Abstract
Purpose
We evaluated the differential impact of stress-associated versus high, pharmacologic concentrations of hydrocortisone pre-treatment on heart rate variability (HRV) during a subsequent systemic inflammatory stimulus.
Materials and Methods
Healthy volunteers were randomized to receive placebo, hydrocortisone at 1.5 mcg/kg/min (STRESS), or at 3.0 mcg/kg/min (PHARM) as a six-hour infusion. The STRESS dose was chosen to replicate the condition of physiological adrenal cortical output during acute systemic stress. The PHARM dose was chosen to induce a supra-physiological concentration of cortisol. The next day all subjects received 2 ng/kg E. coli endotoxin (lipopolysaccharide, LPS). HRV was analyzed with the statistic Approximate Entropy (ApEn). A lower ApEn correlates with decreased HRV.
Results
At the three-hour nadir the decrease in ApEn in the STRESS group was significantly less compared to placebo (p<0.03), while ApEn in the PHARM group was not statistically different. We also found that the maximal decrease in ApEn preceded maximal increase in heart rate in all groups. The decrease in RR interval was maximal at 4 hours while the ApEn nadir was one hour earlier at 3 hours.
Conclusions
Pretreatment with a stress dose of hydrocortisone but not a higher pharmacologic dose maintained a significantly higher ApEn after endotoxin exposure when compared to a placebo. In addition, decreases in ApEn preceded increases in HR.
Keywords: Heart rate variability, Glucocorticoid, Experimental Endotoxemia, Sepsis, Human No reprints will be requested
INTRODUCTION
All systemic physiologic processes represent the end result of a series of interactions between multiple organ systems. Physiologic interconnectedness, which is often best represented by feedback loops, typically behaves in a non-linear fashion due to complex temporal and spatial relationships. One popular and evolving technique to evaluate a complex system is to analyze a time series of an appropriate variable, such as beat-to-beat intervals between successive cardiac cycles. Physiological time series have been shown repeatedly to have inherent variability. Heart Rate Variability (HRV), which is a common example of a physiologic time series, may be simply defined as the variation over time of the period between consecutive heartbeatsi.
The variability of many time series, including HRV, has been shown to correlate with the state of health of an organismii. HRV is to a great extent dependent upon extrinsic, as opposed to intrinsic, factors that control heart rateiii. As such, it reflects to a great extent the interplay between various inputs to the cardiac pacemaker system. A decrease in heart rate variability (HRV) is associated with disease onset and poor clinical outcomes in critically ill patientsiv. For example, a decrease in HRV predicts rapid changes in blood pressure and elevated levels of inflammatory cytokines in patients with sepsisv, and predicts outcomesvi and mortality following a septic episodevii. While numerous studies have examined the ability of changes in HRV to predict poor outcomes, little work has been done examining the varying effects of therapeutic interventions on HRV.
The hypothalamic-pituitary-adrenal axis has a vital role in controlling a wide variety of physiological processes in both humans and animals. It has also been shown to correlate with clinical outcomes in sepsisviii. In particular, cardiovascular responsiveness has been shown to be affected by supplementation with hydrocortisone during human sepsis, leading to a reduction in the duration of vasopressor requirementsix. We have recently shown that preconditioning with hydrocortisone affects the immunological response in a human model of experimental endotoxemia (EE)x. Therefore, to the extent that glucocorticoids can alter disease progression, they might also be expected to alter potential markers of disease such as HRV. With this background, we examined the impact of pretreatment with hydrocortisone on the HRV response to EE in healthy human volunteers. In this study, we pretreated experimental group subjects with 1 of 2 doses of hydrocortisone for 6 hours on the day before the onset of a systemic inflammatory stimulus (EE). These treatments were based on the observation that systemic stress has a ‘priming’ effect that augments subsequent responses to a second stressor (‘2nd hit’ hypothesis in critical care). We hypothesized that cortisol alone (an ‘endocrine stress’) would alter the systemic inflammatory response and the heart rate variability response to a subsequent, delayed systemic inflammatory stress.
METHODS
This study was approved by the Dartmouth College Committee for the Protection of Human Subjects (Institutional Review Board) and written informed consent was obtained from all participants.
