ABSTRACT
BACKGROUND:
Increasing evidence supports the hypothesis that chronic and persistent inflammation contributes to cancer development. However, the molecular mechanisms that lead to cancer in chronic inflammation and the role of angiogenesis in inflammation-associated cancer remain poorly understood.
METHODS:
Ninety patients were enrolled: 30 cases of CHC without cirrhosis, 28 cases of CHC with liver cirrhosis, and 32 cases of HCC and hepatitis C virus infection. Ten wedge liver biopsies, taken during laparoscopic cholecystectomy, served as normal controls. Serum TNF-α levels were measured using the ELISA technique; in situ hybridization and immunohistochemical studies were used to detect hepatic levels of messenger RNA (mRNA) transcripts and mature protein, respectively, for both TNF-α and VEGF.
RESULTS:
The highest hepatic expression of TNF-α was noticed in liver cirrhosis specimens compared to noncirrhotic CHC and HCC. Hepatic expression of VEGF and serum level of TNF-α revealed significant increases in the progression of the disease. Moreover, cases with higher grades of inflammation or stages of fibrosis showed significant increases in serum TNF-α and expression of TNF-α and VEGF. Expression of mRNA of both TNF-α and VEGF shows increasing expression with positive correlation to progression of viral hepatitis to cirrhosis with more positivity in cases developed HCC.
CONCLUSIONS:
VEGF signaling could be one of the molecular signaling pathways involved in TNF-α induced angiogenesis which might pose an important link between inflammation and fibrosis in CHC and HCC development and progression. Moreover, serum inflammatory biomarkers can be used to monitor the disease progression.
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related deaths worldwide.1 It is an inflammation-related cancer and representsa paradigm of the relation between tumor microenvironment and tumor development.2 An unresolved inflammation due to any failure in the precise control of the immune response can continue to perturb the cellular microenvironment, thereby leading to alterations incancer-related genes and posttranslational modification in crucial cellular proteins.3 Data indicate that leukocyte infiltration can promote tumor phenotypes, such as angiogenesis, growth, and invasion. These inflammatory cells may influence cancer promotion by secreting the cytokines, growth factors, chemokines, and proteases that stimulate the proliferation and invasiveness of cancer cells.4 Among the proinflammatory gene products involved in such interactions is tumor necrosis factor (TNF)-α. Induced by a wide range of pathogenic stimuli, TNF-α plays a crucial role in the initiation and amplification of inflammatory reactions, with actions directed toward both tissue destruction and recovery from damage. Hence, when dysregulated and secreted in the circulation, TNF-α can mediate a wide variety of diseases, including cancer.5
Angiogenesis is also a complex multistep process of growth and remodeling involving degradation of the extracellular matrix, cell migration and proliferation, and tube formation.6 Under normal conditions, this process must have a balance between pro- and antiangiogenic factors. Angiogenesis also involves the activation of many receptors by their cognate ligands. Vascular endothelial growth factor (VEGF) is one of the ligands that are known to play the most central role in angiogenesis.7 Given that angiogenesis is essential for tumor growth and metastasis, controlling tumor-associated angiogenesis is a promising tactic in limiting cancer progression.8
The molecular mechanisms that lead to cancer in the setting of chronic inflammation and the role of angiogenesis in inflammation-associated cancer remain poorly understood. In this study, we measured levels of messenger (m)RNA transcripts and mature protein for TNF-α and VEGF, to assess the implication that TNF-α-induced angiogenesis provides a molecular link between inflammation and the development of HCC in patients with chronic hepatitis C (CHC). In addition, we evaluated the possible use of the serum level of circulating TNF-α as an early predictor of HCC development and tumor progression.
MATERIALS AND METHODS
Patients
Ninety patients with CHC (54 men and 36 women; age range, 24–66 years; mean age, 48.32 ± 7.65), who were admitted to the Department of Tropical Medicine, Kasr El Aini Hospital, were enrolled in the study. They included 30 patients with CHC without liver cirrhosis (LC), 28 with LC who had undergone liver biopsy, and 32 from whom tumor specimens were taken by endoscopy and surgical specimens obtained during partial hepatectomies performed at the hospital. Patients presenting with schistosomiasis, other chronic viral diseases, nonalcoholic steatohepatitis, biliary disorders, or other malignancies were excluded. Ten control wedge liver biopsies were taken from age- and sex-matched individuals who underwent laparoscopic cholecystectomy (6 men and 4 women; age range, 37–48 years; mean age, 42.21 ± 4.54). Written, informed consent was obtained from all participants, and the study was approved by the local ethics committee.
Laboratory Investigations
A complete, automated hemogram was performed (ACT Differential; Beckman-Coulter, Roissy, France). Liver function tests were performed with commercially available kits (Abbott Laboratories, North Chicago, IL). Circulating anti-hepatitis C virus (HCV) antibodies were detected by ELISA (Murex anti-HCV, Version V; Murex Diagnostics, Dartford, UK). HCV RNA was detected in patients' sera by real-time polymerase chain reaction (Amplicor test; Roche Diagnostic Systems, Meylan, France).
Measurements of Serum TNF-α Levels
Two milliliters of blood were withdrawn from each patient into plain tubes and centrifuged shortly after clots formed. All samples were stored at −70°C in aliquots and used for analysis of measurement of serum TNF-α with the Quantikine Mouse TNF-α Immunoassay (R&D Systems, Minneapolis, MN), according to the manufacturer's recommended protocol.
