Abstract
Rapid leukocyte motility is essential for immunity and host defense. There has been progress in understanding the molecular signals that regulate leukocyte motility both in vitro and in vivo. However, a gap remains in understanding how complex signals are prioritized to result in directed migration, which is critical for both adaptive and innate immune function. Here we focus on interstitial migration and how external cues are translated into intracellular signaling pathways that regulate leukocyte polarity, directional sensing and motility in three-dimensional spaces.
Introduction
The trafficking of leukocytes into peripheral tissues and lymphoid organs is critical for both innate and adaptive immune function and has been extensively reviewed [1–3]. A key step in this process is the interstitial motility of leukocytes within three-dimensional (3D) spaces, which can be either random or directed. Interstitial motility involves cycles of motility and arrest that is orchestrated by a complex hierarchy of external cues that are translated into changes in intracellular signaling. This function is evolutionarily conserved as demonstrated by the chemotactic interstitial migration of Drosophila hemocytes (immunosurveillance cells) to sites of tissue damage and infection (reviewed in [4]).
The rapid single-cell migration of leukocytes is classically known as “amoeboid” migration. The term “amoeboid” is based on a changing cell morphology, but can encompass different modes of locomotion (reviewed in [5,6]). Pseudopod driven gliding motility is the major mode of locomotion. During this mode of motility, actin polymerization below the leading plasma membrane generates forces for membrane extension. This is coupled with actomyosin contraction in the cell’s rear, which detaches the cell and propels the cell body forward. Leukocytes can also display a blebbing type of migration under some conditions where actomyosin contraction generates hydrostatic pressure to form bleb-based protrusions.
Migration in 3D
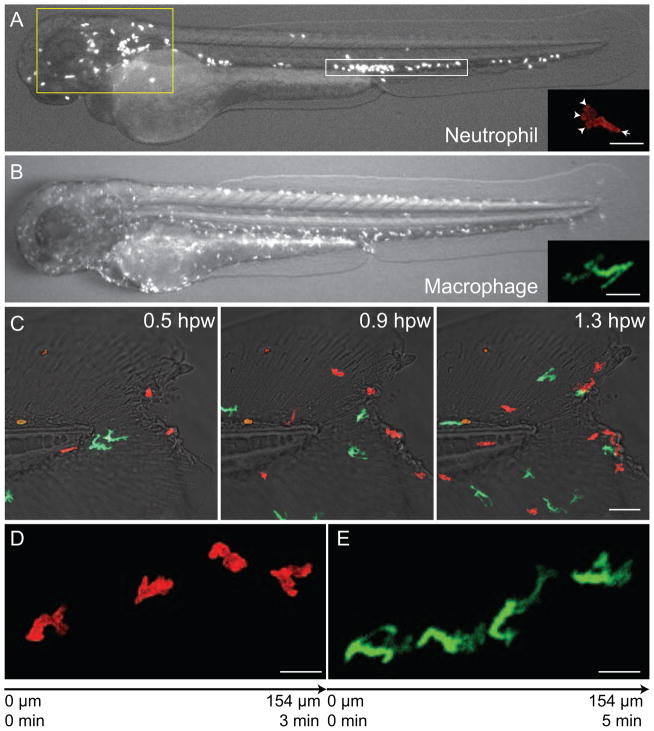
Efficient leukocyte migration through interstitial tissues is a key component of the normal function of the cells of the innate and adaptive immune system. The patrolling function of leukocytes requires cells to maneuver rapidly through complex matrix environments at speeds of up to 20–30 microns per minute. The type of protrusions formed by different leukocytes can be quite distinct, as demonstrated by neutrophils and macrophages migrating in vivo (Figure 1A and B). Neutrophils project small pseudopodia that often bifurcate, while macrophages frequently extend long, thin filopodia-like protrusions in many directions as they maneuver through the interstitium toward damaged tissues within zebrafish embryos (Figure 1C, D and E). T cells, on the other hand, often migrate randomly in their surveillance of lymph nodes in mice, with extension of small pseudopodia at the leading edge [7]. The mechanisms that regulate the generation and maintenance of amoeboid pseudopodia and biased selection are under intense investigation both in vitro and in vivo (reviewed in [8,9]).
Figure 1. Neutrophil and macrophage distribution, morphology and response to wounding in zebrafish embryo at 3 days post fertilization.
(A) Neutrophils distribute mainly in the mesenchymal tissue in the head region (yellow box) and in the caudal hematopoietic tissue (white box). (Inset in A) A typical migrating neutrophil has multiple pseudopodia (arrow heads) and a uropod (arrow). Scale bar = 20 μm. (B) Macrophages distribute in the whole embryo with no clear tissue localization. (Inset in B) A typical migrating macrophage shows multiple spindly protrusions. Scale bar = 20 μm. (C) Time series image of neutrophils (red; lifeact-Ruby) and macrophages (green; dendra) migrate to tail fin wound. Neutrophils have arrived at the wound at 0.5 hours post wounding (hpw) and macrophages arrived at later time points. Scale bar = 50 μm. Time series image of a neutrophil (D) or a macrophage (E) migrating from left to right towards the wound. Scale bar = 20 μm.
