Abstract
Gobionine species belonging to the genera Pseudorasbora, Pseudopungtungia, and Pungtungia (Teleostei; Cypriniformes; Cyprinidae) have been heavily studied because of problems on taxonomy, threats of extinction, invasion, and human health. Nucleotide sequences of three nuclear genes, that is, recombination activating protein gene 1 (rag1), recombination activating gene 2 (rag2), and early growth response 1 gene (egr1), from Pseudorasbora, Pseudopungtungia, and Pungtungia species residing in China, Japan, and Korea, were analyzed to elucidate their intergeneric and interspecific phylogenetic relationships. In the phylogenetic tree inferred from their multiple gene sequences, Pseudorasbora, Pseudopungtungia and Pungtungia species ramified into three phylogenetically distinct clades; the “tenuicorpa” clade composed of Pseudopungtungia tenuicorpa, the “parva” clade composed of all Pseudorasbora species/subspecies, and the “herzi” clade composed of Pseudopungtungia nigra, and Pungtungia herzi. The genus Pseudorasbora was recovered as monophyletic, while the genus Pseudopungtungia was recovered as polyphyletic. Our phylogenetic result implies the unstable taxonomic status of the genus Pseudopungtungia.
1. Introduction
Species of the subfamily Gobioninae (or gudgeons) (Teleostei; Cypriniformes; Cyprinidae) are mostly distributed in East Asia [1–4] except several species belonging to the genera Gobio and Romanogobio in Europe [5]. Among them, the slender topmouth gudgeon Pseudorasbora elongata endemic to China faces a high risk of extinction due to habitat degradation and loss and fishing [6]. Pseudorasbora interrupta was recently erected as novel species [7]. The topmouth gudgeon (or the stone morocco) Pseudorasbora parva, which was first reported from Nagasaki, Japan, is widely distributed in East Asia [1] and has rapidly extended its habitats either naturally or artificially to all of Europe and parts of North Africa during the last 50 years [8, 9]. Moreover, this freshwater fish is notorious as the second intermediate host of the liver fluke Clonorchis sinensis [10] and is a carrier of the rosette agent (Sphaerothecum destruens) which inhibits spawning and causes increased mortality in native European fish species [11]. Pseudorasbora pumila pumila and Pseudorasbora pumila subsp. originally inhabited limited areas in northern and middle parts on Honshu of Japan, respectively, but their distributions have been further restricted to patchily discrete locations due to loss of their habitats and invasion of Pseudorasbora parva into their habitats. Thus, the Japanese Ministry of the Environment designated them as critically endangered subspecies. The striped shiner Pungtungia herzi Herzenstein, which was first reported from Chungju, Korea [1, 4, 12], resides in China, Japan, and Korea [1]. The black shiner Pseudopungtungia nigra endemic to Korea was reported as a novel genus and species by Mori [13]. This species was reported to inhabit the Geum River, the Mangyeong River, and the Ungcheon Stream in Korea [14, 15] but believed to be regionally extinct in the latter due to water impoundment and pollution [4, 16]. Because of the threat of extinction, it was designated as an endangered species in 1997 by the Ministry of Environment of Korea and protected by national legislation. The slender shiner Pseudopungtungia tenuicorpa endemic to Korea, inhabits the upper reaches of the Han and Imjin Rivers [4, 16]. This fish species was also designated as an endangered species since 2005 due to deterioration of its natural habitats.
Despite great concerns on conservation the taxonomic positions of Pseudorasbora elongata, Pseudopungtungia nigra, and Pseudopungtungia tenuicorpa are still unsettled. For example, it was interesting that Pseudorasbora elongata showed closer phylogenetic affiliation to Pungtungia herzi rather than congeneric species based on the mitochondrially encoded cytochrome b gene (mt-cyb) sequences [17]. Meanwhile, Kang [18] suggested transferring two Pseudopungtungia species to the genus Pungtungia based on synapomorphic osteological characters such as jaws supporting the form of mouth, suspensorial elements, and hyoid arch, but they still remain in the former genus [4].
