Abstract
Background
Epithelial permeability is highly dependent upon the integrity of tight junctions, cell-cell adhesion complexes located at the apical aspect of the lateral membrane of polarized epithelial cells. We hypothesize that sinonasal epithelial exposure to Der p 1 house dust mite antigen decreases expression of tight junction proteins (TJPs), representing a potential mechanism for increased permeability and presentation of antigens across the sinonasal epithelial layer.
Methods
Confluent cultured primary human sinonasal epithelial cells were exposed to recombinant Der p 1 antigen versus control, and transepithelial resistance measurements were performed over 24 hours. Antibody staining for a panel of tight junction proteins was examined with immunofluorescence/confocal microscopy and Western blotting. Tissue for these experiments was obtained from 4 patients total.
Results
Der p 1 exposed sinonasal cells showed a marked decrease in transepithelial resistance when compared to control cells. In addition, results of Western immunoblot and immunofluorescent labeling demonstrated decreased expression of TJPs claudin-1 and junction adhesion molecule-A (JAM-A) in Der p 1 exposed cultured sinonasal cells versus controls.
Conclusion
Der p 1 antigen exposure decreases sinonasal epithelium TJP expression, most notably seen in JAM-A and claudin-1 in these preliminary experiments. This decreased TJP expression likely contributes to increased epithelial permeability and represents a potential mechanism for transepithelial antigen exposure in allergic rhinitis.
Keywords: House dust mite, rhinitis, tight junction, allergic rhinitis, epithelium, sinonasal, junction adhesion molecule, claudin-1
Introduction
Sensitization to allergens such as house dust mite (HDM) has a primary role in the development of conditions such as allergic rhinitis and asthma.1,2,3 The HDM is a complex organism which possesses various proteins than can induce IgE antibodies in allergic individuals.4 Of these potentially allergenic proteins, Der p 1 was the first major HDM allergen described.5 Der p 1 is a cysteine protease whose enzymatic activity is believed to be relevant in the development of allergic sensitization.6 In its native form Der p 1 is an inactive pro-enzyme that gains activity after cleavage of its pro-peptide.6,7 The exact mechanism that triggers the conversion from a pro-enzyme to active enzyme is not clearly understood, although it appears that the pro-domain behaves an inhibitor of the Der p 1 enzyme and competitive proteolysis leads to extensive degradation of this pro-piece, even by low concentrations of Der p 1.8,9 When HDM allergens come in contact with the sinonasal epithelial barrier, they must traverse the epithelium in order to interact with antigen presenting cells.1 Because of this, understanding how allergens cross the mucosal barrier is a key step in the pathophysiology of allergic rhinitis and asthma; however, the exact mechanism by which this occurs remains unknown.
Tight junctions (TJs) are transmembrane protein complexes located at the apical surface of the lateral membrane of polarized epithelial cells and are involved in the regulation of paracellular transport.10 Dysfunction of TJ proteins has been linked to multiple diseases such as inflammatory bowel disease, multiple sclerosis, and cystic fibrosis.10 Prior TJ research has evaluated the interaction of environmental antigens with lower airway epithelia.11–13 These studies have shown that in vitro exposure of human bronchial epithelial cell lines to house dust mite and pollen antigens decreases TJ expression or directly disrupts epithelial TJs. This suggests a mechanism for allergen antigen delivery across the respiratory epithelium, thus contributing to the development of antigen sensitization and allergic responses to antigens. TJ alterations in lower airway epithelium upon antigen exposure may also contribute to permeability and inflammatory changes associated with allergic asthma.
Compared to the existing studies on the effect of allergens on the lower airway epithelial TJs, little to no research has been performed examining TJ alterations in the sinonasal epithelia upon allergen exposure.14 The aim of our study was to evaluate the effect of Der p 1 antigen exposure on sinonasal TJ function and expression. Our hypothesis is that sinonasal cells exposed to Der p 1 will demonstrate increased epithelial permeability 15 and decreased expression of specific TJ proteins.
Materials and Methods
Human tissue collection
This study was performed with the approval of the Emory University Institutional Review Board. Following written informed consent, patients undergoing endoscopic transnasal surgical approaches to orbital or skull base pathology underwent biopsy of sinonasal mucosa for primary epithelial cell culture. Inclusion criteria for study participants were: 18 years of age or older, and no clinical or radiographic evidence of inflammatory rhinosinusitis. Tissue for this study was not collected from patients with cystic fibrosis, immune deficiency, autoimmune conditions, granulomatous disorders, aspirin-exacerbated respiratory conditions, chronic use of nasal or systemic steroids for other vulnerable populations. Of note, patients on baseline hydrocortisone replacement for pre-operative hypopituitarism were not excluded from tissue collection. Primary sinonasal epithelial cultures described in this study were conducted using tissue from a total of 4 patients.
