Abstract
Background/Aims
No standard chemotherapy has been established for advanced gallbladder cancer. The authors studied the activity and tolerability of a gemcitabine and oxaliplatin (GEMOX) combination in unresectable gallbladder cancer (GBC).
Methods
Adult patients with pathologically confirmed unresectable GBC were prospectively recruited at three centers. No patient had received prior chemotherapy or radiotherapy. Patients received cycles of gemcitabine at 1,000 mg/m2 on day 1, followed by oxaliplatin at 100 mg/m2 on day 2, every 2 weeks. The primary study endpoint was time to progression.
Results
Forty patients with unresectable GBC were enrolled. The median age was 60 years (range, 38 to 79 years). All patients showed good performance status. Of the 33 analyzable patients, 12 achieved partial response (36%), 17 stable disease (52%), and four progressive disease (12%). No patient achieved a complete response. The tumor control rate was 88%. At a median follow-up of 6.8 months, the median time to progression was 5.3 months (95% confidence interval [CI], 3.7 to 6.9), and median overall survival was 6.8 months (95% CI, 6.1 to 7.5). Nine of the 40 patients (23%) experienced at least a grade-3 adverse event, but no patient experienced a grade-4 adverse event.
Conclusions
GEMOX combination therapy is a feasible option and is well tolerated in unresectable GBC.
Keywords: Gallbladder neoplasms, Gemcitabine, Oxaliplatin
INTRODUCTION
Biliary tract cancers comprise intrahepatic cholangiocarcinoma (CC), extrahepatic CC, gallbladder cancer (GBC), and papilla of Vater cancer. Of these cancers, GBC shows marked geographic and ethnic variations in incidence.1,2 GBC is an uncommon disease in the West, but its incidence is relatively high in East Asia, for example, it accounts for 1.3% of all malignancies in South Korea.3 Early metastasis, late diagnosis, and frequent recurrence after resection are important features of this disease, and 5-year survival for GBC patients is less than 10%.1,4,5
Although chemotherapy for overall biliary tract cancer has been reported to be beneficial over best supportive care,6 no standard chemotherapeutic regimen has been established. In a recent multicenter phase III trial, it was concluded that the gemcitabine-cisplatin combination is superior to gemcitabine alone in advanced biliary tract cancer,7 and the subgroup analysis conducted showed that combination therapy can also prolong the survival of GBC patients. However, no phase III trial has been conducted exclusively in GBC patients. In the majority of studies, cohorts were composed of highly heterogenous patient groups containing intrahepatic and extrahepatic CC, GBC, papilla of Vater cancer, and even pancreatic cancer. However, it is evident that GBC differs from other bile duct cancers at both the molecular and clinical levels.8,9 One pooled analysis of 104 chemotherapy trials in biliary tract cancer revealed that response rates (RRs) of GBC are higher than those of CC (34.4% vs 20.2%, respectively), but that GBC patients have shorter overall survival (OS) than CC patients (7.2 months vs 9.3 months, respectively).10
Furthermore, the tumor biology of GBC is likely to differ by ethnicity and etiology. Eighty percent of GBC cases are associated with a GB stone in the West, whereas anomalous union of the pancreatobiliary duct is more frequently associated with GBC in East Asia.11,12 In addition, genetic alterations in GBC are dependent on its etiology.13
Despite the lack of a randomized controlled study, single gemcitabine has been used as a chemotherapeutic agent in GBC for a decade, though results have been unremarkable. Five phase II studies14-18 that evaluated the activity of gemcitabine and oxaliplatin (GEMOX) in advanced biliary tract cancer or GBC, concluded that gemcitabine-oxaliplatin is beneficial and has tolerable adverse effects in biliary tract cancer and in GBC. Recently, a phase III study19 of GEMOX with or without erlotinib in advanced biliary tract cancer showed that RR and median OS for gemcitabine plus oxaliplatin group were 16% and 9.5 months, respectively. The aim of the present study was to evaluate gemcitabine-oxaliplatin activity and tolerability exclusively in patients with GBC in Korea, where the etiology of the disease differs appreciably from that found in the West. When this study was initiated, no information was available regarding the efficacy of gemcitabine-oxaliplatin in GBC.
