Abstract
About 40% of children with childhood absence epilepsy develop generalized tonic-clonic seizures. It is commonly held that polyspike–wave pattern on the electroencephalogram (EEG) can predict this development of generalized tonic-clonic seizures. However, there is no firm evidence in support of this proposition. To test this assumption, we used survival analysis and compared the incidence of generalized tonic-clonic seizures in 115 patients with childhood absence epilepsy having either isolated 3-Hz spike–wave or coexisting 3 Hz and polyspike–waves and other variables. There was no evidence that polyspike–waves predicted development of generalized tonic-clonic seizures in patients with childhood absence epilepsy. Later age of onset (≥8 years) and family histories of generalized tonic-clonic seizures were the only independent predictors. These results have implications for counseling and in the choice of first-line antiepileptic drugs used for childhood absence epilepsy, especially if valproate is chosen based on the observation of polyspike–waves.
Keywords: childhood absence epilepsy, prognosis, generalized tonic-clonic seizures, antiepileptic drugs
Childhood absence epilepsy is an idiopathic generalized epilepsy that typically occurs in otherwise healthy children between the ages of 3 and 12 years. Childhood absence epilepsy is characterized by the presence of short absences or frequent and sudden losses of consciousness of variable duration (typically between 2 and 30 seconds). These frequent absences are often described as “staring” or “daydreaming.” The electrophysiological correlates of a typical absence seizure are a bilateral, synchronous, and symmetrical 3-Hz spike and wave complexes on the electroencephalogram (EEG).
Childhood absence epilepsy is considered a relatively benign form of epilepsy with regard to seizure prognosis1–3 because 70% to 80% of patients with childhood absence epilepsy are well controlled with antiepileptic drugs,4–6 and a similar proportion will remit by adulthood7 and remain seizure free even after antiepileptic drug withdrawal.5,8 However, recent studies provided evidence for a less benign prognosis, as long-term cognitive and neuropsychiatric comorbidities have been reported.9–11 In addition, children with childhood absence epilepsy have a high probability of developing generalized tonic-clonic seizures.12 This evolution occurs in about 40% of all cases, either concurrently or in adolescence following the remission of absence seizures, usually within 5 to 10 years of childhood absence epilepsy onset.8,12,13
Childhood absence epilepsy overlaps clinically with juvenile myoclonic epilepsy,14,15 another form of age-related idiopathic generalized epilepsy with minor seizures. Roughly 3% to 21% of patients with childhood absence epilepsy eventually develop juvenile myoclonic epilepsy.7,16 However, about one third of patients with juvenile myoclonic epilepsy have concomitant absence seizures. Only a small fraction of juvenile myoclonic epilepsy patients with absences (ie, 25%) have absence seizures starting in childhood and with a high seizure frequency, similar to those of childhood absence epilepsy.17
Juvenile myoclonic epilepsy is often considered less benign in terms of seizure prognosis than childhood absence epilepsy. The syndrome, which has a later age of onset than childhood absence epilepsy and persists into adulthood,13,15 has a higher incidence of generalized tonic-clonic seizures (90% as opposed to about 40% in childhood absence epilepsy).17 Generalized tonic-clonic seizures in juvenile myoclonic epilepsy typically occur in the morning (previously known as “awakening grand mal”) and are often preceded by a series of myoclonic jerks. The typical ictal EEG pattern of myoclonic jerks in juvenile myoclonic epilepsy is polyspike and waves, a pattern that can also be observed in some patients with childhood absence epilepsy.16,18
Because of the higher probability of patients with juvenile myoclonic epilepsy to develop generalized tonic-clonic seizures, the presence of polyspike–waves, the typical juvenile myoclonic epilepsy pattern, in a patient with childhood absence epilepsy is commonly regarded as a risk factor for the later development of myoclonic seizures or generalized tonic-clonic seizures. Although several attempts have been made to find factors that would predict the outcome of childhood absence epilepsy,19,20 the predictive value of polyspike–waves in the EEG of patients with childhood absence epilepsy has, to our knowledge, never been investigated.
