel 2006). The animal can have access to ethanol in one or several bottles in the home cage and thereby be given a free choice to drink from the preferred bottle/s. A common protocol includes a two-bottle free choice between ethanol and water. In such voluntary drinking paradigms, the animals can have either continuous or intermittent access to ethanol. A drawback with continuous access to ethanol is that the delay between consumption and onset of effects often results in overall low voluntary ethanol consumption (Meisch and Lemaire 1993). Rats establish a higher voluntary ethanol intake when they are provided intermittent access to ethanol (Wayner and Greenberg 1972; Wise 1973). Recent studies using an intermittent access protocol with free choice (20 % ethanol and water on Mondays, Wednesdays and Fridays) reported higher intake and preference as compared to continuous access (Simms et al. 2008). However, in the continuous as well as the intermittent paradigms, ethanol is available for 24 h and during this time period the animals drink in sporadic bouts. Of relevance for the achieved blood ethanol concentration is the number of drinking bouts that occur in a unit of time and the amount of ethanol consumed on each occasion. Despite similar intake during 24 h, high volume and short duration bouts that occur frequently may result in different blood ethanol patterns when compared to lower volume, longer duration bouts occurring less frequently (Samson and Czachowski 2003). There are also limited access paradigms in which ethanol is available only a limited period over a 24-h period. In limited access paradigms, it has been stressed that the animals drink more when access to ethanol is given during their active phase, i.e. during the dark period, also known as “drinking in the dark” (Rhodes et al. 2005; Sprow and Thiele 2012). Other protocols use sweetened ethanol, for example by addition of sucrose, to increase voluntary intake but that requires extra controls for the rewarding effects of the sweet solution. In forced drinking paradigms, the animals have no water available and thus no choice but to drink ethanol. Forced paradigms are less suitable whenever the animal’s propensity to drink ethanol is of interest.
Most rats like to drink ethanol in low concentrations such as 2–6 % (Meisch and Lemaire 1993; Richter and Campbell 1940) possibly because low concentrations have a mild-sweet taste (Sanchis-Segura and Spanagel 2006). In continuous access paradigms, it is therefore common to use schedules with a gradually increasing ethanol concentration for example from 2 to 10 % ethanol over a couple of weeks. Voluntary drinking paradigms also include free choice protocols with more than one ethanol bottle in addition to water, for example, three- or four-bottle choice paradigms. With these protocols, it is possible to examine the individual preference for lower or higher concentrations of ethanol, for example in animals that have been exposed to different early-life environmental conditions.
Operant techniques can also be used for self-administration of ethanol. The animals learn to work for a reward and the self-administration of a drug and motivation to take the drug can be assessed. Operant techniques are used primarily for heroin and central stimulants and less for ethanol but can be used for oral delivery of ethanol, or intravenous or intragastric self-administration (see Crabbe et al. 2011; Sanchis-Segura and Spanagel 2006).
Effects of maternal separation on ethanol consumption
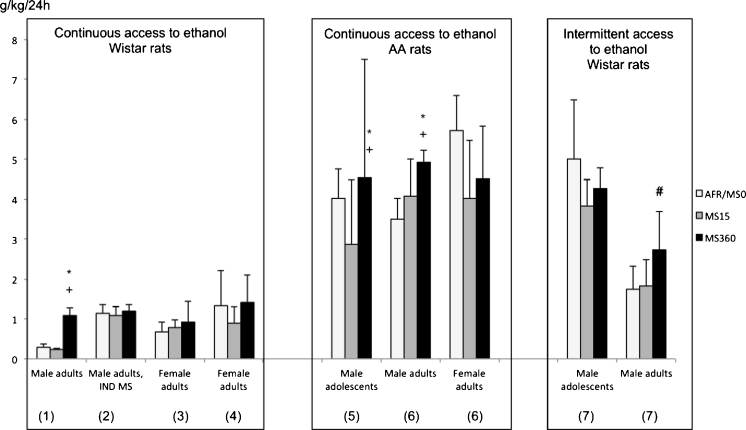
Several lines of evidence from rodent MS studies support the notion that early-life rearing conditions have long-term consequences for ethanol consumption (see reviews by Becker et al. 2011; Miczek et al. 2008; Moffett et al. 2007; Roman and Nylander 2005). As reported in many studies, repeated longer separations from the dam result in higher ethanol intake or preference in a two-bottle free choice between ethanol and water (see Table 2 for details). These studies use litter-wise MS, that is, a protocol where the pups are placed together during the separation from the dam, and they provide evidence for higher ethanol consumption after daily longer maternal separations, i.e. 60 min (Hilakivi-Clarke et al. 1991), 180 min (Huot et al. 2001; Jaworski et al. 2005) and 360 min (Ploj et al. 2003a). The results are similar in Wistar (Hilakivi-Clarke et al. 1991; Ploj et al. 2003a) and Long-Evans rats (Huot et al. 2001; Jaworski et al. 2005), and similar despite different duration of access to ethanol (Table 2). Higher consumption of ethanol after prolonged MS was also seen in those studies that were using sweetened (2.5 % sucrose) ethanol (Huot et al. 2001; Jaworski et al. 2005). Studies on mice also reported higher consumption of sweetened ethanol after daily MS180; the MS180 mice had higher intake of 10 %, but not 6 %, ethanol in a three-bottle choice for 2 × 5 days and, in addition, they achieved higher intake of 10 % ethanol in an operant self-administration paradigm as compared to AFR (Cruz et al. 2008). Finally, in a recent study, an intermittent ethanol intake paradigm was used to examine the ethanol consumption in adult MS15 and MS360 rats. The rats were given access to a free choice between 5 % ethanol, 20 % ethanol and water Mondays, Wednesdays and Fridays over a time period of 5 weeks. These results confirmed that MS360 is a risk environment as evidenced by an increase in total ethanol intake and preference over time in the MS360 rats but not in the MS15 rats (Daoura et al. 2011; Fig. 2).
Table 2.