MATERIALS AND METHODS
1. Eligibility criteria
The eligibility criteria applied were as follows. An age of 18 to 80 years with histologically confirmed, locally advanced or metastatic GBC. In addition, patients had not received prior chemotherapy or radiotherapy, had a WHO performance status (PS) of 0 to 2, no central nervous system metastases, no uncontrolled infection, a life expectancy of >3 months, adequate hematological parameters (neutrophils ≥1,500/mm3, platelets ≥100,000/mm3), and adequate renal and liver functions (serum creatinine <1.5 of the upper limit of normal [ULN] and bilirubin <2.5 ULN). Written informed consent was obtained from each patient before enrollment and the study protocol was approved by the institutional review boards of three centers (Seoul National University Hospital, Seoul; the National Cancer Center, Goyang; and Seoul National University Bundang Hospital, Seongnam, Korea).
2. Pretreatment evaluation
Baseline biological analyses (peripheral blood cell count, serum creatinine, bilirubin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and CA19-9 level) were performed within 1 week of the first cycle and tumor size was assessed by computed tomography (CT) less than 3 weeks before starting the first cycle. A physical examination, complete peripheral blood cell count analysis, and chemical tests were performed within 3 days of starting each cycle.
3. Treatment
All 40 patients were treated using the GEMOX regimen, which comprised gemcitabine (Yuhan Co., Ltd., Seoul, Korea) 1,000 mg/m2 as a 10 mg/m2/min (100 minutes) infusion on day 1, followed by oxaliplatin (Yuhan Co., Ltd.) 100 mg/m2 as a 2-hour infusion on day 2. Treatment was repeated fortnightly. If a nonneurological toxicity occurred of severity greater than grade 2 according to the National Cancer Institute Common Toxicity Criteria (NCI CTC) version 3.0, subsequent cycles were administered after recovery and the gemcitabine dose was decreased to 800 mg/m2 (given as an 80-minute infusion) and oxaliplatin to 85 mg/m2. Oxaliplatin was discontinued when a cumulative peripheral sensory neuropathy of NCI CTC grade 3 occurred; affected patients received only gemcitabine thereafter. When laryngopharyngeal dysesthesia occurred, the duration of oxaliplatin infusion was prolonged to 6 hours, but discontinued if symptoms recurred during subsequent cycles. Patients received GEMOX until there was evidence of disease progression or unacceptable toxicity or they refused further treatment. A chemotherapy delay was defined as one of more than 7 days after the scheduled date.
4. Assessment of efficacy
The primary end point of this study was tine to progression (TTP) and secondary endpoints were safety, RR, and OS. Tumor evaluations were performed every 2 months (every four cycles) during therapy using the Response Evaluation Criteria for Solid Tumors,20 or earlier when clinically indicated. TTP was defined as the time from first day of treatment until evidence of clinical progression or tumor progression by CT. OS was defined as time from first day of treatment until death.
5. Statistical analysis
Sample size was calculated on the assumptions that TTP was 3 months for 5-fluorouracil (FU) and that gemcitabine plus oxaliplatin would result in an improvement to 6 months. A one-sided significance test and a 5% type-I error, and a statistical power of 80% were chosen. Estimating a dropout rate of 10%, at least a total of 35 patients were planned to be accrued for this study. An accrual period of 24 months and a follow-up period of 12 months required the inclusion of 35 patients. TTP and OS data were analyzed using the Kaplan-Meier method.
RESULTS
1. Patient characteristics
Forty patients (16 men and 24 women) with unresectable GBC were enrolled from May 2006 to October 2007 (Table 1). The date cutoff for data analysis was October 7, 2008. Median patient age was 60 years (range, 38 to 79 years) and all had a good PS. All patients had locally advanced disease with/without metastasis and no patient had received prior chemotherapy or radiotherapy for GBC.
Table 1.
