Abstract
Context
The modern practice of anesthesia is highly dependent ona group of anesthetic drugs which many of them are metabolized in the liver.
Evidence Acquisition
The liver, of course, usually tolerates this burden. However, this is not always an unbroken rule. Anesthetic induced apoptosis has gained great concern during the last years; especially considering the neurologic system.
Results
However, we have evidence that there is some concern regarding their effects on the liver cells. Fortunately not all the anesthetics are blamed and even some could be used safely, based on the available evidence.
Conclusions
Besides, there are some novel agents, yet under research, which could affect the future of anesthetic agents' fate regarding their hepatic effects.
Keywords: Liver, Apoptosis, Anesthesiology, Pharmacology
1. Context
Anesthesia is a modern human invention which was clinically introduced for the first time in October 1846 by William Morton, though the clinical effects of nitrous oxide had been discovered in 1844 (1).The introduction and utilization of anesthetic drugs has passed a long way, introducing newer generations of more effective drugs with less unwanted side effects; however, this process is not completed yet and the available anesthetic agents have their current side effects of course with a very low incidence (2).Liver is one of the main body organs performing drug metabolism among its many specific and unique functions. However, drug detoxification would create a spectrum of biochemical by-products imposed to the liver cells; while many pharmaceuticals, includingmost anesthetics, are metabolized, totally or partially, in the liver. This is why the cellular mechanisms for liver injury have a great impact in development and introduction of newer and more "liver friendly" anesthetic drugs.
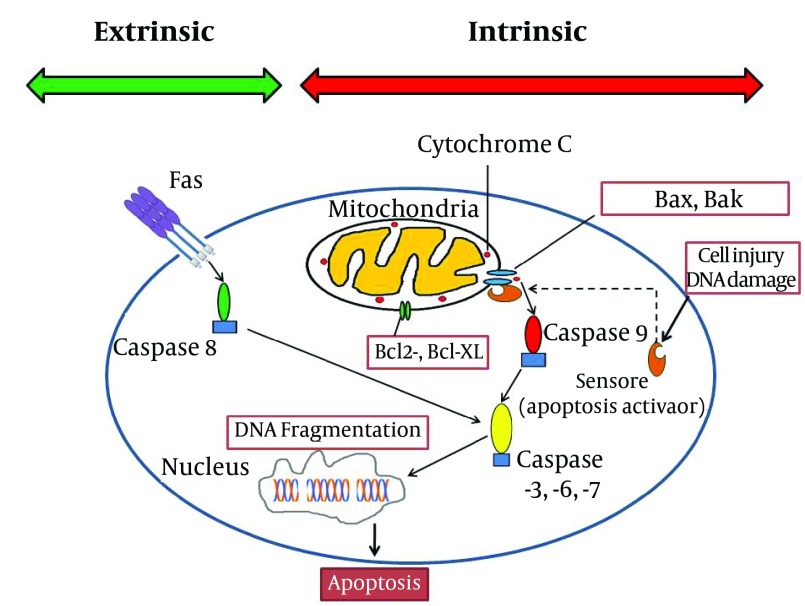
Hepatic cells -during their metabolic functions- continuously produce reactive oxygen species. Reactive oxygen species are reduced to other forms of oxygen by mitochondria; this process may be deficient innonhealthyliver or when the liver is exposed to an extraordinary unwanted burden of toxins ( 3 - 6 ). This oxidant damage would disturb many parts of the cell structure in hepatocytes. Apoptosis (and not necrosis) is the main mechanism of liver injury, especially after drug related -including anesthetics- or viral injuries ( 6 - 14 ) at times ending in massive apoptosis ( 10 ). Apoptosis (programmed cell death) could be induced in two ways: intrinsic and extrinsic ( 9 , 11 ). Although both of pathways resulted in similar consequence (elimination of stressed cells), the initiation mechanisms are different. Intrinsic factors such as lack of growth mediators, DNA damage and cytoplasm detachment could accumulateproapoptoticmembers of Bcl-2 family (Bax and Bak) in mitochondrial membrane ( 4 , 9 , 10 , 15 , 16 ). This phenomenon could increase mitochondrial permeability and accomplish with displacement of cytochrome c and certain proapoptotic proteins from mitochondria to cytoplasm. These mediators activate caspases 9 ( 17 - 19 ) and other subsequent caspases ( 6 , 8 , 20 - 24 ). These enzymes induce DNA fragmentation, plasma membrane blebbing which finally result in formation of apoptotic bodies ( 7 , 20 ). Extrinsic pathway could be triggered by involvement of cell surface receptors which are consisted of a broad spectrum of death receptorsespeciallyFas (CD95) and TNF-RI ( 3 - 6 , 9 , 13 , 15 , 23 - 31 ). Activation of death receptors by related ligands induces recruitment of cytoplasmic adaptor proteins such as TRADD (TNF receptor-associated death domain) and FADD (Fas-associated death domain). This signal transduction would result in caspase 8 and subsequent cascade of caspases activation ( 4 , 15 , 17 , 18 , 23 , 24 , 28 , 29 ). Apoptotic bodies which formed after terminal activation of caspase 3 in both pathways are cleared by phagocytes without inducing inflammation ( 9 ). Liver cell structure remains the main location for the above interactions including the Kupffer cells, dendritic cells, natural killer (NK) cells, NKT cells, neutrophils, mast cells and T cells ( 4 , 12 - 14 , 16 , 27 , 32 - 38 ). The final fate of apoptosis cascade is determined by interaction between proapoptotic and antiapoptotic proteins in Bcl-2 family in cell structure (Figure 1) ( 4 , 9 , 15 , 23 , 32 , 39 , 40 ).
Figure 1. A General Schematic Presentation of the Apoptosis Pathway.
2. Evidence Acquisition
2.1. The Effect of Anesthetic Drugs on Hepatic Apoptosis
During the recent years, it has been demonstrated in a great number of studies that most current anesthetic agents such as intravenous anesthetic agents (like ketamine, barbiturates, propofol, midazolam, diazepam, clonazepam), volatile agents (like halothane, isoflurane, desflurane, sevoflurane), xenon and even, muscle relaxants have been labeled as having apoptotic properties in animal studies, exerting their effect in a dose-dependent manner which its effects would be created as "time-dependent neurodegenerative effects especially in the developing animal brain"; while, a number of other studies have claimed these agents as being neuroprotective which preserve the brain tissue from unwanted adverse effects, like "apoptosis, degeneration, inflammation and energy failure"; however, one important point is that nearly all of these results are from animal models since performing such studies on the human brain is not an easy task (41-43). Sufficient proof (especially regarding lab evidence) for occurrence of neuroapoptosis in the developing brain is scarce yet, and the results gained from nearly all of the human studies demonstrate "associative, not causal relationships" (41, 42). This mandates further research clearing the possibility of previous animal findings in human.
On the other hand, there is a widespread research on the role of anesthetic agents in neuroapoptosis (41), mainly focusing on the neonatal brain; however, the possible role of anesthetic agents in liver apoptosis is not very clear yet and also, would it be real, it is not age limited as it is seen in neonatal apoptosis. Previous assessments have demonstrated that the deeper the level of anesthesia, the more severe the resultant neuroapoptosis; for example, a combined "nitrous oxide and isoflurane, or ketamine and propofol" would result in much severe neuroapoptosis compared toany single anesthetic administered alone (44).The anesthetic agents are many and diverse; however, according to a general classification used in many anesthesia texts and also, in this manuscript, the anesthetics could be classified as 4 groups according to their anesthetic function: hypnotics, analgesics, amnestic agents, and muscle relaxants.
