Abstract
Purpose
To quantify the risk of second primary breast cancer in the contralateral breast (CB) following radiation therapy (RT) for first breast cancer.
Methods and Materials
The study population included participants in the Women’s Environmental, Cancer, and Radiation Epidemiology (WECARE) study: 708 cases (women with asynchronous bilateral breast cancer) and 1399 controls (women with unilateral breast cancer) counter-matched on radiation treatment. Participants were < 55 years of age at first breast cancer. Absorbed doses to quadrants of the CB were estimated. Rate ratios (RR) and 95% confidence intervals were calculated using multivariable-adjusted conditional logistic regression models.
Results
Across all patients, the mean radiation dose to the specific quadrant of the CB tumor was 1.1 Gy. Women < 40 years of age who received > 1.0 Gy of absorbed dose to the specific quadrant of the CB had a 2.5-fold greater risk for CB cancer than unexposed women (RR=2.5, 95% CI= 1.4 – 4.5). No excess risk was observed in women >40 years of age. Women < 40 years of age with followup periods > 5 years had a RR of 3.0 (95% CI=1.1–8.1), and the dose response was significant (excess RR per Gy of 1.0, 95% CI=0.1–3.0).
Conclusions
Women < 40 years of age who received a radiation dose > 1.0 Gy to the CB had an elevated, long-term risk of developing a second primary CB cancer. The risk is inversely related to age at exposure and is dose dependent.
Keywords: Contralateral breast, Radiation risk, Secondary breast cancer
INTRODUCTION
Improvements in breast cancer treatment over the past few decades have resulted in longer survival and increased use of radiation therapy (RT) (1, 2). However, RT for breast cancer inevitably results in scattered radiation dose to the contralateral breast (CB). Radiation is a well-known breast carcinogen (3–10), but it is unclear to what extent radiation to the CB increases the risk for a second primary tumor. Most studies do not report significant increases in CB cancer after RT. When increases are found they are seen generally in large studies where risk is apparent among young women treated with RT under age 45 years and followed for more than 5 years (11, 12). Several randomized trials (13), registry-based studies (12, 14–17), and hospital-based studies (3, 18–20) have evaluated the risk of second breast cancer in the CB following RT (21). Few studies, however, have estimated risk in terms of radiation dose to the CB (11, 22, 23), and only 1 reported a significant dose-response relationship (11).
The goal of this investigation was to quantify the radiation dose to the CB after RT for primary breast cancer and to estimate the associated risk of a primary CB tumor. The study population was composed of participants in the Women’s Environmental, Cancer, and Radiation Epidemiology (WECARE) study, a multi-center, population based, case-control study designed to examine the interaction of radiation and genetic factors and their effect on development of breast cancer (24).
METHODS AND MATERIALS
Study Population
The WECARE study included 708 women with asynchronous bilateral breast cancer (cases) and 1399 women with unilateral breast cancer (controls). Participants were identified, recruited, and interviewed through 5 population-based cancer registries: 4 in the United States (Iowa; Los Angeles County, CA: Orange and San Diego Counties, CA; and Seattle, WA) and 1 in Denmark. Of the eligible cases (998) and controls (2112), 71% of the cases and 66% percent of the controls agreed to participate. The study design is described in detail elsewhere (24, 25).
Cases met the following criteria: (a) diagnosis of first primary breast cancer between January 1, 1985, and December 31, 1999, initially staged “local only” or “local plus regional lymph node disease” and diagnosis of a second primary tumor (in situ or invasive disease) diagnosed in the CB at least 1 year after the diagnosis of the first primary breast tumor; (b) a resident of the same study reporting area during both diagnoses; (c) no previous or intervening cancer diagnoses; (d) age <55 years at diagnosis of the first primary tumor; and (e) alive at the time of contact and able to provide informed consent to complete the interview.
Controls were individually matched to cases in a 2 to 1 ratio by age at first breast cancer diagnosis (5-year strata), year of first cancer diagnosis (4-year strata), registry region, and race. Controls met the following criteria: (a) diagnosed since January 1, 1985, with first primary invasive breast cancer occurring while residing in one of the study reporting areas; (b) residing in the same study reporting area on the reference date (date of first diagnosis plus the time period between the first and second diagnosis [“at-risk interval”] of the matched case); (c) alive at time of contact; (d) never diagnosed (by reference date) as having had a second primary breast cancer or any other cancer diagnosis; and (e) no prior prophylactic mastectomy of the CB. Controls were counter-matched to the cases on registry-reported radiation exposure to improve statistical efficiency: each case and 2 matched control formed a triplet, with 2 members of each triplet exposed to RT and 1 member unexposed. Counter-matching decreased the chance that a triplet was all RT-exposed or all non-exposed and, therefore, uninformative regarding radiation-induced breast cancer risks (25).
Data Collection
The data collection protocol was approved by the Institutional Review Board at each site, and each patient signed an informed consent form. Participants were interviewed by telephone using a pre-tested, structured questionnaire. The questionnaire was designed to obtain information about events occurring prior to the diagnosis of the first primary cancer, events occurring within the at-risk period, and known and suspected risk factors for breast cancer.
Radiation treatment information was sought for each patient whom the cancer registry, questionnaire, or medical record indicated had received RT for initial primary breast cancer, metastases, recurrences, or benign conditions. Radiation treatment details were obtained from the basic RT record, RT summary, RT notes, medical record notes, surgery reports (for brachytherapy), and physician correspondence. RT records were retrieved for 1497 women: 1479 with RT for breast cancer and 18 who received RT for other conditions (metastases, benign conditions, and/or ovarian ablation) Ninety-one percent of the records received were complete for radiation details, 8.5% were missing some radiation details but doses could be estimated ,and 0.5% were inadequate for dosimetry.
For cases, we collected all available information concerning the CB tumor, using pathology reports, medical records, physician notes, and mammography reports. The tumors were classified according to their quadrant location, areolar region (1 cm radius), and/or a clock position. For 609 of the 708 cases, the location of the CB tumor was known. Of the case sets with known CB location, 3 cases and 3 controls were missing dose information and were eliminated from the dose-response analysis. Analysis of the quadrant-specific doses for this study was based on 606 cases and 1200 matched controls for whom RT and CB tumor information were available. The final analytic set of 1806 patients consisted of: 591 complete triplet sets, 12 case-control pairs, 3 control only pairs (matched to cases with missing dose information), and 3 cases only. Of the 1806 patients, 1277 were treated with RT: 1174 (92%) with external-beam therapy, 101 (8%) with external-beam therapy plus brachytherapy, and 2 with an unknown technique. Of the 1277 patients who received RT, 1266 received breast irradiation.
