Abstract
Establishing and maintaining healthy physical activity (PA) levels is important throughout life. The purpose of this study was to determine the extent of PA tracking between ages 3 and 7 y. Objective measures of PA (RT3, triaxial accelerometer) were collected every 4 mo from ages 3 to 7; data from 234 children with PA measures available during each year of age were analyzed. Mean PA (total, moderate/vigorous(MV), and inactivity(IA)) was calculated for each year of age and adjusted for wear time. Correlations with age 3 PA were moderate at age 4 (r=0.42–0.45) but declined by age 7 (r=0.19–0.25). After classification into sex-specific tertiles of PA at age 3, boys in the High age 3 MVPA tertile maintained significantly higher PA at all subsequent ages, while girls in the High age 3 MVPA tertile were not significantly higher at age 6 and 7. Boys and girls in the High age 3 IA tertile had significantly higher IA at multiple subsequent years of age (P<0.05 at ages 5 and 6). In conclusion, boys who were relatively more active at age 3 remained more active for several subsequent years. These findings highlight early childhood differences in physical activity patterns between boys and girls.
Regular physical activity has numerous benefits to health in children (48). Unfortunately, many children have difficulty attaining recommended levels of physical activity and limiting inactivity (46), and there is concern that during key periods of development, physical activity may decline in some youth (19, 28).
Even with the vast developmental changes that occur throughout childhood and adolescence, there is evidence to indicate that some early life behaviors continue during later stages (42). The term “tracking” is used to describe when people or groups of people maintain their relative position of a behavior or measure over time compared to peers (30). Tracking of health-related behaviors, such as physical activity, can have important implications. For instance, if physical activity levels are established early in life, there may be key periods of time when interventions may be most effective. In addition, maintaining a relatively high level of physical activity can protect against development of obesity (51). Conversely, if physical activity levels do not track over time, interventions to increase physical activity may be better implemented at many age periods.
Several studies have shown moderate tracking of physical activity in older children and adolescents (3, 11, 12, 25, 26, 29, 39, 40, 44, 45). Kelder et al found that adolescents with the lowest and highest reported physical activity in sixth grade remained significantly different in each of the 6 years of follow up (26). Studies focused on physical activity tracking in early childhood (< 7 years old) using questionnaire, direct observation, and heart rate monitoring have reported low to moderate tracking, with correlation coefficients between 0.15 and 0.66 (37, 41). Studies using accelerometers for measuring physical activity have reported correlation coefficients between 0.29 and 0.51, but the duration of the studies have been limited to a couple years (18, 23, 27, 35, 43) or had at least 3 years between measurements, and therefore could not examine changes from year to year (24). In addition, while stronger physical activity tracking in boys has been shown in adolescents (11) and older children (34), it is unclear whether these sex differences in tracking exist in early childhood. The aim of this study was to evaluate physical activity measured by accelerometry longitudinally over 5 years in young boys and girls. Our hypothesis was that physical activity behaviors would exhibit moderate tracking from age 3 to age 7 in both sexes, with stronger tracking in boys than girls.
METHODS
Population and Design
This study enrolled a convenience sample of 372 healthy 3-year-old children from the metropolitan area surrounding Cincinnati Children’s Hospital Medical Center (CCHMC) and evaluated them every 4 months for a total of 13 visits between 2001 and 2006. The aims of the original study were to determine the age of BMI rebound and the effects of diet and physical activity on body composition. Informed consent and assent were obtained from the families and participants after the protocol was approved by the CCHMC IRB.
Measures
Physical activity was measured using a triaxial accelerometer (RT3, Stayhealthy, Inc., Monrovia, CA). The RT3 has been validated against room calorimetry in children 3 to 5 years of age(1). Cutoffs to define physical activity intensity from accelerometry counts accurately classify 65%–79% of individuals(1). Participants were instructed to wear the accelerometer at the waist above the right hip with an elastic belt for 3 days, specifically 2 weekdays and 1 weekend day, every 4 months. They were instructed to remove the device while sleeping or during activities that may damage the device and to keep a written log of device wear. The epoch on the device was set to 60 seconds (as opposed to the alternative on the RT3: 1 second) in order to measure multiple days of activity given the memory constraints of the device. Data from the devices were downloaded at each study visit and excluded if output appeared invalid. Accelerometers suspected of malfunctioning were returned for repair.
Accelerometer wear time was defined as recorded minutes that were not within a period of 60 minutes of zero counts, excepting up to 2 consecutive minutes with counts less than 100 (46). The 60-minute limit was chosen to be consistent with several other studies (15, 32, 33, 46), and after examination of other limits (10 and 20 minutes) that resulted in higher automated exclusion of seemingly valid measurements. Days were defined as starting and ending at 2:00 AM. A day with greater than 8 hours of wear time was considered to be valid in order to account for early childhood sleep patterns (greater number of hours at night and higher frequency of naps during the day) and to be similar to other studies in this age group (6–8, 10, 13, 20, 33). The following criteria were used to flag days for further inspection: 1) wear time > 18 hours, 2) mean counts per minute (cpm) >1200, 3) mean cpm < 200, 4) greater than 2 hours of wear time between 11:00 PM and 7:00 AM. All flagged days were visually inspected in the context of the physical activity record for the entire visit and compared with the written log and excluded from analysis if the accelerometer data were consistent with: 1) malfunction, 2) night wear, 3) implausibly high or low counts for most of the day, or 4) incomplete based on written log. Serial numbers from malfunctioning devices were used to inspect other days recorded with the same devices shortly before or after the malfunction. In order to clearly present descriptive longitudinal data on a consistent study cohort, analyses were limited to participants with at least 1 valid day of accelerometry during each age period. Among this subset of participants, in any given age period over 94% of participants had greater than 1 valid day of accelerometry data.
In addition to calculating total physical activity (TPA) for each day (counts per day), each minute was categorized as moderate and vigorous physical activity (MVPA, ≥1400 counts), light physical activity (LPA, ≥175 and <1400 counts), or inactivity (IA, <175 counts) according to Adolph et al (1). For each day, total minutes in each category were calculated. The mean of each PA measure was calculated for each year of age for each participant. Participants who had at least one valid day at each year of age (N = 234) were included in analyses. Participants with at least one valid day of accelerometry at each year of age were significantly older at enrollment and a higher proportion were white compared with those who did not have at least one valid day of accelerometry each year of age, but the two groups were similar in height, weight, BMI (Table 1), total daily accelerometry counts, and hours of wear time (data not shown).
