Abstract
Background/Aims
Cochinchina momordica seed extract (SK-MS10) has a gastric protective effect. We aimed to assess the effect of SK-MS10 on gastric acid secretion with morphologic changes in the aged rat.
Methods
Acid secretions were evaluated in the male F344 rats of four different ages (6-, 31-, 74-week, and 2-year). The 31-week-old rats were divided to three groups and continuously administered chow containing vehicle, SK-MS10 and lansoprazole, respectively. At the age of 74 weeks and 2 years, basal and stimulated acid was measured and the expression of mRNA and protein of H+-K+-ATPase were determined. The area of connective tissue of lamina propria was measured.
Results
Basal and stimulated gastric acid significantly decreased and connective tissue of lamina propria increased with age. The expression of mRNA and protein of H+-K+-ATPase significantly decreased with age. However, 74-week-old rats in the SK-MS10 group had higher stimulated gastric acid secretion than those in the vehicle and lansoprazole groups. In 2-year-old rats of SK-MS10 group, there was no increase of connective tissue.
Conclusions
As SK-MS10 kept the capacity of acid secretion as well as connective tissue area to comparable to young rats, it might valuable to perform further research regarding mechanism of SK-MS10 as an antiaging agent in the stomach.
Keywords: Gastric acid, Hydrogen-potassium-activated ATPase, Cochinchina momordica seed, Aging, Connective tissue
INTRODUCTION
Until the early 1990s, acid secretion was regarded to decrease progressively with increasing age in human due to a loss of parietal cells that occurred with atrophic gastritis.1 The fall of acid secretion and the atrophic change of gastric mucosa with age have been also observed in rodents.1-4 However, since Helicobacter pylori was found in 1982, a paradigm has changed through a lot of research. It is known that H. pylori infection caused atrophic change of gastric mucosa, and gastric acid secretion does not in old people who have no atrophic change of gastric mucosa and the H. pylori infection.5-8
Cochinchina momordica seed extract (SK-MS10) is composed mainly of momordica saponins. In our previous study, SK-MS10 was found to enhance gastric ulcer healing and angiogenesis in the acetic acid-induced ulcer model.9 In addition, we have shown that SK-MS10 has an effect of gastric protection via up-regulation of calcitonin gene-related peptide (CGRP).10 There have been reports that CGRP suppresses acid output.11,12 However, our preliminary study showed that the SK-MS10 did not inhibit acid secretion, suggesting that SK-MS10 might have some role in acid secretion through other mechanism besides CGRP.
The aim of this study was to evaluate the gastric acid secretions in the aging process and to assess the effects of SK-MS10 on gastric acid secretion and morphologic changes with age. In addition, the expression of mRNA and protein of a proton pump, hydrogen-potassium-activated ATPase (H+-K+-ATPase) were determined together with acid secretion.
MATERIALS AND METHODS
1. Preparation and composition of SK-MS10
SK-MS10 was supplied by Life Science R&D Center of the SK Chemicals Co., Ltd. (Seongnam, Korea). SK-MS10 was prepared as follows.9,10 Five liter of aqueous ethanol solution was added to 1 kg (dry weight) of C. momordica, purchased at an herb market in Korea. Extraction was performed for 4 hours at 80℃, and this process was performed twice. The extract was filtered and concentrated under reduced pressure at 60℃ using a rotary evaporator. After complete removal of the solvent in a vacuum oven, 60 g of ethanol extract in powder form (SK-MS10) was obtained. SK-MS10 was dissolved in carboxymethylcellulose sodium salt (Sigma Aldrich C-4888; Sigma Chemical Co., St Louis, MO, USA) during the experiment.
2. Animals
Male Fisher 344 rats, H. pylori free and virus free, were purchased from Orient Co., Ltd., Seoul, Korea, and were raised at our institute. The 31-week aged rats were divided into three groups: vehicle, SK-MS10 and lansoprazole group and housed in a cage maintained at 23℃, with 12:12-hour light-dark cycles under specific pathogen-free conditions. The vehicle group was fed with ad libitum only Purina rat chow and SK-MS10 and lansoprazole group were fed with rat chow containing SK-MS10 (100 mg/kg) and lansoprazole (5 mg/kg) per day until sacrifice. All experimental procedures described here were approved by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital (IACUC No.: BA0903-040/013-01).
3. Measure of gastric acid secretion
The level of acid secretion was measured in each basal and stimulated rat. In the vehicle group, basal and stimulated acid secretion level were measured at 6-, 31-, 74-week-, and 2-year-old rats. In SK-MS10 and lansoprazole group, those were measured at 74-week- and 2-year-old rats. Actually, the feeding period of SK-MS10 or lansoprazole was 43 weeks in the 74-week-old rats and 73 weeks in the 2-year-old (104-week-old) rats, respectively, because the feeding was started at the age of 31 weeks. There were no abnormal signs such as weight loss or side effects in SK-MS10 groups compared to vehicle group. They grew well and no side effects were observed until the experiments. The rats were starved but allowed water for 24 hours prior to the experiments. After measurement of body weight, the rats were anesthetized by zoletil and rompun mixture. The abdomen was gently opened and the esophagogastric junction and pyloric ring were ligated. An overhead lamp was used to maintain core body temperature at 36℃ to 38℃. Following ligation the animals were injected (s.c.) with either phosphate-buffered solution or histamine (40 mg/kg) with carbachol (10 µg/kg), respectively. After incubation for 2 hours, stomach was extracted, and gastric juice was collected using 50 mL conical tube. The acid concentration (mmol H+) was determined by titration with 0.1 N NaOH to pH 7.0.
