Abstract
OBJECTIVE:
The role of Ulinastatin in neuronal injury after cardiopulmonary resuscitation has not been elucidated. We aim to evaluate the effects of Ulinastatin on inflammation, oxidation, and neuronal injury in the cerebral cortex after cardiopulmonary resuscitation.
METHODS:
Ventricular fibrillation was induced in 76 adult male Wistar rats for 6 min, after which cardiopulmonary resuscitation was initiated. After spontaneous circulation returned, the rats were split into two groups: the Ulinastatin 100,000 unit/kg group or the PBS-treated control group. Blood and cerebral cortex samples were obtained and compared at 2, 4, and 8 h after return of spontaneous circulation. The protein levels of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) were assayed using an enzyme-linked immunosorbent assay, and mRNA levels were quantified via real-time polymerase chain reaction. Myeloperoxidase and Malondialdehyde were measured by spectrophotometry. The translocation of nuclear factor-κB p65 was assayed by Western blot. The viable and apoptotic neurons were detected by Nissl and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).
RESULTS:
Ulinastatin treatment decreased plasma levels of TNF-α and IL-6, expression of mRNA, and Myeloperoxidase and Malondialdehyde in the cerebral cortex. In addition, Ulinastatin attenuated the translocation of nuclear factor-κB p65 at 2, 4, and 8 hours after the return of spontaneous circulation. Ulinastatin increased the number of living neurons and decreased TUNEL-positive neuron numbers in the cortex at 72 h after the return of spontaneous circulation.
CONCLUSIONS:
Ulinastatin preserved neuronal survival and inhibited neuron apoptosis after the return of spontaneous circulation in Wistar rats via attenuation of the oxidative stress response and translocation of nuclear factor-κB p65 in the cortex. In addition, Ulinastatin decreased the production of TNF-α, IL-6, Myeloperoxidase, and Malondialdehyde.
Keywords: Cardiopulmonary Resuscitation, Ulinastatin, Oxidative Stress, Inflammatory Response, Neuronal Injury
INTRODUCTION
Patients who have been successfully resuscitated after cardiac arrest (CA) typically have a poor prognosis and a high risk of post-resuscitation disease that shares many features with severe sepsis (1). As early as 3 hours after CA, sharp rises in various cytokines occur in the bloodstream. High levels of cytokines in the bloodstream may contribute to the genesis of tissue damage that can lead to multiple organ dysfunction (1-3). The levels of cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) have deleterious effects on cerebral ischemia and are closely correlated with CA prognosis (3,4). Clinical data indicate that cytokines play a role in ischemic stroke injury and reveal a correlation between cytokine concentrations and neurological outcome (5,6).
Oxidant injury occurs during CA as reactive oxygen species (ROS) increase rapidly after the return of spontaneous circulation (ROSC). It has been shown that ROS stimulate the activation of nuclear factor κB (NF-κB), which is believed to be associated with the rapid transcription of TNF-α and the induction of apoptosis (7,8). NF-κB is translocated from the cytoplasm to the nucleus, and its ratio increases after cerebral ischemia and reperfusion (9), therefore contributing to ischemia-reperfusion injury (10). In contrast, inhibition of NF-κB is associated with neuroprotective effects (11,12); however, it is unclear whether the activation of NF-κB and increased production of inflammatory cytokines play a role in neuronal injury of the brain after ROSC.
Ulinastatin (UTI), a protease inhibitor derived from human urine, has been shown to exhibit organ-protective effects against ischemia-reperfusion injury in the brain (13,14). Previous studies have shown that UTI's protective effect is due to its anti-inflammatory and anti-oxidative stress responses (15,16). UTI reduces the activation of NF-κB and downregulates the expression of its related mediators (17,18). The majority of previous studies investigated the effects of UTI on local organ ischemia-reperfusion injury; however, because CA/cardiopulmonary resuscitation (CPR) CPR is a type of systemic ischemia-reperfusion injury, the role of UTI may be more complicated than what occurs in local organ ischemia-reperfusion injury.
Therefore, in this study, we aimed to evaluate the effects of UTI on the injured cerebral cortex in Wistar rats after CA/CPR and to observe its potential therapeutic effect to provide a scientific basis for its use in the treatment of neuronal injury after ROSC.
MATERIALS AND METHODS
This study was approved by the Animal Investigation Committee of Sun Yat-sen University and was performed in accordance with the Animal Research Reporting In Vivo Experiments guidelines on animal research (19).
Animal preparation
After overnight fasting (with free access to water), 76 healthy male Wistar rats aged 15-16 months and weighing 360.5-416.5 g were anesthetized by intraperitoneal injection of pentobarbital (30 mg/kg; Sigma, Natick, MA, USA). Tracheal intubation was performed under direct vision using indirect illumination to initiate mechanical ventilation (Harvard Rodent Ventilator; Harvard Apparatus, Holliston, MA). Ventilation parameters were adjusted according to arterial blood gas results to maintain the pCO2 in the 35-45 mmHg range.
