Abstract
Background: Tobacco dependence is a chronic, relapsing condition that may require extended treatment.
Objective: To assess whether extended transdermal nicotine therapy increases abstinence from tobacco more than standard duration therapy in adult smokers.
Design: Parallel randomized placebo-controlled trial from September 2004 to February 2008 (small block randomization scheme, not stratified). Study participants and all research personnel except for the database manager were blinded to randomization. (NCT00364156)
Setting: Academic center.
Participants: 568 adult smokers.
Intervention: Participants were randomized to: standard (8 weeks 21mg Nicoderm CQ, 16 weeks placebo) or extended (24 weeks 21mg Nicoderm CQ) therapy.
Measurements: The primary outcome was biochemically-verified point prevalence abstinence at weeks 24 and 52. Secondary outcomes were continuous and prolonged abstinence, lapse and recovery events, cost/additional quitter, and side effects and adherence.
Results: At 24 weeks, extended therapy produced higher rates of point prevalence abstinence (31.6% versus 20.3%; Odds Ratio [OR] = 1.81 [1.23-2.66], p = 0.002), prolonged abstinence (41.5% versus 26.9%; OR = 1.97 [1.38-2.82] p = 0.001), and continuous abstinence (19.2% versus 12.6%; OR = 1.64 [1.04-2.60] p = 0.032), versus standard therapy. Extended therapy reduced the risk for a lapse (Hazard Ratio [HR] = 0.77 [0.63-0.95], p = 0.013) and increased the chances of recovery from lapses (HR = 1.47 [1.17-1.84], p = 0.001). Time to relapse was slower with extended versus standard therapy (HR = 0.50 [0.35-0.73], p < 0.001). At week 52, extended therapy produced higher quit rates for prolonged abstinence only (p = 0.027). There were no group differences in side effects and adverse events at the extended treatment phase assessment.
Limitations: The generalizability of the findings may be limited because participants were treatment-seeking smokers without medical comorbidity and differences in adherence across treatment arms were detected.
Conclusion: Compared to 8 weeks of transdermal nicotine, 24 weeks of transdermal nicotine increased biochemically-confirmed point prevalence abstinence and continuous abstinence at week 24, reduced the risk of smoking lapses, and increased the likelihood of post-lapse recovery to abstinence.
Introduction
The transdermal nicotine patch is one of the most widely used forms of tobacco dependence treatment in the U.S. (1, 2) and Europe (3, 4). Current guidelines for transdermal nicotine recommend 8 weeks of treatment (5). However, the evidence for this recommendation is limited. A meta-analysis comparing nicotine patch trials with ≤ 8 weeks versus those with > 8 weeks of treatment found no difference in quit rates; however, almost all of the “extended therapy” trials were shorter than 12 weeks duration (6). Only one large placebo-controlled randomized trial has compared standard (8 weeks) versus extended (22 weeks) transdermal nicotine therapy (7). Although this trial found no difference in rates of continuous abstinence between treatment arms, more recent guidelines view continuous abstinence measures as too conservative, because smokers who have early lapses but recover are counted as treatment failures (8). This may be particularly important for “rescue therapies”, such as transdermal nicotine, that increase the likelihood of recovery from smoking lapses (9).
There is a growing recognition that nicotine dependence is a chronic, relapsing condition that may require extended therapy to treat effectively (10-12). Further, the 2008 Public Health Service Guidelines call for research on the efficacy of extended therapy for nicotine dependence (5). This parallel randomized placebo-controlled trial evaluated the relative efficacy of extended (24 weeks) versus standard (8 weeks) transdermal nicotine therapy for promoting biochemically-confirmed point prevalence abstinence at weeks 24 and 52 among adult smokers.
Methods
Design Overview
Treatment-seeking smokers enrolled October 2004 to March 2008 into a parallel randomized placebo-controlled trial of standard (8 weeks of transdermal nicotine and 16 weeks of placebo patches) vs. extended (24 weeks of transdermal nicotine) therapy. Participants and all study personnel except for the database manager were blinded to randomization. The primary outcome was biochemically-verified point prevalence abstinence at weeks 24 and 52. Assessments were completed in March 2009. Participants provided informed consent and procedures were approved by the University of Pennsylvania Institutional Review Board (IRB).
Setting and Participants
The trial was conducted at the University of Pennsylvania. Participants were recruited through advertisements for a free smoking cessation program. They were eligible if they were ages 18-65 and smoked ≥ 10 cigarettes/day for at least the past year. Exclusions were: pregnancy or lactation, uncontrolled hypertension, unstable angina, heart attack or stroke within the previous six months, recent diagnosis of cancer or kidney/liver failure, a history of organ transplant, current diabetes, drug or alcohol dependence, history of an Axis I psychiatric disorder, current use of a concomitant medication, or current treatment for nicotine addiction.
Randomization and Interventions
Once eligibility was confirmed (by phone and in person), participants were randomized at week -2 using a computer-based randomization table provided by a statistician using Stata (Version 8, Stata Corporation, College Station, TX) and a computer program overseen by the database manager. A non-stratified randomization scheme was generated by sampling without replacement using small blocks (n = 20). The 24-week supply of patches was pre-packaged and coded with participant ID. The computer program linked the randomization to the patch supply and only the database manager could link ID with treatment allocation.