4. Real-time polymerase chain reaction for ATP4A
ATP4A mRNA, the gene encoding a-subunit of H+-K+-ATPase, were measured by real-time polymerase chain reaction (PCR). Briefly, RNA was extracted from the mucosa-scrapped tissue of body of rat stomach using the RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. RNA samples were diluted to a final concentration of 0.5 mg/mL in RNase-free water and stored at -80℃ until use. Synthesis of the cDNA was performed with 1 mg of total RNA with M-MLV reverse transcription reagents (Invitrogen, Carlsbad, CA, USA). The 20 µL reverse transcription reaction was consisted of 4 µL of first-strand buffer, 500 mM deoxynucleoside triphosphate mixture, 2.5 mM oligo 12 to 18 primer, 0.4 U/mL ribonuclease inhibitor, and 1.25 U/mL Moloney murine leukemia virus reverse transcriptase (Invitrogen). The thermal cycling parameters for the reverse transcription were 10 minutes at 65℃, 50 minutes at 37℃, and 15 minutes at 70℃. Real-time PCR amplification and determination were performed using the ABI PRISM 7000 sequence detection system, TaqMan universal PCR master mix, commercially available predesigned, gene-specific primers, and FAM labeled probe sets for quantitative gene expression (TaqMan Gene Expression Assays; Applied Biosystems, Foster City, CA, USA). All of the probes used in these experiments spanned an exon-intron boundary. The ATP4A and β-actin mRNA were quantified by parallel estimation. The thermal cycler conditions were 2-minute hold at 50℃ and 10-minute hold at 95℃, followed by 40 cycles of 15 seconds at 95℃ and 1 minute at 60℃. The relative expression of target genes was normalized by dividing the target Ct values by the endogenous Ct values.
5. Western blotting for H+-K+-ATPase
The protein expression of α-subunit of H+-K+-ATPase was determined by Western blotting according to the previously reported method.13,14 Briefly, the mucosa-scrapped tissue of body of stomach was homogenized with lysis buffer containing 25 mM Tris-HCL pH 7.4, ethylene glycol tetraacetic acid 1 mM, dithiothreitol 1 mM, leupeptin 10 µg/mL, aprotinin 10 µg/mL, phenylmethylsulfonyl fluoride 1 mM, and Triton X-100 0.1%. The proteins (each sample, 50 µg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (8% wt/wt gel) and transferred to polyvinylidene fluoride membranes. All procedures were performed in Tris buffer (40 mM, pH 7.55) containing 0.3 M of NaCl and 0.3% Tween 20. The membranes were then blocked with dried milk (5% wt/vol) and subsequently incubated with anti-H+-K+-ATPase α-subunit antibody (polyclonal, 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4℃ overnight. The blots were incubated with secondary antibody (rabbit polyclonal antibody, 1:2,000; Santa Cruz Biotechnology) and an imaging analyzer was used to measure the band densities.
6. Mucosal histology
The obtained gastric specimens were fixed in 10% buffered formalin for histology. The specimens were embedded in paraffin and routinely processed and stained with hematoxylin and eosin (H&E). The gastric mucosal histology was evaluated on coded gastric specimens stained with H&E by two independent experts (R.H.N. and H.C.). The area of the connective tissue was quantified in the lower one-third of the mucosa and the total area of each specimen (n=10) by using the MetaMorph 7.0 video image analysis system (Molecular Devices, Downingtown, PA, USA).15 The connective tissue area was expressed as a % of the total area.
7. Measurement of salt soluble collagen, sulfated GAG, lipid hydroperoxide
The gastric mucosal level of salt-soluble collagen, sulfated GAG (sGAG) and lipid hydroperoxide (LPO) were measured by the previously published protocol.15
8. Statistical analysis
All statistical calculations were performed using SPSS software version 18.0 (IBM Co., Armonk, NY, USA). The results were compared using the Mann-Whitney U test and the Wilcoxon rank sum test. All values are reported as means±standard errors. Statistical significance was set at p<0.05.
