Abstract
Background/Aims
The differential diagnosis of pancreatic solid lesions remains challenging. The aim of this study was to investigate the accuracy of contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) in differentiating pancreatic cancer from benign lesions.
Methods
We prospectively evaluated 37 patients with pancreatic solid lesions. After intravenous injection of a contrast agent (SonoVue), CEH-EUS was performed using a radial echoendoscope. Pancreatic solid lesions were classified into three vascular patterns (hyperintense, isointense, and hypointense) on the basis of CEH-EUS imaging, and these patterns were compared to the histological diagnosis.
Results
The lesions were hypervascular (n=6), isovascular (n=3), or hypovascular (n=28). Histological diagnosis was confirmed by EUS-FNA in 26 patients (22 adenocarcinomas, two focal pancreatitis, one pancreatic neuroendocrine tumor [NET], and one pancreatic tuberculosis); by surgery in 10 patients (four adenocarcinomas, three pancreatic NETs, two invasive intraductal papillary mucinous neoplasms, and one acinar cell carcinoma); and by both methods in one patient. Among pancreatic carcinomas, 28 out of 30 lesions (93%) had persistent hypovascular signals in the early and late phase, which indicates a sensitivity and diagnostic accuracy of 93% and 92%, respectively for the diagnosis of pancreatic cancer.
Conclusions
CEH-EUS was useful for characterization of pancreatic solid masses with high sensitivity and accuracy.
Keywords: Pancreatic neoplasms, Endosonography, Contrast-enhanced
INTRODUCTION
Differential diagnosis of pancreatic solid lesions remains challenging. Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) allows real-time perfusion imaging without Doppler-related artifacts, and visualizes not only parenchymal perfusion but also the microvasculature of the pancreas.1 Several studies have reported the usefulness of CEH-EUS in distinguishing pancreatic tumors from benign lesions.2-4 Although CEH-EUS has also been introduced in Korea, it is performed in few institutions.
The gold standard of diagnosis of pancreatic solid lesions is a pathological examination of histological specimens by surgery or EUS-guided fine-needle aspiration (FNA). However, there are cases where the diagnosis is still difficult using EUS-FNA because the aspirant contains insufficient tumor material, and there are contraindications to EUS-FNA, such as coagulopathy. CEH-EUS, therefore, is a promising method of detecting malignancy due to its noninvasiveness. We have prospectively evaluated CEH-EUS for the investigation of patients with pancreatic solid lesions since 2010. The aim of this study was to investigate the accuracy of CEH-EUS in differentiating pancreatic cancer from other types of pancreatic solid lesions by imaging the microvasculature of the pancreas.
MATERIALS AND METHODS
1. Patients
Between November 2010 and June 2012, a total of 37 consecutive patients with solid pancreatic lesions were prospectively examined using B-mode EUS and CEH-EUS. This study was approved by our local ethics committee.
Inclusion criteria for the study were an undetermined, solitary, predominantly solid lesion with a definite histologically proven diagnosis. The lesions were first detected by transabdominal ultrasound, computed tomography (CT), or magnetic resonance imaging.
Exclusion criteria were the presence of a cystic component greater than 50% of the total volume of the lesion, pregnancy, lactation, age under 18 years, and a contraindication to SonoVue (Bracco, Milan, Italy).
2. Gold standard of diagnosis
The final diagnosis was obtained either surgically or by aspiration cytology/histology with EUS-FNA. Both techniques were performed if needed. When the result of the first EUS-FNA was not diagnostic, the procedure was repeated.
3. Standard EUS and CEH-EUS techniques
A commercially available radial echoendoscope (GIF-UE260; Olympus, Tokyo, Japan) was used for B-mode EUS and CEH-EUS. The ultrasound processor was the Aloka α-10 (Aloka Co., Ltd., Tokyo, Japan), which incorporates dedicated software for the CEH-EUS. The EUS examinations were performed under conscious sedation using meperidine and midazolam. All patients in the study underwent conventional B-mode scanning before CEH-EUS was performed. The location, size and EUS characteristics of the lesion were documented.