Such information would not only allow physicians to make more accurate predictions regarding prognosis but would also have an impact on the initial treatment preference in childhood absence epilepsy. The first antiepileptic drug of choice in childhood absence epilepsy is generally ethosuximide. Ethosuximide is usually well tolerated and is associated with few adverse side effects, however it is only effective for absence seizures.21 When other seizure types occur, other antiepileptic drugs have to be added to, or substituted for, ethosuximide.22 If a risk of myoclonic seizures or generalized tonic-clonic seizures is perceived, a more broad spectrum antiepileptic drug (eg, valproate or lamotrigine) is generally recommended.23 These antiepileptic drugs may have more severe adverse effects or may be less efficacious than ethosuximide for absence seizures. Although it makes reasonable sense to use the presence of polyspike–waves as a risk factor for developing myoclonic seizures or generalized tonic-clonic seizures, and treat accordingly, this assumption needs to be evaluated. This is particularly important in the light of differences in tolerability between drug regimens.
The current study thus aims toward a clarification of poly-spike–waves as a predictor for the development of myoclonic seizures or generalized tonic-clonic seizures in childhood absence epilepsy. The ability to predict the outcome and evolution of absence seizures has implications for patients and their families, as well as for the clinician’s plan of treatment. Because polyspike–waves in the EEG of patients with childhood absence epilepsy are widely inferred to be predictors of generalized tonic-clonic seizures, it is important that the validity of this assumption be further explored.
Patients and Methods
Patient Selection
We used a cohort design to measure the incidence of myoclonic seizures or generalized tonic-clonic seizures in patients with childhood absence epilepsy and to assess whether polyspike–waves modify this risk. We collected follow-up seizure history on 115 children seen for childhood absence epilepsy during a time period of 11 years in the Division of Pediatric Neurology of Robert Wood Johnson Medical School. The study was approved by the Institutional Review Board of Robert Wood Johnson Medical School, and all participating patients/guardians gave their informed consent.
Patients were identified retrospectively and systematically through a computerized divisional database using keyword searches on “Childhood Absence Epilepsy, CAE, Absence Epilepsy, Absences, Petit mal, and/or Zarontin/Ethosuximide.” We then verified the diagnosis of childhood absence epilepsy by searching the medical records of the individual. A total of 115 patients (60% female) satisfied the International League Against Epilepsy criteria for childhood absence epilepsy.24 We searched the charts for treatment and EEG data (poly-spike–waves, focal abnormalities, and background activity), in addition to seizure history and family history. All EEGs reviewed were performed as part of standard care and are routine recordings with electrodes placed according to the International 10–20 system.25 Routine pediatric EEG recordings at Robert Wood Johnson Medical School are, when possible, of 25 minutes duration, performed in the awake state and include hyperventilation and photic stimulation. All available EEGs of each participant were considered. On average each patient had at least 2.1 EEGs (median = 2).
Follow-Up
Outcome data were collected in a 2-step process. First, we sent out questionnaires by mail to ascertain the occurrence of seizures until the assessment time. The questionnaire contained lay descriptions of febrile, typical absence, myoclonic seizures, and generalized tonic-clonic seizures, as well as a request for a full treatment history. After 4 weeks, nonresponders were sent a second copy of the questionnaire. In the second step, we telephoned all remaining nonresponders and interviewed them directly. The final response rate was 84% (97 of 115). Outcome data on nonresponders was censored according to their status at last clinical follow-up.
Definitions and Measures
Seizures and epilepsy syndromes followed the International League Against Epilepsy-classification (1989), and lay descriptions were worded accordingly. Time at risk was defined as the elapsed days from childhood absence epilepsy onset until the first myoclonic seizure or generalized tonic-clonic seizures, or last follow-up date.
Statistical Analysis
We first tabulated the clinical characteristics of the study sample, in terms of the seizure type, EEG abnormalities, family history of seizures, and treatment. We used Kaplan-Meier survival methods to plot and compare the incidence of generalized tonic-clonic seizures in patients with childhood absence epilepsy according to the 2 EEG subgroups (3 Hz vs 3 Hz with polyspike–wave). We examined univariate risks of generalized tonic-clonic seizures associated with age of onset (≥8 years vs <8 years), gender, family history of seizures, and focal EEG abnormalities. Finally, we calculated the risk of generalized tonic-clonic seizures or myoclonic seizures using a Cox proportional hazards model, adjusting for potential confounders: gender, age of onset, and family history of epilepsy. Analyses were performed using Stata 8.0 for Macintosh.
