Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy. The incidence of hepatocellular carcinoma has increased dramatically by 80% over the past two decades in the United States. Numerous basic science and clinical studies have documented a strong association between hepatocellular carcinoma and the metabolic syndrome. These studies have documented that, in most patients, non-alcoholic fatty liver disease is the hepatic manifestation of the metabolic syndrome, which may progress to hepatocellular carcinoma through the cirrhotic process. However, minority of patients with non-alcoholic fatty liver disease may progress to hepatocellular carcinoma without cirrhosis. This review summarizes the current literature of the link between hepatocellular carcinoma and metabolic syndrome with special emphasis on various components of the metabolic syndrome including risk of association with obesity, diabetes mellitus, hyperlipidemia, and hypertension. Current understanding of pathophysiology, clinical features, treatments, outcomes, and surveillance of hepatocellular carcinoma in the background of metabolic syndrome and non-alcoholic fatty liver disease is reviewed. With the current epidemic of metabolic syndrome, the number of patients with non-alcoholic fatty liver disease is increasing. Subsequently, it is expected that the incidence and prevalence of HCC will also increase. It is very important for the scientific community to shed more light on the pathogenesis of HCC with metabolic syndrome, both with and without cirrhosis. At the same time it is also important to quantify the risk of hepatocellular carcinoma associated with the metabolic syndrome in a prospective setting and develop surveillance recommendations for detection of hepatocellular carcinoma in patients with metabolic syndrome.
Keywords: Liver, Hepatocellular carcinoma, Metabolic syndrome, Non-alcoholic fatty liver disease, Obesity
Core tip: Hepatocellular carcinoma is a common malignancy with dismal outcome. The metabolic syndrome has been implicated for the recent increase in hepatocellular carcinoma. Numerous studies have shown a strong association between hepatocellular carcinoma and the metabolic syndrome. This review summarizes the current literature linking hepatocellular carcinoma and the metabolic syndrome.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy with increasing incidence and prevalence both nationally and internationally that was evident more than a decade ago[1]. According to International Agency for Research on Cancer (IARC), HCC has high fatality worldwide with overall ratio of mortality to incidence of 0.93[2]. The incidence of HCC has increased dramatically by 80% in the last two decades in the United States[3]. This phenomenon was also observed in many of the developed countries of the world[4]. The trend of increased incidence of HCC from hepatitis C virus is predicted to plateau by 2020 with no significant changes of other known causes[5]. Most of the high risk entities of HCC including hepatitis B virus, hepatitis C virus, and alcohol are well defined. However, 5%-30% of the HCC cases do not have any identifiable risk factor[6]. Moreover, some studies have indicated that even up to 50% cases of HCC may not have any readily identifiable risk factor[7,8]. The majority of these “cryptogenic” HCC in the United States and many other developed countries are now widely attributed to the metabolic syndrome, specially its hepatic manifestation non-alcoholic fatty liver disease (NAFLD)[9].
Metabolic syndrome (MetS), a cluster of metabolic abnormalities is now considered a major public health issue worldwide. It is particularly important in the developed countries because of the alarming obesity epidemic. MetS is also considered to be the central association of the current epidemic of diabetes and cardiovascular diseases[10]. It is estimated that 25% of the United States population meet the diagnostic criteria of MetS[11]. According to the Third National Health and Nutrition Examination Survey (NHANES III) criteria, about 47 million people have metabolic syndrome in the United States and the number is increasing at an alarming rate[12]. In the background of increasing “cryptogenic” HCC, MetS and NAFLD, it is important to review the relationship between HCC and the MetS.
EPIDEMIOLOGY OF HCC
Worldwide, HCC is the fifth most common cancer in men and the seventh most common cancer in women according to the IARC. Most of the disease burden of HCC resides in developing countries such as East Asia, South East Asia, and sub-Saharan Africa, where almost 85% of the cases occur. The overall gender ratio of male: female is 2.4. Low incidence rates are estimated in developed countries, with the exception of Southern Europe where the incidence in men is significantly higher than in other developed regions. Worldwide, there was an estimated 694000 deaths from HCC in 2008 (477000 in men, 217000 in women), making it the third most common cause of death from cancer. The geographical distribution of HCC mortality rates is similar to that observed for the incidence rates indicating more or less similar outcomes across the world[13].
In the United States, the average age of diagnosis for HCC is 63 years (62 years for males, and 69 years for females). The age-adjusted incidence rate is 7.7/100000 per year. The age-adjusted death rate from HCC is 5.5/100000 per year. It is estimated that the median age at death for HCC is 68 years of age. However, gender, race and ethnic disparities exist in the incidence and mortality rates of HCC in the United States. Table 1 summarizes the incidence and mortality rates of HCC according to race, ethnicity and gender based on cases diagnosed in 2006-2010 from 18 Surveillance Epidemiology and End Results (SEER) geographic areas[14,15].