RESULTS
1. Gastric acid secretion and expression of H+-K+-ATPase with age
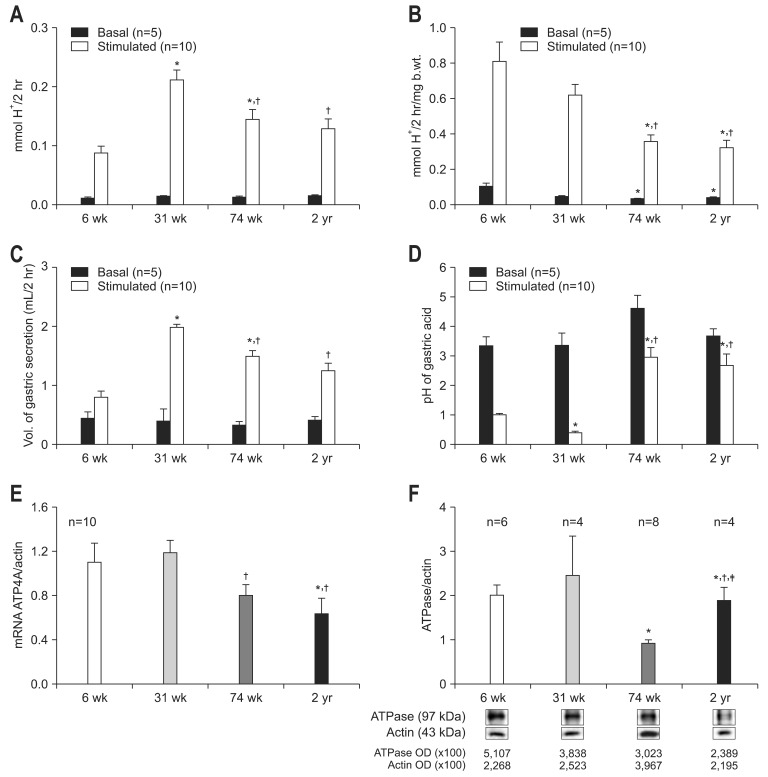
There were no significant differences in basal gastric acid concentration among each age group (basal gastric acid concentration, 0.011±0.002 in 6-week; 0.014±0.001 in 31-week; 0.013±0.002 in 74-week; 0.015±0.001 mmol H+/2 hr in 2-year-old rats). However, the stimulated acid concentration (stimulated gastric acid concentration, 0.087±0.012 in 6-week; 0.212±0.017 in 31-week; 0.145±0.017 in 74-week; 0.129±0.017 mmol H+/2 hr in 2-year-old rats) showed the pattern of decrease with age after 31 weeks (74 weeks, p=0.025 compared to 31 weeks; 2 years, p=0.011 compared to 31 weeks) (Fig. 1A). As there were significant differences of the mean body weight of F344 rats by age (106 g in 6-week-old, 341 g in 31-week-old, and 409 g in 74-week- and 2-year-old rats), the results of acid concentration were corrected by body weight. Weight corrected basal and stimulated gastric acid concentration significantly decreased with age (weight corrected basal and stimulated gastric acid secretion, 0.102±0.019, 0.809±0.108 in 6-week; 0.042±0.004, 0.616±0.061 in 31-week; 0.031±0.004, 0.353±0.040 in 74-week; 0.038±0.003, 0.321±0.043 mmol H+/2 hr/mg body weight in 2-year-old rats). Weight corrected basal gastric acid concentration of 74-week and 2-year-old rats was significantly reduced as compared to 6-week-old rats (74 weeks, p=0.014; 2 years, p=0.005). In addition, weight corrected stimulated gastric acid secretion in 74-week and 2-year-old rats was significantly lower than that in 6- and 31-week-old rats (74 weeks, p=0.010 to 6 weeks, p=0.006 to 31 weeks; 2 years, p=0.002 to 6 weeks, p=0.003 to 31 weeks) (Fig. 1B). The change of volume and pH of gastric acid with age was shown in Fig. 1C and D.
Fig. 1.
Gastric acid secretion and expression of H+-K+-ATPase with age. (A) Basal and stimulated acid concentration. (B) Basal and stimulated acid concentration corrected by body weight. (C) The volume of gastric acid secretion. The volume of basal gastric secretion was not different among each age group. The volume of stimulated gastric secretion peaked in the 31-week-old rat group and decreased with age. (D) pH of gastric acid. pH of stimulated gastric acid of 74-week- and 2-year-old rats was significantly higher than that of young age groups. (E) Expression of ATP4A mRNA. (F) Expression of H+-K+-ATPase protein. For the measurement of basal or stimulated acid secretion, either phosphate-buffered solution or histamine (40 mg/kg) and carbachol (10 µg/kg) was injected (s.c.), respectively. Results are expressed as mean±SEM.
b.wt., body weight; OD, optical density.
*p<0.05 compared with 6-week of age; †p<0.05 compared with 31-week of age; ‡p<0.05 compared with 2-year-aged F344 rats.
The mean expression of ATP4A mRNA of 6-, 31-, 74-week-, and 2-year-old rats were 1.100±0.171, 1.185±0.111, 0.799±0.099, and 0.633±0.141, respectively. In the basal condition, the expression of ATP4A mRNA of 74-week was lower than that of 31-week-old rats (p=0.023), the expression of ATP4A mRNA of 2-year-old rats decreased compared to 6- and 31-weak-old rats (p=0.020, p=0.003, respectively) (Fig. 1E). In the basal condition, the mean expression of H+-K+-ATPase protein 6-, 31-, 74-week-, and 2-year-old rats were 2.0±0.23, 2.46±0.87, 0.93±0.07, and 1.89±0.30, respectively. The 74-week-old rats showed lower expression of H+-K+-ATPase protein than 6-week and 2-year-old rats (p=0.015 to 6 weeks, p=0.011 to 2 years) and 2-year-old rats had lower expression of H+-K+-ATPase protein than 6-week and 31-week-old rats (p=0.015 to 6 weeks, p=0.05 to 31 weeks) (Fig. 1F).