Despite problems with taxonomy, threats of extinction, invasion, and human health, there are not enough molecular data for Pseudorasbora, Pseudopungtungia, and Pungtungia species to provide compelling answers to questions about their intergeneric and interspecific phylogenetic relationships (e.g., Yang et al. [17]), genetic variation for conservation (e.g., Konishi and Takata [19]), phylogeography (e.g., Watanabe et al. [20]), divergence time estimation (e.g., Liu et al. [21]), and developing monitoring markers for tracing their dispersal route. The genetic data available for those species up to date are mostly composed of nucleotide sequences from a single mitochondrially encoded gene: the mt-cyb, which is maternally inherited and thus provides insufficient evidence for resolving their phylogenetic relationships.
Recently, phylogenetic markers of nuclear genes were deciphered and successfully applied for reconstructing phylogenetic trees across diverse Cypriniform species [22–25]. In this study, we analyzed multiple nuclear gene sequences of eight species and subspecies of Pseudorasbora, Pseudopungtungia, and Pungtungia residing in China, Japan, and Korea to elucidate their molecular phylogenetic relationships.
2. Materials and Methods
2.1. Specimen and Genomic DNA Extraction
Fish specimens used in this study were captured with a spoon net (mesh size: 4 × 4 mm) from river drainages of China, Japan, and Korea. The specimens we used were transported to the laboratory alive and killed rapidly with formaldehyde after anaesthetizing them by submerging into a solution containing a fish anaesthetic agent, Tricaine Methane Sulphonate (MS222) (Aqualife TMS, Syndel Laboratories, Ltd., Canada). The specimens were deposited in the fish collection of Soonchunhyang University (SUC; Asan, Republic of Korea), Chinese Academy of Sciences (Wuhan, China), and South China Normal University (Guangzhou, China). Their detailed sampling information was provided in Table 1.
Table 1.
Sampling information of gobionine species used in the phylogenetic analyses.
| Species | Voucher no. | Sampling site | Drainage | GenBank acc. no. | ||
|---|---|---|---|---|---|---|
| rag1 | rag2 | egr1 | ||||
| Pseudopungtungia nigra | SUC-0777 | Geumsan, Korea | Geum River | KF468619 | KF468608 | KF468597 |
| Pseudopungtungia tenuicorpa | SUC-0683 | Yangpyeong, Korea | Han River | KF468618 | KF468607 | KF468596 |
| Pseudorasbora e lo ng at a* | — | China | — | KF468621 | KF468610 | KF468599 |
| Pseudorasbora i nt er ru pt a* | — | China | — | KF468623 | KF468612 | KF468601 |
| Pseudorasbora parva | SUC-6658 | Kasumigaura, Ibaraki Pref., Japan | A small tributary of Lake Kasumigaura | KF468626 | KF468615 | KF468604 |
| Pseudorasbora pumila pumila | SUC-6656 | Nagano, Nagano Pref., Japan | An irrigative pond | KF468624 | KF468613 | KF468602 |
| Pseudorasbora pumila subsp. | SUC-6657 | Bred in Lake Biwa Museum, Shiga Pref., Japan | — | KF468625 | KF468614 | KF468603 |
| Pungtungia herzi | SUC-0706 | Yangpyeong, Korea | Han River | KF468620 | KF468609 | KF468598 |
| Sarcocheilichthys nigripinnis morii | SUC-1833 | Seocheon, Korea | Gilsan Stream | KF468617 | KF468606 | KF468595 |
| Sarcocheilichthys variegatus wakiyae | SUC-0774 | Geumsan, Korea | Geum River | KF468616 | KF468605 | KF468594 |
*Detailed information is not provided by the authors for protecting their natural habitats.
A small piece of a pectoral or anal fin tissue was excised from each specimen to extract genomic DNA (gDNA). It was incubated in 500 μL of TNES-Urea buffer (10 mM Tris-HCl, pH 8.0; 125 mM NaCl; 10 mM EDTA, pH 8.0; 1% SDS; 6 M urea; [26]) containing 100 μg of proteinase K (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for a week, followed by separation with phenol : chloroform : isoamyl alcohol (25 : 24 : 1) solution and ethanol precipitation. The extracted gDNA was finally resuspended in 50 μL of TE buffer (10 mM Tris-HCl, pH 8.0; 1 mM EDTA, pH 8.0). Its quantity and quality were checked using a spectrophotometer, NanoDrop 1000 (Thermo Fisher Scientific, Wilmington, DE, USA) and by electrophoresis in a 0.7% agarose gel after staining with GelRed Nucleic Acid Gel Stain (Biotium, Hayward, CA, USA).