Behavioral counseling (13) was provided at weeks -2, 0, 1, 4, 8, 12, 16, and 20. Five counselors provided the same counseling to both treatment arms; they were trained using a manual and supervised to ensure adherence. At week -2, counseling focused on developing techniques for preparing to quit. At week 0, the quit date, counseling focused on managing withdrawal. The remaining sessions focused on relapse prevention. Patch use was initiated on the quit date (week 0). Participants received: 8 weeks of 21mg transdermal nicotine patch (Nicoderm CQ; GlaxoSmithKline) plus 16 weeks of placebo patch (standard) or 24 weeks of 21mg transdermal nicotine patch (extended).
Outcomes and Measurements
Screening/Pre-treatment Measures
Medication use and history of medical illnesses were assessed. The Structured Clinical Interview screened for Diagnostic and Statistical Manual of Mental Disorders Volume IV psychiatric illnesses (14). Demographic information (e.g., age) and smoking rate were assessed as was nicotine dependence level using the Fagerström Test for Nicotine Dependence (FTND; 15).
Outcome Measures
Daily smoking, from the quit date to week 52, was assessed by a timeline follow-back measure (16). Participants who reported no smoking for the 7 days prior to weeks 8, 24, and 52 were asked to visit the study site to provide a breath sample for assessment of carbon monoxide levels (17). As recommended (8), the primary outcome was 7-day point prevalence abstinence at weeks 24 and 52 and secondary measures of abstinence were included to capture different quitting patterns (e.g., continuous and prolonged abstinence), including the timing and processes of lapse, relapse, and recovery events (8, 9, 18, 19).
Primary Outcome
Seven-day point prevalence abstinence is defined as self-report of no smoking for seven days prior to the assessment, verified by carbon monoxide (≤ 10ppm; 17). Participants were assumed to be smoking if they were lost to follow-up, failed to provide a carbon monoxide sample, or had carbon monoxide levels > 10ppm (17).
Secondary Outcomes
Continuous abstinence, a stringent measure of abstinence, refers to self-reports of no smoking (not even a puff) from the quit date to a follow-up assessment (8). Prolonged abstinence refers to sustained abstinence from the quit date to a follow-up assessment (8). A key aspect of prolonged abstinence is a grace period, which captures delayed treatment effects (8). For prolonged abstinence, relapse is defined as 7 consecutive days of self-reported smoking between the quit date and week 24 and 52 after a 2-week grace period (which means that smoking during the first 14 days post-quit date was not considered a lapse; 8). Time to relapse refers to the duration of time (in days) from the quit day until a relapse (defined as 7 consecutive days of self-reported smoking). This measure was used in survival models. For lapse and recovery events, a smoking lapse was defined as any day between the quit date and the week 24 and week 52 assessments on which participants smoked (even a puff); recovery was defined as any 24-hour period of self-reported abstinence post-lapse (19). The outcome of interest was time to transition between runs of smoking days and runs of abstinent days. Participants were asked at each assessment if they incurred any direct costs (e.g., physician fee or co-pay, prescription or non-prescription medications) or indirect costs (e.g., lost wages to seek medical attention) between the time-points as a result of participating in this study. The primary cost outcome was the incremental cost per additional quitter by treatment arm at week 24.
Follow-up Procedures and Monitoring
Research assistants blind to randomization collected self-report measures in person at weeks 0, 1, 4, 8, 12, 16, and 20, and by phone at weeks 24 and 52.
Side Effects
Side effects were assessed using a 15-item symptom checklist (13) at weeks 0, 1, 4, 8, 12, 16, 20, 24, and 52. This checklist contained symptoms, rated from 1 (none) to 4 (severe), that may occur from nicotine patch use (e.g., nausea, skin reaction). Self-reported serious symptoms were those rated severe. Participants were asked to contact study personnel if they experienced any serious medical problems and Research Assistants used an open-ended question after the checklist to identify additional serious adverse events. Adverse events were considered serious if the participant considered them debilitating or if they required hospitalization. Serious adverse events were reported to the University of Pennsylvania IRB and were classified as related or unrelated to treatment arm allocation.
Adherence
Patch adherence was based on self-report during weeks 0-24. Participants were classified as compliant if they used of ≥ 6 patches/week (20). Treatment arm comparisons in weekly patch adherence were conducted for weeks 1, 4, 8, 12, 16, 20, and 24. Counseling attendance (weeks -2, 0, 1, 4, 8, 12, 16, and 20) was also tracked. At weeks 24 and 52, use of other forms of nicotine replacement therapy (NRT) or bupropion was queried.
A senior data manager oversaw quality control. Microsoft ACCESS was used for data entry, validation, storage, retrieval, and security; data integrity was ensured through range and validity checks during data entry. Data quality assurance reports were generated weekly to summarize production activity and to monitor quality control.