Results
Our cohort comprised of 115 cases with childhood absence epilepsy with an average age of childhood absence epilepsy onset of 6.5 years and a mean duration of follow-up of 5.4 years (median = 4.2 years, range = 1–18 years). The clinical characteristics of the study sample are summarized in Table 1.
Table 1.
General Characteristics of the Study Population
| Gender | 60% female |
| Mean duration of follow-up | 5.4 years |
| Mean age of onset of absence | 6.5 years |
| Mean age of onset of generalized tonic-clonic seizures | 11.4 years |
| Generalized tonic-clonic seizures | 17% |
| Myoclonic jerks | 2% |
| Febrile seizures | 11% |
| EEG | |
| Polyspike–wave pattern | 26% |
| Focal abnormalities | 16% |
| Abnormal background | 3% |
| Antiepileptic drug history | |
| 1st antiepileptic drug ethosuximide | 70% |
| 1st antiepileptic drug valproate acid | 17% |
| Only one antiepileptic drug | 61% |
| >1 antiepileptic drug | 39% |
| Positive family history of any seizure | 34% |
| Of generalized tonic-clonic seizures | 24% |
| Of absence | 5% |
| Of febrile seizures | 5% |
NOTE: EEG, electroencephalogram.
Incidence of Seizures
Twenty patients (17%) developed generalized tonic-clonic seizures; generalized tonic-clonic seizures showed an incidence rate of 8 per 100 000 person-days of follow-up. The mean age of generalized tonic-clonic seizures onset was 11.4 years (SD = 4 years). Only 2 patients with childhood absence epilepsy developed myoclonic seizures. Thirteen patients (11 %) had febrile seizures preceding the absence seizures
Electroencephalogram
Thirty patients (26%) had coexisting polyspike–waves on their EEG, and 85 (74%) had only isolated 3-Hz spike and wave. Four (3%) cases were found to have abnormal slow background activity, while 18 (16%) cases had focal abnormalities on the EEG (9 frontal or frontotemporal and 9 occipital or parietal). In 15 cases (13%) posterior slow waves of youth were noted.
Treatment
Eighty-eight (70%) patients were first prescribed ethosuximide. For 20 patients (17%), valproate was the first drug, and seven (6%) were prescribed other antiepileptic drugs. Seventy (61%) were treated with only 1 drug, while 45 (39%) were treated with 2 or more antiepileptic drugs.
Family History
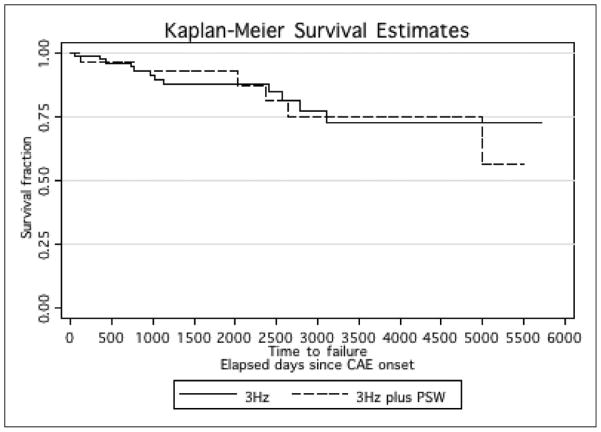
Thirty-nine patients (34%) had a family history of seizures. Six patients (5%) had a family history of absence seizures but no generalized tonic-clonic seizures and 6 other patients had a family history of febrile seizures. The remaining 27 patients (24%) had a family history of generalized tonic-clonic seizures and/or other seizures (the specific classification, other than absence and generalized tonic-clonic seizures could not be determined from medical records; Figure 1).
Figure 1.
Incidence of generalized tonic-clonic seizures over time since the onset of childhood absence epilepsy (in days) by EEG. EEG, electroencephalogram; PSW, polyspike and waves. CAE, childhood absence epilepsy.