3. Results
3.1. Hypnotics
The hypnotic agents with apoptotic activity could be classified as two main groups: N-methyl-D-aspartate (NMDA) antagonist, like ketamine; and gamma-aminobutyric acid (GABA) receptor agonists, like propofol or thiopental (45-50). Of course, hypnotic agents and amnestic agents have some overlapped clinical use; but we have discussed them under two different subtitles.
a)Ketamine: Among the intravenous hypnotics, there are many studies discussing the apoptotic effects of ketamine; especially potentiating hepatic apoptosis. Among all the proposed mechanisms for ketamine apoptosis, the most main ones are “up-regulation of NMDA receptors causing overestimation of glutamatergic system" and "hydroquinone toxicity" which is a metabolite of the drug (51). Ketamine also could "suppress phosphorylated extracellular signal-regulated protein kinase" (52)and "induce the formation of hyperphosphorylated tau", a "hallmark of Alzheimer's disease". S-(+)-ketamine, one of the main ketamine isomers creates "apoptosis in human HepG2 cells" causing hepatocyte and Kupffer cell injury; the potential ketamine injury occurs much sooner than pentobarbital injury (20, 53). Chronic ketamine use has been demonstrated to create hepatic cellular damage; while it did not affect the intestinal mucosa as much as the liver (54); also, ketamine would cause much more severe liver fibrosis. It could be concluded that adverse apoptotic effects of ketamine are seen more frequently when administered concomitantly with other anesthetics like benzodiazepines or when ketamine is used as a chronic drug; often as an abused drug; so, ketamine added to lidocaine could increase the apoptotic effects of lidocaine in an additive manner; some drugs like clonidine might have potential effects in prevention of apoptotic effects of ketamine (55-63).
b) Thiopental: is the prototype barbiturate used for anesthesia with an apoptotic mechanism by GABA-A agonistic action (64); also, through inducing lymphocyte death by "a CD95-independent mechanism" (65) and by "attenuated staurosporine-induced apoptosis and caspase-like activity" (66, 67); of course, the latter effect might be cardioprotective and against cardiomyocyte apoptosis.
c) Propofol: is one of the most common intravenous anesthetic agents and might attenuate " caspase-3 activation"; so, attenuating apoptotic effects of some anesthetics (68); also, propofol might have hepatic protective effects by reducing “the population of apoptotic cells and Caspase-3 and PARP cleavage in hepatic L02 cells in a dose-dependent manner” (69); though there are controversies (30, 52).
d) Etomidate: another intravenous anesthetic agent having in vitro apoptotic and cytotoxic effects in leukemia RAW264.7 cells (70); currently, no animal or clinical evidence for hepatic apoptosis is available.
e) Alpha 2 adrenergic receptor agonists: are used primarily as adjuvant to hypnotics; two main drugs belong to this classification, clonidine and dexmedetomidine, the 2nd one is synthetic, both having antiapoptotic effects, possibly against ketamine and isoflurane. Dexmedetomidine may prevent isoflurane-induced apoptosis in brain and some other organs. In clinic, dexmedetomidine could attenuate apoptotic effects of isoflurane in a dose dependent manner through a number of proposed mechanisms though; there is some controversy (62, 71-76).
f) Volatile anesthetics: these hydrophobic halogenated inhalational agents are often used for general anesthesia. Being among the most common inhalational anesthetics, have potential protective and also unwanted effects. Development of neuroapoptosis in animal brain (41), potential hepatotoxicity (1, 77, 78), attenuation of "antioxidant activity in plasma and erythrocytes", inhibition of "apoptosis in neutrophils", increasing DNA breaks" and "cell death" are among their effects, “cytotoxic effects on treated tumor cells” having a time dependent manner (79-81). Volatile anesthetics have "genotoxicity, cytotoxicity or teratogenicity" effects (82).The adverse effects of anesthetic drugs on liver cells could be altered by the process of liver cell apoptosis; which might be triggered by "concurrent viral infection" which in turn may "inhibit cytochrome (CYP) 450 activities and activate the hepatic innate immune system to proapoptotic factors" (8).
Isoflurane related apoptosis is GABA-A independent and could be prevented by dexmedetomidine; however, some controversies exist; especially when considering the administration dose or concomitant use of other anesthetics especially NMDA antagonists or GABA agonist anesthetics: Isoflurane has been shown to create apoptosis in human neuroglioma cell line with clinically relevant concentrations; emulsified form of isoflurane has protective effects against liver injury through the hepatic levels of malondialdehyde (MDA) and superoxide dismutase, suggesting "inhibition of cell death and improvement of antioxidation in mitochondria" as the potential protective mechanisms (53, 83-87).
Halothane can create apoptosis in vivo and in vitro in liver; Halothane has cytotoxic effects on treated tumor cells in a "time and cell line dependent" and even with low doses could impair irreversibly the cell genome; although it does not directly interact with DNA; also, clinical doses of halothane in animal model could decrease the viability of cells, impair DNA, and trigger "stress-induced apoptosis"; both halothane anesthesia and epidural anesthesia in animal models could induce apoptosis with a fall in lymphocyte counts (8, 19, 80, 82, 88-92).
g) Xenon belongs to noble gas family and is really an ideal inhalational anesthetic in clinical setting with efficient and satisfactory anesthetic properties not only for liver but also for other main organs of the body; many of its features are unique among all the anesthetic agents. No unwanted hepatic effects of xenon are reported. It is "a high-affinity glycine-site NMDA receptor antagonist" with both cardio protective and neuroprotective properties unique for xenon. The unique properties of xenon are possibly related to its interaction regarding the "dopaminergic pathways". It may even suppress the unwanted effects of ketamine and nitrous oxide and the apoptogenic activity of isoflurane. The only problems of xenon are potential pulmonary hypertension and also, the cost of the drug due to the especial technology of anesthesia machine needed for delivery of xenon to patients; a full discussion of the xenon, regarding its merits and possible problems could be found elsewhere (93).
3.2. Analgesics
Opioids might trigger the apoptosis process in a different number of cells; although a few studies have claimed some controversies regarding the role of opioids in apoptosis; also, δ-opioid receptors which are very much frequently found in the cellular membranes of liver cells, have an important role in "oncogenesis and progression of hepatic neoplasia, viral hepatitis and hepatic cirrhosis"; besides, it has been demonstrated in liver cells that "activation of δ-opioid receptor" would result in inhibition of mitochondrial apoptotic pathway through specialized interactions" involving protein kinase C: in this process, the dose and the time of administration of opioidshavekey roles: "higher levels of endogenous opioids" was shown to "increase hepatocyte apoptosis" which might be related to decreased level of antioxidant defense system in liver cells; the following few paragraphs are based on the studies related to opioids (21, 22, 94-112):
a)Morphine: chronic and repeated opioid administration, especially morphine, could induce apoptosis in hepatocytes through the main opioid receptors; these repeated high doses could contribute to oxidative stress in the cells of liver, especially considering that blockade of opioid receptors could be in confrontation of Fas-induced hepatitis, strengthen the mice liver, and also repeated morphine doses might possibly be in relation with deterioration of the host defense chains in mice and rat; these findings are much more important when considering the possible role of opioids in hepatitis pathophysiology;though some controversies exist.
b) Met-enkephalin: it has been proposed that opioid growth factor (i.e. [Met(5)]-enkephalin) and its receptor have an important role in "endogenous pathways controlling cell growth"; the concentration of the opioid growth factor is higher in metastasis-positive human liver tissue than the normal liver tissue; liver met-enkephalin has been demonstrated to have antitumor activity.
c) Methadone: kills leukemia cells through an apoptotic mechanism through mechanisms independent of caspases accompanied with cellular depletion of ATP stores and a critical state in cell bioenergetics; methadone has also been demonstrated to have a similar role in the treatment of small cell lung carcinoma through apoptosis.
d) Fentanyl: is a potent synthetic opioid agonist which could trigger lymphocyte apoptosis in a time-dependent manner.
e) Sufentanil: its protective effects with an antiapoptotic mechanism and modulating Bax and Bcl-2 expressionhavebeen demonstrated and similar results with up-regulation of p-FADD resulting in antiapoptotic effects for other opioids are demonstrated.
f) Remifentanil: is among the opioid compounds used for anesthesia with a number of specialpharmacologic properties. Due to its specific method of metabolism, mainly through cholinesterase metabolism in plasma, it has an ultrashorthalf-life, necessitating its mode of use to be only through intravenous infusion. There are some studies demonstrating the antiapoptotic effects of remifentanil in CNS and myocardium. In one animal study, the anti-inflammatory andantiapoptoticeffects of remifentanil were hepatic protective in rat models; this study demonstrated that "pretreatment with remifentanil can attenuate liver injury both in vivo and in vitro"; also, this study demonstrated that this protective effect is produced through NOS production which is not related to the activation of "opioid receptors" but is maintained by "exhausting reactive oxygen species"; so, it seems that remifentanil has some specific features, regarding its effect pathways and the mode of metabolism (i.e. somewhat different from other anesthetics) that might have promising features for improved liver effects of opioids as experienced in other tissues.