Sinonasal Culture at the Air-Liquid Interface (ALI)
Sinonasal biopsies were minced into ~2 mm3 pieces and placed into phenol-free RPMI 1640 media (Invitrogen, Carlsbad, CA) with antibiotic/antimycotic (Invitrogen, Carlsbad, CA) at 2X concentration. Tissue was digested with type XIV protease isolated from Streptococcus griseus (Sigma-Aldrich, St. Louis, MO) for 2 hours at 37°C. The reaction was stopped with heat inactivated fetal bovine serum. Supernatant was mechanically separated from large tissue pieces and centrifuged (5 minutes, 950 rpm), and the cell pellet was resuspended in Bronchial Epithelial Growth Medium (BEGM): Bronchial Epithelial Basal Medium (BEBM) and BEBM SingleQuot additives (Lonza, Walkersville, MD) with antibiotic/antimycotic (Invitrogen, Carlsbad, CA) and nystatin (Sigma-Aldrich, St. Louis, MO). Epithelial cells were then separated from fibroblasts by incubating in a collagen coated P100 tissue culture treated petri dish at 37°C for 2 hours. Supernatant containing sinonasal epithelial cells was transferred to collagen-coated T75 cell culture flasks (Corning, Corning, NY) and grown in BEGM at 37°C, 5%CO2, 95% humidity. BEGM media was changed every 48–72 hours.
Once the cell layer reached 85% confluency, the cells were washed with Hanks Balanced Salt Solution without Mg2+ or Ca2+ (Sigma-Aldrich, St. Louis, MO) and trypsinized with 1X Trypsin-EDTA (Invitrogen,, Carlsbad, CA). The trypsin reaction was stopped with soybean trypsin inhibitor, the cells were centrifuged (5 minutes, 950 rpm) and resuspended in BEGM. Cell suspensions were plated onto collagen coated Transwell inserts of 6.5 or 24 mm diameter (0.4 μm pore size, Corning Incorporated, Corning, NY).
Cells were grown to confluence with BEGM on the apical and basal surfaces. Again, media was changed every 48–72 hours. Once confluence was confirmed by light microscopy, media was removed from the apical surface and basal media was changed to air-liquid interface (ALI) media. ALI media consisted of a 50:50 mixture of BEBM (Lonza, Basel, Switzerland) and DMEM high glucose (Invitrogen, Carlsbad, CA), along with BEBM SingleQuots, antibiotic/antimycotic, retinol, and bovine serum albumin (Sigma-Aldrich, St. Louis, MO). Cells layers were grown until full polarization and differentiation at the air-liquid interface, which was confirmed by evidence of beating cilia under phase-contrast light microscopy. Once cell differentiation and beating cilia were confirmed, the cells were allowed to continue to grow and stabilize for at least 7–10 days prior to being used in the described experiments.
Preparation of Der p 1 and exposure to sinonasal ALI cultures
Fresh frozen recombinant Der p 1 house dust mite antigen (Indoor Biotechnologies, Charlottesville, VA) was activated with 5mM dithiothreitol (DTT, Fisher Scientific, Fair Lawn, NJ) for 20 minutes at room temperature. DTT is a reducing agent that activates the enzymatic activity of Der p 1. Cells were then incubated with 400nM Der p 1 + 250μM DTT (experimental group) or with 250μM DTT (control group), in the basolateral media chamber for the indicated time points.
Epithelial barrier permeability measurements
Transepithelial electrical resistance (TER) was measured as an indicator of epithelial barrier integrity using an EVOMX voltometer (World Precision Instruments, Sarasota, FL). Resistance measurements were taken at the indicated time points after alcohol sterilization and drying of the electrodes.
Antibodies and other reagents
The following antibodies were used to evaluate a panel of TJ and adherens junction proteins: anti-occludin and anti-claudin-1 (Invitrogen, Carlsbad, CA), anti-E-cadherin (Sigma-Aldrich, St. Louis, MO). The murine monoclonal antibody to JAM-A used in this study was described previously.16 Alexa-488 or Alexa-546 conjugated donkey anti-rabbit and goat anti-mouse secondary antibodies were obtained from Invitrogen (Carlsbad, CA); horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse secondary antibodies were obtained from Jackson Immunoresearch Laboratories. All other immunofluorescent staining and Western blot reagents were of the highest analytical grade and obtained from Sigma-Aldrich (St. Louis, MO).
