Abstract
Background/Aims
Erlotinib and gemcitabine combined chemotherapy is becoming the treatment of choice in advanced pancreatic cancer. We evaluated the effectiveness of treatment with erlotinib plus gemcitabine and the prognostic factors for chemotherapeutic response in Korean pancreatic cancer patients.
Methods
Sixty-nine patients with advanced pancreatic cancer who were treated with daily erlotinib 100 mg orally and gemcitabine 1,000 mg/m2/30 min intravenous infusion on days 1, 8, and 15 of each 4-week cycle from 2006 to 2009 were included in this study. This study was a phase II single-center trial.
Results
All 69 patients with advanced pancreatic cancer were chemotherapy-naïve. The objective response rate was 18.8%, and the overall tumor-stabilization rate was 49.2%. The median overall survival was 7.7 months (95% confidence interval [CI], 6.0 to 9.4 months). The median progression-free survival was 1.9 months (95% CI, 1.4 to 2.5 months). Prognostic factors for good chemotherapeutic response were good performance status and the presence of skin rash during chemotherapy. Patients with lower performance scores showed worse chemotherapeutic responses (odds ratio [OR], 7.6; 95% CI, 2.4 to 24.8). Poor responses were predicted by the absence of skin rash during chemotherapy (OR, 3.0; 95% CI, 1.4 to 6.3).
Conclusions
Erlotinib and gemcitabine chemotherapy is a tolerable treatment regimen and has a favorable therapeutic effect in Korean patients with advanced pancreatic cancer.
Keywords: Gemcitabine, Erlotinib, Pancreatic neoplasms
INTRODUCTION
Pancreatic cancer is a devastating disease and one of the major causes of cancer-related deaths worldwide. It has also been reported as the fifth leading cause of cancer-related mortality in Korea. Furthermore, only 20% of patients with pancreatic cancer have a resectable state at the time of diagnosis.1 Recently, the incidence of pancreatic cancer has increased in Korea. A modern life style and cigarette smoking are the main factors underlying the increase in incidence of pancreatic cancer in Asian patients.2
Since gemcitabine was approved in 1996 by Food and Drug Administration, this purine analog has become the standard of treatment for advanced pancreatic cancer, and has been shown to improve survival.3,4 However, patients who undergo gemcitabine-based chemotherapy for advanced pancreatic cancer still have an overall survival of under 6 months.5,6
Recently, novel molecular agents that target specific biologic pathways that are activated in cancer have been developed to treat solid tumors. Epidermal growth factor receptor (EGFR)-mediated cell signaling is one of the main therapeutic targets of these novel molecular agents. EGFR is a member of the ErbB family of membrane receptors that are involved in cell differentiation, proliferation, apoptosis, invasion, and metastasis.7 Erlotinib (EGFR tyrosine kinase inhibitor [TKI]) is an orally bioavailable small molecule that inhibits the enzymatic activity of EGFR by binding at the adenosine triphosphate site of the receptor's tyrosine kinase region.7,8
EGFR targeting has shown promising results in patients with advanced pancreatic cancer and nonsmall cell lung cancer (NSCLC).9 In particular, subgroup analysis of previous clinical trials in NSCLC showed that certain patients with distinct clinical and histologic characteristics, namely East Asian patients, women, and those with adenocarcinoma, responded favorably to EGFR TKIs, gefitinib, or erlotinib.9-11 In a recent randomized phase III trial in patients from Western countries, those with advanced pancreatic cancer treated with erlotinib plus gemcitabine combined chemotherapy showed better survival than those patients that received gemcitabine monotherapy.12
In this study, we evaluated the effectiveness of treatment with erlotinib plus gemcitabine chemotherapy, and also identified prognostic factors of chemotherapeutic response in Korean patients with advanced pancreatic cancer to see if there is a similar ethnical advantage to treatment as has been demonstrated for NSCLCs.