A 24-Gauge retained needle (BD Intima IITM VeinTM; SuZhou BD Medical Appliance Co., Ltd., SuZhou, P.R. China) was inserted into the right femoral vein to establish a transfusion passage. The right femoral artery was cannulated using a needle filled with a physiological salt solution containing 5 IU/mL of bovine heparin (Jiangsu biochemistry company, Nanjing, China). Arterial blood pressure was continuously measured with a high-sensitivity pressure transducer (Kombidyn Monitoring Set; Braun, Melsungen, Germany). All data were recorded for subsequent analysis using a BIOPAC MP150 data acquisition system (model MP150, version 3.8.1; Goleta, CA).
Induction of ventricular fibrillation
The methods for induction of ventricular fibrillation (VF) were described previously (20-22). Briefly, two acupuncture needles were trans-cutaneously inserted into the epicardium between the fourth rib of the left sternal border and the third rib of the right sternal border. VF was induced by applying an external transthoracic alternating current (50 Hz, 6 V) to the needles for 10 seconds. After successful induction of VF, the ventilator was disconnected. Six minutes after VF, manual chest compression and mechanical ventilation were initiated. Rats were randomly divided into two groups (n = 38 in each group): the VF + PBS group and the VF + UTI group. Animals designated to the VF + UTI group received 100,000 units/kg UTI (Tianpu Biochemistry and Medicine Corporation, Guangzhou, P. R. China) intravenously. The dose of 100,000 units/kg UTI has been used for organ-protective effects in prior studies (23-25). Animals in the VF + PBS group received a volume of PBS equivalent to the volume of UTI received by animals in the VF + UTI group. External chest compression was performed using an external chest compression machine for small animals (developed in our laboratory) at a rate of 200 compressions per min with equal compression-relaxation and a depth of compression equal to one-third of the anteroposterior chest diameter. If resuscitation was not achieved after 2 min of CPR, a single dose of epinephrine at 20 μg/kg was given every 3 min. ROSC was defined as the return of supraventricular rhythm with a mean aortic pressure of 60 mmHg or higher for a minimum of 10 min. If ROSC was not achieved by 15 min after resuscitation, resuscitation efforts were discontinued.
Six rats per group were randomly chosen and were painlessly sacrificed by over-anesthetization 2, 4, and 8 h after ROSC. The brains were removed and the cerebral cortices dissected. Subsequently, samples were flash-frozen in liquid nitrogen and transferred to a -80°C freezer for storage.
Cytokine measurement
Before CPR and at 2, 4, and 8 h after ROSC, blood samples (0.5 ml) were collected in sodium citrate tubes and immediately centrifuged at 1,500 x g for 5 min. The plasma levels of TNF-α and IL-6 were analyzed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Rapidbio; West Hills, CA, USA).
Quantitation of TNF-α and IL-6 concentrations and MPO and MDA activities in the cerebral cortex
Tissue from the cerebral cortex was homogenized, and proteins were extracted using a lysis buffer (Product No. 2355; Cell Signaling Technology, USA). Protein concentrations were determined with a bicinchoninic acid (BCA) assay kit (Bioteke Corporation, Beijing, P. R. China). The tissue concentrations of TNF-α and IL-6 were quantified by enzyme-linked immunosorbent assay (ELISA) as described above. Myeloperoxidase (MPO) activity was determined by measuring the absorbance at 460 nm using a custom assay kit (Institute of Jiancheng Biological Engineering, Nanjing, P. R. China). The Malondialdehyde (MDA) concentration was measured with an MDA detection kit (Institute of Jiancheng Biological Engineering, Nanjing, P. R. China) by diluting the samples 1:10 in assay reagents or in cool normal saline (for an absorbance blank) and measuring the absorbance at 586 nm using a spectrophotometer.
Total RNA extraction, reverse transcription, and quantitative RT-PCR
Total RNA was isolated from the cerebral cortex using Trizol (Invitrogen, Shanghai, P. R. China) according to the manufacturer's instructions. Briefly, 50 mg of cerebral cortex was homogenized in a buffered phenol solution. Nucleic acids were recovered from the lysate by the addition of chloroform (Sigma), centrifugation, and removal of the aqueous layer. Nucleic acids were then precipitated from the aqueous layer by adding absolute ethanol, and the sample was passed through the provided glass fiber filter column by centrifugation. The sample was washed and eluted with 50 μl of RiboPure solution. Next, 1 μl (40 units) of RNase inhibitor (Promega, Madison, WI, USA) was added to each sample. The RNA quality was checked by agarose gel electrophoresis, and 2 μg of total RNA was treated with DNase Turbo (Ambion, Roche, West End, Australia) according to the manufacturer's instructions. After DNase inactivation, cDNA was synthesized by reverse transcription (RT) with/without the SuperScript® III One-Step RT-PCR System with Platinum® Taq High Fidelity (Invitrogen) and 10 μM random hexamer primers. Quantitative real time polymerase chain reaction (qPCR) was performed with an iCycler iQ Real-Time PCR Detection System (Bio-Rad) using SYBR Green I as a double-stranded DNA-specific dye. RT-PCR was performed using the following primer sequences: β-actin-F: GCGCTCGTCGT CGACAAC and β-actin-R: ATACCCACCA TCACACCCT; TNF-α-F: CCACCACGCTCTTCTGTC and TNF-α-R: GCCCATTT GGG AACTTCT; and IL-6-F: TCACAGAGGATACCACCCA and IL-6-R: AGAATTGCCATTGCACAAC. RT-PCR was performed under the following conditions: 50°C for15 min; 95°C for 15 min; 40 cycles of 94°C for 15 sec, 55°C for 45 sec, 72°C for 1 min; and a final 10 min extension at 72°C. RT-PCR was performed at the minimum cycle numbers necessary to observe bands (40 cycles for TNF-α and IL-6; 28 for β-actin) and to avoid saturation of the PCR products (9700 Sequence Detection System and ABI PRISM 310 Genetic Analyzer, Applied Biosystems, USA). The expression of mRNA was quantified as the ratio of 2ΔCt(TNF-α or IL-6)/2ΔCt(β-actin), where ΔCt(TNF-α or IL-6) and ΔCt(β-actin) represent differences between the threshold cycle (Ct) values of the UTI-treated and UTI-untreated RNA samples for TNF-α or IL-6 and β-actin, respectively.
