Abstract
Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a non-flavonoid polyphenol that may be present in a limited number of food-stuffs such as grapes and red wine. Resveratrol has been reported to exert a plethora of health benefits through many different mechanisms of action. This versatility and presence in the human diet have drawn the worldwide attention of many research groups over the past twenty years, which has resulted in a huge output of in vitro and animal (preclinical) studies. In line with this expectation, many resveratrol-based nutraceuticals are consumed all over the world with questionable clinical/scientific support. In fact, the confirmation of these benefits in humans through randomized clinical trials is still very limited. The vast majority of preclinical studies have been performed using assay conditions with a questionable extrapolation to humans, i.e. too high concentrations with potential safety concerns (adverse effects and drug interactions), short-term exposures, in vitro tests carried out with non-physiological metabolites and/or concentrations, etc. Unfortunately, all these hypothesis-generating studies have contributed to increased the number of ‘potential’ benefits and mechanisms of resveratrol but confirmation in humans is very limited. Therefore, there are many issues that should be addressed to avoid an apparent endless loop in resveratrol research. The so-called ‘Resveratrol Paradox’, i.e., low bioavailability but high bioactivity, is a conundrum not yet solved in which the final responsible actor (if any) for the exerted effects has not yet been unequivocally identified. It is becoming evident that resveratrol exerts cardioprotective benefits through the improvement of inflammatory markers, atherogenic profile, glucose metabolism and endothelial function. However, safety concerns remain unsolved regarding chronic consumption of high RES doses, specially in medicated people. This review will focus on the currently available evidence regarding resveratrol’s effects on humans obtained from randomized clinical trials. In addition, we will provide a critical outlook for further research on this molecule that is evolving from a minor dietary compound to a possible multi-target therapeutic drug.
Keywords: Resveratrol, clinical trials, cardiovascular, cancer, nutraceutical, polyphenol.
INTRODUCTION
Polyphenols and Health
Phenolic compounds are plant secondary metabolites with a large variability in their structure and occurrence. These phytochemicals include simple phenolics such as phenolic acids (caffeic acid, gallic acid, etc., which are not polyphenols strictly speaking, because they have only one phenolic group) and large polymers like condensed and hydrolyzable tannins with large molecular weight [1]. Phenolic compounds are chemically classified in to two main groups: flavonoids and non-flavonoids [2]. Flavonoids include flavonols (quercetin, kaempferol, etc.), flavones (apigenin, luteolin, etc.), flavan-3-ols (catechin, epicatechin, etc.), proanthocyanidins (procyanidins B1, B2, etc.), flavanones (hesperidin, naringenin, etc.), anthocyanins (malvidin, cyanidin, etc.) and isoflavones (genistein, daidzein, etc.). Non-flavonoids include hydroxycinnamic acids (chlorogenic acid, caffeic acid, etc.), hydrolyzable tannins such as ellagitannins (punicalagin, pedunculagin, etc.) and gallotannins (pentagalloyl glucose, etc.), hydroxybenzoic acids (ellagic acid, gallic acid, etc.) and stilbenes (resveratrol, piceid, viniferins, etc.). Phenolics are found in most of the 350 plant-derived foods regularly consumed by humans [3] and different epidemiological studies as well as clinical trials have correlated phenolic compounds with the prevention of chronic degenerative diseases [4]. For example, a number of foodstuffs have been acknowledged with health benefits, at least partially due to their polyphenolic content, such as cocoa [5] and tea [6] (both rich in procyanidins), and extra virgin olive oil [7,8] (rich in hydroxytyrosol). Another classical foodstuff with cardioprotective benefits is red wine. Upon moderate red wine intake, both ethanol and polyphenolic content have been correlated with these benefits [9]. Red wine is rich in phenolic compounds mainly in the form of polymeric condensed tannins and pigmented tannins [2]…and, sometimes, it also contains resveratrol [10].
Why Resveratrol?
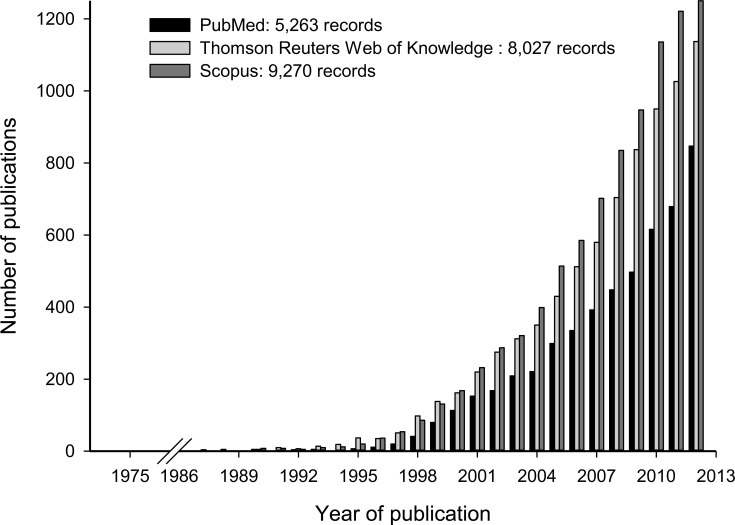
Amongst stilbenes, trans-resveratrol (3,5,4’-trihydroxy-trans-stilbene; RES) is the most relevant compound due to its well-known bioactivity. However, its presence in the human diet is limited to a few foodstuffs including grapes, red wine, peanuts and some types of berries. RES has been studied in different scientific fields (Chemistry, Plant Science, Medicine, Food Science and others). This means that the bibliographical retrieval using the word ‘resveratrol’ yields substantial different figures depending on the database. At the time of submission of the present review, 5,263, 8,027 and 9,270 publications were found according to PubMed (US National Library of Medicine; National Institutes of Health), Thomson Reuters Web of KnowledgeSM (formerly ISI Web of Knowledge) and Scopus (Elsevier B.V.), respectively (Fig. 1). All these publications dealt specifically with resveratrol or included this term in their keywords/abstracts.
Fig. (1).
Number of publications that include the term ‘resveratrol’ as a function of year.
Takaoka [11] isolated this molecule for the first time from hellebore roots (Veratrum grandiflorum O. Loes) and Nomomura [12] did it so from the Japanese knotweed Polygonum cuspidatum. Later on, this compound was detected in wine [13] and it was attributed some cardioprotective effects [14]. But it was not until the publication in Science by Jang et al. [15] on resveratrol’s anticancer potential that the scientific community really became interested in resveratrol and, consequently, reports on the effects and properties of this compound started accumulating exponentially (Fig. 1). Since then, and with different degrees of scientific evidence, RES has been described as a compound that could prevent or reduce a wide range of diseases including neurodegenerative diseases, cancer and cardiovascular diseases [16-18].
About twenty years ago, the consumption of red wine was linked to the low mortality of the French population due to cardiovascular diseases (CVD), in comparison with other Western countries despite sharing CVD risk factors [19]. This apparent contradiction, i.e. CVD risk factors but low mortality, gave rise to the term ‘French Paradox’. The beneficial effects of red wine were further associated with the reduction of oxidative stress and improvement of endothelial function both in healthy people [20] and in patients with acute coronary syndrome [21]. Initially, RES was identified as the potential cause of the beneficial properties of red wine [13,14,22]. Since then, the red wine/RES association has been recurrently used to establish a kind of dogma: red wine contains highly bioactive resveratrol which justifies the former’s benefits. However, some concepts should be taken into consideration before such linear conclusions are drawn. RES is a phytoalexin, i.e. a plant stress-inducible metabolite that is only induced by plants to face pathogen attacks as a part of a number of defensive mechanisms conceived to overcome unfavorable conditions [23]. Thus, typical phytoalexin levels are, by definition, very low and dependent of environmental variables. Stilbenes are grape (Vitis vinifera)-produced phytoalexins and their content, including that of resveratrol, is very low unless grapes have to overcome adverse challenges [24-26]. In this regard, the RES content of wine is usually low, highly variable and thus unpredictable [10,26]. Although red wine is the most important dietary source of RES, its presence in human diet is almost negligible in comparison with other phytochemicals [27]. Therefore, the biological significance of RES, as red wine compound, has been rather overestimated.
The vast majority of studies dealing with the biological activity of RES have been carried out in vitro and in a lesser extent in animal models. Despite the intensive research, the huge output of publications, and the media success of RES, relatively few human clinical trials have been performed so far.
Bearing all the above in mind, the aim of this review is to present an update of the current available evidence regarding RES and randomized clinical trials in humans. In order to show the current dimensions of the research on RES and the insufficient number of human studies, we will approach this topic by showing a summary of the main ‘preclinical’ studies, including both in vitro and animal assays, human studies on bioavailability and metabolism, and finally we will focus on the human randomized clinical trials available so far.
PRECLINICAL STUDIES
In vitro Models
The numerous in vitro studies conducted so far have contributed to increase the existent vast list of potential effects of RES as well as to establish many possible direct or indirect molecular targets and mechanisms of action mediating them, which often overlap. In this section we review recent available basic in vitro research regarding potential mechanisms and molecular targets of RES possibly involved in the prevention or slowing down of infirmities like cardiovascular diseases, cancer and neurodegenerative diseases, or in other RES reported capacities such as aging delaying.
During the last two decades the great majority of reported in vitro data have been obtained after exposing cells to micromolar range (up to 200 µM) concentrations of RES. Dietetic doses of RES are very low and consequently human physiological concentrations found for this molecule and its metabolites do not usually go over 50 nM and 2 µM, respectively [28,29], although exceptional higher plasma RES concentrations (4.2 µM) and derived metabolites (18 µM) have been reported upon ingestion of a high micronized RES dose (5 g) [30]. Taking this into account, nanomolar range concentrations of RES have been employed in recent in vitro studies in order to better reproduce physiological conditions.
Since Jang et al. in 1997 [15] showed a cancer preventive effect of RES on all three phases of skin and breast cancer development: initiation, promotion, and progression, in a mice model, the anticancer activity of RES has been the subject of a vast number of researches. As a result, a wide range of molecular targets whose modulation leads to growth arrest and death on cancer cells have been revealed. Numerous in vitro studies have shown consistent anticancer effects of RES in a variety of human cancer cell lines, including colon, prostate, breast, melanoma, liver, glioma cells, etc. Some of the most referent works are listed in (Supplementary Table 1 (713.7KB, pdf) ). For further information, some recent works have globally [31-34, among others] and specifically (breast [35], liver [36], and colon cancer [37]) reviewed the anticancer activity of RES and its anticarcinogenic mechanisms.
Most in vitro studies have indicated that RES exerts an antiproliferative activity via the induction of apoptosis and/or cell cycle arrest, in different cell lines, which arrested their proliferative cycle mainly in the G0/G1 phase (Supplementary Table 1 (713.7KB, pdf) ). Several molecular targets and/or mechanisms like signal transduction pathways and cell cycle regulating proteins associated with RES-induced cell cycle arrest have been identified. Among these, RES modified the balance of cyclins and cyclin-dependent kinases (cdks) leading to cell cycle arrest in a specific phase. For example, the inhibition of cyclin D1/cdk4 by RES has been correlated with the arrest at G0/G1 phase in different cancer cells [38-42]. In addition, RES has been reported to increase cyclins A and E levels on cancer cell lines with cycle arrest in S and G2/M phases [43,44]. Similar findings have shown that RES exerts cell cycle arrest and activation of the p53-dependent pathway [45-48]. RES has been also shown to inhibit the expression of retinoblastoma protein (Rb), another tumor suppressor protein involved in the G1/S transition in a normal status [41,44,48] (Supplementary Table 1 (713.7KB, pdf) ).
Regarding the apoptosis induction exerted by RES on a wide range of cancer cells, it has been reported that it can activate caspases, primarily -3 and -9 [39,49-52]. Moreover, in cancer cell lines the inhibition by RES of antiapoptotic Bcl-2 family proteins, such as Bax, Bak or Bad, and of the inhibitors of apoptosis (IAPs) protein family, such as cIAP-2 or XIAP, has also been showed as a mechanism of caspase activation and cytochrome C release [50,53]. Other molecular mechanisms involved in the antiproliferative effects of RES against cancer cells include the suppression of the PI3K/Akt/mTOR pathway [41,50,54-60]; the inhibition of nuclear factor-kappa B (NF-κB), a transcription factor involved in the regulation of proliferation and apoptotic stress response [61-64]; the inhibition of the Wnt signalling pathway [65,66]; and the modulation of mitogen-activated protein kinases family members (MAPKs), mainly the activation of extracellular signal regulated kinases (ERKs) and p38 [48,57,67,68]. RES has been also suggested to have a role in the inhibition of angiogenesis-dependent processes, such as tumor growth, cell migration and invasion, and metastasis. Notably, it has been reported that RES decreases leukotriene B4, and the expression of matrix metalloproteinases (MMP) (mainly MMP-9) [62-64,69-71] and angiogenesis markers like VEGF, EGFR or FGF-2 [41,72] (Supplementary Table 1 (713.7KB, pdf) ). Contrary to what was described in many cancer cell models, RES has been shown to rescue neurons from apoptosis (Supplementary Table 2 (713.7KB, pdf) ). In studies with neurotoxicans, like the Aβ peptide or 1-methyl-4-phenylpyridinium (MPP+), RES exerts anti-apoptotic effects by avoiding caspase-3 activation, increasing Bcl-2 protein [73] and activating protein kinase C [74]. Furthermore, in cells exposed to 6-hydroxydopamine (6-OHDA) or Aβ, RES promotes neuron survival in a sirtuin 1 (SIRT1)-dependent way [61]. Finally, RES also has an anti-amyloidogenic effect facilitating the clearance of Aβ in the brain by modulating proteasome activity [75] and inhibiting the formation of Aβ [76] (Supplementary Table 2 (713.7KB, pdf) ).
Many in vitro studies have been focused on identifying targets and mechanisms by which RES exerts beneficial cardiovascular and neuroprotective effects, including its antioxidant and anti-inflammatory capacity. The antioxidant ability of RES has been supported by many in vitro studies where several neurological- and vascular-related cellular models were exposed to low µM concentrations of this polyphenol (Supplementary Tables 2 (713.7KB, pdf) and 3 (713.7KB, pdf) , respectively). RES has been shown to scavenge hydroxyl, superoxide, metal-induced radicals [77]. Nevertheless, its capacity of inhibiting oxygen free radical formation may come from the inhibition of reactive oxygen species (ROS) production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) [78,79] and by the induction of antioxidative enzymes or their substrates, such as superoxide dismutase (SOD) [78,80-83], thioredoxin [84], heme oxygenase-1 (HO-1) [84- 87], glutathione peroxidase-1 (GPx1) [78,80] and catalase [81]. According to the published evidence, RES has the capacity to improve endothelium function mainly by stimulating endothelial production of nitric oxide (NO) [88-91] through multiple mechanisms, particularly by enhancing endothelial nitric oxide synthase (eNOS) expression and/or activity [88,92-94] even at physiological concentrations [89,90,95] (Supplementary Table 3 (713.7KB, pdf) ). It has been suggested that RES enhances eNOS activity via 5’-adenosine monophosphate-activated protein kinase (AMPK) or extracellular signal-regulated kinase 1/2 (ERK1/2)-mediated phosphorylation at Ser1177 [90,96]. The upregulation of eNOS expression is, at least in part, mediated by the histone/protein deacetylase SIRT1 [80,88,92,94]. Recent studies suggest that RES could activate SIRT1 indirectly through AMPK [97] and that NFE2-related factor 2 (Nrf-2) activation is a key mechanism by which RES confers its cytoprotective effects in the cardiovascular system [98] (Supplementary Table 3 (713.7KB, pdf) ).
One of the main properties attributed to RES is anti-inflammatory action. In vitro assays showed that RES was able to attenuate monocyte adhesiveness to the endothelium [99], even at nanomolar concentrations [100] and to prevent spontaneous endothelial cell migration through a Rho kinase-dependent mechanism [101]. RES can reverse H2O2-, tumor necrosis factor-alpha (TNF()- and cigarette smoke extract-induced ROS production [102,103], NF-κB activation [61,100,102-106] and upregulation of inflammatory markers like IL-6, TNF(, MCP-1 and iNOS [101,102]. It also reduces the expression of adhesion molecules such as VCAM-1 and ICAM-1 [100,106], probably by inhibiting the p38 MAPK signalling pathway [107] (Supplementary Table 3 (713.7KB, pdf) ). In addition, RES targets COX-1, suppresses the expression and activity of COX-2 [71] and the downstream signals like prostaglandin [108,109]. Furthermore, RES has been shown to elevate proteoglycan synthesis in chondrocytes [109]. RES has also been associated with having a role in lipid modulation. In vitro, µM range concentrations of RES reduced the synthesis of lipids in 3T3-L1 adipocytes, and decreased lipid accumulation and cell viability in maturing 3T3-L1 preadipocytes [110,111]. Moreover, in mature 3T3-L1 adipocytes, µM range concentrations of RES increased lipolysis and reduced lipogenesis, contributing to reduce lipid accumulation in vitro; in addition, RES decreased cell viability dose dependently and induced apoptosis [111,112]. A study in human preadipocytes and mature adipocytes suggested that RES (>10 µM) influences adipose tissue mass by inhibition of preadipocyte proliferation, inhibition of adipogenic differentiation, and inhibition of de novo lipogenesis in a SIRT1-dependent manner. In addition, RES influences the secretory profile of human preadipocytes in a way that may positively interfere with the development of obesity-associated comorbidities [113]. Another study in human adipocytes showed that in SIRT1-independent way RES synergistically enhanced TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis of human adipocytes [114]. A recent study showed for the first time the delipidating effect of RES metabolites in maturing pre-adipocytes and glucuronide metabolites in mature adipocytes, although the concentrations necessary to produce such effect were not in physiological range [115].
An additional effect of RES has to do with its capacity of inhibiting platelet aggregation. Platelet aggregation inhibition was seen with µM [116] and nM [117] concentrations of RES and it possibly involves both the inhibition of the p38 MAPK pathway and the activation of NO/cyclic guanosine monophosphate, resulting in the inhibition of phospholipase C and/or protein kinase C activation, thereby leading to reduced intracellular calcium concentration or free radical formation, and finally to platelet aggregation inhibition. On the other hand, it has been suggested that RES induces platelet apoptosis at higher concentrations which, in part, is due to stimulation of mitochondrial membrane potential dissipation, activation of caspase-3,-9 and -8, and cytochrome C release [118].
Although scarce, studies addressing the anti-aging effects of RES generally suggest that they are exerted via inhibition of oxidative stress, downregulation of inflammation levels, enhancement of SIRT1 expression and sirtuin-regulated downstream pathways rather than SIRT1 activity [119,120] (Supplementary Table 4 (713.7KB, pdf) ). Additionally, many of these signalling networks are closely related; SIRT1 levels, for example, are regulated by the energy homeostasis and oxidative stress in the hippocampus and cerebral cortex of rats, thus SIRT1 modulation also regulates ROS levels [121].
In conclusion, the in vitro research carried out so far concerning exposure to RES, despite unfinished, has led to great progress on unravelling its molecular targets and mechanisms of action (Fig. 2). Available in vitro evidence on the protective mechanisms of RES contributes to support its role as an anti-oxidant and anti-inflammatory modulator of several signalling pathways and transcriptional factors. This modulation leads to important cellular processes such as apoptosis, induction of cell cycle arrest, migration, adhesion and invasion cell inhibition, lipidic modulation, etc. Nevertheless, it has to be pointed out that in vitro studies where the dose ranges of RES tested are comparable to the physiologically ones found in humans provide more trustworthy evidence.
Fig. (2).
Preclinical effects for resveratrol. AMPK, adenosine monophosphate activated protein kinase; APAF, apoptotic protease activating factor; Apo, apolipoprotein; Bad, Bcl-2-associated death promoter; Bak, Bcl-2-antagonist killer; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra large; BFL-1/A1, Bcl-2-related protein A1; Bid, BH3 interacting-domain death agonist; BH4, tetrahydrobiopterin; Bim, Bcl-2-like 11 apoptosis facilitator; CAT, catalase; cdk, cyclin-dependent kinase; cIAPs, inhibitor of apoptosis proteins; COX, cyclooxigenase; CREB, cAMP response elementbinding; CRP, C-reactive protein; Cyt-c, cytochrome C; E2F, transcription factor E2F; EGFR, endothelial growth factor receptor; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal regulated kinase; FFA, free fatty acids; FGF, fibroblast growth factor; FOXO, forkhead transcription factor; GCH1, GTP cyclohydrolase 1; GCLC, glutamate-cysteine ligase catalytic subunit; GPx, glutathione peroxidase; GRO-α/CXCL1, chemokine (C-X-C motif) ligand 1; GSH, glutathione; HDL-c, high density lipoprotein cholesterol; HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; HO-1, heme oxygenase-1; HOMA-IR, homeostasis model of insulin resistance; ICAM-1, intercellular adhesion molecule 1; IFN-γ, interferon-gamma; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; LDL-c, low-density lipoprotein cholesterol; MAPKs, mitogen-activated protein kinases; MCP, monocyte chemotactic protein; MDA, malondialdehyde; MI, myocardial infarct; MIP, macrophage inflammatory protein; MMP, matrix metallopeptidase; MPO, myeloperoxidase; MTA, metastasis-associated protein; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; NO, nitric oxide; NOX, nicotinamide adenine dinucleotide phosphate oxidase; NQO, nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase; Nrf, nuclear factor-E2-related factor; p21Waf1/Cip1, cyclin-dependent kinase inhibitor 1A; p27, cyclin dependant kinase inhibitor 27; p53, tumor protein 53; p70S6K, p70S6 kinase; PGC, peroxisome proliferatoractivated receptor-gamma coactivator; PKB, protein kinase B; PGE2, prostaglandin E2; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PPAR, peroxisome proliferator-activated receptor; PTEN, phosphatase and tensin homolog; Rb, retinoblastoma tumor suppressor gene; ROS, reactive oxygen species; sCD40L, soluble CD40 ligand; SIRT1, sirtuin 1; SOD, superoxide dismutase; STAT, signal transducer and activator of transcription; TG, triglycerides; TGF, transforming growth factor; TH, tyrosine hydroxylase; TIMP, tissue inhibitors of MMP; TNFα, tumor necrosis factor alfa; Total-c, total cholesterol; TRAF, TNF receptor associated factor; TRAIL, TNF-related apoptosis inducing ligand; Trx, thioredoxin; VCAM-1, vascular cell adhesion protein 1; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis protein; XO, xanthine oxidase. Effect is indicated by ↓ : reduction; ↑ : induction; p-: phosphorylate status.
Animal Models
In the last couple of decades research carried out on in vivo animal models has grown exponentially and the outcomes have contributed to filter the over flow of in vitro-obtained data. The potential effects of RES seen in vitro along with the various possible direct or indirect molecular targets and mechanisms of action mediating them, which often overlap can nowadays be confronted with in vivo evidence. In this section we review available in vivo evidence concerning the potential mechanisms and molecular targets of RES regarding the prevention or deceleration of cancer, cardiovascular and neurodegenerative diseases, and aging.