2.2. PCR Amplification and Sequencing
For phylogenetic analysis, three nuclear genes, that is, recombination activating gene 1 (rag1), recombination activating gene 2 (rag2), and early growth response 1 gene (egr1), were selected based on previous studies [23, 24]. Information for the primers used in this study is shown in Table 2. PCR reactions were carried out in a 20 μL reaction volume using AccuPower PCR Premix (Bioneer, Daejeon, Republic of Korea), including 50 ng of gDNA and 0.2 μM of forward and reverse primers.
Table 2.
Information of PCR primers used in this study.
| Gene | Primer | Sequence (5′ → 3′) | Reference |
|---|---|---|---|
| Recombination activating gene 1 (rag1) | RAG1-1495f3 | CAGTAYCAYAAGATGTACCG | Kim and Bang [27] |
| RAG1-3067r | TTGTGAGCYTCCATRAACTT | Kim and Bang [27] | |
| Recombination activating gene 2 (rag2) | RAG2-108f | CCVARACGCTCATGTCCAAC | This study |
| RAG2-1324r | TGGARCAGWAGATCATKGC | This study | |
| Early growth response 1 gene (egr1) | EGR1-291f | CACAGGMCGTTTCACCCTYG | Modified from Chen et al. [24] |
| EGR1-1456r | GACAGGRGARCTGTAGATGTT | Modified from Chen et al. [24] |
PCR was run with the following thermal cycling profile in a DNA Engine DYAD Peltier Thermal Cycler (MJ Research Inc., Waltham, MA, USA): an initial denaturation at 94°C for 3 min, 25–35 cycles of denaturation at 94°C for 30 s, annealing at 50–52°C for 30 s, and elongation at 72°C for 1 min. The reaction was completed with a final elongation at 72°C for 7 min. The PCR product was purified with the AccuPrep PCR Purification Kit (Bioneer). After cycle sequencing with the ABI PRISM BigDye Terminator v3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems Inc., Foster City, CA, USA), the purified product was directly sequenced on an ABI 3730xl DNA Analyzer (Applied Biosystems Inc.) with PCR primers by a commercial company, Macrogen Inc. (Seoul, Republic of Korea). Electropherograms were assembled in BioEdit 7.0.5 [28] and corrected manually. The sequences analyzed in this study were deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) under accession numbers KF468594-KF468626 (Table 1).
2.3. Phylogenetic Analyses
Nucleotide sequences of the rag1, rag2, and egr1 genes of eight Pseudorasbora, Pseudopungtungia, and Pungtungia species analyzed in this study (Table 1) were aligned with ClustalW in BioEdit [28]. Two Sarcocheilichthys species were used as outgroups, based on previous molecular phylogenetic [17, 25, 29] and morphological [18] studies. The three nuclear genes were concatenated according to genes. There were no indels in the nucleotide matrix that consisted of 1,488, 1,120, and 1,087 bp for each gene, respectively. The nucleotide matrix is available upon request.
Maximum likelihood (ML) analysis was performed with RAxML 7.0.4 [30, 31]. The concatenated nucleotide matrix was partitioned according to genes. The RAxML search was executed for the best-scoring ML tree in one single program run (the “-f a” option) instead of the default maximum parsimony starting tree. The best-scoring ML tree of a thorough ML analysis was determined under the GTRMIX model in 200 inferences. Statistical support was evaluated with 1,000 nonparametric bootstrap inferences.
Bayesian inference (BI) analysis was carried out in MrBayes 3.1.2 [32] after partitioning the nucleotide matrix according to genes. MrModeltest 2.3 [33] in PAUP* 4.0b10 [34] was used to determine the best-fit evolutionary model by Akaike Information Criterion (AIC) for each gene and selected the SYM+Γ, K80+I, and HKY+I models, for the rag1, rag2, and egr1 genes, respectively. All model parameters were unlinked across partitions, and all partitions were allowed to have different rates. Two independent Metropolis-coupled Markov chain Monte Carlo (MCMCMC) runs were performed with four simultaneous chains (three heated and one cold) and random starting trees for 5,000,000 generations, sampling parameters, and topologies every 100 generations. Burn-in was determined by checking the convergence of likelihood values across MCMCMC. A total of 500 out of 50,001 resulting trees were discarded as “burn-in.” The last trees after convergence were used to construct a 50% majority-rule consensus tree and to summarize posterior probability support for each node.