Characteristics of Patients
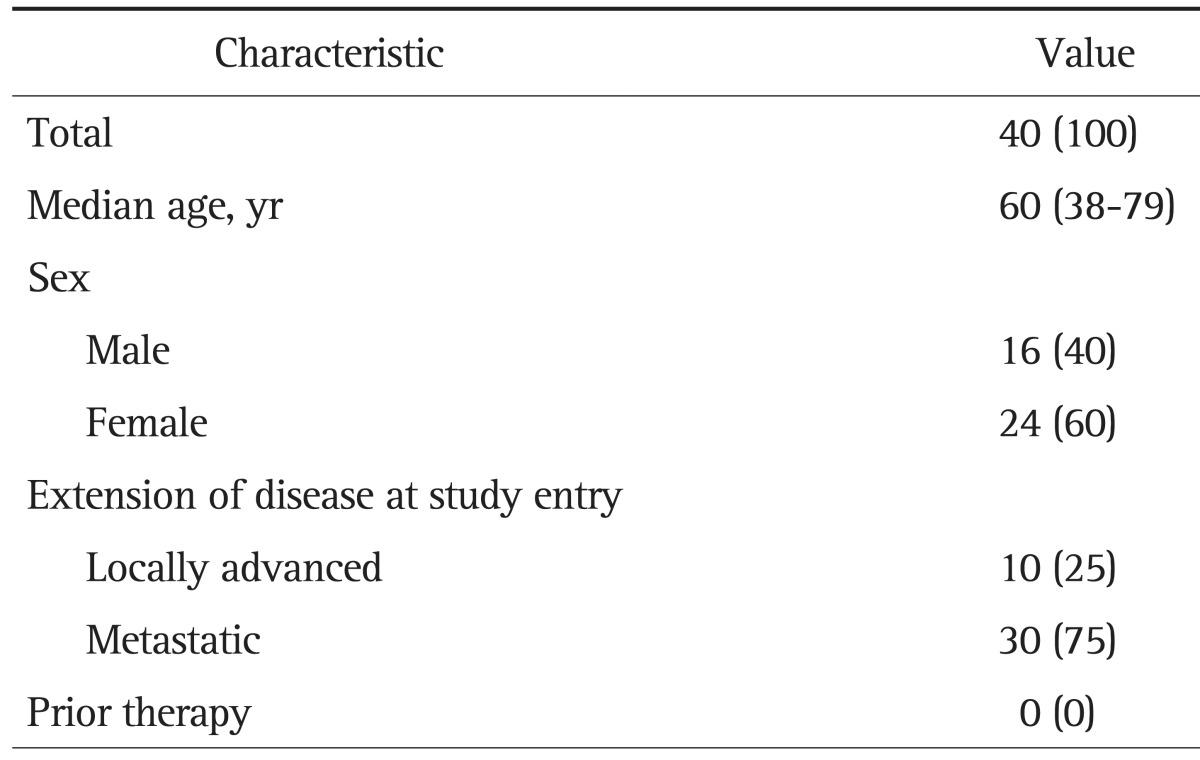
Data are presented as number (%) or median (range).
2. Drug delivery
Patients received a total of 257 cycles of chemotherapy (a median of 5.5 cycles [range, 1 to 12 cycles] per patient). Thirty patients (75%) completed at least four cycles of therapy. The mean cumulative doses of GEMOX received were 9,951 mg (±6,425) and 997 mg (±616), respectively. The average relative dose intensities of GEMOX were 93.2% and 91.6%, respectively. All 257 cycles were administered as scheduled. Gemcitabine dosage was reduced by 25% for one cycle (0.3%) in one patient. Oxaliplatin dosage was reduced for nine cycles (2.7%) in six patients (a 25% reduction for two cycles and by discontinuation for seven cycles).