3.3. Amnestic Agents
Among the amnestic agents, benzodiazepines are the most well-known ones used much frequently in the perioperative period and also, for sedation of patients in ICU wards or during invasive procedures; benzodiazepines have the potential to induce apoptosis in a number of different cell lines, both in vitro and in vivo; the unwanted apoptotic effects of benzodiazepines (especially in the liver) are more expressed when they are coadministered with other anesthetics like ketamine; other benzodiazepines have also well studied apoptotic effects; the apoptotic effects of benzodiazepines could be suppressed by administration of vitamin C; which could restore the cellular reservoirs of glutathione. Midazolam is the prototype of these agents in anesthesia. Midazolam has apoptotic effects with a dose dependent manner, which is independent of GABA receptor and leads to necrosis with increased plasma concentrations; though it has varying degrees of intensity between different benzodiazepine compounds; midazolam or ketamine when added to lidocaine could increase the apoptotic effects of lidocaine in an additive or subadditive manner (9, 18, 63, 64, 113-121).
3.4. Muscle Relaxants
Muscle relaxants are a classification of anesthetic drugs which are used for prevention of movements during surgery (i.e. akinesia during operationor inside ICU for improvement of assisted ventilation). However, it does not seem that these agents would express liver apoptosis (122-125):
a) Pancuronium: an old nondepolarizing long acting muscle relaxant is demonstrated to have apoptotic effects on "peripheral blood lymphocytes" when used at clinical concentrations.
b) Cisatracurium: an intermediate acting muscle relaxant; acrylate esters are produced during its metabolism and could induce oxidative stress which is a well-known and very potent triggering factor for apoptosis in human cell lines.
c) Neostigmine: a muscle relaxant reversal agent, which has not been demonstrated to have apoptotic activity.
4. Conclusions
Future opportunities and potential therapeutics for prevention of anesthetic agents-induced hepatic apoptosis.There are some studies that might help us direct future studies for prevention of anesthetics-induced apoptosis, including hepatic apoptosis.
a)Normal hepatocytes counteract apoptosis by neutralizing free oxygen species using agents like vitamin C and vitamin E; ferritin, glutathione, and a number of enzymes; as mentioned before, administration of vitamin C could suppress the apoptotic effects of benzodiazepines through "restoration of cellular glutathione reservoirs" (4, 118, 126-129).
b) Beta estradiol, a sex hormone being the predominant estrogen during the active female reproduction years; however, it is also observed in male blood as a product of testosterone; this hormone protects against neuroapoptosis by anesthetics; mainly by up regulating serum levels of one of the members of protein kinase B family (Akt); which would protect Brain-derived neurotropic factor (BDNF); anesthetics can cause BDNF imbalance in "cerebral cortex and thalamus in time-dependent fashion"; 17 beta-estradiol pretreatment is also demonstrated to protect the liver from injuries with "heat-shock protein 70 overexpression " mechanism; however, beta-estradiol might improve apoptosis possibly in liver cells (19, 130-132).
c) One of the main agents that could inhibit the apoptotic effects of anesthetic agents is melatonin which could inhibit the "mitochondria-dependent apoptotic" (133).
d) Alpha2A-adrenoceptors including clonidine could negatively regulate the expression of caspase-3 in the neonatal cerebral cortex; exerting their protective roles against anesthetics; so clonidine could be used both as an anesthetic adjuvant and an antiapoptotic agent; its analog, dexmedetomidine, can implement its protective roles to prevent against the apoptotic effects of some anesthetics like isoflurane; their possible role in liver could be the topic of many future researches (62, 71, 73-76, 134).
e) Lithium has been shown to effectively counteract the apoptotic effects of ketamine and propofol; hence lithium has the possible role in prevention of anesthetic induced apoptosis, possibly in brain or liver (52).
f) Xenon has been demonstrated to haveboth cardioprotective and neuroprotective properties which are unique properties of the gas and mimicking the natural cellular protection pathways through K(ATP) channels; also, it can prevent “isoflurane, nitrous oxide and ketamine” apoptotic activity; possible future protective effects of xenon for patients at risk of hepatic apoptosis needing anesthesia and surgery could be promising (93).
g) Heme oxygenase 1 has been shown to "improve neurologic outcome" in rats by "protecting neurons against apoptosis". Possibly Heme oxygenase 1 could be tried to see if it could be useful for prevention of hepatic cell apoptosis after anesthetic administration (135).
h) Antithrombin decreases hepatic injury through releasing calcitonin gene-related peptide or CGRP; CGRP improves hepatic cellular tolerance to cell injury, including apoptosis; CGRP can in turn increase the production of insulin-like growth factor-I (IGF-I); on the other hand, capsaicin, increases the release of CGRP, which might increase IGF-I production, and thereby reduce liver apoptosis(9, 21, 134-138).
i) Gamma-hydroxybutyrate has been shown to protect liver against injury through different mechanisms (138).
j) Pentapeptide V5, with its full name as "pentapeptide Val-Pro-Met-Leu-Lys, V5" is an antiapoptoticpolypeptide (120, 137) which has been shown to be liver protective.
k) Also, a number of pan-caspase inhibitors, namely IDN-6556 (N-[(1,3-dimethylindole-2-carbonyl)valinyl]-3-amino-4-oxo-5-fluoropentanoic acid) and IDN-1965 are in preclinical phase and have been shown to be liver protective andantiapoptotic; which may have some role in prevention of anesthetics-induced apoptosis (including liver) in future years (17, 136, 139-142).
l) Depletion of Kupffer cells from the liver increases the risk of hepatic injury (as seen after acetaminophen model hepatic damage), reduction in IL 6, IL 10, TNFα production and number of Kupffer cells are seen in this condition accompanied with averted activity of Fas/Fas ligand; possibly, these phenomena are attenuated byIbuprofen; though the exact mechanism is controversial; also, Cannabinoid CB2 agonists have similar role in protecting Kupffer cells, being possible antiapoptotic agents in liver (9, 34, 39, 127, 143-150).
m) Also, "exogenous administration of S-adenosyl-l-methionine" prevents hepatotoxicity by the same mechanism (151).
n) After exposure of the liver cells to the viral agents or haptens, there would be an increase in the interleukin 1-β and δ levels which demonstrate Toll like receptor 4 (TLR 4) involvements. TLR 4 could enhance the presence of TNF-αin the Kupffer cells after hepatic cell insults (6, 37, 53).
Acknowledgments
The authors would like to acknowledge the efforts of Anesthesiology Research Center colleagues for their kind support; also, the comments mentioned by Professor Marek Jan Los, Professor in Regenerative Medicine, Linköping University, Department of Clinical and Experimental Medicine, Divison of Cell Biology, S-581 85 Linköping, Sweden for the comments presented in 11th International Congress of Immunology and Allergy, Tehran, Iran, April 2012.
Footnotes
Implication for health policy/practice/research/medical education:
This article discusses the implications of anesthetic agents used clinically worldwide; their effects on the hepatic cells especially their role on hepatic cell apoptosis is discussed and some of the new anesthetic agents with less untoward effects are discussed more; also, some of the drugs under further research with anesthetic properties and less apoptotic effects are presented. The target readers are anesthesiologists, gastroenterologists physiologists and pharmacologists.