Predictors of Generalized Tonic-Clonic Seizures
We first examined our hypothesis that polyspike–waves predicted generalized tonic-clonic seizures in childhood absence epilepsy. Seven (20%) of 30 participants with polyspike–wave developed generalized tonic-clonic seizures, compared with 13 (14%) of 85 with only 3-Hz spike and wave. The incidence rate ratio for generalized tonic-clonic seizures in participants with 3 Hz plus polyspike–wave was 0.99 (95% CI: 0.37–2.64). Next, we examined other possible predictors of generalized tonic-clonic seizures: age at onset, gender, focal EEG abnormalities, and family history of epilepsy. In univariate analyses, age at onset (risk ratio 2.28, 95%CI 0.87–5.97), gender (risk ratio 1.70, 95% CI 0.60–4.79), and EEG focality (risk ratio 2.34 95% CI 0.85–6.46) were not significantly predictive. Family history of seizures was also not predictive of generalized tonic-clonic seizures (risk ratio 1.76 95% CI 0.69–4.47). However, when only a family history of generalized tonic-clonic seizures and nonabsence afebrile seizures were examined, there was a significant association (risk ratio 2.72 95%CI 1.07–6.93). Interestingly, a family history of absence or febrile seizures, but without generalized tonic-clonic seizures, did not predict a higher risk of generalized tonic-clonic seizures. We, therefore, entered age group, gender, EEG group, and family history of generalized tonic-clonic seizures and nonabsence afebrile seizures into the multivariate model.
In the Cox proportional hazards model, we identified 2 variables, age of onset after the age of 8 years, (hazard ratio 3.86) and family history of generalized tonic-clonic seizures (HR 3.60), that increased the risk of generalized tonic-clonic seizures independently of confounding variables (Table 2). Age of onset was not by itself associated with polyspike–wave (18% vs 30 % in <8 years and >8 years age groups, χ2 = 1.78, P = .18).
Table 2.
Cox Proportional Hazards for Generalized Tonic-Clonic Seizures in Childhood Absence Epilepsy Cohort
| Variable | Hazard ratio | 95% confidence interval |
|---|---|---|
| Age group ≥8 years vs <8 years | 3.86 | 1.31–11.34 |
| Gender, female vs male | 2.72 | 0.90–8.20 |
| Family history generalized tonic-clonic seizures | 3.60 | 1.33–9.78 |
| EEG group polyspike–wave vs 3 Hz | 1.54 | 0.55–4.37 |
NOTE: EEG, electroencephalogram.
Lastly, we examined prescribing data to see whether EEG pattern was associated with the probability of valproate or val-proic acid treatment. Although there was a higher percentage of children with polyspike–waves treated with valproic acid, this difference did not reach statistical significance (26% vs 14%, χ2 = 2.54, P = .28).
Discussion
About 40% of children with childhood absence epilepsy develop generalized tonic-clonic seizures later in life.12,26 Not only does this potential risk present a psychological burden on newly diagnosed patients and their families, the occurrence of generalized tonic-clonic seizures in childhood absence epilepsy is often associated with a lower probability of seizure remission. Bouma et al4 found that while over 78% of patients with childhood absence epilepsy without generalized tonic-clonic seizures became seizure free by adulthood, only 35% of patients with generalized tonic-clonic seizures did the same.
Due to the association of polyspike–waves with juvenile myoclonic epilepsy, the occurrence of polyspike–waves in childhood absence epilepsy has often been perceived as a predictor for the later occurrence of generalized tonic-clonic seizures or myoclonic seizures in those children with absence seizures. However, the value of polyspike–waves as a predictor variable has not, to our knowledge, been evaluated previously. The current study attempted to fill this gap. Our data show no evidence to support the hypothesis that polyspike–waves on the EEG predicted the onset of generalized tonic-clonic seizures in patients with typical childhood absence epilepsy. The incidence rate for generalized tonic-clonic seizures in patients with a 3-Hz spike and wave plus polyspike–waves was identical to that of patients with a 3-Hz spike and wave EEG pattern only. As such, we found that polyspike and wave discharges are not an appropriate marker to predict generalized tonic-clonic seizures or myoclonic seizures in childhood absence epilepsy.