3. Efficacy and survival
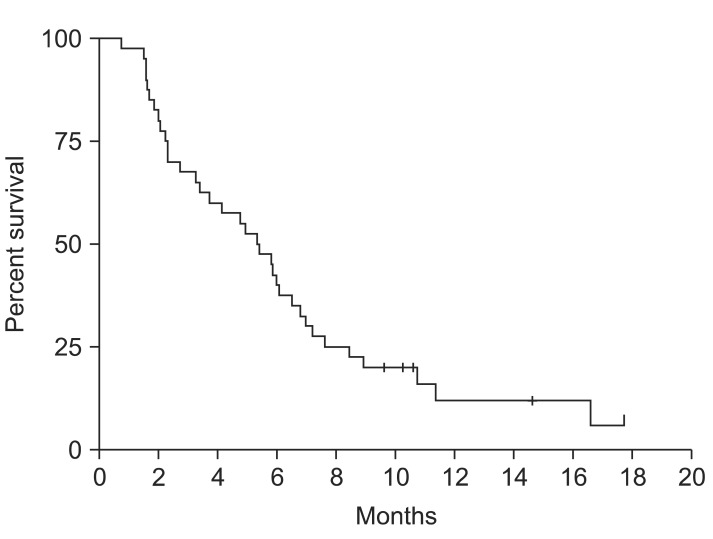
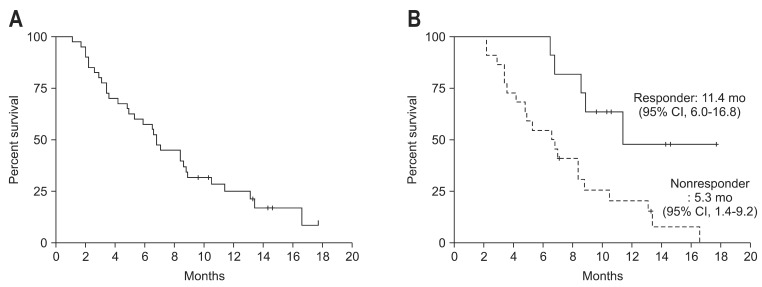
Of the 40 patients, 33 were analyzed for tumor response. Four patients dropped out from the study because of cholangiohepatitis and three refused further chemotherapy after two cycles of chemotherapy without any apparent toxic effect or evidence of disease progression. Of these 33 patients, none achieved complete response (CR) and 12 achieved partial response (PR) (Table 2). Seventeen patients had stable disease (SD) and four progressive diseases. The tumor RR was 36% and the tumor control rate was 88%. Median time to response (CR/PR/SD) was 8.0 weeks (range, 1.7 to 26.7 weeks) from start of therapy. The overall median response-duration was 4.3 months (range, 0.7 to 15.2 months) and the median response-duration of patients that achieved PR was 6.6 months (range, 2.3 to 12.4 months). During a median follow-up of 6.8 months, median TTP was 5.3 months (95% confidence interval [CI], 3.7 to 6.9) (Fig. 1). For these 33 patients, median OS was 6.8 months (95% CI, 6.1 to 7.5) (Fig. 2A), and the 1-year survival rate was 25.0%. Median TTPs for responders and for those with disease stabilization were 10.4 months (95% CI, 6.7 to 14.8) and 5.3 months (95% CI, 3.5 to 7.1), respectively, and median OSs for these two patient group were 11.4 months (95% CI, 6.0 to 16.8) and 7.0 months (95% CI, 3.9 to 10.0), respectively. Median survival of patients who achieved PR was longer than that of nonresponders (SD or PD) (11.4 months vs 5.3 months; p<0.05) (Fig. 2B). Gender, age, and initial disease presentation patterns did not affect OS or TTP.
Table 2.
Best Response according to RECIST Criteria
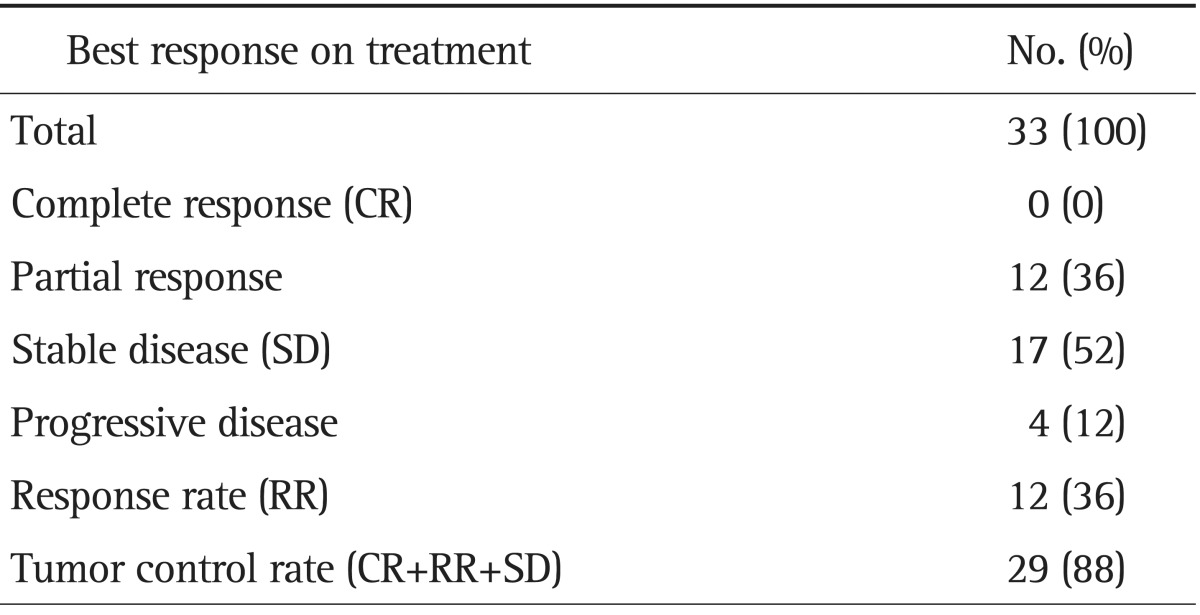
RECIST, Response Evaluation Criteria for Solid Tumor.