Quantification of Radiation Dose
For external-beam therapy, dose estimates to the CB were measured with thermoluminescent dosimeters in tissue-equivalent phantoms. The phantoms were molded with a plastic shell placed on patients in the treatment position. The dosimeters were lithium fluoride powder in tissue-equivalent flat packs. Dosimeters (approximately 300) were placed on a 3-dimensional grid throughout the CB. Measurements were made with and without medial wedge compensators for tangential fields. CB dose was also measured from supraclavicular, axilla, and internal mammary chain fields. For brachytherapy, dose estimates to the CB were derived using standard treatment planning techniques (1). The dosimetry techniques are described in detail elsewhere (24, 26).
Doses were estimated to the 4 quadrants and the central portion (areola) of the CB (Fig. 1). Dose to each quadrant was calculated as the average of doses to points on a 3-dimensional grid. Because some tumors were located at the boundary between quadrants, dose was estimated also to 4 clock positions (3, 6, 9, and 12), which define the dividing lines between the quadrants. For cases with multifocal tumors (n = 67), the dose was calculated to the tumor of origin, assuming the largest tumor or the invasive tumor came first. All radiation dosimetry was performed without knowledge of whether the patient was a case or a control.
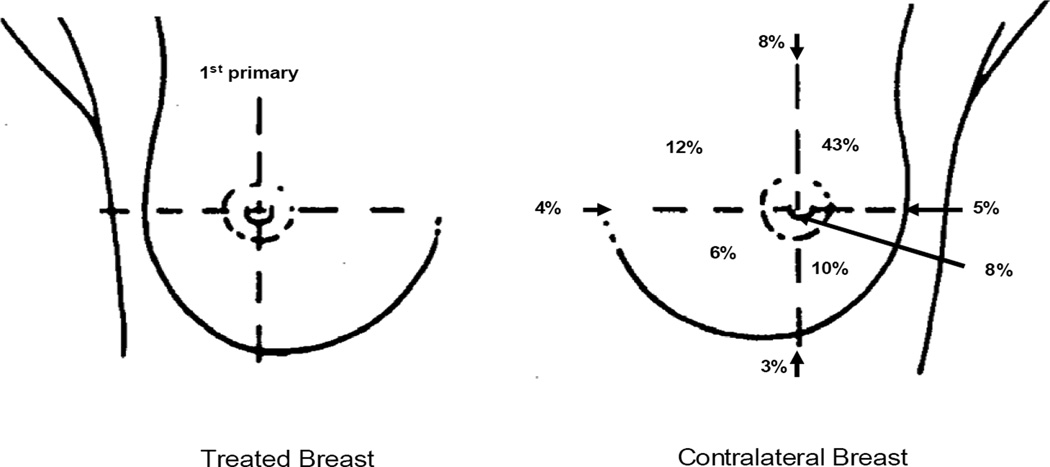
Fig. 1.
Distribution of the location of the contralateral breast primaries (n=606).
Statistical Methods
Rate ratios (RR) and corresponding 95% confidence intervals (CI) were estimated by fitting univariate and multivariable-adjusted conditional logistic regression models, accounting for the counter-matched sampling (25). The multivariable models were adjusted for factors found to be statistically significant in the univariate models and those known to be associated with breast cancer, including: exact age at diagnosis of first primary breast cancer, age at menarche (<13/13+), menopausal status (premenopausal / age at menopause <45/45+), number of full-term pregnancies (0,1,2,3,4+), family history of breast cancer among first degree relatives (yes/no), lobular histology (yes/no) and stage (local/regional) of the first primary, and treatment history (chemotherapy (yes/no) and/or hormonal therapy (yes/no)).
Counter-matching on RT status allows modeling of main effects of radiation as well as interactions and is more efficient than simple random sampling of controls for these analyses (24). Further, because controls are independently sampled from the failure time risks sets, the estimated parameters are rate ratios in the proportional hazards model for cohort data (27) and standard likelihood methods apply (28). Proportions were estimated by computing the factor distribution in controls by RT status, then taking a weighted average of the within RT-status values where the weights are the overall proportions in the risk sets (Table 1).
Table 1.