TABLE 1.
Participant characteristics at enrollment.
| Participants with accelerometry measurement in each year of age (N = 234) |
Participants without accelerometry measurement in each year of age (N = 138) |
P-value | |
|---|---|---|---|
| Age, y | 3.4 (0.3) | 3.3 (0.3) | <0.0001 |
| Sex, % girls | 47.0 | 49.3 | 0.67 |
| Race, % white | 84.6 | 67.4 | 0.0001 |
| Height, cm | 98.6 (4.4) | 97.9 (4.7) | 0.14 |
| Weight, kg | 15.9 (2.0) | 15.5 (2.1) | 0.10 |
| BMI, kg/m2 | 16.3 (1.3) | 16.1 (1.3) | 0.25 |
| BMI z-score | 0.38 (0.97) | 0.21 (0.97) | 0.11 |
Values presented as mean (standard deviation) except proportions. Test statistics are associated with t-tests for continuous variables and chi-square for proportions.
Demographic information, including sex and race, was collected by questionnaire from a parent. At each study visit, height (cm) was measured using a custom stadiometer, and weight (kg) was measured using a Health-O-Meter (Alsip, IL) electronic scale. Body mass index (BMI) was calculated using the formula weight/height2 (kg/m2). Z-scores were assigned to BMI values according to the CDC 2000 growth charts (9).
Analysis
Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). Means and proportions of the demographic, anthropometric, and PA variables were calculated. Since device wear time was significantly correlated with TPA (r = 0.25), MVPA (r = 0.13), LPA (r = 0.56), and IA (r = 0.49), adjustment for wear time was deemed necessary to reduce potential confounding and to improve precision. Each PA measure was adjusted for wear time using the residual method (49). This method involves regressing PA minutes on wear time, and then adding each participant’s residual PA to the predicted PA corresponding to the group’s average wear time. All PA data presented in this paper are adjusted for wear time using this approach. The residual method of adjustment, compared with adjusting in a multivariate model, allows clearer description of the variability (including standard deviations) and a simpler display of the data.
Spearman rank correlation coefficients between PA measures at age 3 and later ages were calculated for the purpose of assessing tracking of PA measures over time. General linear modeling (GLM) with multivariate analysis of variance (MANOVA) was used to examine trends in PA measures over time, differences in PA measures between sexes, and interaction between time and sex, a test of parallelism. GLM with MANOVA with a profile option was also used to test for trends over time and differences between tertile groups based on PA measures at age 3. An alpha level of 0.05 was used to indicate significance and all tests were two-sided.
RESULTS