A wide range of in vivo studies on the anticancer effects of RES in different rodent models have demonstrated that oral administration, topic application and/or injection can prevent induced carcinogenesis, including the ability to decrease cell proliferation, induce apoptosis, and inhibit angiogenesis, metastasis, and tumor growth. RES’s effects are involved in a variety of cellular pathways against numerous types of cancer models such as melanoma, lung, liver, breast, glioma, prostate, and colorectal (Supplementary Table 5 (713.7KB, pdf) ). However, the promising in vitro anticancer results were not confirmed in some studies where no overall differences were observed between RES and controls, suggesting that other factors such as dosage, diet, methods and type of tumor could also influence its efficacy [122-127]. RES can inhibit tumor growth from different tissues (prostate, skin, colon, liver, etc.) and avoid cell proliferation due to cell cycle arrest, mainly at the G2/M phase [128,129], and to apoptosis induction [130-132]. Consistent with in vitro observations in some animal studies RES treatment caused apoptosis inhibition and cell cycle arrest, which involved an increase of pro-apoptotic markers and cell cycle regulator proteins such as Bax, caspase 3, p38, ERK, as well as a decrease in cyclins D1 and B1, Bcl-2, c-fos, and c-jun [133-138]. Furthermore, the anti-cancer effects of RES also involved the inhibition of apoptosis extrinsic and/or intrinsic pathways such as NF-κB, MAPK and PI3K-Akt [134,138-141].
Other molecular targets of RES’s chemoprotective action that have been corroborated in animal studies involve the inhibition of metastasis and angiogenesis, through the inhibition of MMP-2, -9 and EFGR-2 expression and/or activity [130,142,143]. Animal studies have indicated that RES enhanced the cytotoxic effects of several chemotherapeutic agents such as 5-fluorouracil, placitaxel, cisplatin and temozolomide, in the same way in vitro studies did [52, 133,144-146], and decreased or delayed carcinogenesis induced by well-known carcinogens such as doxorubicin, diethylnitrosamine, benzo[a]pyrene, 1,2-dimethylhydrazine, 7,12-dimethyl-benz[a]anth-racene, N-nitrosomethylbenzylamine and azoxyme-thane [36,135, 138,143,147-151].
RES also acts in various facets of CVD, including hypertension, ischemia/reperfusion injury, heart failure and atherosclerosis via multiple mechanisms which lead to risk reduction of cardiovascular events. (Supplementary Tables 6 (713.7KB, pdf) -8 (713.7KB, pdf) ) resume the recent in vivo data obtained from research carried out on different cardiovascular disease-induced animal models. A number of studies where myocardial infarction was induced either by surgery or diabetes (Streptozotocin, STZ, injection or high glucose diet) demonstrated that RES contributed to reduced infarct size (Supplementary Table 6 (713.7KB, pdf) ). In rats, preconditioning with RES significantly reduced infarct area [152] and ameliorated LAD occlusion-induced MI with enhanced cardiac function, enhanced stem cell survival and proliferation, and reduced oxidative stress [153]. Also in rats, RES reduced infarct size in diabetes-induced myocardial infarction after exposure for 5 to 15 days with doses of 1 to 5 mg/kg bw/day (Human Equivalent Dose, HED, of ≈11 to 56 mg of RES for a 70 kg person) [86,154,155] in a process perhaps dependent on NO [86] and related to the induction of eNOS expression in the ischemic heart [156]. Moreover, RES doses of 2.5 and 5 mg/kg bw/day for 14 days improved post-ischemic ventricular recovery, and reduced myocardial infarct size and cardiomyocyte apoptosis, whereas doses >25 mg/kg bw/day (HED= ≈282 mg for a 70 kg person) were found to depress cardiac function and increase myocardial infarct size and apoptotic cells number [157]. The anti-apoptotic action of RES in cardiomyocytes may involve the SIRT1-FOXO1 pathway [158]. In swine models, 100 mg RES/kg bw/day (HED= 7 g for a 70 kg person) for 7 weeks reduced inferolateral function induced by a hypercholesterolemic diet [159], whereas a 437-fold lower dose (0.23 mg/kg bw/day; HED= 16 mg for a 70 kg person) for one year diminished aortic elastic fibers disruption and alteration, and reduced aortic tissue accumulation of fatty cells and O2- [160].
The reductive effects of RES on hypertension were described in several studies using rodents as in vivo models (Supplementary Table 6 (713.7KB, pdf) ). A reduction on blood pressure was found in mouse [161] and rat [162-169] models with doses ranging from 1 to 800 mg RES/kg bw/day and exposure durations ranging from a few hours up to 10 weeks. On the other hand, some studies on rat models reported no effect on blood pressure with low RES doses (2.5 mg/kg/day) [170-172]. After exposure to RES, cardiac dysfunctions were found to be reduced [171-173], as well as unfavourable vascular remodelling [92,161] and cardiac contractility [163,174]. Left ventricular function was improved in rat [175,176] and in swine [177] models. Furthermore, many studies carried out on rat models showed a reduction on hypertrophy [162,166,171,178-180] even with 2.5 mg RES/kg bw/day (HED= ≈28 mg for a 70 kg person). The anti-hypertensive properties of RES seen in rats were often associated with endothelium-dependent vascular relaxation [166,167,169,181], enhanced eNOS activity [166,167,169], and increased NO [167] and glutathione [182-184] levels. In addition, rodent studies have shown an increase in the activities of SOD [167,182,184,185], catalase [184,185] and GPx [182], and a decrease in myeloperoxidase activity [183,186]. It is noteworthy that a low dose of RES reduced cardiac hypertrophy without affecting blood pressure [170,171] which suggests alternative mechanisms may be associated its cardiac hypertrophy inhibitory effect. For example, this has been proposed to occur via AMP-activated protein kinase pathway activation and Akt pathway inhibition [187].
Circulating lipid levels after exposure to RES have been analysed in several in vivo studies (Supplementary Table 7 (713.7KB, pdf) ). In mice, a reduction of total cholesterol, triglycerides and free fatty acids was reported in high-fat diet (HFD)-induced adipogenesis models (7.08-400 mg RES/kg bw/day for 6-10 weeks; HED from ≈4 mg up to >2 g for a 70 kg person) [188,189] and apo E-deficient models showed a decrease in LDL- and total-cholesterol [190]. In rats, significant decreases were observed in total cholesterol (dose range: 10-45 mg RES/kg/day for 6-8 weeks) [165,191], LDL-cholesterol [172,192] and triglycerides [165,169,172,184,185,192] (2.5-10 mg/kg bw/day for 4-10 weeks) and an increase in HDL-cholesterol (2.5-15 mg/kg bw/day for 4-8 weeks) [172,191,192]. In swine models given 100 mg of RES/kg bw/day (HED= 7 g for a 70 kg person) for 7 [159] and 11 weeks [193] a decrease was reported in total- and LDL-cholesterol. Moreover, a reduced grade of steatosis was seen in mice exposed to 200 mg RES/kg bw/day (HED >1 g for a 70 kg person) for 20 weeks [194] and in rats (15-44 mg/kg bw/day for 4-6 weeks) [185,191,195]. Reduced abdominal fat was observed in rats at a dose of 10 [165] (HED= ≈113 mg for a 70 kg person) and 100 mg RES/kg bw/day (HED >1 g for a 70 kg person) [196] for 8-10 weeks. Whereas the administration of high levels of RES (>200 mg/kg bw/day; HED >1 g for a 70 kg person) reduced body weight increase caused by HFD in mice [197] and grey mouse lemurs [198], doses <60 mg/kg bw/day (HED <340 mg for a 70 kg person) did not affect body weight variations [164,165,199].
In the majority of in vivo studies where insulin sensitivity was diminished (as a consequence of HFD or STZ-induced diabetes), RES lowered glucose and/or insulin levels and/or improved insulin sensitivity independently of dose and exposition time (Supplementary Table 7 (713.7KB, pdf) ), particularly in rodents [165,184, 194,196,197,199-205]. In one study, AMPK seemed to be required for the insulin sensitivity enhancing effect of RES in mice [206]. Improved insulin sensitivity and lower glucose levels were found in grey mouse lemurs with 200 mg/kg bw/day for 33 months [207]. In a swine model, 100 mg RES/kg bw/day for 11 weeks [193] improved insulin sensitivity and, in rabbits, insulin levels were reduced after 10 weeks (≈1.5 and 17 mg/kg bw/day; HED= ≈34 and 384 mg for a 70 kg person), although glucose levels were not affected [208]. Metabolic rate was increased in mice after treatment with 400 mg RES/kg bw/day (HED >2 g for a 70 kg person) for 12 weeks [206] and metabolic dysregulation was ameliorated with exposure doses ranging from 1 to 50 mg/kg bw/day (HED= ≈11 up to 560 mg for a 70 kg person) (5 days-15 weeks) in rat models [180,204].
RES has also been show to modulate inflammation, or at least to influence the levels of several inflammatory response markers. Significant changes on the level of these markers were reported in various studies performed on rodents regarding the administration of RES, where doses ranged from 1 mg up to more than 1 g/kg bw/day (HED from ≈11 mg to >5 g for a 70 kg person) and exposure times varied from a few days up to 30 weeks (Supplementary Table 8 (713.7KB, pdf) ). With few exceptions RES reversed the rise in the levels of important pro-inflammatory cytokines and other inflammation-related markers in several disease-induced models, such as induced-obesity, -hypertension, -diabetes and colon colitis, and exposure to carcinogens, among others. RES treatment was generally found to reduce TNFα 146,165,195,209-220, IL-1β 209,210,213,217, 218,220, IL-6 102,210,216,218-222, MCP-1 223,224, COX-2 [209,210,215] and iNOS [102,209,210,217]. In addition, RES exposure diminished the activity of T- and B-cells, and macrophages, due to a significant inhibition of their proliferation, antibody production, and lymphokine secretion [194,221,225]. Moreover, RES inhibited NF-κB, and NF-κB-related inflammatory and autoimmune markers [182,215,216].
Neurodegenerative diseases are a group of progressive disorders characterized by a common inflammatory status and increased ROS levels which primarily produce the loss of neurons’ normal function and also neuron death. The broad-spectrum actions exerted by RES resulting from the modulation of a number of signalling networks and cellular-effector mechanisms related to inflammation, oxidant status and apoptosis, make it a candidate for treating neurodegenerative diseases [226] (Supplementary Table 9 (713.7KB, pdf) ). If the doses required for RES to exert its effect in vivo are somewhat lower than those used in vitro or whether it could have an effect on the central nervous system via the enteric nervous system is something that is still unknown. In neurodegenerative disease models (Alzheimer, Parkinson and Huntington) RES improves the pathological damage of neurons, increasing cell survival by inhibiting apoptosis, inflammation and oxidative stress and thus improving the cognitive impairment and the decline in motor function that accompany these diseases [216,227]. The possible mechanisms responsible for this neuroprotection appear to involve the regulation of HO-1 and peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), the upregulation of the Nrf-2 expression or the activation of the SIRT1-uncoupling protein 2 pathway [228-231].
Extension of lifespan is one of the most fascinating effects exerted by RES (Supplementary Table 10 (713.7KB, pdf) ). In 2003, Howitz and colleagues [232] showed that RES was able to extend yeast replicative lifespan (70%) but not the chronological one of Saccharomyces cerevisiae by the activation of SIRT1. Soon after, some studies conducted in metazoans (Caenorhabditis elegans and Drosophila melanogaster) and the short lived seasonal fish Nothobranchius furzeri showed that RES extended medium and maximal lifespan by modulating SIRT1 [233,234]. In murine models in which RES was administrated with a HFD, a 31% decrease in the risk of death was shown [199]. Also, RES mimicked calorie restriction extended lifespan by increasing survival and improving health quality (motor function, insulin sensitivity and organ pathology) probably by inducing the PGC-1α-SIRT1 pathway [197,199]. Although there is a respectable amount of data supporting the role of RES in SIRT1-mediated lifespan, some controversy still persists. Some studies have not found any effect on survival or locomotor activity in mice [235], or that the effect is sex-dependent in flies [236,237]. Nevertheless, RES improves the symptoms of aging-related chronic diseases in animal models and could be an optimal candidate for treating aging-related diseases.
In conclusion, the growing number preclinical studies have been providing encouraging results concerning RES’s beneficial properties against cancer, cardiovascular and neurodegenerative diseases, and aging retardation. Some of these properties have been observed even at dietetic doses, and often corroboration between in vitro and in vivo data is found; nevertheless, further studies are required to elucidate some variableness found in RES actions, for example regarding its hormetic behavior (i.e. both beneficial and detrimental effects depending on the dose and time of exposure). Despite hopeful advances have been made in order to achieve the final objective of confirming RES as a beneficial and safe molecule for human consumption, the definite test of human clinical trials is just now beginning to bear fruit.
HUMAN INTERVENTION STUDIES
Despite abundant preclinical studies have been carried out in animal models, mostly in rodents, investigations regarding the safety and beneficial effects of RES in humans are scarce [17]. There are many reviews and ‘perspective’ papers about RES but limited human clinical evidence [9,16,17,238-245, among others]. In this context, most research dealing with RES in humans has been related to its pharmacokinetics and metabolism. Therefore, the heading of this section is specifically conceived to include the two clearly different approaches to human studies, i.e. evaluation of pharmacokinetic parameters and metabolic profiles, and randomized clinical trials (evaluation of effects).
Pharmacokinetics and Metabolism
Table 1 summarizes the current knowledge on pharmacokinetics and metabolism of RES, which has been the main topic linking RES research and humans. We have completed here the information that has been reviewed recently in different publications [239-242,246]. We will highlight the main current knowledge about this topic, but the reader is advised to check recent reviews or specific cited references for additional, more detailed, information (Table 1).
Table 1.
Main Published Human Studies Dealing with the Pharmacokinetics, Metabolism and Disposition of Resveratrol.
| Resveratrol (RES) formulation and dosage | Volunteers and sample size (n) | Main outcome | Reference |
|---|---|---|---|
| Single intake of 25 mg/kg RES dissolved in white wine, grape juice or vegetal juice. | Healthy males (n=12). | RES absorption was broadly equivalent in the three matrices. | [28] |
| Single intake of 0.03, 0.5 or 1 mg/kg RES in whisky:water (5 mL:50 mL), and 0.32, 0.64, 0.96 or 1.92 mg RES in grape juice (200, 400, 600 or 1200 mL). | Healthy males (n=3). | Pharmacokinetic, urinary and plasma profile. Absorption of RES-glucoside is lower than that of RES. Similar relative RES absorption all the matrices. | [323] |
| Single oral (25 mg) and intravenous (1.5 mg) administration of 14C-RES. | Healthy females (n=3) and males (n=3). | Pharmacokinetic and metabolic profile. High absorption but very low bioavailability. Dihydroresveratrol could be a relevant RES-derived metabolite. | [246] |
| Single intake (250 mL) of red wine containing 0.4 mg trans-RES and 0.4 mg cis-RES). | Healthy males (n=11). | Detection of RES and some metabolites in LDL particles. | [324] |
| Three dietary approaches: Single ingestion of 300 or 600 mL red wine after fasting, a standard meal, or a meal with different lipid load (total RES from 0.25 mg to 1.9 mg). | Healthy females (n=11) and males (n=14). | Pharmacokinetic and metabolic profile. Food or lipid content did not exert influence on RES bioavailability. | [259] |
| Daily consumption of 300 mL sparkling wine (0.36 mg RES), 200 mL white wine (0.4 mg RES) or 200 mL red wine (2.6 mg RES) for 28 days. | Healthy females (n=10) and males (n=10). | Total RES metabolites increased upon increasing RES ingestion. RES metabolites could be used as biomarkers of wine intake. | [325] |
| Single intake of 1 g RES (capsule). | Adult (unspecified gender) (n=1). | Urinary and plasma metabolic profile. | [254] |
| Single intake of 0.5, 1, 2.5 and 5 g RES (capsules). | Healthy males (n=18) and females (n=22). | Pharmacokinetic and metabolic profile. The highest RES amount ever ingested by humans. No adverse effects upon single intake. | [255] |
| Single intake of 500 mL low-fat milk containing RES previously dissolved in hydroalcoholic solution. RES dose: 85.5 mg/70 kg. | Healthy males (n=9). | Pharmacokinetics and metabolic profile. New plasma RES-C/O-glucuronide metabolites are described. High binding affinity to plasma proteins. | [248] |
| Daily intake of 300 mL white wine (75 mg phenolics) or red wine (540 mg phenolics) for 15 days. | Healthy females (n=11) and males (n=9). | Plasma RES concentration increased from 0.72 mM for white wine to 1.33 mM for red wine. | [326] |
| Single intake of 0.4 g RES following either a standard high-fat meal or 8 h fasting. | Healthy subjects (n=24). | RES absorption was delayed by the food (Tmax was 4-fold higher than after fasting). However AUC values were similar. | [257] |
| Six-daily intake of RES capsules (25, 50, 100 or 150 mg) at 4 h intervals for 13 days. | Healthy males (n=20) and females (n=20). | AUC values were no directly proportional to RES intake. High interindividual variability. Bioavailability was higher after morning administration. | [327] |
| Single plus multiple RES capsules (0.2 g) intake at 8 h intervals for 3 days. | Healthy young (n=6) and elderly (n=6) females and young (n=6) and elderly males (n=6). | Pharmacokinetic and metabolic profile. No gender or age-dependent differences were observed. | [253] |
| Single intake of 250 mL red wine, 10 tablets or 1 L grape juice. Average RES dose 0.014 mg/kg. | Healthy males (n=11). | Bioavailability from wine and grape juice was around 6-fold higher than that from tablets. | [328] |
| Twice daily intake of 2 g RES with a standard breakfast, a high-fat breakfast, quercetin 500 mg twice daily and 5% alcohol 100 mL. | Healthy females (n=5) and males (n=3). | Steady-state 12-hour pharmacokinetics of RES. High-fat meal decreased RES absorption. Quercetin or alcohol did not influence RES plasma concentrations. | [258] |
| Daily intake of capsules containing 0.5, 1, 2.5 or 5 g micronized RES for 29 days. | Healthy females (n=18) and males (n=22). | Micronized RES formulation increased plasma Cmax of RES and derived-metabolites versus published values for standard RES. | [30] |
| Daily ingestion of capsules containing 0.5 g or 1 g micronized RES for 8 days. | Colon cancer male (n=9) and female (n=11) patients. | RES and four derived glucuronide and sulfate derivates were quantified in colon tissues. Higher levels were detected in the right side of the colon. | [261] |
| Single ingestion of 375 mL red wine (6.3 mg total RES content as aglycone and glucoside in both trans- and cis- forms) or 10 capsules containing grape extract (total RES content 4.7 mg RES). | Healthy men (n=10). | Seventeen RES-derived metabolites were identified. RES from grape extract showed delayed absorption versus red wine and remained longer in the organism yielding higher dihydroresveratrol-derived metabolites than in the case of red wine. | [249] |
| Single oral dose of 0.5 mg RES/kg body weight in the form of a grapevine-shoot supplement. Registered at the German Clinical Trials Register as DRKS00004311, Universal Trial Number (WHO) UTN: U1111-1133-4621. | Healthy volunteers (n=12). | Besides DHRES, 2 new metabolites were identified: 3,4'-dihydroxy-trans-stilbene and 3,4'-dihydroxybibenzyl. RES metabolism by human gut microbiota had pronounced interindividual differences. | [252] |
The polyphenol RES is highly absorbed, metabolized and excreted yielding a rather low bioavailability [29]. The first evaluation of RES absorption in humans was carried out by Goldberg et al. [28] who also explored the effect of different food matrices on RES bioavailability. Initially, studies were focused on the analysis of total RES content by treating plasma and urine samples with glucuronidase/sulfatase enzymes due to the lack of suitable standards of phase II-derived metabolites for proper identification and quantification. With time, the knowledge about this topic has substantially increased and up to nearly 20 RES-derived metabolites have been described in plasma, urine and some tissues according to different studies in animals [247] and humans [248,249]. Among these metabolites, there are trans- and/or cis- forms of mono- and diglucuronides, mono- and disulfates and sulfoglucuronides from parent RES, as well as equivalent conjugations of the microbiota-derived metabolite dihydroRES (DHRES). The possible significance of the last-named metabolite had already been pointed out by Walle et al. [29] and was further confirmed in animals [247,250] and humans [249]. The activity of any specific RES circulating metabolite is still under debate, and in the case of circulating DHRES metabolites, the existing gap in this knowledge is almost complete [251]. In this regard, Bode et al [252] tried to elucidate interindividual differences in RES metabolism by human gut microbiota and to identify bacterial strains involved. This recent investigation identified new RES-microbiota derived metabolites using in vitro fermentation experiments with feces samples from 7 healthy volunteers and also with a controlled intervention study with 12 healthy volunteers upon consuming RES. After a washout period, all subjects received a one-time oral dose of 0.5 mg RES/kg body weight in the form of a grapevine-shoot supplement, and 24-h urine samples were analyzed. Besides DHRES, 2 previously unknown bacterial trans-RES metabolites were identified in vitro and in vivo: 3,4'-dihydroxy-trans-stilbene and 3,4'-dihydroxybibenzyl (lunularin). Two strains, Slackia equolifaciens and Adlercreutzia equolifaciens, were identified as DHRES producers. Gut bacteria able to produce dehydroxylated metabolites could, however, not be identified. Authors conclude that RES metabolism by human gut microbiota shows pronounced interindividual differences, which should be taken into account during the investigation of health-related effects of this stilbene.
RES bioavailability shows a high interindividual variability, although gender and age have been found not to have a direct effect on this [253]. In this context, investigations as those commented above dealing with the role of gut microbiota in the production of RES metabolites are welcome and could contribute to clarify the interinvidual variability in RES bioavailability. The plasma concentration of phase II-derived metabolites is much higher than that of parent RES. The highest plasma Cmax (967 ng/mL, around 4.2 µM) was reported by Brown et al. [30] upon ingestion of 5 g micronized RES in comparison to that obtained (539 ng/mL, around 2.3 µM) after standard RES intake [254,255]). The most abundant circulating RES metabolite in humans is RES-3-O-sulfate (maximum reported Cmax was around 18 µM, which was coincident after intake of both 5 g micronized and standard RES [30,255]). In contrast, the most abundant circulating metabolite in rats and pigs is RES-3-O-glucuronide [256].
Another interesting topic is the possible influence of the food matrix on RES bioavailability. The main conclusion is that RES-containing liquid formulations (either added or endogenous RES) such as grape juice, vegetable juice, wine (white, red, sparkling) show similar absorption figures. RES absorption is delayed when provided in capsules but remains longer in the organism giving rise to higher DHRES-derived metabolites [249]. Food has been reported to delay RES absorption but not plasma AUC values [257] whereas high-fat foods either delayed RES absorption [258] or did not exert a significant effect on RES bioavailability, independently of their lipid content [259]. Neither alcohol nor the flavonol quercetin affected RES bioavailability [258].
Very interestingly, Patel et al. [260] reported the detection of RES and derived metabolites in human tissue for the first time. RES and five derived metabolites (3-glucuronide, 4’-glucuronide, sulfoglucuronide, 3-sulfate and 4’ sulfate) were identified in both normal (proximal or distal to the tumor) and in tumor tissue samples obtained from the right or left side of the colorectum. There was a logical very high variability in the content of RES and derived metabolites depending on the RES dose, type of tissue (normal or tumor), and location (proximal or distal to tumor in normal samples, and left or right sides in both normal and tumor tissues). Both parent and RES metabolites were mainly detected in right-sided colorectal tissues (feces are liquid in the right-sided colorectum, and interact earlier with this part of the gut [261]). After 0.5 and 1 g doses, maximal RES mean concentrations of 18.6 and 674 nmol/g were found in normal (proximal to tumor) tissue, respectively. As for tumor tissues, maximal RES mean concentrations found were 8.3 and 94 nmol/g, respectively. In the case of metabolites, 86 nmol/g of RES-3-O-glucuronide were quantified at the 0.5 g dose and 67.2 nmol/g RES-3-O-sulfate at the 1 g dose in normal tissue proximal to tumor, and in both cases, in the right side. Regarding metabolites and tumor tissues, the highest concentration was detected for RES-sulfoglucuronide (29 nmol/g) in the right side. The main plasma RES metabolite was the sulfoglucuronide conjugate with a concentration around 40-fold higher than that of RES-3-O-sulfate [260]. No explanation was given for this finding. Perhaps, this could be explained by the overexpression of phase II enzymes (both glucuronyl- and sulfatetransferases) in colon cancer [262]. Unfortunately, the presence of DHRES was not explored in this study, which is surprising when the objective of the trial was to evaluate the disposition of RES and derived metabolites in colon tissue. DHRES has been reported to be the most abundant RES-derived metabolite in colon from rats [250] and pigs [247]. Whereas there is an increasing body of evidence regarding the potential of RES as cancer chemopreventive molecule [37], however, little is known about the biological relevance of DHRES [251].