MATERIALS AND METHODS
1. Eligibility
Sixty-nine patients with advanced pancreatic cancer who were treated with daily erlotinib and gemcitabine on day 1, 8, and 15 of each 4 weeks between December 2006 and March 2009 at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea were included in this phase II trial. Following inclusion criteria was used for enrollment. Patients with histologically or cytologically confirmed metastatic pancreatic cancer or locally advanced pancreatic cancer were enrolled. In addition, patients who had received previous chemotherapy were not included. Further eligibility criteria included age >20 years, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3, and adequate organ function. In detail, bone marrow function was adequate as indicated by a white blood cell count >3,000/µL, a hemoglobin level >9 g/dL, and a platelet count >100,000/µL. Adequate hepatic function was satisfied to a total bilirubin level <3 mg/dL with adequate biliary decompression, and a serum transaminase level <5 times the upper limit of normal, and renal function was adequate (a creatinine level <1.5 mg/dL). All patients were informed of the investigational nature of the study.
2. Treatment
All patients received oral administration of erlotinib (100 mg) daily, and gemcitabine 1,000 mg/m2 was infused intravenously over 30 minutes weekly (days 1, 8, and 15) followed by a 1-week rest per 4 weeks cycle.
The primary endpoint was overall survival. The secondary endpoints were progression-free survival and response rate. Toxicities and risk factors for chemotherapeutic response were also evaluated.
3. Response assessment and toxicity profiles
Patients' medical history, physical examination results, and ECOG performance status were evaluated before chemotherapy. Laboratory tests, including hematologic, biochemical profiling, and tumor marker profiling, were performed, as was computed tomography or magnetic resonance imaging. Chemotherapy efficacy was assessed by monitoring the patients' responses after every 8 weeks of chemotherapy. According to the Response Evaluation Criteria In Solid Tumors (RECIST) criteria, a complete response (CR) was defined as the disappearance of all measurable disease for 8 weeks, a partial response (PR) as at least a 30% decrease in the sum of the products of maximum diameter and a perpendicular diameter of all measurable lesions, stable disease (SD) as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD), and PD as at least a 20% increase in the sum of the products of maximum diameter and a perpendicular diameter of all measurable lesions since the treatment started or the appearance of one or more new lesions.
Time to progression was defined as the interval between the initiation of therapy and the occurrence of disease progression. Survival duration was defined as time from the initiation of therapy to death, or to last contact.