Statistical Analysis
The a priori sample size was 600 which provided 80% power to detect an odds ratio for a treatment arm effect of 1.72. All eligible randomized participants were included in the analyses conducted using Stata (Version 8, Stata Corporation, College Station, TX). Longitudinal logistic regression using generalized estimating equations was used to analyze week 24 and 52 abstinence rates. Time to relapse was modeled using Cox regression (stratified by time: weeks 1-8, 9-24, 25‐52). All models included terms for age, FTND, and sex, as these factors predicted study retention. Multivariate time-to-event models determined whether treatment arm predicted transitions from abstinence to lapse and from lapse to recovery (19). This type of alternating state multivariate data consists of times to transition between runs of smoking days (≥ one day) and runs of abstinent days (≥ one day; 21). Up to 8 cycles of lapse and recovery events were evaluated, participants could cycle through multiple events, and risk sets (those currently abstinent for lapse events and those currently smoking for recovery events) were stratified as: week 1-8, week 9-24, and week 25-52. Weibull parametric survival models were examined to account for treatment arm effects and other covariates (22), and standard errors were adjusted for repeated measures using the cluster-correlated robust variance estimate (23). Schoenfeld residuals tested the proportional hazards assumption. Participants lost to follow-up on the timeline follow-back (for time to event analyses) were censored at that time (removed from the risk-set without registering an event). Chi-square tests assessed differences between treatment arms in side effects, adherence, and use of other smoking cessation treatments. To capture the periods when symptoms peak (24), analyses of side effects focused on the first week following the quit date (week 1) and one month following the transition to placebo versus extended therapy (week 12). Direct and indirect costs incurred up to week 52 across treatment arms were compared and the incremental cost per additional quitter at week 24 by treatment arm was estimated, computing a confidence interval by Fieller's method (25). Treatment cost estimates used were: $120 for counseling (both arms); $140 for standard therapy; and $420 for extended therapy (www.drugstore.com).
Role of the Funding Source
The National Institutes of Health had no involvement in the study design, collection and analysis of data, writing of this report, or the decision to submit this paper for publication.
Results
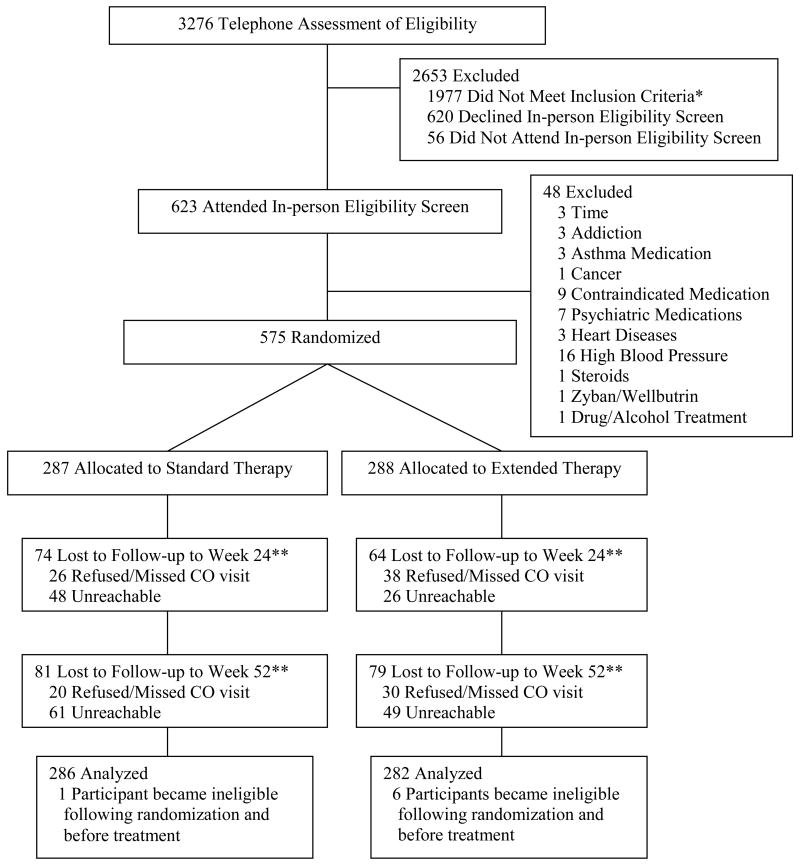
Two hundred eighty seven participants were randomized to standard therapy and 288 to extended therapy; 7 individuals (1 in standard and 6 in extended therapy) were ineligible due to medical contraindications after randomization, but before treatment began, and were excluded from the intent-to-treat (ITT) sample (Figure 1). Participant baseline characteristics were similar across the treatment arms (Table 1). There were no cross-overs of assignments during the trial.
Table 1. Participant Characteristics by Treatment Arm Assignment.
| Characteristic | Standard (n = 286) | Extended (n = 282) | Overall (n = 568) |
|---|---|---|---|
|
|
|
|
|
| Sex (% Female) | 45.1 | 44.3 | 44.7 |
| Age (Mean, SD) | 44.9 (10.4) | 44.8 (10.2) | 44.8 (10.3) |
| Education (% Beyond High School) | 79.0 | 77.7 | 78.4 |
| Race (% European Ancestry) | 86.7 | 82.6 | 84.7 |
| FTND (Mean, SD) | 5.3 (2.1) | 5.2 (2.2) | 5.3 (2.1) |
| Cigarettes per Day (Mean, SD) | 21.3 (9.0) | 21.1 (9.5) | 21.2 (9.2) |
| Plasma Cotinine, ng/mL (Mean, SD) | 272.7 (110.2) | 267.0 (122.9) | 269.8 (116.6) |
Note. FTND = Fagerström Test for Nicotine Dependence; n = Number of Participants; SD = Standard Deviation; ng/mL = nanograms per milliliter.