After performance of a complete EUS examination of the pancreas in B-mode, the echoendoscope was switched to the CEH-EUS mode. For CEH-EUS, SonoVue was used as the contrast agent. The extended pure harmonic detection mode with a transmitting frequency of 3.4 MHz was used for CEH-EUS. After intravenous injection of 2.4-mL SonoVue, CEH-EUS was begun immediately. A second dose of 2.4-mL SonoVue was administered if conclusions could not be drawn from the first examination.
For visualization of the lesion, the echoendoscope was placed in front of the pancreatic area of interest. Real-time continuous observation of the entire lesion was done progressively. The examination lasted until 150 seconds after SonoVue bolus injection for complete observation of the arterial and venous phases. A 30 seconds after SonoVue was regarded as the early phase, followed by the late phase (30 to 120 seconds).
CEH-EUS images were recorded on a digital video system. Three expert endoscopists (C.S.S., Y.K.C., and T.Y.L.) in our hospital evaluated the imaging findings. In all pancreatic lesions, vascularity was determined in comparison with the surrounding pancreatic tissue, and was classified as hypovascular, isovascular, or hypervascular in the early and late phase on the basis of CEH-EUS imaging. We classified the change of enhancement level according to vascularity in the early and late phase into three types; type I (persisted hypovascular pattern in the early and late phase), type II (isovascular or hypervascular in the early phase and hypovascular in the late phase), type III (isovascular or hypervascular in the early phase and isovascular or hypervascular in the late phase). These patterns were compared with the final diagnosis made from surgery or EUS-FNA. We also described three patterns of pancreatic mass enhancement compared to the normal pancreatic tissue (fast, simultaneous, or slow), two types of washout (fast or slow) and two types of distribution (homogeneous, inhomogeneous).
4. Statistical analysis
Statistical analyses were performed using the PASW software version 18.0 (IBM Co., Armonk, NY, USA). Data are presented as means, ranges, and 95% confidence intervals. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for CEH-EUS.
RESULTS
A total of 37 patients with a solid pancreatic lesion were included (24 males, 13 females; mean age 62.3±13.3 years; range 24 to 82 years). Average lesion size was 3.4±0.8 cm (range, 1.7 to 5.6 cm). The tumor involved the head of the pancreas in 20 patients, the body in 10, and the tail in seven. The echo features with B-mode EUS were hyperechoic (n=6) or hypoechoic (n=31). The final diagnoses were: pancreatic adenocarcinoma, 27; neuroendocrine tumor (NET), four; invasive intraductal papillary mucinous tumor (IPMN), two; focal pancreatitis, two; acinar cell carcinoma, one; and pancreatic tuberculosis, one. A histological specimen was obtained in 26 patients by EUS-FNA (24 by first EUS-FNA, two by repeated EUS-FNA); 10 patients by surgery; and one patient by both methods. EUS-FNA was inadequate for histological diagnosis in one patient with pancreatic adenocarcinoma. This patient underwent subsequent surgery that allowed pathological diagnosis. The final diagnosis in each case according to surgical pathology or EUS-FNA is shown in Table 1. In conventional B-mode EUS, the lesions were hyperechoic (n=2), isoechoic (n=2), or hypoechoic (n=33).
Table 1.