Characteristics of study participants
| All WECARE participants (n=2107) | Subset of WECARE participants in present study (n=1806)* |
|||
|---|---|---|---|---|
| Characteristic | Bilateral breast cancer (cases,n=708) |
Unilateral breast cancer (controls,n=1399)† |
Bilateral breast cancer (cases,n=606) |
Unilateral breast cancer (controls,n=1200)† |
| MATCHING CHARACTERISTICS | ||||
| (n and % except where noted) | ||||
| Registry | ||||
| Iowa | 113 (16%) | 222 (16%) | 88 (15%) | 172 (14%) |
| Orange and San Diego Counties, California | 118 (17%) | 231 (17%) | 99 (16%) | 194 (16%) |
| Los Angeles County, California | 199 (28%) | 390 (28%) | 175 (29%) | 343 (29%) |
| Seattle, Washington | 99 (14%) | 198 (14%) | 83 (14%) | 169 (14%) |
| Denmark | 179 (25%) | 358 (26%) | 161 (27%) | 322 (27%) |
| Race | ||||
| White | 649 (92%) | 1288 (92%) | 550 (91%) | 1096 (91%) |
| White of Spanish surname | 24 (3%) | 48 (3%) | 22 (4%) | 43 (4%) |
| Black | 21 (3%) | 39 (3%) | 20 (3%) | 37 (3%) |
| Asian | 6 (1%) | 8 (1%) | 6 (1%) | 8 (1%) |
| Filipino | 7 (1%) | 14 (1%) | 7 (1%) | 14 (1%) |
| Other | 1 (<1%) | 2 (<1%) | 1 (<1%) | 2 (<1%) |
| 1st Breast cancer diagnosis (y) | ||||
| 1985 – 1989 | 303 (43%) | 580 (41%) | 260 (43%) | 492 (41%) |
| 1990 – 1995 | 317 (45%) | 638 (46%) | 269 (44%) | 558 (47%) |
| 1996 – 1999 | 88 (12%) | 181 (13%) | 77 (13%) | 150 (13%) |
| Mean age (y) at first breast cancer (range) | 46 (24 – 55) | 46 (23 – 55) | 46 (24 – 55) | 46 (23 – 55) |
| Mean age (y) at reference date (range)‡ | 51 (27– 71) | 51 (27– 69) | 51 (28 – 71) | 51 (27 – 69) |
| Mean at-risk period (y) (range) | 5 (1 – 16) | 5 (1 – 16) | 5 (1 – 16) | 5 (1 – 16) |
| TREATMENT CHARACTERISTICS | ||||
| (primary / recurrent breast cancer) | ||||
| Radiation§ | ||||
| No | 362 (51%) | 266 (50%) | 302 (50%) | 238 (51%) |
| Yes | 346 (49%) | 1133 (50%) | 304 (50%) | 962 (49%) |
| Chemotherapy | ||||
| No | 386 (55%) | 629 (43%) | 330 (54%) | 546 (44%) |
| Yes | 322 (45%) | 770 (58%) | 276 (46%) | 654 (56%) |
| Radiation and chemotherapy | ||||
| No | 541 (76%) | 779 (71%) | 464 (77%) | 677 (72%) |
| Yes | 167 (24%) | 620 (29%) | 142 (23%) | 523 (28%) |
| Hormone therapy | ||||
| No | 511 (72%) | 909 (66%) | 447 (74%) | 774 (66%) |
| Yes | 197 (28%) | 488 (34%) | 159 (26%) | 424 (34%) |
| Unknown | 0 (0%) | 2 (<1%) | 0 (0%) | 2 (<1%) |
| Mastectomy | ||||
| No | 258 (36%) | 851 (36%) | 231 (38%) | 717 (35%) |
| Yes (total or partial) | 450 (64%) | 548 (64%) | 375 (62%) | 483 (65%) |
| OTHER CHARACTERISTICS | ||||
| Age at menarche | ||||
| 8 −13 years | 338 (48%) | 611 (41%) | 289 (48%) | 525 (41%) |
| 13+ years | 367 (52%) | 782 (58%) | 315 (52%) | 670 (58%) |
| Unknown | 3 (<1%) | 6 (1%) | 2 (<1%) | 5 (1%) |
| Age at menopause (as of reference date) | ||||
| Premenopausal | 124 (18%) | 273 (18%) | 104 (17%) | 227 (18%) |
| <45 years | 209 (30%) | 455 (34%) | 186 (31%) | 385 (34%) |
| 45–54 years | 374 (53%) | 665 (47%) | 316 (52%) | 584 (47%) |
| Unknown | 1 (<1%) | 6 (1%) | 0 (0%) | 4 (1%) |
| Nulliparous at reference age | ||||
| No | 575 (81%) | 1172 (84%) | 492 (81%) | 1003 (83%) |
| Yes | 133 (19%) | 225 (16%) | 114 (19%) | 195 (17%) |
| Unknown | 0 (0%) | 2 (<1%) | 0 (0%) | 2 (<1%) |
| Number of full-term pregnancies | ||||
| None | 133 (19%) | 225 (16%) | 114 (19%) | 195 (17%) |
| 1 | 121 (17%) | 204 (15%) | 104 (17%) | 170 (15%) |
| 2 | 270 (38%) | 545 (36%) | 229 (38%) | 463 (36%) |
| 3 | 128 (18%) | 263 (22%) | 111 (18%) | 225 (22%) |
| 4 or more | 56 (8%) | 160 (11%) | 48 (8%) | 145 (11%) |
| Unknown | 0 (0%) | 2 (<1%) | 0 (0%) | 2 (<1%) |
| Family history of breast cancer | ||||
| Adopted (family history unknown) | 11 (2%) | 26 (2%) | 10 (2%) | 22 (2%) |
| None | 472 (67%) | 1088 (78%) | 408 (67%) | 933 (78%) |
| Any first-degree female relative | 225 (32%) | 285 (20%) | 188 (31%) | 245 (20%) |
| FIRST PRIMARY BREAST CANCER | ||||
| CHARACTERISTICS | ||||
| Histology | ||||
| Ductal | 505 (71%) | 1048 (76%) | 438 (72%) | 902 (76%) |
| Lobular | 90 (13%) | 131 (9%) | 69 (11%) | 112 (8%) |
| Medullary | 33 (5%) | 51 (3%) | 32 (5%) | 41 (3%) |
| Other | 79 (11%) | 165 (12%) | 66 (11%) | 141 (12%) |
| Unknown | 1 (<1%) | 4 (1%) | 1 (<1%) | 4 (1%) |
| Stage | ||||
| Localized | 506 (71%) | 916 (64%) | 434 (72%) | 784 (65%) |
| Regional | 202 (29%) | 483 (36%) | 172 (28%) | 416 (35%) |
| Estrogen receptor status | ||||
| Positive | 338 (48%) | 746 (54%) | 287 (47%) | 658 (55%) |
| Negative | 193 (27%) | 338 (24%) | 168 (28%) | 274 (23%) |
| Unknown | 177 (25%) | 315 (22%) | 151 (25%) | 268 (23%) |
| Progesterone receptor status | ||||
| Positive | 287 (41%) | 616 (43%) | 233 (38%) | 536 (44%) |
| Negative | 172 (24%) | 318 (24%) | 157 (26%) | 262 (23%) |
| Unknown | 249 (35%) | 465 (33%) | 216 (36%) | 402 (34%) |
Cases for whom information on location of the tumor and dose were available and their matched controls.
Proportions estimated accounting for counter-matched sampling.
Reference date for the cases is defined as the diagnosis date of the second primary; for the controls it is defined as the date of the first breast cancer diagnosis plus the “at-risk interval” of matched case.
Actual RT as determined by medical records and interviews. Counter-matching was based only on registry-recorded RT information, which may not be the same as actual RT information.
For the analyses, we used three different measures of radiation, the location specific dose, average dose to the breast and ever/never RT to the breast. The “location-specific dose” was calculated as the dose to the quadrant where the second primary for the cases developed; for the controls it was calculated as the dose received to the same location as the case. The average dose was calculated, for both cases and controls, as the average of the quadrant and central region dose estimates. Dose effects were estimated for categories 0, >0 – 1 Gy and 1+ Gy and as an excess relative risk (ERR) trend. A missing data indicator was used to account for missing covariate data. Analyses were conducted using SAS (SAS Institute, Cary, NC) and Epicure (Hirosoft International Corp., Seattle, WA) (29).