The rate of missing survey data by time and treatment was assessed (Supplemental Table 1; see www.annals.org). Week 24 completion rates were higher for extended (91%) versus standard (83%) (p = 0.007) therapy, but week 52 completion rates were similar (extended = 83%; standard = 79%; p = 0.23); 97 participants (17.1%) had timeline follow-back data that ended prior to week 52 but this was unrelated to treatment arm. The number of missing cost measures per participant ranged from 0 (226 participants) to all 10 (7 participants). A greater proportion of standard therapy participants had missing cost measures versus extended therapy. The seven participants who had no cost measures were excluded from the cost analysis. For the remaining 335 participants who had one to nine cost measures missing, an average for cost per item was imputed. Non-responders to the week 24 survey were younger, had higher FTND scores, and reported smoking more cigarettes/day. Non-responders to the week 52 survey were younger. No additional participant characteristic (Table 1) was related to survey completion.
Abstinence
The odds of point prevalence abstinence were ∼2 times greater for extended versus standard therapy at week 24 (31.6% versus 20.3%; Odds Ratio [OR] = 1.81 [1.23-2.66], p = 0.002; Table 2). There was also an advantage for extended versus standard therapy for prolonged (41.5% versus 26.9; OR = 1.97 [1.38-2.82] p = 0.001) and continuous (19.2% versus 12.6%; OR = 1.64 [1.04-2.60] p = 0.032) abstinence at week 24. In contrast, at week 52 there was no difference between treatment arms in point prevalence abstinence (extended = 14.5%, standard = 14.3%; OR = 1.01 [0.63-1.62], p = 0.95) or continuous abstinence (extended = 0.7%, standard = 1.0%; OR = 0.67 [.11-4.05], p = 0.67), but there was a difference by treatment arm in prolonged abstinence at week 52 (extended = 29.1%, standard = 21.3%; OR = 1.55 [1.05-2.28], p = 0.027).
Table 2. Point Prevalence Abstinence at Weeks 8, 24, and 52.
| Predictor | Standard (n = 286) n (%) | Extended (n = 282) n (%) | OR | 95% CI | p |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Treatment Arm (week 8) | 86 (30.1%) | 98 (34.8%) | 1.23 | 0.87 - 1.76 | 0.25 |
| Treatment Arm (week 24) | 58 (20.3%) | 89 (31.6%) | 1.81 | 1.23 - 2.66 | 0.002 |
| Treatment Arm (week 52) | 41 (14.3%) | 41 (14.5%) | 1.01 | 0.63 - 1.62 | 0.95 |
| Time-point (Week 24 vs. 8) | 0.59 | 0.45 - 0.77 | < .001 | ||
| Time-point (Week 52 vs. 24) | 0.39 | 0.29 - 0.52 | < .001 |
Note. OR = Odds Ratio; CI = Confidence Interval; n = Number of Participants; an OR > 1 favors the extended treatment group; the model controlled for age and sex, which were not related to quit rates, as well as level of nicotine dependence, which was related to quit rates (OR = 0.68, 95% CI: 0.49-0.94, p = 0.021); participants who reported lower nicotine dependence were more likely to be abstinent across time-points.
Time to relapse (any self-reported smoking) was similar across treatment arms up to week 8 when all participants were receiving active treatment (Hazard Ratio [HR] = 0.79 [0.58-1.07], p = 0.133; Figure 2). From week 9-24, the rate of relapse was slower up for those receiving extended versus standard therapy (HR = 0.50 [0.35-0.73], p < 0.001). Time to relapse did not differ across treatment arms between week 25-52 (HR = 1.52 [0.84-2.74], p = 0.163).
In the analysis of transitions from alternating periods of abstinence and smoking, extended therapy also reduced the speed at which participants lapsed during weeks 9 to 24 (HR = 0.77 [0.63-0.95], p = 0.013) and increased the speed of recovery from a lapse during this time period (HR = 1.47 [1.17-1.84], p = 0.001) (Supplemental Table 2; see www.annals.org).
Side Effects and Adherence
Table 3 shows the adverse event data. Three participants in extended therapy reported a serious adverse event (heart attack, hypertension, fainting from blood draw) and one participant in standard therapy reported a serious adverse event (skin redness). All adverse events occurred before week 8 and the heart attack occurred before starting transdermal nicotine; thus, all serious adverse events were not related to extended therapy. Only the participant who experienced the heart attack withdrew from the trial. Participants in extended therapy had a greater frequency of sleep problems at week 1, vs. standard therapy; no other differences were detected.