Participants
Participants (n=36) were healthy male and female volunteers who were between the ages of 18 and 55.
Interventions
On Day 1 of the study, participants were randomized in a double-blind manner to one of 3 groups identified as Control, STRESS, or PHARM (‘pharmacological’). Participants were treated in a hospital outpatient facility during Day 1 where a peripheral intravenous catheter was inserted upon arrival. From 0900 to 1500 hours, Control Group participants received intravenous normal saline at 10 ml/hr; STRESS Group participants received 1.5 mcg/kg/min hydrocortisone (SoluCortef®, Upjohn) in normal saline at 10 ml/hr, and PHARM Group participants received 3.0 mcg/kg/min hydrocortisone in normal saline at 10 ml/hr. Preliminary work showed that the STRESS dose achieved an average cortisol concentration of 35-50 mcg/dl total plasma cortisol, and the PHARM dose achieved an average concentration of 80-100 mcg/dl. The STRESS dose was chosen to replicate the condition of physiological adrenal cortical output during acute systemic stress. The PHARM dose was chosen to induce a supra-physiological concentration of cortisol. The peripheral intravenous catheter was removed at 1500 hours. The following day (Day 2) at 0700 participants were admitted to a hospital acute care facility where a peripheral intravenous catheter was again inserted in a proximal arm vein. Continuous electrocardiography and pulse oximetry measurements were initiated and blood pressure and core temperature were measured non-invasively every 15 minutes for the next five hours and every 30 minutes for the succeeding 4 hours. Participants received 10 ml/kg lactated Ringer’s solution intravenously over the first 2 hours after which the infusion rate was decreased to 1.5 ml/kg/hr. At 0800 participants were injected with 2 ng/kg E. coli endotoxin (US Standard Reference Endotoxin; Lot #67801; Pharmacy Development Section, National Institutes of Health) intravenously over 2 minutes. Participants remained in the acute care facility for the remainder of the day with continuous nursing care and under the direct supervision of a physician.
Starting at 0800 hr R wave to R wave (RR) intervals were digitized at 250 Hz with an analog-to-digital converter (National Instruments) using custom software (National Instruments) and stored for the succeeding 8 hours. Prior to subsequent analysis, hourly 1000 beat epochs of RR intervals were analyzed visually for artifactual data.
Statististical Analysis: Standard deviation and variability were calculated using standard techniques. Regularity of the data sets was assessed with approximate entropy (ApEn). ApEn is a measure of variability that attempts to quantify repeating patterns in a time series. ApEn, as described by Pincusxi, is a group of statistics expressed as ApEn (m,r), where m is the run length and r is an index of similarity. The latter can be normalized to the standard deviation (SD) to correct for the effects of changes in frequency on approximate entropy. To calculate approximate entropy for each epoch, a run of data points of a certain length (determined by m) was compared to every other run of equal length in the data set. Two runs were considered similar when the maximum difference between comparable intervals was smaller than r, the defined similarity parameter. Data were analyzed with m = 2 and r = 15% of the SD.
Continuous physiological variables were compared using ANOVA techniques or a paired student’s t-test, as appropriate. Tests for normality of data were performed using the Kolmogorov-Smirnov/Lilliefor test. ApEn measurements were compared between baseline values and subsequent hourly epochs. A p-value less than 0.05 was considered to represent statistical significance. Statistical analysis was performed with StatPlus from AnalystSoft.