Immunofluorescent Labeling and Confocal Microscopy
Immunofluorescence labeling and confocal laser microscopic evaluation was used to examine tight and adherens junction protein expression. Samples were fixed and permeabilized in 100% ethanol for 20 minutes at −20°C. All subsequent steps were performed at room temperature. Samples were washed with Hanks Balanced Salt Solution with Mg2+ and Ca2+ (HBSS+) and blocked in HBSS+ with 3% bovine serum albumin (BSA) for 1 hour. Primary antibodies were diluted in blocking buffer and added to samples for 1 hour. Primary antibody concentrations were as follows: anti-claudin-1 (1:250), anti-occludin (1:500), anti-JAM-A (1:100), and anti-E-cadherin (1:100). These concentrations have been previously determined in our laboratory to be the optimal concentration for visualization by confocal microscopy. Samples were washed in HBSS+ and incubated with Alexa Fluor secondary antibodies diluted 1:500 in blocking buffer for 1 hour. Samples were again washed with HBSS+ and incubated with Topro-3 iodide (diluted 1:1000) for 5 minutes. After a final wash, samples were mounted on slides with p-phenylenediamine anti-quench reagent and sealed. Stained cell monolayers and tissue sections were examined using a Zeiss LSM510 laser scanning confocal microscope (Zeiss Microimaging Inc., Thornwood, NY) coupled to a Zeiss 100M Axiovert with a 63× Pan-Apochromat oil lens. The fluorescent dyes were imaged sequentially in frame-interlace mode to eliminate cross talk between channels. Images were processed using Zeiss LSM5 image browser software and Adobe Photoshop (San Jose, CA).
Cell Lysis and Immunoblotting
Cell monolayers were washed twice with HBSS+ to remove media and debris before being scraped into RIPA buffer (20 mM Tris, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Na deoxycholate, 1% Triton X-100, 0.1% SDS, pH 7.4) with a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were sonicated on ice, and rotated for 30 minutes to ensure complete cell lysis. Post-nuclear supernatants (1,000 ×g, 5 minutes centrifugation) were normalized for protein concentration using a commercial bicinchoninic acid assay (Thermo Fisher Scientific, Waltham, MA). Lysates were boiled in reducing SDS sample buffer for 10 minutes, and equal amounts of protein were loaded onto SDS-polyacrylamide gels for standard Western blot analysis.
Results
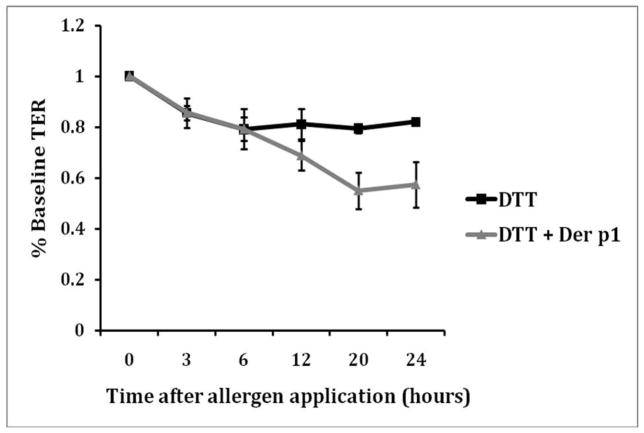
Cultured sinonasal cells exposed to Der p 1 + DTT showed a marked decrease in TER compared to controls cells (Figure 1). At the 12 hour time point, the TER in the control cells was 81% of baseline, compared to 68% in the Der p 1 + DTT group on average. The maximum drop was seen at 24 hours, the final time point measured. The TER was 57% of baseline in the Der p 1 + DTT group. In the control cells, there was no further drop in TER at 24 hours; the level stabilized at 82% of baseline. TER measurements were performed in quadruplicate and repeated to ensure reproducibility.
Figure 1.
Twenty-four hour TER measurements during exposure of cultured sinonasal epithelial cells exposed to Der p 1 + DTT (experimental group) versus cells exposed to DTT only (control). Due to some inherent variability in baseline TER measurements across samples, all measurements were normalized to a value of 1.0.