Howells et al. [263], reported for the first time the capability of micronized RES to reach distant organs such as liver, which was demonstrated in 3 colorectal cancer patients with hepatic metastasis. RES was detected in hepatic tissue following 5 g RES daily administration for approximately 14 days. Unfortunately, no hepatic metabolic profile of RES was provided.
Randomized Clinical Trials
The perfect clinical trial does not exist. The best trial ever designed would be soon criticized by stating that 'additional and complementary trials with higher sample size and/or longer follow-up should be carried out to confirm/complete the results'. Everything cannot be evaluated in a single trial. In other words, no definitive conclusions can be obtained from any single trial but from a set of well-designed and performed trials, and this is a long lasting process. In the following pages, we have critically reviewed the human clinical trials dealing with RES or RES-containing products available in the main three databases (PubMed, Thomson Reuters Web of KnowledgeSM and Scopus). We have also included those studies where effects were evaluated but random allocation of subjects in the study groups was not specified, and trials which consisted of a single group to check post-intervention changes versus baseline without comparison with other compounds or products.
In this section we describe lights and shadows for these trials, i.e. main characteristics, objectives, outcomes, strengths, limitations and, in some cases, possible specific future studies in relation to the trial being described. We have divided this section in two: 1) trials with medicated patients (either at risk or with established disease), and 2) trials with non-medicated subjects either healthy or at risk for disease.
Subjects Under Medication (at Risk or with Established Disease)
The first published human study dealing with RES and cancer was carried out by Nguyen et al. [264] (Table 2). They performed a 4-arm pilot trial to evaluate the effects of different plant preparations on patients with colon cancer. Two patients ingested 20 mg Polygonum cuspidatum extract (containing 3.9 mg RES) plus 120 mg quercetin (flavonol), one patient ingested 80 mg P. cuspidatum extract (containing 15.5 mg RES) plus 480 mg quercetin; three patients consumed 80 g grape powder (containing 0.07 mg grape RES) and 2 patients ingested 120 g grape powder containing 0.11 mg grape RES. Patients ingested these preparations from cancer diagnosis until surgery every day for around 14 days. Normal colonic mucosa and colon cancer tissues were evaluated by Wnt pathway-specific microarrays and RT-PCR, pre- and post-ingestion of the study products. The most significant effects were obtained after ingesting 80 g of grape powder in normal colonic mucosa. The expression of both CD133 and LGR5, markers of colon cancer and colonic stem cells [265], and Wnt target genes (also important in colorectal cancer, [266]) were decreased in normal mucosa. However, no effects were found in colon cancer tissue. No effect was observed with the rest of study products, including those with higher RES dose plus quercetin. Authors suggested that associations of different phenolics as occur in grapes could explain the results obtained. The specific constituent responsible for the effects was not identified but the contribution of RES to these effects seemed to be negligible according to its low amount versus the rest of phenolics. Another limitation of the study is that other dietary compounds could exert effects since no dietary restrictions were approached in the trial. The small sample size and the possible confounding effect of medications also limited the conclusions reached.
Table 2.
Published Clinical Trials Dealing with the Effects of Resveratrol on Human Health
| Cohort and sample size (n) | Trial design, resveratrol dose and time of intervention | Objective | Main outcome | Reference |
|---|---|---|---|---|
| Subjects with established disease and/or under medication | ||||
| Patients with colon cancer (n=8). | Four parallel arms, randomized trial. Placebo-uncontrolled. Unblinded. Daily ingestion (for 19 days) of: 80 g grape powder (GP) with 0.07 mg RES, n=3; or 120 g GP with 0.11 mg RES, n=2; or 3.9 mg RES + 120 mg quercetin, n=2; or 15.5 mg RES + 480 mg quercetin, n=1. GP was dissolved in water. RES+quercetin in capsules. Registration number: NCT00256334a. | Changes in Wnt pathway in normal and cancer colon tissues after surgery. | Inhibition of some genes from Wnt pathway only in normal tissue. | [264] |
| Patients with colorectal cancer (n=20). | Two parallel arms, non-randomized, placebo uncontrolled. Blinded-analysis was performed. Daily ingestion (for 8 days) of 0.5 g, n=10; or 1 g micronized RES, n=10. RES in capsules. | Detection of RES and derived metabolites in colorectal tissue, and effect on proliferation marker Ki-67. | Ki-67 level was reduced by 5% and 7% in cancer and normal tissue, respectively. | [260] |
| Patients with colorectal cancer and hepatic metastasis (n=6). | Two parallel arms, randomized, placebo-controlled trial. Preoperative daily ingestion (from 10 to 21 days) of a sachet containing 5 g of micronized RES or placebo. Registration number: NCT00920803 | Pharmacokinetics, tissue disposition and effect on apoptosis marker (cleaved caspase-3). | Detection of RES in hepatic tissue and increased (39%) content of cleaved caspase-3 in this tissue. RES was well tolerated. Higher RES bioavailability than that reported for standard RES. | [263] |
| Type 2 male diabetics (n=19). | Two parallel arms, randomized, double-blind, placebo-controlled trial. Follow-up: 4 weeks. Daily ingestion of 10 mg RES, n=10 or placebo, n=9. RES in capsules. | Effect on insulin sensitivity and explore the possible related mechanisms. | Decrease of insulin resistance possibly due to a decrease of oxidative stress and improvement of insulin signaling via the Akt pathway. | [267] |
| Patients with metabolic syndrome (n=34). | Randomized, cross-over, unblinded, and placebo- uncontrolled. Follow-up for 6 months but effective treatment with RES was for 3 months and then discontinued, i.e. first 3 months group A, n=17, daily ingested 100 mg RES and for the second 3 months did not. The inverse pattern in group B, n=17. RES in capsules | Improvement of endothelial function in medicated patients with metabolic syndrome. | In both groups, FMD increased approximately from 4% to 9% and returned to baseline values after discontinuation of RES treatment. No effects were observed on some inflammatory and atherogenic markers. | [269] |
| Patients on statin treatment and at high risk of CVD (n=75). | Three parallel arms, randomized, triple-blind, placebo-controlled trial. Follow-up: 6 months daily ingestion of 350 mg placebo (n=25), resveratrol-containing grape extract (GE-RES, grape phenolics + 8 mg RES, n=25) or conventional grape extract lacking RES (GE). Study products in capsules. Registration number: NCT01449110. | Effects on atherogenic makers, i.e. serum lipid profile, ApoB and LDLox. | GE-RES nutraceutical decreased ApoB (-9.8%) and LDLox (-20%) in patients beyond their treatment according to standard guidelines for primary prevention of CVD. No drug interactions were detected. No adverse effects on hematological profile, hepatic, thyroid and renal functions. | [270] |
| Type 2 diabetics (n=62). | Randomized, 2 parallel arms trial. Placebo uncontrolled. Unblinded. Follow up: 3 months daily ingestion of hypoglucemic drugs + 250 mg RES (n=28) or only hypoglucemic drugs in control group (n=29). Study products in capsules. Registration number (India): CTRI/2011/05/001731. | Effects on glycemic control and associated risk markers. | RES improved systolic and diastolic blood pressures, HbA1c (-5%), total cholesterol and LDLc concentrations. | [274] |
| Overweight/obese and moderately insulin resistant older adults (n=10). | Randomized assignment to take RES capsules for 4 weeks in one of the three doses: 1, 1.5, and 2 g/day, taken in divided doses. Open-label, uncontrolled study design. Registration number: NCT01375959. | Glucose metabolism and vascular function. | Improved insulin sensitivity and postmeal plasma glucose. Results did not differ by dose. No drug interactions were observed during the study. | [276] |
| Stable CAD patients (n=40). | Randomized, 2 parallel arms, double-blind, placebo-controlled trial. Follow up: 3 months daily ingestion of 10 mg RES in one of the groups. RES in capsules. | Cardioprotective effects after myocardial infarction. | RES decreased versus baseline LDLc (8%) and improved endothelial function (50%), left ventricular diastolic function (2%), and protected from unfavorable hemorheological changes. No effect on CRP and TNFa. | [275] |
| Patients on statin treatment and at high risk of CVD (n=75). (Same cohort as in Tomé-Carneiro et al. 2012a). | Three parallel arms, randomized, triple-blind, dose-response, placebo-controlled trial. Follow-up: 12 months daily ingestion of 350 mg placebo (n=25), resveratrol-containing grape extract (GE-RES, grape phenolics + 8 mg RES, n=25) or conventional grape extract lacking RES (GE) for 6 months and the double dose for the following 6 months. Study products in capsules. Registration number: NCT01449110. | Effect on inflammatory and fibrinolytic status of patients. | GE-RES nutraceutical decreased hsCRP (-26%), TNFa (-19.8%), PAI-1 (-16.8%) and IL-6/IL-10 ratio (-24%), and increased IL-10 (19.8%). No drug interactions were detected. No adverse effects on hematological profile, hepatic, thyroid and renal functions. | [273] |
| Patients taking an oral contraceptive (n=12 + n=42). | Unmasked and unrandomized trial. Two separate experiments: 1) Follow-up: 2 months with 30 mg of RES in addition to oral contraceptive (containing 3 mg drospiredone plus 30 ug ethinylestardiol) previously taken for 6 months; 2) Follow-up: After submittion to laparoscopy and hysteroscopy for the management of endometriosis. Sixteen patients on oral contraceptives alone for at least 2 months prior to hospital admission, while 26 were using them in combination with RES. | Experiment 1: Effect on the management of endometriosis-related pain in patients who failed to obtain pain relief during the use of an oral contraceptive drospiredone + ethinylestardiol. Experiment 2: Effect on aromatase and cyclo-oxygenase-2 expression in endometrial tissue. | RES significant reduced pain scores (82% of patients reporting complete resolution of dysmenorrhea and pelvic pain after 2 months of use). Inhibition of both aromatase and cyclo-oxygenase-2 expression was significantly greater in the eutopic endometrium of patients taking RES compared with oral contraceptives alone. | [277] |
| Patients with multiple myeloma (n=24). | Two-arm, unrandomized and unmarked phase 2 clinical trial. Follow up: 5g of SRT501 following breakfast for 20 days in a 21-day cycle up to 12 cycles. Registration number: NCT00920556. | Effect of SRT501 with or without bortezomib in multiple myeloma patients who had relapsed or were refractory to at least one prior therapy. | Unacceptable safety profile and minimal efficacy in patients with relapsed/refractory MM highlighting the risks of novel drug development in such populations. | [278] |
| Patients with stable angina pectoris (n=116) | Randomized, double-blinded, active-controlled, and parallel trial with 3 groups of subjects who received the test drugs and 1 control group of subjects who were not randomized. Follow up: inclusion, 30 and 60 days of oral supplementation with calcium fructoborate (CF) (112 mg/day), RES (20mg/day), and their combination. Registration number: ISRCTN02337806. | Effect on inflammation biomarkers (hsCRP), left ventricular function markers (N-terminal prohormone of brain natriuretic peptide (BNP)), and lipid markers (total cholesterol, LDL-c, HDL-c, and triacylglycerols). | Significant hsCRP decrease in all groups at the 30-d and 60-d visits: 39.7% at 60 d for the CF group and 30.3% RES plus CF, at 60 d. The N-terminal prohormone of BNP was significantly lowered by RES (59.7% at 60 d) and by CF (52.6% at 60 d). However, their combination was the most effective and induced a decrease of 65.5%. Lipid markers showed slight changes from baseline in all groups. | [279] |
| Patients with stable CAD (n=75). | Three parallel arms, randomized, triple-blind, dose-response, placebo-controlled trial. Follow-up: 12 months daily ingestion of 350 mg placebo (n=25), resveratrol-containing grape extract (GE-RES, grape phenolics + 8 mg RES, n=25) or conventional grape extract lacking RES (GE) for 6 months and the double dose for the following 6 months. Study products in capsules. Registration number: NCT01449110. | Effect on inflammatory and fibrinolytic status of patients. | Significant increase in adiponectin levels (10%) in GE-RES group in addition to a decrease in PAI-1 levels. Non-HDL cholesterol decreased significantly in both GE and GE-RES groups. Downregulation of pro-inflammatory genes expression in PBMCs isolated from GE-RES group patients. No drug interactions were found and no adverse effects were observed on the hematological profile or on the hepatic, thyroid and renal functions. | [280] |
| Healthy subjects (no medication) | ||||
| Healthy subjects (n=20). | Two parallel arms, randomized, placebo-controlled trial. Unmasked. Follow-up: 6 weeks daily ingestion of 200 mg P. cuspidatum extract (containing 40 mg RES, n=10) or placebo (n=10). Study products in capsules. | Effects on oxidative stress and inflammatory status. | RES-containing extract decreased ROS, p47phox, INFKB, IKKb and JNK, PTP-1B and SOCS-3 in PBMCs. In addition, plasma concentrations of TNFa (-33%) and CRP (-29%) also decreased. | [284] |
| Healthy subjects (n=42). | Placebo-uncontrolled, non-randomized, unblinded. Cohort with a single arm to evaluate effects after 4 weeks upon daily ingestion of 1 g RES (capsules). | Effect of RES on CYPs and phase II enzymes. | RES inhibited the activity of CYP3A4, CYP2D6, and CYP2C9 and induced CYP1A2. | [288] |
| Healthy subjects (n=40). | Four parallel arms, non-randomized, placebo uncontrolled. Blinded for analysis. Daily ingestion of 0.5 g (n=10), 1 g (n=10), 2.5 g (n=10) and 5 g (n=10) micronized RES for 29 days. RES in capsules. | Safety, pharmacokinetics and effects on circulating IGF-1 and IGFBP-3. | IGF-1 levels were decreased on the 2.5 g dose and IGFBP-3 on the 1 and 2.5 g doses. No linear dose-response was observed between RES plasma AUC values and effects on IGF-1 and IGFBP-3. | [30] |
| Healthy subjects (n=10). | 2-arms, crossover, placebo-controlled, non-randomized, unblinded. Ingestion of a single dose of either a nutraceutical containing 100 mg RES from P. cuspidatum and 75 mg muscadine grape phenolics or placebo 10 min before a high-fat, high carbohydrate (HFHC) meal. Effects were measured for 5 hours after meal. Study products in capsules. | Effects on HFHC meal-induced oxidative and inflammatory stress. | The nutraceutical reduced meal-induced elevations of plasma LPS, and improved the expression of different oxidative and inflammatory-related genes in PBMNCs (p47phox, Nrf-2, TLR-4, CD14, IL-1b, SOCS-3, Keap-1, NQO-1 and GST-P1). The highest effects occurred from 3 to 5 hours after meal. | [290] |
| Healthy overweight/obese men or postmenopausal women with mildly elevated blood pressure (n=19). | Randomized, crossover, double-blind trial, single dose, placebo-uncontrolled. Single ingestion of 30, 90, 270 mg synthetic RES or placebo at weekly intervals. Analyses were performed 1 h after consumption of study products (capsules). | Acute, dose-dependent effect of RES on FMD. | FMD improved by 65% 1 h after consuming 30 or 90 mg RES and by 88% with 270 mg RES. | [293] |
| Obese men (n=11). | Randomized, crossover, double-blind, placebo-controlled trial. Daily ingestion of 150 mg synthetic RES for 1 month. RES in capsules. Registration number: NCT00998504. | To assess whether RES induce metabolic changes in obese men. | RES induced modest but consistent metabolic changes that mimic calorie restriction. A number of effects and mechanisms were reported such as reduction of sleeping and resting metabolic rate, activation of AMPK and increase of SIRT1 and PGC-1α in muscle, among others. | [294] |
| Healthy subjects (n=22). | Randomized, crossover, double-blind, dose-response, placebo-controlled trial. Single intake of placebo, 250 mg or 500 mg RES. Analyses were performed 45 min after the ingestion. RES in capsules. Registration number: NCT01010009. | Acute effect on brain functions by improving blood flow. | RES increased dose-dependently cerebral blood flow. Cognitive function was not affected. | [289] |
| Women at increased breast cancer risk (n=31). | Randomized, 3-arm, double-blind, placebo-controlled trial. Daily ingestion of placebo, 5 mg RES or 50 mg RES for 3 months. Study products in capsules. | Effect on DNA methylation and prostaglandin E2 | No significant effect was found on the 4 genes studies (RASSF-1a, APC, CCND2 and p16). A correlation was found between the decrease of RASSSF-1a methylation and serum RES concentration. | [298] |
| Obese subjects (n=32). | Randomized, 3-arm, single-blind, placebo controlled trial. Daily ingestion of 150 mg RES (n=10), 300 mg catechin-rich grape seed extract (CGSE, n=12) or 400 mg RES phosphate (RTP, n=10) for 28 days. Study products in capsules. | Comparison of study products on oxidative stress in obese subjects. | Low density microarrays in whole blood showed preliminary changes in some genes related to oxidative stress, mainly affected by CGSE and RTP. However, data were not validated by RT-PCR. Serum oxidative stress markers were mainly improved by RTP and CGSE. | [295] |
| Healthy subjects (n=12). | Non-randomized, placebo-uncontrolled, unblinded. Daily consumption of 3 capsules to provide a total content of 6 mg RES, 300 mg dried grape extract, 150 mg of dried extract from olive oil, 9 mg lycopene, 300 mg vitamin C and 90 mg bioflavonoids from citrus fruits. Follow-up: 5 days. | Effects on against oxidative DNA-damage and alters their redox status. | No significant effects were found on lymphocytes DNA-stability parameters, and serum CRP and LDLox values after 5 days. | [296] |
| Nonobese, postmenopausal women (n=45). | Randomized, double-blind, placebo-controlled trial. Follow up: 12 weeks of RES supplementation (75 mg/day). Registration number: NCT00823381. | Evaluate the metabolic effects in nonobese, postmenopausal women with normal glucose tolerance. | No change in body composition, resting metabolic rate, plasma lipids, or inflammatory markers. No increase in liver, skeletal muscle, or adipose tissue insulin sensitivity. No affect in RES putative molecular targets, including AMPK, SIRT1, NAMPT, and PPARGC1A, in either skeletal muscle or adipose tissue. | [299] |
| Obese healthy men (n=24). | Randomized, placebo-controlled, double-blinded, and 2-arm parallel. Follow up: either RES or placebo treatment for 4 weeks. | Metabolic effects of high-dose RES in obese human subjects. | Insulin sensitivity deteriorated insignificantly in both groups. Endogenous glucose production and the turnover and oxidation rates of glucose remained unchanged. No effect on blood pressure, resting energy expenditure, oxidation rates of lipid, ectopic or visceral fat content, or in inflammatory and metabolic biomarkers. | [300] |
| Healthy adult smokers (n=50). | Randomized, double-blind, cross-over trial. Follow up: patients were allocated to either "resveratrol-first" group (30 days of 500 mg RES/day, 30 days wash-out, 30-days placebo) or to "placebo-first" group (30 days placebo, 30 days wash-out, 30 days 500 mg RES/day). Registration number: NCT01492114. | Effects on markers of inflammation and oxidative stress in smokers. | Significant CRP and triglyceride concentrations reduction, and increased Total Antioxidant Status values. Uric acid, glucose, insulin, cholesterol, liver enzyme concentrations, and weight, waist circumference, and blood pressure values did not change significantly. | [301] |
| Volunteers with acne vulgaris (n=20). | Single-blind, vehicle-controlled, 1-arm trial. Non-randomized. Daily administration of hydrogel-containing RES and only vehicle in each face’s volunteer for 2 months. | Therapeutic effects of RES on acneic skin. | Global acne grading system (GAGS) score decreased by 53.7% on the RES-treated sides of the face. Histological analyses showed a decrease of 66.7% in the area of micromedones. No adverse effects were observed. | [302] |
| Healthy females (n=15). | 1-arm, vehicle-controlled, trial. Non-randomized. Topical application of 4 different moisturizing cream formulations on six sites on the non-exposed dorsal skin for 4 days. RES triphosphate (RTP), RTP+antioxidant blend, antioxidant blend alone and vehicle alone were applied to each participant. Histological analyses were blinded to the investigators. | Effects of RTP on UV-induced skin damage. | UV-induced erythema was mainly prevented by RTP and inhibited sunburn cell formation and melanin content. | [303] |
| Healthy subjects (n=50). | Randomized, unmasked, placebo-controlled trial. Follow up: 1 capsule of either placebo or a grape extract supplement (133 mg) containing 8 mg RES for 60 days. | Topical and systemic effects on age-related alterations to the skin, the skin antioxidant pool, and systemic oxidative stress levels. | Systemic oxidative stress, plasmatic antioxidant capacity, and skin antioxidant power increased significantly. Skin moisturization and elasticity improved, while skin roughness and depth of wrinkles diminished. Intensity of age spots decreased significantly. | [304] |
As mentioned above, Patel et al. [260] reported the detection of RES and derived metabolites in human colon tissues. The study was unrandomized, placebo uncontrolled, and consisted of two parallel arms. Blinded-analyses were performed. Twenty patients with colorectal cancer daily consumed 0.5 g (n=10) or 1 g (n=10) micronized RES for 8 days before surgery. The main aim of this study was the characterization of RES’ metabolic profile in colon tissues after surgery. However, this study has been included in this section because authors also investigated the effect of RES administration on the proliferation marker Ki-67, commonly used as a surrogate marker of cell growth. High variability was found and a borderline significant reduction of 5% versus baseline was observed when results were analyzed collectively, in all patients (n=20). On the contrary, the separate analysis of RES dosages (0.5 and 1 g) did not render significant differences. Presumably, a larger sample size in each arm would have also yielded significant differences.
In the study carried out by Howells et al. [263], six patients daily consumed either RES (5 g, n=3) or placebo (n=3) for a minimum of 10 days and a maximum of 21 days before surgery. As stated in the previous section, RES was detected in hepatic tissue, and interestingly, the apoptotic marker cleaved caspase-3 increased by 39% in malignant hepatic tissue from patients that consumed micronized RES. No effects were observed in the circulating levels of PGE2 and VEGF. In addition, no effects were found in the hepatic tissue levels of IGF-1, Ki-67, phospho-Akt, Akt1, phospho-GSK3, GSK3, phospho-ERK, ERK, phospho-JNK, JNK, β-catenin, survivin, Bcl-2, Bax or Poly (ADP-ribose) polymerase (PARP), all of them well-known cancer-related markers. Daily ingestion of 5 g for 14 days was well tolerated by the patients, however, safety concerns still persist with such high concentrations, short exposure time and usual medical treatments for this type of patients. A higher RES bioavailability was claimed using a specific micronized formulation versus conventional RES. However, taking into account the high interindividual variability in RES bioavailability, this comparison was made with previous published data and no direct comparison with standard RES was carried out in the same trial.