Separation of cytoplasmic and nuclear proteins
Tissue extracts were prepared using CERA cytoplasmic and nuclear extraction reagents (Bioteke Corporation, Beijing, P. R. China) according to the manufacturer's instructions.
Western blot analysis of NF-κB expression
The expression of NF-κB protein in the cytoplasm and nucleus was determined by Western blot analysis. Cytoplasmic and nuclear protein samples containing 50 μg of total protein for each lane were mixed with loading buffer and boiled for 5 min. Samples were then loaded onto gradient sodium dodecyl sulfate polyacrylamide gels, and electrophoresis was performed using a Mini Protean II Dual-Slab Cell (Bio Rad, Hercules CA, USA). Membranes were subsequently incubated with primary rabbit anti-rat NF-κB p65 antibodies (Santa Cruz Biotechnology, CA, USA) at a dilution of 1:500, anti-PCNA (Proteintech Group, Newcastle-upon-Tyne, UK) at a dilution of 1:500, and glyceraldehyde 3-phosphate dehydrogenase (anti-GAPDH) (Sigma-Aldrich Company, St. Louis, MO, USA) at a dilution of 1:1000 overnight at 4°C. Afterwards, membranes were washed for 15 min and incubated with horseradish peroxidase-conjugated secondary polyclonal goat antibodies raised against rat IgG (Sevac, Prague, Czech Republic) at a dilution of 1:1000 for 1 h at room temperature. The membranes were then washed for 15 min in TBST, incubated with chemiluminescence substrate (Applygen Technologies Inc., Beijing, P. R. China), and exposed to radiographic film. Densities at specific molecular weights were measured using National Institutes of Health image analysis software.
Nissl and TUNEL staining
Animals were painlessly sacrificed while under deep anesthesia at 72 h after ROSC. The brains were removed for cryofixation before embedding and sectioning. Afterwards, 4-μm-thick brain sections were cut coronally in the cortex at the temporal lobe level. Nissl staining was performed to count the number of living neurons in the cortex. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was performed using the In Situ Cell Death Detection Kit (Roche Applied Science, Mannheim, Germany). Three sections from each animal and six standardized fields in each section were randomly selected and analyzed. The Nissl- and apoptotic-positive neurons in each cortex section were counted per 400 × 400-pixel area under 40X magnification by light microscopy (OLYMPUS BX51; OLYMPUS IMAGING CORP, Japan) (20).
Statistical analysis
The experimental data were analyzed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). All data were expressed as medians±SD; the Mann-Whitney rank-sum test was used to determine statistical significance between groups. A two-tailed value of p<0.05 was considered statistically significant.
RESULTS
Outcome of the induction of VF and CPR
Alternating current stimulation caused an immediate drop of blood pressure and a loss of pulse in all 76 rats; all animals were successfully resuscitated. Unconscious animals required mechanical ventilation and intravenous glucose infusion for 20-182 min after ROSC. Ten rats in the VF + PBS group and seven rats in the VF + UTI group died of refractory cardiac shock within 24 h. Except for the six rats that were sacrificed 2, 4, and 8 h after ROSC in the two groups, the remaining four rats in the VF + PBS group and the remaining seven rats in the VF + UTI group survived for 72 h. No difference was observed in the survival rate between the two groups (4/10 versus 7/13, p = 0.159). No statistically significant differences were identified between the two groups in physiological variables, including body weight and heart rate, or in CPR-associated parameters, including duration of base life support and time of defibrillation (Table 1).
Table 1.