2. Gastric acid secretion and expression of H+-K+-ATPase according to drug
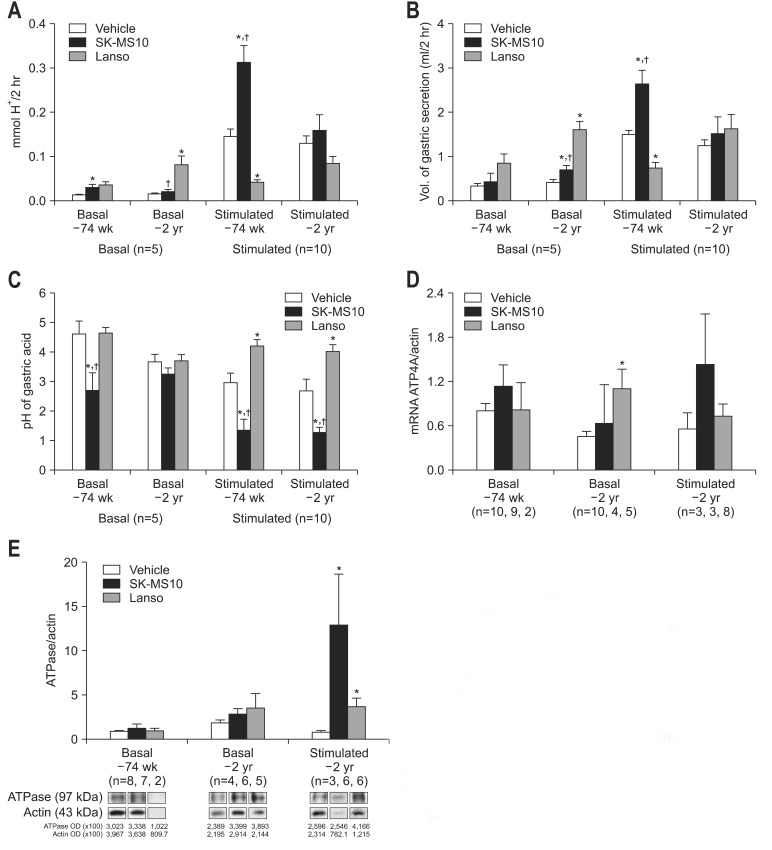
The basal and stimulated gastric acid concentrations of SK-MS10 group (0.030±0.007, 0.313±0.037 in 74-week-old rats; 0.020±0.005, 0.159±0.035 mmol H+/2 hr in 2-year-old rat) were higher than vehicle group. In 74-week-old rats, the elevation of gastric acid secretion was statistically significant (p=0.019 at basal, p=0.001 at stimulated condition) (Fig. 2A). The basal acid concentration of lansoprazole group was higher than that of vehicle or SK-MS10 groups, while the stimulated acid concentration of lansoprazole group was lower than that of other two groups. Especially, there were statistical significance in basal acid concentration of 2-year-old rats and stimulated acid concentration of 74-week-old rats. The stimulated acid secretion in lansoprazole group was very similar to the label of basal condition, which could be explained by pharmacological action (basal and stimulated gastric acid secretion of lansoprazole group, 0.035±0.007, 0.042±0.005 at the age of 74-week-old rat; 0.081±0.020, 0.084±0.016 at the age of 2-year-old rat, respectively) (Fig. 2A). Under the stimulated condition, acid concentration of SK-MS10 group was decreased with age (p=0.025), while acid concentration of lansoprazole group was increased with age in both basal and stimulated conditions (p=0.045 in basal condition, p=0.013 in stimulated condition) (Fig. 2A). In 74-week-old rats, the volume of stimulated gastric secretion of SK-MS10 group was higher compared to other treatment groups with statistical significance (Fig. 2B). The pH of gastric acid of SK-MS10 group was also lower than that of other two groups with statistical significance (Fig. 2C).
Fig. 2.
Gastric acid secretion and expression of H+-K+-ATPase according to drug. (A) Basal and stimulated acid concentration. (B) The volume of gastric acid secretion. The volume of basal gastric secretion of 74-week group was not different among each treatment group. The volume of stimulated gastric secretion of 74-week SK-MS10 was higher compared to other treatment groups with statistical significance. (C) pH of gastric acid. The basal pH of gastric acid of 74-week SK-MS10 group was lower than that of other two groups (p=0.027 compared to vehicle, p=0.016 compared to lansoprazole). In the 2-year-old rat groups, the basal pH of gastric acid did not show statistical significant difference among each treatment groups. pH of stimulated gastric acid show the lowest level in 74-week- and 2-year-old SK-MS10 groups. (D) Expression of ATP4A mRNA. (E) Expression of H+-K+-ATPase protein. The feeding period of SK-MS10 or lansoprazole was 43 weeks in the 74-week-old rats and 73 weeks in the 2-year-old (104-week-old) rats because the feeding was started at the age of 31 weeks. Results are expressed as mean±SEM.
OD, optical density.
*p<0.05 compared with vehicle; †p<0.05 compared with lansoprazole.
Although the mean expression of ATP4A mRNA was increased in SK-MS10 compared to vehicle group both in the basal condition and in 2-year-old rat of stimulated condition, there was no statistical significance. The mean expression of ATP4A mRNA of lansoprazole group was also higher than that of vehicle group, there was significant difference in 2-year-old rat of basal condition (p=0.031) (Fig. 2D). In the basal condition, the mean expression of H+-K+-ATPase protein showed no statistical difference among three drug groups. In 2-year-old rat of stimulated condition, the level of H+-K+-ATPase protein was significantly higher in SK-MS10 group when compared to that of vehicle group (p=0.039). The expression of H+-K+-ATPase protein of lansoprazole group was higher than that of group (p=0.041) (Fig. 2E).