Leukocyte amoeboid motility, in general, occurs in the absence of strong adhesive interactions with surrounding cells or tissues (reviewed in [10]), although leukocyte arrest often requires integrin-mediated adhesion, in particular during transendothelial migration (reviewed in [11]). Integrin-mediated adhesion is essential for migration on two-dimensional surfaces. However, integrins can be dispensable for leukocyte migration in interstitial tissues [12] or in 3D collagen lattices [13]. The idea that movement within confined spaces can be integrin-independent was paradigm shifting and highlights the key role for adhesion in limiting leukocyte migration speed and mediating leukocyte arrest rather than an essential role in motility under many in vivo conditions.
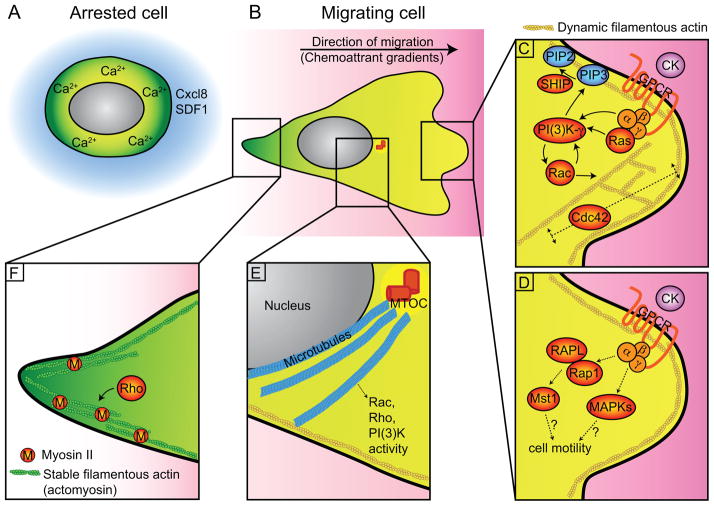
Interstitial environments contain both soluble and tissue-bound cues that help guide leukocytes to migrate or mediate cell arrest. Here, we review the key steps in interstitial leukocyte migration including sensing of environmental cues and the intracellular signaling mechanisms that orchestrate leukocyte motility and cell arrest (Figure 2A and B).
Figure 2. Cell Signaling and morphology in leukocyte interstitial migration in vivo.
Detailed in vitro findings are reviewed elsewhere [72]. Here, we highlight the in vivo data. (A) A round and non-polarized morphology of leukocytes during arrest. (B) A polarized, amoeboid morphology during directed or random motility. (C) Signaling events and cytoskeletal structure at the leading edge. Positive feedback between PI(3)K and Rac support the formation of the leading edge while Cdc42 regulates the actin flow and stability of the protrusion. SHIP hydrolyzes PI(3)K product PIP3 into PIP2 and limits leukocyte motility. (D) GPCR can also activate the small GTPase Rap1; Rap1-RAPL-Mst1 signaling likely contributes to both integrin-dependent and independent leukocyte migration. The regulation of MAPKs on overall leukocyte motility is less clear. (E) Microtubules regulate Rac, Rho and PI(3)K activities, and thus leukocyte motility. (F) Signaling events and cytoskeletal structure at the uropod. Stable filamentous actin present in uropod mediates retraction. CK, chemokines; GPCR, G-protein coupled receptor; MAPKs, Mitogen-activated protein kinases including p38, ERK and JNK; PIP2, PI(3,4)P2; PIP3, PI(3,4,5)P3; ROCK, Rho effector Rho-associated protein kinase; SDF1, stromal cell-derived factor 1 (or CXCL12); SHIP, SH2 domain-containing inositol 5′-phosphatase.
Cell signaling underlying leukocyte motility
Chemoattractants transmit signals through heterotrimeric G-protein-coupled receptors (GPCRs) [14], which activate a plethora of effectors [15,16]. One of the key effectors is the class Ib phosphatydylinositol-3-kinase (PI(3)K) (Figure 2C). Although the role of PI(3)K during in vitro chemotaxis is controversial, PI(3)Kγ is required for cell polarity and motility in vivo [17–19]. PI(3)K promotes Rac-mediated actin polymerization at the leading edge and generates F-actin anteroposterior polarity (dynamic F-actin at the leading edge and stable F-actin at the rear; Figure 2C and F). Inhibition of PI(3)K results in impaired F-actin polarity and neutrophil recruitment to both wounds and infection in zebrafish [17,20]. The importance of PI(3)K signaling is further illustrated by studies showing that the modulation of PI(3)K signaling can result in an inflammation phenotype. The PI(3)K products PI(3,4,5)P3-PI(3,4)P2 are localized to the leading edge of zebrafish neutrophils in vivo [17,21]. PI(3,4,5)P3 can be hydrolyzed to PI(3,4)P2 by SH2-domain-containing inositol 5-phosphatases (SHIP). SHIP limits myeloid cell motility through the modulation of PI(3)K signaling in vitro [22] and in vivo in zebrafish [21]. In addition, SHIP knockout mice show increased myeloid infiltration into vital organs [23,24]. Taken together, the modulation of PI(3,4,5)P3 levels through SHIP can negatively regulate cell motility.