Table 3. Frequency of Side Effects and Adverse Events by Treatment Arm.
| Variable | Standard Therapy (No. %) | Extended Therapy (No. %) |
|---|---|---|
|
|
|
|
| Deaths | 0/286 (0) | 0/282 (0) |
| Withdrawal due to Adverse Event | 0/286 (0) | 1/282 (0.4) |
| Any Self-reported Serious Symptom | 143/286 (50) | 142/282 (50.4) |
| Serious Adverse Event | 1/286 (0.4) | 3/282 (1.1) |
| Week 1 Self-reported Serious Symptoms | ||
| Headache | 5/252 (2.0) | 3/247 (1.2) |
| Nausea | 1/252 (0.4) | 3/247 (1.2) |
| Alertness | 11/252 (4.4) | 16/247 (6.5) |
| Coughing | 5/252 (2.0) | 3/247 (1.2) |
| Pounding Heart | 3/252 (1.2) | 2/247 (0.8) |
| Diarrhea | 2/252 (0.8) | 0/247 (0) |
| Sleep Problems | 7/252 (2.8) | 19/247 (7.7)* |
| Skin Irritation or Rash | 4/252 (1.6) | 3/247 (1.2) |
| Calmness | 5/252 (2.0) | 9/247 (3.6) |
| Dizziness | 1/252 (0.4) | 1/247 (0.4) |
| Light Headedness | 1/252 (0.4) | 2/247 (0.8) |
| Sweating | 8/252 (3.2) | 3/247 (1.2) |
| Good or “High” Feeling | 4/252 (1.6) | 7/247 (2.8) |
| Watery Eyes | 3/252 (1.2) | 3/247 (1.2) |
| Coldness of Hands/Feet | 7/252 (2.8) | 6/247 (2.4) |
| Week 12 Self-reported Serious Symptoms | ||
| Headache | 2/134 (1.5) | 0/182 (0) |
| Nausea | 1/134 (0.7) | 1/182 (0.5) |
| Alertness | 9/134 (6.7) | 17/182 (9.3) |
| Coughing | 3/134 (2.2) | 5 (2.7) |
| Pounding Heart | 2/134 (1.5) | 0/182 (0) |
| Diarrhea | 0/134 (0) | 0/182 (0) |
| Sleep Problems | 6/134 (4.5) | 2/182 (1.1) |
| Skin Irritation or Rash | 1/134 (0.7) | 1/182 (0.5) |
| Calmness | 4/134 (3.0) | 5/182 (2.7) |
| Dizziness | 0/134 (0) | 1/182 (0.5) |
| Light Headedness | 1/134 (0.7) | 1/182 (0.5) |
| Sweating | 2/134 (1.5) | 4/182 (2.2) |
| Good or “High” Feeling | 3/134 (2.2) | 4/182 (2.2) |
| Watery Eyes | 2/134 (1.5) | 1/182 (0.5) |
| Coldness of Hands/Feet | 2/134 (1.5) | 3/182 (1.6) |
Note. Any Self-reported Serious Symptom refers to the total number of participants who reported a severe side effect on the checklist across all time-points where side effects were assessed; a Serious Adverse Event refers to the total number of participants who reported a serious adverse event. Week 1 and week 12 Self-reported Serious Symptoms are those rated as 4 (severe) on the side effects checklist; No. = number of participants; * refers to difference between standard and extended treatment (p = 0.014); all items were scored such that higher values reflected greater severity of the item, even for positive items such as good or high feeling, alertness, and calmness
There were no differences in rates of adherence to the patch between treatment arms at weeks 1, 4 and 8. Compared to standard therapy participants, extended therapy participants reported higher patch adherence at weeks 12 (p < 0.001), 16 (p < 0.001), 20 (p = 0.005), and 24 (p < 0.001). Adherence rates at these weeks were 86%, 69%, 56%, 38%, 34%, 32%, and 25% for standard therapy, and 85%, 68%, 64%, 53%, 49%, 44%, and 40% for extended therapy. There was no difference in rates of counseling adherence at weeks -2, 0, 1, and 4 across treatment arms. Compared to standard therapy participants, extended therapy participants showed higher rates of counseling adherence at weeks 8 (p = 0.014), 12 (p < 0.001), 16 (p = 0.001), and 20 (p = 0.001; Supplemental Table 1). There were no differences across treatment arms at week 24 in use of other NRTs or use of bupropion; at week 52, three participants in extended therapy reported use of nicotine lozenges, versus zero participants in the standard therapy arm (p = 0.033).
Costs
Twenty-four participants (4.2%) reported non-zero out-of-pocket costs from treatment. Thirteen participants (2.3%) reported direct costs for visiting a health professional (e.g., co-pays), ranging from $15-$200; four participants (0.7%) reported indirect costs of seeing a health professional (e.g., lost wages), ranging from $10–$700. Seventeen participants (3.0%) reported medication and other healthcare costs ranging from $3-$300. With adjustment for session, sex and treatment arm, the average total per-person cost incurred beyond protocol-related counseling and drug treatment was $3.92. There was no difference between treatment arms. Because these costs were modest and did not differ across arms, they were excluded from subsequent analyses.