Authors’ Contribution:
Dr Ali Dabbagh developed the original idea and the protocol, abstracted and analyzed data, wrote the manuscript, and is guarantor. Dr Samira Rajaei helped in data collection, writing manuscript and preparing manuscript.
Financial Disclosure:
Authors declare that they have no conflict of interest.
Funding/Support:
This study was not supported by any financial grant or financial support.
References
- 1.Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis. Hepat Mon. 2011;11(1):3–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Walker SM, Yaksh TL. Neuraxial analgesia in neonates and infants: a review of clinical and preclinical strategies for the development of safety and efficacy data. Anesth Analg. 2012;115(3):638–62. doi: 10.1213/ANE.0b013e31826253f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen Jessica I, Roychowdhury Sanjoy, McMullen Megan R, Stavitsky Abram B, Nagy Laura E. Complement and Alcoholic Liver Disease: Role of C1q in the Pathogenesis of Ethanol-Induced Liver Injury in Mice. Gastroenterology. 2010;139(2):664–6740. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottschalk S, Zwingmann C, Raymond VA, Hohnholt MC, Chan TS, Bilodeau M. Hepatocellular apoptosis in mice is associated with early upregulation of mitochondrial glucose metabolism. Apoptosis. 2012;17(2):143–53. doi: 10.1007/s10495-011-0669-y. [DOI] [PubMed] [Google Scholar]
- 5.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84(6):1410–21. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yano A, Higuchi S, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. Involvement of immune-related factors in diclofenac-induced acute liver injury in mice. Toxicology. 2012;293(1-3):107–14. doi: 10.1016/j.tox.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38(5):1188–98. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, You Q, Yin H, Holt M, Franklin C, Ju C. Effect of polyI:C cotreatment on halothane-induced liver injury in mice. Hepatology. 2009;49(1):215–26. doi: 10.1002/hep.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghavami S, Hashemi M, Kadkhoda K, Alavian SM, Bay GH, Los M. Apoptosis in liver diseases--detection and therapeutic applications. Med Sci Monit. 2005;11(11):RA337–45. [PubMed] [Google Scholar]
- 10.Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24(2):85–9. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- 11.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, et al. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15(12):1504–13. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 12.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 13.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134(6):1641–54. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sitia G, Iannacone M, Aiolfi R, Isogawa M, van Rooijen N, Scozzesi C, et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 2011;7(6):e1002061. doi: 10.1371/journal.ppat.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dejean LM, Ryu SY, Martinez-Caballero S, Teijido O, Peixoto PM, Kinnally KW. MAC and Bcl-2 family proteins conspire in a deadly plot. Biochim Biophys Acta. 2010;1797(6-7):1231–8. doi: 10.1016/j.bbabio.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kam PC, Ferch NI. Apoptosis: mechanisms and clinical implications. Anaesthesia. 2000;55(11):1081–93. doi: 10.1046/j.1365-2044.2000.01554.x. [DOI] [PubMed] [Google Scholar]
- 17.Deaciuc IV, D'Souza NB, de Villiers WJ, Burikhanov R, Sarphie TG, Hill DB, et al. Inhibition of caspases in vivo protects the rat liver against alcohol-induced sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2001;25(6):935–43. [PubMed] [Google Scholar]
- 18.Stevens MF, Werdehausen R, Gaza N, Hermanns H, Kremer D, Bauer I, et al. Midazolam activates the intrinsic pathway of apoptosis independent of benzodiazepine and death receptor signaling. Reg Anesth Pain Med. 2011;36(4):343–9. doi: 10.1097/AAP.0b013e318217a6c7. [DOI] [PubMed] [Google Scholar]
- 19.Woodside KJ, Song J, Song W, Hu M, Meng T, Hunter GC, et al. Immunomodulation of hepatic ischemic injury via increased Bcl-XL and decreased Bcl-XS1,2 1. The Journal of surgical research. 2003;112(1):59–64. doi: 10.1016/s0022-4804(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee ST, Wu TT, Yu PY, Chen RM. Apoptotic insults to human HepG2 cells induced by S-(+)-ketamine occurs through activation of a Bax-mitochondria-caspase protease pathway. Br J Anaesth. 2009;102(1):80–9. doi: 10.1093/bja/aen322. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Alvarez S, Cuenca-Lopez MD, de Mera RM, Puerta E, Karachitos A, Bednarczyk P, et al. Methadone induces necrotic-like cell death in SH-SY5Y cells by an impairment of mitochondrial ATP synthesis. Biochim Biophys Acta. 2010;1802(11):1036–47. doi: 10.1016/j.bbadis.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Alvarez Sergio, Solesio Maria E, Cuenca-Lopez Maria D, Melero-Fern, #xE1, ndez de Mera Raquel M, et al. Pharmacological Characterization of the Mechanisms Involved in Delayed Calcium Deregulation in SH-SY5Y Cells Challenged with Methadone. International Journal of Cell Biology. 2012;2012:8. doi: 10.1155/2012/642482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pradelli LA, Beneteau M, Ricci JE. Mitochondrial control of caspase-dependent and -independent cell death. Cell Mol Life Sci. 2010;67(10):1589–97. doi: 10.1007/s00018-010-0285-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werneburg NW, Bronk SF, Guicciardi ME, Thomas L, Dikeakos JD, Thomas G, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein-induced lysosomal translocation of proapoptotic effectors is mediated by phosphofurin acidic cluster sorting protein-2 (PACS-2). J Biol Chem. 2012;287(29):24427–37. doi: 10.1074/jbc.M112.342238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardier JE, Erickson-Miller CL. Fas (CD95)- and tumor necrosis factor-mediated apoptosis in liver endothelial cells: role of caspase-3 and the p38 MAPK. Microvasc Res. 2002;63(1):10–8. doi: 10.1006/mvre.2001.2360. [DOI] [PubMed] [Google Scholar]
- 26.Chida Y, Sudo N, Sonoda J, Sogawa H, Kubo C. Electric foot shock stress-induced exacerbation of alpha-galactosylceramide-triggered apoptosis in mouse liver. Hepatology. 2004;39(4):1131–40. doi: 10.1002/hep.20158. [DOI] [PubMed] [Google Scholar]
- 27.Connolly MK, Ayo D, Malhotra A, Hackman M, Bedrosian AS, Ibrahim J, et al. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology. 2011;54(3):959–68. doi: 10.1002/hep.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng Z, Yan H, Hu J, Zhang S, Peng P, Liu Q, et al. Hepatitis C virus sensitizes host cells to TRAIL-induced apoptosis by up-regulating DR4 and DR5 via a MEK1-dependent pathway. PLoS One. 2012;7(5):e37700. doi: 10.1371/journal.pone.0037700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19(12):2003–14. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osman ES, Khafagy HF, Samhan YM, Hassan MM, El-Shanawany FM, Fathallah AR, et al. In vivo effects of different anesthetic agents on apoptosis. Korean J Anesthesiol. 2012;63(1):18–24. doi: 10.4097/kjae.2012.63.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schattenberg JM, Schuchmann M. Diabetes and apoptosis: liver. Apoptosis. 2009;14(12):1459–71. doi: 10.1007/s10495-009-0366-2. [DOI] [PubMed] [Google Scholar]