Table 1.
Incidence and mortality rates of hepatocellular carcinoma according to race/ethnicity and gender, reported in Surveillance Epidemiology and End Results database 2006-2010
| Race/Ethnicity |
Incidence rate per
100000 |
Mortality rate per
100000 |
||
| Male | Female | Male | Female | |
| All races | 11.9 | 4.0 | 8.3 | 3.4 |
| Non-Hispanic White | 10.4 | 3.5 | 7.6 | 3.2 |
| African American | 15.1 | 4.5 | 11.8 | 4.1 |
| Hispanics | 18.3 | 6.9 | 12.3 | 5.4 |
| Asian/Pacific Islander | 21.4 | 8.2 | 14.4 | 6.0 |
| American Indian/Alaska Native | 20.6 | 7.7 | 13.2 | 6.1 |
The Centers for Disease Control and Prevention (CDC) examined all HCC cases (48596) diagnosed during 2001-2006 that were reported to the National Program of Cancer Registries (NPCR) or SEER from 45 cancer registries (covering 90.4% of the United States population). As shown in Table 2[16], the data document that the incidence rate of HCC is on the rise in both genders. During this period, the annual percentage change (APC) for males (3.6%) was significantly higher than the APC for females (2.3%). The largest significant increase in HCC incidence rates were among Non-Hispanic Whites (APC = 3.8%), African American (APC = 4.8%), and persons aged 50-59 years (APC = 9.1%)[16].
Table 2.
Changes in incidence rate of hepatocellular carcinoma from 2001 to 2006
| Incidence rate/100000 | 2001 | 2006 |
| Overall | 2.7 | 3.2 |
| Male | 4.5 | 5.4 |
| Female | 1.2 | 1.4 |
METABOLIC SYNDROME
The World Health Organization (WHO) was the first to identify MetS as a global problem and took initiative to propose a definition and diagnostic criteria. The WHO used insulin resistance (IR) as the major criteria for defining the MetS. However, in clinical practice it was difficult to quantify or qualify insulin resistance across the world[17]. Subsequently in 2001, the National Cholesterol Education Program expert panel on the detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III or ATP III) defined the metabolic syndrome by the presence of three parameters of the following criteria: hyperglycemia, hypertriglyceridemia, low HDL, abdominal obesity and hypertension[18]. Recently, the International Diabetes Federation (IDF) adopted a definition with emphasis on central obesity in MetS such that central obesity plus two additional factors are required in order to diagnose the MetS[19]. Table 3 summarizes the criteria used to define the metabolic syndrome over time.
Table 3.
Criteria used to define the metabolic syndrome
| Diagnostic criterion | WHO (1999) | ATP (2005) | IDF (2006) |
| Abdominal obesity | BMI - Waist/hip ratio > 0.9 (men) or > 0.85 (women) or BMI ≥ 30 kg/m2 | Central - Waist ≥ 102 cm (men) or ≥ 88 cm (women) | Central - Waist ≥ 102 cm (men) or ≥ 88 cm (women) |
| Hypertension | ≥ 140/90 mmHg | ≥ 130/85 mmHg or drug treatment for hypertension | ≥ 130/85 mmHg or drug treatment for hypertension |
| Fasting glucose | IPG/HOMA | ≥ 5.6 mol/L | ≥ 6.1 mol/L |
| Hypertriglyceridemia | ≥ 1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides | ≥ 1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides | ≥ 1.7 mmol/L (150 mg/dL) or drug treatment for elevated triglycerides |
| Low HDL cholesterol | Not used | < 1.0 mmol/L (40 mg/dL) (men); < 1.3 mmol/L (50 mg/dL) (women) or drug treatment for low HDL | < 1.0 mmol/L (40 mg/dL) (men); < 1.3 mmol/L (50 mg/dL) (women) or drug treatment for low HDL |
| Micro albuminuria | Used | Not used | Not used |
ATP: Adult Treatment Panel; BMI: Body mass index; HDL: High-density lipoprotein; IDF: International Diabetes Federation; IPG/HOMA: Impaired plasma glucose/homeostatic model assessment; WHO: World Health Organization.
The prevalence of MetS varies worldwide depending on the geographic location, socioeconomic background, culture and ethnicity. It is estimated that the prevalence of MetS is about 14% in China, 26% in South Asia, 19% in Australia, 9% in France and 18% in Italy. Although prevalence of obesity as defined by the WHO is relatively low in Asia compared to western countries, metabolic syndrome is growing into a significant public health problem. Comparative studies indicate that metabolic responses to obesity may be greater in South and East Asians than their western counterparts at given body mass indexes (BMI)[10,20].