Our results are in agreement with the finding that certain generalized interictal EEG patterns may be nonspecific with regard to clinical symptoms. Janz and Waltz17,27 found poly-spike–waves interictally in 47% of patients with childhood absence epilepsy and in 45% of patients with juvenile absence epilepsy. Even though polyspike–waves are the ictal pattern of myoclonic jerks in juvenile myoclonic epilepsy, polyspike–waves are seen interictally in only 54%of juvenile myoclonic epilepsy cases. Similarly, the ictal pattern of absence seizures in childhood absence epilepsy, the classical 3-Hz spike and wave complex, is also seen interictally in both juvenile myoclonic epilepsy (in 60%) and juvenile absence epilepsy (in 55%). Thus, it seems not possible to delineate the specific clinical syndromes from the interictal EEG results alone.
We were able to determine 2 variables that were associated with generalized tonic-clonic seizures or myoclonic seizures in absence patients. First, in accord with other studies,2,7,28 we found evidence that a family history of epilepsy increases the risk of generalized tonic-clonic seizures in patients with childhood absence epilepsy. More specifically, we found that it was not simply a family history of epilepsy, but a family history of generalized tonic-clonic seizures (but not absence seizures) that was correlated with the occurrence of generalized tonic-clonic seizures in our patients with childhood absence epilepsy. The notion that generalized tonic-clonic seizures in childhood absence epilepsy is a familial phenomenon supported by previous findings.29–31 For example, Doose30 found that in the case of children with both early onset absence seizures (between age 1 and 5) and generalized tonic-clonic seizures, relatives also had predominantly generalized tonic-clonic seizures, whereas in relatives of children without generalized tonic-clonic seizures, absence seizures prevailed.
We hypothesize that childhood absence epilepsy with generalized tonic-clonic seizures may be a genetically different form of childhood absence epilepsy than childhood absence epilepsy without generalized tonic-clonic seizures. Alternatively, it is possible that generalized tonic-clonic seizure-specific genes are required for the development of generalized tonic-clonic seizures in childhood absence epilepsy. When those are not present, and a negative family history for generalized tonic-clonic seizures might be an indicator for this, a child with childhood absence epilepsy would, therefore, be less likely to develop generalized tonic-clonic seizures.
Second, our findings suggest that a later age of onset is predictive of the development of generalized tonic-clonic seizures, a result that has been corroborated in several other studies.1,2,32,33 Although Callenbach et al19 recently reported no predictive value of age of onset, the difference between their and our findings might be due to the small number of children with generalized tonic-clonic seizures. Only 6 of their 47 participants developed generalized tonic-clonic seizures during follow-up.
In our study, 17% of all participants developed generalized tonic-clonic seizures during the follow-up period. This is in contrast to previous studies that reported an evolution of generalized tonic-clonic seizures between 32% and 49%.26,32–34 This discrepancy might be due to our relatively short average follow-up period of 5.4 years, because there is a second manifestation peak of generalized tonic-clonic seizures usually 5 to 10 years after onset.33 In addition, our strict adherence to the criteria of the International League against Epilepsy and the exclusion of children with atypical absences might have further reduced the number of generalized tonic-clonic seizures in our sample.
Our results raise questions about the utility of separating the 2 absence syndromes into childhood absence epilepsy and juvenile absence epilepsy based on their age of onset (with a somewhat arbitrary cut off of 10 years, or before and after puberty35). Initially, Janz36 delineated 2 different absence syndromes based on the frequency and morphology of the absence seizures. He called the epilepsy with earlier age of onset “pyknolepsy,” based on the frequent (several a day) occurrence of absence seizures (from the Greek word pyknos = often). He differentiated this form from the epilepsy with simple and infrequent absence seizures that manifest mostly in adolescence, calling this second form epilepsy with “spanio-leptic” or indifferent absences. While there is a certain overlap between childhood absence epilepsy and pyknolepsy, and juvenile absence epilepsy and epilepsy with spanioleptic absence, it seems more prudent to define these 2 syndromes by their age of onset and clinical symptoms. Based on different seizure remission patterns, Trinka et al37 argue that these 2 syndromes should be differentiated not only by their seizure frequency (pyknoleptic vs nonpyknoleptic) but also by the development of myoclonic jerks or generalized tonic-clonic seizures. Our data suggest that a family history of generalized tonic-clonic seizures could also help to differentiate these 2 types of disorders. Even with these prognostic tools, however, it may not be possible to completely untangle one disease type from the other. Berkovic et al13 have even suggested that the 3 disorders of absence seizures (childhood absence epilepsy, juvenile absence epilepsy, and juvenile myoclonic epilepsy) have blurred nosological boundaries and form a spectrum.