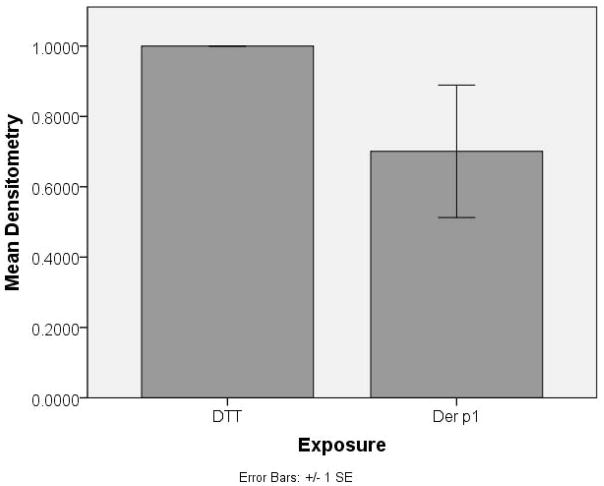
After 24 hrs of exposure to Der p 1 + DTT, image analysis of Western immunoblot results by densitometry revealed a mean decreased expression of tight junction proteins claudin-1 (Figure 2 a and c) and JAM-A (Figure 2 b and c) in cultured sinonasal cells of 70% and 43%, respectively, versus control cells. No changes in protein expression were seen in proteins E-cadherin or occludin. In addition, blots were also probed for poly-ADP ribose polymerase (PARP), as a marker of apoptosis and cell death. There was no significant staining for PARP, indicating no significant apoptosis as a result of Der p 1 or DTT exposure. Western blot immunoblot experiments were performed in a total of 4 cell samples (from 3 patient cultures) to ensure reproducibility.
Figure 2.
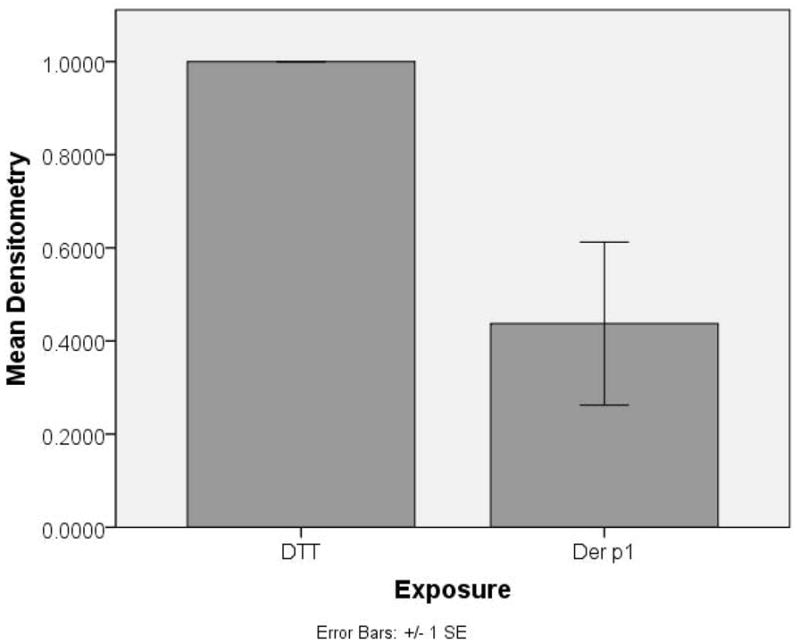
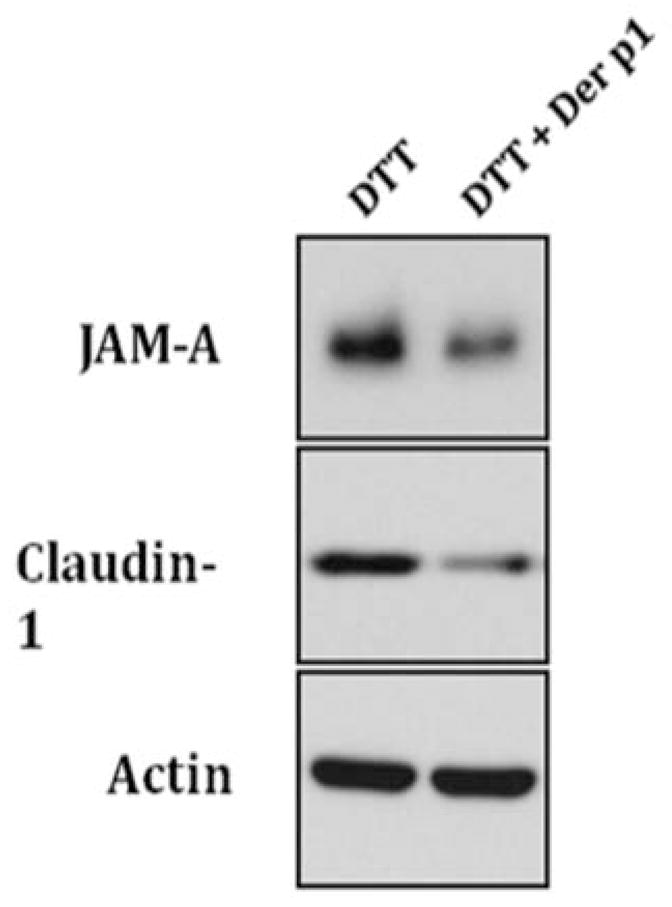
Western immunoblot densitometric analysis graphs for (a) claudin-1 and (b) JAM-A tight junction protein in cultured sinonasal epithelial cells exposed to Der p 1 + DTT (experimental group) and DTT (control) for 24 hours. An example Western immunoblot for claudin-1 and JAM-A, along with the actin protein loading control is also shown (c).