The prevalence of MetS was evaluated in adults in the United States participating in the third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1994). The overall prevalence was 22%, with an age-dependent increase (6.7, 43.5, and 42.0% for ages 20 to 29, 60 to 69, and > 70 years, respectively)[12]. Data from NHANES 1999 to 2000 demonstrate that the prevalence has continued to increase, particularly in women. The unrelenting increase in the prevalence of obesity in the United States suggests that the current prevalence of the metabolic syndrome is now very likely higher than that estimated from 1988-1994 NHANES IIIdata[11]. According to CDC in 2008, the obesity rate among adult Americans was estimated at 32.2% for men and 35.5% for women; these rates were roughly confirmed again for 2009-2010. Recent data indicate that 34% of the adult in the United States met the criteria for MetS and the rate of increment was equal in both sexes[21]. This rapidly increasing prevalence of obesity among adults in the United States will lead to even higher rates of the MetS now and in the near future[22]. Study has shown that susceptibility to obesity cannot simply be attributed to the combination of genetic and environmental factors, but can also be triggered by influences on a baby’s development during intrauterine period, including mother’s dietary habit. A mother’s nutrition while pregnant can cause important epigenetic changes that contribute to her offspring’s risk of obesity during childhood[23].
HEPATOCELLULAR CARCINOMA AND THE METABOLIC SYNDROME
HCC is one of the most common malignancies in the world, with more than 500000 new cases per year[24]. Data from SEER which covers 28% of the United States population, reported a total of 4032 cases of HCC in 2001. This number increased by 27% to 5122 in 2005 and by 60% to 6464 in 2009[14]. It is predicted that the total number of HCC will continue to increase in the future[25]. Since the association is not readily identifiable in a significant percentage of HCC cases, it was postulated, and now well established, that the MetS is contributing to the development of HCC. With the current rising epidemic of obesity and MetS in the general population, it is established that MetS is responsible for HCC cases with unaccounted association[26]. The prevalence of MetS is paralleling the epidemic of obesity in the United States. Obesity and MetS are well documented risks factors associated with NAFLD, a metabolic hepatic disorder that can progress to nonalcoholic steatohepatitis (NASH) and fibrosis. A subset of aggressive NAFLD can lead to cirrhosis and HCC. Worldwide, it is estimated that there are 400 million obese individuals, among whom 75% have NAFLD. Up to 20% have NASH and over 5-10 years, 33% of whom will develop cirrhosis[27,28]. In another study, among patients with NAFLD followed for a mean of 8 years, the occurrence of cirrhosis was 20% and the incidence of HCC was 1%[29]. It is estimated that the prevalence of NAFLD is 3-10 times higher than the prevalence of hepatitis C virus (HCV) in the United States ranging from 5.5% to 31%[30,31]. Besides being the most rapidly increasing cause of cancer death in the United States, the economic burden of HCC is also enormous with an estimated cost of more than 437 million dollars per year[32]. It is very imperative for the medical care providers to appreciate the association of MetS and HCC with appropriate surveillance for the high risk population groups.
HEPATOCELLULAR CARCINOMA RISK FACTORS ASSOCIATED WITH THE METABOLIC SYNDROME
Obesity
Many malignancies have been directly or indirectly associated with obesity and HCC is among these established malignancies[33,34]. Meta-analysis of 11 studies conducted in United States, Europe and Asia demonstrated that both overweight (RR = 1.07, 95%CI: 1.01-1.15) and obesity (RR = 1.85, 95%CI: 1.44-2.37) were associated with development of HCC[35]. Even in patients with chronic HBV and HCV, coexisting obesity has been associated with increased risk for HCC by more than 100-fold[36]. A Large prospective trial showed that obesity has influenced disease progression, and increased weight is associated with overall cancer mortality. In a prospective study of United States adults, BMI > 35 kg/m2 negatively impacted overall mortality from HCC with a relative risk (RR) of 1.68 times in women and 4.52 times in men. This was the highest for any malignancy analyzed in the study[37]. Comparable conclusions were drawn in both Danish and Korean studies analyzing large cohorts of obese patients[38,39]. SEER-Medicare data analysis from 1993 to 2005 showed that adjusted odd ratio (OR) of obesity for HCC was 1.93 (95%CI: 1.71-2.18, P < 0.0001)[40]. The Metabolic Syndrome and Cancer Project (Me-Can) from Norway, Sweden and Austria examining 578700 subjects showed RR of 1.39 (95%CI: 1.24-1.58) for obesity in the development of HCC[25]. Obesity is also an independent predictor of HCC in obese transplanted patients (4% vs 3.4%)[34].
Moreover, in a large retrospective cohort of 342 consecutive patients who underwent liver transplantation for hepatocellular carcinoma, BMI was found to be an independent predictor of micro vascular invasion[41]. Nonetheless, review of the United Network of Organ Sharing database on all liver transplantations performed in the United States, showed that obesity was an independent predictor of HCC in patients with alcoholic cirrhosis and cryptogenic cirrhosis, but not for those with cirrhosis of other associations[34].