Two hundred thirty-four participants had ≥1 valid day of accelerometry during each year of age; 47% (109) were girls and 85% (199) were white. At enrollment, the mean age was 3.4 ± 0.3 years (± standard deviation), height was 99 ± 4 cm, weight was 16 ± 2 kg, BMI was 16 ± 1 kg/m2, and BMI z-score was 0.4 ± 1. Thirty-two percent of the days were weekend days (Saturday or Sunday), and the proportion of weekend days did not vary significantly by age period.
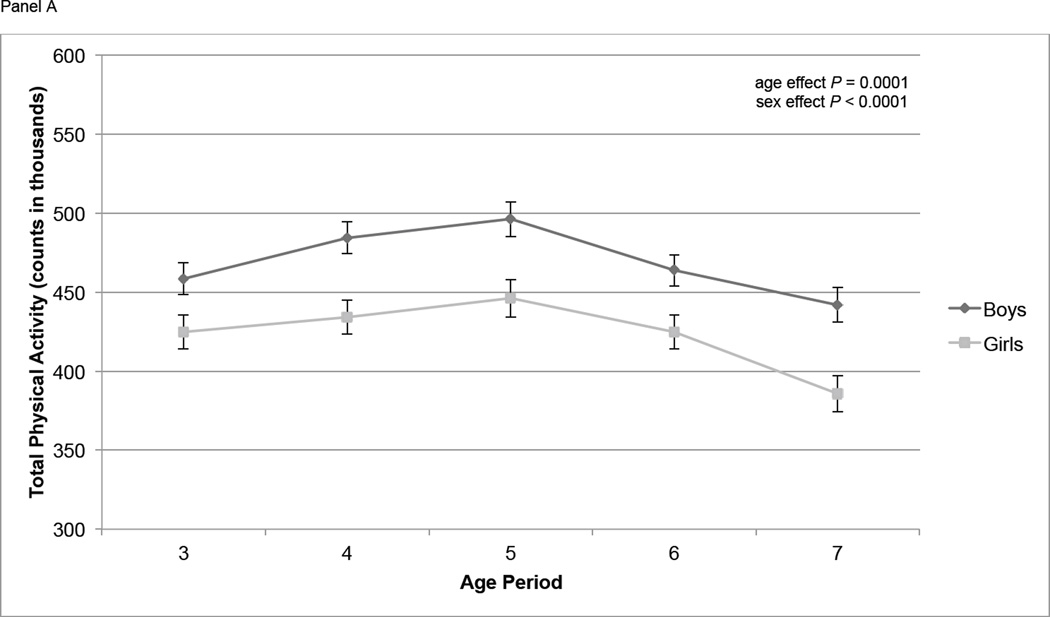
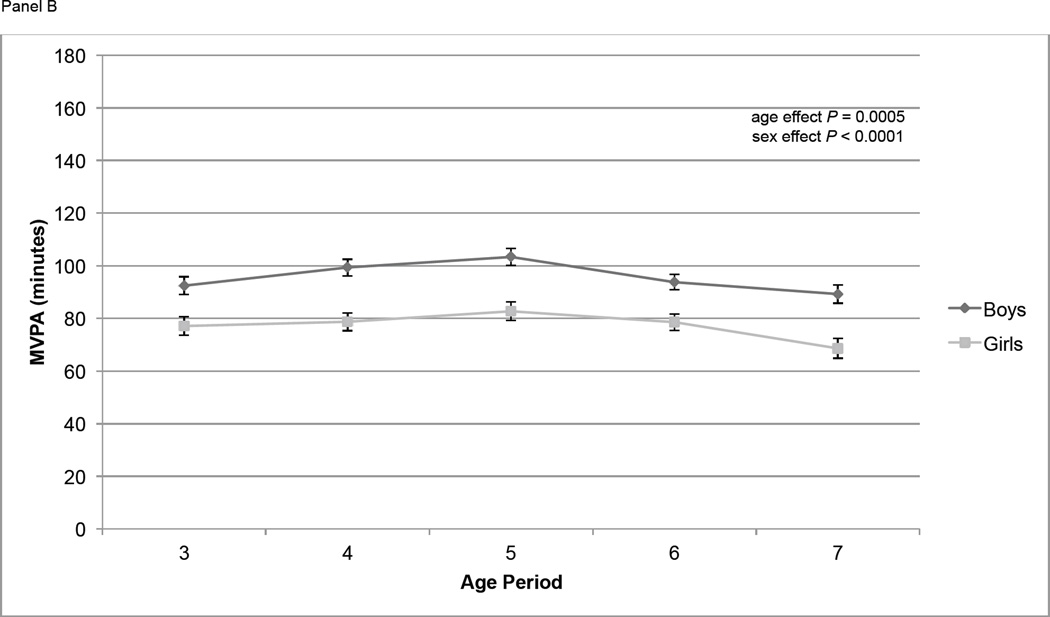
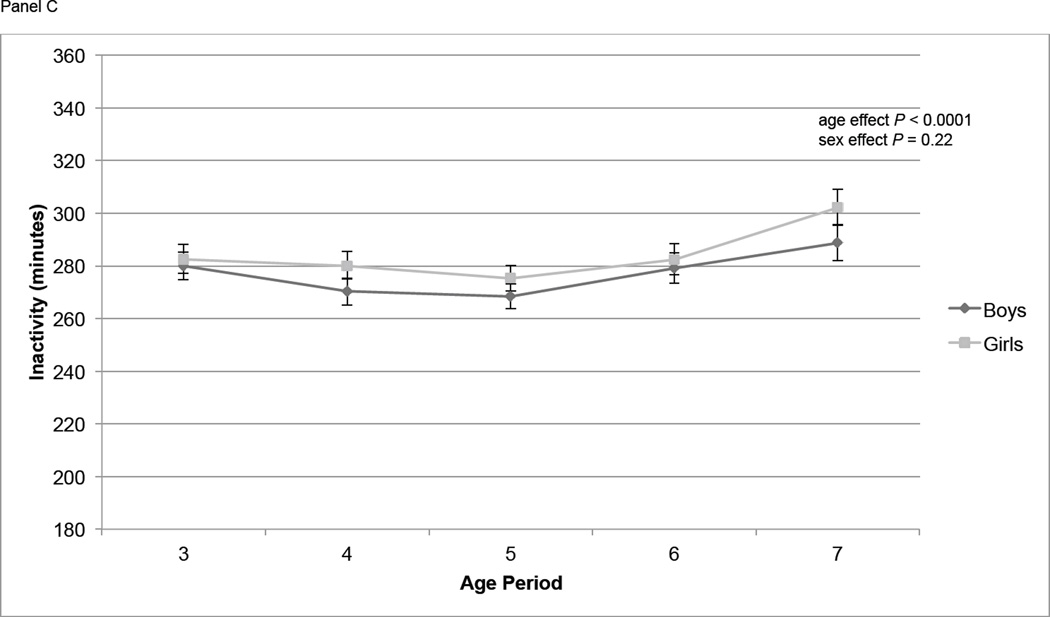
At age 3, mean TPA was 443,000 counts per day, and mean MVPA and IA were 85 and 281 minutes/d respectively (Table 2). At age 7, mean TPA and MVPA had decreased to 416,000 counts per day and 80 minutes/d, respectively, and IA had increased to 295 minutes/d. The proportion of participants whose mean MVPA was ≥ 60 minutes was between 68% and 84% in each year of age. Overall, TPA increased significantly from age 3 to 4 (P = 0.03), increased from age 4 to 5 (P = 0.16), then declined significantly from age 5 to age 6 (P = 0.0061) and from age 6 to age 7 (P = 0.0011) (Figure 1, Panel A). MVPA followed a similar pattern, increasing slightly from age 3 to age 4 (P = 0.11) and age 4 to age 5 (P = 0.11), then declining significantly from age 5 to age 6 (P = 0.0096) and age 6 to age 7 (P = 0.0091) (Figure 1, Panel B). IA did not change significantly from age 3 to age 4 (P = 0.12) or age 4 to age 5 (P = 0.29) but did increase significantly from age 5 to age 6 (P = 0.03) and age 6 to age 7 (P = 0.003) (Figure 1, Panel C). At all ages TPA and MVPA were significantly greater in boys than girls (P < 0.0001) (Figure 1, Panels A and B). IA did not differ by sex over the course of the study (Figure 1, Panel C).
Table 2.
Physical activity measures (per day) at ages 3 through 7 years*. (N = 234)
| Age 3 | Age 4 | Age 5 | Age 6 | Age 7 |
P-value for trend across time |
|
|---|---|---|---|---|---|---|
| Wear time, min per day | 735 ± 77 | 729 ± 65 | 750 ± 57 | 760 ± 64 | 751 ± 82 | |
| Physical Activity† (PA), counts per day | 443,000 ± 114,000 | 461,000 ± 114,000 | 473,000 ± 124,000 | 446,000 ± 111,000 | 416,000 ± 124,000 | <0.0001 |
| Moderate or vigorous PA†, min per day | 85 ± 38 | 90 ± 37 | 94 ± 37 | 87 ± 33 | 80 ± 40 | 0.0005 |
| Light PA†, min per day | 380 ± 45 | 382 ± 42 | 381 ± 42 | 380 ± 47 | 372 ± 55 | 0.15 |
| Inactivity†, min per day | 281 ± 59 | 275 ± 57 | 272 ± 51 | 281 ± 63 | 295 ± 74 | <0.0001 |
| Proportion of participants with mean MVPA† ≥ 60 min per day | 74% | 79% | 84% | 81% | 68% | |
| Proportion of participants with mean LMVPA† ≥ 25% of wear time per day | 100% | 99.6% | 100% | 98.7% | 96.6% |
All values (except proportions) presented as mean ± standard deviation.
Adjusted for wear time.
Figure 1.