3. Mucosal histology
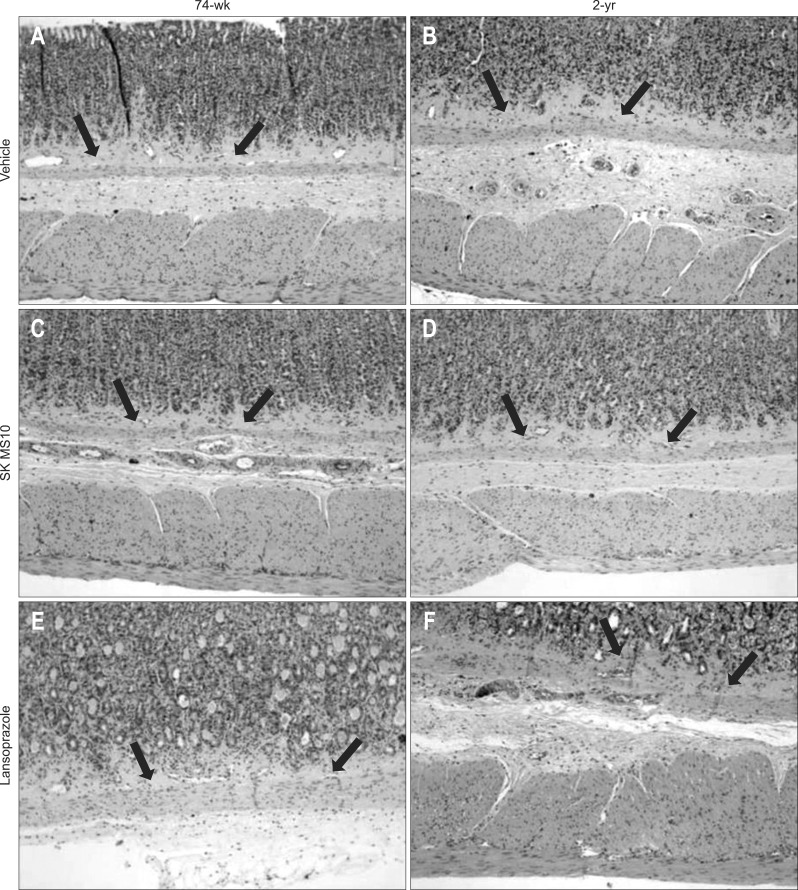
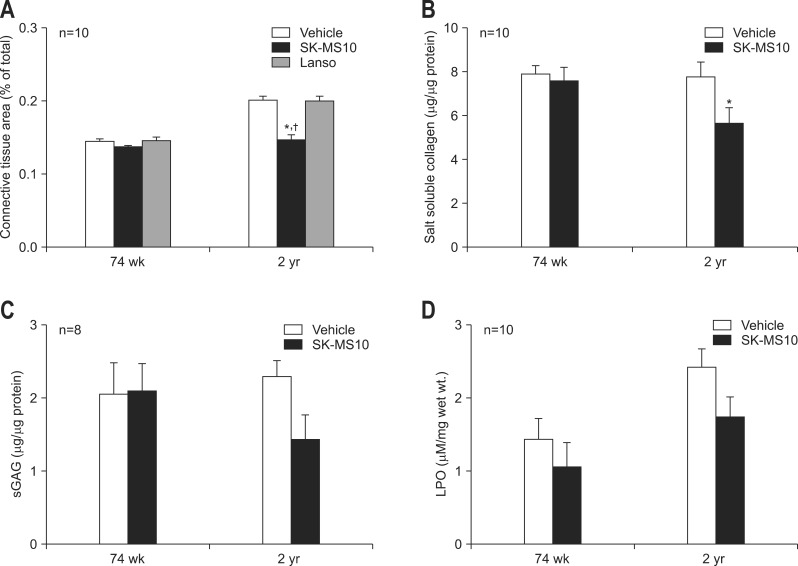
The area of connective tissue of lamina propria was compared between 74-week- and 2-year-old rats of three groups (Fig. 3). The results of vehicle, SK-MS10 and lansoprazole group were 14.4%, 13.6%, 14.6% in 74-week-old rats and 20.1%, 14.7%, 20% in 2-year-old rats, respectively. In vehicle and lansoprazole group of 2-year-old, the area of connective tissue of lamina propria increased with aging since 74-week-old (p<0.001 in both vehicle and lansoprazole group). However, in SK-MS10 group, the connective tissue area of lamina propria of 2-year-old rats was very similar to that of 74-week-old (p=0.242) The connective tissue area of lamina propria in SK-MS10 group of 2-year-old was significantly lower than those of vehicle (p<0.001) and lansoprazole group (p<0.001) of 2-year-old (Fig. 4A).
Fig. 3.
Photomicrographs of gastric mucosa (H&E stain, ×400). (A) Vehicle of 74-week. (B) Vehicle of 2-year. (C) SK-MS10 of 74-week. (D) SK-MS10 of 2-year. (E) Lansoprazole of 74-week. (F) Lansoprazole of 2-year. Arrows indicate the layer of lamina propria.