Diabetes mellitus
Diabetes mellitus (DM) is an independent risk factor for the development of HCC. Analysis of 2061 patients with HCC showed a significant increase in the development of HCC (OR 2.87, 95%CI: 2.49-3.3) in the background of DM regardless of the presence of other risk factors. There was a significant positive interaction between obesity and HCV (P < 0.0001) for HCC[42]. Multiple European studies showed RR of 4.5 of HCC in male patients, with a lower, but still significant RR of 1.86 in female patients with DM[43-45]. Moreover, a large longitudinal study analyzing 173643 DM and 650620 non-DM controls over a period of 10- to 15-year revealed a RR of 2. This risk estimation even persisted after exclusion of the patients with viral hepatitis, alcohol use, or fatty liver disease[46]. DM was established as an independent risk factor for the development of HCC in 12 cohort studies after adjustment for infectious and alcoholic associations[47]. In a large population based study with 615532 DM patients and 614871 controls, the overall hazard rate for the development of HCC in males and females was 32.76 and 17.41 per 10000 patients-years, respectively. Furthermore, in a recent Italian case-control study including 185 HCC cases and 404 controls, diabetes and obesity were positively associated with HCC risk, with ORs of 4.33 (95%CI: 1.89-9.86) and 1.97 (95%CI: 1.03-3.79), respectively[48]. DM with cirrhosis demonstrated the highest risk of HCC development (RR = 82.25, 95%CI: 76.84-94.58)[49]. SEER-Medicare data analysis from 1993 to 2005 showed that adjusted OR of DM for HCC was 2.9 (95%CI: 2.71-3.1, P < 0.0001)[40]. The Me-Can from Norway, Sweden and Austria examining 578700 subjects showed RR of 2.13 (95%CI: 1.55-2.94) for DM in the development of HCC[25].
Hyperlipidemia
Hyperlipidemia is an integral part of the MetS. Although there have been several studies examining the association of HCC with MetS, only few studies reported the association of HCC with hyperlipidemia individually. Hyperlipidemia, in many instances is closely related with the central mechanism of insulin resistance in MetS. SEER-Medicare data analysis from 1993 to 2005 showed that adjusted OR of dyslipoproteinemia for HCC was 1.35 (95%CI: 1.26-2.45, P < 0.0001)[40]. The Me-Can from Norway, Sweden and Austria examining 578700 subjects showed RR of 0.85 (95%CI: 0.65-1.10) in the development of HCC[25]. Although not well understood, this discrepancy between Me-Can study[25] and SEER-Medicare data analysis[40] could be secondary to the short follow up period in the Me-Can study.
Hypertension
Similar to hyperlipidemia, hypertension is one of the parameters of the metabolic syndrome. Very few studies examined the individual association of HCC with hypertension. SEER-Medicare data analysis from 1993 to 2005 showed that adjusted OR of hypertension for HCC was 2.22 (95%CI: 2.04-2.42, P < 0.0001)[40]. In a study from a single center, among 209 NBNC-HCC patients, 38% had hypertension, and 11% had hyperlipidemia[50]. The Me-Can from Norway, Sweden and Austria examining 578700 subjects showed RR of 2.08 (95%CI: 0.95-4.73) for hypertension in the development of HCC[25].
PATHOPHYSIOLOGY OF HEPATOCELLULAR CARCINOMA ASSOCIATED WITH THE METABOLIC SYNDROME
Hepatocellular carcinoma generally arises in the background of cirrhosis (Figure 1). Factors that are associated with development of the metabolic syndrome and HCC maybe inherently linked (Table 4). It is well postulated and established that insulin resistance is the principal dominator that links all the components of MetS. Insulin resistance exerts a major role in the development of NAFLD even in lean subjects with appropriate glycemic control[51]. Insulin resistance leads to fat accumulation in the hepatocytes by lipolysis and hyperinsulinemia. Aberrant adipose tissue accumulation, release of pro-inflammatory cytokines, inhibition of anti-inflammatory cytokines and lipotoxicity collectively promote and propagate both systemic and hepatic insulin resistance, leading to hyperinsulinemia[52]. Multiple mechanisms have been proposed that may work simultaneously and complementary to each other to provide a tumor promoting environment in MetS. This may distinguish the pathogenesis of HCC related to NAFLD from that of infectious and alcoholic associations[53,54]. Hyperinsulinemia results in increased insulin growth factor-1 (IGF-1) which has important proliferative and antiapoptotic effects. IGF-1 promotes angiogenesis through increased vascular endothelial growth factor production, which in turn leads to cancer cell proliferation. Upregulation in IGF-1/IRS1 pathway has been shown to contribute to the pathogenesis of HCC[55]. Likewise, peroxisome proliferator-activated receptors (PPARs) regulate a network of genes encoding protein involved in fatty acids uptake, enzymes required for the β-oxidation of fatty acids, and enzymes required for ketogenesis. PPARs play an important role in fatty liver, and its involvement in carcinogenesis has been clarified. Abnormal stimulation of PPAR-α has been shown to induce HCC in animal models[56].