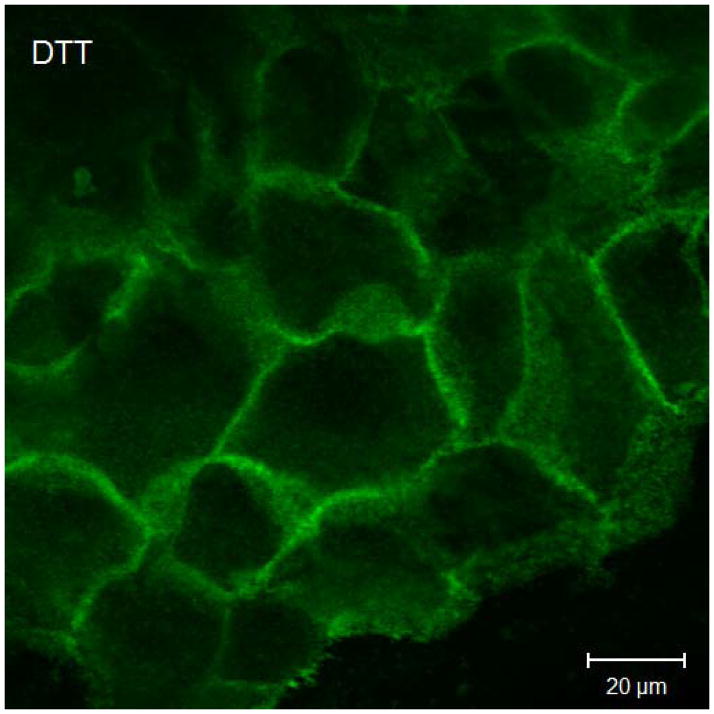
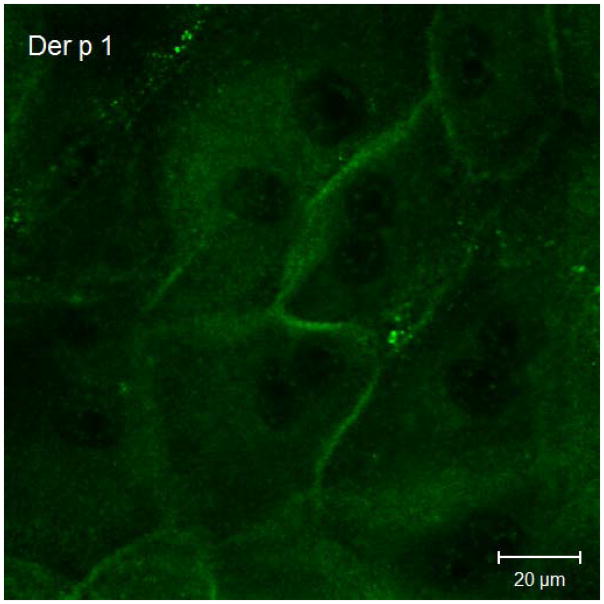
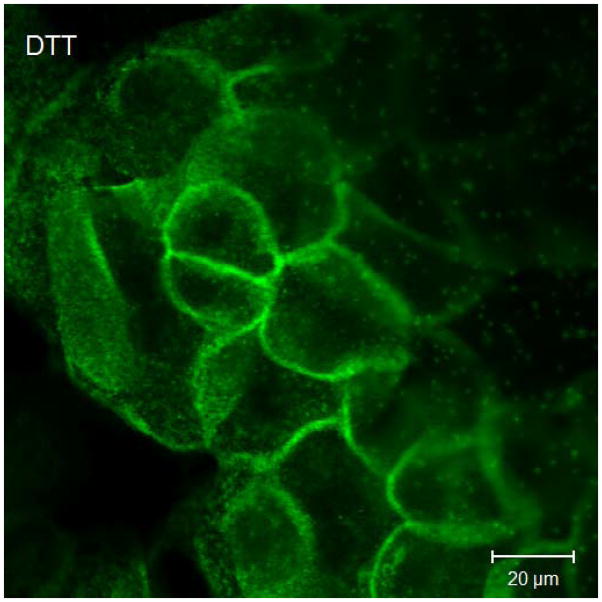
Results of immunofluorescent labeling and confocal microscopy were consistent with the Western immunoblots. Immunofluorescent labeling confirms decreased claudin-1 and JAM-A signaling in cultured sinonasal epithelial cells exposed to Der p 1 + DTT compared to control cells (Figure 3).