Fig. 4.
(A)Comparison of connective tissue area in the laminar propria. The proportion of connective tissue area of the 2-year-old F344 rats was significantly lower in the SK-MS10 group compared to vehicle or lansopraozole group. (B) Salt soluble collagen. (C) Sulfated GAG (sGAG). (D) Lipid hydroperoxide (LPO) according to continuous administration of SK-MS10 (100 mg/kg/day) from 31-week-old up to 74-week- or 2-year-old.
*p<0.05 compared with vehicle; †p<0.05 compared with lansoprazole.
4. Salt soluble collagen, sulfated glycosaminoglycan, and lipid hydroperoxide
As there was no remaining tissue of lansorpazole group salt soluble collagen, sGAG and LPO were measured only in the vehicle and SK-MS10 group. The salt soluble collagen level of SK-MS10 group was significantly lower than vehicle group in 2-year-old rats (5.63 µg/µg protein vs 7.76 µg/µg protein, p=0.028) (Fig. 4B). The sGAG level of SK-MS10 group in 2-year-old rat was lower than that of vehicle group, but not significantly (1.43 µg/µg protein vs 2.09 µg/µg protein, p=0.083) (Fig. 4C). LPO level of 2-year-old rat was higher than 74-week-old rats in vehicle group (2.41 µgM/mg wet wt. vs 1.44 µgM/mg wet wt., p=0.02), but there was no statistically significant difference between 2-year- and 74-week-old rats in SK-MS10 group (1.74 µgM/mg wet wt. vs 1.05 µgM/mg wet wt., p=0.129). When the LPO level was compared between vehicle and SK-MS10 group, LPO levels of SK-MS10 group were lower than those of vehicle groups but it did not reach statistical significance (1.05 vs 1.44, p=0.399 in 74 weeks; 1.74 vs 2.41, p=0.09 in 2 years) (Fig. 4D).
DISCUSSION
There have been reports that gastric acid secretion decreased with increasing age in human as well as animal models.1,3,16 However, in more recent studies, gastric acid secretion has been found to remain stable in old people without atrophy of gastric mucosa and the H. pylori infection.5-8 Furthermore, other studies have shown that acid secretion increased with age.17,18 Consequently, it was thought that the decline of gastric acid was not the simple effect of physiological aging, but the result of gastric mucosal atrophy or H. pylori infection.7,19,20 H. pylori infection of human gastric mucosa down-regulates the activity of the a-subunit of H+-K+-ATPase of the parietal cell, and significantly inhibits acid secretion.21,22 In this study, basal and stimulated gastric acid amounts significantly decreased with aging and the expression of mRNA and protein of H+-K+-ATPase were definitely decreased in the aged rats. As a specific pathogen-free rat model was used in this study, the effect of H. pylori on gastric acid secretion can be excluded. In the previous study, we also have clearly shown that there was atrophy of the gastric glands in the basal mucosa and they were replaced by connective tissues in the aging F344 rats at four points (6, 31, 74 weeks, and 2 years) similar to the present study.9 Together with the results of the present study the decrease of basal and stimulated gastric acid secretion seems to be related to the gastric mucosal atrophy with aging. Furthermore, these results were partially supported by the similar decreasing tendency of mRNA and protein of H+-K+-ATPase in the aged rats although it was not the same in the present study.
C. momordica is the dried ripe seed of Momordica cochinchinensis, a perennial vine that grows in Southern China and Vietnam; it has traditionally been used for its anti-inflammatory activity and for suppurative skin infections. Chemical analysis shows that the C. momordica seeds are composed of compounds including fatty acids, saponins, proteins, α-spinasterol, oleanolic acid, and momordica acid. Among these compounds, momordica saponin I, glycoside, a triterpenoid saponin containing disaccharide chain, has been found to be a major active ingredient.10 The saponins were found to inhibit gastric mucosal injuries induced by ethanol or indomethacin in rats.23 We have previously shown that SK-MS10 enhanced the healing of gastric ulcer and the angiogenesis in the acetic acid-induced ulcer model.9 Also, we demonstrated that the gastric protective effect of SK-MS10 was related with up-regulation of CGRP-nitric oxide pathway.10
CGRP has been known as one of the neurotransmitter of gastric afferent nerve. When the gastric mucosa is endangered the afferent sensory nerve, stimulated by gastric acid, secretes the CGRP. Consequently, via CGRP receptor of G-cell and D-cell of stomach, the acid secretion of gastric parietal cell is reduced.24 Although there were some reports that CGRP suppress acid output11,12 our preliminary study using 6-week-old Spraque-Dawley rats showed that the SK-MS10 did not significantly inhibit acid secretion, suggesting that SK-MS10 might play some role in acid secretion through different mechanisms other than CGRP. In the present study, the decrease of gastric acid secretion in the aged rat was prevented by continuous administration of SK-MS10 via chow (100 mg/kg/day). Moreover, the pH of aged rat who took SK-MS10 continuously was similar to that of 6-week-old rat in vehicle group. Although we have not evaluated the long term effect of SK-MS10 administration in young rats in the present study, the effect of acid secretion of SK-MS10 might be little in the young rat because it had become influential in the aged rat under the decreased potential of acid secretion. These results might be explained in several ways. For instance, SK-MS10 has some antiaging potential in the F344 rat. Or continuous administration of SK-MS10 provoked the increase of CGRP production with age, which might have acted to protect the gastric mucosa.10,15 However, as we did not measure the CGRP concentration neither the other antiaging factors in the present manuscript, further studies are necessary to investigate underlying mechanism of our results.