Histopathologic Study
Four-micrometer-thick sections were cut from formalin-fixed, paraffin-embedded tissue blocks of core liver specimens and stained with hematoxylin and eosin and Masson trichrome stains for proper evaluation of tissue fibrosis according to the METAVIR9 histology scoring system. Two separate scores were used, one for the necroinflammatory activity (A) grade: A1, minimal, A2, moderate; and A3, severe. The other was for the stage of fibrosis (F) on a scale of F0 to F4. A score of F1 to F2 signified significant fibrosis, and a score of F3 and F4 signified advanced fibrosis.
Immunohistochemistry
Immunohistochemistry (IHC) was performed by using an avidin-biotin complex (ABC) immunoperoxidase technique,10 with antihuman primary antibodies against TNF-α and VEGF (Santa Cruz Biotechnology Inc., Santa Cruz, CA) diluted at 1:100 and 1:150, respectively, in phosphate-buffered saline (PBS). We used a streptavidin-biotin-peroxidase preformed complex and peroxidase-DAB (3,3′-diaminobenzidine) (Dako, Glostrup, Denmark), according to the manufacturer's instructions. The cell nuclei were counterstained with Mayer's hematoxylin and mounted with DPX medium. Positive and negative control slides for each marker were included in each session. As a negative control, a liver tissue section was processed as described, but with the primary antibody omitted.
TNF-α Expression
TNF-α in liver tissue was scored by a semiquantitative technique, relating the score of 0 to 4 points to the fraction of stained cells: 0, 0% positive cells; 1, less than 5% positive; 2, 5–20% positive; 3, 20–40% positive; and 4, more than 40% positive.11 Liver sections were examined by light microscopy (Zeiss, Jena Germany) at ×400 power for both markers. The number of positively stained cells with the highest expression was counted in 10 microscopic fields per section, and the results expressed as the mean of the 10 fields. Unstained sections were scored as 0%.
VEGF Expression
All brown-stained endothelial cells or endothelial cell clusters that were clearly separate from the connective tissue elements were considered microvessels. Cell clusters were counted as one microvessel. Stained sections were observed at ×100 magnification, and the areas with the highest number of positive cells were identified. Counts were performed in 10 regions at ×400 magnification. The results for VEGF were classified as follows: <5%, negative for VEGF; 5–25%, weakly positive; 26–50%, mildly positive; 51–75%, moderately positive; and >75%, intensely positive.12
In Situ Hybridization for mRNA VEGF and mRNA TNF-α
Paraffin-embedded sections were deparaffinized by incubation in xylene followed by dehydration and washing in diethylpyrocarbonate-treated water (DEPC-H2O) for 1 minute. The sections were fixed in 4% paraformaldehyde (PFA) in PBS (pH 7.4) for 20 minutes, washed in 3× PBS for 5 minutes, and rehydrated. They were then treated with 0.2 M HCl at room temperature (RT) for 20 minutes and washed in DEPC-H2O for 5 minutes. For proteolysis, the sections were incubated with Pronase (0.125 mg Pronase in 100 mL PBS; Sigma-Aldrich, Saint-Quentin Fallavier, France) at RT for 10 minutes, and the reaction was stopped with 0.1 M glycine DEPC-H2O. The sections were washed twice in 1× PBS for 30 seconds, fixed again in PFA, and washed in 1× PBS for 3 minutes. Acetylation was performed in 0.25% to 0.5% acetic anhydride in 0.1 M triethanolamine for 10 minutes, after which the sections were washed in 1× PBS and air dried. The sections were treated for 2 hours at 42°C with prehybridization mixture containing 10 mM dithiothreitol (DTT; Sigma-Aldrich), 10 mM Tris-HCl, 5 mM EDTA, 0.3 M NaCl, 0.02% (w/v) NaCl, 0.75 mg/mL yeast tRNA, 50% deionized formamide, 12.5% (w/v) dextran sulfate, 1.5% (w/v) salmon sperm DNA, Ficoll, 0.02% (w/v) polyvinylpyrrolidone, 0.02 mg/mL bovine serum albumin (BSA; Sigma-Aldrich), and 6.5% (v/v) DEPC-H2O.
Approximately 1 μg VEGF-digoxigenin in 30 μL hybridization solution and 1 μg TNF-α digoxigenin in 30 μL hybridization solution (the same as the prehybridization solution, but without DEPC-H2O) were first denatured by heating at 95°C for 10 minutes and placed on ice for 5 minutes. ISH was performed at 42°C overnight. A human VEGF-cDNA probe and TNF-α-cDNA was kindly provided by Bruno Voss (Professional Associations' Research Institute for Occupational Medicine BGFA, Ruhr-University, Bochum, Germany).
After hybridization, the slides were washed twice for 20 minutes each in formamide solution (50% deionized formamide, 1% 2-mercaptoethanol, 10% 20× standard saline citrate [SSC; 3 M NaCl, 3 mM Na citrate], and 39% distilled water [DW]) at 47°C; 20 minutes in 2× SSC at 58°C; 3 minutes in 1× PBS at RT; 30 minutes in 0.5% H2O2 in PBS at RT, and 5 minutes in 1× PBS at RT. The slides were blocked by a 30-minute incubation in blocking buffer (1% BSA and 0.2% skimmed milk in PBS). Antidigoxigenin horseradish peroxidase Fab fragment (1:100 [v/v]; Boehringer Mannheim, Mannheim, Germany) in blocking buffer was applied for 30 minutes at RT. The slides were washed 3 times for 5 minutes in PBS at RT. The reaction signal was amplified by the use of a tyramide signal amplification (TSA) kit (Invitrogen, Grand Island, NY) in which TSA fluorescein 1:60 was applied in the dark for 5 minutes at RT and washed 3 times for 5 minutes with PBS in the dark. Counterstaining was performed with propidium iodide (1:10,000) in PBS, and the slides were washed for 10 minutes with PBS. The sections were rehydrated and kept moist with glycerol.