Figure 1.
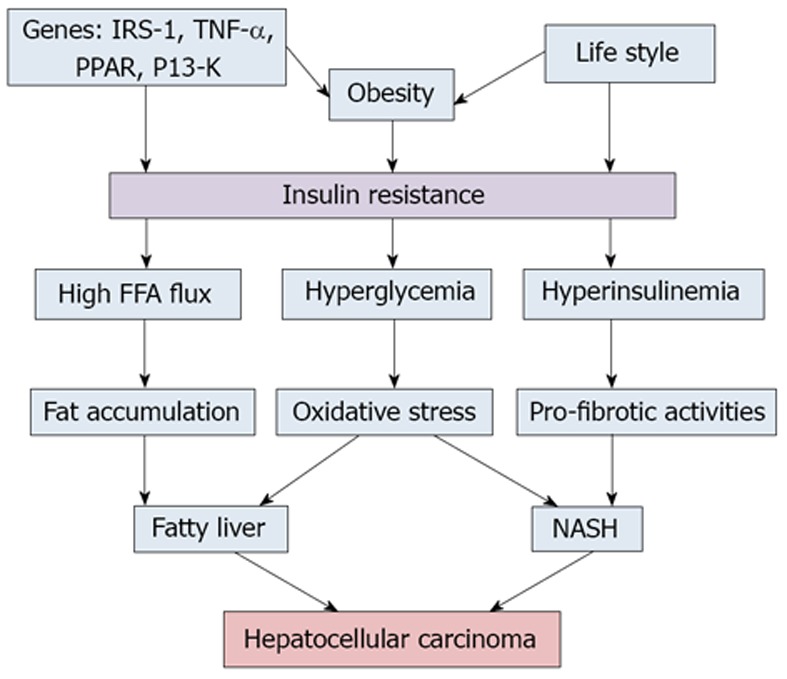
Pathogenesis of hepatocellular carcinoma in the background of metabolic syndrome. PPAR: Peroxisome proliferator-activated receptors; NASH: Nonalcoholic steatohepatitis; FFA: Free fatty acid; IRS-1: Insulin receptor substrate 1; TNF-α: Tumor necrosis factor α.
Table 4.
Association of different components of metabolic syndrome and the development of hepatocellular carcinoma
| Author | Type of Study | Risk Parameter | Obesity | DM | Hyperlipidemia | HTN |
| Larsson et al[35] | Meta analysis | RR | 1.85 | |||
| Calle et al[37] | Prospective | RR | 4.52 (Male) | |||
| 1.68 (Female) | ||||||
| Welzel et al[40] | Retrospective | OR | 1.93 | 2.9 | 1.35 | 2.2 |
| Borena et al[25] | Prospective | RR | 1.39 | 2.13 | 0.85 | 2.08 |
| Turati et al[48] | Retrospective | OR | 1.97 | 4.33 | ||
| Davila et al[42] | Retrospective | OR | 2.87 | |||
| Lagiou et al[43] | Prospective | RR | 4.5 (Male) | |||
| 1.86 (Female) | ||||||
| El-Serag et al[46] | Prospective | RR | 2 | |||
| Tomimaru et al[55] | Prospective | RR | 82.2 (with cirrhosis) |
RR: Relative risk; OR: Odd ratio; DM: Diabetes mellitus; HTN: Hypertension.
Expansion of adipose tissue in obesity may lead to release of pro-inflammatory cytokines. Visceral fat accumulation has been shown to be an independent risk factor for HCC recurrence after curative treatment[57]. Further, Interleukin-6 (IL-6) has been linked to obesity-associated inflammatory response such that it activates STAT3 potentiating cell proliferation and anti-apoptotic mechanisms. Tumor necrosis factor (TNF) activates pro-oncogenic pathways including JNK, NF-κB, mTOR, and the extracellular signal-regulated kinases[58,59]. In experimental models, both TNF and IL-6 strongly promote HCC growth induced by diethyl nitrosamine in mice. Both dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression[60].
Adiponectin (an anti-inflammatory cytokine) is expressed at reduced levels in MetS and NAFLD, which may not sufficient to suppress endotoxin-mediated inflammatory signaling. On the other hand, high circulating levels of leptin in NAFLD exert pro-inflammatory and pro-fibrogenic effects in NAFLD[61-63]. In addition, there is evidence that lipid peroxides and free radicals are elevated in MetS, which may cause oxidative injury, endoplasmic reticulum stress, mitochondrial dysfunction, and apoptosis[64].