Baseline Patient and Lesion Characteristics
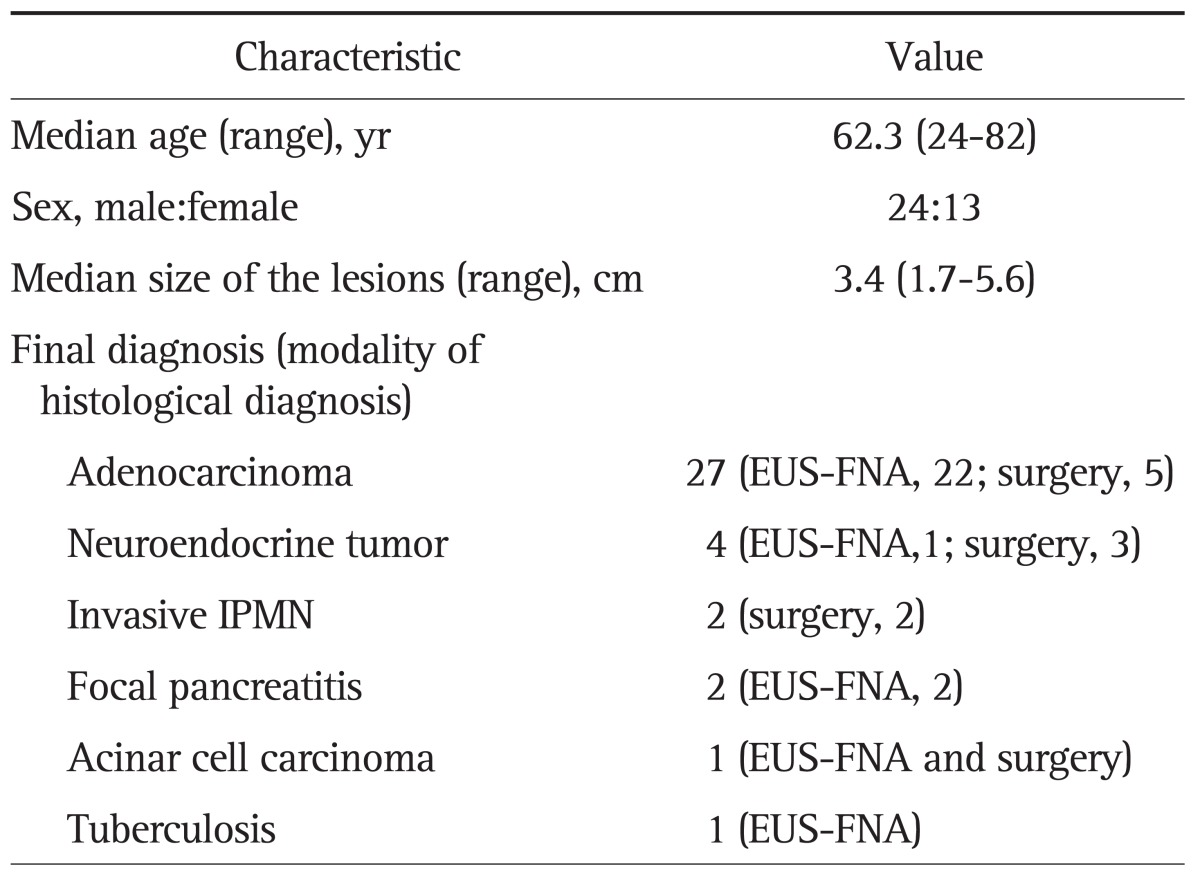
EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; IPMN, intraductal papillary mucinous neoplasm.
The microvasculature of pancreatic lesions and the surrounding pancreas was visualized using the harmonic imaging mode after SonoVue infusion. Injection of 4.8-mL SonoVue was needed in one patient. No complications occurred related to the use of SonoVue. Interpretation of CEH-EUS images was possible for all patients.
Primary pancreatic carcinoma, including adenocarcinoma (Fig. 1), invasive IPMN, and acinar cell carcinoma (Fig. 2), was the most frequent lesion (81%). Comparison of CEH-EUS findings with the final diagnoses showed that 28 of 30 pancreatic carcinomas had hypointense echo signals in early phase (Table 2). One patient with pancreatic carcinoma who showed isointensity in the early phase revealed hypointense echo signal in the late phase. One patient with acinar cell carcinoma showed a heterogenous, hypoenhancing pattern. Regarding invasive IPMN, one invasive IPMN case with a mural nodule showed branch-shaped enhancement in the mural nodule and hypoenhancement of the cyst wall and the other case without a mural nodule showed hypoenhancement of the cyst wall only. Twenty-eight of 30 pancreatic carcinomas (94%) showed type I enhancement (persisted hypointensity). Regarding speed of mass enhancement, 26 of 30 pancreatic carcinomas (87%) had slow enhancement compared to surrounding pancreatic tissue. In 30 pancreatic carcinomas, 28 (94%) showed fast washout and 28 turned out to be inhomogenous enhancement pattern. CEH-EUS revealed hypointensity and thus made a correct diagnosis in one adenocarcinoma with a false-negative result by EUS-FNA.