Fig. 1.
Kaplan-Meier survival curve for time to progression (n=40).
Fig. 2.
Kaplan-Meier survival curve for overall survival (n=40). (A) Overall survival. (B) Survival curves according to response.
CI, confidence interval.
4. Toxic effects
All 40 patients were analyzed for toxic effects (Table 3). Ten of the 40 (25%) experienced at least a grade 3 adverse event. Adverse events were predominantly grade 1 or 2. Grade 3 hematologic toxic effects were uncommon. The most common grade 3/4 toxicity was neutropenia in three patients (1.2% per cycle). Two patients experienced febrile neutropenia and required brief episodes of hospitalization and empiric antibiotic administration. Grade 3/4 nonhematologic toxic effects were nausea/vomiting in four patients (10%). There were no treatment-related deaths. Cholangiohepatitis without neutropenia developed in four patients after the first or second cycle of chemotherapy and was considered to be due to intrahepatic duct stricture.
Table 3.
Grade-3 or -4 NCI CTC Toxicity per Cycle and per Patient
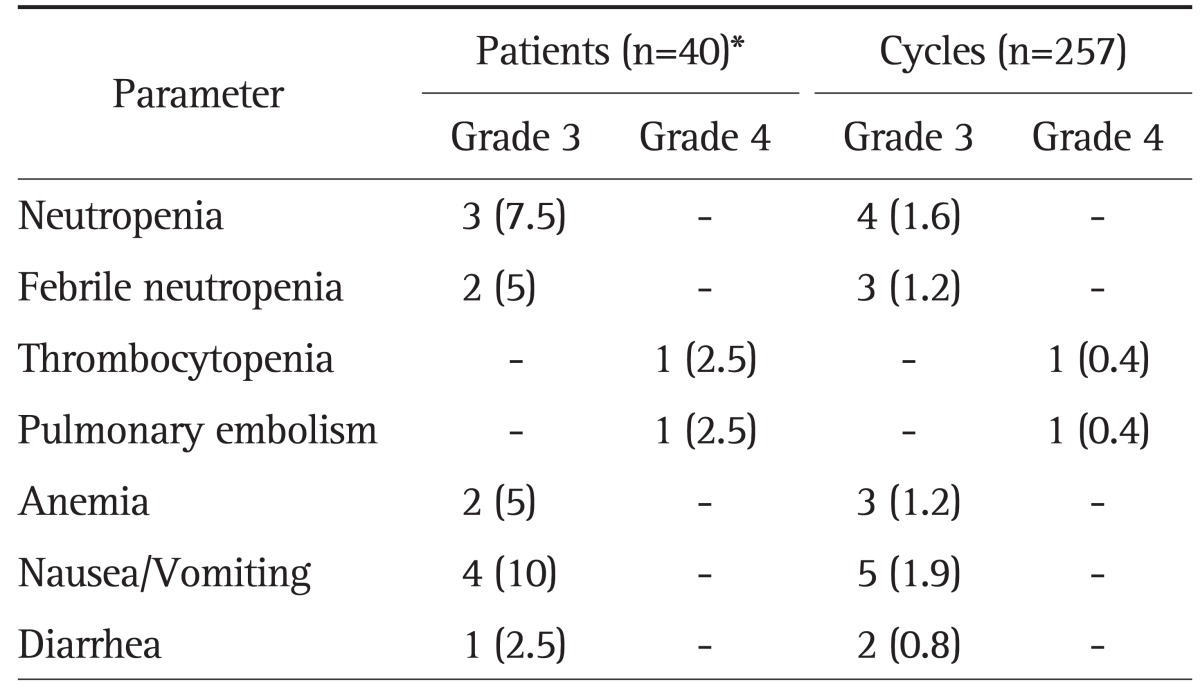
Data are presented as number (%).