Scoring for VEGF mRNA and TNF-α mRNA was as follows: +, normal or weak expression; ++, moderate expression; and +++, overexpression.
Statistical Analysis
SPSS, version 12, was used to evaluate the data. The results are expressed as the mean ± SD or the number with the percentage of the total. Comparisons between the mean of different parameters in the different groups were made by 1-way analysis of variance (ANOVA) with the post hoc least significant difference test. The correlation between parameters was performed with Spearman's rank correlation coefficient (r). P < .05 was considered significant and P < .01 highly significant.
RESULTS
TNF-α protein was observed in the cytoplasm of hepatocytes and inflammatory cells. The pattern of VEGF immunoreactivity in hepatocytes and endothelial cells lining blood vessels was diffuse. In the current study, normal liver specimens showed no detectable TNF-α protein expression and faint VEGF protein expression. Both antigens were significantly higher in the diseased groups (P < .01) than in the control specimens. The highest expression of TNF-α was noticed in LC specimens compared with that in the noncirrhotic CHC and HCC sections (P < .01). The expression of VEGF increased significantly with the progression of the disease (P < .01). The serum level of TNF-α showed a significant increase in all the diseased groups compared with the control (P < .01). A significant increase was also observed with progression of the disease (P < .01) (Tables 1, 2, and 3 and Figures 1 and 2).
Table 1.
Protein expression in all groups
| Protein/location | Controls (n = 10) | CHC without cirrhosis (n = 30) | Cirrhosis (n = 28) | HCC (n = 32) |
|---|---|---|---|---|
| Tissue TNF-α | 0.0 ± 0.0 | 24.8 ± 14.8* | 51.28 ± 15.99*† | 36.93 ± 18.83*‡§ |
| Tissue VEGF | 1.3 ± 0.5 | 14.03 ± 5.46* | 25.42 ± 13.48*† | 66.56 ± 11.7*†§ |
| Serum TNF-α (pg/mL) | 10.2 ± 2.65 | 34.5 ± 15.33* | 61.67 ± 27.37*† | 108.28 ± 43.37*†§ |
Tissue data are the mean percentage ± SD of positive-staining cells in 10 successive microscopic fields (×400) per tissue section.
P < .01 vs. control group.
P < .01.
P < .05, vs. CHC without cirrhosis group.
P < .01, vs. cirrhosis group.
Table 2.
Expression of TNF-α in liver tissue
| Histopathologic diagnosis (n) | Positive cases |
Range |
Intensity (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | 5–20% | 20–40% | >40% | + | ++ | +++ | |
| Control (n = 10) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CHC without cirrhosis (n = 30) | 12 | 40* | 0 | 10 | 2 | 12 | 0 | 0 |
| CHC with cirrhosis (n = 28) | 23 | 82.1*† | 0 | 9 | 14 | 7 | 8 | 8 |
| HCC (n = 32) | 22 | 68.7*†‡ | 0 | 13 | 9 | 0 | 10 | 12 |
P < .01, vs. control group.
P < .01, vs. CHC without cirrhosis.
P < .05, vs. CHC with cirrhosis.
Table 3.
Expression of VEGF in liver tissue
| Histopathologic diagnosis (n) | Positive cases |
Range |
Intensity (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | 5–25% | 26–50% | 51–75% | >75% | + | ++ | +++ | |
| Control (n = 10) | 2 | 20 | 2 | — | — | — | 2 | — | — |
| CHC without cirrhosis (n = 30) | 10 | 33.3* | 3 | 4 | 3 | — | 3 | 7 | — |
| CHC with cirrhosis (n = 28) | 16 | 55.5*† | — | 9 | 4 | 3 | 10 | 6 | — |
| HCC (n = 32) | 24 | 75*†‡ | 2 | 10 | 7 | 5 | — | 15 | 9 |
P < .01 vs. control.
P < .05 vs. CHC without cirrhosis.
P < .05 vs. CHC with cirrhosis.
Figure 1.