Physiologic and CPR requisite parameters in the two groups.
| VF + PBS | VF + UTI | p-value | |
| BW (g) | 320 (304, 337) | 318 (304, 327) | 0.419 |
| HR (times/min) | 413 (381, 422) | 381 (370, 422) | 0.215 |
| MAP (mmHg) | 1.40 (1.05, 2.48) | 2.43 (1.48, 2.82) | 0.167 |
| BLS (min) | 83 (71, 94) | 80 (72, 86) | 0.754 |
| DF (times) | 1 (0, 2) | 2 (0, 2) | 0.603 |
| AD dose (μg) | 20.7 (17.7, 27.5) | 19.0 (16.7, 28.5) | 0.351 |
BW: body weight, HR: heart rate, MAP: mean arterial pressure, BLS: basic life support, DF: defibrillation, AD: adrenalin.
UTI decreased the plasma levels of TNF-α and IL-6 after ROSC
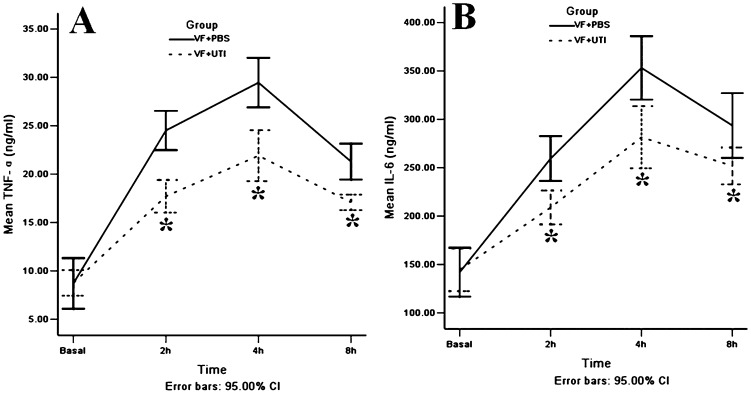
Before VF, no difference was noted in the plasma levels of TNF-α between the VF + PBS and VF + UTI groups; however, UTI treatment significantly decreased plasma levels of TNF-α by 2-, 2.5-, and 2-fold at 2, 4, and 8 h after ROSC, respectively, when compared to the VF + PBS group (Figure 1A); p = 0.009, 0.008, and 0.009, respectively). UTI treatment also significantly reduced plasma levels of IL-6 in the VF + UTI group by 1.5-, 1.2-, and 1.7-fold at 2, 4, and 8 h after ROSC, respectively (Figure 1B); p = 0.009, 0.012, and 0.028, respectively).
Figure 1.
UTI decreased TNF-α and IL-6 plasma levels after ROSC. Treatment with UTI after ROSC effectively decreased TNF-α and IL-6 production in the VF + UTI group; the TNF-α and IL-6 plasma levels were lower in the VF + UTI group than in the VF + PBS group at each time point after ROSC (n = 22 at 2 h, 16 at 4 h, and 10 at 8 h after ROSC in the VF + PBS group; n = 32 at 2 h, 26 at 4 h, and 20 at 8 h after ROSC in VF + UTI group). * VF + PBS versus VF + UTI, p<0.05.
UTI decreased TNF-α, IL-6, and MDA production in the cerebral cortex after ROSC
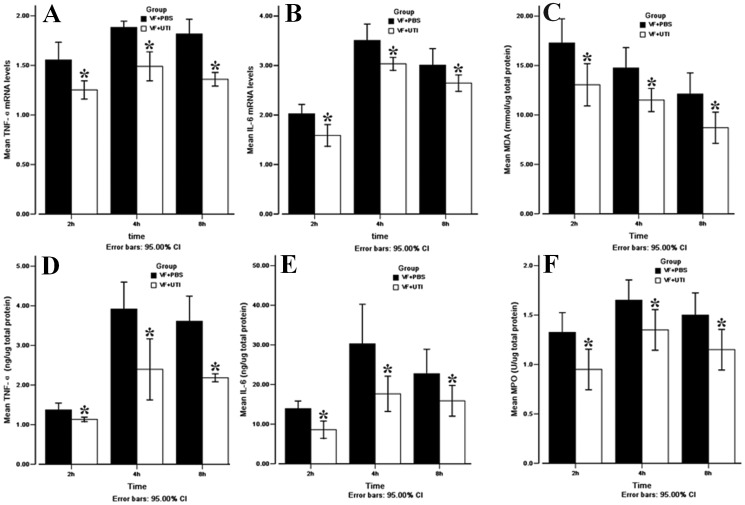
UTI treatment significantly decreased mRNA levels of both TNF-α and IL-6 when compared to the VF + PBS group at 2, 4, and 8 h after ROSC (Figure 2A, B); p = 0.003, <0.001, and <0.001 for TNF-α and p = 0.003, 0.006, and 0.021 for IL-6 at 2, 4, and 8 h after ROSC, respectively). The protein levels of both TNF-α and IL-6 were also lower in the VF + UTI group than in the VF + PBS group (Figures 2D and 2E); p = 0.020, 0.021, and 0.021 for TNF-α and p = 0.023, 0.021, and 0.043 for IL-6 at 2, 4, and 8 h after ROSC, respectively).
Figure 2.