RESULTS
Reported in Table 1 are the patient characteristics of all 2107 WECARE study participants and of the subset of 1806 cases and controls included in the analyses. There were no systematic differences between the overall WECARE study population and the subset of participants in our analyses. For both groups, cases and controls were similar with regard to race, age at diagnosis of the first primary, and length of the at-risk period.
The most common RT technique was external-beam radiation to the whole breast with opposing tangential fields. Most of the tangential fields were delivered with wedge compensators using angles ranging from 15 – 60 degrees; 91% of the patients were treated with both medial and lateral wedges. Ninety-four percent of the treatments were delivered with high-energy photons (87% of these were 4 MV – 8 MV). Some patients also were treated with peripheral lymphatic fields (supraclavicular, axilla, and/or internal mammary chain). All external-beam treatments were delivered at a tumor dose of 1.5 – 2.0 Gy per day for 5 to 6 weeks. These treatments are typical of the patterns of care provided for breast cancer patients from 1985 through 1999.
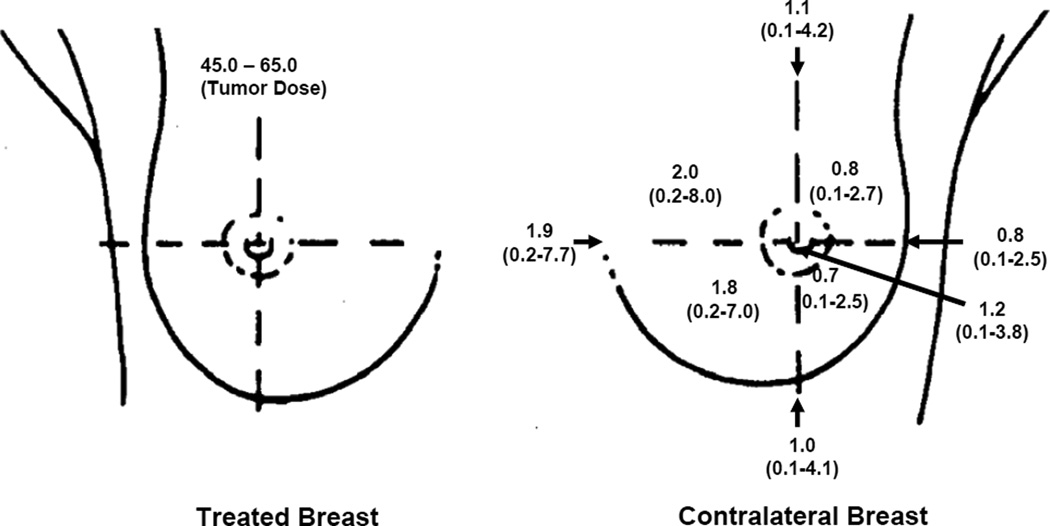
For all cases, the most common site for the second cancer was the upper-outer quadrant of the breast (43%)(Fig.1). The distribution of CB tumors across the breast (inner, central, and outer) was compared between irradiated and unirradiated women. For irradiated women, a significantly higher proportion of CB tumors was located in the inner and central quadrants (p = 0.03). There was a 2- to 3-fold variation in mean dose across the CB quadrants, with the inner quadrants receiving the highest doses (Fig. 2). The mean dose and range of doses for the inner, central, and outer regions of the CB for the various treatment regimens are shown in Table 2. Across all patients undergoing RT, the mean dose for the quadrant location in the CB was 1.1 Gy (range, 0.02–6.2 Gy). There was no difference in the mean quadrant location CB dose by year of treatment. The average dose for the entire breast, i.e. mean of all breast quadrants and central region, was 1.3 Gy for our population.
Fig. 2.
Dose (Gy) to contralateral breast. Mean and range of doses among patients treated with breast RT (n=1266).
Table 2.
Mean radiation dose (Gy) to the inner, central, and outer regions of the contralateral breast
| Method of External Treatment |
N | Dose to Inner Region | Dose to Central Region | Dose to Outer Region | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | 10%ile | mean | 90%ile | 10%ile | mean | 90%ile | 10%ile | mean | 90%ile | |
| Tangential breast fields without peripheral lymphatics |
178 | 542 | 1.2 | 1.8 | 2.5 | 0.8 | 1.3 | 1.8 | 0.5 | 0.8 | 1.1 |
| Without wedges | 11 | 26 | 1.0 | 1.2 | 1.6 | 0.7 | 0.8 | 1.0 | 0.4 | 0.5 | 0.6 |
| With wedges* | 167 | 516 | 1.3 | 1.8 | 2.5 | 0.9 | 1.3 | 1.8 | 0.5 | 0.8 | 1.1 |
| Tangential breast fields with peripheral lymphatics† |
71 | 228 | 1.7 | 2.5 | 3.8 | 1.0 | 1.6 | 2.3 | 0.7 | 1.1 | 1.6 |
| Without wedges | 9 | 23 | 1.4 | 2.2 | 4.6 | 0.9 | 1.3 | 2.4 | 0.6 | 0.9 | 1.7 |
| With wedges* | 62 | 205 | 1.8 | 2.6 | 3.7 | 1.1 | 1.7 | 2.3 | 0.7 | 1.1 | 1.6 |
| Direct breast field without peripheral lymphatics |
11 | 34 | 0.3 | 0.4 | 0.4 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Direct breast field with peripheral lymphatics** |
31 | 111 | 1.1 | 1.5 | 1.8 | 0.4 | 0.5 | 0.6 | 0.4 | 0.4 | 0.5 |
| Other fields | 13 | 47 | 1.1 | 1.7 | 2.1 | 0.7 | 1.2 | 1.4 | 0.4 | 0.7 | 0.9 |
| All Patients | 304 | 962 | 1.1 | 1.9 | 2.8 | 0.6 | 1.2 | 1.9 | 0.4 | 0.8 | 1.2 |
The majority of patients were treated with medial and lateral wedges using angles ranging from 15 to 60 degrees.
Peripheral lymphatic fields included supraclavicular fields, axilla fields, and internal mammary chain fields.