Patients were divided into two groups according to their chemotherapeutic response. Patients with CR, PR, and SD were defined as good responders, whereas those with progressed disease were classified as poor responders. Toxicity assessment was based on the World Health Organization toxicity criteria.
4. Statistical analysis
Continuous data are expressed as means±SD or medians when appropriate. Categorical data are expressed as the number of subjects (or proportion) with a specified condition or clinical variable. The significance of differences between continuous variables was evaluated using Student t-test, while the chi-square test was used for categorical data. Median overall survival was estimated by Kaplan-Meier analysis. The association between progression-free survival and risk variables was assessed by multivariate Cox regression analysis. All analyses were performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). A p<0.05 was considered significant.
RESULTS
1. Patient characteristics
All 69 patients who had not received prior chemotherapy after diagnosis were confirmed to have advanced pancreatic cancer. Advanced pancreatic cancer was defined as inoperable locally advanced cancer (six patients, 8.7%) or distantly metastatic cancer (63 patients, 91.3%) at the time of diagnosis. The median age was 62 years (range, 31 to 82 years), and 50 patients (72.5%) were male. Levels of CA19-9 were elevated in 55 patients (79.7%); the median CA19-9 level was 1,990 U/mL (range, 45.3 to 20,000 U/mL). The clinical characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics of Patients
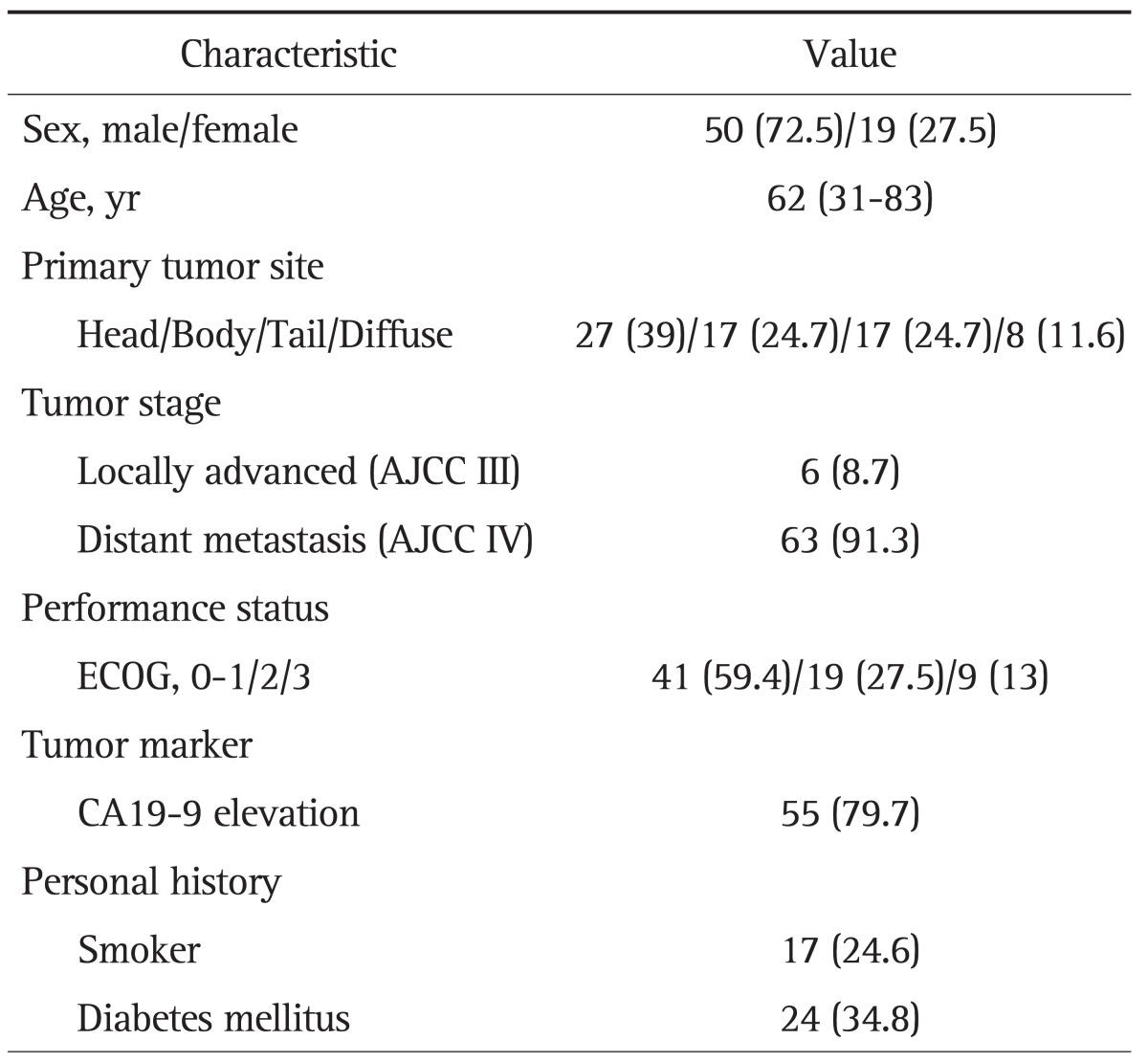
Data are presented as number (%) or median (range).
AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group.
2. Objective responses and overall survival
The objective response rate (ORR) is described in Table 2. There were no CR, but 13 patients showed PR. The overall ORR means percentage of patients whose cancer shrinks or disappears after treatment. ORR was 18.8%, and the tumor stabilization rate, including ORR and SD, was 49.3%. The median time to progression of disease was 1.9 months (95% CI, 1.4 to 2.5 months) (Fig. 1). The median overall survival was 7.7 months (95% CI, 6.0 to 9.4 months), as shown in Fig. 2. Although patients with skin rash tended to have better survival, the number of overall survival days according to the presence of skin rash was not significantly different between the two groups in this study (9.1 months in patients with skin rash vs 6.3 months in patients without skin rash; p=0.32). Patients were divided into two groups according to their response to chemotherapy. The good responder group showed better progression-free survival than the poor responder group (4.2 months vs 1.4 months; p=0.001).
Table 2.