A summary of maternal separation studies of voluntary ethanol consumption in rodents
| Experimental conditions | EtOH intake | References | ||||
|---|---|---|---|---|---|---|
| Animals | MS procedure | EtOH paradigm | MS <15 vs control | MS >60 vs control | ||
| Studies in support of prolonged MS as risk and short MS as protection | Long-Evans rats adult male | LW PND 2–14 | 8 % EtOH + sucrose 3 days | 15 = AFR | 180 > 15, AFR | Huot et al. (2001) |
| Wistar rats adult male | LW PND 1–21 | 2–8 % EtOH 4 weeks | 15 < AFR (2–8 %) 15 = AFR (8 %) | 360 > 15 (2–8 %) 360 > AFR (8 %) | Ploj et al. (2003a) | |
| Long-Evans rats adult male | LW PND 2–14 | 8 % EtOH + sucrose 5 days | 15 = AFR, MS0 15 < NH | 180 > 15 180 = AFR, MS0 180 < NH | Jaworski et al. (2005) | |
| Wistar rats adult male | LW PND 1–21 | 5 + 10 + 20 % EtOH 8 weeks | 15 = AFR (tot intake) 15 < AFR (20 %) | 360 = 15 (tot intake) 360 > 15 (20 %) | Gustafsson and Nylander (2006) | |
| Wistar rats adult male | LW PND 1–21 | 5 + 20 % EtOH 5 weeks | 15 = MS0 | 360 = 15, MS0 (tot intake) 360 ↑ intake over time | Daoura et al. (2011) | |
| Wistar rats adult male | LW; IND PND 5–10; 11–20 | 5 % EtOH 12 days | 15 < AFR | 60 > 15 60 = AFR | Hilakivi-Clarke et al. (1991) | |
| AA rats late adolescent male | LW PND 1–21 | 2–10 % EtOH 3 weeks | 15 < AFR (8–10 %) | 360 > 15 (8–10 %) 360 = AFR | Roman et al. (2003) | |
| AA rats adult male | LW PND 1–21 | 2–10 % EtOH 6 weeks | 15 = AFR (8–10 %) | 360 > 15, AFR (8–10 %) | Roman et al. (2005) | |
| CFW mice young adult male | LW PND 1–14 | 6 + 10 % EtOH + saccharin 10 days | – | 360 = AFR (6 %) 360 > AFR (10 %) | Cruz et al. (2008) | |
| CFW mice young adult male | LW PND 1–14 | 6 or 10 % EtOH operant self-adm | – | 360 > AFR (6 %) 360 > AFR (10 %) | Cruz et al. (2008) | |
| Wistar rats adult male | IND PND 2–15 | Saccharin fading; forced + voluntary 10 % EtOH | – | 2 × 180 > AFR (forced) 2 × 180 > AFR (voluntary) | Romano-Lopez et al. (2012) | |
| SD rats adult male and female | IND PND 1–14 | 2–9 % EtOH 7 weeks | 3 < AFR (5 % EtOH) | – | Weinberg (1987) | |
| Studies reporting no differences | Wistar rats adult male | LW PND 1–15 | 2–6 % EtOH 3 weeks | – | 240 = 5 | Marmendal et al. (2004) |
| Wistar rats adult female | LW PND 1–21 | 2–8 % EtOH 4 weeks | 15 = AFR | 360 = 15, AFR | Roman et al. (2004) | |
| Wistar rats adult female | LW PND 1–21 | 2–8 % EtOH 4 weeks | 15 = AFR | 360 = 15, AFR | Gustafsson et al. (2005) | |
| AA rats adult female | LW PND 1–21 | 2–10 % EtOH 7 weeks | 15 = AFR | 360 = 15, AFR | Roman et al. (2005) | |
| Wistar rats adult male | IND PND 1–21 | 2–8 % EtOH 7 weeks | 15 = AFR | 360 = 15, AFR | Oreland et al. (2011) | |
| Long-Evans rats adolescent male + female | IND PND 1–7 | 5 % EtOH; beer 9 weeks | 15 = NH 15 > NH (peripubertal males) | – | Lancaster (1998) | |
| Wistar rats adolescent male | LW PND 1–21 | 5 % + 20 % EtOH 5 weeks | 15 = MS0 | 360 = 15, MS0 | Daoura et al. (2011) | |
| C57BL/6J mice adult male + female | LW PND 2–14 | 5–20 % EtOH 9 weeks | – | 180 = 15 | Advani et al. (2007) | |
Maternal separation experiments included short (3–15 min) and prolonged (60–360 min) separation from the dam. All numbers represent the duration of the separation in minutes. <, >, and = indicate lower, higher or no difference in ethanol intake as compared to other groups
AA Alko Alcohol (selectively bred alcohol-preferring line), AFR animal facility rearing, CFW Swiss Webster, EtOH ethanol, IND individual MS, LW litter-wise MS, MS maternal separation, NH non-handling, PND postnatal day, SD Sprague–Dawley
Fig. 2.
A comparison of the voluntary ethanol consumption in maternal separation (MS) experiments. The results are examples from MS studies performed in the same lab, using the same Wistar supplier and consistently employing daily maternal separations (15 min, MS15, and 360 min, MS360) during the first three postnatal weeks. AFR was included in studies 1–6 and MS0 was used in study 7. Duration of drinking period, concentration of ethanol and ethanol intake paradigms differed between studies. The ethanol consumption was higher in male Wistar rats and Alko Alcohol (AA) rats that were exposed to MS360. The difference was observed both with continuous and intermittent access to ethanol. No differences were seen in adolescent Wistar rats, in female rats or in rats subjected to individual (IND) MS, i.e. separated both from the dam and the littermates. Data from 1 (Ploj et al. 2003a); 2 (Oreland et al. 2011); 3 (Roman et al. 2004); 4 (Gustafsson et al. 2005); 5 (Roman et al. 2003); 6 (Roman et al. 2005); 7 (Daoura et al. 2011). Asterisk, significantly different from MS15; plus sign, significantly different from AFR; number sign, significant increase over time
These above-mentioned studies clearly show that early-life stress, here induced by interference with vital social interactions between dam and offspring, is associated with higher ethanol preference in adulthood. Importantly, the higher intake was evident when comparing with shorter separations and not when compared to non-handled animals. It is also evident that short periods of MS can serve as a protective environment resulting in low ethanol intake in adulthood (see Table 2 for details). Lower ethanol intake is seen in rats subjected to 3–15 min MS compared to non-handled rats reared similar to what is herein referred to as AFR (Hilakivi-Clarke et al. 1991; Weinberg 1987). These studies indicate that the MS-induced effects are robust in several strains as long as the protocols are similar.