Small GTPases are another class of key effectors downstream of GPCR signaling (reviewed in [25]). They function as molecular switches and play a critical role in the generation and maintenance of cell polarity and motility [25,26]). We will focus on some recent in vivo findings highlighting the role of different small GTPases (i.e., Ras and Rho subfamily) in leukocyte interstitial motility. The Ras subfamily Rap1 GTPase is important for leukocyte migration in vivo (Figure 2D). Interstitial motility of B and T cells in the mouse lymph node is dependent on a Rap1 effector molecule RAPL (Rap1-GTP binding protein) [27]. The complex of RAPL and GTP-bound Rap1 activate the serine-threonine kinase Mst1 (Ste20-like kinase) [28] and Mst1 is required for T-cell and B-cell motility within lymph nodes [29].
The Rho subfamily GTPases including Rac, Cdc42 and Rho are also critical for leukocyte interstitial migration (Figure 2E and F). In agreement with in vitro studies [30,31], Rac is required for neutrophil polarity and motility in zebrafish [32]. Rac2 depletion results in impaired neutrophil motility to wounds. In addition, dominant negative Rac2 expression in neutrophils impairs PI(3,4,5)P3-PI(3,4)P2 polarization and pseudopod formation [32]. Local activation of Rac can mediate protrusion, induce the polarity of F-actin dynamics, activate PI(3)K signaling and is sufficient to direct neutrophil migration in zebrafish [17]. In mature dendritic cells (DCs), Rac1 and Rac2 deletion in mice also impairs migration to lymph nodes [33]. It is becoming increasingly clear that both Rac and PI(3)K are required for leukocyte interstitial motility through a positive feedback loop.
Cdc42 regulates pseudopod stability [30] and controls neutrophil polarity and migration in vitro via crosstalk between WASp, CD11b and microtubules [34]. Although Cdc42 deficient DCs retain the ability to sense chemotactic cues and form protrusions, these protrusion are temporally and spatially dysregulated resulting in impaired interstitial migration in mice [35] (Figure 2C). The Cdc42 GEF, DOCK8, regulates interstitial DC migration in mice at least in part by controlling Cdc42 activity. DOCK8 deficiency impairs Cdc42 activation at the leading edge and results in defective migration [36]. The accumulating evidence suggests that Cdc42 activity maintains pseudopod protrusion.
Rho regulates cell polarity and helps propel the cell forward (Figure 2F). Expression of constitutively active Rho or dominant-negative RhoA impairs neutrophil F-actin polarity in zebrafish [17]. Inhibition of the Rho effector, Rho-associated protein kinase (ROCK), impairs neutrophil migration in zebrafish [17] and DC migration within the interstitium in mice [37]. Overall, Rho GTPases play an important role in leukocyte motility through the regulation of cell protrusion and retraction.
Mitogen-activated protein kinases (MAPKs) are other downstream effectors of GPCR signaling [38,39] that regulate leukocyte interstitial migration in vivo (Figure 2D). Understanding the role of MAPKs has been difficult, in part, due to some conflicting results. An in vivo study using mice reports that p38 positively regulates neutrophil interstitial chemotaxis to a keratinocyte-derived cytokine [40]. However, in zebrafish, p38 has been reported to negatively regulate myeloid cell migration, or may be dispensable [41,42]. In zebrafish, ERK has been shown to be required for neutrophil recruitment [43] but is dispensable for macrophage recruitment to wounds [42]. The requirement of JNK in leukocyte migration in vivo is also controversial [41,42]. These discrepancies may be due to different regulatory roles played by MAPKs depending on the in vivo conditions, and will be questions for future investigation.
The role of microtubules in interstitial motility
Microtubules suppress the polarity and motility of many leukocytes in vitro [44,45] and in vivo [46,47]. Depolymerization of microtubules using nocodazole enhances neutrophil polarity and motility but impairs directional migration in zebrafish [46]. In macrophages, microtubule depolymerization does not affect migration speed in zebrafish, although directional migration is impaired [47]. It is known that microtubules regulate Rho GTPase activity (Figure 2E). Microtubule depolymerization can activate RhoA in vitro in part through the release of microtubule associated Rho GEF [44,48,49]. Both microtubule polymerization and depolymerization activate Rac in vitro [46,50]. It is likely that the dysregulation of Rho and Rac activity upon microtubule depolymerization contributes to impaired directional motility in vivo. These observations suggest that the interconnected relationship between microtubules and the Rho GTPases plays a role in regulating leukocyte directional motility. In addition, microtubules may regulate PI(3)K signaling (Figure 2E) which is required for leukocyte polarization and motility [17]. It has been shown that microtubule depolymerization inhibits PI(3)K activation at the leading edge of neutrophils in zebrafish [46]. These observations highlight the essential role for microtubules in leukocyte directional motility in vivo.