Figure 3.
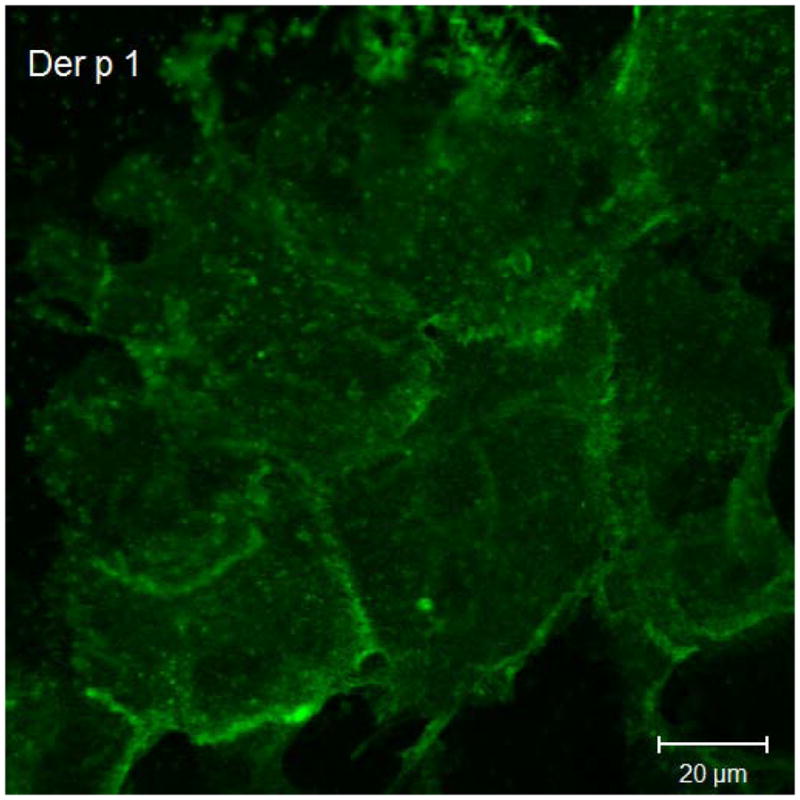
Representative claudin-1 and JAM-A immunofluorescent staining in cultured sinonasal epithelial cells exposed to Der p 1 + DTT versus DTT only. Figure 3a demonstrates claudin-1 staining following exposure to DTT for 24 hours. In this figure, cell borders are easily discernable, with distinct claudin-1 staining. Figure 3b shows claudin-1 staining following Der p 1 + DTT exposure for 24 hours; cell borders are less distinct and claudin-1 staining at cell-cell junctions is significantly diminished. Similarly, in Figure 3c, JAM-A staining following DTT exposure for 24 hours shows distinct cell-cell junctions. JAM-A staining in Figure 3d after Der p 1 + DTT exposure for 24 hours exhibits poorly defined cell-cell junctions.
Discussion
The effect of Der p 1 on TJs in the lower airways has been previously studied13,17,18. However scant research has been performed on the effects of allergen exposure on TJs in the upper airways. This study suggests that the sinonasal epithelium is an important target in the biological response to HDM allergen Der p 1.
Herbert et al. were the first to suggest the importance of Der p 1 cysteine protease activity directed against airway epithelia in an in vitro model.19,20 Bovine bronchial mucosa showed increased permeability 3 hours after apical exposure to Der p 1 and DTT. No effect was seen with Der p 1 in the absence of DTT, suggesting that the permeability defect was a direct result of Der p1 cysteine protease activity.20 Our results showed both a decrease in TER and an accompanying reduction in TJ proteins claudin-1 and JAM-A in the presence of Der p 1 and DTT. This supports the idea that cysteine protease activity may also play a role in the sinonasal epithelial response to Der p 1 antigen.
More recent studies on bronchial epithelial cells seem to correlate in part with our results. Baker et al. showed that Der p 1 causes loss of claudin-1 and occludin from TJ rings in human bronchial epithelial cell cultures.17 The results of this group suggest that Der p 1 may also act by inducing apoptosis, independent of its cysteine protease activity. However, the results of our preliminary study failed to show clear evidence of induced apoptosis in cultured sinonasal cells exposed to Der p 1.