Together with cancer chemopreventive activity, the possible role on cardiovascular protection has been the most acknowledged health benefit of RES. In this regard, to the best of our knowledge, the first human clinical trial in patients dealing with RES and cardioprotection was conducted by Brasnyo et al. [267] in male type 2 diabetics. The design was a randomized, double-blind, placebo-controlled trial with two parallel arms. On a daily basis, patients consumed RES capsules containing either RES (10 mg, n=10) or placebo (n=9) for 1 month. Therefore, this study joined two remarkable characteristics, the first evaluation of RES cardioprotective effects in humans, and importantly, the use of a low RES dose (achievable with dietary supplements). The main objective was to evaluate the effect on insulin sensitivity and assess a possible related mechanism. Overall, RES improved insulin resistance, decreased blood glucose levels and delayed the appearance of glucose peaks after a test meal. These effects were correlated with a decrease of oxidative stress, measured as urinary ortho-tyrosine: creatinine excretion, together with an increase of Akt phosphorylation in platelets, since the activation of Akt pathway is a well-known step of insulin signalling [268]. However, a number of concerns arise from this first trial. The sample size was small (n=19) and the follow-up was short for the low RES dose assayed (1 month). Some markers such as glycated hemoglobin (HbA1c) and hsCRP, among others, were measured at baseline but no post-intervention data was provided. All patients were male and on angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker medication. However, no covariates (age, medication, etc.) were taken into consideration in the statistical analysis. In addition, possible effects on the serobiochemical and hematological profile of patients were not explored. Although patients were instructed to abstain from alcoholic beverages and possible RES containing foods, no dietary records were provided by the patients. Therefore, the influence of each individual’s diet on the results cannot be ruled out, especially when the follow-up is short.
Fujitaka et al. [269] evaluated the effect of RES on endothelial function in 34 patients with metabolic syndrome. The design was a randomized, crossover, unblinded, and placebo-uncontrolled trial. Patients consumed RES for 3 months and then discontinued consumption for the same time, i.e. group A (n=17) ingested 100 mg RES per day for the first 3 months whereas for the following 3 months it did not. The inverse pattern was followed by group B, n=17. In both groups, flow mediated dilatation (FMD), a procedure to measure endothelial function, increased approximately from 4% to 9% and returned to baseline values after discontinuation of RES treatment. No effects were observed on blood pressure nor on some inflammatory (hsCRP, IL-6) and atherogenic (serum lipid profile and others) markers. Endothelial function was assessed after overnight fasting and plasma RES metabolic profile was not characterized. Therefore, the possible direct role (if any) of circulating metabolites on FMD improvement remains unanswered. A limitation of the study is that the specific medication in each group was not specified and no covariates, including this medication, were considered in the statistical analysis. In addition, the hematological profile and the hepatic, renal and thyroid functions were not assessed by determining routine serobiochemical variables.
Tomé-Carneiro et al. [270] investigated the effects of a RES-containing grape nutraceutical on atherogenic markers in patients on statin treatment, at high risk of CVD, and treated according to current guidelines. The design was a three-arm parallel, randomized, triple-blind, placebo-controlled trial. Patients (n=75) daily ingested 1 capsule containing either 350 mg placebo (n=25), conventional grape extract lacking RES (GE, containing around 150 mg phenolics) or RES-containing grape extract (GE-RES, containing around 150 mg grape phenolics, including 8 mg RES, n=25) for 6 months. The trial was divided into 3 arms to include a placebo treatment and, importantly, to evaluate the specific contribution of RES against the rest of grape phenolic content. The main outcome of this trial was the reduction of both apolipoprotein B (ApoB) and the oxidized fraction of LDLc (LDLox) by 9.8% and 20%, respectively, in the RES-containing grape group. On the contrary, no significant effects were observed in the placebo and conventional grape extract (lacking RES) groups. It is known that residual risk can persist in patients with optimized LDLc concentrations, because the size of these LDL particles is also important. There is a linear correlation between high ApoB values and small sized LDL particles which are easily oxidized to yield LDLox and thus higher CVD risk [271]. Whereas statins are effective to control LDL levels, their effects on ApoB and LDLox are not so relevant [272]. Therefore, these results suggest that the effects of the grape complement containing RES go beyond the gold-standard medication in patients undergoing primary prevention of cardiovascular disease and are associated with a decrease on the atherogenicity of LDLc particles via reduction of ApoB and LDLox levels. Circulating RES metabolites could not be detected, probably due to the overnight fasting period prior the blood withdrawal and the low RES amount consumed (8 mg). No adverse effects were found as confirmed by the evaluation of a complete set of serobiochemical and hematological variables. However, some limitations can be found in this trial. No stratification by gender was done and more females were randomly allocated in the group consuming GE-RES. However, a number of covariates (medication, age, gender, etc.) were used in the ANCOVA statistical analysis with Bonferroni posthoc test to discard the possible influence of these factors in the results obtained. In addition, sample size (n=75) was low to draw clinical conclusions from this intervention.
The same authors further evaluated the inflammatory and fibrinolytic status in the same cohort of patients with the same study products [273]. In this case, the trial design was similar to that described above [272], but with a 1 year follow-up and a dose increase (2 capsules/day) for the final 6 months. This is the longest human clinical trial dealing with RES reported to date. Patients’ characteristics and baseline clinical values were thoroughly detailed. After adjustment for multiple covariates, the main outcome was a dose-dependent decrease of high-sensitivity C-reactive protein (hsCRP) by 26% after 12 months which was well correlated with the decrease of both TNFα (-19.8%,) and the thrombogenic plasminogen inhibitor activator type-1 (-16.8%, PAI-1). In addition, the anti-inflammatory cytokine IL-10 increased (19.8%) and the IL-6/IL-10 ratio, commonly used to express the inflammatory status, decreased (-24%). These changes were also supported by a clear trend towards the improvement of other markers such as adiponectin, IL-18 and soluble intercellular adhesion molecule type 1 (sICAM-1). The pathophysiological association of these changes was confirmed by significant correlations for each patient, i.e. hsCRP vs TNFα, hsCRP vs PAI-1, sICAM-1 vs IL-18 and adiponectin vs sICAM-1 [273]. No significant effects were observed in placebo and conventional grape extract groups. No drug interactions were found and no adverse effects were observed on the hematological profile or on the hepatic, thyroid and renal functions. Overall, this RES-containing grape nutraceutical exerted effects beyond the gold-standard medication in CVD primary-prevention patients. Once again, the presence of RES in the grape extract seemed essential to exert the effects. A possible, though unconfirmed, synergistic action between statins and the grape nutraceutical was hypothesized. As expected, more studies are needed to confirm these results. A possible and challenging next step will be to perform a multicenter trial with much higher sample size and longer follow-up to evaluate the possible clinical impact of this RES-containing grape product as coadjuvant in the therapeutic treatment of CVD.
Bhatt et al. [274] conducted a randomized, 2-arm parallel, unblinded and placebo uncontrolled trial. Type 2 diabetic subjects ingested hypoglycemic drugs plus 250 mg RES (n=28) or only hypoglucemic drugs (control group (n=29) daily for 3 months. The main outcomes upon RES consumption were the decrease of systolic blood pressure (-8.4%), glycated hemoglobin HA1c (-5%) and total cholesterol (-8.5%). At the end of the trial, significant differences were obtained between RES group and control group for fasting blood glucose, HbA1c, systolic and diastolic blood pressures, total cholesterol, triglycerides, LDLc, urea nitrogen, creatinine, and total protein levels. No covariates were used in the statistical analysis. The proportion of female/male was not homogeneous and possible gender-dependent effects cannot be ruled out. Only partial safety follow-up was assessed (i.e. creatinine, urea and total proteins).
Magyar et al. [275] recently performed a randomized, 2-arm parallel, placebo-controlled, double-blind trial in which 40 stable coronary artery disease (CAD) patients (26 men, 14 women) were followed-up for 3 months. One group (n=20) ingested 10 mg RES and the other group (n=20) placebo, both on a daily basis. RES intake improved a number of markers and features in CAD patients versus baseline such as the decrease of LDLc by 8%, a noteworthy improvement of endothelial function (approximately from 2% to 4.5%) and left ventricular diastolic function (2%), and also provided protection against some unfavorable hemorheological changes. Inflammatory markers such as CRP and TNFα increased around 50% in both placebo and RES groups after 3 months. In this case, whereas the standard error in baseline values of both inflammatory markers was rather low, this error dramatically increased in the final measurements, especially in CRP values, and thus significant statistical difference was not obtained for this important increase after 3 months. No explanation was given for these results and no details were provided about the technique used to measure these parameters. Baseline LDLc values for placebo and RES groups seemed to be very different. Despite the medication of CAD patients, hepatic, thyroid and renal functions were monitored to discard any possible side-effect. No covariates were used in the statistical analysis. Regarding the improvement of endothelial function measured as FMD, the amelioration observed with the low RES dose assayed (10 mg) is remarkable. The improvement was similar to that observed by Fujitaka et al. [269] in the same follow-up time (3 months), although a 10-fold lower RES concentration was used. A rapid conclusion when comparing these studies is that FMD was improved in two very different pathological statuses (metabolic syndrome and CAD) and that this improvement was not proportional to RES dosage. This introduces the controversy about RES dosage and effects that will be approached in a subsequent section.
One more recent pilot trial on insulin sensitivity and glucose tolerance was carried out by Crandall et al. [276]. Overweight/obese and moderately insulin resistant adults (n=7 females and 3 males, 72±3 years) ingested 1, 1.5 or 2 g RES (capsules) per day, for 1 month. Some participants were under anti-hypertensive (n=4) and statin (n=3) treatments. Although subjects were randomly chosen to take one of the three RES doses, the study was open-labeled and uncontrolled. Overall, after 4 weeks, peak postmeal and 3-hour glucose AUC decreased and the Matsuda index for insulin sensitivity improved by 18.4%. Despite the high RES doses assayed, no changes in hsCRP and serum lipid profile were observed versus baseline. There are some limitations in this trial concerning the design, follow-up and sample size. However, these results confirmed the improvement of insulin sensitivity upon RES intake and it also included the specific search for possible drug interactions and the evaluation of some enzymes related to hepatic function. Interestingly, as mentioned above, Brasnyo et al. [267] also described an improvement in insulin sensitivity in diabetics upon intake of only 10 mg RES for the same follow-up period (1 month).
Recently, Maia et al. [277] have evaluated the effect of RES on the management of endometriosis-related pain in 12 patients of reproductive age who failed to obtain pain relief during the use of an oral contraceptive containing 3 mg drospiredone plus 30 µg ethinylestradiol for 6 months. The addition of 30 mg of RES to the contraceptive regimen resulted in a significant reduction in pain scores, with 82% of patients reporting complete resolution of dysmenorrhea and pelvic pain after 2 months of use. In a separate experiment, aromatase and cyclo-oxygenase-2 (COX-2) expression were investigated in the endometrial tissue of 42 patients submitted to laparoscopy and hysteroscopy for the management of endometriosis. Sixteen of these patients had been using drospirenone + ethinylestradiol in an extended regimen for at least 2 months prior to surgery while the remaining 26 were using the same contraceptive regimen associated with 30 mg of RES. Inhibition of both aromatase and COX-2 expression was significantly greater in the eutopic endometrium of patients using combined drospirenone + RES therapy compared with the endometrium of patients using oral contraceptives alone. This was an unmasked and unrandomized trial. Authors acknowledge that better designed clinical trials are required to provide level 1 evidence that the combination of oral contraceptives with RES is superior to the use of either progestins or combined oral contraceptives alone in the management of endometriosis-related pain symptoms. In addition, both aromatase and COX-2 expression were evaluated only by immunohistochemical methods, with no further confirmatory studies using molecular biology techniques, such as polymerase chain reaction. No covariates were considered in the statistical analysis.
Not long ago, Popat et al. [278] conducted a 2-arm, unrandomized and unblinded phase 2 clinical trial on the effects of the RES formulation SRT501 with or without bortezomib in multiple myeloma patients who had relapsed or were refractory to at least one prior therapy. Twenty four patients were enrolled into this trial. Inclusion criteria included adequate renal/bone marrow function, and prior bortezomib was permitted irrespective of response. Patients received 5 g of SRT501 following breakfast for 20 days in a 21-day cycle up to 12 cycles. After two cycles, those with stable disease (SD) received two additional cycles, those with progressive disease (PD) had bortezomib added. All 24 patients commenced SRT501 monotherapy, and 9 had bortezomib added for PD. The mean duration on study was 92.8 days and patients received a mean cumulative dose of 336 g SRT501 (range 5–1505 g). Eleven patients discontinued before first response assessment and, according to study protocol, were not evaluable. The modified intention-to-treat population (mITT) comprised 13 patients. Nine patients received SRT501 and bortezomib combination, achieving an overall response rate (ORR) by mITT of 22% or, by intention-to-treat analysis, an ORR of 8%. The predominant study finding was unexpected renal toxicity, with 5 serious adverse events of renal failure leading to early study termination. The most commonly reported adverse events were: nausea (79%) and diarrea (71%). As SRT501 is extensively metabolized, renal failure seemed specific to MM patients. Renal failure was not observed for SRT501 and bortezomib despite low efficacy. However disease stabilization by bortezomib may have prevented renal failure whereas low efficacy of SRT501 with nausea and vomiting may have resulted in disease progression and dehydration, leading to renal failure. This study demonstrated an unacceptable safety profile and minimal efficacy in patients with relapsed/refractory MM highlighting the risks of novel drug development in such populations.
Militaru et al. [279] evaluated the effect on the clinical and biological statuses of subjects with stable angina pectoris of a short-term (60 days) oral supplementation with calcium fructoborate (CF), RES, and their combination. The clinical trial was randomized, double-blinded, active-controlled and paralleled with three groups of subjects who received the test drugs and one control group of subjects who were not randomized. All groups received their usual medical care and treatment. In addittion, one group received a single daily capsule (20 mg/day) of a powdered extract standardized to 50% RES (trans-RES 10 mg); another group received the same RES dose combined with CF (112 mg/day); and a third group received CF. The non-randomized control group received only their usual medical care and treatment.Of the total number of subjects included in study (n=166), 87 completed the 60 days test treatment study period and 29 followed in parallel their usual medical care and treatment. There was a significant decrease of hsCRP in all groups at the 30 and 60 days visits. This decrease was greater (39.7% at 60 days) for the CF group, followed by the RES + CF group (30.3% at 60 days). The N-terminal prohormone of brain natriuretic peptide (BNP) was significantly lowered by the RES group (59.7% at 60 days) and by CF (52.6% at 60 days). However, their combination was the most effective and induced a decrease of 65.5%. Lipid markers showed slight changes from baseline in all groups. The improvement in the quality of life was best observed for subjects who received the RES and CF mixture. No covariates were considered in the statistical analysis. RES seemed to be well tolerated as no adverse effects were reported by the volunteers, although safety evaluation focusing on serobiochemical and hematological parameters was not carried out.
Very recently, Tomé-Carneiro et al. [280] explored the effects of a RES-containing grape nutraceutical on patients with stable CAD, in a one year dietary intervention. The trial design was equivalent to a previously conducted on primary prevention patients of CVD [273]. Very briefly, it was a three-arm parallel, randomized, triple-blind, placebo-controlled trial, using the same study products (placebo, grape extract (GE) and grape extract containing RES (GE-RES)), doses and treatment duration. Authors evaluated the inflammatory and fibrinolytic status in these patients. The main outcome of this trial was a significant increase in adiponectin levels (10%) in patients included in the GE-RES group. Also, a decrease in PAI-1 levels was found in the GE-RES group, in addition to a remarkable but non-statistically significant reduction in hsCRP values (-25% vs. placebo). The atherogenic lipid load, measured as cholesterol’s non-high density lipoprotein load, decreased significantly in both GE and GE-RES groups. The placebo group presented a statistically significant reduction in IL-10 and adiponectin, together with an increase in PAI-1 levels. This study also showed a downregulation of pro-inflammatory genes expression in PBMCs isolated from GE-RES group patients. Six inflammatory transcription factors were found to be modulated, including Kruppel like factor 2, NF-κB, activator protein-1, c-Jun, activating transcription factor 2, and CREB-binding protein. No drug interactions were found and no adverse effects were observed on the hematological profile or on the hepatic, thyroid and renal functions. Covariates as age, gender and medication, among others, were used in the statistical analysis. This was the longest exploratory trial dealing with RES in patients with stable CAD; nevertheless authors acknowledge that the small sample size and follow-up limit the clinical impact of this dietary intervention. In addition, the gene expression microarray-based profile encountered provides preliminary evidence. Finally, authors conclude that these overall results warrant further research on this nutraceutical as a possible safe coadjuvant food supplement in the follow-up of these types of patients.
Non-medicated Subjects (Healthy or at Risk for Disease)
Pharmaceutical drugs are prescribed by physicians and are consumed to treat mild or serious pathological processes. Nevertheless, plant-based extracts, phytochemical compounds, including RES, or nutraceutical preparations are initially conceived to be consumed by the vast majority of individuals looking for 'prevention'. How can prevention be measured? The homeostasis of healthy subjects, by definition, is supposed to be stable. Therefore, what marker, mechanisms or processes would be the right targets in healthy people? Do healthy people need to consume this type of preparations? For example, should a decrease in the normal values of LDLc, CRP, total cholesterol or blood pressure be targeted in healthy people? The desired effect should be ‘to prevent the alteration of the normal values range’. Perhaps, the targeted 'healthy' people should present a borderline status with mild disease, i.e. non-medicated and non-established disease, such as obese (but healthy) people, non-medicated mild hypertensive subjects, etc. In addition, healthy people that consume this type of products are those reaching an age at which the risk of incidence for chronic-degenerative pathologies such as cardiovascular, cancer and neurodegenerative diseases increases [281-285]. Although this is an interesting debate, there is no universal and easy answer for all these questions.
One of the first studies with a RES-containing extract was carried out by Ghanim et al. [284] (Table 2). They conducted a randomized, unblinded, placebo-controlled, two-arm parallel, 6-weeks follow-up trial. Remarkable results on CRP and TNFα were obtained in healthy subjects after consuming an herbal extract containing 40 mg RES for 3 weeks. These results were spectacular taking into account that basal levels of both CRP and TNFα are very low in healthy subjects. Very slight changes at these concentration levels can be only measured when analysing high-sensitivity (hs)CRP and hsTNFα, which was not specified in the study. Increased prevention of these pro-inflammatory markers can be a target in subjects at risk or with established diseases, or upon specific inflammatory stresses. However, it is unclear whether decreasing these important mediators in the inflammatory homeostasis below normal values is desirable in healthy, non-medicated people. A significant decrease of both markers was detected after 3 weeks, whereas following the same dosage, the values tended to be restored after 6 weeks and no significant difference versus baseline was observed for CRP. An important limitation is that the possible influence of lifestyles, including the diet, was not taken into account in the follow-up. The male/female ratio was not specified in the study. This is relevant since the expression of some genes, such as SOCS-3, is gender-dependent [285]. No covariates were used in the statistical analysis (age, gender, etc.). In addition, the relevance of RES in the effects observed is unclear since a similar herbal extract lacking RES was not assayed (the herbal extract had a 20% RES content and no characterization of the remaining 80% was provided).
Brown et al. [30], as mentioned above, evaluated the pharmacokinetics of RES (see above, Table 1) and also the effects on circulating IGF-1 and IGFBP-3 upon consumption of 0.5, 1, 2.5 and 5 g RES for 29 days. The IGF axis signalling has been reported to play a crucial role in the development of cancer [286]. The most remarkable decrease of IGF-1 was found in volunteers consuming the 2.5 g dose whereas the effect on IGFBP-3 was mainly observed for the 1 and 2.5 g doses. Interestingly, the highest RES dose (5 g) failed to exert effects. Therefore, no clear relationship was found between the effects and plasma metabolites concentration. These high RES doses were well tolerated by the four cohorts. However, the term 'safe' cannot be unequivocally used with such short assay times and in the absence of any medication. In addition, no serobiochemical or hematological variables were determined to support this safety. Perhaps, we can conclude that higher concentration does not necessarily mean higher effect [287].
Chow et al. [288] carried out an essential human study (11 men and 31 women) about the effect of RES on phase I isoenzymes (cytochrome P450, CYPs) and phase II detoxification enzymes upon the daily consumption of 1 g RES for 1 month. The CYP phenotypic index decreased by 50%, 75% and 175% in CYP3A2, CYP2D6 and CYP2C9, respectively, whereas it was increased by 20% in CYP1A2. No significant effect was observed in both GST and UGT1A1 activities. The interpretation of these results is dual. On one hand, these results could support the cancer chemopreventive activity of RES by modulating the enzyme systems involved in carcinogen activation and detoxification. On the other hand, RES inhibited CYPs isoenzymes that are critically involved in the bioavailability, metabolism, activation and detoxification of many drugs, including those used for treating cancer. Further studies are needed to ascertain whether high RES doses for a long period can affect the CYP system, especially in the presence of therapeutic drugs. This is a challenging but necessary evaluation. Otherwise, if safety concerns are not clarified, the future of RES as potential multitarget pharmacological drug is rather obscure.
As previously mentioned in the section of preclinical effects of RES, a number of studies have reported promising results regarding RES and brain functions (Supplementary Table 9 (713.7KB, pdf) ). However, to date, the only human trial dealing with this topic was carried out by Kennedy et al. [289]. In a randomized, crossover, double-blinded, dose-response, placebo-controlled trial, 22 participants ingested a single dose of placebo, 250 mg or 500 mg RES. Initially, mean RES and derived metabolites were determined in plasma. Maximum concentration of RES metabolites was found between 90 and 120 min. After a 45-minutes resting absorption period, the volunteers performed a number of cognitive tasks that activated the frontal cortex for 36 additional minutes. Blood flow and hemodynamics in the brain were measured. Interestingly, RES increased cerebral blood flow and oxygen extraction in a dose-dependent fashion. Although no effect was found in cognitive function, this cannot be ruled out upon chronic ingestion. Therefore, future studies should assess the lowest RES concentration that affects cerebral blood flow (to minimize unsafety concerns) and to perform intermittent cognitive tasks in healthy individuals upon chronic (months) RES intake.
Ghanim et al. [290] reported a logical further step in their investigations after their first study in healthy subjects [283] in which RES was reported to affect a number of oxidative and inflammatory-related genes in the absence of any type of induced stress. In this case, the objective was to evaluate the effects of a single ingestion of a nutraceutical containing 100 mg RES from P. cuspidatum plus 75 mg phenolics from muscadine grape after a high fat, high carbohydrate (HFHC) meal (930 kcal). In accordance with their previous study, a number of oxidative and inflammatory-related genes were also improved, including p47phox, IL-1b, CD14 and TLR4 gene expressions and lipopolysaccharide (LPS) and lipopolysaccharide-binding protein (LBP) concentrations. In addition, the authors also reported that the antioxidant transcription factor Nrf-2 was induced, and the expression of some related Nrf-2 target genes, such as NQO-1 and GST-1P, was also improved. All these results became statistically significant due to the homogeneous gene expression results from isolated mononuclear and polymorph nuclear cells, which is not common due to the high interindividual variability, the gender-dependent expression of many genes (presence of 4 males and 6 females in the assay) and the small sample size under study (n=10). Interestingly, in accordance with the prevention of SOCS-3 increase in long-term pigs fed an atherogenic diet and supplemented with a RES-rich grape supplement [160], the increase of SOCS-3 was prevented in this study, which could play a role in obesity and the prevention of insulin and leptin resistance [291,292]. In addition, despite the significant result obtained on hsCRP in their previous trial [284], this important inflammatory mediator was not explored here. In fact, if the decrease of normal hsCRP values in healthy people can be questionable, the prevention of its increase after a HFHC meal is a common target to attenuate a postprandial pro-inflammatory status. In addition, the specific relevance of RES in the effects was not explored since a parallel experiment with the same nutraceutical but lacking RES was not carried out. No phenolic characterization of muscadine grape was included in the study. No covariates were used in the statistical analysis.