Directional sensing and interstitial migration
Leukocyte gradient sensing and subsequent biased migration are necessary steps in directed migration. Directional migration can be guided by soluble or tissue-bound chemoattractant signals (Table 1). These directional cues can work separately or, in some cases, in concert.
Table 1.
Signaling molecules (soluble or tissue-bound) mediate leukocyte directed migration in vivo
| Tissue/Type of injury | Signaling Molecule | Mode of function | Distance from injury/Destination | Leukocyte | Receptor/Direct downstream target | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Epithelium injury | H2O2 | Signal peaks at ~20 min after wounding | Extends to ~100 to 200 μm from wound | Neutrophils | Lyn | Initial recruitment to tissue damage | [51] [43] |
| (TNF) | -- | -- | Neutrophils, Macrophages | FAN | Directed interstitial migration | [55] | |
| (Purine nucleotides) | -- | -- | Neutrophils | cADPR production | Amplification phase of recruitment | [61] | |
|
| |||||||
| Oncogene-transformed cells | H2O2 | Stochastic and transient | In transformed cells and their immediately neighboring cells | Neutrophils, Macrophages | -- | Leukocyte recruitment | [52] |
|
| |||||||
| Normal lymphatic endothelium | CCL21 | Tissue-bound gradient | ≤ 90 μm from lymphatic vessel | DCs | CCR7 | Directed migration from dermal interstitium into afferent lymphatic vessels | [59] |
|
| |||||||
| Normal lymph nodes | CCL21 | Tissuebound gradient | Increasing gradient from follicle region to the B/T boundary | B cells | CCR7 | Migration from follicle zone to the B/T boundary | [60] |
|
| |||||||
| Sterile skin inflammation | ICAM-1 | Cell surface protein | On NG2+ pericytes | Monocytes, Neutrophils, Macrophages | -- | Increases chemotaxis | [62] |
| MIF | Secreted by NG2+ pericytes | On NG2+ pericytes | Monocytes, Neutrophils, Macrophages | -- | Increases chemotaxis | [62] | |
|
| |||||||
| Sterile hepatic inflammation | MIP-2 | Intravascular gradient | Decreasing gradient from ~150 μm to 650 μm from the injury border | Neutrophils | CXCR2 | Directed intravascular migration | [63] |
| Formyl peptides | Released from necrotic cells | Within ~150 μm around the injury | Neutrophils | FPR1 | Serves as end-target chemoattractant | [63] | |
|
| |||||||
| Local bacterial infection | (TNF) | -- | -- | Neutrophils/Macrophages | FAN | Directed interstitial migration | [55] |
| Cxcl8 | Tissue-bound gradient | ≤ 50 μm from the producing cells | Neutrophils | -- | Mediates directional bias and restricts cell motility near the source | [58] | |
| Cxcl8 | -- | Systemic | Neutrophils | Cxcr2 | Neutrophil mobilization from hematopoietic tissue and recruitment to infection sites | [71] | |
cADPR, cyclic ADP ribose
DCs, dendritic cells
FAN, Factor associated with neutral sphingomyelinase activity
FPR1, formyl-peptide receptor 1
H2O2, hydrogen peroxide
ICAM-1, intercellular adhesion molecule 1
MIF, macrophage migration-inhibitory factor
MIP-2, macrophage inflammatory protein 2, the closest IL-8 homologue in the mouse
TNF-R1, p55 TNF receptor
Injured epithelial cells generate hydrogen peroxide (H2O2) through an NADPH oxidase (DUOX), which mediates rapid neutrophil wound detection in zebrafish [51]. Similarly, H2O2 is generated by oncogene-transformed cells, and this also mediates neutrophil and macrophage recruitment in zebrafish [52]. These observations suggest that H2O2 serves as an important signal for leukocyte detection of tissue damage. Recently, a Src family kinase (Lyn), was identified as a redox sensor that neutrophils use to detect the H2O2 gradients in injured tissues in zebrafish [43]. H2O2 directly oxidizes and activates Lyn. Activation of Lyn, in turn, leads to the phosphorylation and activation of the MAPK Erk but not p38 or JNK [43]. Interestingly, H2O2 is not required for neutrophils to detect localized bacterial infections in zebrafish, since inhibition of H2O2 production does not inhibit neutrophil recruitment to localized Pseudomonas aeruginosa or Streptococcus iniae infection [20].