When comparing rats subjected to prolonged MS with AFR rats, the results are less consistent. Some studies report the same ethanol intake in AFR rats and rats subjected to 60 or 180 min separations whereas other studies show similar intake in AFR and MS15 rats (see Table 2). The handling of the rats in the AFR setting varies between labs (Chesler et al. 2002; Crabbe et al. 1999; Wahlsten et al. 2003) and the variability in outcome when comparing prolonged MS with AFR strongly support the notion that AFR rats are less suitable as the only control in MS studies.
It has also been shown that early-life environment interact with genetic propensity for high ethanol intake. In the selectively bred alcohol-preferring Alko Alcohol (AA) rats (Bell et al. 2012; Sommer et al. 2006), exposure to MS360 adds to genetic risk and results in increased ethanol intake (Roman et al. 2003, 2005; Fig. 2). AA rats that were subjected to repeated MS15 instead had lower ethanol consumption than AFR AA rats, further pointing to the fact that short periods of maternal absence is beneficial for the offspring and can counteract genetic predisposition for high ethanol consumption (Roman et al. 2003, 2005).
Studies on the consequences of interference with social interactions post-weaning in young rats on later ethanol consumption were recently reviewed by Nieswander et al. The compiled results generate a mixed picture; isolation immediately after weaning resulted either in increased, decreased or no effect on later ethanol consumption, for details see (Neisewander et al. 2012). However, these studies aim to interfere with social behaviour in adolescent rats and interactions with littermates and not the dam and thus not further described in detail herein. Of interest for MS-induced effects is a study on mice combining MS with post-weaning isolation (Advani et al. 2007). No differences were shown in ethanol consumption between MS15 and MS180 mice, and social isolation after weaning increased ethanol intake independent on previous early-life environment (Advani et al. 2007). That is, the increase in ethanol intake induced by disturbance of social interactions post-weaning was not potentiated in animals reared in the potential stressful MS180 condition. However, females responded differently as described later (see “Sex differences”).
From the studies on MS-induced effects on ethanol consumption later in life, it is evident that the ethanol intake is affected by early-life rearing conditions, indicating good face and construct validity. However, although several studies report similar outcome such as increased propensity for higher ethanol consumption after prolonged MS, there are studies that report no MS-induced effects on ethanol intake or preference (see Table 2). It is clear that the experimental conditions affect the outcome. Some experimental factors, and their putative mechanisms, that can influence the outcome of a MS experiment and thereby contribute to differences between MS studies are described in detail below.
Factors that influence the effects of MS on ethanol consumption
Influence of separation conditions
In contrast to the consistent findings in litter-wise paradigms, it was recently shown that there were no differences in adult ethanol consumption between repeated MS15 and MS360 when the rat pups were placed individually during the separation (Oreland et al. 2011; Fig. 2 and Table 2). The rats that were subjected to individual MS15 had higher ethanol consumption than usually seen after litter-wise MS15 and not different from the intake seen in rats subjected to MS360 or AFR. However, it is worth noting that even though the ethanol intake was similar, the ethanol-induced effects differed in MS15 and MS360 rats (Oreland et al. 2011). Thus, the rearing conditions were of importance for later ethanol response. In the study by Hilakivi-Clarke et al., the rat pups were separated in litters postnatal day (PND) 5–10 and then individually from PND 11 to 20. With this paradigm, there was a difference between prolonged (60 min) separations and MS15 (Hilakivi-Clarke et al. 1991) indicating that it is most important to keep the litters intact during the first ten postnatal days.
The findings of no differences in ethanol consumption after individual MS are in agreement with previous results showing different neurobiological outcome after individual and litter-wise separations (Gustafsson et al. 2008; Oreland et al. 2010). The individual and litter-wise separations in those reports were performed in one and the same MS study to exclude other confounding factors and show that the tactile contact during separations from the dam is of vital importance for the outcome of MS. Deprivation of littermate contact seem to be stressful, even though it is just for 15 min, which can explain why the loss of this sensory input also results in loss of the protective influences from short periods of separations from the dam such as the litter-wise MS15 condition.
In contrast, a recent study reported increased ethanol consumption in adult Long-Evans rats subjected to prolonged MS (Romano-Lopez et al. 2012). In this study, the rats were individually separated from the dam twice daily for 180 min or remained with the dam and handled like AFR rats. Notably, another ethanol intake paradigm was used to assess consumption. The animals were introduced to ethanol in a fading saccharin schedule and then first exposed to forced consumption followed by voluntary drinking of 10 % ethanol. These results indicate that although no differences can be seen using voluntary drinking protocols, differences in ethanol consumption may emerge when using forced consumption paradigms.