- 32.Ghavami S, Mutawe MM, Sharma P, Yeganeh B, McNeill KD, Klonisch T, et al. Mevalonate cascade regulation of airway mesenchymal cell autophagy and apoptosis: a dual role for p53. PLoS One. 2011;6(1):e16523. doi: 10.1371/journal.pone.0016523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38(2):355–63. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CM, Wang JS, Liu CH, Chen LW. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock. 2002;17(4):280–5. doi: 10.1097/00024382-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Locatelli I, Sutti S, Vacchiano M, Bozzola C, Albano E. NF-kappaB1 deficiency stimulates the progression of non-alcoholic steatohepatitis (NASH) in mice by promoting NKT-cell-mediated responses. Clin Sci (Lond). 2013;124(4):279–87. doi: 10.1042/CS20120289. [DOI] [PubMed] [Google Scholar]
- 36.Schneider EM, Lorenz I, Muller-Rosenberger M, Steinbach G, Kron M, Janka-Schaub GE. Hemophagocytic lymphohistiocytosis is associated with deficiencies of cellular cytolysis but normal expression of transcripts relevant to killer-cell-induced apoptosis. Blood. 2002;100(8):2891–8. doi: 10.1182/blood-2001-12-0260. [DOI] [PubMed] [Google Scholar]
- 37.Traeger T, Mikulcak M, Eipel C, Abshagen K, Diedrich S, Heidecke CD, et al. Kupffer cell depletion reduces hepatic inflammation and apoptosis but decreases survival in abdominal sepsis. Eur J Gastroenterol Hepatol. 2010;22(9):1039–49. doi: 10.1097/MEG.0b013e32833847db. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Wang J, Sheng Y, Zou Y, Bo L, Wang F, et al. Baicalin improves survival in a murine model of polymicrobial sepsis via suppressing inflammatory response and lymphocyte apoptosis. PLoS One. 2012;7(5):e35523. doi: 10.1371/journal.pone.0035523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Njoku DB, Talor MV, Fairweather D, Frisancho-Kiss S, Odumade OA, Rose NR. A novel model of drug hapten-induced hepatitis with increased mast cells in the BALB/c mouse. Exp Mol Pathol. 2005;78(2):87–100. doi: 10.1016/j.yexmp.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Panigrahi S, Stetefeld J, Jangamreddy JR, Mandal S, Mandal SK, Los M. Modeling of molecular interaction between apoptin, BCR-Abl and CrkL--an alternative approach to conventional rational drug design. PLoS One. 2012;7(1):e28395. doi: 10.1371/journal.pone.0028395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth. 2011;21(7):716–21. doi: 10.1111/j.1460-9592.2010.03506.x. [DOI] [PubMed] [Google Scholar]
- 42.Istaphanous GK, Loepke AW. General anesthetics and the developing brain. Curr Opin Anaesthesiol. 2009;22(3):368–73. doi: 10.1097/aco.0b013e3283294c9e. [DOI] [PubMed] [Google Scholar]
- 43.Schifilliti D, Grasso G, Conti A, Fodale V. Anaesthetic-related neuroprotection: intravenous or inhalational agents? CNS Drugs. 2010;24(11):893–907. doi: 10.2165/11584760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Cattano D, Williamson P, Fukui K, Avidan M, Evers AS, Olney JW, et al. Potential of xenon to induce or to protect against neuroapoptosis in the developing mouse brain. Can J Anaesth. 2008;55(7):429–36. doi: 10.1007/BF03016309. [DOI] [PubMed] [Google Scholar]
- 45.Dong Y, Wu X, Zhang G, Xu Z, Zhang Y, Gautam V, et al. Isoflurane Facilitates Synaptic NMDA Receptor Endocytosis in Mice Primary Neurons. Current Molecular Medicine. 13(4):488–98. doi: 10.2174/1566524011313040003. [DOI] [PubMed] [Google Scholar]
- 46.Jin J, Gong K, Zou X, Wang R, Lin Q, Chen J. The blockade of NMDA receptor ion channels by ketamine is enhanced in developing rat cortical neurons. Neurosci Lett. 2013;539:11–5. doi: 10.1016/j.neulet.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu CL, Gao M, Jin GZ, Zhen X. GABA neurons in the ventral tegmental area responding to peripheral sensory input. PLoS One. 2012;7(12):e51507. doi: 10.1371/journal.pone.0051507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F, Patterson TA, Sadovova N, Zhang X, Liu S, Zou X, et al. Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol Sci. 2013;131(2):548–57. doi: 10.1093/toxsci/kfs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trudell JR, Bertaccini E, Maciver MB. Teaching an old GABA receptor new tricks. Anesth Analg. 2012;115(2):270–3. doi: 10.1213/ANE.0b013e31824a0b3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang S, Tian Y, Song L, Lim G, Tan Y, You Z, et al. Exacerbated mechanical hyperalgesia in rats with genetically predisposed depressive behavior: role of melatonin and NMDA receptors. Pain. 2012;153(12):2448–57. doi: 10.1016/j.pain.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wai MS, Luan P, Jiang Y, Chan WM, Tsui TY, Tang HC, et al. Long term ketamine and ketamine plus alcohol toxicity - what can we learn from animal models? Mini Rev Med Chem. 2013;13(2):273–9. doi: 10.2174/1389557511313020009. [DOI] [PubMed] [Google Scholar]
- 52.Straiko MM, Young C, Cattano D, Creeley CE, Wang H, Smith DJ, et al. Lithium protects against anesthesia-induced developmental neuroapoptosis. Anesthesiology. 2009;110(4):862–8. doi: 10.1097/ALN.0b013e31819b5eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson JS, Brown SA, Khurdayan V, Zeynalzadedan A, Sullivan PG, Scheff SW. Early effects of tribromoethanol, ketamine/xylazine, pentobarbitol, and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp Med. 2002;52(1):63–7. [PubMed] [Google Scholar]
- 54.Wong YW, Lam LH, Tang HC, Liang Y, Tan S, Yew DT. Intestinal and liver changes after chronic ketamine and ketamine plus alcohol treatment. Microsc Res Tech. 2012;75(9):1170–5. doi: 10.1002/jemt.22045. [DOI] [PubMed] [Google Scholar]
- 55.Bai X, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Zaja I, et al. Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species-mediated mitochondrial pathway. Anesth Analg. 2013;116(4):869–80. doi: 10.1213/ANE.0b013e3182860fc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bosnjak ZJ, Yan Y, Canfield S, Muravyeva MY, Kikuchi C, Wells CW, et al. Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Curr Drug Saf. 2012;7(2):106–19. doi: 10.2174/157488612802715663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116(2):372–84. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brambrink AM, Orfanakis A, Kirsch JR. Anesthetic neurotoxicity. Anesthesiol Clin. 2012;30(2):207–28. doi: 10.1016/j.anclin.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Braun S, Gaza N, Werdehausen R, Hermanns H, Bauer I, Durieux ME, et al. Ketamine induces apoptosis via the mitochondrial pathway in human lymphocytes and neuronal cells. Br J Anaesth. 2010;105(3):347–54. doi: 10.1093/bja/aeq169. [DOI] [PubMed] [Google Scholar]
- 60.Braun S, Werdehausen R, Gaza N, Hermanns H, Kremer D, Kury P, et al. Benzethonium increases the cytotoxicity of S(+)-ketamine in lymphoma, neuronal, and glial cells. Anesth Analg. 2010;111(6):1389–93. doi: 10.1213/ANE.0b013e3181f690e4. [DOI] [PubMed] [Google Scholar]
- 61.Persson J. Wherefore ketamine? Curr Opin Anaesthesiol. 2010;23(4):455–60. doi: 10.1097/ACO.0b013e32833b49b3. [DOI] [PubMed] [Google Scholar]
- 62.Ponten E, Viberg H, Gordh T, Eriksson P, Fredriksson A. Clonidine abolishes the adverse effects on apoptosis and behaviour after neonatal ketamine exposure in mice. Acta Anaesthesiol Scand. 2012;56(8):1058–65. doi: 10.1111/j.1399-6576.2012.02722.x. [DOI] [PubMed] [Google Scholar]