Other cytokines are up-regulated with wounding or infection, including Tumor Necrosis Factor (TNF) expression in zebrafish [53,54]. The adaptor protein FAN (Factor associated with neutral sphingomyelinase activity) binds to the TNF receptor and plays a role in leukocyte interstitial directed migration in response to wounds and bacterial infections in zebrafish. FAN knockdown results in disorganized pseudopod generation in leukocytes, leading to impaired navigational capacity [55]. FAN mediates TNF-induced Cdc42 activation and actin reorganization in vitro [56]. WASP is one of the effectors of Cdc42, and WASP depletion impairs neutrophil and macrophage wound responses in zebrafish, similar to FAN knockdown [57].
In a zebrafish infection model, zCxcl8 (also known as IL-8) expression is induced upon local Escherichia coli infection. A zCxcl8 tissue-bound gradient is established by binding to heparan sulfate proteoglycans on the tissue surface. This zCxcl8 gradient mediates neutrophil directional bias by altering the relationship between cell speed and directionality [58]. DCs have been shown to use a similar strategy for interstitial migration in mice. The chemokine CCL21 generated on the lymphatic endothelium forms a tissue-bound gradient, which guides DCs to migrate from dermal tissue into afferent lymphatic vessels [59]. Antigen-engaged B cells in mice also sense the increasing CCL21 gradients from the follicle zone to the B-zone-T-zone boundary for directional migration, where early B-cell-T-cell interactions occur. This CCL21 gradient and its G-protein coupled receptor CCR7 are required for B cell directional migration [60].
The hierarchy and integration of signaling that govern leukocyte chemotaxis in vivo is further illustrated in several sterile inflammation models in which inflammation occurs in the absence of any microorganisms. In a sterile skin inflammation model in mice, a three-step cascade of neutrophil responses can occur with 1) initial scouting neutrophils followed by 2) waves of arriving neutrophils (amplification phase) and subsequently 3) stabilization of neutrophils at injured tissues. The involvement of GPCRs in initial recruitment is followed by a role for cyclic ADP ribose in the amplification phase [61]. In another mouse sterile skin inflammation model, pericytes that wrap around the capillaries regulate leukocyte interstitial migration through their effects on leukocyte responsiveness to interstitial migratory cues and their effector functions [62]. Upon local sterile inflammation, proteoglycan NG2+ pericytes increase ICAM-1 expression and secrete the chemoattractant macrophage migration-inhibitory factor (MIF) that increases myeloid cell migration. It is suggested that the NG2+ pericytes form a ‘highway’ for the rapid migration of extravasted leukocytes. By contrast, in a hepatic sterile inflammation mouse model, leukocyte chemotaxis is guided within an intravascular ‘highway’ instead of the extravascular space mediated by pericytes [63]. In this model, a multi-step process of directional cues guide neutrophils to sites of sterile inflammation with neutrophil chemotaxis in the vasculature directed by macrophage inflammatory protein 2 (MIP-2) gradients. Closer to the tissue injury, formyl peptides released from necrotic cells serve as end-target chemoattractants. Different insults, therefore, can trigger distinct signaling cascades that mediate leukocyte recruitment. It appears that leukocytes can utilize a variety of chemotactic signaling pathways for directional migration in response to tissue damage that is context dependent.
The requirement for proteases in interstitial leukocyte motility is less clear. In 3D collagen matrices, the tyrosine-kinase collagen receptor discoidin domain receptor 2 regulates neutrophil chemotaxis through the induction of metalloproteinase secretion and the subsequent generation of collagen-derived chemotactic peptides [64]. It has been shown that knockdown of Mmp13 metalloproteinase decreases macrophage wound recruitment in zebrafish, indicating that protease activity is required for proper leukocyte interstitial migration [42]. In addition, migrating neutrophils in zebrafish can deform the collagen matrix, suggesting the involvement of proteases in interstitial migration [65].
Interstitial Leukocyte arrest
Localized leukocyte arrest is also a key component of interstitial leukocyte function. It limits leukocyte motility to regions where the effector immune function is needed. This is perhaps best demonstrated in the context of sequestered zones of leukocyte function in lymph nodes that are guided by specific chemokine signatures (reviewed in [66]). Leukocyte arrest/immobilization appears to be an active process that is mediated by specific chemokines and downstream effector pathways (Figure 2A). In support of this idea, there has been extensive work showing an essential role for calcium signaling in T cell arrest, including developing T cells in 3D mouse thymic tissues [67] through intracellular Ca2+ flux mediated by calcium release-activated calcium (CRAC) channels, such as Orai (reviewed in [68]). Evidence suggests that intracellular calcium influx through CRAC channels also mediates neutrophil arrest (reviewed in [69]), likely downstream of chemokine signaling. During tissue injury in zebrafish, the chemokine Cxcl8 has been implicated in mediating neutrophil retention [58]. Deficiencies in Cxcl8 result in decreased neutrophil retention at sites of infection. CXCR4-Rac2 signaling is also required for neutrophil retention in hematopoietic tissues in zebrafish [32]. Lack of Rac2 function results in decreased neutrophil motility but increased neutrophil release into the circulation, suggesting that retention is an active process mediated by Rac signaling. Stromal cell-derived factor 1 (SDF1, CXCL12) has also been implicated in the retention of leukocytes in hematopoietic tissue in zebrafish [70]. These observations suggest that leukocyte arrest in interstitial tissues, including wounds and hematopoietic tissues, is an active process. The regulation of leukocyte mobility versus arrest allows leukocytes to perform proper immune functions at the right place and at the right time, and is a fundamental part of leukocyte directional decision making during leukocyte interstitial motility.