Influence of ethanol concentrations
Many MS studies use a two-bottle free choice between ethanol and water to assess MS-induced effects on voluntary ethanol intake and ethanol preference. However, the concentration of ethanol varies between studies, which may affect the outcome (Meisch and Lemaire 1993; Richter and Campbell 1940; Sanchis-Segura and Spanagel 2006). Differences between short and longer periods of MS have commonly been shown with the use of a continuous free choice paradigm with ethanol in concentrations up to 10 % (Table 2). MS studies using ethanol in the lower range, for example 5 % (Lancaster 1998) or 6 % (Marmendal et al. 2004) in rats, and 5 % in mice (Advani et al. 2007) have not been able to show robust effects on ethanol consumption or preference. In addition, it has been questioned whether ethanol intake at these concentrations are pharmacologically relevant (Sanchis-Segura and Spanagel 2006). The preference for different ethanol concentrations in animals subjected to MS was examined using a four-bottle paradigm with a continuous free choice between 5, 10 and 20 % ethanol in addition to water. The results showed that there were no differences in total ethanol consumption between rats subjected to MS15 and MS360 with this paradigm (Gustafsson and Nylander 2006). The notion that rats like to drink ethanol in lower concentrations (Meisch and Lemaire 1993; Richter and Campbell 1940) was confirmed in this study where the access to 5 % in addition to the 10 and 20 % ethanol resulted in higher intake in MS15 (Gustafsson and Nylander 2006) as compared to previous studies when they only had access to less palatable 8 % ethanol (Ploj et al. 2003a). Thus, the choice of 5 % resulted in higher intake in the MS15 rats and no protective effect of being reared in this setting as compared to MS360. However, whilst there were no differences in the total ethanol intake in a free choice between 5, 10 and 20 % ethanol in addition to water, there were differences in preference for the different concentrations over time depending on the early environmental conditions. A subgroup of the MS360 rats increased their preference for 20 % ethanol over the 8-week drinking period, whereas the MS15 rats predominantly consumed 5 % ethanol (Gustafsson and Nylander 2006). Thus, the MS360 setting is related to a risk environment in the sense that only MS360 rats acquired a preference for the 20 % ethanol in a continuous intake paradigm (Gustafsson and Nylander 2006).
A study on mice reported that MS180 mice subjected to a three-bottle choice between 6 % ethanol, 10 % ethanol and water in a limited access (2 h) paradigm had higher intake of 10 % but not 6 % as compared to AFR mice as controls (Cruz et al. 2008). Thus, the difference was also in this study observed in the higher concentration. In the same study, it was shown that the MS180 mice reached a higher intake of both 6 and 10 % in an operant paradigm showing that the consummatory behaviour also in this paradigm is higher after prolonged MS (Cruz et al. 2008).
However, with the use of an intermittent ethanol intake paradigm and a three-bottle choice between 5 % ethanol, 20 % ethanol and water, it was shown that the observed increase in ethanol consumption and preference over time in the MS360 rats was due to increased intake of 5 and not 20 % ethanol (Daoura et al. 2011). It was the first report that rats prefer 5 % ethanol over 20 % ethanol in the intermittent paradigm, which was confirmed in another study (Palm et al. 2011b). The animals were only drinking 5 weeks and this may be too short to be able to see an increase in consumption of 20 % ethanol. The results also indicated that even though MS360 rats increase their ethanol consumption their preferred choice of ethanol concentration differs in the continuous and intermittent schedules.
Differences in response to MS
It is well known that although traumatic experiences early in life are related to vulnerability for psychiatric disorders (Danese and McEwen 2012; McCrory et al. 2011; Teicher et al. 2003), it is not all individuals that are affected by adverse early-life conditions. Some seem to be resilient and not respond to these harmful environmental factors (Atkinson et al. 2009; Kim-Cohen 2007), whereas others are vulnerable and respond with negative consequences. Genotype is important as described in the excellent work by Caspi et al. that reported a MAO genotype associated with resilience to develop antisocial behaviour after exposure to early-life trauma (Caspi et al. 2002). MAO gene variation is also important for alcohol-related problem behaviour as evidenced by an interaction between genotype and early-life psychosocial environment (Nilsson et al. 2007, 2008, 2011). Other gene–environment interactions of interest for AUD are the finding of higher alcohol consumption in peer-reared but not mother-reared female non-human primates carrying a 5HTT-LPR allele (Barr 2013; Barr et al. 2003), an allele that also is related to predisposition for high ethanol intake in humans (Todkar et al. 2013).
Individual differences in the susceptibility for early adversity may also be true for rodents, and when using outbred rats, this could lead to differences in response to MS. Interestingly, previous studies have shown that the higher ethanol intake seen in the MS360 group of rats was not observed in all MS360 rats; some of these rats consumed similar amounts of ethanol (Ploj et al. 2003a) and in a pattern (Gustafsson and Nylander 2006) close to MS15 rats. These studies indicate the presence of rats that are sensitive to the supposedly stressful MS360 environment and they were denoted responders in contrast to the non-responders that had similar ethanol intake as the MS15 rats (Ploj et al. 2003a). Recently, it was also reported that there are responders and non-responders to treatment with naltrexone depending on early-life environment. The MS360 rats decreased their ethanol consumption after naltrexone, whereas the MS15 rats did not respond to this treatment (Daoura and Nylander 2011). Variability in the effects of naltrexone such as the ability to reduce ethanol consumption is well known, and besides the previously described influence of genotype (Mague and Blendy 2010; Oslin et al. 2003), this study shows that early-life conditions also affect the outcome of naltrexone treatment.
The presence of these subgroups enables studies of interactions between innate factors and early environmental factors that can shed light on the basis for vulnerability and resilience to AUD and other psychiatric disorders. The factors that predispose for responder or non-responder, i.e. vulnerability or resilience to early-life stress, are not clear and strongly merit further examination. The presence of responders and non-responders is also a matter of concern in MS studies since it is an additional factor to consider with respect to variation in outcome. In a given group of animals, for example a certain delivery from the animal supplier, the innate responder or non-responder characterization of individuals is unknown, as is the proportion of sample size that is comprised of each group.
Influence of age
The majority of the studies investigating MS-induced effects on voluntary ethanol consumption have examined the effects in adult rats and thereby also shown that the effects induced by early-life events during the first postnatal weeks clearly are evident for a long time, maybe persistent. However, it is also of interest to examine the effects on ethanol consumption in younger rats. Lancaster investigated ethanol intake in adolescent rats that were subjected to daily individual MS15 the first postnatal week. Higher intake of ethanol (5 % ethanol prepared in alcohol-free beer) was observed during the peripubertal period in male rats but a higher preference was seen only occasionally (in 8 out of 60 days) compared to non-handled male rats (Lancaster 1998). In another study, the effects of daily litter-wise MS on voluntary ethanol consumption initiated during adolescence and adulthood was compared (Daoura et al. 2011). The effects were compared in one single experiment, i.e. the animals were from the same batch from the supplier, subjected to the same MS protocol and the same experimental conditions including experimenter handling. Interestingly, it was shown that the increase in ethanol intake over time was only seen in MS360 rats and only when the rats initiated their drinking in adulthood. When the animals were provided free access to ethanol during adolescence, no increase in ethanol consumption was seen (Daoura et al. 2011; Fig. 2 and Table 2). Finally, when assessing effects of MS in alcohol-preferring AA rats, the differences between MS15 and MS360 rats were consistent but larger individual differences were seen in late adolescent/young adult MS360 rats (Roman et al. 2003) compared to adult MS360 rats (Roman et al. 2005; Fig. 2 and Table 2).