Physical activity measures (means ± standard errors) over time by sex. Physical activity is adjusted for wear time using the residual method. Overall age and sex effects tested using repeated measures ANOVA. Panel A contains total physical activity (counts); age effect: P < 0.0001; sex effect: P < 0.0001. Panel B contains moderate and vigorous physical activity (MVPA, minutes); age effect: P = 0.0005; sex effect: P < 0.0001. Panel C contains inactivity (IA, minutes); age effect: P < 0.0001; sex effect: P = 0.22.
Spearman correlation coefficients between measures of PA at age 3 with those at ages 4 ranged from 0.42 to 0.45 (Table 3). These correlations declined in subsequent years as the age interval increased. By age 7, the correlation with age 3 PA measures ranged between 0.19 for TPA and 0.25 for MVPA. All correlation coefficients were significantly greater than zero (P < 0.05). Similar results were obtained when correlations were calculated for the superset of the analysis group, all participants who had ≥ 1 valid day at age 3 and at least one other age period (data not shown).
TABLE 3.
Correlation of physical activity measures at ages 4 through 7 with age 3 years. (N = 234)
| Age 4 | Age 5 | Age 6 | Age 7 | |
|---|---|---|---|---|
| Physical Activity* (PA), counts per day, correlation with age 3 | 0.42 | 0.33 | 0.26 | 0.19 |
| Moderate or vigorous PA*, min per day, correlation with age 3 | 0.43 | 0.35 | 0.27 | 0.25 |
| Light PA*, min per day, correlation with age 3 | 0.45 | 0.33 | 0.29 | 0.23 |
| Inactivity*, min per day, correlation with age 3 | 0.45 | 0.35 | 0.31 | 0.20 |
All p-values ≤ 0.01.
Adjusted for wear time.
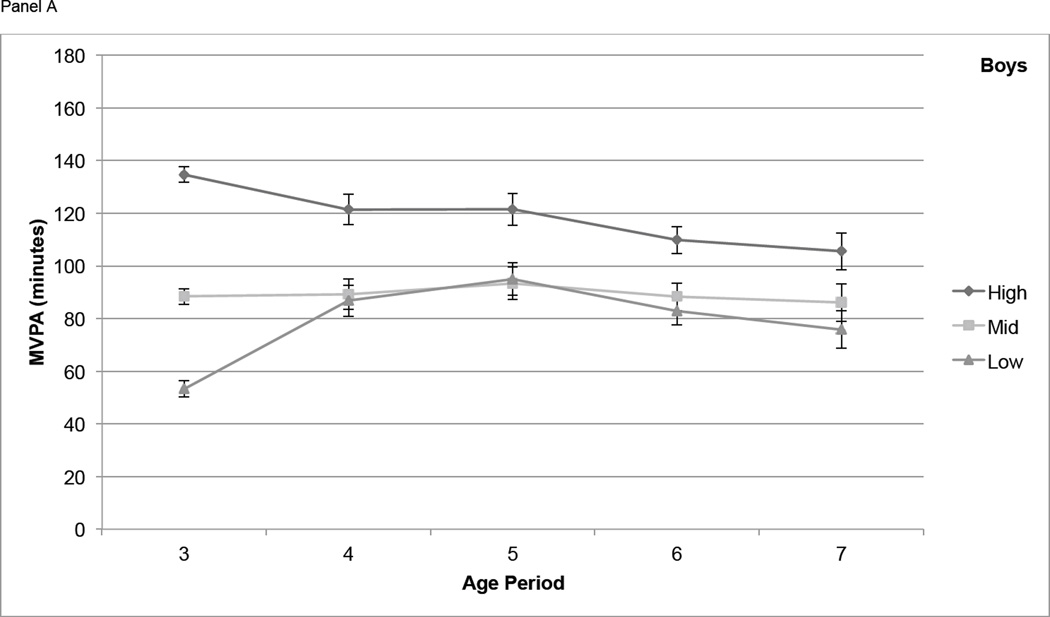
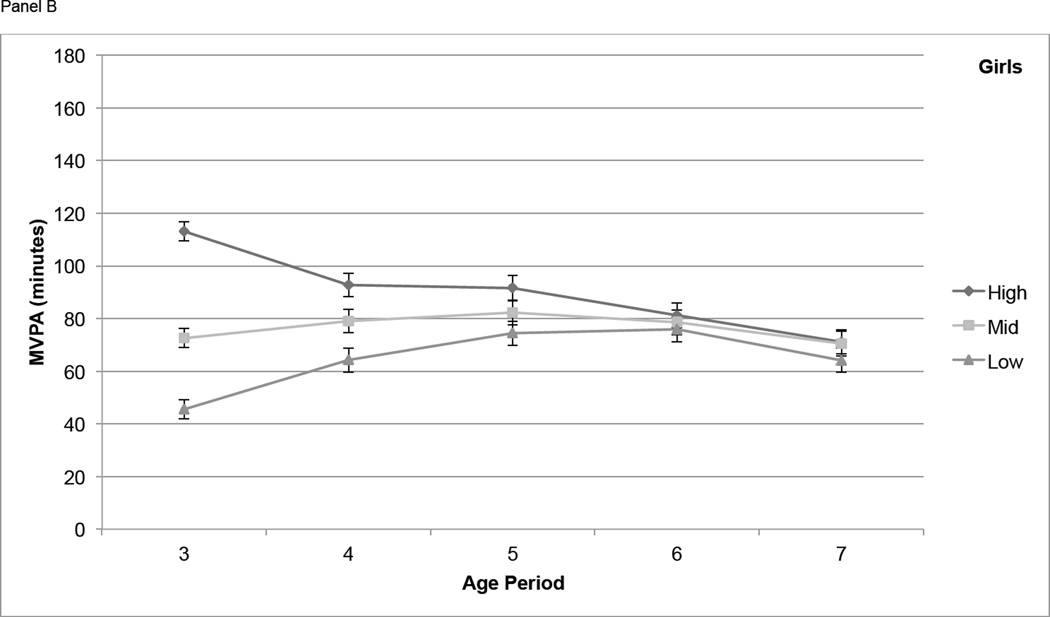
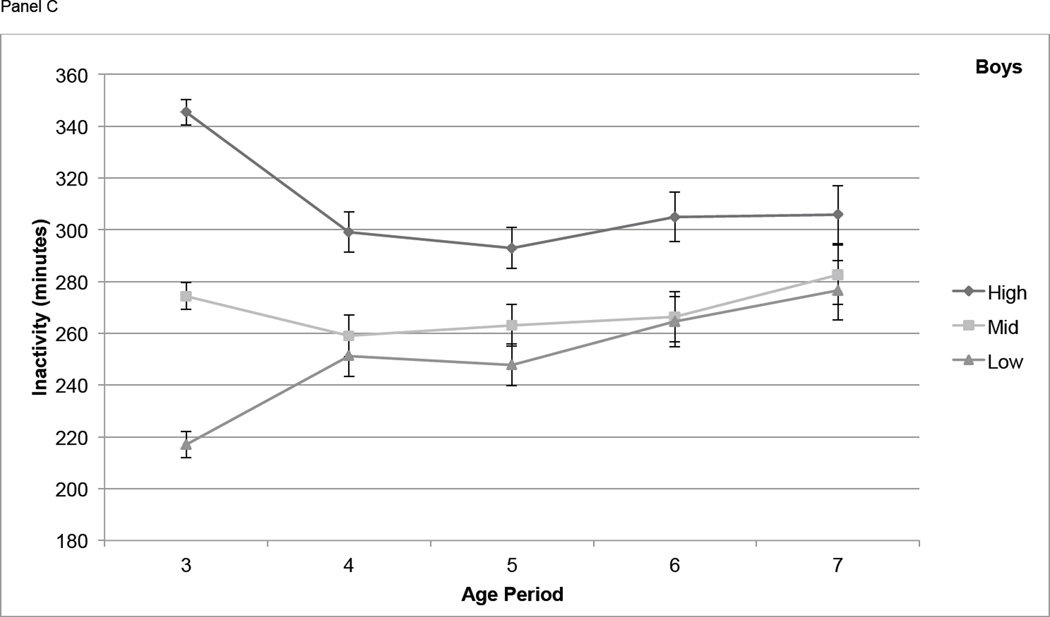
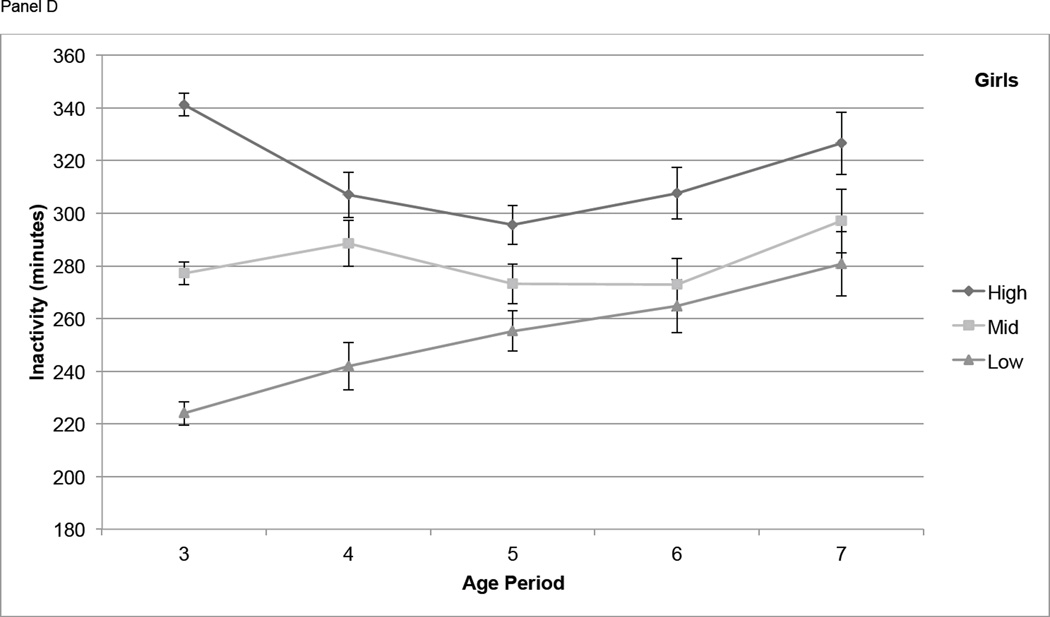
For boys, differences in MVPA among tertiles based on activity at age 3 narrowed from age 3 to age 4, but participants in the High tertile maintained significantly higher MVPA than those in the Mid and Low tertiles at each age from 4 through 7 (P < 0.01) with the exception of the difference between High and Mid at age 7 (P = 0.051) (Figure 2, Panel A). In girls, differences in MVPA among the High, Mid, and Low age 3 tertiles remained significant at age 4, narrowed at age 5, and were not significant at age 6 and 7 (Figure 2, Panel B). Boys in the High IA tertile at age 3 had significantly higher IA (P < 