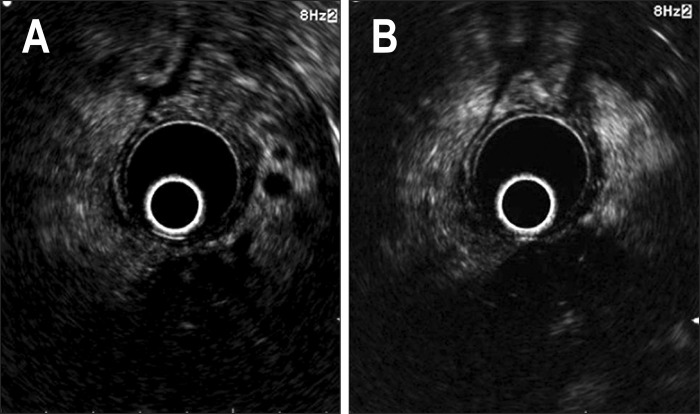
Fig. 1.
Pancreatic adenocarcinoma. (A) The tumor shows a hypointense echo signal with fine branches in the early phase. (B) Persistent hypointensity was noted in the late phase.
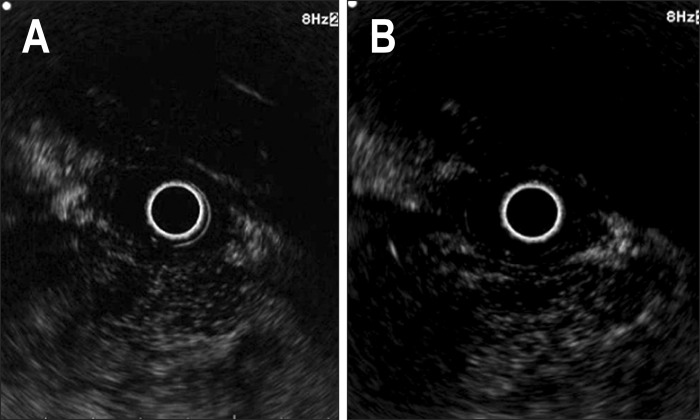
Fig. 2.
Pancreatic acinar cell carcinoma. (A) The tumor shows a hypointense echo signal with heterogeneous enhancement in the early phase. (B) Persistent hypointensity and heterogeneous enhancement were noted in the late phase.
Table 2.
Comparison of Contrast-Enhanced Harmonic Endoscopic Ultrasound and Final Diagnoses
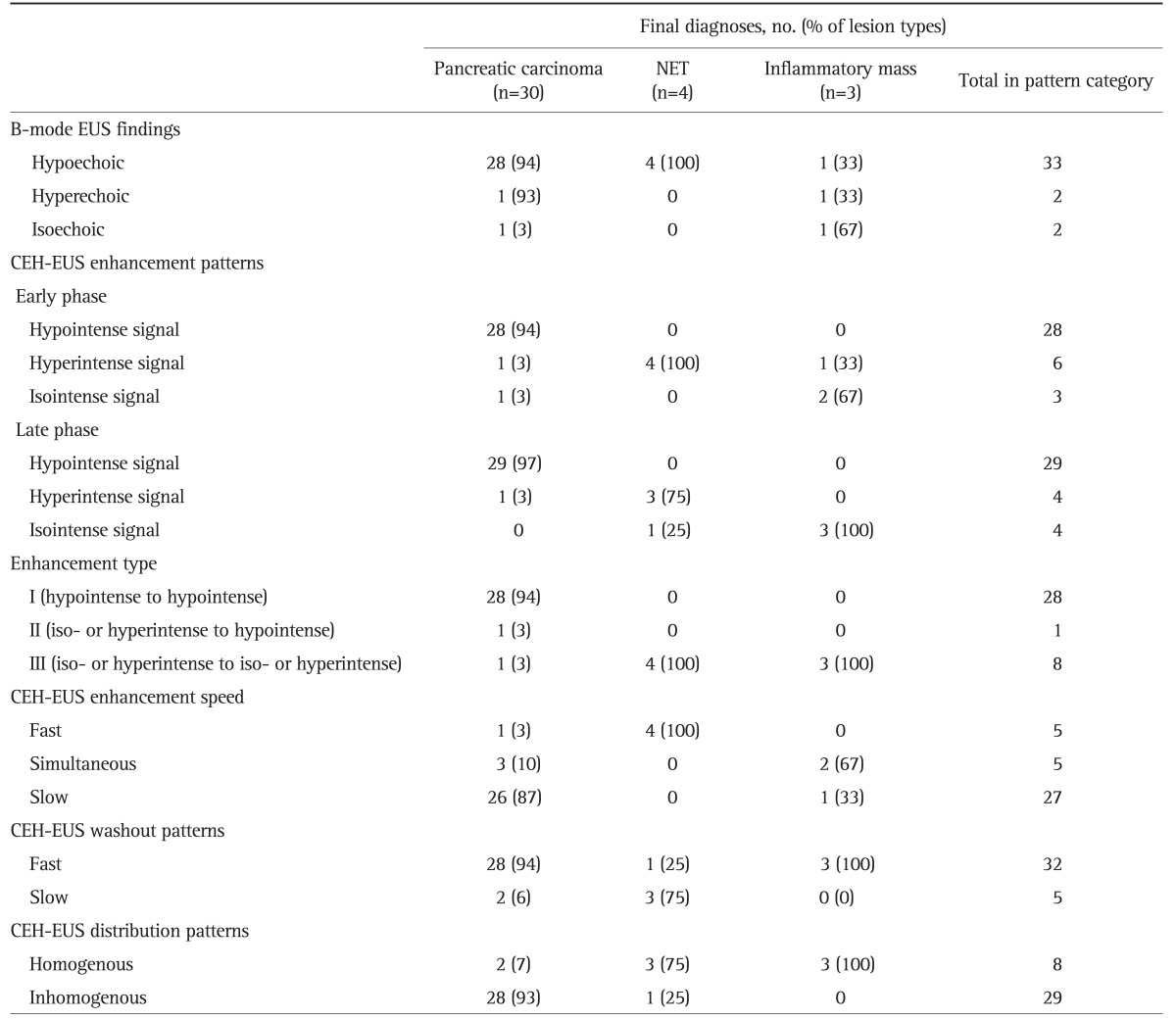
NET, neuroendocrine tumor; CEH-EUS, contrast-enhanced harmonic endoscopic ultrasound.
All four NETs (Fig. 3) showed hyperintense signals in early phase and three of those maintained hyperintense signals in the late phase (Table 2). All patients with NETs