Abstract
While the potential roles of endothelial cells (ECs) in the microvascules of prostate cancer (PCa) during angiogenesis have been documented, their direct impacts on the PCa metastasis remain unclear. We found that the CD31-positive and CD34-positive ECs are increased in PCa compared to the normal tissues and these ECs cells were decreased upon castration, gradually recovered with time, and become increased after PCa progresses into the castration resistant stage, suggesting a potential linkage of these ECs with androgen deprivation therapy. The in vitro invasion assays demonstrated that the co-culture of ECs with PCa cells significantly enhanced the invasion ability of the PCa cells. Mechanism dissection found that co-culture of PCa cells with ECs led to increased IL-6 secretion from ECs, which might result in down-regulation of AR signaling in PCa cells, and then the activation of TGF-β/MMP9 signaling. The consequences of the IL-6→androgen receptor→TGFβ→MMP9 signaling pathway might then trigger the increased invasion of PCa cells. Blocking the IL-6→androgen receptor→TGFβ→MMP9 signaling pathway either by IL-6 antibody, AR-siRNA, or TGF-β1 inhibitor all interrupted the ability of ECs to influence PCa invasion. These results, for the first time, revealed the important roles of ECs within the PCa microenvironment to promote the PCa metastasis, and provide new potential targets of IL-6→androgen receptor→TGFβ→MMP9 signals to battle the PCa metastasis.
Keywords: prostate cancer metastasis, endothelial cells, androgen receptor, microvascules, invasion
Introduction
Once prostate cancer (PCa) progresses into the castration resistant stage, PCa cells may rapidly gain the ability to invade and metastasize to lymph nodes and distant organs (1, 2). The tumor microenvironment (TME) of PCa includes different types of cells such as non-malignant cells, activated fibroblasts, infiltrated macrophages, and other immune cells, as well as microvasculature containing endothelial cells (ECs) (3). How each component of this TME responds to the androgen deprivation therapy (ADT) and contributes to the PCa progression into metastasis is largely unknown.
Emerging evidence indicates that ECs may contribute to the development and progression of PCa (4, 5). Upon ADT, the earliest event is the perturbation of prostatic blood flow (6, 7) and subsequent decreased numbers of microvascules. As a result of this process, ECs are subjected to apoptosis and their numbers decrease. Interestingly, it was shown that the ECs of prostate only, not the ECs of any other organs, respond to ADT. More interestingly, the perturbed microvascules are regenerated rapidly (4, 5) and their numbers are increased in castration resistant PCa (CRPC), which is frequently accompanied by a high incidence of distal metastasis.
Up-to-date, the focus of ECs role in PCa has been in the angiogenesis process. However, the recent reports suggest there is a mutual interaction of PCa cells and ECs (4, 5) and a high number of microvascules may be associated with higher incidence of metastatic cancer (8, 9). Moreover, a higher permeability of the microvascules was observed in the metastatic tumors (10). These results suggest the importance of ECs residing in microvascules to affect PCa cells.
In this study, we used multiple in vitro and in vivo strategies to demonstrate that, other than their angiogenesis functions, ECs can secrete cytokines to inhibit AR function and induce PCa metastasis. The mechanisms by which these ECs contribute to the enhanced metastatic potential of PCa cells were also investigated.
Materials and Methods
Cell lines and co-culture experiments
Human umbilical vein ECs (HUVECs), human dermal microvascular ECs (HMECs), LNCaP, C4-2, C81, and CWR22Rv1 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). HUVECs were cultured in EC medium supplemented with growth factors (ATCC) and HMECs were cultured in MCDB131 (Gibco, Grand Island, NY) supplemented with 1 μg/ml hydrocortisone, 10 ng/ml EGF and 10% fetal bovine serum (FBS). LNCaP, C4-2, C81, and CWR22Rv1 cells were cultured in RPMI 1640 with 10% FBS. Cells were maintained in a humidified 5% CO2 environment at 37°C. Six-well (3 μm) and 24-well (8 μm) transwell plates (Corning, Lowell, MA) were used for co-culture and invasion assay, respectively. Cell lines used in these studies were authenticated.
Lentiviral infection
For incorporation of AR-siRNA or scramble control plasmids into PCa cells, lentivirus carrying either control (pLVTHM-scramble) or AR-siRNA (pLVTHM-AR-siRNA), was transfected into HEK293T cells with a mixture of pLVTHM-scramble/ pLVTHM-AR-siRNA, psPAX2 (virus packaging plasmid), and pMD2G (envelope plasmid) (4:3:2 ratio) by calcium-phosphate transfection. Culture medium containing virus was collected 32 h after transfection and filtrated through a 0.4 μm filter to remove cell debris or cells. The collected virus were added to the target cells in the presence of polybrene (2 μg/ml) to incubate for 24 hr. Cells were refreshed with culture medium and cultured for another 3 days to allow target protein expression. Since the lentiviral vectors express green fluorescence protein, fluorescence microscopy was used to monitor the infection efficiency via checking the green fluorescence signal.
Cell invasion assay
For in vitro invasion assays, the upper chambers of the transwells were pre-coated with diluted matrigel (1:3) (BD Biosciences, Sparks, MD). Before the invasion assays, PCa cells were co-cultured with HUVECs (ECs culture medium for control) for 48 hrs in transwell plates. 105 PCa cells (in serum free media) and 10% serum containing media were plated in the upper and lower chambers, respectively. After 24 to 48 hrs of incubation, the cells in the upper chamber were removed. The insert membranes were fixed in ice cold methanol, stained with crystal violet, and the positively stained cells were counted under the microscope. The numbers of cells were averaged from counting of six random fields. Each sample was run in triplicate and in multiple experiments and values are expressed as mean ± SD.
Cytokine Array and ELISA
Conditioned medium (CM) was collected from HUVECs culture or HUVECs-PCa co-culture and used for cytokine arrays and ELISA analyses. The levels of a selected panel of cytokines were determined using the Human Antibody Array kit (Affymetrix, Santa Clara, CA) while the IL-6 ELISA kit (eBioscience, San Diego, CA) was applied to measure IL-6 level in the CM. The protocols were followed according to the manufacturer’s instructions.