Further, our results show that patients with focal EEG abnormalities were not significantly more likely to develop generalized tonic-clonic seizures. In agreement with this result, Yoshinaga et al38 found that the proportion of focal abnormalities is the highest in patients with childhood absence epilepsy that are not only well controlled for seizures but also no longer have epileptiform EEGs. The rate of focal abnormalities was lowest in patients that either continued to have seizures or were seizure-free but still had abnormal EEGs. However, Hedström and Olsson39 reported that focal abnormalities and brief episodes of spike and wave without a clinical correlation predicted the onset of generalized tonic-clonic seizures. Several long-term follow-up EEG studies have shown that focal EEG abnormalities in general are not uncommon in idiopathic generalized epilepsy. Lombroso40 showed that 56% of patients with idiopathic generalized epilepsy showed focal abnormalities. Leutmezer et al41 found focal discharges in 35% of patients with idiopathic generalized epilepsy and in 40% of patients with absence seizures.
Our findings have important implications for treatment. Patients with juvenile myoclonic epilepsy show good responses to valproate or lamotrigine. Ethosuximide is considered very specific for the treatment of absence seizures but ineffective for the treatment of generalized tonic-clonic seizures or myoclonic seizures. Valproate is often recommended for the treatment of absence seizures when polyspike–waves are seen on the EEG, even in the absence of clinical generalized tonic-clonic seizures.23 This practice has to be questioned based on our findings that childhood absence epilepsy patients with polyspike–wave are as likely to develop generalized tonic-clonic seizures as patients that have only 3-Hz spike and wave on EEG. Ethosuximide should still be considered a first-line antiepileptic drug for pure absence epilepsy because of its favorable side effect and toxicity profile,42,43 especially in cases with an early age of onset of absences and a negative family history for myoclonic seizures or generalized tonic-clonic seizures.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: Research was supported by grants from the National Institutes of Health: NS37466 (MD), NS27941, NS047530 (DKP), DK31775, through a generous donation from the Charles L. Shor Foundation for Epilepsy Inc. (DKP), and the Partnership for Pediatric Epilepsy Research (DKP).
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
The data was collected at the Division of Pediatric Neurology of Robert Wood Johnson Medical School.
References
- 1.Currier RD, Kooi KA, Saidman LJ. Prognosis of “pure” petit mal; a follow-up study. Neurology. 1963;13(11):959–967. doi: 10.1212/wnl.13.11.959. [DOI] [PubMed] [Google Scholar]
- 2.Gibberd FB. The prognosis of petit mal. Brain. 1966;89(3):531–538. doi: 10.1093/brain/89.3.531. [DOI] [PubMed] [Google Scholar]
- 3.Gordon N. The natural history of petit mal epilepsy. Dev Med Child Neurol. 1965;7(5):537–542. doi: 10.1111/j.1469-8749.1965.tb10962.x. [DOI] [PubMed] [Google Scholar]
- 4.Bouma PA, Westendorp RG, van Dijk JG, Peters AC, Brouwer OF. The outcome of absence epilepsy: a meta-analysis. Neurology. 1996;47(3):802–808. doi: 10.1212/wnl.47.3.802. [DOI] [PubMed] [Google Scholar]