There may be several explanations for these differences. Ethanol may induce other effects in young rats with hormonal and neuronal systems still undergoing development. The adolescent rats consumed more ethanol than the adults, as also shown in other studies (Doremus et al. 2005; Garcia-Burgos et al. 2009; Lancaster et al. 1996; Vetter et al. 2007), and there may be other factors, for example behavioural, neuronal and hormonal factors (Spear 2000; Vanderschuren et al. 1997), which are more important determinants for ethanol consumption than the early-life conditions. Furthermore, depending on when adolescent rats are given access to ethanol, it is possible that individual housing has a larger impact than the rearing environment and perhaps masked the previously seen differences between these groups as social play behaviour during adolescence is shown to be critical (Trezza et al. 2010; Vanderschuren et al. 1997). Finally, the consequences of being subjected to MS may not be detectable until adulthood when the brain is fully developed. This theory is supported by detection of different neurobiological effects of MS depending on the age when analysing the brain. Differences between short and prolonged separations in reward-related brain areas were detectable in adult rats but not in young rats that were analysed immediately after the MS period (Gustafsson et al. 2008; Nylander and Roman 2012).
Influence of rodent strain and animal supplier
The strain of rat is of vital importance for the outcome in a specific animal model. The use of rat strains such as Wistar, Sprague–Dawley or Long-Evans is known to contribute to between-study variation and differences in stress-induced effects have been described depending on strain (Becker et al. 2011). However, studies including Sprague–Dawley, Wistar and Long-Evans have resulted in similar effects, for example higher ethanol consumption after prolonged MS compared to short periods of MS (Hilakivi-Clarke et al. 1991; Huot et al. 2001; Jaworski et al. 2005; Ploj et al. 2003a) and lower ethanol intake in short (3–15 min) MS compared to rats similar to the AFR condition (Hilakivi-Clarke et al. 1991; Weinberg 1987). These studies indicate that the MS-induced effects are robust in several strains, including selectively bred animals (Roman et al. 2003, 2005), and that the variability seen between studies more is a result of other experimental factors. The use of inbred or outbred rats will also affect variability and can, for example, influence the presence of responder and non-responder subgroups and thereby affect the outcome in a MS study. Furthermore, the animal supplier is unfortunately a factor that can cause differences in experimental outcome as exemplified in recent comparative studies of outbred Wistar rats from different animal suppliers. Striking differences in ethanol intake and consumption patterns (Palm et al. 2011b) and in ethanol-induced effects (Palm et al. 2012) were reported in addition to pronounced behavioural differences depending on supplier (Palm et al. 2011a) which can contribute to differences in outcome in studies of early-life events on ethanol intake.
Sex differences
The majority of MS studies include only male rats but the question about possible sex differences in the outcome of MS has been partially addressed. Weinberg et al. compared male and female Sprague–Dawley rats and reported no sex differences; all rats exposed to handling for 3 min showed lower ethanol consumption compared to control rats similar to AFR (Weinberg 1987). In the study by Lancaster et al., the females were affected differently than the males with no differences in ethanol intake between female handled and non-handled rats (Lancaster 1998). In other studies, it was evident that female rats were not affected by postnatal environmental manipulations; no differences were seen when comparing the ethanol intake in freely cycling Wistar female MS15 and MS360 rats (Gustafsson et al. 2005; Roman et al. 2004) or in female AA MS15 and MS360 rats (Roman et al. 2005; Fig. 2 and Table 2). In one of these studies, the oestrous cycle was followed and no differences in ethanol intake between the different phases were observed (Roman et al. 2005). In contrast to these studies, 180 min of MS followed by post-weaning isolation increased ethanol preference in female MS180 but not in MS15 mice while higher intake and preference was found in males regardless of pre-weaning rearing conditions (Advani et al. 2007). That is, even though MS did not affect ethanol consumption the vulnerability for higher intake seemed to be dependent on previous early-life environment in female mice. Moreover, in female mice, MS180 resulted in faster behavioural sensitization to ethanol relative to MS15 whereas no difference between MS groups was seen in male mice (Kawakami et al. 2007).
Summary
This review provides some guidance with regard to factors that influence the outcome of a MS study. The aims were to discriminate between the factors and experimental conditions that either result or do not result in higher ethanol intake and/or preference and clarify what the experimental parameters are that render MS a good model versus a less suitable model. Taken together, the compiled studies provide evidence that repeated prolonged separations (e.g. litter-wise MS180 or MS360) between the dam and her litter result in a propensity for higher ethanol consumption in adult male offspring as compared to offspring subjected to shorter periods of separations (e.g. MS15). The higher ethanol consumption is observed in several rat strains and in mice, and with the use of both continuous and intermittent ethanol access paradigms and with operant self-administration. A choice between low and high ethanol concentrations in a continuous access paradigm caused acquisition of a preference for higher ethanol concentrations in MS360 rats but not MS15 rats even though the total ethanol consumption was similar. These studies all support the notion that prolonged periods of MS are associated with risk and can be used to study consequences of early-life adversity on ethanol consumption behaviour. This finding is in agreement with the notion that ethanol intake is robust across labs, in contrast to behavioural measures (Crabbe et al. 1999; Wahlsten et al. 2003). Other MS-induced effects like HPA axis activity and behavioural alterations show less conclusive results and cannot fully explain the conclusive finding of elevated ethanol intake after prolonged MS.