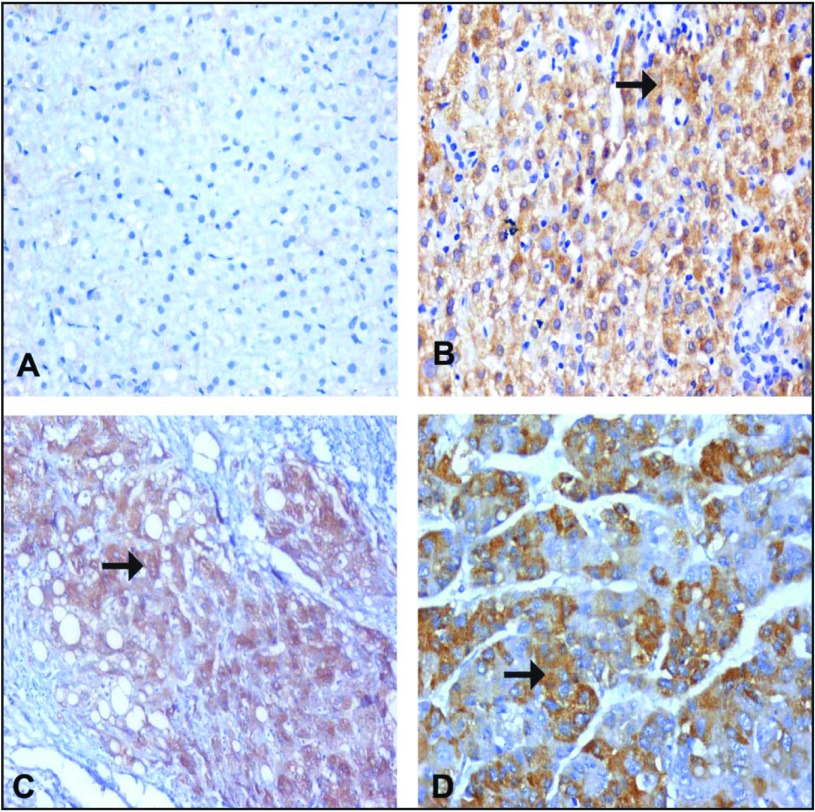
Immune staining for TNF-α. (A) Normal hepatocytes from a control patient, showing negative expression of TNF-α in the cytoplasm of the hepatocytes. (B) CHC specimen without cirrhosis (A1F1) showing moderate expression of TNF-α in the cytoplasm of a hepatocyte (arrow). (C) A CHC specimen with cirrhosis (A2F3), showing a cirrhotic nodule with moderate to marked expression of TNF-α in the cytoplasm of a hepatocyte (arrow). (D) A case of moderately differentiated HCC, showing moderately expressed TNF-α in the cytoplasm of a hepatocyte (arrow). (A–D) IHC: DAB, ×200.
Figure 2.
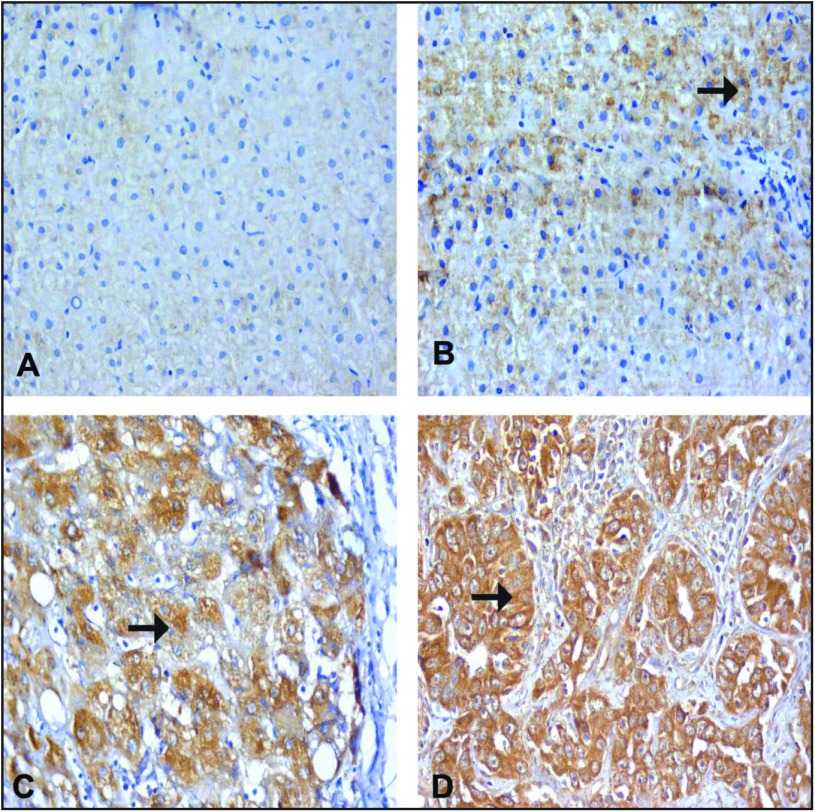
Immune staining for VEGF. (A) Normal hepatocyte from a control case, showing faint expression of VEGF as a cytoplasmic stain in the hepatocytes. (B) A case of CHC without cirrhosis (A1F1), showing mild expression of VEGF in the cytoplasm of a hepatocyte (arrow). (C) A case of CHC with cirrhosis (A2F3), showing a cirrhotic nodule with moderate expression of VEGF in the cytoplasm of a hepatocyte (arrow). (D) A case of moderately differentiated HCC, showing marked expressed VEGF in the cytoplasm (arrow) of a hepatocyte. (A–D) IHC: DAB, ×200.
Patients with CHC with higher grades of inflammation or stages of fibrosis showed significant increases in the serum level of TNF-α and expression of both TNF-α and VEGF, than did those with lower scores (Tables 4 and 5).
Table 4.
Inflammatory activity scores according to the METAVIR system in the CHC with or without cirrhosis groups combined
| Protein/location | A1 (n = 25) | A2 (n = 23) | A3 (n = 10) |
|---|---|---|---|
| Tissue TNF-α | 18.42 ± 15.91 | 32.84 ± 20.11* | 59.91 ± 17.41*† |
| Tissue VEGF | 10.39 ± 4.0 | 18.46 ± 2.14 | 28.66 ± 3.89*† |
| Serum TNF-α (pg/mL) | 15.84 ± 3.31 | 38.3 ± 6.58* | 73.58 ± 8.32*† |
n = 58. Tissue data are the mean percentage ± SD of positive-staining cells in 10 successive microscopic fields (×400) per liver tissue section.
P < .05, vs. A1 group.
P < .05, vs. A2 group.
Table 5.