Tumor Responses of Patients
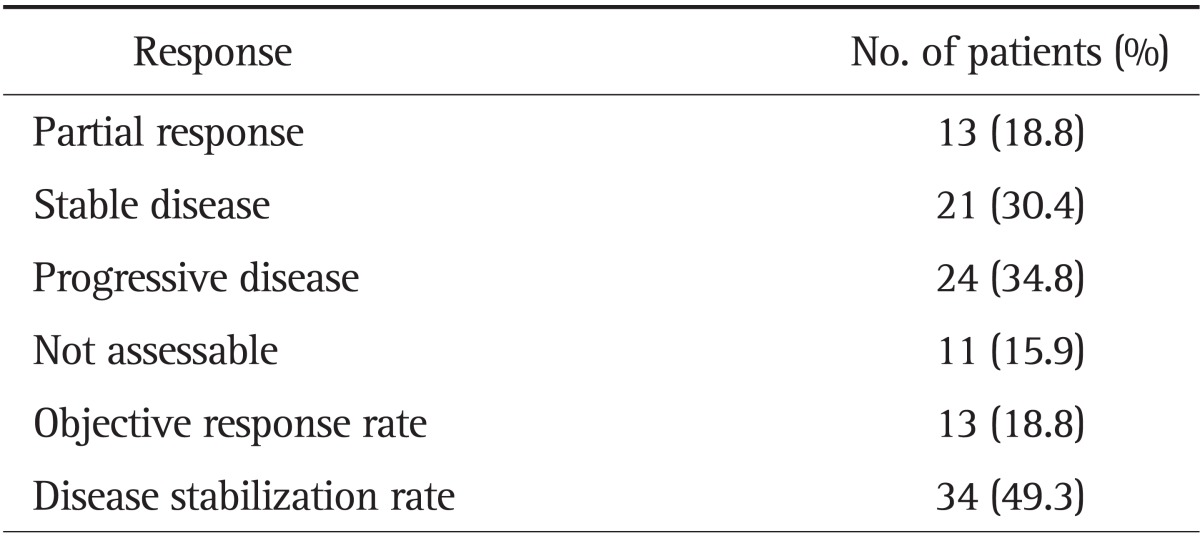
Fig. 1.

Progression-free survival (PFS) estimated by Kaplan-Meier analysis. PFS (black line) in good responders (orange line) and in poor responders (green line; p=0.001).
Fig. 2.
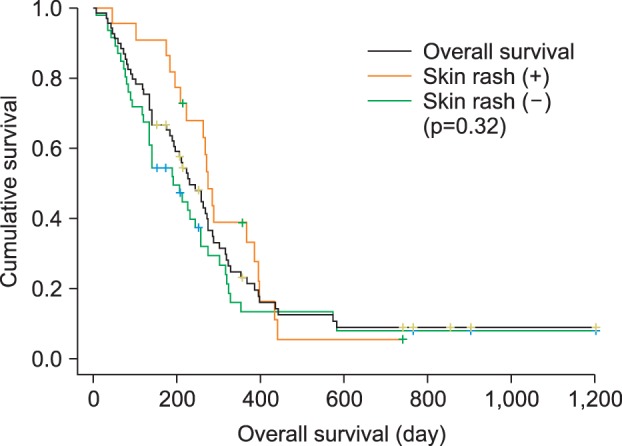
Overall survival (OS) estimated by Kaplan-Meier analysis. OS (black line) in patients with skin rash (orange line) and in patients without skin rash (green line; p=0.32).
3. Toxicity data
All patients were assessed for toxicity. The most common symptoms of toxicity were skin rashes, diarrhea, and thrombocytopenia. The most common adverse event was skin toxicity (31.8%). One patients developed chemotherapy-related interstitial lung disease (ILD)-like syndrome. Except for anemia and ILD, most of the toxicities were grade 1 or grade 2. All of toxicity profiles are summarized in Table 3.
Table 3.