CLINICAL FEATURES OF HCC IN THE BACKGROUND OF METABOLIC SYNDROME
NAFLD leading to cirrhosis can predispose to HCC. There are several reports that patients may even develop HCC from steatosis without cirrhosis. However, the degree of developing HCC from NAFLD is less in comparison to infectious and alcoholic associations[65]. Patients who develop HCC in the background of MetS are predominantly males. The average age of diagnosis is older than HCC secondary to other causes; and HCC secondary to NAFLD is generally well differentiated with early stages at diagnosis[53]. An analysis of 87 Japanese patients with HCC in the background of MetS showed a median age of 72 years, and the male patients appear to develop HCC at a less-advanced stage of liver fibrosis[66]. Similar results were also confirmed by another Japanese study[67]. Male predominance, older age at diagnosis, and early stages were also found in a prospective study comparing 34 NASH cases with HCC with 348 NASH patients without HCC[68]. A recent study from China examined 169 patients with NAFLD associated HCC. The result showed 73% male predominance with average age of diagnosis of 67 years, 99% had at least one component of MetS, 76% with solitary nodule (mean 3.4 cm) and most of the patients were well or moderately differentiated. In more than 40% patients, HCC developed in the absence of cirrhosis[69]. Comparable results were reported by different studies with different population groups[70-72].
TREATMENT AND OUTCOMES OF HCC IN THE BACKGROUND OF METABOLIC SYNDROME
Unfortunately there is no specific recommendation for treatment of HCC developing in the background of MetS. It is thought that HCC secondary to MetS may have better prognosis than its other counterparts partly because of early diagnosis with favorable prognostic markers. Because of lack of specific guidelines at present, HCC secondary to MetS are treated like HCC from other major associations. Insulin-sensitizing therapy may also improve the outcome of HCC. Metformin therapy is associated with lower mortality in diabetic patients with early stage HCC after radiofrequency ablation[73]. In another study, 100 diabetic patients with hepatitis C virus and cirrhosis were prospectively followed for 2.3-8.3 years and evaluated for the development of HCC, liver-related death, or liver transplantation. The 5-year HCC development was lower in the group receiving metformin than in the group without metformin (9.5% vs 32.1%; P = 0.001). Multivariate analysis showed that metformin treatment was independently associated with decreased HCC development (HR = 0.19; P = 0.023) and liver-related death or transplantation in those patients (HR = 0.22; P = 0.049)[74]. Several other studies also demonstrated that the use of insulin-sensitizing agents in diabetes may reduce the risk of HCC development[75-77]. The role of rosuvastatin and ursodeoxycholic acid in the treatment of NASH or NAFLD and in prevention of NASH or NAFLD associated HCC is well studied[78,79]. A recent systematic review and meta-analysis showed a 50% reduction in HCC incidence with metformin use (OR = 0.50, 95%CI: 0.34-0.73). However, thiazolidinediones did not modify the risk of HCC (OR = 0.54, 95%CI: 0.28-1.02)[80]. Moreover, post-hoc analysis of randomized controlled trials did not reveal any significant association between antidiabetic medication use and risk of HCC although there was considerable heterogeneity across studies[80].
SURVEILLANCE FOR HCC IN METABOLIC SYNDROME
HCC still remains one of the malignancies with higher mortality ratio[2]. With better imaging studies, improvement of surgical techniques, and targeted therapy, the prognosis for early stage HCC is improving. But for advanced HCC the prognosis still remains poor. There are well established recommendations for surveillance of the patients with infectious risk factors and cirrhosis for HCC[81]. Evidence on metabolic syndrome as a risk factor for development of HCC, especially in the background of DM and obesity, is growing rapidly. With the ongoing epidemic of obesity, increased number of patients with DM, and the overall increase in the incidence and prevalence of MetS, it is imperative to identify the high risk groups for the development of HCC with MetS and provide appropriate surveillance strategies. The patients, who develop cirrhosis in the background of NAFLD, may be under surveillance as per current recommendations. But the question remains whether surveillance in patients who have NAFLD, without evidence of cirrhosis, is appropriate.
Most of the time, NAFLD will progress to cirrhosis before the development of HCC. But in a very small number of cases, it may progress to HCC without cirrhosis in the background of MetS. With about one third (34%) of the United States adult population meeting the criteria of MetS, surveillance for HCC may cause a significant health related economic issue in this regard[22]. But nevertheless, there should be an urgent and collaborative process to resolve this issue in the near future.
SUMMARY
HCC is a common malignancy with dismal outcome. The associations of HCC have been identified in great details. With the recent increase in the incidence of HCC without any indefinable causes, major efforts have been undertaken to identify more causative factors. The metabolic syndrome, especially with obesity and DM, has been implicated for the recent increase in HCC. Numerous basic science and clinical studies have shown a strong association between HCC and the metabolic syndrome. These studies have documented that NAFLD, to a large extent, is the hepatic manifestation of MetS and may progress to HCC through the cirrhotic process. It has also been shown that in a very small fraction of patients with NAFLD, HCC may develop without evidence of cirrhosis.