Fibrosis scores according to the METAVIR system in the CHC with or without cirrhosis groups combined
| Protein/location | F1 (n = 18) | F2 (n = 12) | F3 (n = 20) | F4 (n = 8) |
|---|---|---|---|---|
| Tissue TNF-α | 14.33 ± 7.03 | 24.96 ± 14.45* | 30.28 ± 5.16* | 50.28 ± 15.99*†‡ |
| Tissue VEGF | 13.47 ± 9.43 | 16.66 ± 5.77 | 27.42 ± 3.48*† | 48.42 ± 6.06*†‡ |
| Serum TNF-α (pg/mL) | 12.55 ± 3.12 | 24.0 ± 5.29* | 61.67 ± 2.37*† | 82.67 ± 7.10*†‡ |
n = 58. Tissue data are the mean percentage ± SD of positive-staining cells in 10 successive microscopic fields (×400) per liver tissue section.
P < .01, vs. F1 group.
P < .01 vs. F2 group.
P < .05 vs. F3 group.
The expression of TNF-α mRNA was higher in all diseased specimens than in the controls (P < .01). A significant increase (P < .05) was also noticed in the LC and HCC groups compared with the noncirrhotic CHC group. Moreover, the expression of VEGF mRNA increased and showed a positive correlation with progression from viral hepatitis to cirrhosis, with more patients developing HCC (Table 6 and 7 and Figures 3 and 4).
Table 6.
Expression of TNF-α mRNA in liver tissue
| Histopathologic diagnosis (n) | Positive cases |
Range |
Intensity% |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | 5–20% | 20–40% | >40% | + | ++ | +++ | |
| Control (n = 10) | 0 | 0 | — | — | — | — | — | — |
| CHC without cirrhosis (n = 30) | 15 | 50* | 2 | 13 | — | 10 | 5 | — |
| CHC with cirrhosis (n = 28) | 23 | 82.1*† | 0 | 10 | 13 | 5 | 10 | 8 |
| HCC (n = 32) | 26 | 81.3*† | 0 | 13 | 13 | 0 | 16 | 10 |
P < .01 vs. control.
P < .05 vs. CHC without cirrhosis.
Table 7.
Expression of VEGF mRNA in liver tissue
| Histopathologic diagnosis (n) | Positive cases |
Range |
Intensity% |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | 5-25% | 26-50% | 51-75% | >75% | + | ++ | +++ | |
| Control (n = 10) | 3 | 30 | 3 | 0 | 0 | 0 | 3 | — | — |
| CHC without cirrhosis (n = 30) | 12 | 40 | 7 | 2 | 2 | 1 | 8 | 4 | — |
| CHC with cirrhosis (n = 28) | 18 | 64.2*† | 6 | 2 | 6 | 4 | 2 | 10 | 6 |
| HCC (n = 32) | 26 | 81.2*†‡ | 0 | 9 | 7 | 10 | 2 | 14 | 10 |
P < .01 vs. control.
P < .01 vs. CHC without cirrhosis.
P < .05 vs. CHC with cirrhosis.
Figure 3.
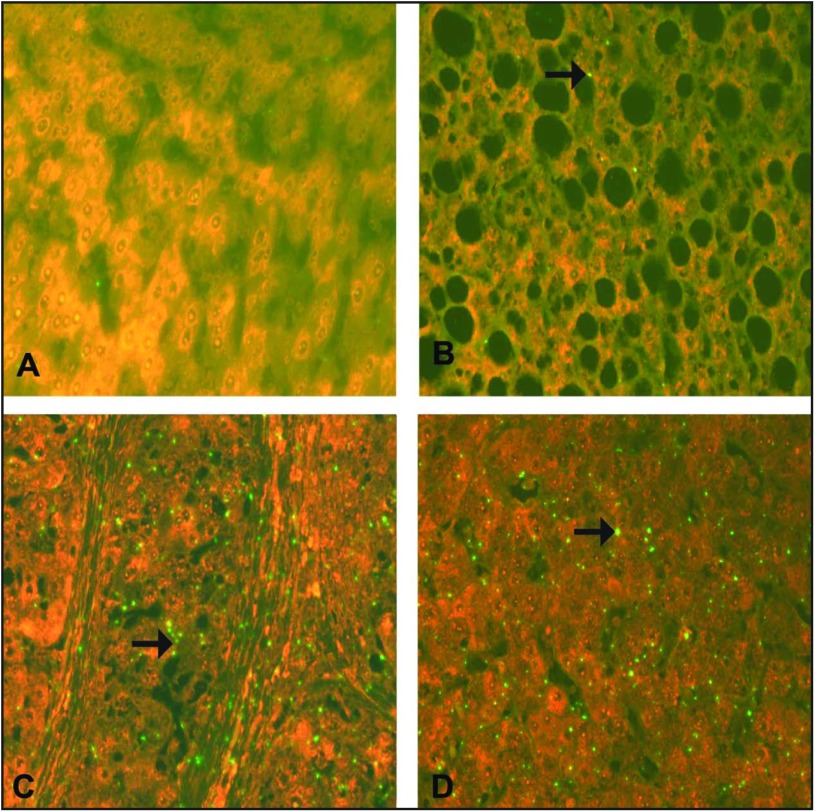
ISH staining for TNF-α mRNA. (A) Normal hepatocytes from a control case, showing negative expression of TNF-α mRNA. (B) A case of CHC without cirrhosis (A1F1), showing mild (+) green signal for TNF-α mRNA in a hepatocyte (arrow). (C) A case of CHC with cirrhosis (A2F3) showing a cirrhotic nodule with moderate (++) green signal for TNF-α mRNA in a hepatocyte (arrow). (D) A case of moderately differentiated HCC, showing intense (+++) green signal for TNF-α mRNA in a hepatocyte (arrow). (A–D) ISH; ×625.