showed fast enhancement, and three of four revealed a slow washout pattern and homogenous enhancement pattern. One case of pancreatic tuberculosis had hyperintensity in the early phase and isointensity in the late phase (Fig. 4). Regarding focal pancreatitis, all two cases were isointense in the early and late phase. Two of three inflammatory masses revealed a simultaneous enhancement speed pattern. All inflammatory masses, including tuberculosis and focal pancreatitis, showed homogenous enhancement and a slow washout pattern. All NETs and inflammatory masses showed type III (isointense or hyperintense to isointense or hyperintense) enhancement pattern.
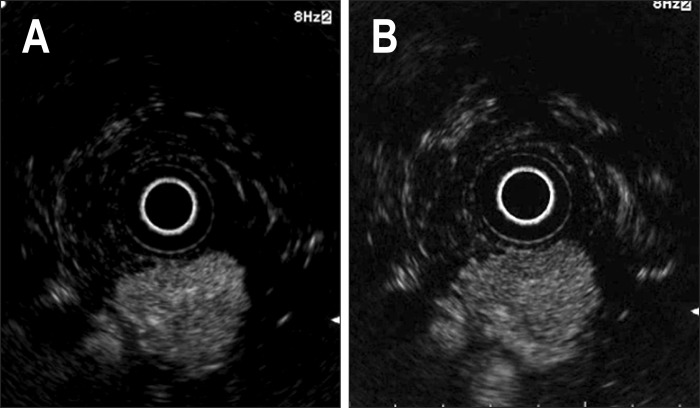
Fig. 3.
Pancreatic neuroendocrine tumor. (A) The tumor shows a diffuse hyperintense echo signal in the early phase. (B) Hyperintensity was persistent in the late phase.