RNA Extraction and Quantitative Real-Time PCR Analysis
Total RNAs were isolated using Trizol reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. One μg of total RNA was subjected to reverse transcription using Superscript III transcriptase (Invitrogen, Grand Island, NY). Quantitative real-time PCR (qRT-PCR) was conducted using a Bio-Rad CFX96 system with SYBR green to determine the level of mRNA expression of a gene of interest. Primers used were: AR sense, 5′-TATCCTGGTGGAGTTGTG-3′; antisense, 5′-CAGAGTCATCCCTGCTTC-3′; GAPDH sense, 5′-AATGTCACCGTTGTCCAGTTG-3′, antisense, 5 ′-GTGGCTGG GGCTCTACTTC-3′; CCL5 sense, 5′-ATCCTCATTGCTACTGCCCTC-3′, antisense, 5′-GCCACTGGTGTAGAAATACTCC-3′; IL-6 sense, 5′-AAATTCGGTACATCCTCGACGG-3′, antisense, 5′-GGAAGGTTCAGGTTGTTTTCTGC-3′; IL-8 sense, 5′-TGGGGACTGTCTATGAATCTGT-3′, antisense, 5′-GCAACACCATCCGCCATTTT-3′; E-cadherin sense, 5′-CGAGAGCTACACGTTCACGG-3′, antisense, 5′-GTGTCGAGGGAAAAATAGGCTG-3′; TGF-β1 sense, 5′-TTGCTTCAGCTCCACAGAGA-3′, and antisense, 5′-TGGTTGTAGAGGGCAAGGAC-3′. Expression levels were normalized to the expression of GAPDH RNA.
Western Blot Analysis
Cells were lysed in cell lysis buffer (50 mM Tris–HCl/pH 7.4; 1% NP-40; 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 mM Na3VO4; 1 mM NaF; 1 mM okadaic acid; and 1 mg/ml aprotinin, leupeptin, and pepstatin). Proteins (20-40 μg) were separated on 8–10% SDS/PAGE gel and then transferred onto PVDF membranes (Millipore, Billerica, MA). After blocking the membranes with 5% fat free milk in TBST for 1 hr at room temperature, the membranes were incubated with appropriate dilutions of specific primary antibodies overnight at 4°C. After washing, the blots were incubated with HRP-conjugated secondary antibodies for 1 hr and visualized using the ECL system (Thermo Fisher Scientific, Rochester, NY).
Hematoxylin and eosin (H&E) Staining
The tissue sections were de-waxed and rehydrated routinely. The sections were stained in hematoxylin for 5 min, and washed in running tap water for 5 min. Then the sections were stained in eosin for 30 sec, dehydrated, and mounted by routine methods. We then examined and photographed at least 10 fields per each slide. The consistent and representative fields were presented in the figures.
Histology and Immunohistochemistry
Prostate tissues were fixed in 10% (v/v) formaldehyde in PBS, embedded in paraffin, and cut into 5-μm sections. Immunostaining was performed as described previously (11). For systematic counting of ECs, six ocular measuring fields within a tissue were randomly chosen under a microscope at 400× magnification. The mean number of human CD31-positive (CD+) and CD34-positive (CD+) cells was determined as the ECs count. For AR, TGF-β1, and MMP-9 quantitation, the German Immunoreactive Score (0–12) was calculated by multiplying the percentage of immunoreactive cells (0%=0; 1–10%=1; 11–50%=2; 51–80%=3; 81–100%=4) by the staining intensity (negative=0; weak=1; moderate=2; strong=3). Scores were considered negative (0–1), weakly positive (2–4), moderately positive (6–8), and strongly positive (9–12).
Luciferase assay
PCa cells were plated in 24-well plates and transfected with MMTV-luc containing ARE sequence using Lipofectamine (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. After transfection, RPMI media containing charcoal stripped FBS were added with addition of various concentrations of DHT, 0 (ethanol as vehicle control), 1, and 10 nM, and incubated for 48 hrs. pRL-TK was used as internal control. Luciferase activity was measured using Dual-Luciferase Assay (Promega, Madison, WI) according to the manufacturer’s manual.
In vivo animal studies
Male 6- to 8-week old nude mice were used. CWR22rv1 cells were engineered to express luciferase reporter gene (REN/luc, PPM-Mill/luc) by stable transfection and the positive stable clones were selected and expanded in culture (12). 20 mice were injected with PCa cells (106 luciferase expressing cells with Matrigel, 1:1) and 10 mice were co-injected with PCa cells co-cultured with HUVECs (105) into the anterior prostate (AP). Metastasis in live mice was monitored using a Fluorescent Imager (IVIS Spectrum, Caliper Life Sciences, Hopkinton, MA) at 6 different time points. After monitoring with the Imager, mice were sacrificed and the metastases in lung, lymph node, and bone were further examined by H&E and IHC staining using anti-firefly Luciferase antibody. To culture metastatic cancer cells from peritoneal ascites, the ascites were collected and immediately diluted into 5 ml PBS before coagulation, were washed 3 times in PBS, and then cultured (13, 14). All animal studies were performed under the supervision and guidelines of the University of Rochester Medical Center Animal Care and Use Committee.
Statistics
The data values were presented as the mean ± SD. Differences in mean values between two groups were analyzed by two-tailed Student’s t test. p ≤ 0.05 was considered statistically significant.
Results
ECs/Microvascules are increased in PCa compared to normal prostate tissues
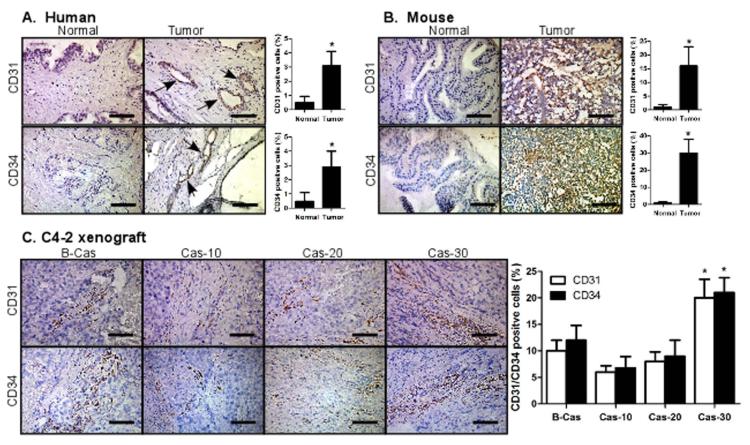
We performed immunohistochemical (IHC) staining of the human and mouse originated normal prostate/PCa tissues using ECs specific antibodies, CD31 and CD34, and found that the CD31+ and CD34+ cell numbers were very low in normal prostate tissues. In contrast, significantly increased CD31+ and CD34+ cells were identified in human PCa tissues (Fig. 1A) and TRAMP mouse PCa tissues (Fig. 1B). However, when we compared CD31+ or CD34+ cells in human PCa C4-2 xenografted tumors, we found that these cells decreased after castration, but gradually increased in tissues of the castration resistant tumors (Fig. 1C). Together, results from Fig. 1A-C suggest the existence of ECs in PCa microenvironment may be linked to PCa progression and can be influenced by androgen deprivation.
Fig. 1. Endothelial cells/microvasucles are increased in PCa vs normal tissues.