3. Results
ML and BI trees inferred from the multiple nuclear gene sequences generated identical tree topologies. In the phylogenetic tree, species belonging to the genera Pseudorasbora, Pseudopungtungia, and Pungtungia formed a monophyletic group with the highest level of confidence with respect to the Sarcocheilichthys outgroups (Figure 1).
Figure 1.
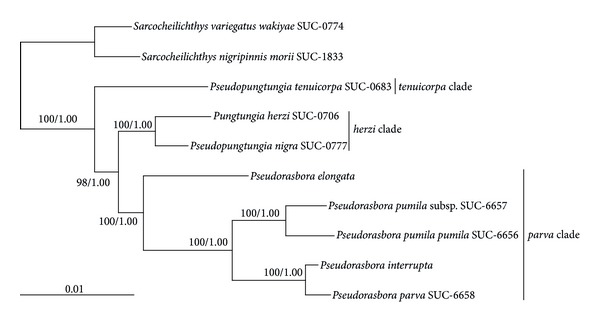
Maximum likelihood (ML) trees of gobionine species belonging to the genera Pseudorasbora, Pseudopungtungia, and Pungtungia inferred from multiple nuclear genes, that is, the recombination activating gene 1 (rag1), recombination activating gene 2 (rag2), and early growth response 1 gene (egr1). ML and Bayesian inference (BI) trees were reconstructed after partitioning the concatenated nucleotide matrix according to genes. Bootstrap values above 50% of ML analysis and posterior probabilities above 0.90 of BI analysis were shown at each branch node. The scale bar indicates substitutions/site.
In the phylogenetic tree, Pseudorasbora, Pseudopungtungia, and Pungtungia species were ramified into three distinct clades; the “tenuicorpa” clade composed of a single species, Pseudopungtungia tenuicorpa, the “herzi” clade of Pseudorasbora nigra and Pungtungia herzi, and the “parva” clade of Pseudorasbora elongata, Pseudorasbora interrupta, Pseudorasbora parva and Pseudorasbora pumila pumila, Pseudorasbora pumila subsp. (Figure 1). Among those clades, “tenuicorpa” clade placed at the basal position, giving rise to two ramifying “herzi” and “parva” clades, supported by 98% bootstrap value in ML tree and 1.00 posterior probability in BI tree. The “herzi” and “parva” clades were supported with the highest statistical supports. Within the “parva” clade, Pseudorasbora elongata formed the sister-group relationship to the lineage composed of Pseudorasbora pumila pumila, Pseudorasbora pumila subsp., Pseudorasbora interrupta, and Pseudorasbora parva. The former two consistently separated from the latter two.
4. Discussion
In the phylogenetic tree, the genus Pseudorasbora was recovered as monophyletic, but the genus Pseudopungtungia was recovered as polyphyletic; the monotypic Pungtungia herzi was closely affiliated to Pseudopungtungia nigra with highest statistical supports, and Pseudopungtungia tenuicorpa was placed at the basal position among Pseudorasbora, Pseudopungtungia, and Pungtungia species.
Previous molecular phylogenetic studies inferred from nuclear or mitochondrial gene sequences [17, 25, 28] clearly revealed the monophyly of the genera Pseudorasbora and Pungtungia. However, those studies did not include a closely related genus (i.e., Pseudopungtungia) and all Pseudorasbora species (i.e., Pseudorasbora interrupta and both Pseudorasbora pumila subspecies). Overall tree topologies generated in this study after including all those species revealed the clear monophyletic nature of the three genera Pseudorasbora, Pungtungia, and Pseudopungtungia from China, Japan, and Korea with respect to Sarcocheilichthys outgroups. This is completely or partially congruent with previous phylogenetic assumptions based on osteology [18, 35] and anatomy (vertebral formula; [36]). Meanwhile, our phylogenetic trees recovered the genus Pseudorasbora as monophyletic and the genus Pseudopungtungia as polyphyletic. Pseudopungtungia nigra showed the closest phylogenetic affiliation to Pungtungia herzi, and Pseudopungtungia tenuicorpa was clearly separated not only from those two species but also from the five Pseudorasbora species and subspecies.