NCI CTC, National Cancer Institute Common Toxicity Criteria.
*Ten of 40 patients (25%) experienced a grade-3 or -4 adverse event.
DISCUSSION
No standard chemotherapeutic regimen has been established for advanced GBC. Clinical trials on chemotherapies for GBC have not been actively pursued because it is rare in the West, the difficulties of obtaining an adequate biopsy specimen, and high incidences of complications, such as, cholangiohepatitis and jaundice. A pooled analysis of clinical trials showed that the mean number of GBC patients per clinical trial was only 16.7.10
In the present study, we enrolled 40 GBC patients, which is a relatively large number. Gemcitabine-oxaliplatin resulted in a tumor RR of 36% and temporary abrogation of progression in 88%. Median TTP and OS were 5.3 and 6.8 months, respectively. Only five phase II trials have been conducted exclusively in GBC to determine the activities of palliative chemotherapies;17,21-24 results are summarized in Table 4 and compared with those of present study. The regimens used in these studies were single gemcitabine, 5 FU-cisplatin, or gemcitabine plus platinum agents. However, Malik's study22 should be excluded from such comparisons because the patient number was too small and the regime used was heterogeneous. Taking the four remaining studies and the present study, RRs ranged from 21.2% to 36.6% and median OS from 5 to 7.5 months. However, because none of these studies had a control group, it is difficult to determine the merits and demerits of the regimens used. Hematologic and nonhematologic toxicity of grade 3 or 4 occurred more frequently during gemcitabine-cisplatin than gemcitabine-oxaliplatin studies. Sharma et al.17 used a gemcitabine-oxaliplatin combination like the present study, but the doses and schedules used differed. Whereas they infused gemcitabine at 900 mg/m2 and oxaliplatin at 80 mg/m2 on days 1 and 8 every 3 weeks, we infused gemcitabine at 1,000 mg/m2 on day 1 and oxaliplatin at 100 mg/m2 on day 2 every 2 weeks. However, incidences of grade 3/4 toxicities were similar in the two studies (22% vs 25%).
Table 4.
Results of Phase II Studies Conducted Only on Gallbladder Carcinoma Patients
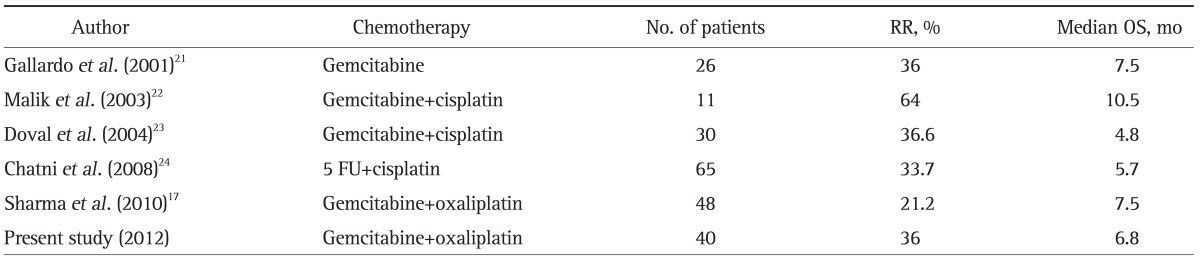
RR, response rate; OS, overall survival.