Figure 4.
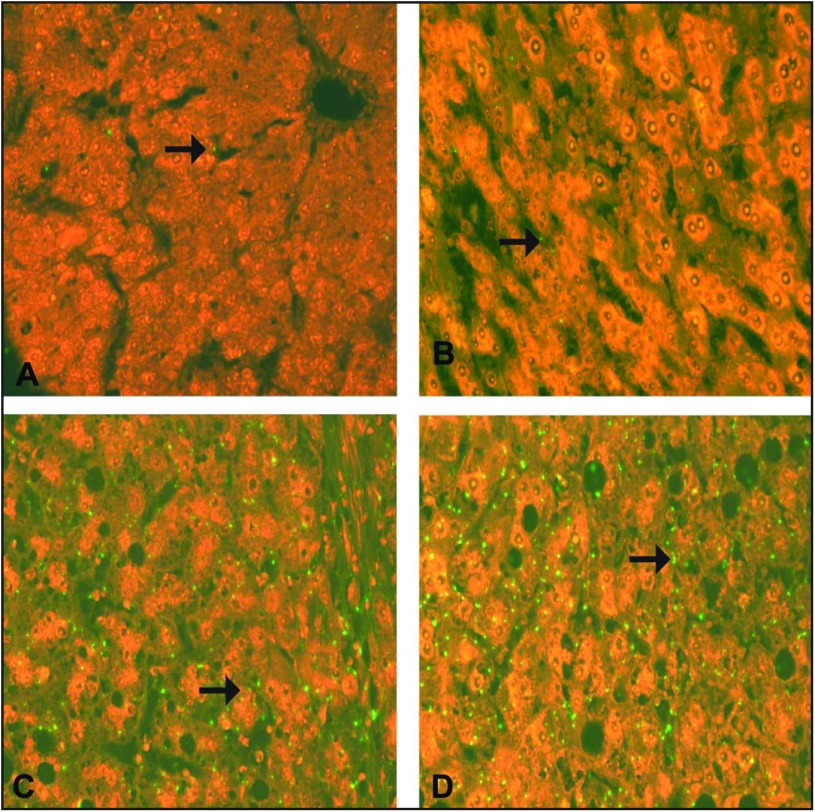
ISH staining for VEGF mRNA. (A) Normal hepatocyte (arrow) from a control case, showing faint expression of VEGF mRNA. (B) A case of CHC without cirrhosis (A1F1), showing mild (+) green signal for VEGF mRNA in a hepatocyte (arrow). (C) A case of CHC with cirrhosis (A2F3) with a cirrhotic nodule showing moderate (++) green signal for VEGF mRNA in a hepatocyte (arrow). (D) A case of moderately differentiated HCC, showing intense (+++) green signal for VEGF mRNA in a hepatocyte (arrow). (A–D) ISH; ×625.
A correlation analysis of the TNF-α and VEGF expression and their mRNA levels in tissue and serum is presented in Table 8.
Table 8.
Correlation analysis
| r | P | |
|---|---|---|
| Tissue VEGF vs. tissue TNF-α | 0.633 | >.01 |
| Tissue VEGF vs. tissue TNF-α mRNA | 0.251 | >.05 |
| Serum TNF-α vs. tissue TNF-α mRNA | 0.582 | >.01 |
DISCUSSION
Angiogenesis is an integral part of tumor progression. It also plays a major role in chronic inflammation. Accumulation of inflammatory infiltrate and development of fibrosis increases resistance of the tissue to blood flow and the delivery of oxygen.13 Genetic changes and local hypoxia in tumors cause increased expression of several proteins and secretion of soluble angiogenic factors that activate a complex interplay among the different cells.14
TNF-α is mainly produced by macrophages in inflammatory tissues and has been implicated in angiogenesis during inflammation, wound repair, and growth of tumors.4 The results of this study showed no detectable TNF-α protein or TNF-α mRNA expression in normal liver specimens. In the CHC and LC specimens, elevated expression of TNF-α and its transcripts was observed. This increased expression was mainly found in hepatocytes and in cells of liver sinusoids (macrophages and endothelial cells). Our data are consistent with the results of studies that showed increased TNF-α production in chronic liver disease.15,16 TNF-α is an integral part of inflammation in CHC infection.17 Proinflammatory TNF-α is produced in response to tissue injury by an overwhelming number of infiltrating TNF-α-secreting monocytes and is associated with an increase in cell cycle progression and oxidative stress through the formation of 8-oxo-deoxyguanosine, an established marker of the DNA damage associated with chronic hepatitis in the human liver.18 In addition, HCV induces TNF-α expression in the human liver and human hepatoma cell lines.19 Our results revealed significant increases in the expression of TNF-α and its transcripts in patients with higher stages of fibrosis and in LC specimens than in CHC specimens. In liver fibrosis, one of the first events is the activation of resident innate inflammatory cells and the recruitment of additional inflammatory monocytes/macrophages and the liver-resident Kupffer cells.20 In accordance with our results, Wang et al21 noticed an increased number of TNF-α positive cells in liver tissues from patients with LC compared with those with CHC and suggested that TNF-α is related to and may promote liver fibrosis. The data also showed a significant increase in the expression of TNF-α and TNF-α mRNA in HCC specimens compared with control and CHC specimens. ISH studies revealed no significant difference in TNF-α mRNA between the HCC and LC specimens. Although a higher proportion of patients with LC had cells that expressed TNF-α than did those with HCC, the intensity of TNF-α and its transcript was higher in HCC specimens. There is increasing evidence that the inflammatory process is inherently associated with many different cancer types, including HCC.22 A high level of proinflammatory TNF-α has been associated with carcinogenesis and was detected in HCC patients, especially those with recurrence.23 In addition, TNF-α levels were found to be lower in HCC tumor tissue vs. the cirrhotic tissue surrounding the tumor.24
The serum TNF-α level was significantly higher in all the patient groups than in the healthy volunteers. Moreover, a highly significant increase in serum TNF-α was noticed with disease progression and HCC development and correlated well with its liver IHC pattern, suggesting that peripheral levels could be used as surrogate markers of local TNF expression in patients with CHC. Our results are in accordance with findings in other studies25,26 that showed similar correlations in patients with CHC. Serum TNF-α was found to be positively associated with both inflammation and fibrosis in liver biopsies of CHC, with and without cirrhosis. Even patients with mild liver inflammation had elevated serum TNF-α levels, suggesting that this cytokine could be used as a sensitive predictor of liver inflammation.