In accordance with the polyphyletic nature of the genus Pseudopungtungia, Kim [37] mentioned significant morphological differences between Pseudopungtungia nigra and Pseudopungtungia tenuicorpa in the body shape and crossbars in fins except the mouth shape and the unstable taxonomic status of the genus Pseudopungtungia. Mori [13] morphologically differentiated Pseudopungtungia nigra from Pungtungia herzi by the mouth shape and fin coloration and erected the former as a novel genus and species. However, Banarescu [38] described their similarities in the mouth shape, lips, and jaws that are congruent with our molecular phylogenetic result. The close relationship can also be explained by the occurrence of a natural hybrid between them [39]. Independently, Kim et al. [40] carried out the polyacrylamide gel electrophoresis of muscle proteins extracted from Korean gobionine species to investigate their systematic relationships. Their result revealed many similarities among species of Coreoleuciscus, Pseudorasbora, Pseudopungtungia, and Pungtungia and the close relationship between Pseudopungtungia nigra and Pungtungia herzi among them, which is also congruent with our results.
The monophyly of the genus Pseudorasbora reflected the current taxonomic classification, which includes Pseudorasbora elongata, Pseudorasbora interrupta, Pseudorasbora parva, Pseudorasbora pumila pumila, and Pseudorasbora pumila subsp. Banarescu and Nalbant [41] mentioned that Pseudorasbora elongata has a notably distinct taxonomic position from other Pseudorasbora species, because of its elongated body and snout and longitudinal blackish stripes as Pungtungia. This is congruent with our phylogenetic tree, because Pseudorasbora elongata was placed at the basal position separated from other Pseudorasbora species and subspecies. This is also congruent with the result of Yang et al. [17] based on the mt-cyb gene. However, Yang et al. [17] and Liu et al. [21] showed that Pseudorasbora elongata consistently clustered with Pungtungia herzi and clearly separated from congeneric Pseudorasbora parva and Pseudorasbora pumila in their mt-cyb trees. Xiao et al. [7] mentioned the close relationship of Pseudorasbora interrupta to Pseudorasbora parva and Pseudorasbora pumila, which is congruent with our phylogenetic tree. In this study, Pseudorasbora interrupta has a closer phylogenetic relationship to Pseudorasbora parva than the two Pseudorasbora pumila subspecies.
Our result shows the unstable taxonomic status of the genus Pseudopungtungia and suggests a novel genus should be erected to accommodate Pseudopungtungia tenuicorpa in a future taxonomic study. Besides resolving phylogenetic relationships, the nucleotide sequence information presented in this study will provide useful baseline data for developing recovery plans of endangered species and subspecies investigated in this study (i.e., Pseudopungtungia nigra and Pseudopungtungia tenuicorpa, Pseudorasbora elongata and Pseudorasbora pumila subsp.) because clarification of their phylogenetic positions is the prerequisite for such efforts.
Acknowledgments
The authors express their sincere thanks to Drs. Koji Tojo and Keisuke Takata (Shinshu University) and Dr. Masanari Matsuda (Lake Biwa Museum) for their assistance in collecting Pseudorasbora pumila and providing useful information on their biology. This work was supported by the Soonchunhyang University Research Fund.