RESULTS
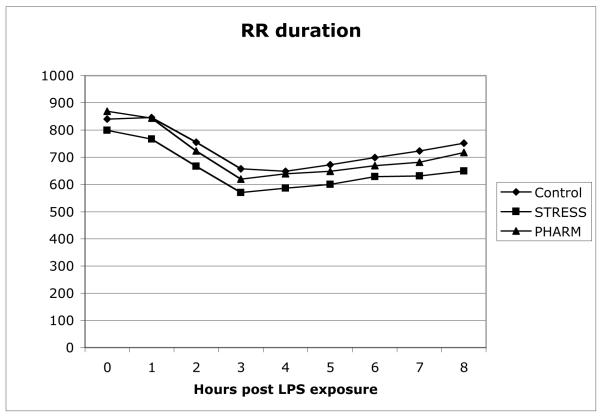
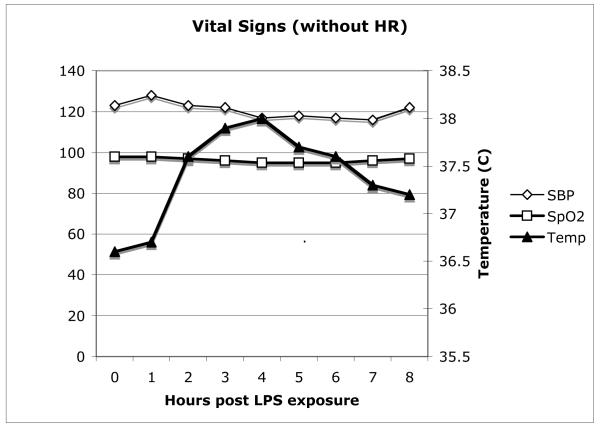
Subjects in the three groups were similar in age, gender, and weight (see Table 1.) RR intervals decreased (increase in heart rate) in a similar fashion in all three groups (Figure 1). Combined vital sign data for all 3 groups are shown in Figure 2. As with the HR data, all of the groups displayed similar changes.
Table 1.
Participant characteristics. Participants were randomized in a double-blind manner into one of 3 groups: Control, STRESS, or PHARM. Control = intravenous saline for 6 hours on Day 1; STRESS = intravenous hydrocortisone @ 1.5 mcg/kg/min for 6 hours on Day 1; PHARM = intravenous hydrocortisone @ 3ug/kg/min for 6 hours on Day 1. Data presented as mean (S.E.).
| SEX (M/F) |
AGE (years) |
WEIGHT (kg) |
|
|---|---|---|---|
|
|
|||
| CONTROL | 5/6 | 35.5 (4.3) |
75.8 (3.6) |
| STRESS | 5/7 | 33.0 (2.5) |
78.7 (4.1) |
| PHARM | 6/7 | 36.3 (3.5) |
77.5 (3.7) |
Figure 1.
Hourly R-to-R (RR) duration (milliseconds) by group assignment. No significant differences between groups were noted.
Figure 2.
Combined hourly vital sign data. SBP=systolic blood pressure, SpO2=Pulse oximetry (% saturation), Temp=temperature (°C). No significant differences between groups were observed.
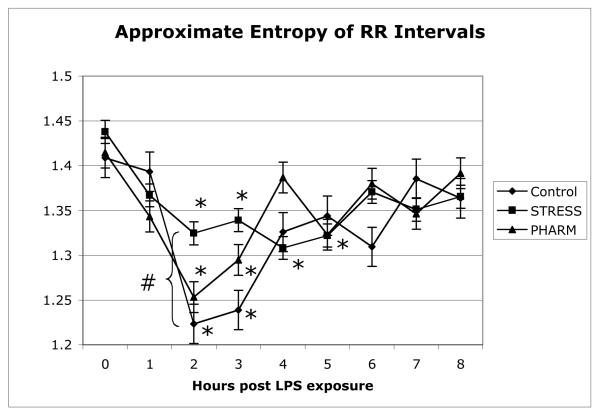
ApEn data were found to be normally distributed. ApEn decreased in all groups, with the nadir at three hours after the onset of EE (Figure 3). However, at the three hour nadir the PHARM group was not statistically different from the Control group, while the STRESS group differed in that it decreased to a significantly lesser degree (p<0.05).
Figure 3.
Approximate Entropy (ApEn) decreased in all three groups. Each measurement point represents hourly epochs of 1000-beat R-to-R wave intervals by group assignment, ± standard error. (*) represents a significant difference (p < 0.05) from baseline within groups. (#) represents a significant difference (p < 0.05) between the Control group and the STRESS group. Differences not marked were not significantly different.
The maximal decrease in ApEn preceded maximal decreases in RR interval (increase in heart rate) in all three groups. The decrease in RR interval was maximal at 4 hours, but the ApEn nadir was observed one hour earlier at 3 hours.