Comparisons of the three methods of estimating dose (ever/never RT, average dose across breast, and location-specific dose) were conducted. Among all women, no method showed a statistically significant elevation in risk associated with radiation (Table 3). However, certain subgroups of women had statistically significant risks. In women < age 40, those who received > 1 Gy of radiation had a 2.5-fold greater risk than unexposed women (95% CI=1.4 – 4.5), whereas no excess risk was observed for women over age 40 treated with RT (Table 4). When latency was considered with age, women , 40 years of age ,treated with RT, with 5+ years latency had an RR=3.0 (95% CI =1.1 – 8.1). Women <40 years of age, treated with RT, with < 5 years latency had a lower but significantly elevated risk (RR = 2.3, 95% CI = 1.1 – 4.6). There was a significant trend with dose among all women under age 40 (ERR/Gy = 0.6, 95% CI=0.1 −1.5) and for the subgroup of women <age 40 who had at least a 5+ year latency (ERR/Gy = 1.0, 95% CI=0.1 – 3.0). CIs in all of these subgroups were wide, reflecting the small numbers of participants.
Table 3.
Risk of developing cancer in the contralateral breast
| Method of Estimating Dose | Dose to CB* |
Number of Cases |
Number of Controls |
Rate Ratio† | 95% Confidence Interval |
|---|---|---|---|---|---|
| Yes/ No Radiotherapy | |||||
| Never RT | ─ | 362 (51%) | 266 (50%) | 1.0 | |
| Ever RT | ─ | 346 (49%) | 1133 (50%) | 1.1 | 0.9 – 1.3 |
| Average Dose to the CB | 0 Gy | 353 (50%) | 259 (49%) | 1.0 | |
| 0<1.0 Gy | 100 (14%) | 276 (13%) | 1.2 | 0.9 – 1.6 | |
| ≥ 1.0 Gy | 251 (36%) | 860 (38%) | 1.0 | 0.9 – 1.3 | |
| Trend ERR/Gy | 0.0 | −0.1 – 0.2 | |||
| Location-specific Dose to the CB | 0 Gy | 297 (49%) | 232 (50%) | 1.0 | |
| 0<1.0 Gy | 169 (28%) | 536 (28%) | 1.2 | 0.9 – 1.5 | |
| ≥ 1.0 Gy | 140 (23%) | 432 (23%) | 1.2 | 1.0 – 1.6 | |
| Trend ERR/Gy | 0.1 | −0.1 – 0.3 |
Scatter radiation dose estimated by dosimetry received to the CB.
Adjusted for age at first diagnosis of breast cancer, age of menarche, family history of breast cancer, total number of full-term pregnancies, age at menopause, chemo/hormonal treatment, histology, and stage.
Table 4.
Risk of developing a contralateral breast cancer after RT according to age at time of exposure, time since exposure, and estimated radiation dose delivered to the site of CB cancer (cases=606, controls=1200)
| Variable | Dose (Gy) to CB | Number (%) of Cases |
Number (%)** of Controls |
Rate Ratio* |
95% Confidence Interval |
|---|---|---|---|---|---|
| Time (y) since first breast cancer | |||||
| <5 | 0 | 167 (49%) | 134 (46%) | 1.0 | |
| 0<1.0 | 94 (27%) | 313 (31%) | 0.9 | 0.7 −1.3 | |
| ≥ 1.0+ | 82 (24%) | 238 (23%) | 1.2 | 0.9 −1.7 | |
| Trend ERR/Gy | 0.1 | −0.1 – 0.3 | |||
| 5–9 | 0 | 101 (49%) | 78 (54%) | 1.0 | |
| 0<1.0 | 57 (27%) | 176 (23%) | 1.3 | 0.8 – 2.0 | |
| ≥ 1.0+ | 50 (24%) | 154 (23%) | 1.4 | 0.9 – 2.3 | |
| Trend ERR/Gy | 0.2 | −0.1 – 0.6 | |||
| 10–14 | 0 | 29 (53%) | 20 (64%) | 1.0 | |
| 0<1.0 | 18 (33%) | 47 (23%) | 1.8 | 0.8 – 4.1 | |
| ≥ 1.0+ | 8 (15%) | 40 (12%) | 0.7 | 0.3 −2.0 | |
| Trend ERR/Gy | −0.2 | <<0 – 0.5‡ | |||
| Age (y) at first breast cancer | |||||
| <40 | 0 | 47 (41%) | 43 (45%) | 1.0 | |
| 0<1.0 | 31 (27%) | 112 (36%) | 0.9 | 0.5 −1.6 | |
| ≥ 1.0+ | 36 (32%) | 68 (19%) | 2.5 | 1.4 – 4.5 | |
| Trend ERR/Gy | 0.6 | 0.1 – 1.5 | |||
| 40–44 | 0 | 73 (50%) | 53 (49%) | 1.0 | |
| 0<1.0 | 46 (32%) | 140 (29%) | 1.3 | 0.8 −2.0 | |
| ≥ 1.0+ | 26 (18%) | 89 (22%) | 1.0 | 0.6 −1.8 | |
| Trend ERR/Gy | −0.2 | <<0 −0.3‡ | |||
| 45–54 | 0 | 177 (51%) | 136 (52%) | 1.0 | |
| 0<1.0 | 92 (27%) | 284 (25%) | 1.2 | 0.9 −1.7 | |
| ≥ 1.0+ | 78 (22%) | 275 (24%) | 1.0 | 0.7 −1.4 | |
| Trend ERR/Gy | 0.0 | −0.1 −0.3 | |||
| Age (y) at and time (y) since first breast cancer | |||||
| <40 and <5 | 0 | 27 (42%) | 26 (41%) | 1.0 | |
| 0<1.0 | 17 (26%) | 61 (37%) | 0.8 | 0.4 −1.8 | |
| ≥ 1.0+ | 21 (32%) | 43 (22%) | 2.3 | 1.1 −4.6 | |
| Trend ERR/Gy | 0.4 | −0.1 −1.4 | |||
| <40 and 5+ | 0 | 20 (41%) | 17 (51%) | 1.0 | |
| 0<1.0 | 14 (29%) | 51 (32%) | 1.0 | 0.4 −2.4 | |
| ≥ 1.0+ | 15 (31%) | 25 (17%) | 3.0 | 1.1 −8.1 | |
| Trend ERR/Gy | 1.0 | 0.1 −3.0 | |||
| 40+ and <5 | 0 | 140 (50%) | 108 (47%) | 1.0 | |
| 0<1.0 | 77 (28%) | 252 (29%) | 1.0 | 0.7 −1.4 | |
| ≥ 1.0+ | 61 (22%) | 195 (24%) | 1.0 | 0.7 −1.5 | |
| Trend ERR/Gy | 0.0 | −0.2 −0.3 | |||
| 40+ and 5+ | 0 | 110 (51%) | 81 (57%) | 1.0 | |
| 0<1.0 | 61 (29%) | 172 (21%) | 1.7 | 1.1 −2.5 | |
| ≥ 1.0+ | 43 (20%) | 169 (22%) | 1.0 | 0.6 −1.6 | |
| Trend ERR/Gy | 0.0 | <<0 −0.4‡ | |||
Adjusted for age at first diagnosis of breast cancer, age of menarche, family history of breast cancer, total number of full-term pregnancies, age at menopause, chemo/hormonal treatment, histology, and stage.