Another acute effect of RES was reported by Wong et al. [293] in non-medicated overweight/obese individuals with mildly elevated blood pressure. FMD increased by 65% 1 h after consumption of a 30 and 90 mg dose and by 88% with a 270 mg RES dose. Therefore, FMD did not improve proportionally to RES concentration. A significant (P<0.01) but poor (R2=0.08) correlation was found between FMD and the logarithm of RES plasma concentration. However, 'total' RES concentration was calculated by using glucuronidase/sulfatase treatments and thus the possible specific individual metabolite contribution to FMD improvement (if any) remained unanswered. In addition, FMD was measured only 1 h after ingestion of RES doses. Taking into account that RES bioavailability shows high interindividual variability and that pharmacokinetics parameters can change depending on the RES formulation, FMD should be measured at different times and correlated with the specific pharmacokinetic profile for each RES preparation assayed. In fact, RES capsules usually delay absorption and metabolism [249] and a higher effect on FMD cannot be ruled out. In the previously commented trial conducted by Fuikata et al. in patients with metabolic syndrome [269], daily consumption of 100 mg RES for 3 months improved FMD by 100%. Interestingly, patients consumed the RES capsule after dinner and endothelial function was determined in the morning after overnight fasting. Although the plasma metabolic profile of RES was not provided in that study, according to previous reports, the clearance of RES and derived metabolites is almost complete at that time [249,270]. A similar rationale can be applied to the study of Magyar et al. [275] using 10 mg RES per day in CAD patients for 3 months. Therefore, taking into account these trials [269,275,293], the specific contribution of circulating metabolites to modify endothelial function cannot be inferred. Overall, this indicates that RES metabolite-activity relationship is far from being established and FMD improvement is not proportional to RES dose.
Timmers et al. [294] described the calorie restriction-like effects of RES in humans for the first time. A single dose (150 mg RES) was ingested daily by 11 healthy obese men for 1 month and a thorough evaluation of the metabolic profile and energy metabolism of participants was performed. RES ingestion reduced sleeping and resting metabolic rate. Systolic blood pressure decreased by 5% and HOMA index was improved. TNFa significantly decreased whereas CRP, IL-1b, IL-6 and IL-8 did not reach statistical significant changes. RES supplementation improved muscle mitochondrial respiration, and increased intramyocellular lipid levels whereas intrahepatic lipid content and other serum biochemical variables decreased. Gene expression profiling using microarrays in muscle biopsies revealed the differential expression of 469 genes, including the upregulation of mitochondrial oxidative phosphorylation pathways and the downregulation of inflammatory pathways. RES ingestion also activated AMPK and increased SIRT1 and PGC-1 protein levels in protein extracts from muscle biopsies. In general, the effects were modest but consistent. Authors suggested that the unfavorable metabolic profile in obese people could be overcome by RES supplementation. Perhaps, longer treatments could amplify these effects. The results were quite promising but the exposure time was very short to discard possible adverse effects in longer lasting trials, especially if medicated subjects are included. Finally, only 11 obese men participated in this trial, thus future investigations should confirm the above results with longer follow-up periods and larger cohorts, including males and females.
De Groote et al. [295] performed a randomized, 3-arm, single-blinded, placebo controlled trial in obese subjects who ingested daily doses of 150 mg RES (n=10), 300 mg catechin-rich grape seed extract (CGSE, n=12) or 400 mg RES phosphate (RTP, n=10) for 28 days. Doses were adjusted at molar level (0.66 mmol in each case). A low density (200 genes) microarray was used to evaluate RNA transcript profiling in whole blood. Ten, fifteen and twenty eight genes were affected by RES, RTP and CGSE, respectively, after 28 days. Affected genes were classified into ‘antioxidant’, ‘inflammatory’, ‘stress response’ and ‘other’. However, this preliminary microarray data were not further validated by RT-PCR and/or at protein level. A number of serum determinations were carried out: ‘total antioxidant power’, endogenous antioxidant systems such as glutathione peroxidase (GPx), reduced (GSH) and oxidized (GSSG) glutathione and superoxide dismutase (SOD), lipid peroxides (LOOH), oxidized LDL (LDLox), and antibodies against LDLox (anti-LDLox). Statistically significant improvements were observed mainly for CGSE and RTP in GSSG, GPx and anti-LDLox.
Recently, Heger et al. [296] have evaluated the effect of a dietary supplement against oxidative DNA damage and redox status of healthy subjects. The study presented a number of important limitations such as lack of randomization; no blinding, no placebo-control, small sample size (7 females and 5 males) and short follow-up (only 5 days). Volunteers consumed 3 capsules/day. Each capsule contained 2 mg RES, 100 mg dried grape extract, 50 mg of dried extract from olive oil, 3 mg lycopene, 100 mg vitamin C and 30 mg ‘bioflavonoids’ from citrus fruits. No short-term significant effects were found on the DNA-stability parameters from peripheral blood cells. Surprisingly, the study focused especially on the lack of effects referring to RES intake (6 mg/day) when other significant antioxidant molecules or products such as vitamin C, lycopene, olive oil extract, grape extract etc. were also present in the supplement. It is known that RES antioxidant in vivo activity is discrete in comparison to other phenolics [160]. In addition, the detailed phytochemical analysis of the grape and olive extracts was not provided. No effects were found on LDLox and CRP values either. Even though these markers have been reported to be affected by a grape supplement in patients after a 6 or 12 months follow-up [270,273], the lack of effect seen here is not surprising, particularly due to the healthy state of subjects, without any specific induced stress (inflammatory, high-fat meals, etc.) and to the short follow-up time. In the case of CRP the lack of effect is even more logical since this protein levels were in the limit of quantification for the method used [297]. Although not specified, blood withdrawal was apparently carried out each day, in the morning, and therefore a complete clearance of compounds (especially RES) was expected. This means that effects on DNA-stability parameters in the first hours after supplement intake cannot be ruled out.
Zhu et al. [298] conducted a randomized, double-blinded, 3-arm, placebo-controlled trial in healthy women but at increased risk of breast cancer. Volunteers consumed either placebo, 5 mg RES or 50 mg RES for 3 months. Thirty-nine participants started the trial but only 31 completed it. Unfortunately the distribution of women that completed each arm was not provided. In addition, not all the biomarkers were analyzed at all time points for women. Methylation assessment of 4 cancer-related genes (p16, RASSF-1α, APC and CCND2) was carried out in the mammary ductoscopy samples of 22 participants, and prostaglandin E2 (PGE2) levels were measured in serum and nipple aspirates. No significant changes were observed on the above genes using a quantitative methylation specific (qMS)-PCR technique. No effects were observed on PGE2 either. Total serum RES concentration increased with RES dose and the logarithm of serum RES concentration poorly but statistically significant correlated with the decrease of methylation of the tumor suppressor gene RASSF-1a (P=0.047; r2=-0.14). Unfortunately, RES metabolic profiles of serum, aspirate fluids and ductoscopy samples were not evaluated. Unfortunately, the possible effects were focused only on 4 genes whereas microarray and/or antibodyarray assays would probably have yielded interesting results. Despite the limitations of the study, this trial is the first one to evaluate the effects of RES on women at high risk of breast cancer.
Yoshino et al [299] conducted a randomized, double-blind, placebo-controlled trial to evaluate the metabolic effects of RES supplementation in nonobese, postmenopausal women with normal glucose tolerance. A total of 45 lean and overweight, Caucasian, postmenopausal women were randomly assigned to one of three groups: 1) placebo treatment for 12 weeks (n=15), 2) RES supplementation (75 mg/day) for 12 weeks (n=15), or 3) calorie restriction targeted to achieve a 5% weight loss within 12 weeks (n=15). Although RES supplementation increased plasma RES concentration, it did not change body composition, resting metabolic rate, plasma lipids, or inflammatory markers. A two-stage hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically labeled tracer infusions, demonstrated that RES did not increase liver, skeletal muscle, or adipose tissue insulin sensitivity. Consistent with the absence of in vivo metabolic effects, RES did not affect its putative molecular targets, including AMPK, SIRT1, NAMPT, and PPARGC1A, in either skeletal muscle or adipose tissue. Pretreatment values were used as covariates. Addition of the CR weight loss group to the analysis of outcome measures did not change the comparisons between the placebo and RES groups. The authors conclude that these findings demonstrate that RES supplementation does not have beneficial metabolic effects in nonobese, postmenopausal women with normal glucose tolerance. Nevertheless, the authors point out the important difference between this study and others where several metabolic benefits were reported, in that the subjects in these studies had more severe pre-existing metabolic dysfunction, such as obesity, type 2 diabetes, and impaired glucose tolerance. Further adding that studies conducted in rodent models of diet induced obesity have shown that RES improves insulin sensitivity, lipids, and mitochondrial function but did not show beneficial metabolic effects in normal rodents. In this context, authors raise the possibility that RES only improves metabolic outcomes in obese and metabolically abnormal people, and not in nonobese glucose tolerant women. After reading this study one can be drawn to the idea that RES is useless for healhty people. However, it should be highlighted that the real objective is to prevent the impairment of disease-risk markers or to decrease their abnormal high values. In this regard, the above results are rather logical and not surprising at all. It seems clear that the challenge in which studies should be focused on is evaluating 'preventive effects' in healthy, homeostatic, subjects.
Another study focused on the metabolic effects of high-dose RES (1.5 g RES/day) in obese human subjects was carried out by Poulsen et al [300]. In a randomized, placebo-controlled, double-blinded, and parallel-group design, 24 obese but otherwise healthy men were randomly assigned to 4 weeks of RES or placebo treatment. Extensive metabolic examinations including assessment of glucose turnover and insulin sensitivity (hyperinsulinemic euglycemic clamp) were performed before and after treatment. The primary outcome measure was insulin sensitivity which deteriorated insignificantly in both groups. Endogenous glucose production and the turnover and oxidation rates of glucose remained unchanged. RES supplementation also had no effect on blood pressure, resting energy expenditure, oxidation rates of lipid, ectopic or visceral fat content, or in inflammatory and metabolic biomarkers. The lack of effects caused authors to question RES as a human nutritional supplement in metabolic disorders. Once again, one-month period is a too short period to draw conclusions regarding the effect of RES, especially on healthy people. The challenge is to evaluate the evaluation of risk markers for a long period and to ascertain whether RES supplementation can prevent the impairment of these markers.
Bo et al. [301] evaluated whether RES had beneficial effects on markers of inflammation and oxidative stress in healthy smokers. A randomized, double-blind, cross-over trial was performed in 50 healthy adult smokers: 25 were randomly allocated to 'resveratrol-first' (30 days of 500 mg RES/day, 30 days wash-out, 30-days placebo) and 25 to 'placebo-first' (30 days placebo, 30 days wash-out, 30 days 500 mg RES/day). RES significantly reduced CRP and triglyceride concentrations, and increased Total Antioxidant Status (TAS) values. After analyzing data with general linear models to assess period and carry-over effects, the ratios of the values after RES to those after placebo were respectively: 0.47 (95% CI 0.38-0.59) -CRP- and 0.71 (95% CI 0.65-0.78) triglycerides-, while TAS increased by 74.2 µmol/L (95% CI 60.8-87.6). Uric acid, glucose, insulin, cholesterol, liver enzyme concentrations, and weight, waist circumference, and blood pressure values did not significantly change after RES supplementation.
Two recent studies have also evaluated the effects of RES preparations on the skin upon topical application. Fabbrocini et al. [302] reported the anti-acneic properties of RES in 20 volunteers with acne vulgaris in a one armed vehicle-controlled trial. A hydrogel-containing RES and vehicle alone were applied daily to the right and left sides of volunteers’ face, respectively, for 2 months. Non-adverse experiences were recorded. Clinical evaluation revealed a mean decrease of 53.7% in the Global Acne Grading System (GAGS) score with the RES-containing formulation versus a 6.1% reduction with the vehicle alone. These results were supported by histological analyses that showed a 66.7% reduction in the area of microcomedones on the hydrogel-containing RES-treated sides versus a 9.7% reduction in the vehicle-treated side. Overall, authors suggest that these results deserve further studies in a larger cohort. Although these results are promising, the therapeutic effectiveness versus other conventional, well-known anti-acneic treatments was not included. This is essential to claim a possible commercial viability of RES formulations to prevent or treat acne vulgaris.
Recently, Wu et al. [303] reported the protective effect of RES triphosphate against UV-induced erythema. Different moisturizing cream preparations were assayed in 15 healthy females. RES triphosphate (1%) plus an antioxidant blend (0.1% ascorbyl phosphate, 0.5% tocopherol acetate, 0.01% Echinacea pallida extract, 0.12% chamomile extract and 0.18% caffeine), RES triphosphate alone, the antioxidant blend alone or only the moisturizing vehicle were applied in different dorsal skin sites that were UV-exposed for 4 consecutive days. Study products were applied immediately after each UV treatment and the effects were compared against positive (only UV treatment) and negative (no UV, no creams) controls. Non-invasive skin color were measured using CIE L*a*b* (CIELAB), which is the most complete color space specified by the International Commission on Illumination. The three coordinates of CIELAB represent the lightness of the color (L*), its position between red/magenta and green (a*) and its position between yellow and blue (b*). In addition, histological analyses (sunburn cells and melanin content) from biopsies were studied for each treatment. Overall, depending on the specific parameter investigated, RES triphosphate alone or in combination with the antioxidant blend showed the best results, i.e., decrease of erythema, number of sunburn cells and melanin content (percentage and optical densities). However, no statistical analyses among groups were carried out and no apparent differences were observed in the light of the results.
Finally, Buonocore et al. [304] wanted to evaluate the topical and systemic effects of a grape supplement containing 8 mg resveratrol and procyanidins on age-related alterations to the skin, the skin antioxidant pool, and systemic oxidative stress levels. An instrumental study was performed in 50 subjects (placebo, n=25, and treatment, n=25) with clinical signs of skin aging (wrinkles, dull complexion, brown spots) to identify clinical features induced by chronoaging or photoaging. Product efficacy was evaluated after 60 day of treatment in terms of in vivo and in situ skin hydration, elasticity, and skin roughness levels, systemic oxidative stress levels by plasmatic derivatives of reactive oxygen metabolites and oxyadsorbent tests, and extent of the skin antioxidant pool. After 60 days of treatment, values for systemic oxidative stress, plasmatic antioxidant capacity, and skin antioxidant power increased significantly. Additionally, skin moisturization and elasticity improved, while skin roughness and depth of wrinkles diminished. Intensity of age spots significantly decreased, as evidenced by improvement in the individual typological angle. No covariates were considered in the statistical analysis.
POTENTIAL CLINICAL EFFECTS VS HUMAN EVIDENCE
There are a number of questions that can be formulated regarding human clinical trials dealing with plant-derived compounds. First of all, what do we really expect from a plant-based molecule such as RES? A future multitarget pharmaceutical drug or a dietary molecule present in foodstuffs or nutraceutical preparations to prevent diseases or to enhance the effectiveness of medical treatments? Both?
As stated in a previous section, the traditional link between red wine consumption, the ‘French Paradox’, RES and the beneficial effects of red wine consumption, has been recurrently used in the past years [305]. After the cancer chemopreventive activity of RES was described in rats [15], Howitz et al. [232] described the life span extension of yeasts by RES and the involvement of SIRT1 activation in the effects. This was further confirmed in worms [233], flies [306] and short-lived fish [234]. The interest in RES peaked with these discoveries. However, the research on RES and its role on life extension became a scientific-commercial battle because despite the abundant studies published in high-impact journals, the exact role of SIRT1 in calorie restriction and life span extension is still under debate [307,308]. Overall, it is difficult to assume that aging is controlled by a so-called on/off button named SIRT1. Therefore, independently of the exact ‘magical’ mechanism involved, there is a well-argued body of evidence related to the beneficial effects of RES against chronic diseases in animal models [16]. However, why are there so many in vitro and animal model assays and so little human evidence? It seems that the weighting scale is not quite balanced. Perhaps, the pharmaceutical industry is not interested in investing funds on human research since RES is ready-available for everyone and general findings are promptly used by competitors who do not perform any type of research. In addition, the activity of RES against so many processes (cancer, inflammation, oxidative stress, cardiovascular and neurodegenerative diseases, aging, etc.) has been very attractive for the scientific community and the irrelevant use of high in vitro or in vivo doses to describe many different mechanism and effects has been recurrently published. This has contributed to a kind of endless loop in RES research thus far. Many in vitro or animal studies, (some human studies also), usually justify the results obtained (regardless of the assayed RES dose) with the presence of RES in the human diet (mainly in red wine), yet RES as a part of human diet is negligible [27]. The most abundant source of RES in the diet is red wine but quantities are low and quite unpredictable [10,26]. Therefore, i) the consumption of wine does not ensure the intake of enough RES to exert beneficial effects, ii) the conjecture that directly correlates the cardiovascular protection of red wine and its RES content is not fully true and iii) the specific contribution of the minor RES content compared to the rest of major polyphenolic compounds in red wine is not fully known.
To date, in a rough classification depending on the time of intervention, acute (hours-days, short-term (weeks-few months, and long-term (1 year) human clinical trials have been reported (Table 2). Perhaps, the first debate regarding this classification would be to determine what are acute, short- and long-term interventions. The terms acute (single dose for a few days), subchronic (repetitive doses for weeks), and chronic (repetitive doses for months) are well established to evaluate toxicological effects. However, it is difficult to establish an unequivocal time range to evaluate either ‘preventive’ or ‘curative’ effects of a dietary compound in which possible adverse effects should be also evaluated. In general, the biological activity of phytochemicals, including RES, is low in comparison with the vast majority of pharmaceutical drugs intended to cure. In addition, in the context of a dietary molecule, the term low or high concentration is not defined anywhere. In general, a RES dose that can be ingested through the diet is considered to be a low dose. A limiting figure for a low RES dose could be 15 or 20 mg RES (hypothetically equivalent to 1 litre of red wine with the highest RES concentration ever described, which is not representative but possible). Since the content of RES in the diet is low and unpredictable, the consumption of a RES-containing nutraceutical with a low standardized RES dose seems reasonable. Higher doses could not be justified through the diet. If the above is assumed, the majority of human trials have involved the use of high RES concentrations (hundred milligrams to grams) and only in a few cases, concentrations closer to a dietary context (Table 2).
In general, from all the mechanisms described for RES in ‘preclinical’ models (Fig. 2), only a few of them have somehow been confirmed in humans, including signalling cascades via Akt pathway [267] and regulation of some target genes or proteins in peripheral blood cells [284,290] or muscle [294]. The highest degree of evidence in humans (although still scarce) deals with the cardioprotective activity of RES through effects related to the improvement of inflammatory markers, atherogenic profile, glucose metabolism and endothelial function (Table 2).
The ‘Resveratrol Paradox’
Goldberg et al. [28], in 2003, already pointed out that the huge output of studies reporting in vitro effects of free RES could be irrelevant as it was absorbed as conjugates. As recently reviewed by Planas et al. [309], the bioavailability and distribution of RES are constrained by ABC transporters [250,310,311]. Enterocytes rapidly metabolize RES to yield glucuronide and sulfate derivatives, which are excreted through the ABC membrane proteins. In this regard, Azorín-Ortuño et al [247] coined the expression ‘Resveratrol Paradox’ to illustrate the high activity exerted by RES despite its low bioavailability. This dramatically hampers i) the identification of the possible real actor responsible for the effects exerted by RES and ii) the right dose for achieving such effects [287]. A logical rationale for relating plasmatic (or tissue) concentrations with specific systemic activity is to assume that parent RES and/or derived metabolites must interact directly with a specific systemic target [287]. However, the direct association between RES and/or specific circulating RES metabolites and the biological activity of RES is far from being demonstrated in vivo. For example, FMD did not improve proportionally with RES concentration in humans [269,275,293]. Patel et al. [260] could not find significant effects on the cancer marker Ki-67 in colon cancer patients when RES dosages (0.5 and 1 g) were analyzed separately. Brown et al. [30] described the highest effects on circulating IGF-1 and IGFBP-3 on the 1 and 2.5 g RES doses, whereas the highest concentration (5 g) failed to exert the effects in the patients. Kennedy et al. [289] reported the dose-dependent increase of cerebral blood in volunteers although cognitive function did not change. Obviously, this could not be correlated with the metabolic profile of RES in the brain but the presence of RES and derived metabolites in the brain is very low [247]. Tomé-Carneiro et al. [273] reported the improvement of inflammatory and fibrinolytic markers in patients undergoing primary prevention of cardiovascular disease upon consumption of a RES-containing grape nutraceutical. After 6 months of intervention, the dose was doubled for 6 additional months. No clear dose-response was observed for all the markers analyzed. No circulating RES metabolites were detected after overnight fasting, suggesting that the effects could be exerted by a low but repetitive-chronic exposure to these metabolites (not necessarily found in a specific time-point). In addition, other mediators not fully identified yet could be the final responsible for the effects observed. In another recent study, the chronic consumption of the same RES-containing nutraceutical prevented early events of atherosclerosis in pigs fed an atherogenic diet [160]. No circulating RES metabolites were detected either. Interestingly, a number of metabolites were found in pig aortic tissue. However, no clear metabolite-activity relationship could be established [160]. In the same line, previous reports supported anti-inflammatory systemic effects after oral administration of low RES doses (human equivalent dose to 10 mg for a 70 kg person) in animal assays in which circulating metabolites were detected at very low concentration or even not detected at all [221,312].
The specific biological activity of circulating metabolites has been scarcely approached due to the lack of suitable standards [313,314]. In this regard, Lasa et al. [115] have recently taken a further step suggesting the anti-obesity effect of some RES metabolites after their in vitro exposure at 25 µM to mouse pre-adipocytes for 24 hours. Unfortunately, the disposition of RES in human adipose tissue is not known, and these rather high metabolite concentrations have not been detected yet in animal adipose tissues after high RES supplementation [247,315]. Azorín-Ortuño et al. [251] described that 100 nM RES or DH-RES, but not their corresponding glucuronide metabolites, decreased fatty acid binding protein type-4 (FABP4) levels in macrophages exposed to oxidized LDL particles. These concentrations are achievable in plasma after RES intake. In addition, an interesting finding was the hormetic-like response detected because the effects were lower when RES concentration was increased [316]. Although this possibility cannot be ruled out, more in vivo evidences are needed to support the hormetic theory for RES.
The evaluation of effects upon the supplementation of low doses of RES is challenging because it requires long-term trials to exert quantifiable effects. It seems that effects on endothelial function [275] and glucose metabolism [267] require neither high concentrations (10 mg/day) nor long-term follow-ups (from 1 to 3 months). On the contrary, effects on inflammatory and other markers as well as the evaluation of safety parameters and drug interactions could require longer interventions [270,273]. To conduct such long-term trials is time-consuming (sequential recruitment of volunteers and follow-up can take several years) and could be risky (instead of spectacular changes, rather moderate or even no statistically significant effects are expected). Scientists need to publish their results and the seductive shortcut of using high RES doses and short follow-ups (to maximize effects in a short time) has been the main strategy followed so far. Surprisingly, many high-impact factor journals are recurrently publishing studies with detailed pathways, magical targets that explain many effects, etc…but using in vitro or in vivo assays with unrealistic, non-physiological RES concentrations (type and amount of metabolites) or with questionable extrapolation to humans due to safety concerns. Consequently, and unfortunately, many of these spectacular effects and pathways have not been confirmed in humans so far.