Wan et al. also found that Der p 1 causes cleavage of TJ proteins occludin and ZO-1 which could be prevented by adding a cysteine protease inhibitor. Our preliminary study did not reveal changes in occludin or ZO-1 proteins, but different claudin-1 and JAM-A TJ protein changes were seen on exposure to Der p 1 in our primary cultured sinonasal epithelial cells.
Previous studies have used a preparation of Der p 1 obtained from HDM culture by a laborious approach which includes steps such as ammonium sulfate fractionation, chromatofocusing, and affinity chromatograpy. For our study, we chose to use recombinant Der p 1 (Indoor Biotechnologies, Charlottesville, VA). Multiple studies have demonstrated that when compared with native HDM Der p 1 allergen, recombinant HDM Der p 1 has indistinguishable enzymatic activity and it is suitable for use in studies such as ours9,21. Of note, in our experiments the recombinant Der p 1 antigen and DTT activator was added to the media in the basolateral chamber of the Transwell culture system. We recognize that this does not precisely mimic the in vivo state in which the antigen would come into contact with the ciliated air-exposed surface of the respiratory epithelium, and we acknowledge this is a limitation of our experimental model. However, the time course of epithelial resistance changes indicates that antigen exposure over multiple hours is required to effect a change in epithelial permeability. Exposure of the polarized air-liquid interface sinonasal epithelial culture model to extended periods of apical surface liquid causes significant alterations in cell morphology in our hands. We therefore elected to maintain the culture model morphology and place the antigen on the basolateral surface instead.
The potential role of TJs in the pathophysiology of upper airway diseases such as nasal polyposis and allergic rhinitis has been studied previously in our laboratory22,23. Zuckerman et al. found that expression of desmosomal junction proteins DSG-2 and DSG-3 were significantly decreased in nasal polyp mucosa compared to normal controls23. Similarly, Rogers et al. found decreased expression of TJ proteins claudin-1 and occludin in nasal polyp mucosa22. Our preliminary results presented here suggest that inhaled allergens such as HDM Der p 1 interfere with TJ expression, function, or stability in primary sinonasal epithelial cells in culture. Further studies will allow us to better elicit the precise effect of Der p 1 and other inhalant allergens on sinonasal epithelial junctions.
The proposed modes of action of Der p 1 on the sinonasal epithelium TJs are believed to happen via two possible routes.18 The first proposed mechanism is direct extracellular cleavage of TJs, followed by intracellular processing of the antigen. Another possibility is an indirect route via intracellular cleavage as a result of a receptor transduction pathway or via the interaction of gap junction such as connexin 26, which has been show have a role in the expression and maintenance of TJs and its expression is diminished by Der p 1. 14,15 In either case, the end result appears to be a more permeable epithelial barrier and increased probability for inhaled allergens to interact with antigen-presenting cells.
Conclusion
This preliminary study shows that Der p 1 antigen exposure increases epithelial permeability and decreases TJ proteins JAM-A and claudin-1 in samples of cultured primary sinonasal epithelial cells. These findings suggest that TJ protein alterations may play a critical role in the pathway for inhaled allergen presentation.
Acknowledgments
The authors would like to thank Dr. Porfirio Nava and Dr. Keli Kolegraff for their critical support with immunofluorescence, and Dr. Susan Voss for her assistance in cell culture.
This work was supported in part by the following funding sources:
Research in Otolaryngology & Allergy Development (ROAD) Scholarship Program Grant of the AAOA (O.A.H. and S.K.W.)
American Rhinologic Society New Investigator Award (S.K.W.)
Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resources KL2 RR025009 and UL1 RR025008 (S.K.W.)
Structure function studies in intestinal epithelial JAM, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases DK061379 (C.A.P.)
Neutrophil interactions with intestinal epithelial cells, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, DK072564 (C.A.P.)
Intestinal epithelial tight junction structure-function, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, DK059888 (A.N.)
Short Term Training in Health Professional Schools, National Institutes of Health T35-HL007473 (K.D.B.)
IRB Statement: Approval for this study was granted by the Institutional Review Board of Emory University.
Footnotes
Presented at the annual meeting of the American Academy of Otolaryngic Allergy (AAOA), San Francisco, California, September 9, 2011.
This manuscript and the data within it have not been previously published and are not under consideration for publication in any other journal.