- 5.Braathen G, Andersson T, Gylje H, et al. Comparison between one and three years of treatment in uncomplicated childhood epilepsy: a prospective study. I. Outcome in different seizure types. Epilepsia. 1996;37(9):822–832. doi: 10.1111/j.1528-1157.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Drury I, Dreifuss FE. Pyknoleptic petit mal. Acta Neurol Scand. 1985;72(4):353–362. doi: 10.1111/j.1600-0404.1985.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 7.Wirrell EC, Camfield CS, Camfield PR, Dooley JM. Long-term prognosis of typical childhood absence epilepsy: remission or progression to juvenile myoclonic epilepsy. Neurology. 1996;47:912–918. doi: 10.1212/wnl.47.4.912. [DOI] [PubMed] [Google Scholar]
- 8.Duncan JS, Shorvon SD, Fish DR. Clinical Epilepsy. New York, NY: Churchill Livingstone; 1995. [Google Scholar]
- 9.Barnes GN, Paolicchi JM. Neuropsychiatric comorbidities in childhood absence epilepsy. Nat Clin Pract Neurol. 2008;4(12):650–651. doi: 10.1038/ncpneuro0947. [DOI] [PubMed] [Google Scholar]
- 10.Caplan R, Siddarth P, Stahl L, et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49(11):1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 11.Mandelbaum DE, Burack GD. The effect of seizure type and medication on cognitive and behavioral functioning in children with idiopathic epilepsy. Dev Med Child Neurol. 1997;39(11):731–735. doi: 10.1111/j.1469-8749.1997.tb07374.x. [DOI] [PubMed] [Google Scholar]
- 12.Gastaut H, Broughton RJ. Epileptic Seizures. Clinical and Electrographic Features, Diagnosis and Treatment. Springfield, IL: Thomas; 1972. [Google Scholar]
- 13.Berkovic SF, Andermann F, Andermann E, Gloor P. Concepts of absence epilepsies: discrete syndromes or biological continuum? Neurology. 1987;37(6):993–1000. doi: 10.1212/wnl.37.6.993. [DOI] [PubMed] [Google Scholar]
- 14.Asconape J, Penry JK. Some clinical and EEG aspects of benign juvenile myoclonic epilepsy. Epilepsia. 1984;25(1):108–114. doi: 10.1111/j.1528-1157.1984.tb04163.x. [DOI] [PubMed] [Google Scholar]
- 15.Janz D. Epilepsy with impulsive petit mal (juvenile myoclonic epilepsy) Acta Neurol Scand. 1985;72(5):449–59. doi: 10.1111/j.1600-0404.1985.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 16.Shian WJ, Chi CS. Evolution of childhood absence epilepsy, juvenile myoclonic epilepsy and epilepsy with grand mal on awakening. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1994;35(2):119–123. [PubMed] [Google Scholar]
- 17.Janz D, Waltz S. Juvenile myoclonic epilepsy with absences. In: Duncan JS, Panayiosopoulos CP, editors. Typical Absences and Related Epileptic Syndromes. London: Churchill; 1995. pp. 174–183. [Google Scholar]
- 18.Oletsky H, Greenfield J, Sato S. 14 and 6 Hz positive spikes preceding 3 Hz generalized spike and wave in a 15 year old patient with absence: a case report. Electroencephalogr Clin Neurophysiol. 1998;106(3):262–264. doi: 10.1016/s0013-4694(97)00127-2. [DOI] [PubMed] [Google Scholar]
- 19.Callenbach PM, Bouma PA, Geerts AT, et al. Long-term outcome of childhood absence epilepsy: Dutch Study of Epilepsy in Childhood. Epilepsy Res. 2009;83(2–3):249–256. doi: 10.1016/j.eplepsyres.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Wolf P. Determinants of outcome in childhood epilepsy. Acta Neurol Scand Suppl. 2005;112(182S):5–8. doi: 10.1111/j.1600-0404.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 21.Myslobodsky MS, Mirsky AF. Elements of Petit Mal Epilepsy: Basic Mechanisms. New York, NY: Peter Lang; 1988. [Google Scholar]
- 22.Wallace SJ. Use of ethosuximide and valproate in the treatment of epilepsy. Neurol Clin. 1986;4(3):601–616. [PubMed] [Google Scholar]
- 23.Santavuori P. Absence seizures: valproate or ethosuximide? Acta Neurol Scand Suppl. 1983;68(97S):41–48. doi: 10.1111/j.1600-0404.1983.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 24.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epileptica. 1989;30(4):389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 25.Jasper HH. The 10–20-electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1958;10(2):370–375. [PubMed] [Google Scholar]