DISCUSSION
This study had 2 important findings: First, pretreatment with a stress dose of hydrocortisone but not a high pharmacologic dose maintained a significantly higher ApEn during EE when compared with placebo. To our knowledge this is the first demonstration of a pharmacologic intervention showing a dose-dependent effect on the HRV response to an endotoxin challenge. Second, it is particularly intriguing to note that decreases in ApEn preceded increases in HR and other physiologic events such as fever.
Multiple aspects of HRV alterations in sepsis have been investigated. Godin et al noted a decrease in HRV during EE in the human LPS modelxii. We later corroborated these results, and also found a generalized decrease in physiological variability of the immunological (leukocyte phagocytosis) and endocrine (plasma cortisol) systems, as well as HRVxiii. Annane et al evaluated frequency-domain measures of HRV of patients in septic shock and found impaired sympathetic modulation, despite the presence of high circulating catecholaminesxiv.
With this background, HRV has been examined as a means to predict poor clinical outcomes. A clinical diagnosis of neonatal sepsis, for example, is preceded by alterations in HRV dynamicsxv,xvi,xvii,xviii. This approach has been validated on a large population of neonatal intensive care unit patients and shown to be predictive of poor clinical outcomes, including deathxvi, xviii. Clinical outcomes in adult patients who are more likely to develop sepsis upon presentation to an emergency department can be predicted by alterations in time-domain or frequency-domain measures of HRVxix. Furthermore, patients admitted to an intensive care unit with a diagnosis of sepsis are likely to develop multiple organ dysfunctions if time-domain or frequency-domain measures of HRV are abnormal upon admissionxx. Frequency-domain techniques of measuring HRV are also able to detect patients at risk for blood pressure instability following induction of general anesthesia. This was observed in patients who would have been predicted to have a low rate of cardiovascular autonomic neuropathy by traditional diagnostic criteriaxxi. Similarly, spectral analysisxxii and point correlation dimension analysisxxiii of HRV predicts hypotension after spinal anesthesia for elective cesarean delivery. In fact, an algorithm to prophylactically manage hypotension after spinal anesthesia for cesarean delivery based on heart rate variability has been createdxxiv.
Despite the potential for HRV to be an indicator of a physiological stress or a predictor of poor clinical outcomes, surprisingly little research has been performed that investigates whether maintaining HRV at a level that is considered to be normal would be associated with altered outcomes. A recent study examined the effects of corticosteroid supplementation on cardiovascular variability in human sepsisxxv. The investigators examined HRV as well as both systolic and diastolic blood pressure variability in a continuous fashion. They observed that the loss in cardiovascular variability in septic shock is more marked in patients with adrenal insufficiency and is partly restored by exogenous administration of corticosteroids, which has led to improved outcomes in some studiesxiv. Interestingly, the parameter that was affected the most was diastolic blood pressure variability and not HRV, again highlighting the complex nature of these interactions.
Variability in successive heart beat intervals has been examined for many years. Multiple reviews have discussed various techniques to analyze physiological time series dataxxvi,xxvii,iv ,xxviii. However, there has been little consensus as to what is the optimal method for evaluating a given set of data. The central issue is that there are multiple aspects of a time series of heart rate intervals that correlate with variability. Time domain, frequency domain, and measures of complexity, such as entropy measures, have all been examined in various settings with varying degrees of success. A consensus conference published an extensive report detailing standards for measurement and analysis of HRV in 1996xxix, but concerned itself only minimally with measures of complexity. Notably, the field was evolving at a rapid rate at that time. A similar effort given to time and frequency domain would benefit the research in this area significantly. We performed the analysis of HRV with the well-known statistic approximate entropy. This approach has the advantages of standardization as well the fact that it is valid with data sets of varying sizes. Criticisms of this statistic center on the fact that ApEn was first calibrated against three mathematically chaotic systems, and not physiologic time series, and that the latter are not fully deterministic and are certainly noisyxxx. Despite these limitations, the weight of the data supports our approach, although there is controversyxxx over the most appropriate analysis of a given set of data involving a given physiological condition.