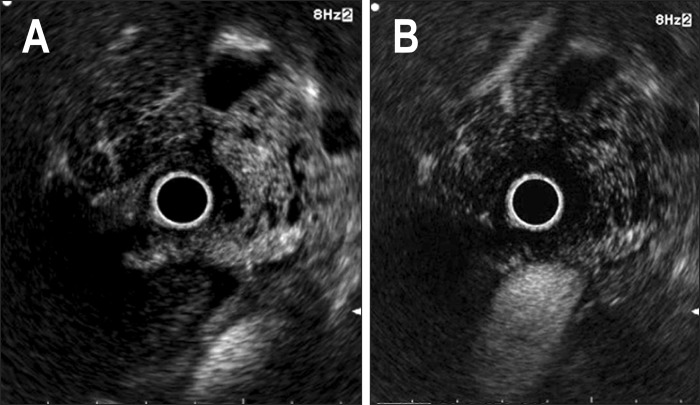
Fig. 4.
Pancreatic tuberculosis. (A) The lesion shows a hyperintense echo signal with septated cysts. (B) Hyperintensity became isointensity in the late phase.
Hypovascular echo signals as a sign of pancreatic carcinoma had a sensitivity of 93%, specificity of 86%, PPV of 93%, NPV of 75%, and accuracy of 92%. Hyperintense or isointense echo signals had a sensitivity of 86%, specificity of 93%, PPV of 75%, NPV of 96%, and accuracy of 92% for the diagnosis of other than pancreatic carcinoma.
DISCUSSION
The differential diagnosis of pancreatic solid lesions is sometimes challenging using conventional imaging modalities. Despite its ability to detect small pancreatic lesions with high sensitivity, EUS alone has limitations in differential diagnosis of pancreatic cancer and non-neoplastic pancreatic masses.5 Recently, CEH-EUS was introduced and has made possible evaluation of the dynamic enhancement of various pancreatic lesions. Contrast-enhanced techniques provide information on vascularity and blood flow in normal and pathological pancreatic tissues.6
To our knowledge, this is the first study in Korea to investigate the performance of CEH-EUS in differentiation of pancreatic solid masses. Although the sample size was relatively small, the present study provides evidence supporting CEH-EUS as a useful tool for the characterization of pancreatic solid lesions. In this study, the sensitivity, specificity, and accuracy of CEH-EUS in the diagnosis of pancreatic carcinoma were 93%, 86%, and 92%, respectively. This is not substantially different from those of previous overseas studies that reported sensitivities of 82% to 97% and specificities of 89% to 100%.
However, the present study provided additional information regarding CEH-EUS for the differentiation of focal pancreatic masses. Most previous studies have used linear prototype EUS to perform CEH-EUS;4,7 however, we used a commercially available radial EUS. Although linear EUS has color-flow Doppler capability and enhanced tissue resolution, newer electronic radial EUS also offers color-flow Doppler and similar tissue resolution for CEH-EUS as for linear methods.8 Moreover, radial EUS provides a cross-sectional image similar to a CT scan and a 360° sonographic view, which may facilitate anatomical distinction and evaluation of CEH enhancement pattern, including the surrounding pancreatic tissue. Our data suggest that radial EUS imaging for CEH-EUS is not inferior to linear imaging.
We observed typical enhancement patterns for most ductal adenocarcinoma with a hypovascular and inhomogenous appearance, for most NETs with a hypervascular and homogenous appearance and for most inflammatory masses with an isovascular or hypervascular and homogenous appearance. We also found the enhancement level in early phase was maintained to late phase in most patients with three diseases category. Twenty-eight cases of 30 pancreatic carcinoma (94%) showed a persisted hypovascularity, three of four NETs (75%) showed a persisted hypervascularity, and two of three inflammatory masses (67%) showed a persisted isovascularity. So the change of enhancement level may be important for the differential diagnosis as well as the enhancement level in the early phase.
CEH-EUS made a correct diagnosis by detecting a hypovascular pattern, even in one patient with a false-negative result in EUS-FNA. Although CEH-EUS is useful for ruling out ductal adenocarcinoma, making the differential diagnosis between NET and pseudotumoral pancreatic mass is difficult because both diseases can have an isovascular or hypervascular appearance.9 We found that contrast enhancement and washout speed differed between carcinoma, NET, and inflammatory masses. Regarding the enhancement speed of pancreatic masses, that of most carcinomas (26/30, 87%) was slower than normal pancreatic tissue; that of inflammatory masses (2/3, 67%) was simultaneous; and that of NETs (100%) was faster. Fast washout was observed in 28 of 30 carcinomas (93%) and all three inflammatory masses, whereas slow washout was observed in three of four NETs. In accordance with this, Fusaroli et al.2 reported that fast washout in the majority of pancreatic carcinoma and inflammatory mass and that slow washout indicated NET. Therefore, among the isovascular/hypervascular masses that were difficult to diagnose differentially, fast enhancement and slow washout suggested NET, and simultaneous enhancement and fast washout indicated an inflammatory mass more likely.