(A-B) Immunohistochemical staining of normal prostate and prostate tumor tissues. (A) Human patient tissues and (B) TRAMP mouse tissues. Human normal prostate and prostate tumor tissues were obtained from young (about 30 years of age) normal organ donors and patients with radical resection of prostate cancer, respectively, at the Shanghai First People’s Hospital (Shanghai, China). TRAMP tumors were obtained from 28 wks old TRAMP mice (B6 background). (C) Immunohistochemical staining of C4-2 orthotopic xenografted tumor tissues, before and after castration. C4-2 cells were orthotopically injected into APs of mice. After tumors developed, mice were castrated and castration resistant tumors allowed to re-develop. Mice were sacrificed 10, 20, and 30 days after castration and tumors were obtained. Tumors from some mice before castration were used as controls. Tumor tissues were immunohistochemically stained with CD31 and CD34 antibodies. Black arrows represent CD31/CD34 positive cells.
Co-culture with ECs enhanced invasion ability of PCa cells
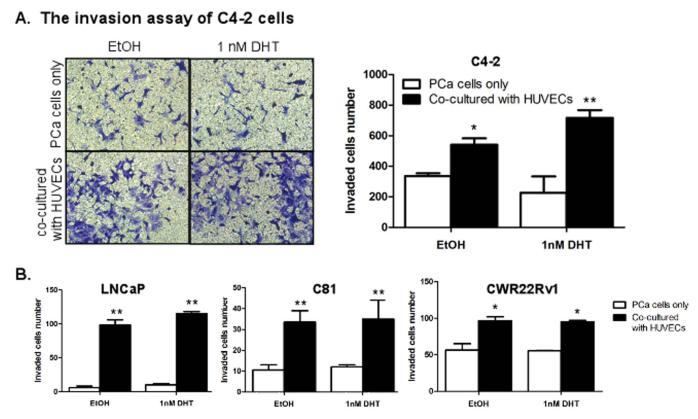
We applied a co-culture system to determine whether the presence of ECs could affect PCa cell invasion ability. C4-2 cells were co-cultured with ECs (media as control) and the invasion abilities were compared. Since the established human prostate ECs are not available, human umbilical vein ECs (HUVECs) and human dermal microvascular ECs (HMECs) were used as two different ECs sources as they have been demonstrated to have similar properties compared to the primary human prostate ECs (5) in various ECs-PCa studies (15-17). We found that the invasion abilities of C4-2 cells were increased upon co-culture with HUVECs (Fig. 2A) and similar results were obtained with LNCaP, C81, and CWR22Rv1 cells (Fig. 2B). The results with HMECs were shown in Supplementary Fig. S1. These tests were done in the presence of 1 nM DHT condition, which is the human PCa in vivo DHT concentration after ADT (18, 19). Similar results were also obtained when we replaced 1 nM DHT with 10 nM DHT (the human PCa in vivo DHT concentration before ADT) (data not shown). Together, results from Fig. 2 suggested that the presence of ECs in the PCa microenvironment might promote PCa invasion before and after ADT in the different PCa cell lines tested.
Fig. 2. Endothelial cells enhance PCa cells invasion in vitro.
(A) C4-2 cells (1 × 105/well) were co-cultured with HUVECs and invasion assay performed in the presence of 0 (EtOH) and 1 nM concentrations of DHT. Invaded cells were stained with toluidine blue and the positively stained cells were counted from six random fields. (B) LNCaP, C81, and CWR22Rv1 cells were used for invasion assay, similar to (A). Results are expressed as mean ± SD. Different numbers between two groups were analyzed by two-tailed Student’s t test. * p < 0.05, **p < 0.01.
ECs co-culture mediates down-regulation of AR signal in PCa cells
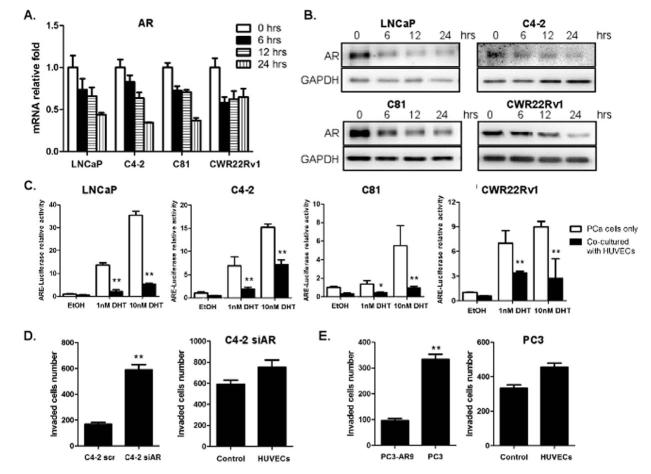
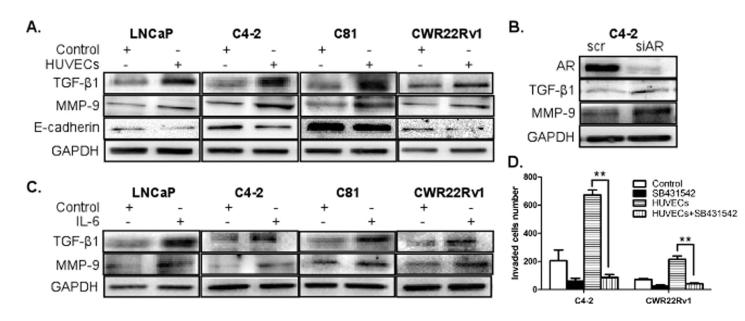
To dissect the potential mechanisms by which ECs enhanced the invasion abilities of PCa cells, the activation of several signaling pathways in PCa cells was investigated after co-culture with HUVECs/HMECs. Surprisingly, we found significantly decreased AR levels in all PCa cells when co-cultured with ECs (Fig. 3A, B, and Supplementary Fig. S2). To test whether the down-regulation of AR expression is an earlier event than the increased invasion, we performed time course experiments and found that this AR mRNA down-regulation was detected as early as 6 hrs after co-culture incubation (Fig. 3A, B, and Supplementary Fig. S2), suggesting that altered AR signaling preceded the increased invasion. AR transactivation was also tested in ARE-driven luciferase assay, and as expected, when PCa cells were co-cultured with ECs, the AR mediated ARE-luciferase activity was significantly decreased (Fig. 3C), suggesting that both the expression level and AR transactivation activity were decreased upon co-culture with ECs.
Fig. 3. Endothelial cells down-regulate AR signaling in PCa cells.
(A-B) LNCaP, C4-2, C81 and CWR22Rv1 cells (1×105/well) were co-cultured with HUVECs (medium for control) in transwell plates (3 μm) for 6, 12, 24 hrs. (A) Total RNAs were extracted and AR mRNAs level was analyzed by qRT-PCR. (B) PCa cell extracts were obtained for Western blot analysis for AR expression. (C) LNCaP, C4-2, C81 and CWR22Rv1 cells were transfected with MMTV-luc containing ARE and co-cultured with HUVECs (medium for control) in the presence of various concentrations of DHT as indicated. After 24 hrs, luciferase activity was measured. (D) C4-2 cells, transfected with either AR-siRNA or scramble control, were used in invasion assays as in 2A. (E) PC3 and PC3AR9 cells were used in invasion assay, similar to (D). All experiments were repeated three times. Data were presented as mean ± SD. (*p < 0.05, **p < 0.01).