Research on glucocorticoids (GC) has traditionally been guided by a one-dimensional paradigm in which GCs are considered to exert an exclusively anti-inflammatory action. Recent work has demonstrated a more complex relationship between GCs and immune-mediated inflammation. It is now increasingly clear that: 1) GCs can exert pro-inflammatory as well as anti-inflammatory effects on key inflammatory processes and, 2) GC regulation of inflammation can vary from anti-inflammatory to pro-inflammatory in a time- and concentration-dependent mannerxxxi. The immediate in vivo effect of both stress-induced and pharmacological GC concentrations is to suppress concurrent inflammation and protect the organism from an excessive or prolonged inflammatory response. However, GCs also appear to regulate adaptation to disease such that transient, stress associated increases in GC concentration will lead to a significantly enhanced inflammatory response to a subsequent inflammatory stimulus when compared higher or lower concentrationsx. The data presented here support this model of GC regulation of adaptational responses to inflammation since pre-treatment with stress-associated concentrations attenuated a subsequent marker of inflammatory illness, HRV.
In this study we showed that HRV can be altered by a specific intervention that preceded the onset of an artificially induced inflammatory disease state. We, and others, have shown that transient in vivo exposure to increased cortisol concentrations induces an adaptational response that is manifested by an enhanced responsiveness to subsequent exposure to bacterial LPSx, xxxii. Whether or not this enhanced response is adaptational in that it leads to improved outcomes is currently unknown. The results reported here suggest, at least, that maintenance of HRV is associated with the more vigorous inflammatory response to bacterial LPS reported earlierx. Finally, the finding that loss of HRV preceded other physiologic events such as tachycardia and hyperthermia suggest further work on this modality as a method of monitoring and treatment of septic patients.
Acknowledgments
All work completed at the Dartmouth-Hitchcock Medical Center, Lebanon, NH 03756 Supported by a grant from the National Institutes of Health, NIAID # AI051547 (PMG) None of the authors has a conflict of interest with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.Seely AJ, Macklem PT. Complex systems and the technology of variability analysis. Crit Care. 2004;8(6):R367–84. doi: 10.1186/cc2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ii.Gang Y, Malik M. Heart rate variability in critical care medicine. Curr Opin Crit Care. 2002;8(5):371–5. doi: 10.1097/00075198-200210000-00002. [DOI] [PubMed] [Google Scholar]
- iii.Seely AJ, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems. Crit Care Med. 2000;28(7):2193–200. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- iv.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47(4):658–87. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- v.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002;8(4):311–5. doi: 10.1097/00075198-200208000-00007. [DOI] [PubMed] [Google Scholar]
- vi.Pontet J, Contreras P, Curbelo A, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. 2003;18(3):156–63. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- vii.Schmidt H, Müller-Werdan U, Hoffmann T, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33(9):1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- viii.Marik PE, Pastores SM, Annane D, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36(6):1937–49. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- ix.Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- x.Yeager MP, Rassias AJ, Pioli PA, et al. Pretreatment with stress cortisol enhances the human systemic inflammatory response to bacterial endotoxin. Crit Care Med. 2009;37(10):2727–32. doi: 10.1097/ccm.0b013e3181a592b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xi.Pincus SM, Gladstone IM, Ehrenkranz RA. A regularity statistic for medical data analysis. J Clin Monit. 1991;7(4):335–45. doi: 10.1007/BF01619355. [DOI] [PubMed] [Google Scholar]
- xii.Godin PJ, Fleisher LA, Eidsath A, et al. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med. 1996;24(7):1117–24. doi: 10.1097/00003246-199607000-00009. [DOI] [PubMed] [Google Scholar]