- 26.Dieterich E, Baier WK, Doose H, Tuxhorn I, Fichsel H. Longterm follow-up of childhood epilepsy with absences. I. Epilepsy with absences at onset. Neuropediatrics. 1985;16(3):149–154. doi: 10.1055/s-2008-1052560. [DOI] [PubMed] [Google Scholar]
- 27.Waltz S, Beck-Mannagetta G, Janz D. Are there syndrome-related genetically determined spike and wave patterns? A comparison between syndromes of generalized epilepsy. Epilepsia. 1990;31(6):819. [Google Scholar]
- 28.Sato S, Dreifuss FE, Penry JK. Prognostic factors in absence seizures. Neurology. 1976;26(8):788–796. doi: 10.1212/wnl.26.8.788. [DOI] [PubMed] [Google Scholar]
- 29.Beck-Mannagetta G, Janz D. Syndrome-related genetics in generalized epilepsy. Epilepsy Res Suppl. 1991;4:105–111. [PubMed] [Google Scholar]
- 30.Doose H. Absence epilepsy of early childhood—genetic aspects. Eur J Pediatr. 1994;153(5):372–377. doi: 10.1007/BF01956423. [DOI] [PubMed] [Google Scholar]
- 31.Matthes A, Weber H. Clinical and electroencephalographic family studies on pyknolepsy. Dtsch Med Wochenschr. 1968;93(10):429–435. doi: 10.1055/s-0028-1105082. [DOI] [PubMed] [Google Scholar]
- 32.Charlton MH, Yahr MD. Long-term follow-up of patients with petit mal. Arch Neurol. 1967;16(6):595–598. doi: 10.1001/archneur.1967.00470240033003. [DOI] [PubMed] [Google Scholar]
- 33.Loiseau P, Pestre M, Dartigues JF, Commenges D, Barberger-Gateau C, Cohadon S. Long-term prognosis in two forms of childhood epilepsy: typical absence seizures and epilepsy with rolandic (centrotemporal) EEG foci. Ann Neurol. 1983;13(6):642–648. doi: 10.1002/ana.410130610. [DOI] [PubMed] [Google Scholar]
- 34.Livingston S, Torres I, Pauli LL, Rider RV. Petit mal epilepsy. Results of a prolonged follow-up study of 117 patients. JAMA. 1965;194(3):227–232. doi: 10.1001/jama.194.3.227. [DOI] [PubMed] [Google Scholar]
- 35.Janz D. Type of seizures and pathogenetic form of epileptic diseases. Nervenarzt. 1955;26(1):20–28. [PubMed] [Google Scholar]
- 36.Janz D. The clinical classification of pyknolepsy. Dtsch Med Wochenschr. 1955;80(38):1392–1400. doi: 10.1055/s-0028-1116209. [DOI] [PubMed] [Google Scholar]
- 37.Trinka E, Baumgartner S, Unterberger I, et al. Long-term prognosis for childhood and juvenile absence epilepsy. J Neurol. 2004;251(10):1235–1241. doi: 10.1007/s00415-004-0521-1. [DOI] [PubMed] [Google Scholar]
- 38.Yoshinaga H, Ohtsuka Y, Tamai K, et al. EEG in childhood absence epilepsy. Seizure. 2004;13(5):296–302. doi: 10.1016/S1059-1311(03)00196-1. [DOI] [PubMed] [Google Scholar]
- 39.Hedström A, Olsson I. Epidemiology of absence epilepsy: EEG findings and their predictive value. Pediatr Neurol. 1991;7(2):100–104. doi: 10.1016/0887-8994(91)90004-5. [DOI] [PubMed] [Google Scholar]
- 40.Lombroso CT. Consistent EEG focalities detected in subjects with primary generalized epilepsies monitored for two decades. Epilepsia. 1997;38(7):797–812. doi: 10.1111/j.1528-1157.1997.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 41.Leutmezer F, Lurger S, Baumgartner C. Focal features in patients with idiopathic generalized epilepsy. Epilepsy Res. 2002;50(3):293–300. doi: 10.1016/s0920-1211(02)00084-0. [DOI] [PubMed] [Google Scholar]
- 42.Posner EB, Mohamed K, Marson AG. Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. Cochrane Database Syst Rev. 2005:CD00303. doi: 10.1002/14651858.CD003032.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996;15(6):378–393. doi: 10.2165/00002018-199615060-00003. [DOI] [PubMed] [Google Scholar]