UTI decreased TNF-α and IL-6 levels, MDA production, and MPO activity in the cerebral cortex after ROSC. TNF-α mRNA (A) and IL-6 mRNA (B) expression levels were lower in the VF + UTI group than in the VF + PBS group at each time point. TNF-α (E) and IL-6 (F) protein levels were also lower in the VF + UTI group than in the VF + PBS group. Oxidative stress damage as expressed by the MDA level was more severe in the VF + UTI group than in the VF + PBS group (C). UTI decreased MPO activity in the cerebral cortex in the VF + UTI group after ROSC (F) (n = 6 at each time point in different groups). * VF + PBS versus VF + UTI, p<0.05.
UTI treatment reduced MDA levels at 2, 4, and 8 h after ROSC when compared to the VF + PBS group (p = 0.020, 0.024, and 0.021, respectively; Figure 2C). However, no significant difference was noted in the MDA levels between the two groups prior to VF.
MPO activity was measured at 2, 4, and 8 h after ROSC with and without UTI; UTI treatment decreased neutrophil infiltration when compared to the VF + PBS group at each time point examined (Figure 2F); p = 0.020, 0.029, and 0.020, respectively).
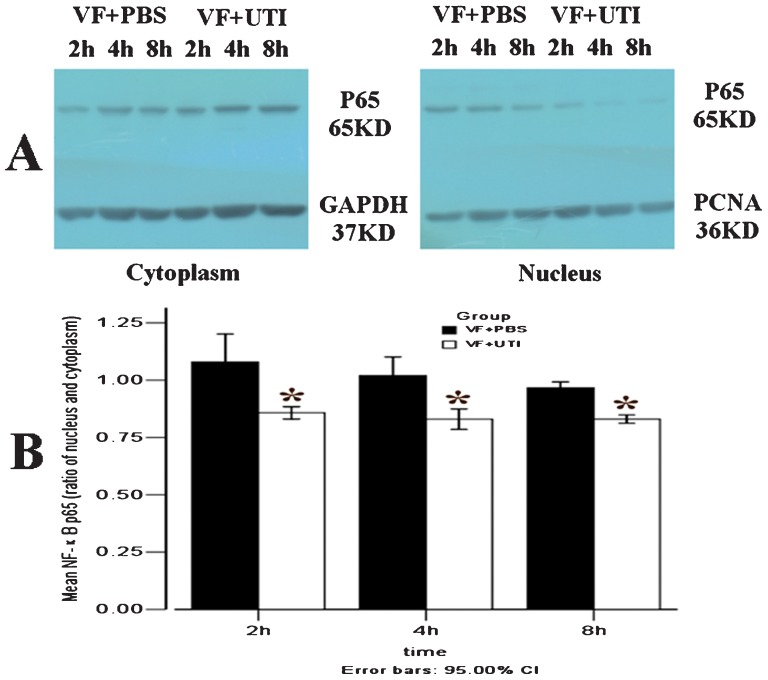
UTI attenuated the translocation of NF-κB p65 from the cytoplasm to the nucleus.
In the VF + PBS group, the ratios of NF-κB p65 translocation were 1.08±0.08, 1.02±0.05, and 0.9±0.02 at 2, 4, and 8 h after ROSC, respectively. The translocation of NF-κB p65 from the cytoplasm to the nucleus was significantly attenuated by UTI at 2, 4, and 8 h after ROSC (Figure 3A, B); p = 0.021, 0.020, and 0.019, respectively).
Figure 3.
UTI attenuated the translocation of NF-κB p65 from the cytoplasm to the nucleus. The ratios of NF-κB p65 translocation in VF + PBS group were 1.08 (1.01, 1.15), 1.02 (0.97, 1.07), and 0.97 (0.95, 0.98) at 2, 4, and 8 h after ROSC, respectively (A). The ratios of NF-κB p65 translocation were significantly attenuated by UTI in the VF + UTI group at 2, 4, and 8 h after ROSC (B) (n = 6 at each time point in different groups). * VF + PBS versus VF + UTI, p<0.05.
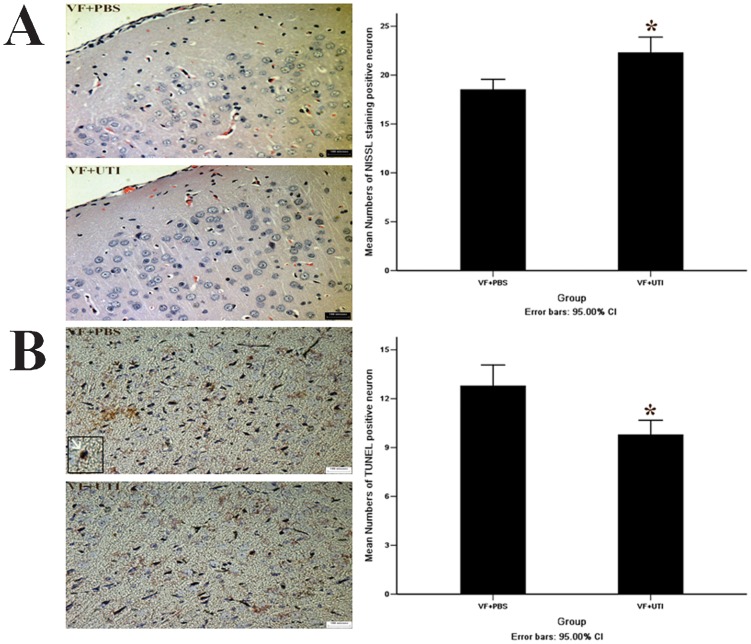
UTI increased the number of viable cells and decreased the number of TUNEL-positive cells in the cortex 72 h after ROSC.