- 63.Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146(2):189–97. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of N-methyl-D-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107(3):427–36. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 65.Keel M, Mica L, Stover J, Stocker R, Trentz O, Harter L. Thiopental-induced apoptosis in lymphocytes is independent of CD95 activation. Anesthesiology. 2005;103(3):576–84. doi: 10.1097/00000542-200509000-00021. [DOI] [PubMed] [Google Scholar]
- 66.Kim HS, Hwang KC, Park WK. Cardioprotection via modulation of calcium homeostasis by thiopental in hypoxia-reoxygenated neonatal rat cardiomyocytes. Yonsei Med J. 2010;51(2):187–96. doi: 10.3349/ymj.2010.51.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roesslein M, Schibilsky D, Muller L, Goebel U, Schwer C, Humar M, et al. Thiopental protects human T lymphocytes from apoptosis in vitro via the expression of heat shock protein 70. J Pharmacol Exp Ther. 2008;325(1):217–25. doi: 10.1124/jpet.107.133108. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Zhen Y, Dong Y, Xu Z, Yue Y, Golde TE, et al. Anesthetic propofol attenuates the isoflurane-induced caspase-3 activation and Abeta oligomerization. PLoS One. 2011;6(11):e27019. doi: 10.1371/journal.pone.0027019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H, Xue Z, Wang Q, Feng X, Shen Z. Propofol protects hepatic L02 cells from hydrogen peroxide-induced apoptosis via activation of extracellular signal-regulated kinases pathway. Anesth Analg. 2008;107(2):534–40. doi: 10.1213/ane.0b013e3181770be9. [DOI] [PubMed] [Google Scholar]
- 70.Wu RS, Wu KC, Yang JS, Chiou SM, Yu CS, Chang SJ, et al. Etomidate induces cytotoxic effects and gene expression in a murine leukemia macrophage cell line (RAW264.7). Anticancer Res. 2011;31(6):2203–8. [PubMed] [Google Scholar]
- 71.Hwang L, Choi I-Y, Kim S-E, Ko I-G, Shin M-S, Kim C-J, et al. Dexmedetomidine ameliorates intracerebral hemorrhage-induced memory impairment by inhibiting apoptosis and enhancing brain-derived neurotrophic factor expression in the rat hippocampus. International journal of molecular medicine. 2013;31(5):1047. doi: 10.3892/ijmm.2013.1301. [DOI] [PubMed] [Google Scholar]
- 72.Kalis M, Kumar R, Janciauskiene S, Salehi A, Cilio CM. alpha 1-antitrypsin enhances insulin secretion and prevents cytokine-mediated apoptosis in pancreatic beta-cells. Islets. 2010;2(3):185–9. doi: 10.4161/isl.2.3.11654. [DOI] [PubMed] [Google Scholar]
- 73.Kishikawa H, Kobayashi K, Takemori K, Okabe T, Ito K, Sakamoto A. The effects of dexmedetomidine on human neutrophil apoptosis. Biomed Res. 2008;29(4):189–94. doi: 10.2220/biomedres.29.189. [DOI] [PubMed] [Google Scholar]
- 74.Koca U, Olguner CG, Ergur BU, Altekin E, Tasdogen A, et al. The Effects of Dexmedetomidine on Secondary Acute Lung and Kidney Injuries in the Rat Model of Intra-Abdominal Sepsis. The Scientific World Journal. 2013;2013:11. doi: 10.1155/2013/292687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang F, Ding T, Yu L, Zhong Y, Dai H, Yan M. Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through the I2 imidazoline receptor-PI3K/AKT pathway in rat C6 glioma cells. J Pharm Pharmacol. 2012;64(1):120–7. doi: 10.1111/j.2042-7158.2011.01382.x. [DOI] [PubMed] [Google Scholar]
- 76.Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao X, et al. Dexmedetomidine administration before, but not after, ischemia attenuates intestinal injury induced by intestinal ischemia-reperfusion in rats. Anesthesiology. 2012;116(5):1035–46. doi: 10.1097/ALN.0b013e3182503964. [DOI] [PubMed] [Google Scholar]
- 77.Dabbagh A, Rajaei S. Halothane: Is there still any place for using the gas as an anesthetic? Hepat Mon. 2011;11(7):511–2. [PMC free article] [PubMed] [Google Scholar]
- 78.Mahboobi N, Esmaeili S, Safari S, Habibollahi P, Dabbagh A, Alavian SM. Halothane: how should it be used in a developing country? East Mediterr Health J. 2012;18(2):159–64. doi: 10.26719/2012.18.2.159. [DOI] [PubMed] [Google Scholar]
- 79.Irwin MG, Trinh T, Yao CL. Occupational exposure to anaesthetic gases: a role for TIVA. Expert Opin Drug Saf. 2009;8(4):473–83. doi: 10.1517/14740330903003778. [DOI] [PubMed] [Google Scholar]
- 80.Kvolik S, Glavas-Obrovac L, Bares V, Karner I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. 2005;77(19):2369–83. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 81.Brozovic G, Orsolic N, Knezevic F, Horvat Knezevic A, Benkovic V, Sakic K, et al. Genotoxicity and cytotoxicity of cisplatin treatment combined with anaesthetics on EAT cells in vivo. Onkologie. 2009;32(6):337–43. doi: 10.1159/000218066. [DOI] [PubMed] [Google Scholar]
- 82.Stephanova E, Topouzova-Hristova T, Hazarosova R, Moskova V. Halothane-induced alterations in cellular structure and proliferation of A549 cells. Tissue Cell. 2008;40(6):397–404. doi: 10.1016/j.tice.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 83.Edmands SD, Ladow E, Hall AC. Microarray analyses of genes regulated by isoflurane anesthesia in vivo: a novel approach to identifying potential preconditioning mechanisms. Anesth Analg. 2013;116(3):589–95. doi: 10.1213/ANE.0b013e31827b27b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim M, Park SW, D'Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against renal ischemia-reperfusion-induced liver and intestine injury. Anesthesiology. 2011;114(2):363–73. doi: 10.1097/ALN.0b013e3182070c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim M, Park SW, D'Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against bilateral nephrectomy-induced liver and intestine dysfunction. Am J Physiol Renal Physiol. 2011;300(1):F167–76. doi: 10.1152/ajprenal.00467.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiang L, Tan JW, Huang LJ, Jia L, Liu YQ, Zhao YQ, et al. Inhalation of hydrogen gas reduces liver injury during major hepatotectomy in swine. World J Gastroenterol. 2012;18(37):5197–204. doi: 10.3748/wjg.v18.i37.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang L, Luo N, Liu J, Duan Z, Du G, Cheng J, et al. Emulsified isoflurane preconditioning protects against liver and lung injury in rat model of hemorrhagic shock. J Surg Res. 2011;171(2):783–90. doi: 10.1016/j.jss.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 88.Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29(7):1002–10. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mongan PD, Capacchione J, West S, Karaian J, Dubois D, Keneally R, et al. Pyruvate improves redox status and decreases indicators of hepatic apoptosis during hemorrhagic shock in swine. Am J Physiol Heart Circ Physiol. 2002;283(4):H1634–44. doi: 10.1152/ajpheart.01073.2001. [DOI] [PubMed] [Google Scholar]
- 90.Stephanova E, Topouzova-Hristova T, Konakchieva R. Mitochondria are involved in stress response of A549 alveolar cells to halothane toxicity. Toxicol In Vitro. 2008;22(3):688–94. doi: 10.1016/j.tiv.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Topouzova-Hristova T, Hazarosova R, Bandreva B, Stephanova E. Halothane does not directly interact with genome DNA of A549 cells. Folia Biol (Praha). 2007;53(5):176–82. [PubMed] [Google Scholar]
- 92.Wu X, Prueckner S, Rollwagen F, Kentner R, Stezoski J, Kochanek PM, et al. Gut damage during hemorrhagic shock: effects on survival of oral or enteral interleukin-6. Shock. 2001;16(6):449–53. doi: 10.1097/00024382-200116060-00008. [DOI] [PubMed] [Google Scholar]