The incremental cost per additional quitter for extended versus standard therapy at week 24 was estimated as the difference in treatment costs between the treatment arms divided by the difference in week 24 point prevalence quit rates, or ($420−$140)/(.316−.203). The incremental cost of extended vs. standard therapy is $2,482 per additional quitter (95% CI $1,519 – $6,781).
Discussion
Smokers who received extended therapy with transdermal nicotine were about twice as likely to be abstinent at 24 weeks post-quit date versus those receiving standard therapy. The benefit of extended therapy to week 24 was robust across all cessation outcomes. Compared to standard therapy, extended therapy with transdermal nicotine slowed the time to relapse, reduced the risk for a lapse, and increased the chances of recovery from lapses. These benefits with extended therapy cost $2,482/quitter, which compares well to other smoking cessation treatments (26). Benefits of extended therapy with transdermal nicotine were evident only while treatment was maintained.
Extended therapy with transdermal nicotine produced end of treatment quit rates that were comparable to those reported for other cessation medications approved by the U.S. Food and Drug Administration, including standard 10-week treatment with bupropion (∼19%) (27) and standard 12-week treatment with varenicline (33-35%) (28, 29). Compared to these medications, transdermal nicotine is accessible over the counter and has fewer contraindications for use.
Compared to the existing literature (English-language PUBMED search to August 2009), our findings diverge from the only other large placebo-controlled randomized trial which reported equivalent efficacy for standard versus extended therapy (7). There are differences in study design and analysis between the two trials. First, the previous trial was performed at 37 sites across 17 countries; quit rates varied from 3-30% across sites. Second, the previous trial compared 15mg and 25mg patch doses with a 16-hour dosing schedule and tapering, whereas the present study used a 24-hour 21mg dose with no tapering. Third, the previous trial used 1-year post-quit date continuous abstinence as the outcome. Continuous abstinence is a conservative measure of treatment efficacy since smokers who have early lapses (e.g., a single puff) but recover are counted as treatment failures (8). This may be particularly important for “rescue therapies”, such as transdermal nicotine, that increase the likelihood of recovery from smoking lapses (9, 30). The current data are consistent with a meta-analysis indicating that ∼50% of relapses could be averted if nicotine patch treatment is continued beyond the recommended duration (31).
Improvement in recovery from lapses is one potential mechanism of efficacy for extended treatment. During the active treatment phase (weeks 9-24), participants in extended therapy lapsed more slowly and, if they did lapse, they were faster in recovering versus those in standard therapy. This is biologically plausible, since chronic nicotine, such as that delivered by the patch, desensitizes α4β2 neuronal nicotinic acetylcholine receptors, potentially reducing nicotine reward from smoking (32). Extended therapy with transdermal nicotine may also help smokers to recover from a lapse by extinguishing the learned reinforcement from smoking similar to the use of transdermal nicotine prior to a quit date (33). This may also explain why the benefit of therapy in terms of point prevalence abstinence was no longer evident at week 52.
Extended therapy with transdermal nicotine increased costs, versus standard therapy, but this cost compares favorably to other smoking cessation treatments (26). Although patch use and efficacy increase when costs are covered (3, 4, 34), only 8.6% of U.S. health plans fully cover nicotine patches (35), and only 33 states cover the nicotine patch under Medicaid (36). Longer studies and/or more comprehensive cost models can address whether the costs of extended nicotine patch therapy are justified by the health and economic benefits of enhanced quit rates. Comparison of the cost per quitter using extended therapy with the cost per quitter using pre-cessation treatment with transdermal nicotine (33) is also warranted. Additionally, misperceptions about concurrent smoking and nicotine patch use may lead to discontinuing treatment prematurely following lapses (37). Recent data show suggest that smoking while using the patch poses minimal risk (38, 39) and smokers are being advised to continue patch use, even after a lapse (40).
This study used strict eligibility criteria to enhance internal validity. Thus, participants may not be representative of the general population of smokers, such as those with medical comorbidities. Since this study evaluated nicotine patch, carbon monoxide was used to verify cessation self-reports. Carbon monoxide has a shorter half-life than cotinine and, thus, misclassification of participant abstinence reports was possible. Patch adherence was lower than desired; however, this study used a conservative definition of adherence, and the present adherence rates during the first 8 weeks of treatment were greater than those reported previously (41). The differences in patch adherence between the treatment arms may also indicate that participants were able to determine their randomization from differences in patch effects. Lastly, 24% of participants at week 24 and 26% of participants at week 52 did not provide abstinence data or a breathe sample. These participants were classified as smokers, as is recommended (17). There was no difference across treatment arms in the rate of completion of these assessments and this rate of completion exceeds those reported in similar previous trials (28, 29).
Overall, this study demonstrates the benefits of extended therapy with transdermal nicotine and encourages a reexamination of the recommended duration of tobacco dependence treatment using nicotine patches. Maintaining smokers on transdermal nicotine for 24 weeks improved end-of-treatment abstinence rates compared to standard 8-week treatment and did not increase adverse events or side effects. However, this benefit was lost when therapy was discontinued. These findings have public health implications for smokers, clinicians, and policy-makers and may translate into reductions in smoking rates. Additional research on the optimal duration of therapy and the possible addition of other treatment components (e.g., more intensive counseling, pre-cessation use of nicotine patches) from an efficacy, patient acceptance, and cost perspective should be a priority.