The number of Nissl-stained cells in the cortices of rats in the VF + PBS group was significantly lower than in the VF + UTI group 72 h after ROSC (Figure 4A); p = 0.001). However, the number of TUNEL-positive neurons in the cortex of the temporal lobe in the VF + PBS group was greater than in the VF + UTI group 72 h after ROSC (Figure 4B); p = 0.001).
Figure 4.
UTI increased the number of Nissl-stained neurons and decreased the number of TUNEL-positive cells in the cortex. Nissl staining indicated that rat cortical cell numbers in the VF + PBS group were significantly lower than in the VF + PBS group 72 h after ROSC (A, B). There was a greater number of TUNEL-positive neurons (indicated by the white arrow) in rat cortices in the VF + PBS group than in the VF + UTI group 72 h after ROSC (C, D) (72 standard fields in the VF + PBS group and 126 standard fields in the VF+UTI group were analyzed). * VF + PBS versus VF + UTI, p<0.05.
DISCUSSION
In this study, we demonstrated that treatment with UTI after ROSC was effective in the prevention of oxidative damage and resulted in a decreased inflammatory response after CA induced by VF. UTI treatment significantly decreased the level of MDA in the cortex, the ratios of NF-κB p65 translocation from the cytoplasm to the nucleus, and the mRNA and protein levels of TNF-α and IL-6. We also found that UTI treatment increased the number of Nissl-positive (i.e., living) neurons while reducing the number of TUNEL-positive (i.e., apoptotic) cells, indicating that UTI decreased neuronal apoptosis. These findings suggest that attenuation of neuronal injury by UTI in a rat model of VF is associated with inhibition of oxidative stress and activation of the NF-κB/TNF-α signaling pathway.
We found that the activity of MPO in the cortex of rats in the VF + UTI group was lower than in the VF + PBS group. It is known that leukocyte adhesion to vessel walls and translocation into tissues is mediated by selectins and interstitial cell adhesion molecules, which are expressed on the surfaces of endothelial cells. Moreover, the intracellular content of MPO is constant in neutrophils, and their accumulation was evaluated by measurement of MPO activity. Therefore, based on our results, leukocyte infiltration to the cortex after ROSC was presumably attenuated by UTI.
The results of this study showed that plasma concentrations of TNF-α and IL-6 increased dramatically shortly after resuscitation from VF cardiac arrest and remained elevated during the early post-resuscitation period. Substantial evidence from animal and human ex-vivo cardiac experiments has supported the hypothesis that acute inflammation associated with ischemia-reperfusion injury is partially caused by TNF-α and IL-6 (26). Support for this hypothesis is based on the finding that TNF-α levels are increased in the peritoneal macrophages collected from rats subjected to myocardial ischemia-reperfusion injury, and TNF-α is primarily produced by circulation-activated macrophages in response to ischemia and reperfusion (27-29). TNF-α acts as an important substance in the recruitment of circulating leukocytes to the sites of inflammatory lesions; additionally, the cytokine may be synthesized during ischemia and released on reperfusion from the ischemic tissue itself (30), as shown in the present study.
IL-6 is another well-known cytokine that is up-regulated following cerebral ischemia. The serum level of IL-6 is associated with the brain infarct volume and is a powerful predictor of early neurological deterioration (31,32). Here, we showed that the levels of TNF-α and IL-6, two pro-inflammatory cytokines, are significantly lower in the plasma and cerebral tissue obtained from UTI-treated animals, which may explain the protective action of UTI against post-ischemic injury.
It has been shown that the inflammatory response is mediated by NF-κB (33), a redox-sensitive transcription factor that can be activated by reactive oxygen and is generated during the acute phase of reperfusion (34,35); therefore, inhibition of NF-κB suppresses the inflammatory response and limits injury. TNF-α and IL-6 were greatly increased in the VF + PBS group, but their levels were significantly lower in the VF + UTI group. This result strongly suggests that UTI might reduce the levels of cytokines via inhibition of NF-κB activation after ROSC; however, direct evidence needs to be acquired with further investigation.
In addition to the anti-inflammatory and anti-oxidative stress effects of UTI demonstrated in this Wistar rat CA/CPR model, other mechanisms may also contribute to the neuroprotective action of UTI on global ischemia-reperfusion in the brain (13,14). The effects of UTI on the preservation of mitochondrial function may also be involved in the protection of neurons from ischemia-reperfusion injury after ROSC (36).