Proportions estimated accounting for counter-matched sampling
<< Indicates a very large negative number that could not be estimated by the method.
DISCUSSION
Overall, in our study, cancer of the CB was not significantly associated with RT for first breast cancer in our large, international, case-control study of women. However, among women < 40 years of age who received RT and were followed for ≥ 5 years, a significant dose-response relationship was observed. Radiation dose was estimated to the quadrant where the second breast cancer occurred, which reduced the uncertainty of analyses based on average dose to the entire CB.
Several studies have assessed the relationship between risk of subsequent primary cancer in the CB and radiation dose (11, 22, 23). The study by Basco et al. (22) involved over 14,000 women treated for breast cancer in British Columbia (mean dose to CB ∼ 1.5 Gy), but the number of CB cancers was small (n=194, with only 30 occurring in women < 40 years). In a nested case-control study conducted by Boice et al. (11) of 41,109 women in Connecticut treated for breast cancer between 1935 and 1982, 655 women developed cancer in the CB ≥ 5 years after initial treatment; no significant radiation risk was observed (RR 1.2; mean dose to CB, 2.8 Gy). However, a significant risk and dose-response relationship were apparent among women < 45 years of age at first treatment (RR 1.6; ERR/Gy =0.2). Storm et al. (23) conducted a nested case-control study of 56,540 women in Denmark with breast cancer treated at any age between 1943 and 1978. Overall, 529 CB cancers occurred ≥ 8 years after initial treatment, but no radiation risk was observed (RR 1.0; mean dose to CB, 2.5 Gy). The Danish study had fewer women < 45 years of age than did the Connecticut study (19% vs. 31%, respectively).
A similarity between our study and that of Boice et al. (11) is that both included a large number of women with early-onset breast cancer among whom a significant doseresponse relationship was seen. However, the Boice study differs from ours in several ways: (a) Boice et al. used average dose to the whole breast, whereas we used dose to the quadrant of the CB tumor, (b) the patients in the Boice study were treated with orthovoltage x-rays and cobalt-60 teletherapy, which resulted in higher doses to the CB than from the high-energy photons in our study, (c) the Boice study had a longer latent period (5 to > 15 years) than did our study (1 – 10 years), (d) the Boice study included women of all ages, including those > 55 years of age, whereas the women in our study were all < 55 years of age at first breast cancer diagnosis. Despite these differences, the results of the 2 studies are consistent. In neither study was radiation risk observed for women > 45 years of age at time of treatment; for women <45 years of age who were followed for ≥ 5 years, the estimates of risk in the 2 studies were the same (ERR/Gy = 0.2 (30)).
A meta-analysis of 78 randomized treatment comparisons (13) showed a higher risk of CB cancer among women over 50 years of age; however, no radiation doses were reconstructed for the CB and the wide range of treatment techniques may have confounded the actual radiation risk to the CB.
Our study adds to the body of knowledge that the risk of developing radiation-induced CB cancer is stronger among young women and that the women > 45 years of age when treated with RT for first breast cancer are not at significant risk (11, 22, 23). This finding is reassuring given that most women are > 45 years of age when breast cancer is first detected. Among younger women (< 45 years of age), our data provide evidence that the lower dose of radiation scatter (mean 1.3 Gy) to the CB from treatments delivered from 1985 through 1999 remains sufficiently high to result in a detectable increase in the risk of radiation-induced breast cancer.
The cases and controls in our study were diagnosed with breast cancer as early as 1985. Cases and controls were restricted to patients who were alive so they could be interviewed. Thus, there is a potential for survival bias if survival depends on both case-control status and radiation dose. We explored this possibility in the Los Angeles registry using the RT information available in the registry records. When all participants were analyzed, the RR contrasting RT with no RT was estimated to be 1.2 (95% CI = 1.0–1.4). Restricting the analysis to women known to be alive in 2000 yielded the same result (RR = 1.2, 95% CI = 1.0–1.5). Among younger patients (< 45 years of age) with longer latency periods (≥ 5 years), similar results were found (RR for all subjects = 1.8 (95% CI = 1.2–2.6), RR restricted to alive in 2000 =1.9 (95% CI = 1.3–2.8)). Thus, it is unlikely that there is significant survival bias in our estimates of radiation effect.
A strength of our study was that we standardized the procedures used to collect information and implemented stringent quality control. There may have been differences in data quality between the registries but because subjects were matched by registry and date of first cancer, cases and controls should have data of similar quality. There was no evidence of large variations in radiation effect over registries or over the calendar years of first cancer diagnosis.
The quadrant of the breast tumor was not available for 99 of the 708 cases, so these patients were omitted from the main dose-response analyses. Although the tumor characteristics (stage, histology, ER/PR status) of the excluded cases did not differ statistically from those of patients included in the analyses, there was a difference in the percent of patients exposed to radiation between the cases with known tumor locations (50%) and those with unknown tumor locations (36%). Therefore, although it seems implausible that the radiation dose response for tumors with unknown location would differ from tumors that could be assigned a location, we can not entirely rule out the possibility of a systematic bias from excluding the cases with unknown tumor location.