With the current epidemic of MetS, the number of patients with obesity and DM is increasing. Subsequently, it is expected that the incidence and prevalence of HCC will also increase. It is very important for the scientific community to shed more light on the pathogenesis of HCC with MetS, both with and without cirrhosis. At the same time it is also important to quantify the risk of HCC associated with the MetS in prospective setting and develop surveillance recommendations to detect HCC for patients with MetS.
Footnotes
Supported by Raymond E and Vaona H Peck Chair in Cancer Research
P- Reviewers Baffy G, Charatcharoenwitthaya P, Niculescu M, Sato K, Tulassay ZJ S- Editor Wen LL L- Editor A E- Editor Liu XM
References
- 1.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. GLOBOCAN 2008. Available from: http://globocan.iarc.fr.
- 3.Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. 2008;14:4300–4308. doi: 10.3748/wjg.14.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43:737–746. doi: 10.1016/j.humpath.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M, Angulo P, Chalasani N, Merriman R, Viker K, Charatcharoenwitthaya P, Sanderson S, Gawrieh S, Krishnan A, Lindor K. Low circulating levels of dehydroepiandrosterone in histologically advanced nonalcoholic fatty liver disease. Hepatology. 2008;47:484–492. doi: 10.1002/hep.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer. 2009;115:5651–5661. doi: 10.1002/cncr.24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosch FX, Ribes J, Cléries R, Díaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211, v. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17:863–869. doi: 10.1016/j.annepidem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 10.McCullough AJ. Epidemiology of the metabolic syndrome in the USA. J Dig Dis. 2011;12:333–340. doi: 10.1111/j.1751-2980.2010.00469.x. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27:2444–2449. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 12.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 13.International Agency for Research on Cancer. Cited 2013-01-07. Available from: http://globocan.iarc.fr/factsheet.asp.
- 14.National Cancer Institute. SEER Stat Fact Sheets: Liver and Intrahepatic Bile Duct. Available from: http://seer.cancer.gov/statfacts/html/livibd.html.
- 15.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 16.Morbidity and Mortality Weekly Report (MMWR) Hepatocellular Carcinoma - United States, 2001-2006. Cited 2013-02-02. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5917a3.htm. [PubMed]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 20.Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, Whelton PK, He J. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 21.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009:1–7. [PubMed] [Google Scholar]
- 22.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991-1998. JAMA. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 23.Godfrey KM, Sheppard A, Gluckman PD, Lillycrop KA, Burdge GC, McLean C, Rodford J, Slater-Jefferies JL, Garratt E, Crozier SR, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60:1528–1534. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 25.Borena W, Strohmaier S, Lukanova A, Bjørge T, Lindkvist B, Hallmans G, Edlinger M, Stocks T, Nagel G, Manjer J, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131:193–200. doi: 10.1002/ijc.26338. [DOI] [PubMed] [Google Scholar]
- 26.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 27.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162–168. doi: 10.1159/000282081. [DOI] [PubMed] [Google Scholar]
- 29.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 30.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 31.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 32.2013 ASCO Annual Meeting. Cited 2013-02-17. Available from: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=53&abstractID=10464.
- 33.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–155. doi: 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 35.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005–1008. doi: 10.1038/sj.bjc.6603932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, Wang LY, Sun CA, Lu SN, Chen DS, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135:111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 37.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 38.Møller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer. 1994;30A:344–350. doi: 10.1016/0959-8049(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 39.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 40.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, Jacobson JS, Hershman DL, Verna EC, Zaretsky J, et al. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539–543. doi: 10.1097/TP.0b013e31825c58ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagiou P, Kuper H, Stuver SO, Tzonou A, Trichopoulos D, Adami HO. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Natl Cancer Inst. 2000;92:1096–1099. doi: 10.1093/jnci/92.13.1096. [DOI] [PubMed] [Google Scholar]
- 44.Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Curr Opin Lipidol. 2011;22:479–488. doi: 10.1097/MOL.0b013e32834c7cfc. [DOI] [PubMed] [Google Scholar]