References
- 1.Banarescu P, Nalbant TT. (Das Tierreich Lieferung).Pisces, Teleostei, Cyprinidae (Gobioninae) 1973;93 [Google Scholar]
- 2.Chen Y, Chu X, Luo Y, et al. Fauna Sinica: Osteichthyes Cypriniformes II. Beijing, China: Science Press; 1998. (Chinese) [Google Scholar]
- 3.Nakabo T. Fishes of Japan with Pictorial Keys to the Species. Tokyo, Japan: Tokai University Press; 2002. [Google Scholar]
- 4.Kim I-S, Choi Y, Lee C-L, Lee Y-J, Kim B-J, Kim J-H. Illustrated Book of Korean Fishes. Seoul, Republic of Korea: Kyohak Publishing; 2005. (Korean) [Google Scholar]
- 5.Nowak M, Koščo J, Popek W. Review of the current status of systematics of gudgeons (Gobioninae, Cyprinidae) in Europe. AACL Bioflux. 2008;1(1):27–38. [Google Scholar]
- 6.DePing K, GuiHua C, JunXing Y. Threatened fishes of the world: Pseudorasbora elongata Wu, 1939 (Cyprinidae) Environmental Biology of Fishes. 2006;76(1):69–70. [Google Scholar]
- 7.Xiao Z, Lan Z-H, Chen X-L. A new species of the genus Pseudorasbora from Guangdong Province, China (Cypriniformes, Cyprinidae) Acta Zootaxonomica Sinica. 2007;32(4):977–980. [Google Scholar]
- 8.Witkowski A. NOBANIS—Invasive Alien Species Fact Sheet—Pseudorasbora parva—From: Online Database of the North European and Baltic Network on Invasive Alien Species—NOBANIS. 2006, http://www.nobanis.org.
- 9.Gozlan RE, Andreou D, Asaeda T, et al. Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions. Fish and Fisheries. 2010;11(4):315–340. [Google Scholar]
- 10.Rim H-J. Clonorchiasis in Korea. Korean Journal of Parasitology. 1990;28, supplement:63–78. doi: 10.3347/kjp.1990.28.suppl.63. [DOI] [PubMed] [Google Scholar]
- 11.Gozlan RE, St.-Hilaire S, Feist SW, Martin P, Kent ML. Biodiversity: disease threat to European fish. Nature. 2005;435(7045):p. 1046. doi: 10.1038/4351046a. [DOI] [PubMed] [Google Scholar]
- 12.Herzenstein SM. Ichthyologische Bemerkungen aus dem Zoologischen Museum der Kaiserlichen Akademie Wissenschaften. III. Mélanges Biologiques, Tirés du Bulletin Physico-Mathématique de l’Académie Impériale des Sciences de St. Pétersbourg. 1892;13, part 2:219–235. [Google Scholar]
- 13.Mori T. Descriptions of two new genera and seven new species of Cyprinidae from Chosen. Annotations of Zoologicae Japonenses. 1935;15(2):161–181. [Google Scholar]
- 14.Choi KC. On the geographical distribution of freshwater fishes south of DMZ in Korea. Korean Journal of Limnology. 1973;6(3):29–36. [Google Scholar]
- 15.Jeon SR. Ecological studies on the Pseudopungtungia nigra from Korea. Korean Journal of Limnology. 1977;10(1):33–46. [Google Scholar]
- 16.Kim I-S, Park J-Y. Freshwater Fishes of Korea. Seoul, Republic of Korea: Kyohak Publishing; 2002. (Korean) [Google Scholar]
- 17.Yang J, He S, Freyhof J, Witte K, Liu H. The phylogenetic relationships of the Gobioninae (Teleostei: Cyprinidae) inferred from mitochondrial cytochrome b gene sequences. Hydrobiologia. 2006;553(1):255–266. [Google Scholar]
- 18.Kang E-J. Phylogenetic study on the subfamily gobioninae (Pisces: Cyprinidae) from Korea as evidenced by their comparative osteology and myology [Ph.D. thesis] Jeonju, Republic of Korea: Chonbuk National University; 1991. (Korean) [Google Scholar]
- 19.Konishi M, Takata K. Isolation and characterization of polymorphic microsatellite DNA markers in topmouth gudgeon, Pseudorasbora (Teleostei: Cyprinidae) Molecular Ecology Notes. 2004;4(1):64–66. [Google Scholar]
- 20.Watanabe K, Iguchi K, Hosoya K, Nishida M. Phylogenetic relationships of the Japanese minnows, Pseudorasbora (Cyprinidae), as inferred from mitochondrial 16S rRNA gene sequences. Ichthyological Research. 2000;47(1):43–50. [Google Scholar]
- 21.Liu HZ, Yang JQ, Tang QY. Estimated evolutionary tempo of East Asian gobionid fishes (Teleostei: Cyprinidae) from mitochondrial DNA sequence data. Chinese Science Bulletin. 2010;55(15):1501–1510. [Google Scholar]