To investigate whether the down-regulation of AR signaling is the key step in mediating the enhanced metastatic potential of PCa cells, invasion abilities of the C4-2 cells were tested after selective knockdown of AR by siRNA strategy. Consistent with previous co-culture studies, the knockdown of AR significantly increased C4-2 cells invasion (Fig. 3D). However, when we co-cultured these AR knocked down PCa cells with HUVECs, we no longer could see the HUVECs effect in promoting PCa invasion ability, indicating that the AR down-regulation is critical in triggering ECs-induced invasion ability of the C4-2 cells. We also compared the HUVECs influence on the invasion ability of the PC3 cells (lack AR expression), vs PC3-AR9 cells (with stably transfected human AR) (20). Consistent with the C4-2 cell results, the HUVECs effect in promoting PCa metastasis was shown lower in PC3 cells compared to the PC3AR9 cells (Fig. 3E). Together, results from Fig. 3A-E suggest that ECs exert their effect on promoting PCa invasion abilities via down-regulation of AR signaling in the PCa cells.
IL-6 is a key mediator for AR down-regulation in ECs-PCa co-culture cells
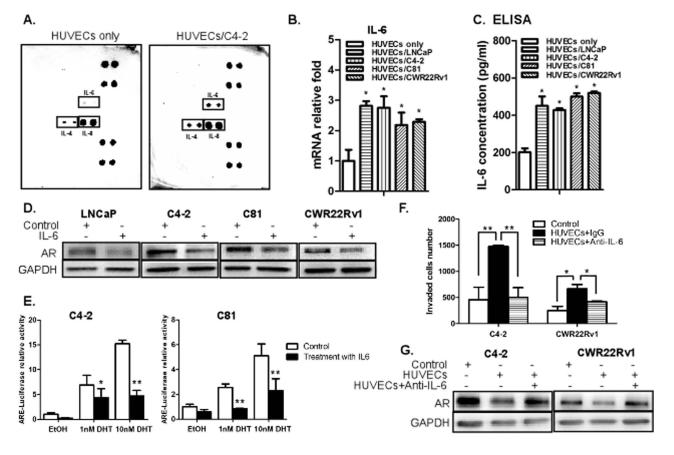
It was reported that ECs secrete chemokines/cytokines/growth factors to exert their paracrine effect (21). Therefore, we speculated that the ECs effect in enhancing the invasion ability of PCa cells could be through the paracrine effect. We performed cytokine array to investigate whether the secreted chemokines/cytokines were changed in HUVECs after co-culture with PCa C4-2 cells, and as shown in Fig. 4A, we found the levels of IL-4, IL-6, and IL-8 were increased. We then independently assayed the mRNA levels of all reported and related cytokines/chemokines/growth factors in HUVECs, with or without co-culture with PCa cells (22, 23). The results showed that the levels of several cytokines and chemokines including CCL5 and IL-6 in ECs were increased upon co-culture with PCa cells (Supplementary Fig. S1A and B).
Fig. 4. IL-6 is a potential mediator to down-regulate AR signaling, which in turn, enhances invasion ability of PCa cells.
(A) Cytokine array analysis. HUVECs were co-cultured with or without C4-2 cells for 2 days and the conditioned media (CM) were collected for cytokine array analysis. (B) qPCR analysis showing mRNA levels of IL-6. Total RNAs were extracted from HUVECs, either with or without co-culture with PCa cells, cDNAs obtained by reverse transcriptase reaction, and the mRNA levels of IL-6 were analyzed. (C) IL-6 ELISA assay result demonstrating increase in IL-6 secretion in PCa cells upon co-culture with HUVECs. HUVECs were co-cultured with LNCaP, C4-2, C81 and CWR22Rv1 cells for 2 days and the CM were collected for ELISA. (D) LNCaP, C4-2, C81 and CWR22Rv1 cells (1 × 105/well) were treated with 20 ng/ml of human recombinant IL-6 (Prospec Bio) for 6 hrs. PCa cell extracts were used for Western blot analysis for AR expression. (E) C4-2 and C81 cells were transfected with MMTV-luc containing ARE. After transfection, cells were treated with 20 ng/ml of IL-6 for 24 hrs in the presence of various concentrations of DHT as indicated, and luciferase activity was measured. (F) C4-2 and CWR22Rv1 cells were co-cultured with HUVECs in the presence of anti-IL-6 neutralizing antibody (R&D systems) (normal IgG as control) for 2 days and invasion assay was performed. (G) PCa cell extracts were obtained under the similar conditions as in (F) and used for the Western blot analysis for AR. Data are mean ± SD. (*p < 0.05, **p < 0.01).
From these two analyses, we speculate that IL-6 and IL-8 are the best possible candidate molecules secreted by ECs to affect the invasion ability of PCa cells. IL-6 has been considered as an important growth-regulatory factor in human PCa (21, 24) and has roles in metastases and morbidity (25). IL-8 has also been reported to be associated with increased metastatic ability of cancer cells (26, 27). In contrast, few reports linked IL-4 to the risk or progression of PCa (28).
To confirm the above points, we tested the effect of IL-6, IL-4, and IL-8 in down-regulating the AR signaling and their ability to increase the invasion ability of PCa cells. As shown in Supplementary Fig. S3C, the AR expression was decreased in C4-2 cells incubated with IL-6, but not with IL-4 or IL-8. In addition, IL-6 could effectively increase invasion, IL-4 had some moderate effects, and IL-8 failed to change invasion capability of PCa C4-2 cells (Supplementary Fig. S3D). Also, analysis of IL-6 mRNA and ELISA demonstrated that the co-culture of PCa cells significantly increased the IL-6 secretion in ECs (Fig. 4B-C). Western blot and luciferase activity analyses revealed that the IL-6 treatment could down-regulate AR expression in androgen-dependent LNCaP cells as well as moderately decreased AR in castration resistant C4-2, C81, and CWR22rv1 cells (Fig. 4D), IL6 treatment also decreased AR transactivation (Fig. 4E). Importantly, adding IL-6 neutralizing antibody in co-cultured ECs-PCa cells reversed the HUVECs effects on AR down-regulation and the increased invasion abilities of C4-2 and CWR22Rv1 cells (Fig. 4F-G). Together, results from Fig. 4F-G suggest that IL-6 is the key molecule secreted from ECs to impact the down-regulation of AR in PCa cells that results in enhanced PCa cell invasion.