Conflict of Interest Disclosure:
| Author | Potential Conflicts of Interest |
|---|---|
| Oswaldo A. Henriquez, M.D. | None |
| Kyle Den Beste, B.S. | None |
| Elizabeth K. Hoddeson, M.D | None |
| Charles Parkos, M.D. | None |
| Asma Nursat, M.D. | None |
| Sarah K. Wise, M.D. | Arthrocare ENT: Unrestricted Educational Grant |
References
- 1.Holgate ST. The epidemic of allergy and asthma. Nature. 1999;402:B2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 2.Sporik R, Chapman MD, Platts-Mills TA. House dust mite exposure as a cause of asthma. Clin Exp Allergy. 1992;22:897–906. doi: 10.1111/j.1365-2222.1992.tb02062.x. [DOI] [PubMed] [Google Scholar]
- 3.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 4.Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- 5.Chapman MD, Platts-Mills TA. Purification and characterization of the major allergen from Dermatophagoides pteronyssinus-antigen P1. J Immunol. 1980;125:587–92. [PubMed] [Google Scholar]
- 6.Robinson C, Kalsheker NA, Srinivasan N, et al. On the potential significance of the enzymatic activity of mite allergens to immunogenicity. Clues to structure and function revealed by molecular characterization. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 1997;27:10–21. [PubMed] [Google Scholar]
- 7.Meno K, Thorsted PB, Ipsen H, et al. The crystal structure of recombinant proDer p 1, a major house dust mite proteolytic allergen. J Immunol. 2005;175:3835–45. doi: 10.4049/jimmunol.175.6.3835. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Hamilton JM, Garrod DR, Robinson C. Interactions between mature Der p 1 and its free prodomain indicate membership of a new family of C1 peptidases. Allergy. 2007;62:1302–9. doi: 10.1111/j.1398-9995.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Saint-Remy JM, Garrod DR, Robinson C. Comparative enzymology of native and recombinant house dust mite allergen Der p 1. Allergy. 2009;64:469–77. doi: 10.1111/j.1398-9995.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 10.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai HY, Tam MF, Chou H, et al. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–8. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 12.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–42. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 13.Wan H, Winton HL, Soeller C, et al. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. 2000;30:685–98. doi: 10.1046/j.1365-2222.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 14.Zheng J, Liu W, Fan Y, et al. Suppression of connexin 26 is related to protease-activated receptor 2-mediated pathway in patients with allergic rhinitis. American journal of rhinology & allergy. 2012;26:e5–9. doi: 10.2500/ajra.2012.26.3740. [DOI] [PubMed] [Google Scholar]
- 15.Kojima T, Murata M, Go M, Spray DC, Sawada N. Connexins induce and maintain tight junctions in epithelial cells. The Journal of membrane biology. 2007;217:13–9. doi: 10.1007/s00232-007-9021-4. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Nusrat A, Schnell FJ, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113 ( Pt 13):2363–74. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 17.Baker SF, Yin Y, Runswick SK, et al. Peptidase allergen Der p 1 initiates apoptosis of epithelial cells independently of tight junction proteolysis. Mol Membr Biol. 2003;20:71–81. doi: 10.1080/0968768021000061150. [DOI] [PubMed] [Google Scholar]
- 18.Wan H, Winton HL, Soeller C, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104:123–33. doi: 10.1172/JCI5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbert CA, Holgate ST, Robinson C, Thompson PJ, Stewart GA. Effect of mite allergen on permeability of bronchial mucosa. Lancet. 1990;336:1132. doi: 10.1016/0140-6736(90)92611-k. [DOI] [PubMed] [Google Scholar]
- 20.Herbert CA, King CM, Ring PC, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. American journal of respiratory cell and molecular biology. 1995;12:369–78. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
- 21.Best EA, Stedman KE, Bozic CM, et al. A recombinant group 1 house dust mite allergen, rDer f 1, with biological activities similar to those of the native allergen. Protein Expr Purif. 2000;20:462–71. doi: 10.1006/prep.2000.1327. [DOI] [PubMed] [Google Scholar]
- 22.Rogers GA, Beste KD, Parkos CA, Nusrat A, DelGaudio JM, Wise S. Epithelial tight junction alterations in nasal polyposis. International Forum of Allergy & Rhinology. 2011;I:50–4. doi: 10.1002/alr.20014. [DOI] [PubMed] [Google Scholar]
- 23.Zuckerman JD, Lee WY, DelGaudio JM, et al. Pathophysiology of nasal polyposis: the role of desmosomal junctions. Am J Rhinol. 2008;22:589–97. doi: 10.2500/ajr.2008.22.3235. [DOI] [PubMed] [Google Scholar]