- 93.Dabbagh A, Rajaei S. Xenon: a solution for anesthesia in liver disease? Hepat Mon. 2012;12(11):e8437. doi: 10.5812/hepatmon.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Allegaert K. The clinical pharmacology of short acting analgo-sedatives in neonates. Curr Clin Pharmacol. 2011;6(4):222–6. doi: 10.2174/157488411798375912. [DOI] [PubMed] [Google Scholar]
- 95.Avella DM, Kimchi ET, Donahue RN, Tagaram HR, McLaughlin PJ, Zagon IS, et al. The opioid growth factor-opioid growth factor receptor axis regulates cell proliferation of human hepatocellular cancer. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R459–66. doi: 10.1152/ajpregu.00646.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boronat MA, Garcia-Fuster MJ, Garcia-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001;134(6):1263–70. doi: 10.1038/sj.bjp.0704364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delogu G, Moretti S, Antonucci A, Marandola M, Tellan G, Sale P, et al. Apoptogenic effect of fentanyl on freshly isolated peripheral blood lymphocytes. J Trauma. 2004;57(1):75–81. doi: 10.1097/01.ta.0000075349.66640.3e. [DOI] [PubMed] [Google Scholar]
- 98.Friesen C, Roscher M, Alt A, Miltner E. Methadone, commonly used as maintenance medication for outpatient treatment of opioid dependence, kills leukemia cells and overcomes chemoresistance. Cancer Res. 2008;68(15):6059–64. doi: 10.1158/0008-5472.CAN-08-1227. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Fuster MJ, Ramos-Miguel A, Miralles A, Garcia-Sevilla JA. Opioid receptor agonists enhance the phosphorylation state of Fas-associated death domain (FADD) protein in the rat brain: functional interactions with casein kinase Ialpha, Galpha(i) proteins, and ERK1/2 signaling. Neuropharmacology. 2008;55(5):886–99. doi: 10.1016/j.neuropharm.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 100.Garcia-Fuster MJ, Ramos-Miguel A, Rivero G, La Harpe R, Meana JJ, Garcia-Sevilla JA. Regulation of the extrinsic and intrinsic apoptotic pathways in the prefrontal cortex of short- and long-term human opiate abusers. Neuroscience. 2008;157(1):105–19. doi: 10.1016/j.neuroscience.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Jaume M, Jacquet S, Cavailles P, Mace G, Stephan L, Blanpied C, et al. Opioid receptor blockade reduces Fas-induced hepatitis in mice. Hepatology. 2004;40(5):1136–43. doi: 10.1002/hep.20428. [DOI] [PubMed] [Google Scholar]
- 102.Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50,000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim HS, Cho JE, Hong SW, Kim SO, Shim JK, Kwak YL. Remifentanil protects myocardium through activation of anti-apoptotic pathways of survival in ischemia-reperfused rat heart. Physiol Res. 2010;59(3):347–56. doi: 10.33549/physiolres.931772. [DOI] [PubMed] [Google Scholar]
- 104.Kuniyasu H, Luo Y, Fujii K, Sasahira T, Moriwaka Y, Tatsumoto N, et al. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut. 2010;59(3):348–56. doi: 10.1136/gut.2009.178376. [DOI] [PubMed] [Google Scholar]
- 105.Luo FC, Zhao L, Deng J, Liang M, Zeng XS, Liu H, et al. Geranylgeranylacetone protects against morphine-induced hepatic and renal damage in mice. Mol Med Rep. 2013;7(2):694–700. doi: 10.3892/mmr.2012.1217. [DOI] [PubMed] [Google Scholar]
- 106.Mani AR, Moore KP. New insights into the role of endogenous opioids in the pathogenesis of gastrointestinal and liver disease. Gut. 2009;58(7):893–5. doi: 10.1136/gut.2007.141648. [DOI] [PubMed] [Google Scholar]
- 107.Park SW, Yi JW, Kim YM, Kang JM, Kim DO, Shin MS, et al. Remifentanil alleviates transient cerebral ischemia-induced memory impairment through suppression of apoptotic neuronal cell death in gerbils. Korean J Anesthesiol. 2011;61(1):63–8. doi: 10.4097/kjae.2011.61.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Payabvash S, Beheshtian A, Salmasi AH, Kiumehr S, Ghahremani MH, Tavangar SM, et al. Chronic morphine treatment induces oxidant and apoptotic damage in the mice liver. Life Sci. 2006;79(10):972–80. doi: 10.1016/j.lfs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Payabvash S, Kiumehr S, Nezami BG, Zandieh A, Anvari P, Tavangar SM, et al. Endogenous opioids modulate hepatocyte apoptosis in a rat model of chronic cholestasis: the role of oxidative stress. Liver Int. 2007;27(4):538–47. doi: 10.1111/j.1478-3231.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Wong GT, Man K, Irwin MG. Pretreatment with intrathecal or intravenous morphine attenuates hepatic ischaemia-reperfusion injury in normal and cirrhotic rat liver. Br J Anaesth. 2012;109(4):529–39. doi: 10.1093/bja/aes209. [DOI] [PubMed] [Google Scholar]
- 111.Wu Q-l, Shen T, Ma H, Wang J-k. Sufentanil postconditioning protects the myocardium from ischemia-reperfusion via PI3K/Akt-GSK-3β pathway. The Journal of surgical research. 2012;178(2):563–570. doi: 10.1016/j.jss.2012.05.081. [DOI] [PubMed] [Google Scholar]
- 112.Yang LQ, Tao KM, Liu YT, Cheung CW, Irwin MG, Wong GT, et al. Remifentanil preconditioning reduces hepatic ischemia-reperfusion injury in rats via inducible nitric oxide synthase expression. Anesthesiology. 2011;114(5):1036–47. doi: 10.1097/ALN.0b013e3182104956. [DOI] [PubMed] [Google Scholar]
- 113.Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochemistry International. 2002;40(6):475–486. doi: 10.1016/S0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 114.Cellai C, Balliu M, Laurenzana A, Guandalini L, Matucci R, Miniati D, et al. The new low-toxic histone deacetylase inhibitor S-(2) induces apoptosis in various acute myeloid leukaemia cells. J Cell Mol Med. 2012;16(8):1758–65. doi: 10.1111/j.1582-4934.2011.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Decaudin D, Castedo M, Nemati F, Beurdeley-Thomas A, De Pinieux G, Caron A, et al. Peripheral benzodiazepine receptor ligands reverse apoptosis resistance of cancer cells in vitro and in vivo. Cancer Res. 2002;62(5):1388–93. [PubMed] [Google Scholar]
- 116.Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res. 2004;153(2):367–76. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 117.Goto Y, O'Malley C, Fanning NF, Wang J, Redmond HP, Shorten GD. Benzodiazepines inhibit the rate of neutrophil apoptosis. Ir J Med Sci. 2003;172(4):191–4. doi: 10.1007/BF02915288. [DOI] [PubMed] [Google Scholar]
- 118.Pavlovic V, Pavlovic D, Kamenov B, Sarac M, Peric Z, Velojic M. Protective role of vitamin C in diazepam-induced apoptosis in rat thymocytes. Bratisl Lek Listy. 2012;113(6):350–3. doi: 10.4149/bll_2012_079. [DOI] [PubMed] [Google Scholar]
- 119.Sarissky M, Lavicka J, Kocanova S, Sulla I, Mirossay A, Miskovsky P, et al. Diazepam enhances hypericin-induced photocytotoxicity and apoptosis in human glioblastoma cells. Neoplasma. 2005;52(4):352–9. [PubMed] [Google Scholar]
- 120.Tanaka K, Kobayashi N, Gutierrez AS, Rivas-Carrillo JD, Navarro-Alvarez N, Chen Y, et al. Prolonged survival of mice with acute liver failure with transplantation of monkey hepatocytes cultured with an antiapoptotic pentapeptide V5. Transplantation. 2006;81(3):427–37. doi: 10.1097/01.tp.0000188693.48882.18. [DOI] [PubMed] [Google Scholar]
- 121.Vitale I, Antoccia A, Crateri P, Leone S, Arancia G, Tanzarella C. Caspase-independent apoptosis is activated by diazepam-induced mitotic failure in HeLa cells, but not in human primary fibroblasts. Apoptosis. 2005;10(4):909–20. doi: 10.1007/s10495-005-2948-y. [DOI] [PubMed] [Google Scholar]