Down-regulation of AR resulted in the decreased E-cadherin level with increased TGF-β1 and MMP-9 levels
To dissect the molecular mechanisms by which IL-6 mediated down-regulation of AR in PCa led to increased PCa cell invasion, we examined expression levels of the epithelial-mesenchymal transition (EMT) markers, E-cadherin (E-cad), N-cad, vimentin, and Snail, since the EMT is known to be an important step in the initiation of early dissemination that leads to metastasis (29). Interestingly, we found that the expression of E-cad was significantly decreased in PCa cells upon co-culture with ECs (Fig. 5A), but we failed to observe an increase in N-cad (Supplementary Fig. S4A), which usually accompanies the E-cad decrease. We also failed to detect expression changes in the other EMT markers, vimentin and Snail (Supplementary Fig. S4A and B). However, while investigating the expressions of TGF-β1 and MMP-9, known as critical molecules in EMT process (30), we found significant increases in PCa cells when co-cultured with HUVECs (Fig. 5A). To further confirm whether TGF-β1 and MMP-9 are the downstream molecules of the AR signaling, we compared their expression in the AR knocked down C4-2 cells and scramble control cells. As shown in Fig. 5B, the expression levels of TGF-β1 and MMP-9 were increased when AR expression was knocked down, indicating that the AR down-regulation is essential in mediating increased expressions of these EMT related molecules in PCa cells.
Fig. 5. Down-regulation of AR signals resulted in decrease in E-cadherin level, but increase in TGF-β1 and MMP-9.
(A) LNCaP, C4-2, C81, and CWR22Rv1 cells (1 × 105/well) were co-cultured with HUVECs (medium for control) for 48 hrs in transwell plates. PCa cell extracts were obtained for Western blot analyses using antibodies for TGF-β1 (Santa Cruz Biotechnology), MMP-9 (Abcam) and E-cadherin (MAB1838, R&D systems) expressions. (B) C4-2 cells were infected with lentivirus carrying AR-siRNA or scrambled control. Cell extracts were obtained for Western blot analysis for the expression analysis of the indicated molecules. (C) LNCaP, C4-2, C81, and CWR22Rv1 cells (1 × 105/well) were treated with 20 ng/ml of IL-6 for 48 hrs. PCa cell extracts were obtained for Western blot analyses for TGF-β1 and MMP-9 expressions. (D) C4-2 and CWR22Rv1 cells were co-cultured with HUVECs (medium for control) in the presence of TGF-β1 inhibitor, SB431542 for 2 days and invasion assays were performed.
It was reported that TGF-β1 up-regulates the expression of MMP-9, which is closely associated with tumor invasion (31, 32). Therefore, we treated PCa cells with the TGF-β1 inhibitor, SB431542, to test whether the inhibition of the TGF-β1 pathway can block the HUVECs-induced PCa cell invasion. Our data showed that this TGF-β1 inhibitor treatment blocked the increased invasion ability of PCa cells significantly (Fig. 5D), confirming the TGF-β1 role in mediating ECs-increased PCa cell invasion.
We then added IL-6 into the ECs-PCa co-culture system to see if the IL-6 can down-regulate AR signaling in PCa cells to alter expressions of TGF-β1 and MMP-9 (Fig. 3 and 4). As shown in Fig. 5C, the IL-6 treatment indeed increased expressions of these molecules, and once again confirmed that IL-6 is a critical ECs secreted factor to mediate down-regulation of AR signaling and the consequent increases of TGF-β1 and MMP-9 in PCa. Together, results from Figs. 4-5 indicated that ECs may influence PCa cell invasion via the IL6→AR→TGF-β1→MMP-9 signaling pathway and blocking these signals (either by IL-6 antibody, AR-siRNA, or TGF-β1 inhibitor) interrupted the ability of ECs to influence PCa invasion.
In vivo xenografted mice demonstrate ECs effect in enhancing PCa metastasis
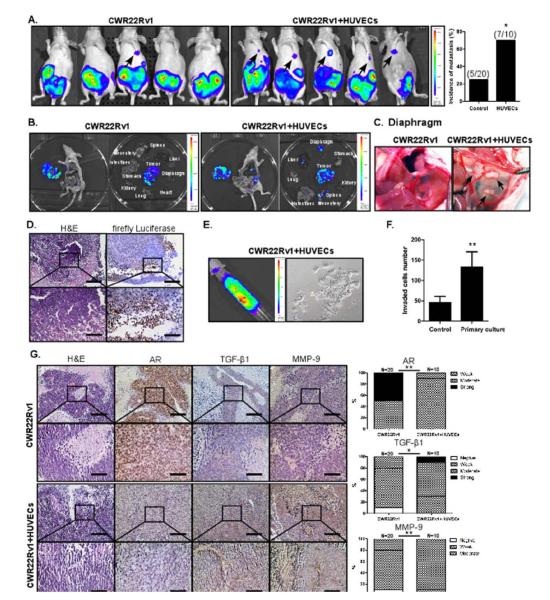
To confirm the above in vitro cell lines results showing ECs promote the metastatic ability of PCa cells in vivo, PCa CWR22Rv1 cells were orthotopically implanted, either alone or co-implanted with HUVECs, into the APs of the nude mice. After injection, the metastatic incidence in these two groups of mice was monitored using in vivo imaging system (IVIS). As shown in Fig. 6A, the metastatic incidence of the co-implantation group was significantly increased showing more tumors mainly in the lymph nodes and diaphragm compared to the control group (Fig. 6A-D). The PCa cells were also detected in the peritoneal ascites fluids in a few cases (Fig. 6E). The morphology of the primary cultured tumor cells isolated from ascites resembled the original CWR22Rv1 cells, indicating that these circulating cells are from the primary tumor site (Fig. 6E). Importantly, we also examined the expression levels of AR, TGF-β1, and MMP-9 in the primary tumors and found that AR expressions were decreased while the expressions of TGF-β1 and MMP-9 were increased in the tissues of the co-implantation group mice compared to the control group mice tissues, which was consistent with our in vitro data (Fig. 6F). We used only one mouse model in the in vivo animal studies, so further studies are needed to confirm the contribution of ECs in PCa metastasis.
Fig. 6. HUVECs treatment enhances PCa metastasis in orthotopic xenografted mice.
CWR22Rv1 cells were transfected with luciferase (Luciferase-pcDNA3, Addgene), stable clones were selected, and their luciferase activity was confirmed before injection. 1 × 106 of these cells, either alone or together with HUVECs (10:1 PCa cells:HUVECs), as a mixture with Matrigel, 1:1, total of 20 μl, were orthotopically implanted into the APs of 8 wks old mice. Tumor growth and metastasis was monitored by examining luminescence using IVIS at 3, 4, 5, and 6 wks after injection. (A) The metastatic incidence shown in two groups of mice. (B) The imaging data showing primary and metastatic tumors of two mice groups. (C) The imaging demonstrating diaphragm metastasis. (D) H&E and IHC staining of metastatic tumors from diaphragm using antibodies of anti-firefly Luciferase antibody (Abcam). (E) The imaging demonstrating the ascites metastases obtained from metastatic mouse (left panel) and primary cultures cells from ascites (right panel). (F) Invasion assay of primary cultured CWR22Rv1 from ascites (parental CWR22Rv1 cells as control). (G) H&E and IHC staining of primary and metastatic tumors using antibodies of AR, TGF-β1, and MMP-9.