0.01) at all subsequent years of age except age 7, but the Mid and Low tertiles were not consistently distinct from each other at ages 4 through 7 (Figure 2, Panel C). For IA in girls, the High, Mid, and Low tertiles at age 3 remained somewhat distinct at ages 4 through 7, with the High tertile remaining significantly higher than both other tertiles at age 5 (P < 0.05) and age 6 (P < 0.05) (Figure 2, Panel D).
Figure 2.
Physical activity measures (means ± standard errors) over time, by tertile (based on PA at age 3). Physical activity is adjusted for wear time using the residual method. Panel A contains moderate and vigorous physical activity (MVPA, minutes) in boys, Panel B contains MVPA in girls, Panel C contains inactivity (IA, minutes) in boys, and Panel D contains IA in girls.
DISCUSSION
The ability to obtain objective measures of physical activity by accelerometry has enabled important advances in our understanding of the natural history of PA in young children and the extent to which PA and inactivity track from the preschool years to mid-childhood. In this study we found that tracking of PA was moderate from age 3 to 4 but declined by age 7. We also found tracking differences by sex. The more active (in terms of MVPA) 3-year-old boys remained more active over the following few years, whereas the most active 3-year-old girls did not remain more active later in the study. In contrast, the tracking of inactivity was similar in boys and girls from age 3 to 7.
Our findings of tracking in physical activity must be considered in the context of the overall physical activity patterns in children that occur during this age period. Overall, physical activity and inactivity remained stable between 3 to 5 years of age. Between ages 5 to 7, however, physical activity significantly decreased and inactivity increased in both boys and girls. The decline in physical activity after age 5 y is consistent with other studies of children of similar age. Janz et al showed a decline in activity and increase in inactivity from age 5 to 8 (24), and Basterfield et al showed a decline in physical activity from age 7 to 9 (5). A possible explanation for the decline in PA after age 5 is entrance into school and being required to sit for longer periods. This study did not record timing of school entrance or types of reported activity during the day, however, so it was not possible to test this hypothesis.