A major strength of our study is the large sample size, with 1806 cases and controls. The counter-matched design (24) further enhanced our statistical power to evaluate risks by ensuring that each triplet had 2 radiation-exposed members and 1 unexposed. The nested case-control design involving patients obtained from cancer registries ensured that controls were representative of the population, so the likelihood of selection bias was minimized. Another strength of our study is the collection of complete RT records for 91% of patients who received RT, which allowed detailed dosimetry information for each quadrant of the CB. The radiation exposure characterization for each patient was determined using the patient’s RT record rather than information from cancer registries, which are not necessarily complete or correct. Finally, dosimetry was performed without regard to a subject’s case-control status, so there was little likelihood of information bias.
Our dosimetry results are compatible with those of prior studies of patients undergoing RT that estimated the CB dose to range between 0.5 and 4 Gy, corresponding to about 1% to 8% of a typical treatment dose of 50 Gy (13, 31, 32). A novel feature of our study, however, was that we estimated radiation dose to the CB quadrant where the second primary breast cancer developed. The mean quadrant-specific dose for all patients was found to be lower than the mean breast dose, which has important implications with regard to estimating dose-specific risk, because second primary tumors, similar to first primary tumors, are more common in the outer quadrants of the breast (33, 34). Basing analyses on average breast dose rather than quadrant-specific dose, accordingly, would underestimate the true dose effect. Thus our dose-response analyses based on quadrant tumor location provide a more valid assessment of radiation risk than has been possible in previous investigations of the CB.
CONCLUSIONS
Our results show that for the majority of women treated for breast cancer with recent RT techniques, RT did not play a significant role in the development of a second breast primary; however, young women with breast cancer had an elevated long-term risk of developing a CB cancer. In addition, this radiation risk was inversely related to age at exposure and was dose dependent. Despite the overall reassuring results and the lower CB doses resulting from recent treatments (35), the newer treatment techniques, such as intensity-modulated RT and field-in-field irradiation, should be investigated with regard to their roles as risk factors for secondary breast cancer in the CB (36).
Acknowledgments
We thank Rita E. Weathers, M.S., for her assistance in computer programming analysis and Barbara Pylate for her help with the manuscript.
Supported in part by the National Institutes of Health, grant numbers U01-CA83178, R01-CA97397, and R01-CA42949.
APPENDIX
Corporate Authorship List
WECARE Study Collaborative Group
P.I.: Jonine L. Bernstein, Ph.D.; Co-investigators named on grant: Hoda Anton-Culver, Ph.D., Colin Begg., Ph.D., Leslie Bernstein, Ph.D., John Boice, Jr., Ph.D., Anne-Lise Børresen-Dale, Ph.D., Marinela Capanu, Ph.D., Patrick Concannon, Ph.D., Richard A. Gatti, Ph.D., Robert W. Haile, Dr.P.H., Ph.D., Bryan M. Langholz, Ph.D., Charles F. Lynch, M.D., Ph.D., Kathleen E. Malone, Ph.D., Jørgen H. Olsen, M.D., DMSc., Barry Rosenstein, Ph.D., Roy E. Shore, Ph.D., Dr.P.H., Marilyn Stovall, Ph.D., Duncan C. Thomas, Ph.D., W. Douglas Thompson, Ph.D.
Coordinating Center: Memorial Sloan-Kettering Cancer Center (New York, NY) Jonine L. Bernstein, Ph.D. (WECARE Study P.I.), Xiaolin Liang, M.D., M.S. (Informatics Specialist), Abigail Wolitzer, M.S.P.H. (Project Director); National Cancer Institute (Bethesda, MD) Daniela Seminara, Ph.D., M.P.H. (Program Officer).
Data Collection Centers*: University of Southern California (Los Angeles, CA) Leslie Bernstein, Ph.D. (P.I.), Laura Donnelly-Allen (Project Manager); Danish Cancer Society (Copenhagen, Denmark) Jørgen H. Olsen, M.D., DMSc. (P.I.), Lene Mellemkjær, Ph.D., MSc. (Project Manager); University of Iowa (Iowa City, IA) Charles F. Lynch, M.D., Ph.D. (P.I.), Jeanne DeWall, M.A. (Project Manager); Fred Hutchinson Cancer Research Center (Seattle, WA) Kathleen E. Malone, Ph.D. (P.I.), Noemi Epstein (Project Manager); University of California at Irvine (Irvine, CA) Hoda Anton-Culver, Ph.D. (P.I.), Joan Largent, Ph.D., M.P.H. (Project Manager).
Radiation Measurement: University of Texas, M.D. Anderson Cancer Center (Houston, TX) Marilyn Stovall, Ph.D. (P.I.), Susan Smith, M.P.H. (Quality Assurance Dosimetry Supervisor); New York University (New York, NY) Roy E. Shore, Ph.D., Dr.P.H. (Epidemiologist); International Epidemiology Institute (Rockville, MD) and Vanderbilt University (Nashville, TN) John D. Boice Jr., Sc.D. (Consultant).
Biostatistics Core: University of Southern California (Los Angeles, CA) Bryan M. Langholz, Ph.D., Duncan C. Thomas, Ph.D.; Memorial Sloan-Kettering Cancer Center (New York, NY) Colin Begg., Ph.D., Marinela Capanu, Ph.D.; University of Southern Maine (Portland, ME) W. Douglas Thompson, Ph.D. (P.I.).
External Advisor: Stanford University (Palo Alto, CA) Alice Whittemore, Ph.D.
Footnotes
Conflict of interest: none
REFERENCES
- 1.Ries LAG, Eisner MP, Kosary CL, et al. SEER cancer statistics review. 2005 1975–2002 Available at: http://seer.cancer.gov/csr/1975_2002/
- 2.Cancer Facts & Figures 2007. Available at: http://www.cancer.org/docroot/STT/content/STT_1x_Cancer_Facts__Figures_2007.asp.