Supplementary Material
Figure 1. Participant Flow.
Note. * A list of the reasons for participant ineligibility can be provided by the authors upon request; inclusion and exclusion criteria listed in the text were identical for phone and in-person eligibility assessments; ** indicates included in intent-to-treat analysis.
Figure 2. Time to Relapse by Treatment Arm to Week 52.
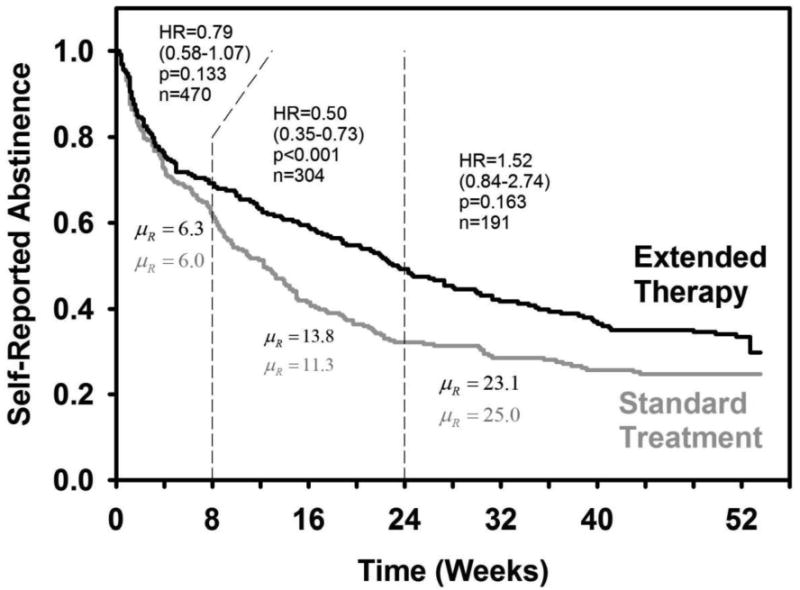
Note. HR = Hazard Ratio (with 95% CI); n = number of participants at risk for relapse; μR = restricted mean number of weeks to relapse (included censored observation times); the model controled for age (HR = 1.00, 95% CI: 0.99-1.01, p = 0.65), sex (HR = 0.96, 95% CI: 0.77-1.19, p = 0.69), and level of nicotine dependence. Nicotine dependence level predicted relapse from 0 to 8 weeks (HR = 1.83, 95% CI: 1.35-2.48, p < 0.001), but not from weeks 9-24 (HR = 0.91, 95% CI: 0.61-1.36, p = 0.65), or weeks 25-52 (HR = 1.04, 95% CI: 0.60-1.70, p = 0.90). The HRs were stable and uniform over the time intervals (p = 0.80). There is a residual decline in abstinence after 24 weeks, but the decline is statistically equivalent across treatment arms.
Acknowledgments
Primary Source of Funding: National Institutes of Health grants P50 CA/DA84718 and P50 CA143187.
This research was supported by a Transdisciplinary Tobacco Use Research Center Grant from the National Cancer Institute and the National Institute on Drug Abuse P50 CA/DA84718 (CL) and P50 CA143187 (CL).
The authors thank the following individuals who assisted with study implementation or with manuscript preparation: Dr. Janet Audrain-McGovern, Dr. Margaret Rukstalis, Angela Pinto, Susan Ware, Kia Kerrin, Janice Biddle, Sean Fleming, Paul Sanborn, and Susan Kerns.
A preliminary report of the study findings was presented at the annual meeting of the Society for Research on Nicotine and Tobacco, February 27, 2008, Portland, OR, USA.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
The protocol is available by emailing Dr. Schnoll at: schnoll@mail.med.upenn.edu. The statistical code used for the analyses is available by emailing Dr. Wileyto at: epw@mail.med.upenn.edu. The dataset used for this study is available by request after January 1, 2011 by emailing Dr. Schnoll at: schnoll@mail.med.upenn.edu.
Contributors: Dr. Lerman was responsible for study conception and design, analysis and interpretation of data, drafting of the manuscript, and obtaining funding. Drs. Schnoll, Wileyto, Heitjan, Asch, and Shields were responsible for analysis and interpretation of data and drafting of the manuscript. Dr. Patterson was responsible for acquisition of data, analysis and interpretation of data, supervision, and drafting of the manuscript. The authors can be contacted using the following addresses:
Conflicts of Interest Statement: Dr. Lerman has served as a consultant to GlaxoSmithKline, one company that manufactures the nicotine patch. She has also served as a consultant or has received research funding from Astra Zeneca, Pfizer, and Novartis. Financial support for this study was not provided from an industry sponsor. Dr. Lerman had full access to the data and had full responsibility for the decision to submit for publication. No other author has additional conflicts of interest to disclose.