Toxicity Profiles during Chemotherapy according to World Health Organization Criteria
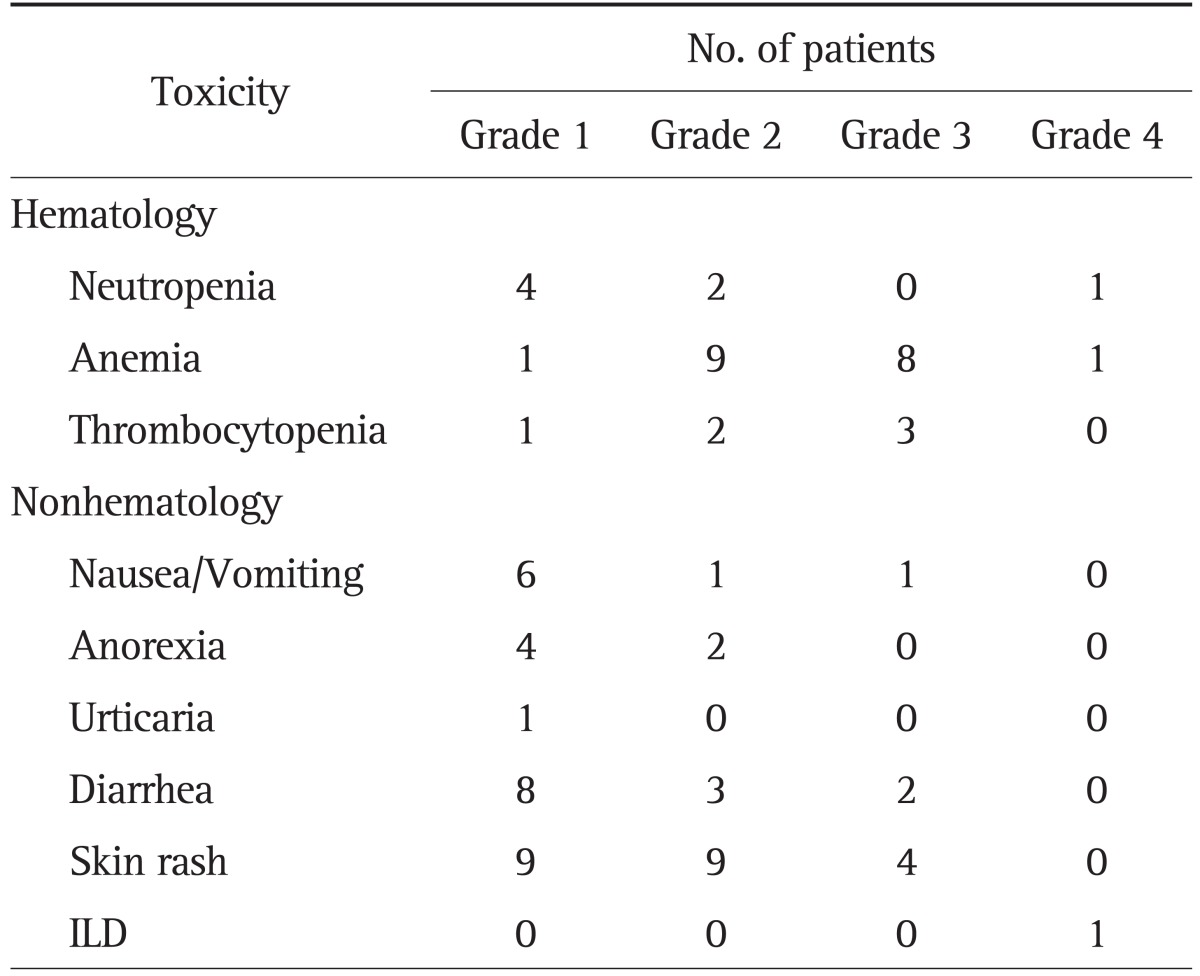
ILD, interstitial lung disease.
4. Prognostic factors for chemotherapeutic response
Prognostic factors for poor chemotherapeutic response were evaluated by multivariate Cox analysis. Comparison of the good and poor responder groups revealed that patients with a tumor location other than the pancreatic head, those with no skin rashes, and those with a poor performance status (ECOG 3) had a poorer chemotherapeutic response (Table 4).
Table 4.