Another finding in this study was the higher total physical activity and MVPA in boys throughout the study period. Although many studies have focused on the issue of lower physical activity in adolescent girls (19), fewer studies have described this difference during early childhood (4, 17). In a cross sectional study of preschool children boys had higher physical activity than girls during a 3 hour sampling period (38). The Iowa Bone Development study used accelerometry to measure physical activity for at least 8 hours a day and found that boys had higher overall and vigorous physical activity compared to girls at age 5 and age 8 (24). Our robust measures of physical activity measured over a median of 6 days per year of age and adjusted for wear time confirm and extend these prior findings.
The correlation coefficient is the most commonly reported measure of tracking in past studies of physical activity. In this study, tracking of the four PA measures was highest from age 3 to age 4, and subsequently declined as the intervening period grew longer. These moderate to low correlation coefficients over 1 to 4 year intervals are consistent with other tracking studies of children in this age group using accelerometry (18, 23, 27, 43) and other PA measurement methods. Sallis et al reported correlation coefficients between 0.15 and 0.36 over 2 years in 4-year-old children whose PA was measured by observation (41). Pate et al estimated PA by heart rate measurement in 47 children ages 3–4 years and reported correlation coefficients between 0.57 to 0.66 from year to year over 3 years (37).
When grouped by tertile at age 3, boys in the High MVPA tertile had significantly higher levels of MVPA at later ages, while girls in the High MVPA tertile at age 3 did not. The finding that tracking of MVPA seems to be more evident in boys than girls during this early age period is consistent with studies of older children (34, 45). Nyberg et al studied 97 children at age 7 and age 9 using accelerometry and found stronger correlation for boys than girls (34). Telama et al studied a wider age range (age 9–30) and larger intervals (3–12 years) and found higher tracking among boys than girls (45). Our findings show that differences in moderate and vigorous physical activity tracking patterns between boys and girls are present even in early childhood. This is in contrast to tracking of inactivity, which did not differ by sex in this study. In the Iowa Bone Development Study the correlation coefficients between inactivity at ages 5 and 8 were also similar in boys (0.41) and girls (0.44) (24).
The evidence of differences by sex in MVPA tracking but not inactivity tracking in our study indicates that MVPA was relatively more stable for boys than for girls in the early childhood period, whereas girls’ patterns suggest a regression to the mean effect (i.e., that tertiles at age 3 are not representative of later periods). The reasons for this sex difference in PA tracking patterns remain unclear. There may be some clues provided in analyses of differences in overall physical activity in early childhood. Yamamoto et al studied 649 children ages 3–6 years old and found that MVPA was associated with extrinsic motivators in girls (e.g. parental PA) and boys’ MVPA was associated with intrinsic motivators like the desire to be active (50). Although beyond the scope of this analysis, further exploration of potential reasons for differences in the tracking of PA between boys and girls could provide avenues for tailored clinical interventions and public health messages to increase PA in youth.
It was encouraging that nearly all the participants had Light or Moderate/Vigorous PA account for more than 25% of their wear time on an average day (which corresponds to the IOM PA recommendations(22)). Furthermore, most of the children accumulated at least 60 minutes of MVPA per day during each age period, corresponding to the recommendations for children 6 years and older in the PA Guidelines for Americans(48). The differences between these two findings (nearly 100% meeting IOM recommendations vs. ~75% for US PA guidelines) reflect the inclusion of Light physical activity in the IOM guidelines. The challenge translating between different sets of guidelines in this age group underscores the need for more accelerometer-based nationally-representative physical activity surveillance data on children in the United States under 6 years old, as well as more studies linking physical activity with health markers in this early age group.
The strengths of this study include the objective measurement of physical activity with a triaxial accelerometer and a high frequency of measurement over the course of this 5-year longitudinal study. Also, a confounding variable (wear time) was addressed by adjustment using a robust method (2, 49). Weaknesses include the selection bias inherent in any longitudinal study; the population in this study was able and willing to complete several study visits over multiple years and thus may differ from other populations. Of note, the study included mostly white participants, so extrapolation to physical activity in non-white populations may be limited. Although Table 1 reveals that those who had data available at all ages were not substantially different in their characteristics at enrollment from those who did not (except for race), the participants who were able to consistently adhere to many study visits over several years may have differed from the population from which it was sampled and this limits the external validity of the study.
Using accelerometry to measure a complex behavior such as physical activity and the subsequent decisionmaking required to analyze the data collected (e.g. assessing non-wear time, minimum time needed, epoch) has many inherent limitations that have been laid out in detail elsewhere (31). Most, if not all, of these limitations apply to this study. First, our choice of 60 minutes of consecutive zero counts to determine non-wear time was made primarily to be consistent with other studies (14, 32, 46), although there is far from universal agreement on this point, and using a higher or lower limit can bias the measures toward higher or lower amounts of physical activity (16, 36). The accelerometer used in this study (RT3) uses a 60 second epoch, which may not accurately reflect the bursts of physical activity that can occur in children (47). This was ameliorated somewhat by collapsing the moderate and vigorous physical activity categories, but epoch-related bias toward lower amounts of MVPA has been reported (21).
In this study we selected 8 hours as a minimum requirement to constitute a “valid” day. Other studies have selected both lower and higher minimum hour thresholds (6–8, 10, 13, 20, 33). A higher threshold may lead to more included days capturing a higher proportion of the day’s physical activity, but in a younger age group with higher sleep requirements, a higher threshold may also exclude more useful data.
In order to present data on a consistent cohort of children, we limited the analysis to participants who had at least 1 valid day of accelerometry data in each age period. Even though over 94% of the participants in this subset had greater than 1 valid day of accelerometry in any given age period, this decision to include some participants with 1 valid day in an age period may increase the likelihood that our estimate of physical activity from a participant may not fairly represent their usual physical activity, limiting our ability to make inferences that are based on that assumption.
In this longitudinal study of 3–7 year-old children, boys were more active than girls and maintained high moderate and vigorous activity more so than girls, while in both sexes, those who were more inactive at age 3 remained more inactive at later ages. These findings highlight the importance of promoting physical activity early in life and reinforce that differences in physical activity patterns by sex are present in elementary school. Exploration of these differences may allow tailored intervention to promote physical activity in youth.