Study limitations
The analysis of NF-κB translocation and TNF-α and IL-6 expression in this study focused on the cerebral cortex, which contains different types of cells, such as inflammatory and endothelial cells, neurons, and glial cells, that all could induce changes in NF-κB, TNF-α, and IL-6 expression after global brain ischemia and reperfusion. Although we found that UTI treatment reduced the tissue expression of TNF-α and IL-6 and the translocation of NF-κB p65 from the cytoplasm to the nucleus, we did not differentiate different cell types in these tissue sections. In future studies, it would be worthwhile to identify specific cellular types that are potentially targeted by UTI after ischemia/reperfusion stimulation.
This study was the first to demonstrate that UTI can preserve neuronal survival and inhibit neuron apoptosis after ROSC in Wistar rats via attenuating oxidative stress and the translocation of NF-κB p65 from the cytoplasm to the nucleus in the cortex, subsequently decreasing the production of TNF-α, IL-6, MDA, and MPO. Our study will provide the scientific basis for UTI treatment in neuronal injury after ROSC.
ACKNOWLEDGMENTS
This study was supported by funding from the TIANPU Fund (01200918), NSFC (81272062), and the Science and Technology Foundation of Guangdong Province, China (2012B031800286). The funders had no role in the study design, data collection, analysis, decision to publish, or the preparation of the manuscript.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome. Curr Opin Crit Care. 2004;10(3):208–12. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 2.Callaway CW RJC, Logue ES MMJ. Hypothermia after cardiac arrest does not alter serum inflammatory markers. Crit Care Med. 2008;36(9):2607–12. doi: 10.1097/CCM.0b013e318184443b. [DOI] [PubMed] [Google Scholar]
- 3.Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation. 2002;106(5):562–8. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Li CS, Gong P, Tang ZR, Hua R, Mei X, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83(7):913–20. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Vila N, Castillo J, Davalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31(10):2325–9. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 6.Sairanen T, Carpen O, Karjalainen-Lindsberg ML, Paetau A, Turpeinen U, Kaste M, et al. Evolution of cerebral tumor necrosis factor-alpha production during human ischemic stroke. Stroke. 2001;32(8):1750–8. doi: 10.1161/01.str.32.8.1750. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Nozari A, Liu XL, Rubertsson S, Wiklund L. Development of a novel biomarker of free radical damage in reperfusion injury after cardiac arrest. FEBS Lett. 2000;470(1):1–6. doi: 10.1016/s0014-5793(00)01279-5. [DOI] [PubMed] [Google Scholar]
- 8.Niemann JT, Rosborough J, Youngquist S, Lewis RJ, Phan QT, Filler S. The proinflammatory cytokine response following resuscitation in the swine model dl depends on the method of ventricular fibrillation induction. Acad Emerg Med. 2008;15(10):939–44. doi: 10.1111/j.1553-2712.2008.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson D, Yin T, Smalstig EB, Hsu MA, Panetta J, Little S, et al. Transcription factor nuclear factor-kappa B is activated in neurons after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20(3):592–603. doi: 10.1097/00004647-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, et al. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35(4):987–91. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- 11.Sironi L, Banfi C, Brioschi M, Gelosa P, Guerrini U, Nobili E, et al. Activation of NF-kB and ERK1/2 after permanent focal ischemia is abolished by simvastatin treatment. Neurobiol Dis. 2006;22(2):445–51. doi: 10.1016/j.nbd.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Valerio A, Dossena M, Bertolotti P, Boroni F, Sarnico I, Faraco G, et al. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke. 2009;40(2):610–7. doi: 10.1161/STROKEAHA.108.528588. [DOI] [PubMed] [Google Scholar]
- 13.Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, et al. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32(9):925–32. doi: 10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- 14.Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98(2):465–73. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Abu-Amara M, Gurusamy K, Hori S, Glantzounis G, Fuller B, Davidson BR. Systematic review of randomized controlled trials of pharmacological interventions to reduce ischaemia-reperfusion injury in elective liver resection with vascular occlusion. HPB (Oxford) 2010;12(1):4–14. doi: 10.1111/j.1477-2574.2009.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga Y, Fujita M, Tsuruta R, Koda Y, Nakahara T, Yagi T, et al. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol Res. 2010;32(9):925–32. doi: 10.1179/016164110X12645013515133. [DOI] [PubMed] [Google Scholar]