Prognostic Factors of a Poor Chemotherapeutic Response
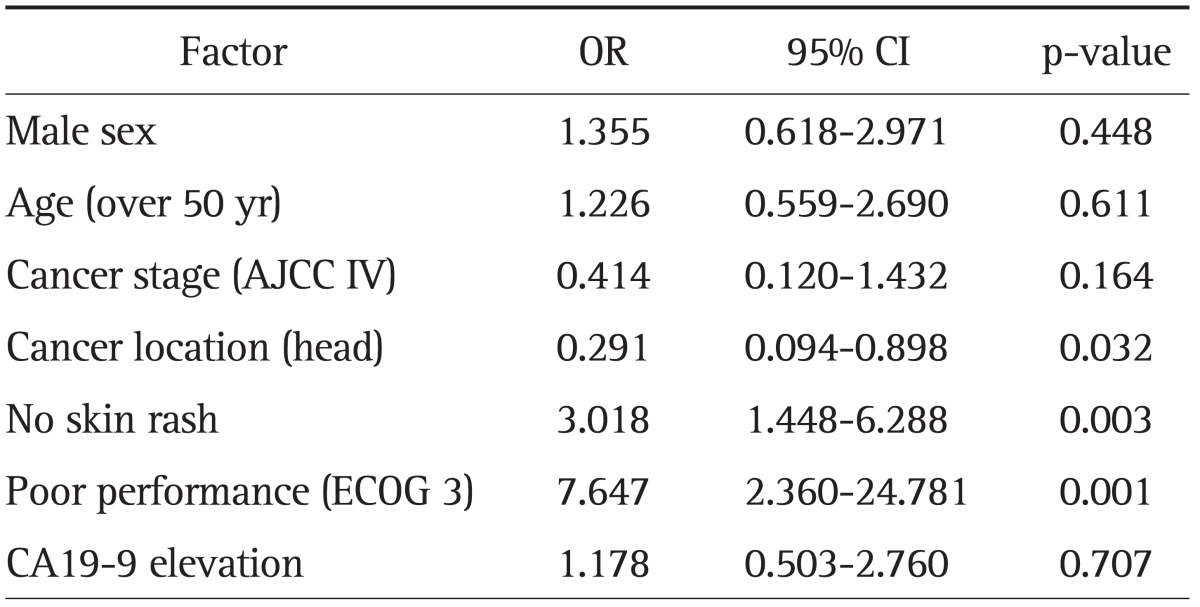
OR, odds ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; ECOG, Eastern Cooperative Oncology Group.
DISCUSSION
Despite the inclusion of patients with poor performance status (ECOG 3), the median overall survival of patients included in this trial was 7.7 months, which represents some improvement in survival, consistent with previous phase III gemcitabine and erlotinib trials in Western patients with advanced pancreatic cancer.12
The main symptoms of EGFR TKI toxicity were diarrhea and skin rash.13,14 In general, skin toxicities occur in over 50% patients treated with EGFR TKI.15 Higher skin toxicity symptoms in pancreatic cancer patients treated with EGFR inhibitors have been reported to be associated with better overall survival and progression-free survival.12 The mechanism of the relationship between the presence of skin rash and a good prognosis is not clear, but it has been proposed that skin toxicity may be a surrogate indicator of an immune-based local inflammatory reaction at the tumor level that provides antitumor activity.9,15 In this trial, one of the risk factors for poor prognosis were patients in the group without skin rash like results of other previous studies,12 despite the similar overall survival between the two groups according to the presence of skin rash (9.1 months vs 6.3 months; p=0.32). Subgroup analysis was performed in the patients with severe skin rash over grade 2 compared to the absence of skin rash. The median overall survival days shows 9.5 months versus 6.3 months (p=0.064). Survival gain by statistics fails to achieve due to small number of patients in the analysis. However, there is significantly better PES in the patients with the presence of skin rash over grade 2 compared to no skin rash (4.8 months vs 1.4 months; p=0.003). In summary, although significant survival benefit was not showed, we could predict the good prognosis in the group of patients with skin rash.
Besides the common toxicities like skin rash or diarrhea, life-threatening pulmonary toxic effects, like ILD, have been reported, albeit rarely. The prevalence of ILD-like syndrome in NSCLC patients treated with EGFR TKI ranges from 1% to 3%.16 Risk factors for this adverse effect have been evaluated in lung cancer patients. And these factors are previous chemotherapy or radiation therapy of the lung, concomitant pulmonary infection, preexisting parenchymal lung disease, and metastatic lung disease.9 In our study, only one patient (1.5%) showed ILD-like syndrome during the fifth cycle of erlotinib and gemcitabine treatment. This patient had no previous pulmonary infection or any specific lung disease. His case was reported as probably-related serious adverse event.