ACKNOWLEDGMENT
This study was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health, through grant R01 HL064022 (Epidemiology of BMI Rebound), and in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 KL2 TR000078-04, and in part by the Heart Institute Research Core at Cincinnati Children’s Hospital Medical Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank the families who participated in the Epidemiology of BMI Rebound study and Patricia M. Herbers, MS and Heidi J. Sucharew, PhD for their invaluable advice and counsel.
References
- 1.Adolph AL, Puyau MR, Vohra FA, Nicklas TA, Zakeri IF, Butte NF. Validation of uniaxial and triaxial accelerometers for the assessment of physical activity in preschool children. J Phys Act Health. 2012;9(7):944–953. doi: 10.1123/jpah.9.7.944. [DOI] [PubMed] [Google Scholar]
- 2.Affuso O, Stevens J, Catellier D, et al. Validity of self-reported leisure-time sedentary behavior in adolescents. Journal of negative results in biomedicine. 2011;10:2. doi: 10.1186/1477-5751-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggett CD, Stevens J, McMurray RG, Evenson KR, Murray DM, Catellier DJ, He K. Tracking of physical activity and inactivity in middle school girls. Med Sci Sports Exerc. 2008;40(11):1916–1922. doi: 10.1249/MSS.0b013e318180c390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranowski T, Thompson WO, DuRant RH, Baranowski J, Puhl J. Observations on physical activity in physical locations: age, gender, ethnicity, and month effects. Res Q Exerc Sport. 1993;64(2):127–133. doi: 10.1080/02701367.1993.10608789. [DOI] [PubMed] [Google Scholar]
- 5.Basterfield L, Adamson AJ, Frary JK, Parkinson KN, Pearce MS, Reilly JJ. Longitudinal study of physical activity and sedentary behavior in children. Pediatrics. 2011;127(1):e24–e30. doi: 10.1542/peds.2010-1935. [DOI] [PubMed] [Google Scholar]
- 6.Basterfield L, Adamson AJ, Pearce MS, Reilly JJ. Stability of habitual physical activity and sedentary behavior monitoring by accelerometry in 6- to 8-year-olds. J Phys Act Health. 2011;8(4):543–547. doi: 10.1123/jpah.8.4.543. [DOI] [PubMed] [Google Scholar]
- 7.Bringolf-Isler B, Grize L, Mäder U, Ruch N, Sennhauser FH, Braun-Fahrländer C. Assessment of intensity, prevalence and duration of everyday activities in Swiss school children: a cross-sectional analysis of accelerometer and diary data. International Journal of Behavioral Nutrition and Physical Activity. 2009;6(1):50. doi: 10.1186/1479-5868-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgi F, Meyer U, Granacher U, Schindler C, Marques-Vidal P, Kriemler S, Puder JJ. Relationship of physical activity with motor skills, aerobic fitness and body fat in preschool children: a cross-sectional and longitudinal study (Ballabeina) Int J Obes (Lond) 2011 Mar 29;35(7):937–944. doi: 10.1038/ijo.2011.54. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [Accessed 2013 March 8];A SAS program for the CDC growth charts. Available at: http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm.
- 10.Cliff DP, Okely AD. Comparison of two sets of accelerometer cut-off points for calculating moderate-to-vigorous physical activity in young children. J Phys Act Health. 2007;4(4):509–513. [PubMed] [Google Scholar]
- 11.Dencker M, Tanha T, Wollmer P, Karlsson MK, Andersen LB, Thorsson O. Tracking of Physical Activity with Accelerometers Over a Two-year Time Period. J Phys Act Health. 2013 Feb;10(2):241–248. doi: 10.1123/jpah.10.2.241. [DOI] [PubMed] [Google Scholar]
- 12.Dumith SC, Gigante DP, Domingues MR, Hallal PC, Menezes AM, Kohl HW., 3rd A longitudinal evaluation of physical activity in brazilian adolescents: tracking, change and predictors. Pediatr Exerc Sci. 2012;24(1):58–71. doi: 10.1123/pes.24.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eijkemans M, Mommers M, de Vries SI, van Buuren S, Stafleu A, Bakker I, Thijs C. Asthmatic symptoms, physical activity, and overweight in young children: a cohort study. Pediatrics. 2008;121(3):e666–e672. doi: 10.1542/peds.2007-1236. [DOI] [PubMed] [Google Scholar]
- 14.Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307(7):704–712. doi: 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekelund U, Sjostrom M, Yngve A. Physical activity assessed by activity monitor and doubly labeled water in children. Med Sci Sports Exerc. 2001;33(2):275–281. doi: 10.1097/00005768-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Evenson KR, Terry JW., Jr Assessment of differing definitions of accelerometer nonwear time. Res Q Exerc Sport. 2009;80(2):355–362. doi: 10.1080/02701367.2009.10599570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finn K, Johannsen N, Specker B. Factors associated with physical activity in preschool children. J Pediatr. 2002;140(1):81–85. doi: 10.1067/mpd.2002.120693. [DOI] [PubMed] [Google Scholar]
- 18.Gabel L, Obeid J, Nguyen T, Proudfoot NA, Timmons BW. Short-term muscle power and speed in preschoolers exhibit stronger tracking than physical activity. Appl Physiol Nutr Metab. 2011;36(6):939–945. doi: 10.1139/h11-118. [DOI] [PubMed] [Google Scholar]
- 19.Goran MI, Gower BA, Nagy TR, Johnson RK. Developmental changes in energy expenditure and physical activity in children: evidence for a decline in physical activity in girls before puberty. Pediatrics. 1998;101(5):887–891. doi: 10.1542/peds.101.5.887. [DOI] [PubMed] [Google Scholar]
- 20.Heelan KA, Eisenmann JC. Physical Activity, Media Time, and Body Composition in Young Children. Journal of Physical Activity & Health. 2006;3(2):200–209. doi: 10.1123/jpah.3.2.200. [DOI] [PubMed] [Google Scholar]
- 21.Hislop JF, Bulley C, Mercer TH, Reilly JJ. Comparison of accelerometry cut points for physical activity and sedentary behavior in preschool children: a validation study. Pediatr Exerc Sci. 2012;24(4):563–576. doi: 10.1123/pes.24.4.563. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine of the National Academies. [Accessed 2013 March 8];Early Childhood Obesity Prevention: Policies Goals, Recommendations, and Potential Actions. Available at: http://www.iom.edu/Reports/2011/Early-Childhood-Obesity-Prevention-Policies/Recommendations.aspx.