Conclusion
Advances in multi-photon vital imaging technology and new model systems such as the zebrafish, have made it possible to study interstitial migration with higher resolution in vivo. What is becoming increasingly clear, however, is that although much of the knowledge acquired from in vitro studies can apply to the in vivo environment, not all mechanisms are similar. Therefore, future challenges will be to apply and combine both in vitro and in vivo analyses to increase our understanding of leukocyte interstitial migration and how responses are prioritized to competing extracellular cues.
Acknowledgments
This work was supported by the National Institutes of Health [grant number GM074827] to AH. We thank our lab members for their critical comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notarangelo LD, Badolato R. Leukocyte trafficking in primary immunodeficiencies. J Leukoc Biol. 2009;85:335–343. doi: 10.1189/jlb.0808474. [DOI] [PubMed] [Google Scholar]
- 3.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 4.Fauvarque MO, Williams MJ. Drosophila cellular immunity: a story of migration and adhesion. J Cell Sci. 2011;124:1373–1382. doi: 10.1242/jcs.064592. [DOI] [PubMed] [Google Scholar]
- 5.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 7.Friedman RS, Jacobelli J, Krummel MF. Mechanisms of T cell motility and arrest: deciphering the relationship between intra- and extracellular determinants. Semin Immunol. 2005;17:387–399. doi: 10.1016/j.smim.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Insall RH. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat Rev Mol Cell Biol. 2010;11:453–458. doi: 10.1038/nrm2905. [DOI] [PubMed] [Google Scholar]
- 9.Van Haastert PJ. Chemotaxis: insights from the extending pseudopod. J Cell Sci. 2010;123:3031–3037. doi: 10.1242/jcs.071118. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt S, Friedl P. Interstitial cell migration: integrin-dependent and alternative adhesion mechanisms. Cell Tissue Res. 2010;339:83–92. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vestweber D. Novel insights into leukocyte extravasation. Curr Opin Hematol. 2012;19:212–217. doi: 10.1097/MOH.0b013e3283523e78. [DOI] [PubMed] [Google Scholar]
- 12*.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. This study is the first to show that integrins are required in 2D but not during interstitial migration. DCs depleted of all integrin receptors can still migrate in interstitial tissues. [DOI] [PubMed] [Google Scholar]
- 13.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Janetopoulos C, Jin T, Devreotes P. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science. 2001;291:2408–2411. doi: 10.1126/science.1055835. [DOI] [PubMed] [Google Scholar]
- 15.Kimmel AR, Parent CA. The signal to move: D. discoideum go orienteering. Science. 2003;300:1525–1527. doi: 10.1126/science.1085439. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya M, Babwah AV, Ferguson SS. Small GTP-binding protein-coupled receptors. Biochem Soc Trans. 2004;32:1040–1044. doi: 10.1042/BST0321040. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 20.Deng Q, Harvie EA, Huttenlocher A. Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell Microbiol. 2012;14:517–528. doi: 10.1111/j.1462-5822.2011.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam PY, Yoo SK, Green JM, Huttenlocher A. The SH2-domain-containing inositol 5-phosphatase (SHIP) limits the motility of neutrophils and their recruitment to wounds in zebrafish. J Cell Sci. 2012;125:4973–4978. doi: 10.1242/jcs.106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol Cell Biol. 2005;25:4211–4220. doi: 10.1128/MCB.25.10.4211-4220.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Sasaki T, Kozieradzki I, Wakeham A, Itie A, Dumont DJ, Penninger JM. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 1999;13:786–791. doi: 10.1101/gad.13.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helgason CD, Damen JE, Rosten P, Grewal R, Sorensen P, Chappel SM, Borowski A, Jirik F, Krystal G, Humphries RK. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–1620. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charest PG, Firtel RA. Big roles for small GTPases in the control of directed cell movement. Biochem J. 2007;401:377–390. doi: 10.1042/BJ20061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parent CA. Making all the right moves: chemotaxis in neutrophils and Dictyostelium. Curr Opin Cell Biol. 2004;16:4–13. doi: 10.1016/j.ceb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Ebisuno Y, Katagiri K, Katakai T, Ueda Y, Nemoto T, Inada H, Nabekura J, Okada T, Kannagi R, Tanaka T, et al. Rap1 controls lymphocyte adhesion cascade and interstitial migration within lymph nodes in RAPL-dependent and - independent manners. Blood. 2010;115:804–814. doi: 10.1182/blood-2009-03-211979. [DOI] [PubMed] [Google Scholar]
- 28.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 29.Katagiri K, Katakai T, Ebisuno Y, Ueda Y, Okada T, Kinashi T. Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J. 2009;28:1319–1331. doi: 10.1038/emboj.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun CX, Downey GP, Zhu F, Koh AL, Thang H, Glogauer M. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. doi: 10.1182/blood-2004-03-0781. [DOI] [PubMed] [Google Scholar]