IHC and ISH studies revealed that tumor and nontumor sections were more positive for VEGF protein and VEGF mRNA than was histologically normal liver samples. In accordance with those in other studies,27,28 our results also demonstrated that this increased positivity matched the development of LC. Moreover, the highest levels of both VEGF mRNA and protein expression were mostly encountered among HCC patients, as was found in another study,29 and correlated strongly with the degree of vascularization.30 VEGF is a key mediator of angiogenesis in various disorders.31 Increased expression of VEGF by hepatocytes in the cirrhotic liver is accompanied by active angiogenesis.32,33 Our results were in accordance with those of Amarapurkar et al,13 who found that VEGF expression was significantly higher in stage 3 and 4 fibrosis than in stages 1 and 2. One of the sources of VEGF in the fibromuscular stroma may be fibroblasts, since the ability of these cells to produce VEGF has been demonstrated.34 Increased vascularization of the cirrhotic stroma may be a part of the formation of liver fibrosis and granulation tissue or a compensatory response to the decreased blood supply and hypoxia in the cirrhotic nodules.30 Hypoxia also stimulates angiogenesis to support tumor growth in HCC.35 Results of the present study revealed significant VEGF expression in cases of higher grades of inflammation, in accordance with a study suggesting that capillarization and phenotypic changes in hepatic sinusoids occur with inflammation and liver fibrosis.36 Moreover, a strong correlation between TNF-α and VEGF at both the protein expression and the mRNA levels was detected in all the diseased groups. The data suggest increased expression of VEGF in response to the proinflammatory cytokine TNF-α and that TNF-α may mediate its angiogenic effect by upregulating VEGF. A well-known association between inflammation and tumor development has been found in HBV, HCV, alcoholic LC, and hepatocarcinoma.37 VEGF expression in cirrhotic liver is modulated by inflammatory cytokines released from infiltrating inflammatory cells.38,39 TNF-α has even been reported to mediate macrophage-induced angiogenesis.40 TNF-α promotes angiogenesis through its ability to synergize VEGF-induced vessel permeability, a prerequisite initial event for plasma exudation and fibrin clot formation, a matrix permissive of angiogenesis.41 TNF-α is also capable of inducing gene expression of the proangiogenic molecules VEGF and its receptors (VEGFRs).42,43 Indeed, the cellular VEGF mRNA level is potently enhanced in response to TNF-α, probably because of the transcriptional activation mediated by the transcription factor SP-1,44 leading to induction of a paracrine loop for neovascularization under pathologic conditions, including cancer.45
In view of all the preceding data, we can sum up that VEGF signaling could be one of the molecular signaling pathways involved in TNF-induced angiogenesis that may be a crucial link between inflammation and fibrosis in CHC and HCC development and progression. Thus, inflammatory biomarkers can be used to monitor disease progression. Moreover, both inflammatory and angiogenic responses in tumor stroma could be targets for development of anticancer therapeutic drugs and the use of anti-inflammatory drugs as an adjuvant to other therapies, such as antiangiogenic or cytotoxic agents, and may provide efficacious therapeutic regimens for the treatment of HCC.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, et al. : Global cancer statistics. CA Cancer J Clin 61:69–90, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Capece D, Fischietti M, Verzella D, et al. : The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int 2013:187–204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eiró N, Vizoso FJ: Inflammation and cancer. World J Gastrointest Surg 4:62–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mantovani A, Schioppa T, Porta C, et al. : Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25:315–322, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Aggarwal BB: Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3:745–756, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P: Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–395, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Ono M: Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 99:1501–1506, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dudek Az, Gupta K, Ramakrishnan S, et al. : Tumor angiogenesis. J Oncol 2010:1–2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The French METAVIR Cooperative Study Group: Intraobserver and interobserver variation in liver biopsy interpretation in patients with chronic hepatitis C. Pathology 20:15–20, 1994 [PubMed] [Google Scholar]
- 10. Hsu SM, Raine L, Fanger H: Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580, 1981 [DOI] [PubMed] [Google Scholar]
- 11. Kasprzak A, Zabel M, Biczysko W, et al. : Expression of cytokines (TNF-α, IL-1α, and IL-2) in chronic hepatitis C: comparative hybridocytochemical and immunocytochemical study in children and adult patients. J Histochem Cytochem 52:29–38, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Zhu H, Chen X, Zhang W, et al. Expression and significance of new inhibitor of apoptosis protein surviving in hepatocellular carcinoma. World J Gastroenterol 11:3855–3859, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amarapurkar AD, Amarapurkar DN, Vibhav S, et al. : Angiogenesis in chronic liver disease. Ann Hepatol 6:170–173, 2007 [PubMed] [Google Scholar]