Discussion
The intense neovascularization surrounding tumors suggest their roles not only in supplying nutrients for the continued tumor growth but also in initiating angiogenesis by seeding tumor cells into the blood stream in microvascules (33). We found that EC numbers were increased in PCa vs normal tissues and following castration/ADT, compared to before castration/ADT treatment, although further studies using a set of sequential specimens in human tissues are necessary to support these findings.
We demonstrated that ECs may also play an important role in enhancing the metastatic potential of PCa both in vitro and in vivo. These new findings will add insights into ECs contribution to PCa metastasis and emphasize the importance of ECs as a component of the TME.
In mediating ECs role in enhancing the metastatic potential of PCa, we showed that the ECs action in enhancing the metastatic ability of PCa was via down-regulation of AR, which may challenge the current understanding that AR plays a positive role to promote PCa progression (34-38). Up-to-date, most of the efforts for decades have applied ADT strategy via suppression of the androgen/AR signaling to battle PCa (39-42), so suggesting the suppressor role of AR in increasing PCa metastasis is novel and challenging. Several recent studies support this idea. The recently published reports on clinical studies suggest that ADT might increase metastases in some patients (43, 44). Increased expressions of the EMT related markers, such as N-cadherin (45), Cadherin-11 (46, 47), and nestin (48), were found in human clinical PCa samples after ADT. Cell line studies also demonstrated that ADT causes EMT transition (49). Since the EMT process is highly correlated with metastases (50, 51), these results supported the idea that ADT enhances PCa metastases. ADT with surgical castration was also demonstrated to lead to increased lymph node (52) or distant (53) metastases. Furthermore, Niu et al (54) found that mice with AR knock down in prostate epithelial cells developed increased metastatic PCa, with mice dying earlier than in the TRAMP mouse model. Therefore, the results showing AR down-regulation in PCa cells upon ECs co-culture and increasing PCa metastasis in this study is consistent with these new emerging concepts.
Among several cytokines/chemokines/growth factors identified from ECs to exert paracrine effects to influence PCa metastasis, we found IL-6 was the strongest candidate molecule and we believe even other cytokines, such as IL-4 and IL-8, might also contribute to enhancing PCa metastases, but they might act via different mechanisms, and not via down-regulation of AR. IL-6 is known to be increased in patients with advanced stages of PCa (55, 56), play an important role in PCa progression (57), and can be secreted from several cell types, including macrophages (58) and adipocytes (59). In this study we found ECs are another source of IL-6.
VEGF has been suggested as a critical molecule to target ECs mediated angiogenesis (60, 61). However, none of our results showed significantly increased VEGF levels when co-cultured with PCa cells nor increased invasion ability of PCa cells upon addition of VEGF (data not shown).
We found TGF-β and MMP9 were key molecules mediating IL-6-AR signals to enhance the metastatic potential of PCa. These two molecules are known to be the multifunctional factors during diverse physiological and pathological processes including development, wound healing, proliferation, and cancer metastasis (62). TGF-β is a growth suppressive cytokine in many normal situations, but becomes an active and important participant in malignant disease functions including angiogenesis, extracellular matrix deposition, immuno-suppression, and metastasis growth promotion (63). Zhang et al (64) investigated the TGF-β role in growth and metastasis of the highly metastatic PC-3MM2 human PCa cells and found that TGF-β signaling enhanced tumor angiogenesis by regulating IL-8 expression in tumor cells. TGF-β1 was also shown to enhance PCa PC3 cell invasion by an uPA/plasmin-dependent mechanism to play a key role in malignant PCa progression (65). Recently, several studies have shown that TGF-β1 can up-regulate MMP-9 expression and activity in other cells, such as human skin (66), corneal epithelial cells (67), and brain astrocytes (62). These results, together with our current findings may allow us to develop a new therapeutic approach based on targeting these two molecules to block ECs-promoted PCa metastasis.
We also found E-cad decreases in PCa cells upon ECs-PCa co-culture, but failed to observe the difference of other EMT markers. It will be interesting to see whether the increase of TGF-β/MMP9 were due to the E-cad level changes or if these two are separate signals.
Based on these studies, we believe that development of a combination therapy to block two processes, tumor proliferation where AR plays positive role, and metastasis in which AR plays negative roles, is essential. The combination therapy targeting tumor growth (by the classical ADT) and angiogenesis (by blocking VEGFR tyrosine kinase) has been attempted (68). A therapy targeting bone and brain metastasis has also been suggested (69). Maybe in the near future, development of an effective therapeutic strategy to interrupt the ECs mediated IL6→AR→TGFβ→MMP9 signaling pathway identified here, to suppress metastasis after classical anti-proliferation therapy to suppress PCa progression, may help us to better battle PCa. Further in vivo mice studies to test therapeutic approaches need to be performed.
Supplementary Material
Acknowledgments
We thank Karen Wolf for help with manuscript preparation.
Grant Support: This work was supported by NIH grants (CA122840 and CA127300, C. Chang) and Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH102-TD-B-111-004, C. Chang) to China Medical University, Taiwan.
Footnotes
Conflict of Interest: There is no conflict of interest issue.
Authors’ Contributions Conception and design: Shujie Xia, Shuyuan Yeh, Chawnshang Chang.
Development of methodology: Soo Ok Lee, Shuyuan Yeh.
Acquisition of data: Xiaohai Wang, Qi Jiang, Jie Luo.
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Xiaohai Wang, Qi Jiang, Soo Ok Lee, Lei Li.
Writing, review, and/or revision of the manuscript: Xiaohai Wang, Soo Ok Lee, Lei Li, Shujie Xia.
Study supervision: Shuyuan Yeh, and Chawnshang Chang.
Conflict of Interest: There is no conflict of interest issue.