- xiii.Rassias AJ, Holzberger PT, Givan AL, et al. Decreased physiologic variability as a generalized response to human endotoxemia. Crit Care Med. 2005;33(3):512–9. doi: 10.1097/01.ccm.0000155908.46346.ed. [DOI] [PubMed] [Google Scholar]
- xiv.Annane D, Trabold F, Sharshar T, et al. Inappropriate sympathetic activation at onset of septic shock: a spectral analysis approach. Am J Respir Crit Care Med. 1999;160(2):458–65. doi: 10.1164/ajrccm.160.2.9810073. [DOI] [PubMed] [Google Scholar]
- xv.Cao H, Lake DE, Griffin MP, et al. Increased nonstationarity of neonatal heart rate before the clinical diagnosis of sepsis. Ann Biomed Eng. 2004;32(2):233–44. doi: 10.1023/b:abme.0000012743.81754.0b. [DOI] [PubMed] [Google Scholar]
- xvi.Griffin MP, Lake DE, Bissonette EA, et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116(5):1070–4. doi: 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- xvii.Griffin MP, O’Shea TM, Bissonette EA, et al. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res. 2003;53:920–926. doi: 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- xviii.Griffin MP, O’Shea TM, Bissonette EA, et al. Abnormal heart rate characteristics are associated with neonatal mortality. Pediatr Res. 2004;55:782–788. doi: 10.1203/01.PDR.0000119366.21770.9E. [DOI] [PubMed] [Google Scholar]
- xix.Chen WL, Chen JH, Huang CC, et al. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med. 2008;26(4):395–401. doi: 10.1016/j.ajem.2007.06.016. [DOI] [PubMed] [Google Scholar]
- xx.Pontetab J, Contrerasa P, Curbelob A, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. Journal of Critical Care. 2003;18(3):156–163. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- xxi.Huang CJ, Kuok CH, Kuo TB, Hsu YW, Tsai PS. Pre-operative measurement of heart rate variability predicts hypotension during general anesthesia. Acta Anaesthesiol Scand. 2006;50(5):542–8. doi: 10.1111/j.1399-6576.2006.001016.x. [DOI] [PubMed] [Google Scholar]
- xxii.Hanss R, Bein B, Ledowski T, et al. Heart rate variability predicts severe hypotension after spinal anesthesia for elective cesarean delivery. Anesthesiology. 2005;102(6):1086–93. doi: 10.1097/00000542-200506000-00005. [DOI] [PubMed] [Google Scholar]
- xxiii.Chamchad D, Arkoosh VA, Horrow JC, et al. Using heart rate variability to stratify risk of obstetric patients undergoing spinal anesthesia. Anesth Analg. 2004;99(6):1818–21. doi: 10.1213/01.ANE.0000140953.40059.E6. [DOI] [PubMed] [Google Scholar]
- xxiv.Hanss R, Bein B, Francksen H, Scherkl W, et al. Heart rate variability-guided prophylactic treatment of severe hypotension after subarachnoid block for elective cesarean delivery. Anesthesiology. 2006;104(4):635–43. doi: 10.1097/00000542-200604000-00005. [DOI] [PubMed] [Google Scholar]
- xxv.Aboab J, Polito A, Orlikowski D, et al. Hydrocortisone effects on cardiovascular variability in septic shock: a spectral analysis approach. Crit Care Med. 2008;36(5):1481–6. doi: 10.1097/CCM.0b013e31816f48f2. [DOI] [PubMed] [Google Scholar]
- xxvi.Laitio T, Jalonen J, Kuusela T, et al. The role of heart rate variability in risk stratification for adverse postoperative cardiac events. Anesth Analg. 2007;105(6):1548–60. doi: 10.1213/01.ane.0000287654.49358.3a. [DOI] [PubMed] [Google Scholar]
- xxvii.Seely AJ. Heart rate variability and infection: diagnosis, prognosis, and prediction. J Crit Care. 2006;21(3):286–9. doi: 10.1016/j.jcrc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- xxviii.Goldstein B, Fiser DH, Kelly MM, et al. Decomplexification in critical illness and injury: relationship between heart rate variability, severity of illness, and outcome. Crit Care Med. 1998;26(2):352–7. doi: 10.1097/00003246-199802000-00040. [DOI] [PubMed] [Google Scholar]
- xxix.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- xxx.Webber CL., Jr The meaning and measurement of physiologic variability? Crit Care Med. 2005;33(3):677–8. doi: 10.1097/01.ccm.0000155772.47288.ec. [DOI] [PubMed] [Google Scholar]
- xxxi.Yeager MP, Guyre PM, Munck AU. Glucocorticoid Regulation of the Inflammatory Response to Injury. Acta Anaesthesiol Scand. 2004;48(7):799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- xxxii.Barber AE, Coyle SM, Marano MA, et al. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150(5):1999–2006. [PubMed] [Google Scholar]