To Consume or not to Consume Resveratrol? That is the Question
The specific RES dose required to maximize effects without safety concerns is not known yet. Therefore, this opens the question of whether chronic ingestion of low RES doses can exert more benefits than short-time exposures to high RES doses. In this context, the current commercial competition to sell (and consume) nutraceuticals with high RES content is not justified. Consumers establish a direct parallelism between the terms ‘natural’ and ‘safe’; however, they should bear in mind that many well-known powerful poisons are ‘very natural’. There is a profound contradiction between the high RES concentrations commonly assayed in vitro and in animal models, and the safety concern derived from the possible use of these high concentrations in long-term human interventions. For example, in addition to many other drugs, the bioavailability of statins is affected by CYPs isoenzymes, including CYP3A4, in addition to uptake and efflux transporters that affect drug disposition [317]. Statins are lipid-lowering drugs of primary choice in the prevention of cardiovascular diseases and millions of people at risk, but healthy, consume statins all over the world. High RES doses can inhibit CYPs and interact with transporters [288,309], which could modify the metabolism of statins provoking serious adverse effects. Therefore, medicated people (with established disease but also those at risk or with mild disease) should be warned that consuming high doses of RES for long periods could be harmful. Indeed, this rationale is also valid for the vast majority of plant-derived preparations commonly consumed without any type of control.
CONCLUSION AND OUTLOOK
The relevance of the huge output of in vitro and animal studies looking at the health benefits of RES is unclear. Scientific literature is filled with plenty of studies where unrealistic assay conditions have lead to many effects and mechanisms that are far from being confirmed in humans. Responsibility lies with scientists but also with journals that rather publish spectacular results, no matter the doubtful extrapolation to humans, instead of moderate, borderline effects obtained using a more realistic physiological context. To date and according to the clinical trials conducted so far, it is becoming evident that RES exerts cardioprotective benefits through the improvement of inflammatory markers, atherogenic profile, glucose metabolism and endothelial function (Table 2). These effects have been observed using both high and low doses of RES and both in healthy volunteers and medicated patients. However, the specific mechanisms by which this may occur are not yet clear. More trials are needed to confirm these and other possible effects and mechanisms. The promising neurodegenerative and cancer chemopreventive effects of RES in animal models have not been yet confirmed in humans. There are a number of ongoing clinical trials dealing with RES (www.clinicaltrials.gov) that will increase the knowledge about the effects of RES on human health in the coming months/years (Supplementary Table 11 (713.7KB, pdf) ).
Despite the effects observed, the poor bioavailability of RES has been a classical drawback for this molecule and even a recurrent criticism used by some physicians or pharmacologists, i.e. 'RES cannot exert benefits because it is rapidly metabolized and its presence in the bloodstream is negligible to justify any effect'. However, the effects exist. More research should focus on identifying the actual metabolite(s) or signals or mediators responsible for these effects. In addition, and to overcome the poor bioavailabilty of RES, intense research has been performed to enhance its bioavailability [318,319]. Most strategies based on encapsulations and modifications have yielded, however, discrete results in vivo. Other more specific strategies previously approached in animals such as the inhibition of CYPs by using known inhibitors like piperine [320] are also under evaluation in humans (Supplementary Table 11 (713.7KB, pdf) ). However, a call of caution should be made when using these approaches due to the significance of CYPs in the metabolism of drugs and dietary carcinogens.
After RES intake, the actual metabolite responsible (if any) for the effects exerted is still unknown. The specific activity associated to known metabolites has been scarcely investigated. However, taken into account the effects observed and the apparent lack of relationship with metabolites concentration, other hypotheses cannot be ruled out and perhaps, we should be looking into other directions, i.e. sensory neuron stimulation in the gastrointestinal tract and triggering of signalling cascades through other intermediates [321].
There are many mechanisms and pathways supposedly regulated by RES but the evidence in humans is very poor. In this regard, marketing and media coverage have moved on much faster than research [322]. All the effects attributed to RES on account of the in vitro and animal results, with disregard for the context and assay conditions, are literally used by sellers to publicise and market their RES-containing products. Food and pharmaceutical companies should be more cautious and look for specific RES formulations with proven significant benefits and ensure the absence of any type of adverse effects or drug interactions. In this regard and independently of the RES concentration used, more long-term trials are needed with special attention to medicated people (not necessarily symptomatic subjects) such as the millions of people under statins treatment. Safety is also an important issue for RES. Like many other plant-based nutraceuticals rich in phenolics and other phytochemicals, RES and RES-containing products may also cause adverse effects and interact with drugs. ‘Natural’ does not equal ‘safe’. It is a matter of dose and time of administration. Efficacy and safety must be demonstrated for each specific product.
The objective of using high doses of RES with a pharmacological use is still unclear. Safety concerns, easy and cheap commercial availability of the molecule, and lack of added value for pharmaceutical companies make the future of RES, as a possible pharmaceutical multitarget drug, rather obscure. On the other hand, the RES molecule could be the scaffold for the development of other synthetic compounds with specific added-value for pharmaceutical companies.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
This work has been supported by the Projects CICYT BFU2007-60576 and ALG2011-22447 (Ministry of Economy and Competitiveness, MINECO, Spain), Fundación Séneca (grupo de excelencia GERM 06 04486, Murcia, Spain) and Consolider Ingenio 2010, CSD2007-00063 (Fun-C-Food, Spain). J.T.-C.is holder of a FPI-predoctoral grant (MINECO, Spain).
SUPPLEMENTARY TABLES (AVAILABLE ONLINE)
Supplementary Table 1 (713.7KB, pdf) . Effects of resveratrol and related mechanisms on human cancer cells.
Supplementary Table 2 (713.7KB, pdf) . Neuroprotective effects of resveratrol in in vitro models.
Supplementary Table 3 (713.7KB, pdf) . Effects of resveratrol on human cells involved in the vascular milieu.
Supplementary Table 4 (713.7KB, pdf) . Anti-aging effects of resveratrol in in vitro models.
Supplementary Table 5 (713.7KB, pdf) . Cancer chemopreventive effects of resveratrol and related mechanisms in animal models.
Supplementary Table 6 (713.7KB, pdf) . Effects of exposure to resveratrol on animal models of cardiovascular disease.
Supplementary Table 7 (713.7KB, pdf) . Resveratrol-exposure effects on insulin, glucose and lipid levels of animal models of obesity, diabetes and metabolic dysfunction.
Supplementary Table 8 (713.7KB, pdf) . Anti-inflammatory targets and related mechanisms dealing with resveratrol in animal models.
Supplementary Table 9 (713.7KB, pdf) . Neuroprotective effects of resveratrol in animal models.
Supplementary Table 10 (713.7KB, pdf) . Anti-aging effects of resveratrol in animal models.
Supplementary Table 11 (713.7KB, pdf) . Human trials dealing with resveratrol registered at clinicaltrials.gov.
ABBREVIATIONS
- Aβ
= Amyloid-beta peptide
- Akt
= Serine/threonine kinase (protein kinase B)
- AMPK
= AMP activated protein kinase
- ApoB
= Apolipoprotein B
- AUC
= Area under the curve
- Bax
= Bcl-2-associated X protein
- Bcl-2
= B-cell lymphoma 2
- CAD
= Coronary artery disease
- COX
= Cyclooxigenase
- CVD
= cardiovascular disease
- CYP
= Cytochrome P450
- eNOS
= Endothelial nitric oxide synthase
- ERK
= Extracellular signal regulated kinase
- FMD
= Flow-mediated dilatation
- FOXO
= Forkhead transcription factor
- GE-RES
= RES-rich grape extract
- GPx
= Glutathione peroxidase
- GST-1P
= Glutathione S-transferase-1P
- HA1c
= Hemoglobin A1c
- HDL
= High density lipoprotein
- HED
= Human equivalent dose
- HFHC
= High fat, high carbohydrate
- HO-1
= Heme oxygenase-1
- hsCRP
= High-sensitivity C-reactive protein
- ICAM-1
= Intercellular adhesion molecule
- IGF-I
= Insulin-like growth factor-I
- IGFBP
= IGF binding proteins
- IKKb
= Inhibitor of êB-kinase-b
- IL
= Interleukin
- INFKB
= Intranuclear nuclear factor-êB-binding
- iNOS
= Inducible nitric oxide synthase
- JNK
= Jun-N-terminal kinase
- LDLc
= Low-density lipoprotein cholesterol
- LDLox
= Oxidized LDL
- MAPK
= Mitogen-activated protein kinase
- MCP
= Monocyte chemotactic protein
- MMP
= Metalloproteinase
- NF-κB
= Nuclear factor kappa B
- NQO-1
= NADPH:quinone oxidoreductase-1
- Nrf-2
= NF-E2-related factor-2
- NO
= nitric oxide
- PAI-1
= Plasminogen inhibitor activator type-1
- PBMCs
= Peripheral blood mononuclear cells
- PGC-1α
= Peroxisome proliferator-activated receptor gamma coactivator-1 alpha
- PGE2
= Prostaglandin E2
- PI3K
= Phosphoinositide 3-kinase
- PTP-1B
= Phosphotyrosine phosphatase-1B
- RES
= trans-resveratrol
- ROS
= Reactive oxygen species
- SIRT1
= Sirtuin type 1 (silent information regulator type 1)
- SOCS-3
= Suppressor of cytokine signalling type 3
- SOD
= Superoxide dismutase
- TLR-4
= Toll-like receptor-4
- TNF
= Tumor necrosis factor
- VCAM-1
= Vascular cell adhesion protein 1
- VEGF
= Vascular endothelial growth factor
REFERENCES
- 1.Tomás-Barberán FA, Espín JC. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric. 2001;81:853–76. [Google Scholar]
- 2.Waterhouse AL. Wine phenolics. Ann N Y Acad Sci. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 3.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 4.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans.II Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 5.Hooper L, Kay C, Abdelhamid A, Kroon PA, et al. Effects of chocolate cocoa and flavan-3-ols on cardiovascular health a systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–51. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 6.Andrade JP, Assunção M. Protective effects of chronic green tea consumption on age-related neurodegeneration. Curr Pharm Des. 2012;18:4–14. doi: 10.2174/138161212798918986. [DOI] [PubMed] [Google Scholar]
- 7.Visioli F, Bernardini E. Extra virgin olive oil's polyphenols biological activities. Curr Pharm Des. 2011;17:786–804. doi: 10.2174/138161211795428885. [DOI] [PubMed] [Google Scholar]
- 8.Urpí-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol Res. 2012;65:577–83. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Förstermann U. Red wine and cardiovascular health. Circ Res. 2012;111:959–61. doi: 10.1161/CIRCRESAHA.112.278705. [DOI] [PubMed] [Google Scholar]
- 10.Stervbo U, Vang O, Bonnesen C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007;101:449–57. [Google Scholar]
- 11.Takaoka MJ. Of the phenolic substances of white hellebore (Veratrum grandiflorum Loes.fil.). J Faculty Sci Hokkaido Imperial University. 1940;3:1–16. [Google Scholar]
- 12.Nonomura S, Kanagawa H, Makimoto A. [Chemical Constituents of Polygonaceous Plants. I. Studies on the Components of Ko-J O-Kon. (Polygonum Cuspidatum Sieb. et Zucc.)]. Yakugaku Zasshi. 1963;83:988–90. [PubMed] [Google Scholar]
- 13.Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49–52. [Google Scholar]
- 14.Bertelli AA, Giovannini L, Giannessi D, et al. Migliori Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- 15.Jang M, Cai L, Udeani GO, Slowing KV, et al. Cancer chemopreventive activity of resveratrol a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 16.Baur JA, Sinclair DA. Therapeutic potential of resveratrol the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 17.Vang O, Ahmad N, Baile CA, Baur JA, et al. What is new for an old molecule.Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6:e19881–0. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li F, Gong Q, Dong H, Shi J. Resveratrol a neuroprotective supplement for Alzheimer's disease. Curr Pharm Des. 2012;18:27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 19.Renaud S, deLorgeril M. Wine alcohol platelets and the French paradox for coronary heart disease. Lancet. 1992;339:1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 20.Micallef M, Lexis L, Lewandowski P. Red wine consumption increases antioxidant status and decreases oxidative stress in the circulation of both young and old humans. Nutr J. 2007;6:27–0. doi: 10.1186/1475-2891-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarda E, Godoy I, Foncea R, Pérez DD, et al. Red wine reduces oxidative stress in patients with acute coronary syndrome. Int J Cardiol. 2005;104:35–8. doi: 10.1016/j.ijcard.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Bertelli A, Bertelli AA, Gozzini A, Giovannini L. Plasma and tissue resveratrol concentrations and pharmacological activity. Drugs Exp Clin Res. 1998;24:133–8. [PubMed] [Google Scholar]
- 23.Langcake P, Pryce RJ. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976;9:77–86. [Google Scholar]
- 24.Cantos E, Espín JC, Fernández MJ, Oliva J, et al. Postharvest UV-C-irradiated grapes as a potential source for producing stilbene-enriched red wines. J Agric Food Chem. 2003;51:1208–14. doi: 10.1021/jf020939z. [DOI] [PubMed] [Google Scholar]
- 25.Cantos E, Espín JC, Tomás-Barberán FA. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J Agric Food Chem. 2002;50:6322–9. doi: 10.1021/jf020562x. [DOI] [PubMed] [Google Scholar]
- 26.Cantos E, Espín JC, Tomás-Barberán FA. Postharvest induction modeling method using UV irradiation pulses for obtaining resveratrol-enriched table grapes a new "functional" fruit. J Agric Food Chem. 2001;49:5052–8. doi: 10.1021/jf010366a. [DOI] [PubMed] [Google Scholar]
- 27.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventós RM, Berenguer T, et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population.European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br J Nutr. 2008;100:188–96. doi: 10.1017/S0007114507882997. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 29.Walle T, Hsieh F, DeLegge MH, Oatis JEJr, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 30.Brown VA, Patel KR, Viskaduraki M, Crowell JA, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers safety pharmacokinetics and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–11. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Athar M, Back JH, Kopelovich L, Bickers DR, et al. Multiple molecular targets of resveratrol.Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction. To die or not to die. Mol Nutr Food Res. 2009;53:115–28. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- 33.Yu W, Fu YC, Wang W. Cellular and Molecular Effects of resveratrol in Health and Disease. J Cell Biochem. 2012;113:752–9. doi: 10.1002/jcb.23431. [DOI] [PubMed] [Google Scholar]
- 34.Aluyen JK, Ton QN, Tran T, Yang AE, et al. Resveratrol potential as anticancer agent. J Diet Suppl. 2012;9:45–56. doi: 10.3109/19390211.2011.650842. [DOI] [PubMed] [Google Scholar]
- 35.LeCorre L, Chalabi N, Delort L, Bignon YJ, et al. Resveratrol and breast cancer chemoprevention molecular mechanisms. Mol Nutr Food Res. 2005;49:462–71. doi: 10.1002/mnfr.200400094. [DOI] [PubMed] [Google Scholar]
- 36.Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36:43–53. doi: 10.1016/j.ctrv.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Juan ME, Alfaras I, Planas JM. Colorectal cancer chemoprevention by trans-resveratrol. Pharmacol Res. 2012;65:584–91. doi: 10.1016/j.phrs.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Wolter F, Akoglu B, Clausnitzer A, Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr. 2001;131:2197–203. doi: 10.1093/jn/131.8.2197. [DOI] [PubMed] [Google Scholar]
- 39.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, et al. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl. 2007;28:282–93. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 40.Gatouillat G, Balasse E, Joseph-Pietras D, Morjani H, et al. Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma. J Cell Biochem. 2010;110:893–902. doi: 10.1002/jcb.22601. [DOI] [PubMed] [Google Scholar]
- 41.Bai Y, Mao QQ, Qin J, Zheng XY, et al. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101:488–93. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanamala J, Radhakrishnan S, Reddivari L, Bhat VB, et al. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-FAK signaling pathways-A proteomic approach. Proteome Sci. 2011;9:49–0. doi: 10.1186/1477-5956-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Dupouy M, et al. Trans-resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23:1327–33. doi: 10.1093/carcin/23.8.1327. [DOI] [PubMed] [Google Scholar]
- 44.Filippi-Chiela EC, Villodre ES, Zamin LL, Lenz G. Autophagy interplay with apoptosis and cell cycle regulation in the growth inhibiting effect of resveratrol in glioma cells. PLoS One. 2011;6:e20849–0. doi: 10.1371/journal.pone.0020849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkhalaf M, Jaffal S. Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells.Novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic Biol Med. 2006;41:318–25. doi: 10.1016/j.freeradbiomed.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Rashid A, Liu C, Sanli T, Tsiani E, et al. Resveratrol enhances prostate cancer cell response to ionizing radiation.Modulation of the AMPK Akt and mTOR pathways. Radiat Oncol. 2011; , 6:144–0. doi: 10.1186/1748-717X-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao PC, Ng LT, Lin LT, Richardson CD, et al. Resveratrol arrests cell cycle and induces apoptosis in human hepatocellular carcinoma Huh-7 cells. J Med Food. 2010;13:1415–23. doi: 10.1089/jmf.2010.1126. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh TC, Wong C, JohnBennett D, Wu JM. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells an in silico and biochemical approach targeting integrin avß3. Int J Cancer. 2011;129:2732–43. doi: 10.1002/ijc.25930. [DOI] [PubMed] [Google Scholar]
- 49.Sareen D, vanGinkel PR, Takach JC, Mohiuddin A, et al. Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2006;47:3708–16. doi: 10.1167/iovs.06-0119. [DOI] [PubMed] [Google Scholar]
- 50.Aziz MH, Nihal M, Fu VX, Jarrard DF, et al. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3'-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther. 2006;5:1335–41. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 51.Li G, He S, Chang L, Lu H, et al. GADD45a and annexin A1 are involved in the apoptosis of HL-60 induced by resveratrol. Phytomedicine. 2011;18:704–9. doi: 10.1016/j.phymed.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 52.Lin X, Wu G, Huo WQ, Zhang Y, et al. Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int J Urol. 2012;19:757–64. doi: 10.1111/j.1442-2042.2012.03024.x. [DOI] [PubMed] [Google Scholar]
- 53.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, et al. Resveratrol inhibits proliferation induces apoptosis and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 54.Faber AC, Dufort FJ, Blair D, Wagner D, et al. Inhibition of phosphatidylinositol 3-kinase-mediated glucose metabolism coincides with resveratrol-induced cell cycle arrest in human diffuse large B-cell lymphomas. Biochem Pharmacol. 2006;72:1246–56. doi: 10.1016/j.bcp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Puissant A, Robert G, Fenouille N, Luciano F, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK- mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–52. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Romigh T, He X, Orloff MS, et al. Resveratrol regulates the PTEN/AKT pathway through androgen receptor-dependent and -independent mechanisms in prostate cancer cell lines. Hum Mol Genet. 2010;19:4319–29. doi: 10.1093/hmg/ddq354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.BanerjeeMustafi S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS One. 2010;5:e8719–0. doi: 10.1371/journal.pone.0008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parekh P, Motiwale L, Naik N, Rao KV. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp Toxicol Pathol. 2011;63:167–73. doi: 10.1016/j.etp.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, Ganapathy S, Singh KP, Shankar S, et al. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS One. 2010;5:e15288–0. doi: 10.1371/journal.pone.0015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He X, Wang Y, Zhu J, Orloff M, et al. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011;301:168–76. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 62.Pozo-Guisado E, Merino JM, Mulero-Navarro S, Lorenzo-Benayas MJ, et al. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-kappaB. Int J Cancer. 2005;115:74–84. doi: 10.1002/ijc.20856. [DOI] [PubMed] [Google Scholar]
- 63.Yu H, Pan C, Zhao S, Wang Z, et al. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed Pharmacother. 2008;62:366–72. doi: 10.1016/j.biopha.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Lee MF, Pan MH, Chiou YS, Cheng AC, et al. Resveratrol modulates MED28 (Magicin/EG-1) expression and inhibits epidermal growth factor (EGF)-induced migration in MDA-MB-231 human breast cancer cells. J Agric Food Chem. 2011;59:11853–61. doi: 10.1021/jf202426k. [DOI] [PubMed] [Google Scholar]
- 65.Hope C, Planutis K, Planutiene M, Moyer MP, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells implications for colon cancer prevention. Mol Nutr Food Res. 2008;52:S52–61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer. 2010;10:238–0. doi: 10.1186/1471-2407-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ulrich S, Loitsch SM, Rau O, vonKnethen A, et al. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res. 2006;66:7348–54. doi: 10.1158/0008-5472.CAN-05-2777. [DOI] [PubMed] [Google Scholar]
- 68.Colin D, Limagne E, Jeanningros S, Jacquel A, et al. Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells. Cancer Prev Res. 2011;4:1095–106. doi: 10.1158/1940-6207.CAPR-10-0274. [DOI] [PubMed] [Google Scholar]
- 69.Castino R, Pucer A, Veneroni R, Morani F, et al. Resveratrol reduces the invasive growth and promotes the acquisition of a long-lasting differentiated phenotype in human glioblastoma cells. J Agric Food Chem. 2011;59:4264–72. doi: 10.1021/jf104917q. [DOI] [PubMed] [Google Scholar]
- 70.Weng CJ, Wu CF, Huang HW, Wu CH, et al. Evaluation of anti-invasion effect of resveratrol and related methoxy analogues on human hepatocarcinoma cells. J Agric Food Chem. 2010;58:2886–94. doi: 10.1021/jf904182y. [DOI] [PubMed] [Google Scholar]
- 71.Annabi B, Lord-Dufour S, Vézina A, Béliveau R. Resveratrol Targeting of Carcinogen-Induced Brain Endothelial Cell Inflammation Biomarkers MMP-9 and COX-2 is Sirt1-Independent. Drug Target Insights. 2012;6:1–11. doi: 10.4137/DTI.S9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vergara D, Valente CM, Tinelli A, Siciliano C, et al. Resveratrol inhibits the epidermal growth factor-induced epithelial mesenchymal transition in MCF-7 cells. Cancer Lett. 2011;310:1–8. doi: 10.1016/j.canlet.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Bournival J, Quessy P. Martinoli MG.rotective effects of resveratrol and quercetin against MPP+ induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol. 2009;29:1169–80. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han YS, Zheng WH, Bastianetto S, Chabot JG, et al. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–82. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 76.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–13. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leonard SS, Xia C, Jiang BH, Stinefelt B, et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–26. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 78.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–7. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 79.Zhang F, Shi JS, Zhou H, Wilson B, et al. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol Pharmacol. 2010;78:466–77. doi: 10.1124/mol.110.064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spanier G, Xu H, Xia N, Tobias S, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1) glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol. 2009;60:111–6. [PubMed] [Google Scholar]
- 81.Xia N, Daiber A, Habermeier A, Closs EI, et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–54. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 82.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–81. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanno M, Kuno A, Yano T, Miura T, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–82. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaga S, Zhan L, Matsumoto M, Maulik N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1 heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–22. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 85.Zhuang H, Kim YS, Koehler RC, Doré S. Potential mechanism by which resveratrol a red wine constituent protects neurons. Ann N Y Acad Sci. 2003;993:276–6. doi: 10.1111/j.1749-6632.2003.tb07534.x. [DOI] [PubMed] [Google Scholar]