- 122.Cui W, Li W, Zhao Y, Mak S, Gao Y, Luo J, et al. Preventing H(2)O(2)-induced apoptosis in cerebellar granule neurons by regulating the VEGFR-2/Akt signaling pathway using a novel dimeric antiacetylcholinesterase bis(12)-hupyridone. Brain Res. 2011;1394:14–23. doi: 10.1016/j.brainres.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 123.Rieder J, Lirk P, Bodrogi F, Sawires M, Gruber G, Hoffmann G. Cisatracurium, but not mivacurium, induces apoptosis in human umbilical vein endothelial cells in vitro. Eur J Anaesthesiol. 2005;22(1):16–9. doi: 10.1017/s0265021505000049. [DOI] [PubMed] [Google Scholar]
- 124.Delogu G, Moretti S, Marcellini S, Antonucci A, Tellan G, Marandola M, et al. Pancuronium bromide, a non-depolarizing muscle relaxant which promotes apoptosis of blood lymphocytes in vitro. Acta Anaesthesiol Scand. 2003;47(9):1138–44. doi: 10.1034/j.1399-6576.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 125.Werdehausen R, Braun S, Hermanns H, Kremer D, Kury P, Hollmann MW, et al. The influence of adjuvants used in regional anesthesia on lidocaine-induced neurotoxicity in vitro. Reg Anesth Pain Med. 2011;36(5):436–43. doi: 10.1097/AAP.0b013e318226ba62. [DOI] [PubMed] [Google Scholar]
- 126.Cazanave S, Berson A, Haouzi D, Vadrot N, Fau D, Grodet A, et al. High hepatic glutathione stores alleviate Fas-induced apoptosis in mice. J Hepatol. 2007;46(5):858–68. doi: 10.1016/j.jhep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 127.Haouzi D, Lekehal M, Tinel M, Vadrot N, Caussanel L, Letteron P, et al. Prolonged, but not acute, glutathione depletion promotes Fas-mediated mitochondrial permeability transition and apoptosis in mice. Hepatology. 2001;33(5):1181–8. doi: 10.1053/jhep.2001.24235. [DOI] [PubMed] [Google Scholar]
- 128.Oishi K, Hagiwara S, Koga S, Kawabe S, Uno T, Iwasaka H, et al. The vitamin E derivative, EPC-K1, suppresses inflammation during hepatic ischemia-reperfusion injury and exerts hepatoprotective effects in rats. J Surg Res. 2012;176(1):164–70. doi: 10.1016/j.jss.2011.03.080. [DOI] [PubMed] [Google Scholar]
- 129.Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21(2):111–27. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 130.Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99(23):15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11(9):1603–15. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 132.Shen SQ, Zhang Y, Xiong CL. The protective effects of 17beta-estradiol on hepatic ischemia-reperfusion injury in rat model, associated with regulation of heat-shock protein expression. J Surg Res. 2007;140(1):67–76. doi: 10.1016/j.jss.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 133.Yon JH, Carter LB, Reiter RJ, Jevtovic-Todorovic V. Melatonin reduces the severity of anesthesia-induced apoptotic neurodegeneration in the developing rat brain. Neurobiol Dis. 2006;21(3):522–30. doi: 10.1016/j.nbd.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 134.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110(5):1077–85. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 135.Zhang B, Wei X, Cui X, Kobayashi T, Li W. Effects of heme oxygenase 1 on brain edema and neurologic outcome after cardiopulmonary resuscitation in rats. Anesthesiology. 2008;109(2):260–8. doi: 10.1097/ALN.0b013e31817f5c2e. [DOI] [PubMed] [Google Scholar]
- 136.Natori S, Higuchi H, Contreras P, Gores GJ. The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury. Liver Transpl. 2003;9(3):278–84. doi: 10.1053/jlts.2003.50019. [DOI] [PubMed] [Google Scholar]
- 137.Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, Okitsu T, Chen Y, et al. Cell-permeable pentapeptide V5 inhibits apoptosis and enhances insulin secretion, allowing experimental single-donor islet transplantation in mice. Diabetes. 2007;56(5):1259–67. doi: 10.2337/db06-1679. [DOI] [PubMed] [Google Scholar]
- 138.Wei ZZ, Xia SS. gamma-hydroxybutyrate protects the liver from warm ischemia-reperfusion injury in rat. Hepatobiliary Pancreat Dis Int. 2004;3(2):245–9. [PubMed] [Google Scholar]
- 139.Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, et al. Clinical Trial of the Pan-Caspase Inhibitor, IDN-6556, in Human Liver Preservation Injury. Am J Transplant. 2007;7(1):218–25. doi: 10.1111/j.1600-6143.2006.01595.x. [DOI] [PubMed] [Google Scholar]
- 140.Hayakawa Y, Chandra M, Miao W, Shirani J, Brown JH, Dorn GW, 2nd, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108(24):3036–41. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 141.Hoglen NC, Hirakawa BP, Fisher CD, Weeks S, Srinivasan A, Wong AM, et al. Characterization of the caspase inhibitor IDN-1965 in a model of apoptosis-associated liver injury. J Pharmacol Exp Ther. 2001;297(2):811–8. [PubMed] [Google Scholar]
- 142.Matsushita T, Yagi T, Hardin JA, Cragun JD, Crow FW, Bergen HR, 3rd, et al. Apoptotic cell death and function of cryopreserved porcine hepatocytes in a bioartificial liver. Cell Transplant. 2003;12(2):109–21. doi: 10.3727/000000003108746696. [DOI] [PubMed] [Google Scholar]
- 143.Cazanave S, Vadrot N, Tinel M, Berson A, Letteron P, Larosche I, et al. Ibuprofen administration attenuates serum TNF-alpha levels, hepatic glutathione depletion, hepatic apoptosis and mouse mortality after Fas stimulation. Toxicol Appl Pharmacol. 2008;231(3):336–43. doi: 10.1016/j.taap.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 144.Hafenrichter DG, Roland CR, Mangino MJ, Flye MW. The Kupffer cell in endotoxin tolerance: mechanisms of protection against lethal endotoxemia. Shock. 1994;2(4):251–6. [PubMed] [Google Scholar]
- 145.Hiramoto T, Chida Y, Sonoda J, Yoshihara K, Sudo N, Kubo C. The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via alpha7 nicotinic acetylcholine receptor. Gastroenterology. 2008;134(7):2122–31. doi: 10.1053/j.gastro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 146.Li G, Han C, Xu L, Lim K, Isse K, Wu T. Cyclooxygenase-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor. Hepatology. 2009;50(3):834–43. doi: 10.1002/hep.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54(4):1217–26. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 148.Matsuki Y, Li L, Hsu HC, Yang PA, Zheng R, Edwards CK, 3rd, et al. Soluble Fas gene therapy protects against Fas-mediated apoptosis of hepatocytes but not the lethal effects of Fas-induced TNF-alpha production by Kupffer cells. Cell Death Differ. 2002;9(6):626–35. doi: 10.1038/sj/cdd/4401016. [DOI] [PubMed] [Google Scholar]
- 149.Ohtaki Y, Yamaguchi K, Yu Z, Kumamoto H, Shimauchi H, Iwakura Y, et al. Hepatic platelet accumulation in Fas-mediated hepatitis in mice. Int Immunopharmacol. 2009;9(9):1071–8. doi: 10.1016/j.intimp.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 150.Yee SB, Ganey PE, Roth RA. The role of Kupffer cells and TNF-alpha in monocrotaline and bacterial lipopolysaccharide-induced liver injury. Toxicol Sci. 2003;71(1):124–32. doi: 10.1093/toxsci/71.1.124. [DOI] [PubMed] [Google Scholar]
- 151.Wang X, Cederbaum AI. S-adenosyl-L-methionine decreases the elevated hepatotoxicity induced by Fas agonistic antibody plus acute ethanol pretreatment in mice. Arch Biochem Biophys. 2008;477(1):1–11. doi: 10.1016/j.abb.2008.04.033. [DOI] [PubMed] [Google Scholar]