Although exocrine tumors other than ductal adenocarcinoma comprise about 10% of all exocrine tumors of the pancreas, their typical imaging pattern has not been well described. We found one acinar cell carcinoma and two IPMNs with invasive adenocarcinoma also had hypovascularity, slow enhancement and fast washout. Two studies including a total of four patients reported that acinar cell carcinoma showed moderate, heterogenous enhancement in contrast-enhanced ultrasound due to its moderate vascularization or slight hypervascularization.10,11 In our study, one patient with acinar cell carcinoma showed heterogenous, hypoenhancing pattern. Since our study included only one acinar cell carcinoma, it is difficult to say why it showed a hypoenhancement pattern, in contrast to previous reports. Regarding invasive IPMN, the cyst walls showed hypoenhancement and the mural nodules revealed branch-shaped enhancement patterns in previous studies.12,13 Kurihara et al.13 reported that the branch-shaped pattern lesion in IPMN was associated with carcinoma. Similarly, one invasive IPMN case with a mural nodule in our study showed branch-shaped enhancement in the mural nodule and hypoenhancement of the cyst wall.
In summary, this study demonstrates that CEH-EUS is a promising adjunctive modality for differential diagnosis of pancreatic solid lesions. By evaluating microvascularization in the early and late phase, enhancement speed, and washout pattern, CEH-EUS may help to distinguish adenocarcinoma from other masses and differentiate between NET and inflammatory masses. A larger prospective study is needed to clarify the clinical role of CEH-EUS in the evaluation of pancreatic solid masses.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 2.Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–634. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–597. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564–570. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 5.Kitano M, Sakamoto H, Komaki T, Kudo M. New techniques and future perspective of EUS for the differential diagnosis of pancreatic malignancies: contrast harmonic imaging. Dig Endosc. 2011;23(Suppl 1):46–50. doi: 10.1111/j.1443-1661.2011.01146.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakamoto H, Kitano M, Kamata K, El-Masry M, Kudo M. Diagnosis of pancreatic tumors by endoscopic ultrasonography. World J Radiol. 2010;2:122–134. doi: 10.4329/wjr.v2.i4.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303–310. doi: 10.1038/ajg.2011.354. [DOI] [PubMed] [Google Scholar]
- 8.Stevens T, Zuccaro G, Jr, Dumot JA, et al. Prospective comparison of radial and linear endoscopic ultrasound for diagnosis of chronic pancreatitis. Endoscopy. 2009;41:836–841. doi: 10.1055/s-0029-1215061. [DOI] [PubMed] [Google Scholar]
- 9.Saftoiu A, Dietrich CF, Vilmann P. Contrast-enhanced harmonic endoscopic ultrasound. Endoscopy. 2012;44:612–617. doi: 10.1055/s-0032-1308909. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Goto H, Hirooka Y, et al. Contrast-enhanced transabdominal ultrasonography in the diagnosis of pancreatic mass lesions. Acta Radiol. 2003;44:103–106. [PubMed] [Google Scholar]
- 11.D'Onofrio M, Malagò R, Zamboni G, et al. Contrast-enhanced ultrasonography better identifies pancreatic tumor vascularization than helical CT. Pancreatology. 2005;5:398–402. doi: 10.1159/000086540. [DOI] [PubMed] [Google Scholar]
- 12.Recaldini C, Carrafiello G, Bertolotti E, Angeretti MG, Fugazzola C. Contrast-enhanced ultrasonograpic findings in pancreatic tumors. Int J Med Sci. 2008;5:203–208. doi: 10.7150/ijms.5.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurihara N, Kawamoto H, Kobayashi Y, et al. Vascular patterns in nodules of intraductal papillary mucinous neoplasms depicted under contrast-enhanced ultrasonography are helpful for evaluating malignant potential. Eur J Radiol. 2012;81:66–70. doi: 10.1016/j.ejrad.2010.11.027. [DOI] [PubMed] [Google Scholar]