- 14. Medina J, Arroyo A, Sanchez-Madrid F, et al. : Angiogenesis in chronic inflammatory liver disease. Hepatology 39:1185–1195, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Larrea E, Garcia N, Qian C, et al. : Tumor necrosis factor alpha gene expression and the response to interferon in chronic hepatitis C. Hepatology 23:210–217, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCaughan GW, Gorrell MD, Bishop GA, et al. : Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev 174:172–191, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Knobler H, Schattner A: TNF-a, chronic hepatitis C and diabetes: a novel triad. Q J Med 98:1–6, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Wheelhouse NM, Chan YS, Gillies SE, et al. : TNF-α induced DNA damage in primary murine hepatocytes. Int J Mol Med 12:889–894, [PubMed] [Google Scholar]
- 19. Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L, et al. : Induction of tumor necrosis factor α production by human hepatocytes in chronic viral hepatitis. J Exp Med 179:841–848, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heymann F, Hammerich L, Storch D, et al. : Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology 55;898–909, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang X, Chen YX, Xu CF, et al. : Relationship between tumor necrosis factor-alpha and liver fibrosis. World J Gastroenterol. 4:18, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Budhu A, Wang XW: The role of cytokines in hepatocellular carcinoma. J Leukoc Biol 80:1197–1213, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Bortolami M, Venturi C, Giacomelli L, et al. : Cytokine, infiltrating macrophage and T cell-mediated response to development of primary and secondary human liver cancer Dig. Liver Dis 34:794–801, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Zekri AR, Ashour MS, Hassan A, et al. : Cytokine profile in Egyptian hepatitis C virus genotype-4 in relation to liver disease progression. World J Gastroenterol 11:6624–6630, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neuman MG, Benhamou JP, Malkiewicz IM, et al. : Cytokines as predictors for sustained response and as markers for immunomodulation in patients with chronic hepatitis C. Clin Biochem 34:173–182, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Neuman M.G, Benhamou JP, Marcellin P, et al. : Cytokine–chemokine and apoptotic signatures in patients with hepatitis C. Transl Res 149:126–136, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Deli G, Jin CH, Mu R, et al. : Immunohistochemical assessment of angiogenesis in hepatocellular carcinoma and surrounding cirrhotic liver tissues. World J Gastroenterol 11:960–963, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iavarone M, Lampertico P, Iannuzzi F, et al. : Increased expression of vascular endothelial growth factor in small hepatocellular carcinoma. J Viral Hepat 14:133–139, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Yamaguchi R, Yano H, Nakashima Y, et al. : Expression and localization of vascular endothelial growth factor receptors in human hepatocellular carcinoma and non-HCC tissues. Oncol Rep 7:725–729, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Brodsky SV, Mendelev N, Melamed M, et al. : Vascular density and VEGF expression in hepatic lesions. J Gastrointestin Liver Dis 16:373–377, 2007 [PubMed] [Google Scholar]
- 31. Carmeliet P: VEGF as a key mediator of angiogenesis in cancer. Oncology 69:4–10, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Corpechot C, Barbu V, Wendum D, et al. : Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 35:1010–1021, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Giatromanolaki A, Kotsiou S, Koukourakis MI, et al. : Angiogenic factor expression in hepatic cirrhosis. Mediators Inflamm 67:1010–187, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beddy D, Watson RW, Fitzpatrick JM, et al. : Increased vascular endothelial growth factor production in fibroblasts isolated from strictures in patients with Crohn's disease. Br J Surg 91:72–77, 2004 [DOI] [PubMed] [Google Scholar]
- 35. von Marschall Z, Cramer T, Hocker M, et al. : Dual mechanism of vascular endothelial growth factor upregulation by hypoxia in human hepatocellular carcinoma. Gut 48:87–96, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park YN, Yang CP, Fernandez GJ, et al. : Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol 22:656–662, 1998; [DOI] [PubMed] [Google Scholar]
- 37. Lu H, Ouyang W, Huang C: Inflammation, a key event in cancer development. Mol Cancer Res 4:656–221, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Feng DY, Shen M, Zheng H, et al. : Relationship between vascular endothelial growth factor expression and microvessel density in hepatocellular carcinoma and their surrounding liver tissue (in Chinese). Hunan Yi Ke Da Xue Xue Bao 25:132–134, 2000 [PubMed] [Google Scholar]
- 39. Jeng KS, Sheen IS, Wang YC, et al. : Prognostic significance of preoperative circulating vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma: a prospective study. World J Gastroenterol 10:643–648, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aggarwal BB, Shishodia S, Sandur SK, et al. : Inflammation and cancer: how hot is the link? Biochem Pharmacol 72:1605–1621, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Lee MJ, Thangada S, Claffey KP, et al. : Vascular endothelial cell adherents junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99:301–312, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Giraudo E, Primo L, Audero E, et al. : Tumor necrosis factor-alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem 273:22128–22135, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Ristimaki A, Narko K, Enholm B, et al. : Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem 273:8413–8418, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Ryuto M, Ono M, Izumi H, et al. : Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells: possible roles of SP-1. J Biol Chem 1996:271:28220–28228, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Guadagni F, Ferroni P, Palmirotta R, et al. : TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo 21;147–161, 2007 [PubMed] [Google Scholar]