A choice to drink 5 % ethanol in addition to higher concentrations and in protocols where only lower concentrations were used resulted in inconsistent results. The consumption of lower ethanol concentrations seemed less dependent on early-life environmental conditions. In an individual MS paradigm or when ethanol access starts during adolescence, there were no differences between short and prolonged early-life separations. Hence, it can also be concluded that the MS15 condition is only associated with low ethanol intake after litter-wise separation, in males, group housed during adolescence and when access to ethanol starts in adulthood. Under these circumstances, the MS15 represents a protective environment. In males exposed to daily individual MS and in single housed, adolescent males, the ethanol intake is similar in rats subjected to short and prolonged periods of MS, that is, the protective conditions characterizing the MS15 environment are lost. Finally, the early-life conditions simulated in MS paradigms seem to have little impact in female rats. The fact that MS is less valid for studies on the impact of early-life environment and propensity for high ethanol consumption in females merits further investigation.
Conclusion
These studies all support the notion that prolonged periods of MS are associated with risk and can be used to study consequences of early-life adversity on ethanol consumption behaviour. The compiled studies report an increased propensity for high ethanol consumption and preference in adult male rodents that previously have been exposed to disturbed mother–pup interactions, indicating good face and construct validity. However, identification of confounding factors and knowledge of the outcome induced by specific experimental protocols are of vital importance to be able to interpret the consequences of MS and to be able to compare results from different laboratories and protocols. It is evident from the compiled studies that there are several MS protocols in use and the results are not uniform. It is therefore not appropriate to talk about “the rodent maternal separation model”; there are several models, they generate different results and the experimental conditions have to be chosen accordingly.
Acknowledgments
Funding from the ERAB The European Foundation for Alcohol Research (EA 11 30; IN and ER), the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly (2011-0056 and 2012-0052 IN; 2011-0062 and 2012-0047 ER) and the Swedish Medical Research Council (K2012-61X-22090-01-3; IN) supported this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ader R, Grota LJ. Effects of early experiences on adrenocortical reactivity. Physiol Behav. 1969;4:303–305. doi: 10.1016/0031-9384(69)90179-6. [DOI] [Google Scholar]
- Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol. 2007;10:595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Zaharia MD, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/S0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Hawks BW, Owens MJ, Plotsky PM, Nemeroff CB. Increased responsiveness of presumed 5-HT cells to citalopram in adult rats subjected to prolonged maternal separation relative to brief separation. Psychopharmacology (Berl) 2004;176:248–255. doi: 10.1007/s00213-004-1883-x. [DOI] [PubMed] [Google Scholar]
- Atkinson PA, Martin CR, Rankin J. Resilience revisited. J Psychiatr Ment Heal Nurs. 2009;16:137–145. doi: 10.1111/j.1365-2850.2008.01341.x. [DOI] [PubMed] [Google Scholar]
- Barr CS. Non-human primate models of alcohol-related phenotypes: the influence of genetic and environmental factors. Curr Top Behav Neurosci. 2013;13:223–249. doi: 10.1007/7854_2011_142. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003;27:812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Becker HC. Animal models of excessive alcohol consumption in rodents. Curr Top Behav Neurosci. 2013;13:355–377. doi: 10.1007/7854_2012_203. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav. 2012;103:119–155. doi: 10.1016/j.pbb.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–923. doi: 10.1016/S0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Alleva E. Early disruption of the mother–infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev. 2003;27:73–82. doi: 10.1016/S0149-7634(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Claessens SE, Daskalakis NP, van der Veen R, Oitzl MS, de Kloet ER, Champagne DL. Development of individual differences in stress responsiveness: an overview of factors mediating the outcome of early life experiences. Psychopharmacology (Berl) 2011;214:141–154. doi: 10.1007/s00213-010-2118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene–environment interaction. Addict Biol. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29:344–357. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Quadros IM, Planeta Cda S, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 2008;201:459–468. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Daoura L, Nylander I. The response to naltrexone in ethanol-drinking rats depends on early environmental experiences. Pharmacol Biochem Behav. 2011;99:626–633. doi: 10.1016/j.pbb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Daoura L, Haaker J, Nylander I. Early environmental factors differentially affect voluntary ethanol consumption in adolescent and adult male rats. Alcohol Clin Exp Res. 2011;35:506–515. doi: 10.1111/j.1530-0277.2010.01367.x. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Claessens SE, Laboyrie JJ, Enthoven L, Oitzl MS, Champagne DL, de Kloet ER. The newborn rat’s stress system readily habituates to repeated and prolonged maternal separation, while continuing to respond to stressors in context dependent fashion. Horm Behav. 2011;60:165–176. doi: 10.1016/j.yhbeh.2011.04.003. [DOI] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/S0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt M. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Faturi CB, Tiba PA, Kawakami SE, Catallani B, Kerstens M, Suchecki D. Disruptions of the mother–infant relationship and stress-related behaviours: altered corticosterone secretion does not explain everything. Neurosci Biobehav Rev. 2010;34:821–834. doi: 10.1016/j.neubiorev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Burgos D, Gonzalez F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33:722–728. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Giachino C, Canalia N, Capone F, Fasolo A, Alleva E, Riva MA, Cirulli F, Peretto P. Maternal deprivation and early handling affect density of calcium binding protein-containing neurons in selected brain regions and emotional behavior in periadolescent rats. Neuroscience. 2007;145:568–578. doi: 10.1016/j.neuroscience.2006.12.042. [DOI] [PubMed] [Google Scholar]