- 86.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes role of nitric oxide thioredoxin and heme oxygenase. Free Radic Biol Med. 2007;43:720–9. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Juan SH, Cheng TH, Lin HC, Chu YL, et al. Mechanism of concentration-dependent induction of heme oygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol. 2005;69:41–8. doi: 10.1016/j.bcp.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 88.Yang J, Wang N, Li J, Zhang J, et al. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine. 2010;37:365–72. doi: 10.1007/s12020-010-9314-8. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi S, Nakashima Y. Repeated and long-term treatment with physiological concentrations of resveratrol promotes NO production in vascular endothelial cells. Br J Nutr. 2012;107:774–80. doi: 10.1017/S0007114511003588. [DOI] [PubMed] [Google Scholar]
- 90.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–97. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 91.Elíes J, Cuíñas A, García-Morales V, Orallo F, et al. Trans-resveratrol simultaneously increases cytoplasmic Ca(2+) levels and nitric oxide release in human endothelial cells. Mol Nutr Food Res. 2011;55:1237–48. doi: 10.1002/mnfr.201100240. [DOI] [PubMed] [Google Scholar]
- 92.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wallerath T, Deckert G, Ternes T, Anderson H, et al. Resveratrol a polyphenolic phytoalexin present in red wine enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–8. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 94.Arunachalam G, Yao H, Sundar IK, Caito S, et al. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, et al. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280:7460–8. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 96.Xu Q, Hao X, Yang Q, Si L. Resveratrol prevents hyperglycemia-induced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase. Bio-chem Biophys Res Commun. 2009;388:389–94. doi: 10.1016/j.bbrc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 97.Park SJ, Ahmad F, Philp A, Baar K, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–33. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ungvari Z, Bagi Z, Feher A, Recchia FA, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moon SO, Kim W, Sung MJ, Lee S, et al. Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol. Pharmacol. 2006;70:112–9. doi: 10.1124/mol.106.022392. [DOI] [PubMed] [Google Scholar]
- 100.Csiszar A, Smith K, Labinskyy N, Orosz Z, et al. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: role of NF-kappaB inhibition. Am J Physiol Heart Circ Physiol. 2006;291:H1694–9. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 101.Cicha I, Regler M, Urschel K, Goppelt-Struebe M, et al. Resveratrol inhibits monocytic cell chemotaxis to MCP-1 and prevents spontaneous endothelial cell migration through Rho kinase-dependent mechanism. J Atheroscler Thromb. 2011;18:1031–42. doi: 10.5551/jat.8136. [DOI] [PubMed] [Google Scholar]
- 102.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, et al. Vasoprotective effects of resveratrol and SIRT1attenuation of cigarette smoke-induced oxidative stress and proin-flammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–35. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sayin O, Arslan N, Altun ZS, Akdogan G. In vitro effect of resveratrol against oxidative injury of human coronary artery endothelial cells. Turk J Med Sci. 2011;41:211–8. [Google Scholar]
- 104.Holmes-McNary M, Baldwin ASJr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–83. [PubMed] [Google Scholar]
- 105.Wang Z, Chen Y, Labinskyy N, Hsieh TC, et al. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem Biophys Res Commun. 2006;346:367–76. doi: 10.1016/j.bbrc.2006.05.156. [DOI] [PubMed] [Google Scholar]
- 106.Ferrero ME, Bertelli AE, Fulgenzi A, Pellegatta F, et al. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am J Clin Nutr. 1998;68:1208–14. doi: 10.1093/ajcn/68.6.1208. [DOI] [PubMed] [Google Scholar]
- 107.Kim SW, Kim CE, Kim MH. Flavonoids inhibit high glucose-induced up-regulation of ICAM-1 via the p38 MAPK pathway in human vein endothelial cells. Biochem Biophys Res Commun. 2011;415:602–7. doi: 10.1016/j.bbrc.2011.10.115. [DOI] [PubMed] [Google Scholar]
- 108.Subbaramaiah K, Chung WJ, Michaluart P, Telang N, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998;273:21875–82. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- 109.Dave M, Attur M, Palmer G, Al-Mussawir HE, et al. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786–97. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 110.Picard F, Kurtev M, Chung N, Topark-Ngarm A, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPARgamma. Nature. 2004;429:771–6. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rayalam S, Yang JY, Ambati S, Della-Fera MA, et al. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytotherapy Res. 2008;22:1367–71. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 112.Szkudelska K, Nogowski L, Szkudelski T. Resveratrol a naturally occurring diphenolic compound affects lipogenesis lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009;113:17–24. doi: 10.1016/j.jsbmb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 113.Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr. 2010;92:5–15. doi: 10.3945/ajcn.2009.28435. [DOI] [PubMed] [Google Scholar]
- 114.Mader I, Wabitsch M, Debatin KM, Fischer-Posovszky P, et al. Identification of a novel proapoptotic function of resveratrol in fat cells.SIRT1-independent sensitization to TRAIL-induced apoptosis. Faseb J. 2010;24:1997–2009. doi: 10.1096/fj.09-142943. [DOI] [PubMed] [Google Scholar]
- 115.Lasa A, Churruca I, Eseberri I, Andrés-Lacueva C, et al. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol Nutr Food Res. 2012;56:1559–68. doi: 10.1002/mnfr.201100772. [DOI] [PubMed] [Google Scholar]
- 116.Shen MY, Hsiao G, Liu CL, Fong TH, et al. Inhibitory mechanisms of resveratrol in platelet activation pivotal roles of p38 MAPK and NO cyclic GMP. Br J Haematol. 2007;139:475–85. doi: 10.1111/j.1365-2141.2007.06788.x. [DOI] [PubMed] [Google Scholar]
- 117.Yang YM, Chen JZ, Wang XX, Wang SJ, et al. Resveratrol attenuates thromboxane A2 receptor agonist induced platelet activation by reducing phospholipase C activity. Eur J Pharmacol. 2008;583:148–55. doi: 10.1016/j.ejphar.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 118.Lin KH, Hsiao G, Shih CM, Chou DS, et al. Mechanisms of resveratrol induced platelet apoptosis. Cardiovasc Res. 2009;83:575–85. doi: 10.1093/cvr/cvp139. [DOI] [PubMed] [Google Scholar]
- 119.Stefani M, Markus MA, Lin RC, Pinese M, et al. The effect of resveratrol on a cell model of human aging. Ann N Y Acad Sci. 2007;1114:407–18. doi: 10.1196/annals.1396.001. [DOI] [PubMed] [Google Scholar]
- 120.Tang Y, Xu J, Qu W, Peng X, et al. Resveratrol reduces vascular cell senescence through attenuation of oxidative stress by SIRT1/NADPH oxidase-dependent mechanisms. J Nutr Biochem. 2012;23:1410–6. doi: 10.1016/j.jnutbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 121.Wu A, Ying Z, Gomez-Pinilla F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur J Neurosci. 2006;23:2573–80. doi: 10.1111/j.1460-9568.2006.04807.x. [DOI] [PubMed] [Google Scholar]
- 122.Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001–5. doi: 10.1006/bbrc.2002.6554. [DOI] [PubMed] [Google Scholar]
- 123.Ziegler CC, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in Apc (Min/+) mice. J Nutr. 2004;134:5–10. doi: 10.1093/jn/134.1.5. [DOI] [PubMed] [Google Scholar]
- 124.Berge G, Ovrebo S, Eilertsen E, Haugen A, et al. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo [alpha]pyrene. Br J Cancer. 2004;91:1380–3. doi: 10.1038/sj.bjc.6602125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Niles RM, Cook CP, Meadows GG, Fu YM, et al. Resveratrol is rapidly metabolized in athymic (Nu/Nu) mice and does not inhibit human melanoma xenograft tumor growth. J Nutr. 2006;136:2542–6. doi: 10.1093/jn/136.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zunino SJ, Storms DH, Newman JW, Pedersen TL, et al. Resveratrol given intraperitoneally does not inhibit the growth of high-risk t (4.1) acute lymphoblastic leukemia cells in a NOD/SCID mouse model. Int J Oncol. 2012;40:1277–84. doi: 10.3892/ijo.2011.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stakleff KS, Sloan T, Blanco D, Marcanthony S, et al. Resveratrol exerts differential effects in vitro and in vivo against ovarian cancer cells. Asian Pac J Cancer Prev. 2012;13:1333–40. doi: 10.7314/apjcp.2012.13.4.1333. [DOI] [PubMed] [Google Scholar]
- 128.Carbó N, Costelli P, Baccino FM, López-Soriano FJ, et al. Resveratrol a natural product present in wine decreases tumor growth in a rat tumor model. Biochem Biophys Res Commun. 1999;254:739–43. doi: 10.1006/bbrc.1998.9916. [DOI] [PubMed] [Google Scholar]
- 129.Asensi M, Medina I, Ortega A, Carretero J, et al. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–98. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 130.Ganapathy S, Chen Q, Singh KP, Shankar S, et al. Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor. PLoS One. 2010;5:e15627–0. doi: 10.1371/journal.pone.0015627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seeni A, Takahashi S, Takeshita K, Tang M, et al. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac J Cancer Prev. 2008;9:7–14. [PubMed] [Google Scholar]
- 132.Zhou HB, Chen JJ, Wang WX, Cai JT, et al. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol. 2005;11:280–4. doi: 10.3748/wjg.v11.i2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuan Y, Xue X, Guo RB, et al. Sun XL, Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS Neurosci Ther. 2012;18:536–46. doi: 10.1111/j.1755-5949.2012.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roy SK, Chen Q, Fu J, Shankar S, et al. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One. 2011;6:e25166–0. doi: 10.1371/journal.pone.0025166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kowalczyk MC, Kowalczyk P, Tolstykh O, Hanausek M, et al. Synergistic effects of combined phytochemicals and skin cancer prevention in SENCAR mice. Cancer Prev Res. 2010;3:170–8. doi: 10.1158/1940-6207.CAPR-09-0196. [DOI] [PubMed] [Google Scholar]
- 136.Bishayee A, Dhir N. Resveratrol-mediated chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis inhibition of cell proliferation and induction of apoptosis. Chem Biol Interact. 2009;179:131–44. doi: 10.1016/j.cbi.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 137.Yang Hl, Chen Wq, Cao X, Du LF, et al. Caveolin-I enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J Transl Med. 2009;7:22–0. doi: 10.1186/1479-5876-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roy P, Kalra N, Prasad S, George J, et al. Chemopreventive Potential of resveratrol in Mouse Skin Tumors Through Regulation of Mitochondrial and PI3K/AKT Signaling Pathways. Pharm Res. 2009;26:211–7. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 139.Mbimba T, Awale P, Bhatia D, Geldenhuys WJ, et al. Alteration of hepatic proinflammatory cytokines is involved in the resveratrol-mediated chemoprevention of chemically-induced hepatocarcinogenesis. Curr Pharm Biotechnol. 2012;13:229–34. doi: 10.2174/138920112798868575. [DOI] [PubMed] [Google Scholar]
- 140.Salado C, Olaso E, Gallot N, Valcarcel M, et al. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of inter-leukin-18. J Transl Med. 2011;9:59–0. doi: 10.1186/1479-5876-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br J Cancer. 2004;91:1742–50. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee HS, Ha AW, Kim WK. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr Res Pract. 2012;6:294–300. doi: 10.4162/nrp.2012.6.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol.Role of nuclear factor-kappa B cyclooxygenase 2 and matrix metalloprotease 9. Can-cer Res. 2002;62:4945–54. [PubMed] [Google Scholar]
- 144.Wu SL, Sun ZJ, Yu L, Meng KW, et al. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048–52. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur J Cancer. 2010;46:1882–91. doi: 10.1016/j.ejca.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.El-Mowafy AM, El-Mesery ME, Salem HA, Al-Gayyar MM, et al. Prominent chemopreventive and chemoenhancing effects for resveratrol unraveling molecular targets and the role of C-reactive protein. Chemotherapy. 2010;56:60–5. doi: 10.1159/000298821. [DOI] [PubMed] [Google Scholar]
- 147.Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21(CIP) expression. Carcinogenesis. 2000;21:1619–22. [PubMed] [Google Scholar]
- 148.Li ZG, Hong T, Shimada Y, Komoto I, et al. Suppression of Nnitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis. 2002;23:1531–6. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 149.Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumor incidence in 1.-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br J Nutr. 2006;96:145–53. doi: 10.1079/bjn20061789. [DOI] [PubMed] [Google Scholar]
- 150.Huderson AC, Myers JN, Niaz MS, Washington MK, et al. Chemoprevention of benzo(a)pyrene-induced colon polyps in Apc (Min) mice by resveratrol. J Nutr Biochem. 2012;24:713–24. doi: 10.1016/j.jnutbio.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oktem G, Uysal A, Oral O, Sezer ED, et al. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol. 2012;64:471–9. doi: 10.1016/j.etp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 152.Saleh MC, Connell BJ, Saleh TM. Resveratrol preconditioning induces cellular stress proteins and is mediated via NMDA and estrogen receptors. Neuroscience. 2010;166:445–54. doi: 10.1016/j.neuroscience.2009.12.060. [DOI] [PubMed] [Google Scholar]
- 153.Gurusamy N, Lekli I, Mukherjee S, Ray D, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86:103–12. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang JP, Huang SS, Deng JY, Chang CC, et al. Insulin and resveratrol act synergistically preventing cardiac dysfunction in diabetes, but the advantage of resveratrol in diabetics with acute heart attack is antagonized by insulin. Free Radic Biol Med. 2010;49:1710–21. doi: 10.1016/j.freeradbiomed.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 155.Lekli I, Szabo G, Juhasz B, Das S, et al. Protective mechanisms of resveratrol against ischemia/reperfusion-induced damage in hearts obtained from Zucker obese rats the role of GLUT-4 and endothelin. Am J Physiol Heart Circ Physiol. 2007;294:H859–66. doi: 10.1152/ajpheart.01048.2007. [DOI] [PubMed] [Google Scholar]
- 156.Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radic Biol Med. 2004;36:774–81. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 157.Dudley J, Das S, Mukherjee S, Das DK. Resveratrol a unique phytoalexin present in red wine delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20:443–52. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 158.Chen CJ, Yu W, Fu YC, Wang X, et al. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FOXO1 pathway. Biochem Biophys Res Commun. 2009;378:389–93. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 159.Robich MP, Osipov RM, Nezafat R, Feng J, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–9. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Azorín-Ortuño M, Yañéz-Gascón MJ, Pallarés FJ, Rivera J, et al. A dietary resveratrol-rich grape extract prevents the developing of atherosclerotic lesions in the aorta of pigs fed an atherogenic diet. J Agric Food Chem. 2012;60:5609–20. doi: 10.1021/jf301154q. [DOI] [PubMed] [Google Scholar]
- 161.Delucchi F, Berni R, Frati C, Cavalli S, et al. Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats. PLoS One. 2012;7:e39836–0. doi: 10.1371/journal.pone.0039836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Biala A, Tauriainen E, Siltanen A, Shi J, et al. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press. 2010;19:196–205. doi: 10.3109/08037051.2010.481808. [DOI] [PubMed] [Google Scholar]
- 163.Chan V, Fenning A, Iyer A, Hoey A, et al. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr Pharm Biotechnol. 2011;12:429–36. doi: 10.2174/138920111794480552. [DOI] [PubMed] [Google Scholar]
- 164.Aubin MC, Lajoie C, Clement R, Gosselin H, et al. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia therapeutic potential of resveratrol. J Pharmacol Exp Ther. 2008;325:961–8. doi: 10.1124/jpet.107.135061. [DOI] [PubMed] [Google Scholar]
- 165.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–63. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 166.Miatello R, Vazquez M, Renna N, Cruzado M, et al. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens. 2005;18:864–70. doi: 10.1016/j.amjhyper.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 167.Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol. 2011;667:258–64. doi: 10.1016/j.ejphar.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 168.Chen C, Yang W, Xueding C, Qi Z, et al. Resveratrol downregulates acute pulmonary thromboembolism-induced pulmonary artery hypertension via p38 mitogen-activated protein kinase and monocyte chemoattractant protein-1 signaling in rats. Life Sci. 2012;90:721–7. doi: 10.1016/j.lfs.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 169.Akar F, Uludag O, Aydin A, Aytekin YA, et al. High-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats protective effect of resveratrol. Food Chem Toxicol. 2012;50:2135–41. doi: 10.1016/j.fct.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 170.Behbahani J, Thandapilly SJ, Louis XL, Huang Y, et al. Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am J Hypertens. 2010;23:1273–8. doi: 10.1038/ajh.2010.161. [DOI] [PubMed] [Google Scholar]
- 171.Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, et al. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23:192–6. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- 172.Louis XL, Thandapilly SJ, Mohankumar SK, Yu L, et al. Treatment with low-dose resveratrol reverses cardiac impairment in obese prone but not in obese resistant rats. J Nutr Biochem. 2012;23:1163–9. doi: 10.1016/j.jnutbio.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 173.Rimbaud S, Ruiz M, Piquereau J, Mateo P, et al. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391–0. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paffett ML, Hesterman J, Candelaria G, Lucas S, et al. Longitudinal in vivo SPECT/CT imaging reveals morphological changes and cardiopulmonary apoptosis in a rodent model of pulmonary arterial hypertension. PLoS One. 2012;7:e40910–0. doi: 10.1371/journal.pone.0040910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin JF, Lin SM, Chih CL, Nien MW, et al. Resveratrol reduces infarct size and improves ventricular function after myocardial ischemia in rats. Life Sci. 2008;83:313–7. doi: 10.1016/j.lfs.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 176.Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, et al. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholes-terolemic rat. J Mol Cell Cardiol. 2007;42:508–16. doi: 10.1016/j.yjmcc.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Al-Dissi AN, Weber LP. Resveratrol preserves cardiac function, but does not prevent endothelial dysfunction or pulmonary inflammation after environmental tobacco smoke exposure. Food Chem Toxicol. 2011;49:1584–91. doi: 10.1016/j.fct.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 178.Wojciechowski P, Juric D, Louis XL, Thandapilly SJ, et al. Resveratrol arrests and regresses the development of pressure overload but not volume overload-induced cardiac hy-pertrophy in rats. J Nutr. 2010;140:962–8. doi: 10.3945/jn.109.115006. [DOI] [PubMed] [Google Scholar]
- 179.Dolinsky VW, Chan AY, RobillardFrayne I, Light PE, et al. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–52. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 180.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARa to protect the heart from hypertrophy metabolic dysregulation and inflammation. Car-diovasc Res. 2011;90:276–84. doi: 10.1093/cvr/cvq376. [DOI] [PubMed] [Google Scholar]
- 181.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol attenuates ovariectomy-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats. J Nutr Sci Vitaminol. 2000;46:78–83. doi: 10.3177/jnsv.46.78. [DOI] [PubMed] [Google Scholar]
- 182.Ramar M, Manikandan B, Raman T, Priyadarsini A, et al. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur J Pharmacol. 2012;690:226–35. doi: 10.1016/j.ejphar.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 183.Alturfan AA, Tozan-Beceren A, Sehirli AO, Demiralp E, et al. Resveratrol ameliorates oxidative DNA damage and protects against acrylamide-induced oxidative stress in rats. Mol Biol Rep. 2012;39:4589–96. doi: 10.1007/s11033-011-1249-5. [DOI] [PubMed] [Google Scholar]
- 184.Bagul PK, Middela H, Matapally S, Padiya R, et al. Attenuation of insulin resistance metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol Res. 2012;66:260–8. doi: 10.1016/j.phrs.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 185.Franco JG, Lisboa PC, Lima NS, Amaral TA, et al. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J Nutr Biochem 2012. doi.org/10.1016/j.jnutbio.2012.06.019. . 0000;00:0–0. doi: 10.1016/j.jnutbio.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 186.Petrat F, deGroot H. Protection against severe intestinal ischemia reperfusion injury in rats by intravenous resveratrol. J Surg Res. 2011;167:e145–55. doi: 10.1016/j.jss.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 187.Chen YR, Yi FF, Li XY, Wang CY, et al. Resveratrol attenuates ventricular arrhythmias and improves the long-term survival in rats with myocardial infarction. Cardiovasc Drugs Ther. 2008;22:479–85. doi: 10.1007/s10557-008-6141-8. [DOI] [PubMed] [Google Scholar]
- 188.Do GM, Jung UJ, Park HJ, Kwon EY, et al. Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol Nutr Food Res. 2012;56:1282–91. doi: 10.1002/mnfr.201200067. [DOI] [PubMed] [Google Scholar]
- 189.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem Pharmacol. 2011;81:1343–51. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 190.Do GM, Kwon EY, Kim HJ, Jeon SM, et al. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun. 2008;374:55–9. doi: 10.1016/j.bbrc.2008.06.113. [DOI] [PubMed] [Google Scholar]
- 191.Gómez-Zorita S, Fernández-Quintela A, Macarulla MT, Aguirre L, et al. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr. 2012;107:202–10. doi: 10.1017/S0007114511002753. [DOI] [PubMed] [Google Scholar]
- 192.Roghani M, Baluchnejadmojarad T. Mechanisms underlying vascular effect of chronic resveratrol in streptozotocin-diabetic rats. Phytother Res. 2010;24:S148–54. doi: 10.1002/ptr.3032. [DOI] [PubMed] [Google Scholar]
- 193.Robich MP, Osipov RM, Chu LM, Han Y, et al. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur J Pharmacol. 2011;664:45–53. doi: 10.1016/j.ejphar.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jeon BT, Jeong EA, Shin HJ, Lee Y, et al. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61:1444–54. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bujanda L, Hijona E, Larzabal M, Beraza M, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC Gastroenterol. 2008;8:40–0. doi: 10.1186/1471-230X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shang J, Chen LL, Xiao FX, Sun H, et al. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 197.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 198.Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol. 2010;10:11–0. doi: 10.1186/1472-6793-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baur JA, Pearson KJ, Price NL, Jamieson HA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ramadori G, Gautron L, Fujikawa T, Vianna CR, et al. Central administration of resveratrol improves diet-induced diabetes. Endocrinology. 2009;150:5326–33. doi: 10.1210/en.2009-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharma S, Misra CS, Arumugam S, Roy S, et al. Antidiabetic activity of resveratrol a known SIRT1 activator in a genetic model for type-2 diabetes. Phytother Res. 2011;25:67–73. doi: 10.1002/ptr.3221. [DOI] [PubMed] [Google Scholar]
- 202.Kang W, Hong HJ, Guan J, Kim DG, et al. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only in vitro and in vivo experi-ments in rodents. Metabolism. 2012;61:424–33. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 203.Gonzalez-Rodriguez A, MasGutierrez JA, Sanz-Gonzalez S, Ros M, et al. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes. 2010;59:588–99. doi: 10.2337/db09-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Deng JY, Hsieh PS, Huang JP, Lu LS, et al. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes. 2008;57:1814–23. doi: 10.2337/db07-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Andersen G, Burkon A, Sulzmaier FJ, Walker JM, et al. High dose of dietary resveratrol enhances insulin sensitivity in healthy rats but does not lead to metabolite concentrations effective for SIRT1 expression. Mol Nutr Food Res. 2011;55:1197–206. doi: 10.1002/mnfr.201100292. [DOI] [PubMed] [Google Scholar]
- 206.Um JH, Park SJ, Kang H, Yang S, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–63. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Marchal J, Blanc S, Epelbaum J, Aujard F, et al. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate, Microcebus murinus. PLoS One. 2012;7:e34289–0. doi: 10.1371/journal.pone.0034289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Akar F, Pektas MB, Tufan C, Soylemez S, et al. Resveratrol shows vasoprotective effect reducing oxidative stress without affecting metabolic disturbances in insulin-dependent diabetes of rabbits. Cardiovasc Drugs Ther. 2011;25:119–31. doi: 10.1007/s10557-010-6255-7. [DOI] [PubMed] [Google Scholar]