- 3.Obedian E, Fischer DB, Haffty BG. Second malignancies after treatment of early-stage breast cancer: lumpectomy and radiation therapy versus mastectomy. J Clin Oncol. 2000;18:2406–2412. doi: 10.1200/JCO.2000.18.12.2406. [DOI] [PubMed] [Google Scholar]
- 4.Haffty BG, Harrold E, Khan AJ, et al. Outcome of conservatively managed early-onset breast cancer by BRCA1/2 status. Lancet. 2002;359:1471–1477. doi: 10.1016/S0140-6736(02)08434-9. [DOI] [PubMed] [Google Scholar]
- 5.Boice JD, Jr, Preston D, Davis FG, et al. Frequent chest X-ray fluoroscopy and breast cancer incidence among tuberculosis patients in Massachusetts. Radiat Res. 1991;125:214–222. [PubMed] [Google Scholar]
- 6.Boice JD., Jr Radiation and breast carcinogenesis. Med Pediatr Oncol. 2001;36:508–513. doi: 10.1002/mpo.1122. [DOI] [PubMed] [Google Scholar]
- 7.Land CE, Tokunaga M, Koyama K, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res. 2003;160:707–717. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- 8.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 9.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preston DL, Mattsson A, Holmberg E, et al. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res. 2002;158:220–235. doi: 10.1667/0033-7587(2002)158[0220:reobcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Boice JD, Jr, Harvey EB, Blettner M, et al. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–785. doi: 10.1056/NEJM199203193261201. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 13.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 14.Harvey EB, Brinton LA. Second cancer following cancer of the breast in Connecticut, 1935–82. Natl Cancer Inst Monogr. 1985;68:99–112. [PubMed] [Google Scholar]
- 15.Horn PL, Thompson WD, Schwartz SM. Factors associated with the risk of second primary breast cancer: an analysis of data from the Connecticut Tumor Registry. J Chronic Dis. 1987;40:1003–1011. doi: 10.1016/0021-9681(87)90114-7. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein JL, Thompson WD, Risch N, et al. Risk factors predicting the incidence of second primary breast cancer among women diagnosed with a first primary breast cancer. Am J Epidemiol. 1992;136:925–936. doi: 10.1093/oxfordjournals.aje.a116565. [DOI] [PubMed] [Google Scholar]
- 17.RE Curtis, DM Freedman, E Ron, et al., editors. New Malignancies Among Cancer Survivors. SEER Cancer Registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. NIH Publ. No. 05-5302. [Google Scholar]
- 18.Kurtz JM, Amalric R, Brandone H, et al. Contralateral breast cancer and other second malignancies in patients treated by breast-conserving therapy with radiation. Int J Radiat Oncol Biol Phys. 1988;15:277–284. doi: 10.1016/s0360-3016(98)90005-0. [DOI] [PubMed] [Google Scholar]
- 19.Parker RG, Grimm P, Enstrom JE. Contralateral breast cancers following treatment for initial breast cancers in women. Am J Clin Oncol. 1989;12:213–216. doi: 10.1097/00000421-198906000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Fowble B, Hanlon A, Freedman G, et al. Second cancers after conservative surgery and radiation for stages I–II breast cancer: identifying a subset of women at increased risk. Int J Radiat Oncol Biol Phys. 2001;51:679–690. doi: 10.1016/s0360-3016(01)01665-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Thompson W, Semenciw R, et al. Epidemiology of contralateral breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:855–861. [PubMed] [Google Scholar]
- 22.Basco VE, Coldman AJ, Elwood JM, et al. Radiation dose and second breast cancer. Br J Cancer. 1985;52:319–325. doi: 10.1038/bjc.1985.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storm HH, Andersson M, Boice JD, Jr, et al. Adjuvant radiotherapy and risk of contralateral breast cancer. J Natl Cancer Inst. 1992;84:1245–1250. doi: 10.1093/jnci/84.16.1245. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein JL, Langholz B, Haile RW, et al. Study design: evaluating gene-environment interactions in the etiology of breast cancer - the WECARE study. Breast Cancer Res. 2004;6:199–214. doi: 10.1186/bcr771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langholz B, Borgan O. Counter-matching: a stratified nested case-control sampling method. Biometrika. 1995;82:69–79. [Google Scholar]
- 26.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life tables. J Roy Statist Soc B. 1972;34:187–220. [Google Scholar]
- 28.Borgan O, Goldstein L, Langholz B. Methods for the analysis of sampled cohort data in the Cox Proportional Hazards Model, Annals of Statistics. 1995;12:1749–1778. [Google Scholar]
- 29.Preston DL, Lubin JH, Pierce DA, et al. Epicure Users Guide. Seattle, WA: Hirosoft International Corporation; 1993. [Google Scholar]
- 30.Langholz B, Thomas DC, Zhang X, Bernstein JL, et al. The WECARE Study Group. Statistical Methods for Analyzing Radiation Dose-Response with Tumor and/or Dose Location-Specific Information with Application to the Wecare Study of Asynchronous Contralateral Breast Cancer. Technical Report 177. 2007 doi: 10.1111/j.1541-0420.2008.01096.x. Department of Preventive Medicine, Biostatistics Division. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraass BA, Roberson PL, Lichter AS. Dose to the contralateral breast due to primary breast irradiation. Int J Radiat Oncol Biol Phys. 1985;11:485–497. doi: 10.1016/0360-3016(85)90179-8. [DOI] [PubMed] [Google Scholar]
- 32.Kelly CA, Wang XY, Chu JC, et al. Dose to contralateral breast: a comparison of four primary breast irradiation techniques. Int J Radiat Oncol Biol Phys. 1996;34:727–732. doi: 10.1016/0360-3016(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 33.Khan AJ, Haffty BG. The location of contralateral breast cancers after radiation therapy. Breast J. 2001;7:331–336. doi: 10.1046/j.1524-4741.2001.20118.x. [DOI] [PubMed] [Google Scholar]
- 34.Hill-Kayser CE, Harris EE, Hwang WT, et al. Twenty-year incidence and patterns of contralateral breast cancer after breast conservation treatment with radiation. Int J Radiat Oncol Biol Phys. 2006;66:1313–1319. doi: 10.1016/j.ijrobp.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Haffty BG. Radiation therapy and the risk of contralateral breast cancer. Int J Radiat Oncol Biol Phys. 2003;56:920–921. doi: 10.1016/s0360-3016(03)00204-9. [DOI] [PubMed] [Google Scholar]
- 36.Borghero YO, Salehpour M, McNeese MD, et al. Multileaf field-in-field forward-planned intensity-modulated dose compensation for whole-breast irradiation is associated with reduced contralateral breast dose: a phantom model comparison. Radiother Oncol. 2007;82:324–328. doi: 10.1016/j.radonc.2006.10.011. [DOI] [PubMed] [Google Scholar]