- Greisen MH, Bolwig TG, Wortwein G. Cholecystokinin tetrapeptide effects on HPA axis function and elevated plus maze behaviour in maternally separated and handled rats. Behav Brain Res. 2005;161:204–212. doi: 10.1016/j.bbr.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Grota LJ, Ader R. Continuous recording of maternal behaviour in Rattus norvegicus. Anim Behav. 1969;17:722–729. doi: 10.1016/S0003-3472(69)80019-9. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Nylander I. Time-dependent alterations in ethanol intake in male Wistar rats exposed to short and prolonged daily maternal separation in a 4-bottle free-choice paradigm. Alcohol Clin Exp Res. 2006;30:2008–2016. doi: 10.1111/j.1530-0277.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Ploj K, Nylander I. Effects of maternal separation on voluntary ethanol intake and brain peptide systems in female Wistar rats. Pharmacol Biochem Behav. 2005;81:506–516. doi: 10.1016/j.pbb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Oreland S, Hoffmann P, Nylander I. The impact of postnatal environment on opioid peptides in young and adult male Wistar rats. Neuropeptides. 2008;42:177–191. doi: 10.1016/j.npep.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32:37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88:7261–7265. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Res. 1991;542:286–292. doi: 10.1016/0006-8993(91)91580-T. [DOI] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology (Berl) 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Kalsi G, Prescott CA, Kendler KS, Riley BP. Unraveling the molecular mechanisms of alcohol dependence. Trends Genet: TIG. 2009;25:49–55. doi: 10.1016/j.tig.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Kawakami SE, Quadros IM, Takahashi S, Suchecki D. Long maternal separation accelerates behavioural sensitization to ethanol in female, but not in male mice. Behav Brain Res. 2007;184:109–116. doi: 10.1016/j.bbr.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Choi SH, Lee YS, Jahng JW. Fasting-induced increases of arcuate NPY mRNA and plasma corticosterone are blunted in the rat experienced neonatal maternal separation. Neuropeptides. 2005;39:587–594. doi: 10.1016/j.npep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J. Resilience and developmental psychopathology. Child Adolesc Psychiatr Clin N Am. 2007;16:271–283. doi: 10.1016/j.chc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM. Responses to maternal separation: mechanisms and mediators. Int J Dev Neurosci. 1998;16:261–270. doi: 10.1016/S0736-5748(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/S0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Lancaster FE. Sex differences in voluntary drinking by Long Evans rats following early stress. Alcohol Clin Exp Res. 1998;22:830–836. doi: 10.1111/j.1530-0277.1998.tb03875.x. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Langeland W, Draijer N, van den Brink W. Psychiatric comorbidity in treatment-seeking alcoholics: the role of childhood trauma and perceived parental dysfunction. Alcohol Clin Exp Res. 2004;28:441–447. doi: 10.1097/01.ALC.0000117831.17383.72. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Freund N, Brenhouse HC, Thompson BS, Andersen SL. Depressive-like behavior in adolescents after maternal separation: sex differences, controllability, and GABA. Dev Neurosci. 2012;34:210–217. doi: 10.1159/000339162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Enduring effects of early experience on adult behavior. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, brain and behavior. London: Academic; 2002. pp. 535–542. [Google Scholar]
- Levine S, Lewis GW. Critical period for effects of infantile experience on maturation of stress response. Science. 1959;129:42–43. doi: 10.1126/science.129.3340.42. [DOI] [PubMed] [Google Scholar]
- Macri S, Würbel H. Developmental plasticity of HPA and fear responses in rats: a critical review of the maternal mediation hypothesis. Horm Behav. 2006;50:667–680. doi: 10.1016/j.yhbeh.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Mague SD, Blendy JA. OPRM1 SNP (A118G): involvement in disease development, treatment response, and animal models. Drug Alcohol Depend. 2010;108:172–182. doi: 10.1016/j.drugalcdep.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmendal M, Roman E, Eriksson CJP, Nylander I, Fahlke C. Maternal separation alters maternal care, but has minor effects on behavior and brain opioid peptides in adult offspring. Dev Psychobiol. 2004;45:140–152. doi: 10.1002/dev.20027. [DOI] [PubMed] [Google Scholar]
- Martisova E, Solas M, Horrillo I, Ortega JE, Meana JJ, Tordera RM, Ramirez MJ. Long lasting effects of early-life stress on glutamatergic/GABAergic circuitry in the rat hippocampus. Neuropharmacology. 2012;62:1944–1953. doi: 10.1016/j.neuropharm.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Matthews K, Hall FS, Wilkinson LS, Robbins TW. Retarded acquisition and reduced expression of conditioned locomotor activity in adult rats following repeated early maternal separation: effects of prefeeding, d-amphetamine, dopamine antagonists and clonidine. Psychopharmacology (Berl) 1996;126:75–84. doi: 10.1007/BF02246414. [DOI] [PubMed] [Google Scholar]
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse. 2001;40:1–10. doi: 10.1002/1098-2396(200104)40:1<1::AID-SYN1020>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. Br J Pharmacol. 2008;154:275–287. doi: 10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry Front Res Found. 2011;2:48. doi: 10.3389/fpsyt.2011.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/S0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Meisch RA, Lemaire GA (1993) Drug self-administration. In: van Haaren F (ed) Methods in behavioral pharmacology. Elsevier, Amsterdam, pp 257–300
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl) 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Wargelius HL, Leppert J, Lindström L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius HL, Sjoberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Aslund C, Nordquist N, Leppert J, Oreland L. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. 2011;16:347–355. doi: 10.1111/j.1369-1600.2010.00238.x. [DOI] [PubMed] [Google Scholar]
- Nylander I, Roman E. Neuropeptides as mediators of the early-life impact on the brain; implications for alcohol use disorders. Front Mol Neurosci. 2012;5:77. doi: 10.3389/fnmol.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Mikuni M, Kuroda Y, Muneoka K, Mori KJ, Takahashi K. Periodic maternal deprivation alters stress response in adult offspring: potentiates the negative feedback regulation of restraint stress-induced adrenocortical response and reduces the frequencies of open field-induced behaviors. Pharmacol Biochem Behav. 1994;49:961–967. doi: 10.1016/0091-3057(94)90250-X. [DOI] [PubMed] [Google Scholar]
- Oreland S, Pickering C, Gokturk C, Oreland L, Arborelius L, Nylander I. Two repeated maternal separation procedures differentially affect brain 5-hydroxytryptamine transporter and receptors in young and adult male and female rats. Brain Res. 2009;1305(Suppl):S37–S49. doi: 10.1016/j.brainres.2009.08.069. [DOI] [PubMed] [Google Scholar]