- 45.Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 46.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 47.Rosmorduc O, Fartoux L. HCC and NASH: how strong is the clinical demonstration? Clin Res Hepatol Gastroenterol. 2012;36:202–208. doi: 10.1016/j.clinre.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Turati F, Talamini R, Pelucchi C, Polesel J, Franceschi S, Crispo A, Izzo F, La Vecchia C, Boffetta P, Montella M. Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer. 2013;108:222–228. doi: 10.1038/bjc.2012.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen HF, Chen P, Li CY. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology. 2010;52:155–163. doi: 10.1002/hep.23641. [DOI] [PubMed] [Google Scholar]
- 50.Nagaoki Y, Hyogo H, Aikata H, Tanaka M, Naeshiro N, Nakahara T, Honda Y, Miyaki D, Kawaoka T, Takaki S, et al. Recent trend of clinical features in patients with hepatocellular carcinoma. Hepatol Res. 2012;42:368–375. doi: 10.1111/j.1872-034X.2011.00929.x. [DOI] [PubMed] [Google Scholar]
- 51.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahsan MK, Okuyama H, Hoshino Y, Oka S, Masutani H, Yodoi J, Nakamura H. Thioredoxin-binding protein-2 deficiency enhances methionine-choline deficient diet-induced hepatic steatosis but inhibits steatohepatitis in mice. Antioxid Redox Signal. 2009;11:2573–2584. doi: 10.1089/ars.2009.2385. [DOI] [PubMed] [Google Scholar]
- 53.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 54.Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303–1307. doi: 10.1136/gut.2009.199661. [DOI] [PubMed] [Google Scholar]
- 55.Tomimaru Y, Koga H, Yano H, de la Monte S, Wands JR, Kim M. Upregulation of T-cell factor-4 isoform-responsive target genes in hepatocellular carcinoma. Liver Int. 2013;33:1100–1112. doi: 10.1111/liv.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakiri L, Wagner EF. Mouse models for liver cancer. Mol Oncol. 2013;7:206–223. doi: 10.1016/j.molonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, Masuzaki R, Goto T, Hamamura K, Kanai F, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58:839–844. doi: 10.1136/gut.2008.164053. [DOI] [PubMed] [Google Scholar]
- 58.Bian Z, Ma X. Liver fibrogenesis in non-alcoholic steatohepatitis. Front Physiol. 2012;3:248. doi: 10.3389/fphys.2012.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikejima K, Okumura K, Kon K, Takei Y, Sato N. Role of adipocytokines in hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S87–S92. doi: 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 62.Tawaramoto K, Kotani K, Hashiramoto M, Kanda Y, Nagare T, Sakaue H, Ogawa W, Emoto N, Yanagisawa M, Noda T, et al. Ablation of 3-phosphoinositide-dependent protein kinase 1 (PDK1) in vascular endothelial cells enhances insulin sensitivity by reducing visceral fat and suppressing angiogenesis. Mol Endocrinol. 2012;26:95–109. doi: 10.1210/me.2010-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51–56. doi: 10.1016/j.bbrc.2009.02.131. [DOI] [PubMed] [Google Scholar]
- 64.Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis. 2012;32:30–38. doi: 10.1055/s-0032-1306424. [DOI] [PubMed] [Google Scholar]
- 66.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433; quiz e50. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 67.Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World J Gastroenterol. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 69.Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:18–27. doi: 10.1016/s1499-3872(11)60120-3. [DOI] [PubMed] [Google Scholar]
- 70.Chagas AL, Kikuchi LO, Oliveira CP, Vezozzo DC, Mello ES, Oliveira AC, Cella LC, Herman P, Bachella T, Caldwell SH, et al. Does hepatocellular carcinoma in non-alcoholic steatohepatitis exist in cirrhotic and non-cirrhotic patients? Braz J Med Biol Res. 2009;42:958–962. doi: 10.1590/s0100-879x2009005000019. [DOI] [PubMed] [Google Scholar]
- 71.Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- 72.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 73.Chen TM, Lin CC, Huang PT, Wen CF. Metformin associated with lower mortality in diabetic patients with early stage hepatocellular carcinoma after radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:858–865. doi: 10.1111/j.1440-1746.2011.06664.x. [DOI] [PubMed] [Google Scholar]
- 74.Nkontchou G, Cosson E, Aout M, Mahmoudi A, Bourcier V, Charif I, Ganne-Carrie N, Grando-Lemaire V, Vicaut E, Trinchet JC, et al. Impact of metformin on the prognosis of cirrhosis induced by viral hepatitis C in diabetic patients. J Clin Endocrinol Metab. 2011;96:2601–2608. doi: 10.1210/jc.2010-2415. [DOI] [PubMed] [Google Scholar]
- 75.Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL, et al. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938–1946. doi: 10.1002/cncr.24982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 77.Chen HP, Shieh JJ, Chang CC, Chen TT, Lin JT, Wu MS, Lin JH, Wu CY. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–615. doi: 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 78.Nakahara T, Hyogo H, Kimura Y, Ishitobi T, Arihiro K, Aikata H, Takahashi S, Chayama K. Efficacy of rosuvastatin for the treatment of non-alcoholic steatohepatitis with dyslipidemia: An open-label, pilot study. Hepatol Res. 2012;42:1065–1072. doi: 10.1111/j.1872-034X.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 79.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 80.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108:881–891; quiz 892. doi: 10.1038/ajg.2013.5. [DOI] [PubMed] [Google Scholar]
- 81.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]