As a proton pump, H+-K+-ATPase is known to be the common pathway mediating secretion of hydrochloric acid and a marker of parietal cell function.25 In the next step we measured mRNA of ATP4A and protein level of ATPase. Similar to the results of gastric acid secretion the expression of mRNA and protein of H+-K+-ATPase were also higher in the SK-MS10 group compared to vehicle group although there was no statistical significance. Thus, the higher acid secretion of SK-MS10 group than vehicle group in the aged rat might be related with the other mechanism than H+-K+-ATPase. In the future, underlying mechanism the up-regulation of gastric acid secretion by SK-MS10 is planned to be undertaken. In contrast to SK-MS10, continuous administration of lansoprazole (5 mg/kg/day) inhibited the acid output of stimulated condition but significantly increased the basal acid output than vehicle group. The increase of basal acid with increase of mRNA of ATP4A in the lansoprazole group over the vehicle group could be explained by the compensation of gastric mucosa for the significant decrease of stimulated acid output by lansoprazole.
After finding this effect of SK-MS10 on acid secretion we checked the effect of SK-MS10 on the connective tissue change of lamina propria with aging because a few previous studies showed the increase of connective tissue area of gastric mucosa of rat with age.2,4,15 Similar to the previous study by our group,15 the connective tissue area of gastric mucosa increased with aging in vehicle and lansoprazole group. Interestingly, there was no statistical significant increment of connective tissue of lamina propria in SK-MS10 group, supported by the lower mucosal collagen level of SK-MS10 group compared to vehicle group in 2-year-old rats. We speculated that continuous administration of SK-MS10 might have an antiaging effect on the gastric mucosa as well as mucoprotective agent in the stomach.
LPO as oxidative products have been shown to participate in tissue injury and chronic inflammation.26 Naturally, the relation between LPO level and aging has been suggested.27,28 Previously we indicated that LPO level of gastric mucosa was increased in aged rat.15 In the present study, LPO level of 2-year-old rats was significantly higher than that of 74-week-old rats (p=0.034). However, this increase of LPO was blocked by continuous administration of SK-MS10 (p=0.139), suggesting the possibility of an antiaging effect of SK-MS10 together with the prevention effect of increase of connective tissue in the lamina propria.
In conclusion, SK-MS10 attenuated connective tissue change with age and kept the capacity of acid secretion to that of young age. These findings suggest that SK-MS10 might be an antiaging agent in the stomach as well as mucoprotective agent. This effect of SK-MS10 that prevents the decrease of gastric acid secretion might be helpful for the inhibition of development of small intestinal bacterial overgrowth, relatively increase in old age. Further study is needed to investigate the mechanism of SK-MS10 related to the prevention effect of decrease of gastric acid secretion and that of increase of connective tissue change.
ACKNOWLEDGEMENTS
This work was supported by grant no 03-2011-018 from the Seoul National University Bundang Hospital Research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Khalil T, Singh P, Fujimura M, Townsend CM, Jr, Greeley GH, Jr, Thompson JC. Effect of aging on gastric acid secretion, serum gastrin, and antral gastrin content in rats. Dig Dis Sci. 1988;33:1544–1548. doi: 10.1007/BF01535944. [DOI] [PubMed] [Google Scholar]
- 2.Majumdar AP, Jasti S, Hatfield JS, Tureaud J, Fligiel SE. Morphological and biochemical changes in gastric mucosa of aging rats. Dig Dis Sci. 1990;35:1364–1370. doi: 10.1007/BF01536742. [DOI] [PubMed] [Google Scholar]
- 3.Maitra RS, Edgerton EA, Majumdar AP. Gastric secretion during aging in pyloric-ligated rats and effects of pentagastrin. Exp Gerontol. 1988;23:463–472. doi: 10.1016/0531-5565(88)90058-7. [DOI] [PubMed] [Google Scholar]
- 4.Hollander D, Tarnawski A, Stachura J, Gergely H. Morphologic changes in gastric mucosa of aging rats. Dig Dis Sci. 1989;34:1692–1700. doi: 10.1007/BF01540046. [DOI] [PubMed] [Google Scholar]
- 5.Yakabi K, Sakurada T, Takabayashi H, Kani K, Kawashima J. Change in function of gastric acid secretion by aging. Nihon Rinsho. 2010;68:2001–2005. [PubMed] [Google Scholar]
- 6.Nakamura K, Ogoshi K, Makuuchi H. Influence of aging, gastric mucosal atrophy and dietary habits on gastric secretion. Hepatogastroenterology. 2006;53:624–628. [PubMed] [Google Scholar]