- Oreland S, Gustafsson-Ericson L, Nylander I. Short- and long-term consequences of different early environmental conditions on central immunoreactive oxytocin and arginine vasopressin levels in male rats. Neuropeptides. 2010;44:391–398. doi: 10.1016/j.npep.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Oreland S, Raudkivi K, Oreland L, Harro J, Arborelius L, Nylander I. Ethanol-induced effects on the dopamine and serotonin systems in adult Wistar rats are dependent on early-life experiences. Brain Res. 2011;1405:57–68. doi: 10.1016/j.brainres.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Palm S, Hävermark Å, Meyerson BJ, Nylander I, Roman E. When is a Wistar a Wistar? Behavioral profiling of outbred Wistar rats from five different suppliers using the MCSF test. Appl Animal Behav Sci. 2011;135:128–137. doi: 10.1016/j.applanim.2011.08.010. [DOI] [Google Scholar]
- Palm S, Roman E, Nylander I. Differences in voluntary ethanol consumption in Wistar rats from five different suppliers. Alcohol. 2011;45:607–614. doi: 10.1016/j.alcohol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Palm S, Roman E, Nylander I. Differences in basal and ethanol-induced levels of opioid peptides in Wistar rats from five different suppliers. Peptides. 2012;36:1–8. doi: 10.1016/j.peptides.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Camarini R, Quadros IM, Miczek KA, Israel Y. Genetic and environmental influences on ethanol consumption: perspectives from preclinical research. Alcohol Clin Exp Res. 2010;34:976–987. doi: 10.1111/j.1530-0277.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Fabio MC, Spear NE. Early maternal separation affects ethanol-induced conditioning in a nor-BNI insensitive manner, but does not alter ethanol-induced locomotor activity. Pharmacol Biochem Behav. 2012;100:630–638. doi: 10.1016/j.pbb.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering C, Gustafsson L, Cebere A, Nylander I, Liljequist S. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Effects of maternal separation on brain nociceptin/orphanin FQ peptide levels in male Wistar rats. Pharmacol Biochem Behav. 2002;73:123–129. doi: 10.1016/S0091-3057(02)00778-5. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/S0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides. 2003;37:149–156. doi: 10.1016/S0143-4179(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharmacol Biochem Behav. 2007;86:234–245. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/S0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Richter CP, Campbell KH. Alcohol taste thresholds and concentrations of solution preferred by rats. Science. 1940;91:507–508. doi: 10.1126/science.91.2369.507. [DOI] [PubMed] [Google Scholar]
- Roman E, Nylander I. The impact of emotional stress early in life on adult voluntary ethanol intake—results of maternal separation in rats. Stress. 2005;8:157–174. doi: 10.1080/10253890500188666. [DOI] [PubMed] [Google Scholar]
- Roman E, Hyytiä P, Nylander I. Maternal separation alters acquisition of ethanol intake in male ethanol-preferring AA rats. Alcohol Clin Exp Res. 2003;27:31–37. doi: 10.1111/j.1530-0277.2003.tb02717.x. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Hyytiä P, Nylander I. Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res. 2005;29:591–601. doi: 10.1097/01.ALC.0000158933.70242.FC. [DOI] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Berg M, Nylander I. Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Behav. 2006;50:736–747. doi: 10.1016/j.yhbeh.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Romano-Lopez A, Mendez-Diaz M, Ruiz-Contreras AE, Carrisoza R, Prospero-Garcia O. Maternal separation and proclivity for ethanol intake: a potential role of the endocannabinoid system in rats. Neuroscience. 2012;223:296–304. doi: 10.1016/j.neuroscience.2012.07.071. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Feldon J, Pryce CR. Circadian- and temperature-specific effects of early deprivation on rat maternal care and pup development: short-term markers for long-term effects? Dev Psychobiol. 2004;45:59–71. doi: 10.1002/dev.20014. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 2005;156:297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–143. doi: 10.1016/S0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Wang XD, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology (Berl) 2011;214:131–140. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Heilig M, Hommer DW, George DT, Ramchandani VA. Childhood trauma exposure and alcohol dependence severity in adulthood: mediation by emotional abuse severity and neuroticism. Alcohol Clin Exp Res. 2013;37:984–992. doi: 10.1111/acer.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20 % ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer W, Hyytiä P, Kiianmaa K. The alcohol-preferring AA and alcohol-avoiding ANA rats: neurobiology of the regulation of alcohol drinking. Addict Biol. 2006;11:289–309. doi: 10.1111/j.1369-1600.2006.00037.x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: evidence from rodent models. Physiol Behav. 2012;106:325–331. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/S0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Todkar A, Nilsson KW, Oreland L, Hodgins S, Comasco E (2013) Serotonin transporter genotype by environment: studies on alcohol use and misuse in non-human and human primates. Translational Neurosci 4
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21:309–326. doi: 10.1016/S0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vazquez V, Penit-Soria J, Durand C, Besson MJ, Giros B, Dauge V. Maternal deprivation increases vulnerability to morphine dependence and disturbs the enkephalinergic system in adulthood. J Neurosci. 2005;25:4453–4462. doi: 10.1523/JNEUROSCI.4807-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140:355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Lopez-Gallardo M, Suarez J, Bermudez-Silva F, De la Fuente M, Rodriguez de Fonseca F, Garcia-Segura LM. Sex-dependent alterations in response to maternal deprivation in rats. Psychoneuroendocrinology. 2009;34(Suppl 1):S217–S226. doi: 10.1016/j.psyneuen.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, II, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene–environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I. Effects of hypothalamic stimulation, acclimation and periodic withdrawal on ethanol consumption. Physiol Behav. 1972;9:737–740. doi: 10.1016/0031-9384(72)90043-1. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Effects of early experience on responsiveness to ethanol: a preliminary report. Physiol Behav. 1987;40:401–406. doi: 10.1016/0031-9384(87)90068-0. [DOI] [PubMed] [Google Scholar]
- Weininger O. Physiological damage under emotional stress as a function of early experience. Science. 1954;119:285–286. doi: 10.1126/science.119.3087.285. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Shartrand AM. Temperature-dependent effects of maternal separation on growth, activity, and amphetamine sensitivity in the rat. Dev Psychobiol. 1992;25:213–226. doi: 10.1002/dev.420250306. [DOI] [PubMed] [Google Scholar]