- 32.Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev Cell. 2011;21:735–745. doi: 10.1016/j.devcel.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi MD. Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules. Blood. 2012;120:3563–3574. doi: 10.1182/blood-2012-04-426981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammermann T, Renkawitz J, Wu X, Hirsch K, Brakebusch C, Sixt M. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood. 2009;113:5703–5710. doi: 10.1182/blood-2008-11-191882. [DOI] [PubMed] [Google Scholar]
- 36.Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. 2012;119:4451–4461. doi: 10.1182/blood-2012-01-407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitschke M, Aebischer D, Abadier M, Haener S, Lucic M, Vigl B, Luche H, Fehling HJ, Biehlmaier O, Lyck R, et al. Differential requirement for ROCK in dendritic cell migration within lymphatic capillaries in steady-state and inflammation. Blood. 2012;120:2249–2258. doi: 10.1182/blood-2012-03-417923. [DOI] [PubMed] [Google Scholar]
- 38.Aomatsu K, Kato T, Fujita H, Hato F, Oshitani N, Kamata N, Tamura T, Arakawa T, Kitagawa S. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology. 2008;123:171–180. doi: 10.1111/j.1365-2567.2007.02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Ilasaca M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol. 1998;56:269–277. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 40.Cara DC, Kaur J, Forster M, McCafferty DM, Kubes P. Role of p38 mitogenactivated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J Immunol. 2001;167:6552–6558. doi: 10.4049/jimmunol.167.11.6552. [DOI] [PubMed] [Google Scholar]
- 41.Taylor HB, Liepe J, Barthen C, Bugeon L, Huvet M, Kirk PD, Brown SB, Lamb JR, Stumpf MP, Dallman MJ. P38 and JNK have opposing effects on persistence of in vivo leukocyte migration in zebrafish. Immunol Cell Biol. 2012;91:60–69. doi: 10.1038/icb.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Bai XT, Zhu KY, Jin Y, Deng M, Le HY, Fu YF, Chen Y, Zhu J, Look AT, et al. In vivo interstitial migration of primitive macrophages mediated by JNK-matrix metalloproteinase 13 signaling in response to acute injury. J Immunol. 2008;181:2155–2164. doi: 10.4049/jimmunol.181.3.2155. [DOI] [PubMed] [Google Scholar]
- 43**.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature. 2011;480:109–112. doi: 10.1038/nature10632. The recruitment of leukocytes to wounds is mediated by a gradient of hydrogen peroxide (Niethammer et al). This study in zebrafish identifies the Src family kinase Lyn as a redox sensor that mediates initial neutrophil recruitment to wounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niggli V. Microtubule-disruption-induced and chemotactic-peptide-induced migration of human neutrophils: implications for differential sets of signalling pathways. J Cell Sci. 2003;116:813–822. doi: 10.1242/jcs.00306. [DOI] [PubMed] [Google Scholar]
- 45.Xu J, Wang F, Van Keymeulen A, Rentel M, Bourne HR. Neutrophil microtubules suppress polarity and enhance directional migration. Proc Natl Acad Sci U S A. 2005;102:6884–6889. doi: 10.1073/pnas.0502106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo SK, Lam PY, Eichelberg MR, Zasadil L, Bement WM, Huttenlocher A. The role of microtubules in neutrophil polarity and migration in live zebrafish. J Cell Sci. 2012;125:5702–5710. doi: 10.1242/jcs.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redd MJ, Kelly G, Dunn G, Way M, Martin P. Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil Cytoskeleton. 2006;63:415–422. doi: 10.1002/cm.20133. [DOI] [PubMed] [Google Scholar]
- 48.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 49.Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19:2147–2153. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 51**.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. This study in zebrafish is the first observation of hydrogen peroxide as a paracrine signal to induce leukocyte recruitment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8:1–19. doi: 10.1371/journal.pbio.1000562. This study demonstrated parallels between leukocyte recruitment to wounds and transformed cells. Similar to the hydrogen peroxide-induced neutrophil recruitment to wounds (Niethammer et al), hydrogen peroxide mediates neutrophil recruitment to transformed cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pressley ME, Phelan PE, 3rd, Witten PE, Mellon MT, Kim CH. Pathogenesis and inflammatory response to Edwardsiella tarda infection in the zebrafish. Dev Comp Immunol. 2005;29:501–513. doi: 10.1016/j.dci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Yan B, Shi YQ, Zhang WQ, Wen ZL. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J Biol Chem. 2012;287:25353–25360. doi: 10.1074/jbc.M112.349126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boecke A, Sieger D, Neacsu CD, Kashkar H, Kronke M. Factor associated with neutral sphingomyelinase activity mediates navigational capacity of leukocytes responding to wounds and infection: live imaging studies in zebrafish larvae. J Immunol. 2012;189:1559–1566. doi: 10.4049/jimmunol.1102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haubert D, Gharib N, Rivero F, Wiegmann K, Hosel M, Kronke M, Kashkar H. PtdIns(4,5)P-restricted plasma membrane localization of FAN is involved in TNF-induced actin reorganization. EMBO J. 2007;26:3308–3321. doi: 10.1038/sj.emboj.7601778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cvejic A, Hall C, Bak-Maier M, Flores MV, Crosier P, Redd MJ, Martin P. Analysis of WASp function during the wound inflammatory response--live-imaging studies in zebrafish larvae. J Cell Sci. 2008;121:3196–3206. doi: 10.1242/jcs.032235. [DOI] [PubMed] [Google Scholar]