- 7.Katelaris PH, Seow F, Lin BP, Napoli J, Ngu MC, Jones DB. Effect of age, Helicobacter pylori infection, and gastritis with atrophy on serum gastrin and gastric acid secretion in healthy men. Gut. 1993;34:1032–1037. doi: 10.1136/gut.34.8.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043–1052. doi: 10.1053/gast.1996.v110.pm8612992. [DOI] [PubMed] [Google Scholar]
- 9.Kang JM, Kim N, Kim B, et al. Enhancement of gastric ulcer healing and angiogenesis by cochinchina Momordica seed extract in rats. J Korean Med Sci. 2010;25:875–881. doi: 10.3346/jkms.2010.25.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang JM, Kim N, Kim B, et al. Gastroprotective action of Cochinchina momordica seed extract is mediated by activation of CGRP and inhibition of cPLA(2)/5-LOX pathway. Dig Dis Sci. 2009;54:2549–2560. doi: 10.1007/s10620-008-0671-6. [DOI] [PubMed] [Google Scholar]
- 11.Lawson DC, Mantyh CR, Pappas TN. Effect of CGRP antagonist, alpha-CGRP 8-37, on acid secretion in the dog. Dig Dis Sci. 1994;39:1405–1408. doi: 10.1007/BF02088041. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Martinez V, St Pierre S, Taché Y. CGRP antagonists enhance gastric acid secretion in 2-h pylorus-ligated rats. Peptides. 1995;16:1257–1262. doi: 10.1016/0196-9781(95)02004-g. [DOI] [PubMed] [Google Scholar]
- 13.Kanai S, Hosoya H, Ohta M, Miyasaka K. Decreased hydrogen-potassium-activated ATPase (H+-K+-ATPase) expression and gastric acid secretory capacity in aged mice. Arch Gerontol Geriatr. 2007;45:243–252. doi: 10.1016/j.archger.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Hamada K, Yamada N, et al. Gastric acid secretion in L-histidine decarboxylase-deficient mice. Gastroenterology. 2002;122:145–155. doi: 10.1053/gast.2002.30312. [DOI] [PubMed] [Google Scholar]
- 15.Kang JM, Kim N, Kim JH, et al. Effect of aging on gastric mucosal defense mechanisms: ROS, apoptosis, angiogenesis, and sensory neurons. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1147–G1153. doi: 10.1152/ajpgi.00218.2010. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara M, Ito M. Influence of aging on gastric ulcer healing activities of cimetidine and omeprazole. Eur J Pharmacol. 2002;444:209–215. doi: 10.1016/s0014-2999(02)01651-5. [DOI] [PubMed] [Google Scholar]
- 17.Goldschmiedt M, Barnett CC, Schwarz BE, Karnes WE, Redfern JS, Feldman M. Effect of age on gastric acid secretion and serum gastrin concentrations in healthy men and women. Gastroenterology. 1991;101:977–990. doi: 10.1016/0016-5085(91)90724-y. [DOI] [PubMed] [Google Scholar]
- 18.Chung SC, Chen TS, Wang JW, et al. Age-related differences in gastric acid secretion and response of gastric inhibitory polypeptide after oral glucose in male rats. Chin J Physiol. 1993;36:219–223. [PubMed] [Google Scholar]
- 19.Trey G, Marks IN, Louw JA, et al. Changes in acid secretion over the years. A 30-year longitudinal study. J Clin Gastroenterol. 1997;25:499–502. doi: 10.1097/00004836-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Haruma K, Kamada T, Kawaguchi H, et al. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277–283. doi: 10.1046/j.1440-1746.2000.02131.x. [DOI] [PubMed] [Google Scholar]
- 21.Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. 2012;47:609–618. doi: 10.1007/s00535-012-0592-1. [DOI] [PubMed] [Google Scholar]
- 22.Saha A, Hammond CE, Beeson C, Peek RM, Jr, Smolka AJ. Helicobacter pylori represses proton pump expression and inhibits acid secretion in human gastric mucosa. Gut. 2010;59:874–881. doi: 10.1136/gut.2009.194795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda H, Li Y, Murakami T, Yamahara J, Yoshikawa M. Protective effects of oleanolic acid oligoglycosides on ethanol- or indomethacin-induced gastric mucosal lesions in rats. Life Sci. 1998;63:PL245–PL250. doi: 10.1016/s0024-3205(98)00426-3. [DOI] [PubMed] [Google Scholar]
- 24.Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- 25.Du GM, Shi ZM, Wei XH, Liu MJ, Zhang L, Zhao RQ. Expression of gastric ghrelin and H(+)-K(+)-ATPase mRNA in weanling piglets and effect of ghrelin on H(+)-K(+)-ATPase expression and activity in gastric mucosal cells in vitro. Res Vet Sci. 2007;82:99–104. doi: 10.1016/j.rvsc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Waki S, Kinoshita Y, Wang HY, et al. Effect of aging on gastrin receptor gene expression in rat stomach. Peptides. 1998;19:225–229. doi: 10.1016/s0196-9781(97)00373-2. [DOI] [PubMed] [Google Scholar]
- 27.Tokumaru S, Iguchi H, Kojo S. Change of the lipid hydroperoxide level in mouse organs on ageing. Mech Ageing Dev. 1996;86:67–74. doi: 10.1016/0047-6374(95)01674-0. [DOI] [PubMed] [Google Scholar]
- 28.Mármol F, Sánchez J, López D, Martínez N, Mitjavila MT, Puig-Parellada P. Oxidative stress, nitric oxide and prostaglandin E2 levels in the gastrointestinal tract of aging rats. J Pharm Pharmacol. 2009;61:201–206. doi: 10.1211/jpp/61.02.0009. [DOI] [PubMed] [Google Scholar]