References
- 1.Akaza H. Current status and prospects of androgen depletion therapy for prostate cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:293–302. doi: 10.1016/j.beem.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Valdespino V, Tsagozis P, Pisa P. Current perspectives in the treatment of advanced prostate cancer. Med Oncol. 2007;24:273–86. doi: 10.1007/s12032-007-0017-9. [DOI] [PubMed] [Google Scholar]
- 3.Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev. 2009;28:177–83. doi: 10.1007/s10555-008-9175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godoy A, Montecinos VP, Gray DR, Sotomayor P, Yau JM, Vethanayagam RR, et al. Androgen deprivation induces rapid involution and recovery of human prostate vasculature. Am J Physiol Endocrinol Metab. 2011;300:E263–75. doi: 10.1152/ajpendo.00210.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godoy A, Watts A, Sotomayor P, Montecinos VP, Huss WJ, Onate SA, et al. Androgen receptor is causally involved in the homeostasis of the human prostate endothelial cell. Endocrinology. 2008;149:2959–69. doi: 10.1210/en.2007-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shabsigh A, Chang DT, Heitjan DF, Kiss A, Olsson CA, Puchner PJ, et al. Rapid reduction in blood flow to the rat ventral prostate gland after castration: preliminary evidence that androgens influence prostate size by regulating blood flow to the prostate gland and prostatic endothelial cell survival. The Prostate. 1998;36:201–6. doi: 10.1002/(sici)1097-0045(19980801)36:3<201::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Shabsigh A, Lee B, Buttyan R. Unique morphological aspects of the rat ventral prostate gland revealed by vascular corrosion casting. The Prostate. 1999;39:240–5. doi: 10.1002/(sici)1097-0045(19990601)39:4<240::aid-pros4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Gustavsson H, Welen K, Damber JE. Transition of an androgen-dependent human prostate cancer cell line into an androgen-independent subline is associated with increased angiogenesis. The Prostate. 2005;62:364–73. doi: 10.1002/pros.20145. [DOI] [PubMed] [Google Scholar]
- 9.Tomic TT, Gustavsson H, Wang W, Jennbacken K, Welen K, Damber JE. Castration resistant prostate cancer is associated with increased blood vessel stabilization and elevated levels of VEGF and Ang-2. The Prostate. 2011 doi: 10.1002/pros.21472. [DOI] [PubMed] [Google Scholar]
- 10.Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp Cell Res. 2004;296:231–44. doi: 10.1016/j.yexcr.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Wu CT, Altuwaijri S, Ricke WA, Huang SP, Yeh S, Zhang C, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12679–84. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen SU, Chou CH, Lin CW, Lee H, Wu JC, Lu HF, et al. Signal mechanisms of vascular endothelial growth factor and interleukin-8 in ovarian hyperstimulation syndrome: dopamine targets their common pathways. Hum Reprod. 2010;25:757–67. doi: 10.1093/humrep/dep432. [DOI] [PubMed] [Google Scholar]
- 13.Penet MF, Pathak AP, Raman V, Ballesteros P, Artemov D, Bhujwalla ZM. Noninvasive multiparametric imaging of metastasis-permissive microenvironments in a human prostate cancer xenograft. Cancer research. 2009;69:8822–9. doi: 10.1158/0008-5472.CAN-09-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He H, Yang X, Davidson AJ, Wu D, Marshall FF, Chung LW, et al. Progressive epithelial to mesenchymal transitions in ARCaP E prostate cancer cells during xenograft tumor formation and metastasis. The Prostate. 2010;70:518–28. doi: 10.1002/pros.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu L, Vogiatzi P, Puhr M, Dagvadorj A, Lutz J, Ryder A, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer. 2010;17:481–93. doi: 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao CH, Guh JH, Chueh SC, Yu HJ. Anti-angiogenic effects and mechanism of prazosin. The Prostate. 2011;71:976–84. doi: 10.1002/pros.21313. [DOI] [PubMed] [Google Scholar]
- 17.Rahim S, Beauchamp EM, Kong Y, Brown ML, Toretsky JA, Uren A. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PloS one. 2011;6:e19343. doi: 10.1371/journal.pone.0019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 19.Mizokami A, Koh E, Fujita H, Maeda Y, Egawa M, Koshida K, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer research. 2004;64:765–71. doi: 10.1158/0008-5472.can-03-0130. [DOI] [PubMed] [Google Scholar]
- 20.Altuwaijri S, Wu CC, Niu YJ, Mizokami A, Chang HC, Chang C. Expression of human AR cDNA driven by its own promoter results in mild promotion, but not suppression, of growth in human prostate cancer PC-3 cells. Asian J Androl. 2007;9:181–8. doi: 10.1111/j.1745-7262.2007.00258.x. [DOI] [PubMed] [Google Scholar]
- 21.Stachon A, Schluter T, Koller M, Weisser H, Krieg M. Primary culture of microvascular endothelial cells from human benign prostatic hyperplasia. The Prostate. 2001;48:156–64. doi: 10.1002/pros.1094. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SS, Lukacs NW, Kunkel SL. Eotaxin/CCL11 suppresses IL-8/CXCL8 secretion from human dermal microvascular endothelial cells. J Immunol. 2002;168:2887–94. doi: 10.4049/jimmunol.168.6.2887. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer research. 2010;70:10411–21. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- 24.Hobisch A, Ramoner R, Fuchs D, Godoy-Tundidor S, Bartsch G, Klocker H, et al. Prostate cancer cells (LNCaP) generated after long-term interleukin 6 (IL-6) treatment express IL-6 and acquire an IL-6 partially resistant phenotype. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:2941–8. [PubMed] [Google Scholar]
- 25.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–15. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, et al. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:2104–19. [PubMed] [Google Scholar]
- 27.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer research. 2007;67:6854–62. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 28.Kesarwani P, Ahirwar DK, Mandhani A, Mittal RD. Association between −174 G/C promoter polymorphism of the interleukin-6 gene and progression of prostate cancer in North Indian population. DNA and cell biology. 2008;27:505–10. doi: 10.1089/dna.2008.0742. [DOI] [PubMed] [Google Scholar]
- 29.Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nature reviews Urology. 2011;8:428–39. doi: 10.1038/nrurol.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–36. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–36. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 32.Goodyear SM, Kheyfets SB, Garcia FU, Stearns ME. Role of the VEGFR3/VEGFD receptor axis in TGFbeta1 activation of primary prostate cell lines. The Prostate. 2009;69:982–90. doi: 10.1002/pros.20945. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee S, Dowsett M, Ashworth A, Martin LA. Mechanisms of disease: angiogenesis and the management of breast cancer. Nature clinical practice Oncology. 2007;4:536–50. doi: 10.1038/ncponc0905. [DOI] [PubMed] [Google Scholar]
- 34.Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer. 1991;67:3057–64. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Hobisch A, Culig Z, Radmayr C, Bartsch G, Klocker H, Hittmair A. Androgen receptor status of lymph node metastases from prostate cancer. The Prostate. 1996;28:129–35. doi: 10.1002/(SICI)1097-0045(199602)28:2<129::AID-PROS9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Mohler JL, Chen Y, Hamil K, Hall SH, Cidlowski JA, Wilson EM, et al. Androgen and glucocorticoid receptors in the stroma and epithelium of prostatic hyperplasia and carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996;2:889–95. [PubMed] [Google Scholar]