- 17.Wu YJ, Ling Q, Zhou XH, Wang Y, Xie HY, Yu JR, et al. Urinary trypsin inhibitor attenuates hepatic ischemia-reperfusion injury by reducing nuclear factor-kappa B activation. Hepatobiliary Pancreat Dis Int. 2009;8(1):53–8. [PubMed] [Google Scholar]
- 18.Wu YJ, Ling Q, Zhou XH, Wang Y, Xie HY, Yu JR, et al. Urinary trypsin inhibitor attenuates hepatic ischemia-reperfusion injury by reducing nuclear factor-kappa B activation. Hepatobiliary Pancreat Dis Int. 2009;8(1):53–8. [PubMed] [Google Scholar]
- 19.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu CL, Wei HY, Liu ZY, Li X, Liao XX, Li YJ, et al. Investigation of the relationship between venticular fibrillation duration and cardiac/neurological damage in a rabbit model of electrically induced arrhythmia. J Trauma. 2010;69(6):1442–7. doi: 10.1097/TA.0b013e3181dbbefc. [DOI] [PubMed] [Google Scholar]
- 21.Chun-Lin H, Jie W, Xiao-Xing L, Xing L, Yu-Jie L, Hong Z, et al. Effects of therapeutic hypothermia on coagulopathy and microcirculation aftercardiopulmonary resuscitation in rabbits. Am J Emerg Med. 2011;29(9):1103–10. doi: 10.1016/j.ajem.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Lin JY, Liao XX, Li H, Wei HY, Liu R, Hu CL, et al. Model of cardiac arrest in rats by transcutaneous electrical epicardium stimulation. Resuscitation. 2010;81(9):1197–204. doi: 10.1016/j.resuscitation.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Wu YJ, Ling Q, Zhou XH, Wang Y, Xie HY, Yu JR, et al. Urinary trypsin inhibitor attenuates hepatic ischemia-reperfusion injury by reducing nuclear factor-kappa B activation. Hepatobiliary Pancreat Dis Int. 2009;8(1):53–8. [PubMed] [Google Scholar]
- 24.Shin IW, Jang IS, Lee SM, Park KE, Ok SH, Sohn JT, et al. Myocardial protective effect by ulinastatin via an anti-inflammatory response after regional ischemia/reperfusion injury in an in vivo rat heart model. Korean J Anesthesiol. 2011;61(6):499–505. doi: 10.4097/kjae.2011.61.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yano T, Anraku S, Nakayama R, Ushijima K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology. 2003;98(2):465–73. doi: 10.1097/00000542-200302000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Wanderer AA. Ischemic-reperfusion syndromes: biochemical and immunologic rationale for IL-1 targeted therapy. Clin Immunol. 2008;128(2):127–32. doi: 10.1016/j.clim.2008.03.514. [DOI] [PubMed] [Google Scholar]
- 27.Caputi AP, Squadrito F. Role of TNF-alpha and therapeutic perspectives in bowel and myocardial ischemia/reperfusion injury. Pharmacol Res. 1992;26 Suppl 2:150–1. doi: 10.1016/1043-6618(92)90640-w. [DOI] [PubMed] [Google Scholar]
- 28.Lucchesi BR. Modulation of leukocyte-mediated myocardial reperfusion injury. Annu Rev Physiol. 1990;52:561–76. doi: 10.1146/annurev.ph.52.030190.003021. [DOI] [PubMed] [Google Scholar]
- 29.Dinerman JL, Mehta JL. Endothelial, platelet and leukocyte interactions in ischemic heart disease: insights into potential mechanisms and their clinical relevance. J Am Coll Cardiol. 1990;16(1):207–22. doi: 10.1016/0735-1097(90)90481-4. [DOI] [PubMed] [Google Scholar]
- 30.Lavine SD, Hofman FM, Zlokovic BV. Circulating antibody against tumor necrosis factor-alpha protects rat brain from reperfusion injury. J Cereb Blood Flow Metab. 1998;18(1):52–8. doi: 10.1097/00004647-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Clark WM, Rinker LG, Lessov NS, Hazel K, Eckenstein F. Time course of IL-6 expression in experimental CNS ischemia. Neurol Res. 1999;21(3):287–92. doi: 10.1080/01616412.1999.11740933. [DOI] [PubMed] [Google Scholar]
- 32.Acalovschi D, Wiest T, Hartmann M, Farahmi M, Mansmann U, Auffarth GU, et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34(8):1864–9. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- 33.Senftleben U, Karin M. The IKK/NF-kappa B pathway. Crit Care Med. 2002;30(1 Suppl):S18–26. [PubMed] [Google Scholar]
- 34.Yu X, Patterson E, Huang S, Garrett MW, Kem DC. Tumor necrosis factor alpha, rapid ventricular tachyarrhythmias, and infarct size in canine models of myocardial infarction. J Cardiovasc Pharmacol. 2005;45(2):153–9. doi: 10.1097/01.fjc.0000151930.12026.b7. [DOI] [PubMed] [Google Scholar]
- 35.Chandrasekar B CJT, Geimer J CD, Freeman GL. Induction of nuclear factor kappaB but not kappaB-responsive cytokine expression during myocardial reperfusion injury after neutropenia. Free Radic Biol Med. 2000;28(11):1579–88. doi: 10.1016/s0891-5849(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 36.Zhao H, Li CS, Gong P, Tang ZR, Hua R, Mei X, et al. Molecular mechanisms of therapeutic hypothermia on neurological function in a swine model of cardiopulmonary resuscitation. Resuscitation. 2012;83(7):913–20. doi: 10.1016/j.resuscitation.2012.01.001. [DOI] [PubMed] [Google Scholar]