In a study by Valle et al.,7 although results were obtained by subgroup analysis of heterogenous biliary cancers rather than by analysis of an exclusive GBC population, gemcitabine-cisplatin was found to be superior to gemcitabine alone in GBC patients, especially when PS was good.7 In a recent phase trial of GEMOX with or without erlotinib, median progression-free survival for the patients with GBC was 4.0 months, consistent with our data. Our results showed that median time to progression was 5.3 months (95% CI, 3.7 to 6.9) with a median follow-up of 6.8 months. These findings indicate that gemcitabine-cisplatin and gemcitabine-oxaliplatin regimens are suitable for the treatment of advanced GBC. Further studies are needed to address this issue.
The encouraging results obtained in the present study for a gemcitabine-oxaliplatin based combination therapy, and in the previous studies,15,18-20 suggest that gemcitabine plus a platinum agent can be used to manage locally advanced or metastatic GBC. Summarizing, the present study shows that GEMOX chemotherapy is feasible and safe in Korean patients with advanced GBC.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003;4:167–176. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 2.Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin. 2001;51:349–364. doi: 10.3322/canjclin.51.6.349. [DOI] [PubMed] [Google Scholar]
- 3.Shin HR, Jung KW, Won YJ, Park JG 139 KCCR-affiliated Hospitals. 2002 Annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006;118:1591–1602. doi: 10.1002/ijc.21683. [DOI] [PubMed] [Google Scholar]
- 5.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 6.Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Jarnagin WR, Klimstra DS, Hezel M, et al. Differential cell cycle-regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcome. J Clin Oncol. 2006;24:1152–1160. doi: 10.1200/JCO.2005.04.6631. [DOI] [PubMed] [Google Scholar]
- 9.Yonemoto N, Furuse J, Okusaka T, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843–851. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 10.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura K, Ohto M, Saisho H, et al. Association of gallbladder carcinoma and anomalous pancreaticobiliary ductal union. Gastroenterology. 1985;89:1258–1265. doi: 10.1016/0016-5085(85)90641-9. [DOI] [PubMed] [Google Scholar]
- 12.Park YK, Kim SW, Park YH. A clinical study of gallbladder carcinoma. Korean J Gastroenterol. 1989;21:113–122. [Google Scholar]
- 13.Hanada K, Itoh M, Fujii K, Tsuchida A, Ooishi H, Kajiyama G. K-ras and p53 mutations in stage I gallbladder carcinoma with an anomalous junction of the pancreaticobiliary duct. Cancer. 1996;77:452–458. doi: 10.1002/(SICI)1097-0142(19960201)77:3<452::AID-CNCR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Andre T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 15.Andre T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99:862–867. doi: 10.1038/sj.bjc.6604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HJ, Lee NS, Lee SC, et al. A phase II study of gemcitabine in combination with oxaliplatin as first-line chemotherapy in patients with inoperable biliary tract cancer. Cancer Chemother Pharmacol. 2009;64:371–377. doi: 10.1007/s00280-008-0883-7. [DOI] [PubMed] [Google Scholar]
- 17.Sharma A, Mohanti B, Raina V, et al. A phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother Pharmacol. 2010;65:497–502. doi: 10.1007/s00280-009-1055-0. [DOI] [PubMed] [Google Scholar]
- 18.Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95:848–852. doi: 10.1038/sj.bjc.6603334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Gallardo JO, Rubio B, Fodor M, et al. A phase II study of gemcitabine in gallbladder carcinoma. Ann Oncol. 2001;12:1403–1406. doi: 10.1023/a:1012543223020. [DOI] [PubMed] [Google Scholar]
- 22.Malik IA, Aziz Z, Zaidi SH, Sethuraman G. Gemcitabine and Cisplatin is a highly effective combination chemotherapy in patients with advanced cancer of the gallbladder. Am J Clin Oncol. 2003;26:174–177. doi: 10.1097/00000421-200304000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Doval DC, Sekhon JS, Gupta SK, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516–1520. doi: 10.1038/sj.bjc.6601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatni SS, Sainani RS, Mehta SA, Mohandas KM. Infusion chemotherapy with cisplatinum and fluorouracil in the treatment of locally-advanced and metastatic gallbladder cancer. J Cancer Res Ther. 2008;4:151–155. doi: 10.4103/0973-1482.43341. [DOI] [PubMed] [Google Scholar]