- 23.Jackson DM, Reilly JJ, Kelly LA, Montgomery C, Grant S, Paton JY. Objectively measured physical activity in a representative sample of 3- to 4-year-old children. Obes Res. 2003;11(3):420–425. doi: 10.1038/oby.2003.57. [DOI] [PubMed] [Google Scholar]
- 24.Janz KF, Burns TL, Levy SM. Tracking of activity and sedentary behaviors in childhood: the Iowa Bone Development Study. Am J Prev Med. 2005;29(3):171–178. doi: 10.1016/j.amepre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Janz KF, Dawson JD, Mahoney LT. Tracking physical fitness and physical activity from childhood to adolescence: the muscatine study. Med Sci Sports Exerc. 2000;32(7):1250–1257. doi: 10.1097/00005768-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Kelder SH, Perry CL, Klepp KI, Lytle LL. Longitudinal tracking of adolescent smoking, physical activity, and food choice behaviors. Am J Public Health. 1994;84(7):1121–1126. doi: 10.2105/ajph.84.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly LA, Reilly JJ, Jackson DM, Montgomery C, Grant S, Paton JY. Tracking physical activity and sedentary behavior in young children. Pediatr Exerc Sci. 2007;19(1):51–60. doi: 10.1123/pes.19.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Kimm SY, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347(10):709–715. doi: 10.1056/NEJMoa003277. [DOI] [PubMed] [Google Scholar]
- 29.Kwon S, Janz KF. Tracking of accelerometry-measured physical activity during childhood: ICAD pooled analysis. Int J Behav Nutr Phys Act. 2012;9(1):68. doi: 10.1186/1479-5868-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malina RM. Tracking of physical activity and physical fitness across the lifespan. Res Q Exerc Sport. 1996;67(3 Suppl):S48–S57. doi: 10.1080/02701367.1996.10608853. [DOI] [PubMed] [Google Scholar]
- 31.Masse LC, Fuemmeler BF, Anderson CB, Matthews CE, Trost SG, Catellier DJ, Treuth M. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37(11 Suppl):S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- 32.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McManus AM, Chu EY, Yu CC, Hu Y. How children move: activity pattern characteristics in lean and obese chinese children. J Obes. 2011;2011:679328. doi: 10.1155/2011/679328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyberg G, Ekelund U, Marcus C. Physical activity in children measured by accelerometry: stability over time. Scand J Med Sci Sports. 2009;19(1):30–35. doi: 10.1111/j.1600-0838.2007.00756.x. [DOI] [PubMed] [Google Scholar]
- 35.Oja L, Jurimae T. Tracking of motor abilities, physical activity, and elementary motor skills during transition from preschool to school. Acta Kinesiologiae Universitatis Tartuensis. 2001;6:91–101. [Google Scholar]
- 36.Oliver M, Badland HM, Schofield GM, Shepherd J. Identification of accelerometer nonwear time and sedentary behavior. Res Q Exerc Sport. 2011;82(4):779–783. doi: 10.1080/02701367.2011.10599814. [DOI] [PubMed] [Google Scholar]
- 37.Pate RR, Baranowski T, Dowda M, Trost SG. Tracking of physical activity in young children. Med Sci Sports Exerc. 1996;28(1):92–96. doi: 10.1097/00005768-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Pate RR, Pfeiffer KA, Trost SG, Ziegler P, Dowda M. Physical activity among children attending preschools. Pediatrics. 2004;114(5):1258–1263. doi: 10.1542/peds.2003-1088-L. [DOI] [PubMed] [Google Scholar]
- 39.Raudsepp L, Neissaar I, Kull M. Longitudinal stability of sedentary behaviors and physical activity during early adolescence. Pediatr Exerc Sci. 2008;20(3):251–262. doi: 10.1123/pes.20.3.251. [DOI] [PubMed] [Google Scholar]
- 40.Raustorp A, Svenson K, Perlinger T. Tracking of pedometer-determined physical activity: a 5-year follow-up study of adolescents in Sweden. Pediatr Exerc Sci. 2007;19(2):228–238. doi: 10.1123/pes.19.2.228. [DOI] [PubMed] [Google Scholar]
- 41.Sallis JF, Berry CC, Broyles SL, McKenzie TL, Nader PR. Variability and tracking of physical activity over 2 yr in young children. Med Sci Sports Exerc. 1995;27(7):1042–1049. doi: 10.1249/00005768-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Singer MR, Moore LL, Garrahie EJ, Ellison RC. The tracking of nutrient intake in young children: the Framingham Children's Study. Am J Public Health. 1995;85(12):1673–1677. doi: 10.2105/ajph.85.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor RW, Murdoch L, Carter P, Gerrard DF, Williams SM, Taylor BJ. Longitudinal study of physical activity and inactivity in preschoolers: the FLAME study. Med Sci Sports Exerc. 2009;41(1):96–102. doi: 10.1249/MSS.0b013e3181849d81. [DOI] [PubMed] [Google Scholar]
- 44.Telama R. Tracking of physical activity from childhood to adulthood: a review. Obes Facts. 2009;2(3):187–195. doi: 10.1159/000222244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Telama R, Leskinen E, Yang X. Stability of habitual physical activity and sport participation: a longitudinal tracking study. Scand J Med Sci Sports. 1996;6(6):371–378. doi: 10.1111/j.1600-0838.1996.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 46.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 47.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 Suppl):S531–S543. doi: 10.1249/01.mss.0000185657.86065.98. [DOI] [PubMed] [Google Scholar]
- 48.United States Dept. of Health and Human Services. 2008 physical activity guidelines for Americans : be active, healthy, and happy! Washington, DC: U.S. Dept. of Health and Human Services; 2008. [Google Scholar]
- 49.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 9S-31S. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto S, Becker S, Fischer J, De Bock F. Sex differences in the variables associated with objectively measured moderate-to-vigorous physical activity in preschoolers. Prev Med. 2011;52(2):126–129. doi: 10.1016/j.ypmed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Yang X, Telama R, Viikari J, Raitakari OT. Risk of obesity in relation to physical activity tracking from youth to adulthood. Med Sci Sports Exerc. 2006;38(5):919–925. doi: 10.1249/01.mss.0000218121.19703.f7. [DOI] [PubMed] [Google Scholar]