- 209.Sanchez-Fidalgo S, Cardeno A, Villegas I, Talero E, et al. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633:78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 210.Singh UP, Singh NP, Singh B, Hofseth LJ, et al. Resveratrol (trans-3,5,4 trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332:829–39. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yao J, Wang JY, Liu L, Li YX, et al. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res. 2010;41:288–94. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 212.Zhang H, Morgan B, Potter BJ, Ma L, et al. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress. Am J Physiol Heart Circ Physiol. 2010;299:H985–94. doi: 10.1152/ajpheart.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sehirli O, Tozan A, Omurtag GZ, Cetinel S, et al. Protective effect of resveratrol against naphthalene-induced oxidative stress in mice. Ecotoxicol Environ Saf. 2008;71:301–8. doi: 10.1016/j.ecoenv.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 214.Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain participation of nitric oxide and TNF-alpha. Phytother Res. 2007;21:278–83. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- 215.Martin AR, Villegas I, Sanchez-Hidalgo M, delaLastra CA. The effects of resveratrol a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147:873–85. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioral changes possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485–92. doi: 10.1097/00008877-200609000-00014. [DOI] [PubMed] [Google Scholar]
- 217.Hong SW, Jung KH, Zheng HM, Lee HS, et al. The protective effect of resveratrol on dimethylnitrosamine-induced liver fibrosis in rats. Arch Pharm Research. 2010;33:601–9. doi: 10.1007/s12272-010-0415-y. [DOI] [PubMed] [Google Scholar]
- 218.Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines hyperglycemia mediated oxidative stress and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010;224:423–32. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 219.Sener G, Topaloglu N, Ozer SA, Ercan F, et al. Resveratrol alleviates bleomycin-induced lung injury in rats. Pulm Pharmacol Ther. 2007;20:642–9. doi: 10.1016/j.pupt.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 220.Ara C, Kirimlioglu H, Karabulut AB, Coban S, et al. Protective effect of resveratrol against oxidative stress in cholestasis. J Surg Res. 2005;127:112–7. doi: 10.1016/j.jss.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 221.Larrosa M, Yanez-Gascon MJ, Selma MV, Gonzalez-Sarrias A, et al. Effect of a low dose of dietary resveratrol on colon microbiota inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem. 2009;57:2211–20. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 222.Inanaga K, Ichiki T, Matsuura H, Miyazaki R, et al. Resveratrol attenuates angiotensin II-induced interleukin-6 expression and perivascular fibrosis. Hypertens Res. 2009;32:466–71. doi: 10.1038/hr.2009.47. [DOI] [PubMed] [Google Scholar]
- 223.Norata GD, Marchesi P, Passamonti S, Pirillo A, et al. Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis. 2007;191:265–71. doi: 10.1016/j.atherosclerosis.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 224.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, et al. Resveratrol (trans-3,5,4'-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72:1508–21. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee M, Kim S, Kwon OK, Oh SR, et al. Anti-inflammatory and anti-asthmatic effects of resveratrol a polyphenolic stilbene in a mouse model of allergic asthma. Int Immuno-pharmacol. 2009;9:418–24. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 226.FotiCuzzola V, Ciurleo R, Giacoppo S, Marino S, et al. Role of resveratrol and its analogues in the treatment of neurodegenerative diseases focus on recent discoveries. CNS Neurol Disord Drug Targets. 2011;10:849–62. doi: 10.2174/187152711798072310. [DOI] [PubMed] [Google Scholar]
- 227.Kumar A, Naidu PS, Seghal N, Padi SS. Neuroprotective effects of resveratrol against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress in rats. Pharmacology. 2007;79:17–26. doi: 10.1159/000097511. [DOI] [PubMed] [Google Scholar]
- 228.Mudò G, Mäkelä J, DiLiberto V, Tselykh TV, et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci. 2012;7:1153–65. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Srivastava G, Dixit A, Yadav S, Patel DK, et al. Resveratrol potentiates cytochrome P450 2 d22-mediated neuroprotection in maneb and paraquat-induced parkinsonism in the mouse. Free Radic Biol Med. 2012;52:1294–306. doi: 10.1016/j.freeradbiomed.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 230.Ren J, Fan C, Chen N, Huang J, et al. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem Res. 2011;36:2352–62. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 231.Della-Morte D, Dave KR, DeFazio RA, Bao YC, et al. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 233.Wood JG, Rogina B, Lavu S, Howitz K, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 234.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 235.Strong R, Miller RA, Astle CM, Baur JA, et al. Evaluation of Resveratrol Green Tea Extract Curcumin Oxaloacetic Acid and Medium-Chain Triglyceride Oil on Life Span of Genetically Heterogeneous Mice. J Gerontol A Biol Sci Med Sci. 2012;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Wang C, Wheeler CT, Alberico T, Sun X, et al. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age (Dordr). 2011;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chandrashekara KT, Shakarad MN. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2011;66:965–71. doi: 10.1093/gerona/glr103. [DOI] [PubMed] [Google Scholar]
- 238.Somoza V. Resveratrol current status and outlook. Mol Nutr Food Res. 2011;55:1127–8. doi: 10.1002/mnfr.201190030. [DOI] [PubMed] [Google Scholar]
- 239.Patel KR, Scott E, Brown VA, Gescher AJ, et al. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–9. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 240.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–41. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 241.Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, et al. Resveratrol pills to replace a healthy diet. Br J Clin Pharmacol. 2011;72:27–38. doi: 10.1111/j.1365-2125.2011.03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging. 2012;4:146–58. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Neves AR, Lucio M, Lima JL, Reis S. Resveratrol in medicinal chemistry a critical review of its pharmacokinetics drug-delivery and membrane interactions. Curr Med Chem. 2012;19:1663–81. doi: 10.2174/092986712799945085. [DOI] [PubMed] [Google Scholar]
- 244.Smoliga JM, Vang O, Baur JA. Challenges of translating basic research into therapeutics: Resveratrol as an example. J Gerontol A Biol Sci Med Sci. 2012;67:158–67. doi: 10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wang H, Yang YJ, Qian HY, Zhang Q, et al. Resveratrol in cardiovascular disease what is known from current research. Heart Fail Rev. 2012;17:437–48. doi: 10.1007/s10741-011-9260-4. [DOI] [PubMed] [Google Scholar]
- 246.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 247.Azorín-Ortuño M, Yáñez-Gascón MJ, Vallejo F, Pallarés FJ, et al. Metabolites and tissue distribution of resveratrol in the pig. Mol Nutr Food Res. 2011;55:1154–68. doi: 10.1002/mnfr.201100140. [DOI] [PubMed] [Google Scholar]
- 248.Burkon A, Somoza V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides two novel resveratrol metabolites in human plasma. Mol Nutr Food Res. 2008;52:549–57. doi: 10.1002/mnfr.200700290. [DOI] [PubMed] [Google Scholar]
- 249.Rotches-Ribalta M, Andres-Lacueva C, Estruch R, Escribano E, et al. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol Res. 2012;66:375–82. doi: 10.1016/j.phrs.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 250.Alfarás I, Pérez M, Juan ME, Merino G, et al. Involvement of breast cancer resistance protein (BCRP1/ABCG2) in the bioavailability and tissue distribution of trans-resveratrol in knockout mice. J Agric Food Chem. 2010;58:4523–8. doi: 10.1021/jf9042858. [DOI] [PubMed] [Google Scholar]
- 251.Azorín-Ortuño M, Yáñez-Gascón MJ, González-Sarrías A, Larrosa M, et al. Effects of long-term consumption of low doses of resveratrol on diet-induced mild hypercholesterolemia in pigs a transcriptomic approach to disease prevention. J Nutr Biochem. 2012;23:829–37. doi: 10.1016/j.jnutbio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 252.Bode LM, Bunzel D, Huch M, Cho GS, et al. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 253.Nunes T, Almeida L, Rocha JF, Falcão A, et al. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J Clin Pharmacol. 2009;49:1477–82. doi: 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]
- 254.Boocock DJ, Patel KR, Faust GE, Normolle DP, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:182–7. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Boocock DJ, Faust GE, Patel KR, Schinas AM, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 256.Azori´n-Ortuño M, Yane´zGasco´n MJ, Pallare´s FJ, Vallejo F, et al. Pharmacokinetic study of trans resveratrol in adult pigs. J Agric Food Chem. 2010;58:11165–71. doi: 10.1021/jf102799m. [DOI] [PubMed] [Google Scholar]
- 257.Vaz-da-Silva M, Loureiro AI, Falcao A, Nunes T, et al. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int J Clin Pharmacol Ther. 2008;46:564–70. doi: 10.5414/cpp46564. [DOI] [PubMed] [Google Scholar]
- 258.laPorte C, Voduc N, Zhang G, Seguin I, et al. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food quercetin and alcohol (ethanol) in healthy human subjects. Clin Pharmacokinet. 2010;49:449–54. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 259.Vitaglione P, Sforza S, Galaverna G, Ghidini C, et al. Bioavailability of trans-resveratrol from red wine in humans. Mol Nutr Food Res. 2005;49:495–504. doi: 10.1002/mnfr.200500002. [DOI] [PubMed] [Google Scholar]
- 260.Patel KR, Brown VA, Jones DJ, Britton RG, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–9. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hebden JM, Gilchrist PJ, Perkins AC, Wilson CG, et al. Stool water content and colonic drug absorption contrasting effects of lactulose and codeine. Pharm Res. 1999;16:1254–9. doi: 10.1023/a:1014805815499. [DOI] [PubMed] [Google Scholar]
- 262.Sheweita SA, Tilmisany AK. Cancer and phase II drug-metabolizing enzymes. Curr Drug Metab. 2003;4:45–58. doi: 10.2174/1389200033336919. [DOI] [PubMed] [Google Scholar]
- 263.Howells LM, Berry DP, Elliott PJ, Jacobson EW, et al. Phase I randomized double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases safety pharmacokinetics and pharmacodynamics. Cancer Prev Res (Phila). 2011;4:1419–25. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nguyen AV, Martinez M, Stamos MJ, Moyer MP, et al. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag Res. 2009;1:25–37. [PMC free article] [PubMed] [Google Scholar]
- 265.Sanders MA, Majumdar AP. Colon cancer stem cells implications in carcinogenesis. Front Biosci. 2011;16:1651–62. doi: 10.2741/3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Saif MW, Chu E. Biology of colorectal cancer. Cancer J. 2010;16:196–201. doi: 10.1097/PPO.0b013e3181e076af. [DOI] [PubMed] [Google Scholar]
- 267.Brasnyó P, Molnár GA, Mohás M, Markó L, et al. Resveratrol improves insulin sensitivity reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–9. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 268.Avogaro A, deKreutzenberg SV, Fadini GP. Oxidative stress and vascular disease in diabetes is the dichotomization of insulin signaling still valid. Free Radic Biol Med. 2002;44:1209–15. doi: 10.1016/j.freeradbiomed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 269.Fujitaka K, Otani H, Jo F, Jo H, et al. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res. 2011;31:842–7. doi: 10.1016/j.nutres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 270.Tomé-Carneiro J, Gonzálvez M, Larrosa M, García-Almagro FJ, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease a triple-blind 6-month follow-up placebo-controlled randomized trial. Mol Nutr Food Res. 2012;56:810–21. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- 271.Ehara S, Ueda M, Naruko T, Haze K, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–60. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 272.Contois JH, Warnick GR, Sniderman AD. Reliability of low-density lipoprotein cholesterol non-high-density lipoprotein cholesterol and apolipoprotein B measurement. J Clin Lipidol. 2011;5:264–72. doi: 10.1016/j.jacl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 273.Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110:356–63. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 274.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–41. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 275.Magyar K, Halmosi R, Palfi A, Feher G, et al. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–87. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- 276.Crandall JP, Oram V, Trandafirescu G, Reid M, et al. Pilot Study of resveratrol in Older Adults With Impaired Glucose Tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–12. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Maia HJr, Haddad C, Pinheiro N, Casoy J. Advantages of the association of resveratrol with oral contraceptives for management of endometriosis-related pain. Int J Womens Health. 2012;4:543–9. doi: 10.2147/IJWH.S36825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Popat R, Plesner T, Davies F, Cook G, et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2012;160:714–7. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- 279.Militaru C, Donoiu I, Craciun A, Scorei ID, et al. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris.Effects on lipid profiles inflammation markers and quality of life. Nutrition. 2013;29:178–83. doi: 10.1016/j.nut.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 280.Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells a triple-blind placebo-controlled one year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Fulop T, Larbi A, Kotb R, deAngelis F, et al. Aging immunity and cancer. Discov Med. 2011;11:537–50. [PubMed] [Google Scholar]
- 282.Wang JC, Bennett M. Aging and atherosclerosis mechanisms functional consequences and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–59. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 283.Ferrer I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol. 2012;97:38–51. doi: 10.1016/j.pneurobio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 284.Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, et al. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Waters MJ. Seeking SOCS and sex steroids. Trends Endocrinol Metab. 2003;14:149–51. doi: 10.1016/s1043-2760(03)00050-x. [DOI] [PubMed] [Google Scholar]
- 286.Sandhu MS, Dunger DB, Giovannucci EL. Insulin insulin-like growth factor-I (IGF-I) IGF binding proteins their biologic interactions and colorectal cancer. J Natl Cancer Inst. 2002;94:972–80. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 287.Scott E, Steward WP, Gescher AJ, Brown K. Resveratrol in human cancer chemoprevention-choosing the 'right' dose. Mol Nutr Food Res. 2012;56:7–13. doi: 10.1002/mnfr.201100400. [DOI] [PubMed] [Google Scholar]
- 288.Chow HH, Garland LL, Hsu CH, Vining DR, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). 2010;3:1168–75. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kennedy DO, Wightman EL, Reay JL, Lietz G, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans a double-blind placebo-controlled crossover investigation. Am J Clin Nutr. 2010;91:1590–7. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 290.Ghanim H, Sia CL, Korzeniewski K, Lohano T, et al. A resveratrol and polyphenol preparation suppresses oxidative and inflammatory stress response to a high-fat, high-carbohydrate meal. J Clin Endocrinol Metab. 2011;96:1409–14. doi: 10.1210/jc.2010-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bjørbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 199;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 292.Emanuelli B, Peraldi P, Filloux C, Chavey C, et al. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J BiolChem. 2003;276:47944–49. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 293.Wong RH, Howe PR, Buckley JD, Coates AM, et al. Acute resveratrol supplementation improves flow mediated dilatation in overweight obese individuals with mildly ele-vated blood pressure. Nutr Metab Cardiovasc Dis. 2011;21:851–6. doi: 10.1016/j.numecd.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 294.Timmers S, Konings E, Bilet L, Houtkooper RH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–22. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.DeGroote D, VanBelleghem K, Devière J, VanBrussel W, et al. Effect of the intake of resveratrol resveratrol phosphate and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann Nutr Metab. 2012;61:15–24. doi: 10.1159/000338634. [DOI] [PubMed] [Google Scholar]
- 296.Heger A, Ferk F, Nersesyan A, Szekeres T, et al. Intake of a resveratrol-containing dietary supplement has no impact on DNA stability in healthy subjects. Mutat Res. 2012;749:82–6. doi: 10.1016/j.mrgentox.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 297.Sisman AR, Kume T, Tas G, Akan P, et al. Comparison and evaluation of two C-reactive protein assays based on particle-enhanced immunoturbidime-try. J Clin Lab Anal. 2007;21:71–76. doi: 10.1002/jcla.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhu W, Qin W, Zhang K, Rottinghaus GE, et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. doi: 10.1080/01635581.2012.654926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Yoshino J, Conte C, Fontana L, Mittendorfer B, et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–64. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, et al. High-Dose Resveratrol Supplementation in Obese Men.An Investigator-Initiated Randomized Placebo-Controlled Clinical Trial of Substrate Metabolism Insulin Sensitivity and Body Composition. Diabetes. 2012;62:1186–95. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bo S, Ciccone G, Castiglione A, Gambino R, et al. Anti-inflammatory and Antioxidant Effects of Resveratrol in Healthy Smokers. Curr Med Chem. 2012;20:1323–31. doi: 10.2174/0929867311320100009. [DOI] [PubMed] [Google Scholar]
- 302.Fabbrocini G, Staibano S, DeRosa G, Battimiello V, et al. Resveratrol-containing gel for the treatment of acne vulgaris a single-blind vehicle-controlled pilot study. Am J Clin Dermatol. 2011;12:133–41. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 303.Wu Y, Jia LL, Zheng YN, Xu XG, et al. Resveratrate protects human skin from damage due to repetitive ultraviolet irradiation. J Eur Acad Dermatol Venereol. 2012. doi: 10.1111/j.1468-3083.2011.04414.x. doi: 10.1111/j.1468-3083.2011.04414.x. [DOI] [PubMed] [Google Scholar]
- 304.Buonocore D, Lazzeretti A, Tocabens P, Nobile V, et al. Resveratrol-procyanidin blend nutraceutical and antiaging efficacy evaluated in a placebocontrolled double-blind study. Clin Cosmet Investig Dermatol. 2012;5:159–65. doi: 10.2147/CCID.S36102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wu JM, Hsieh TC. Resveratrol a cardioprotective substance. Ann N Y Acad Sci. 2011;1215:16–21. doi: 10.1111/j.1749-6632.2010.05854.x. [DOI] [PubMed] [Google Scholar]
- 306.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–43. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 307.Burnett C, Valentini S, Cabreiro F, Goss M, et al. Absence of effects of Sir2 overexpression on lifespan in C elegans and Drosophila. Nature. 2001;477:482–5. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Price NL, Gomes AP, Ling AJ, Duarte FV, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–90. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Planas JM, Alfaras I, Colom H, Juan ME. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch Biochem Biophys. 2012;527:67–73. doi: 10.1016/j.abb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 310.van de, Wetering K, Burkon A, Feddema W, Bot A, et al. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol. 2009;75:876–85. doi: 10.1124/mol.108.052019. [DOI] [PubMed] [Google Scholar]
- 311.Juan ME, González-Pons E, Planas JM. Multidrug resistance proteins restrain the intestinal absorption of trans-resveratrol in rats. J Nutr. 2010;140:489–95. doi: 10.3945/jn.109.114959. [DOI] [PubMed] [Google Scholar]
- 312.Larrosa M, Tomé-Carneiro J, Yáñez-Gascón MJ, Alcántara D, et al. Preventive oral treatment with resveratrol pro-prodrugs drastically reduce colon inflammation in rodents. J Med Chem. 2010;53:7365–76. doi: 10.1021/jm1007006. [DOI] [PubMed] [Google Scholar]
- 313.Hoshino J, Park EJ, Kondratyuk TP, Marler L, et al. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem. 2010;53:5033–43. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Calamini B, Ratia K, Malkowski MG, Cuendet M, et al. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem J. 2010;42:273–82. doi: 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Andres-Lacueva C, Macarulla MT, Rotches-Ribalta M, Boto-Ordóñez M, et al. Distribution of resveratrol metabolites in liver adipose tissue and skeletal muscle in rats fed different doses of this polyphenol. J Agric Food Chem. 2012;60:4833–40. doi: 10.1021/jf3001108. [DOI] [PubMed] [Google Scholar]
- 316.Calabrese EJ, Mattson MP, Calabrese V. Resveratrol commonly displays hormesis occurrence and biomedical significance. Hum Exp Toxicol. 2010;29:980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- 317.Willrich MA, Hirata MH, Hirata RD. Statin regulation of CYP3A4 and CYP3A5 expression. Pharmacogenomics. 2009;10:1017–24. doi: 10.2217/pgs.09.42. [DOI] [PubMed] [Google Scholar]
- 318.Santos AC, Veiga F, Ribeiro AJ. New delivery systems to improve the bioavailability of resveratrol. Expert Opin Drug Deliv. 2011;8:973–90. doi: 10.1517/17425247.2011.581655. [DOI] [PubMed] [Google Scholar]
- 319.Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol.What formulation solutions to bioavailability limitations. J Control Release. 2012;158:182–93. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 320.Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, et al. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55:1169–76. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Harada N, Zhao J, Kurihara H, Nakagata N, et al. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J Nutr Biochem. 2011;22:1150–9. doi: 10.1016/j.jnutbio.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 322.Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 323.Meng X, Maliakal P, Lu H, Lee MJ, et al. Urinary and plasma levels of resveratrol and quercetin in humans mice and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–42. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 324.Urpí-Sardà M, Jáuregui O, Lamuela-Raventós RM, Jaeger W, et al. Uptake of diet resveratrol into the human low-density lipoprotein.Identification and quantification of resveratrol metabolites by liquid chromatography coupled with tandem mass spectrometry. Anal Chem. 2005;77:3149–55. doi: 10.1021/ac0484272. [DOI] [PubMed] [Google Scholar]
- 325.Zamora-Ros R, Urpí-Sardà M, Lamuela-Raventós RM, Estruch R, et al. Diagnostic performance of urinary resveratrol metabolites as a biomarker of moderate wine consumption. Clin Chem. 2006;52:1373–80. doi: 10.1373/clinchem.2005.065870. [DOI] [PubMed] [Google Scholar]
- 326.Gresele P, Pignatelli P, Guglielmini G, Carnevale R, et al. Resveratrol at concentrations attainable with moderate wine consumption stimulates human platelet nitric oxide production. J Nutr. 2008;138:1602–8. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 327.Almeida L, Vaz-da-Silva M, Falcão A, Soares E, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53:S7–15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 328.Ortuño J, Covas MI, Farre M, Pujadas M, et al. Matrix effects on the bioavailability of resveratrol in humans. Food Chem. 2010;4:1123–30. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 (713.7KB, pdf) . Effects of resveratrol and related mechanisms on human cancer cells.
Supplementary Table 2 (713.7KB, pdf) . Neuroprotective effects of resveratrol in in vitro models.
Supplementary Table 3 (713.7KB, pdf) . Effects of resveratrol on human cells involved in the vascular milieu.
Supplementary Table 4 (713.7KB, pdf) . Anti-aging effects of resveratrol in in vitro models.
Supplementary Table 5 (713.7KB, pdf) . Cancer chemopreventive effects of resveratrol and related mechanisms in animal models.
Supplementary Table 6 (713.7KB, pdf) . Effects of exposure to resveratrol on animal models of cardiovascular disease.
Supplementary Table 7 (713.7KB, pdf) . Resveratrol-exposure effects on insulin, glucose and lipid levels of animal models of obesity, diabetes and metabolic dysfunction.
Supplementary Table 8 (713.7KB, pdf) . Anti-inflammatory targets and related mechanisms dealing with resveratrol in animal models.
Supplementary Table 9 (713.7KB, pdf) . Neuroprotective effects of resveratrol in animal models.
Supplementary Table 10 (713.7KB, pdf) . Anti-aging effects of resveratrol in animal models.
Supplementary Table 11 (713.7KB, pdf) . Human trials dealing with resveratrol registered at clinicaltrials.gov.