- 58**.Sarris M, Masson JB, Maurin D, Van der Aa LM, Boudinot P, Lortat-Jacob H, Herbomel P. Inflammatory Chemokines Direct and Restrict Leukocyte Migration within Live Tissues as Glycan-Bound Gradients. Curr Biol. 2012;22:2375–2382. doi: 10.1016/j.cub.2012.11.018. This study in zebrafish shows that a zCxcl8 tissue-bound gradient established upon local bacterial infection mediates neutrophil recruitment and retention at sites of infection. This paper together with (Weber et al) demonstrated a functional haptotactic gradient in vivo for leukocyte directed migration. [DOI] [PubMed] [Google Scholar]
- 59**.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–332. doi: 10.1126/science.1228456. This study in mice demonstrated that tissue-bound chemokine CCL21 gradients produced by lymphatic endothelium guide dendritic cell directional migration to the lymphatic vessels. This paper together with (Sarris et al) demonstrated a functional haptotactic gradient in vivo for leukocyte directed migration. [DOI] [PubMed] [Google Scholar]
- 60.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:1047–1061. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61*.Ng LG, Qin JS, Roediger B, Wang Y, Jain R, Cavanagh LL, Smith AL, Jones CA, de Veer M, Grimbaldeston MA, et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J Invest Dermatol. 2011;131:2058–2068. doi: 10.1038/jid.2011.179. This study utilizes intravital multiphoton microscopy to uncover the three-step cascade of neutrophil attraction (scouting, amplification and stabilization phase) in response to sterile tissue injury. [DOI] [PubMed] [Google Scholar]
- 62*.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. This study uses a local sterile skin inflammation model to show that proteoglycan NG2+ pericytes increase ICAM-1 expression and secrete the chemoattractant MIF (macrophage migration-inhibitory factor) leading to increased myeloid cell migration. The contact of NG2+ pericytes and myeloid cells drives the increased expression of Toll-like receptors, integrins and matrix metalloproteinases in myeloid cells. As a result, myeloid cells become more efficient in directional migration through interstitial space along capillaries and arterioles toward the inflammatory foci. [DOI] [PubMed] [Google Scholar]
- 63*.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. This study uses a local hepatic sterile inflammation model to show that neutrophils migrate into the necrotic site through the vasculature instead of transmigrating out of the vasculature and migrating through the shorter interstitial path. Neutrophil chemotaxis is directed by a macrophage inflammatory protein 2 (MIP-2) and formyl peptides released from necrotic cells. Interestingly, ATP released from necrotic cells does not serve as a chemotactic signal. [DOI] [PubMed] [Google Scholar]
- 64*.Afonso PV, McCann CP, Kapnick SM, Parent CA. Discoidin domain receptor 2 regulates neutrophil chemotaxis in 3D collagen matrices. Blood. 2012;121:1644–1650. doi: 10.1182/blood-2012-08-451575. This study shows that the discoidin domain receptor 2 (DDR2) mediates persistence of 3D neutrophil motility through increased metalloproteinase secretion and generation of collagen-derived chemotactic peptide gradients, independent of its adhesive function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He S, Lamers GE, Beenakker JW, Cui C, Ghotra VP, Danen EH, Meijer AH, Spaink HP, Snaar-Jagalska BE. Neutrophil-mediated experimental metastasis is enhanced by VEGFR inhibition in a zebrafish xenograft model. J Pathol. 2012;227:431–445. doi: 10.1002/path.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 67.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 68.Shaw PJ, Qu B, Hoth M, Feske S. Molecular regulation of CRAC channels and their role in lymphocyte function. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dixit N, Simon SI. Chemokines, selectins and intracellular calcium flux: temporal and spatial cues for leukocyte arrest. Front Immunol. 2012;3:188. doi: 10.3389/fimmu.2012.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng Q, Sarris M, Bennin DA, Green JM, Herbomel P, Huttenlocher A. Localized bacterial infection induces systemic activation of neutrophils through Cxcr2 signaling in zebrafish. J Leukoc Biol. 2013;93:761–769. doi: 10.1189/jlb.1012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]