- 37.Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, Chang C. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. The Journal of urology. 1992;147:798–803. doi: 10.1016/s0022-5347(17)37389-5. [DOI] [PubMed] [Google Scholar]
- 38.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. International journal of cancer Journal international du cancer. 1991;48:189–93. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 39.Kung HJ, Evans CP. Oncogenic activation of androgen receptor. Urol Oncol. 2009;27:48–52. doi: 10.1016/j.urolonc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan X, Li T, Wang H, Zhang T, Barua M, Borgesi RA, et al. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169:682–96. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841–9. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kageyama Y, Hyochi N, Kihara K, Sugiyama H. The androgen receptor as putative therapeutic target in hormone-refractory prostate cancer. Recent Pat Anticancer Drug Discov. 2007;2:203–11. doi: 10.2174/157489207782497172. [DOI] [PubMed] [Google Scholar]
- 43.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan CJ, Shah S, Efstathiou E, Smith MR, Taplin ME, Bubley GJ, et al. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–61. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jennbacken K, Tesan T, Wang W, Gustavsson H, Damber JE, Welen K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr Relat Cancer. 2010;17:469–79. doi: 10.1677/ERC-10-0015. [DOI] [PubMed] [Google Scholar]
- 46.Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ, Chen DT, et al. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res. 2008;6:1259–67. doi: 10.1158/1541-7786.MCR-08-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YC, Cheng CJ, Huang M, Bilen MA, Ye X, Navone NM, et al. Androgen depletion up-regulates cadherin-11 expression in prostate cancer. J Pathol. 2010;221:68–76. doi: 10.1002/path.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, et al. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer research. 2007;67:9199–206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer research. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 50.Shiota M, Zardan A, Takeuchi A, Kumano M, Beraldi E, Naito S, et al. Clusterin Mediates TGF-beta-Induced Epithelial-Mesenchymal Transition and Metastasis via Twist1 in Prostate Cancer Cells. Cancer research. 2012;72:5261–72. doi: 10.1158/0008-5472.CAN-12-0254. [DOI] [PubMed] [Google Scholar]
- 51.Ezponda T, Popovic R, Shah MY, Martinez-Garcia E, Zheng Y, Min DJ, et al. The histone methyltransferase MMSET/WHSC1 activates TWIST1 to promote an epithelial-mesenchymal transition and invasive properties of prostate cancer. Oncogene. 2012 doi: 10.1038/onc.2012.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91. [PubMed] [Google Scholar]
- 53.Tang Y, Wang L, Goloubeva O, Khan MA, Zhang B, Hussain A. Divergent effects of castration on prostate cancer in TRAMP mice: possible implications for therapy. Clin Cancer Res. 2008;14:2936–43. doi: 10.1158/1078-0432.CCR-07-4925. [DOI] [PubMed] [Google Scholar]
- 54.Niu Y, Altuwaijri S, Lai KP, Wu CT, Ricke WA, Messing EM, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12182–7. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iacopino F, Pinto F, Bertaccini A, Calarco A, Proietti G, Totaro A, et al. Soluble E-cadherin and IL-6 serum levels in patients affected by prostate cancer before and after prostatectomy. Oncol Rep. 2012;28:370–4. doi: 10.3892/or.2012.1785. [DOI] [PubMed] [Google Scholar]
- 56.Johnke RM, Edwards JM, Evans MJ, Nangami GN, Bakken NT, Kilburn JM, et al. Circulating cytokine levels in prostate cancer patients undergoing radiation therapy: influence of neoadjuvant total androgen suppression. In Vivo. 2009;23:827–33. [PubMed] [Google Scholar]
- 57.Culig Z, Puhr M. Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol Cell Endocrinol. 2012;360:52–8. doi: 10.1016/j.mce.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee GT, Kwon SJ, Lee JH, Jeon SS, Jang KT, Choi HY, et al. Induction of interleukin-6 expression by bone morphogenetic protein-6 in macrophages requires both SMAD and p38 signaling pathways. The Journal of biological chemistry. 2010;285:39401–8. doi: 10.1074/jbc.M110.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finley DS, Calvert VS, Inokuchi J, Lau A, Narula N, Petricoin EF, et al. Periprostatic adipose tissue as a modulator of prostate cancer aggressiveness. J Urol. 2009;182:1621–7. doi: 10.1016/j.juro.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Pratheeshkumar P, Son YO, Budhraja A, Wang X, Ding S, Wang L, et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PloS one. 2012;7:e52279. doi: 10.1371/journal.pone.0052279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen JP, Frost CD, Lane ML, Skelton WP, Iv, Skelton M, Vesely DL. Novel dual inhibitors of vascular endothelial growth factor and VEGFR2 receptor. Eur J Clin Invest. 2012;42:1061–7. doi: 10.1111/j.1365-2362.2012.02695.x. [DOI] [PubMed] [Google Scholar]
- 62.Hsieh HL, Wang HH, Wu WB, Chu PJ, Yang CM. Transforming growth factor-beta1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK- and JNK-NF-kappaB pathways. Journal of neuroinflammation. 2010;7:88. doi: 10.1186/1742-2094-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinkas J, Teicher BA. TGF-beta in cancer and as a therapeutic target. Biochem Pharmacol. 2006;72:523–9. doi: 10.1016/j.bcp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Zhang F, Lee J, Lu S, Pettaway CA, Dong Z. Blockade of transforming growth factor-beta signaling suppresses progression of androgen-independent human prostate cancer in nude mice. Clin Cancer Res. 2005;11:4512–20. doi: 10.1158/1078-0432.CCR-04-2571. [DOI] [PubMed] [Google Scholar]
- 65.Festuccia C, Angelucci A, Gravina GL, Villanova I, Teti A, Albini A, et al. Osteoblast-derived TGF-beta1 modulates matrix degrading protease expression and activity in prostate cancer cells. International journal of cancer Journal international du cancer. 2000;85:407–15. [PubMed] [Google Scholar]
- 66.Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor-beta - and tumor necrosis factor-alpha -mediated induction and proteolytic activation of MMP-9 in human skin. The Journal of biological chemistry. 2001;276:22341–50. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon GM, Ledee DR, Feuer WJ, Fini ME. Cytokines and signaling pathways regulating matrix metalloproteinase-9 (MMP-9) expression in corneal epithelial cells. Journal of cellular physiology. 2009;221:402–11. doi: 10.1002/jcp.21869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicholson B, Gulding K, Conaway M, Wedge SR, Theodorescu D. Combination antiangiogenic and androgen deprivation therapy for prostate cancer: a promising therapeutic approach. Clin Cancer Res. 2004;10:8728–34. doi: 10.1158/1078-0432.CCR-04-0902. [DOI] [PubMed] [Google Scholar]
- 69.Yin JJ, Zhang L, Munasinghe J, Linnoila RI, Kelly K. Cediranib/AZD2171 inhibits bone and brain metastasis in a preclinical model of advanced prostate cancer. Cancer research. 2010;70:8662–73. doi: 10.1158/0008-5472.CAN-10-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.