The cost-effectiveness of treatment with EGFR TKI has been questioned due to the relatively low response rate and high cost of this treatment.17 Therefore, it is necessary to be able to identify subgroups of patients who are likely to derive clinical benefit from these agents. Clinical trials in East Asian patients, such as this study, are therefore required for anticipating the results of NSCLC research.
In this study, subgroup analysis about treatment prognosis was performed using several clinical characteristics (Table 4). Group of patients with skin rash compared to no rash group, and group of patients showing better performance scale (ECOG 0, 1, or 2) rather than ECOG 3 group are likely to derive clinical benefit from these combination chemotherapy. Furthermore, we might predict much poorer prognosis in patients with pancreatic head cancer than pancreatic body or tail cancer. According to our analysis, the most predictable factor about chemotherapeutic response is patient's performance status, and the next best thing is skin rash.
A limitation of this study is that it was not a large-scale phase III trial. We did not evaluate molecular predictors, such as EGFR expression levels, EGFR mutation status, or K-ras mutation status, which are strongly related to pancreatic cancer. However, a recent study that evaluated EGFR gene copy number and K-ras mutation status as molecular predictive markers in patients with advanced pancreatic cancer enrolled in a previous phase III gemcitabine plus erlotinib trial reported that these markers were not predictive.18 This highlights the need to identify additional molecular predictive markers in the future.
In conclusion, erlotinib plus gemcitabine chemotherapy is a tolerable treatment regimen and has a favorable therapeutic effect in Korean patients with unresectable advanced pancreatic cancer, similar to EGFR-targeted therapy in Asian patients with NSCLC.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Van Cutsem E, Aerts R, Haustermans K, Topal B, Van Steenbergen W, Verslype C. Systemic treatment of pancreatic cancer. Eur J Gastroenterol Hepatol. 2004;16:265–274. doi: 10.1097/00042737-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lowenfels AB, Maisonneuve P. Epidemiology and prevention of pancreatic cancer. Jpn J Clin Oncol. 2004;34:238–244. doi: 10.1093/jjco/hyh045. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., 3rd Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael J, Fink U, Russell RC, et al. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang S, Jeon TJ, Kim MH, et al. Phase II study of cisplatin combined with weekly gemcitabine in the treatment of patients with metastatic pancreatic carcinoma. Pancreatology. 2006;6:635–641. doi: 10.1159/000097784. [DOI] [PubMed] [Google Scholar]
- 7.Bareschino MA, Schettino C, Troiani T, Martinelli E, Morgillo F, Ciardiello F. Erlotinib in cancer treatment. Ann Oncol. 2007;18(Suppl 6):vi35–vi41. doi: 10.1093/annonc/mdm222. [DOI] [PubMed] [Google Scholar]
- 8.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 9.Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364:947–955. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]
- 10.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 12.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 13.Sandler AB. Nondermatologic adverse events associated with anti-EGFR therapy. Oncology (Williston Park) 2006;20(5 Suppl 2):35–40. [PubMed] [Google Scholar]
- 14.Boeck S, Hausmann A, Reibke R, Schulz C, Heinemann V. Severe lung and skin toxicity during treatment with gemcitabine and erlotinib for metastatic pancreatic cancer. Anticancer Drugs. 2007;18:1109–1111. doi: 10.1097/CAD.0b013e3281ceabec. [DOI] [PubMed] [Google Scholar]
- 15.Lynch TJ, Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007;12:610–621. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 16.Ando M, Okamoto I, Yamamoto N, et al. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24:2549–2556. doi: 10.1200/JCO.2005.04.9866. [DOI] [PubMed] [Google Scholar]
- 17.Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506–4507. doi: 10.1200/JCO.2007.13.0401. [DOI] [PubMed] [Google Scholar]
- 18.da Cunha Santos G, Dhani N, Tu D, et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010;116:5599–5607. doi: 10.1002/cncr.25393. [DOI] [PubMed] [Google Scholar]