- 22.Šlechtová V, Bohlen J, Perdices A. Molecular phylogeny of the freshwater fish family Cobitidae (Cypriniformes: Teleostei): delimitation of genera, mitochondrial introgression and evolution of sexual dimorphism. Molecular Phylogenetics and Evolution. 2008;47(2):812–831. doi: 10.1016/j.ympev.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Li J, He S. Molecular evidence for the monophyly of East Asian groups of Cyprinidae (Teleostei: Cypriniformes) derived from the nuclear recombination activating gene 2 sequences. Molecular Phylogenetics and Evolution. 2007;42(1):157–170. doi: 10.1016/j.ympev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Chen W-J, Miya M, Saitoh K, Mayden RL. Phylogenetic utility of two existing and four novel nuclear gene loci in reconstructing tree of life of ray-finned fishes: the order Cypriniformes (Ostariophysi) as a case study. Gene. 2008;423(2):125–134. doi: 10.1016/j.gene.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Mayden RL, Tang KL, Wood RM, et al. Inferring the tree of life of the order Cypriniformes, the earth’s most diverse clade of freshwater fishes: implications of varied taxon and character sampling. Journal of Systematics and Evolution. 2008;46(3):424–438. [Google Scholar]
- 26.Asahida T, Kobayashi T, Saitoh K, Nakayama I. Tissue preservation and total DNA extraction from fish stored at ambient temperature using buffers containing high concentration of urea. Fisheries Science. 1996;62(5):727–730. [Google Scholar]
- 27.Kim K-Y, Bang I-C. Molecular phylogenetic position of Abbottina springeri (Cypriniformes, Cyprinidae) based on nucleotide sequences of RAG1 gene. Korean Journal of Ichthyology. 2010;22(4):273–278. [Google Scholar]
- 28.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;(41):95–98. [Google Scholar]
- 29.Saitoh K, Sado T, Mayden RL, et al. Mitogenomic evolution and interrelationships of the Cypriniformes (Actinopterygii: Ostariophysi): the first evidence toward resolution of higher-level relationships of the world’s largest freshwater fish clade based on 59 whole mitogenome sequences. Journal of Molecular Evolution. 2006;63(6):826–841. doi: 10.1007/s00239-005-0293-y. [DOI] [PubMed] [Google Scholar]
- 30.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 31.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology. 2008;57(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 32.Ronquist F, Huelsenbeck JP. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 33.Nylander JAA. MrModeltest v2.2. Uppsala, Sweden: Evolutionary Biology Centre, Uppsala University; 2004. [Google Scholar]
- 34.Swofford DL. “PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods),” ver.4, Sinauer Associates, Sunderland, UK, 2002.
- 35.Kim I-S, Kang E-J. Comparative study on the urohyal of the subfamily Gobioninae of Korea. Korean Journal of Ichthyology. 1989;1(1):24–34. [Google Scholar]
- 36.Naseka AM. Comparative study on the vertebral column in the Gobioninae (Cyprinidae, Pisces) with special reference to its systematics. Publicaciones Especiales Instituto Español de Oceanografía. 1996;21:149–167. [Google Scholar]
- 37.Kim I-S. The taxonomic study of gudgeons of the subfamily Gobioninae (Cyprinidae) in Korea. Bulletin of Korean Fisheries Society. 1984;17(5):436–448. [Google Scholar]
- 38.Banarescu PM. A critical updated checklist of Gobioninae (Pisces, Cyprinidae) Travaux du Muséum d'Histoire Naturelle “Grigore Antipa”. 1992;32:303–330. [Google Scholar]
- 39.Kim I-S, Choi Y, Shim J-H. An occurrence of intergeneric hybrid cross, Pungtungia herzi × Pseudopungtungia nigra from the Ungcheon River, Korea. Korean Journal of Ichthyology. 1991;3(1):42–47. [Google Scholar]
- 40.Kim J-S, Kim I-S, Shim JW. Electrophoretic study on the muscle proteins and systematic relationships of the gudgeons of the subfamily Gobioninae (Cyprinidae) in Korea. Korean Journal of Limnology. 1984;17(3):55–61. [Google Scholar]
- 41.Banarescu P, Nalbant TT. Studies on the systematic of Gobioninae (Pisces, Cyprinidae) Revue Roumaine de Biologie. 1965;10(4):219–229. [Google Scholar]