References
- 1.Jonk YC, Sherman SE, Fu SS, Hamlett-Berry KW, Geraci MC, Joseph AM. National trends in the provision of smoking cessation aids within the Veterans Health Administration. Am J Manag Care. 2005;11:77–85. [PubMed] [Google Scholar]
- 2.Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA. 2002;288:1260–4. doi: 10.1001/jama.288.10.1260. [DOI] [PubMed] [Google Scholar]
- 3.Tilson L, Bennett K, Barry M. Prescribing trends for nicotine replacement therapy in primary care. Ir Med J. 2004;97:270–3. [PubMed] [Google Scholar]
- 4.West R, DiMarino ME, Gitchell J, McNeill A. Impact of UK policy initiatives on use of medicines to aid smoking cessation. Tob Control. 2005;14:166–71. doi: 10.1136/tc.2004.008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; May, 2008. Treating tobacco use and dependence: 2008 Update. Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care 2000; 45:1196-9. [Google Scholar]
- 6.Stead L, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;21:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Tønnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society Eur Respir J. 1999;13:238–46. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 8.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 9.Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, et al. Analyzing milestones in smoking cessation: illustration in a nicotine patch trial in adult smokers. J Consult Clin Psychol. 2006;74:276–85. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- 10.McKay JR. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100:1594–610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- 11.Partin MR, An LC, Nelson DB, Nugent S, Snyder A, Fu SS, et al. Randomized trial of an intervention to facilitate recycling for relapsed smokers. Am J Prev Med. 2006;31:293–9. doi: 10.1016/j.amepre.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004;161:2100–7. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 13.Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain-McGovern J. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Ann Internal Med. 2004;140:426–33. doi: 10.7326/0003-4819-140-6-200403160-00009. [DOI] [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 15.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–12. [Google Scholar]
- 17.Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 18.Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–97. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- 19.Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine Tob Res. 2005;7:257–68. doi: 10.1080/14622200500055673. [DOI] [PubMed] [Google Scholar]
- 20.Russell MA, Stapleton JA, Feyerabend C, Wiseman SM, Gustavsson G, Sawe U. Targeting heavy smokers in general practice: randomised controlled trial of transdermal nicotine patches. BMJ. 1993;306:1308–12. doi: 10.1136/bmj.306.6888.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hougaard P. Analysis of Multivariate Survival Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 1. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 23.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–46. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 24.Shiffman S, Patten C, Gwaltney C, Paty J, Gnys M, Kassel J. Natural history of nicotine withdrawal. Addiction. 2006;101:1822–32. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- 25.Heitjan DF. Fieller's method and net health benefits. Health Economics. 2000;9:327–35. doi: 10.1002/1099-1050(200006)9:4<327::aid-hec517>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 26.Song F, Raftery J, Aveyard P, Hyde C, Barton P, Woolacott N. Cost-effectiveness of pharmacological interventions for smoking cessation: a literature review and a decision analytic analysis. Med Decis Making. 2002;22:S26–37. doi: 10.1177/027298902237708. [DOI] [PubMed] [Google Scholar]
- 27.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews. 2007;1:CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 29.Jorenby DE, Hays JT, Rigotti NA, Rigotti NA, Azoulay S, Watsky EJ. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 30.Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology (Berl) 2006;184:608–18. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- 31.Medioni J, Berlin I, Mallet A. Increased risk of relapse after stopping nicotine replacement therapies: a mathematical modelling approach. Addiction. 2005;100:247–54. doi: 10.1111/j.1360-0443.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- 32.Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose JE, Herskovic JE, Behm FM, Westman EC. Precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment. Nicotine Tob Res. 2009;11:1067–75. doi: 10.1093/ntr/ntp103. [DOI] [PubMed] [Google Scholar]
- 34.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under 4 insurance plans in a health maintenance organization. NEJM. 1998;339:673–9. doi: 10.1056/NEJM199809033391006. [DOI] [PubMed] [Google Scholar]
- 35.McPhillips-Tangum C, Bocchino C, Carreon R, Erceg CM, Bocchino C. Addressing tobacco in managed care: results of the 2002 survey. Prev Chronic Dis. 2004;1:A04. [PMC free article] [PubMed] [Google Scholar]
- 36.State Medicaid coverage for tobacco-dependence treatments--United States, 2005. MMWR. 2006;55:1194–7. [PubMed] [Google Scholar]
- 37.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them? Nicotine Tob Res. 2004;6:S303–10. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- 38.Benowitz NL. Smoking less as a treatment goal for those who cannot stop smoking. Am J Med. 2004;116:203–5. doi: 10.1016/j.amjmed.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Hatsukami D, Mooney M, Murphy S, LeSage M, Babb D, Hecht S. Effects of high dose transdermal nicotine replacement in cigarette smokers. Pharmacol Biochem Behav. 2007;86:132–9. doi: 10.1016/j.pbb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kozlowski LT, Giovino GA, Edwards B, Difranza J, Foulds J, Hurt R. Advice on using over-the-counter nicotine replacement therapy-patch, gum, or lozenge-to quit smoking. Addict Behav. 2007;32:2140–50. doi: 10.1016/j.addbeh.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Alterman AI, Gariti P, Cook TG, Cnaan A. Nicodermal patch adherence and its correlates. Drug Alc Dep. 1999;53:159–65. doi: 10.1016/s0376-8716(98)00124-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.