Abstract
Recent scientific studies have advanced the notion of chronic inflammation as a major risk factor underlying aging and age-related diseases. In this review, low-grade, unresolved, molecular inflammation is described as an underlying mechanism of aging and age-related diseases, which may serve as a bridge between normal aging and age-related pathological processes. Accumulated data strongly suggest that continuous (chronic) up-regulation of pro-inflammatory mediators (e.g., TNF-α, IL-1β, 6, COX-2, iNOS) are induced during the aging process due to an age-related redox imbalance that activates many pro-inflammatory signaling pathways, including the NF-κB signaling pathway. These pro-inflammatory molecular events are discussed in relation to their role as basic mechanisms underlying aging and age-related diseases. Further, the anti-inflammatory actions of aging-retarding caloric restriction and exercise are reviewed. Thus, the purpose of this review is to describe the molecular roles of age-related physiological functional declines and the accompanying chronic diseases associated with aging. This new view on the role of molecular inflammation as a mechanism of aging and age-related pathogenesis can provide insights into potential interventions that may affect the aging process and reduce age-related diseases, thereby promoting healthy longevity.
Keywords: molecular inflammation, aging, calorie restriction, exercise, cytokines, oxidative stress, inflammatory diseases, age-related diseases, obesity, sarcopenia, dementia, atherosclerosis, cancer, osteoporosis
Introduction
It is now accepted that chronic inflammation is a major underlying condition of many age-related diseases, such as atherosclerosis, arthritis, cancer, diabetes, osteoporosis, dementia, vascular diseases, obesity and metabolic syndrome (Yu and Chung, 2006). However, the involvement of inflammation in the aging process has not been seriously considered. We recently proposed the molecular inflammation hypothesis of aging suggesting that a state of chronic, low-grade inflammation is a possible converging process linking normal aging and the pathogenesis of age-related diseases (Chung et al., 2006). The molecular inflammation hypothesis (Chung et al., 2001; Chung et al., 2002) provides molecular insights into the interactions between age-related physiological changes and the pathogenesis of many age-related diseases during aging, while opening up new avenues for exploring the interrelation between aging and age-related pathological processes (Chung et al., 2006; Yu and Chung, 2006).
The premise of the age-related inflammatory hypothesis is based on two established findings: 1) a dysregulation of the immune system with age, and 2) altered redox status during aging. Both processes lead to increases in a systemic inflammatory status due to the activation of a wide variety of inflammatory mediators through mainly oxidative stress-induced redox imbalance. The age-related redox imbalance is likely caused by the net effect of weakened anti-oxidative defense systems, and incessantly increasing production of reactive species (RS), such as superoxide (O2−), hydroxyl radical (·OH), and hydrogen peroxide (H2O2), reactive nitric oxide (NO), peroxynitrite (ONOO−) and reactive lipid aldehydes. Overproduced and unregulated RS during aging are a major causative factor in the activation of immune systems (Brod, 2000), as exemplified in over-reactive macrophages in the inflammatory process. The salient point of the molecular inflammation hypothesis is that unresolved chronic inflammation during aging may act as the patho-physiologic link that drives normal functional changes to become many of the age-related degenerative diseases (Chung et al., 2002).
In this review, we describe and update recent findings on the role of chronic inflammation in both normal and pathological aging processes. Two aging-intervention paradigms, namely calorie restriction (CR) and physical exercise, are discussed to substantiate the importance of the suppression of inflammatory process to deter age-related chronic diseases. The anti-oxidative action of CR is best known for its ability to maintain redox balance and suppress the activation of various redox-sensitive, pro-inflammatory transcription factors and signaling pathways. Accumulating evidence indicates that anti-oxidative CR significantly attenuates nuclear factor-κB (NF-κB), tumor necrosis factors (TNF-α and TNF-β), interleukins (IL-1β, IL-2, and IL-6), chemokines (IL-8 and RANTES), adhesion molecules (AMs) (Kim et al., 2002; Zou et al., 2004; Chung et al., 2006). Furthermore, CR modulates enzymes, such as inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2), both of which are pro-inflammatory and known to increase with age (Chung et al., 2002). Another well-known anti-aging intervention, physical exercise, has also emerged as an effective anti-inflammatory intervention (Radak et al., 2004; Kalani et al., 2006). Exercise is also known to increase overall anti-oxidant defense systems including SOD, catalase, and GSH peroxidase. Thus, similar to CR, regular exercise enhances the maintenance of the redox status, GSH/GSSG balance, and NF-κB activity, thereby leading to suppression of pro-inflammatory mediators, such as cytokines, chemokines and AMs (Sen, 1995; Seo et al., 2006).
This review begins with a description of two major factors responsible for causing oxidative stress during aging, namely oxidative damage and redox imbalance, and then proceeds to provide details on molecular inflammation and proinflammatory mediators. Chronic inflammation is discussed as the basis of sarcopenia, metabolic syndrome, and obesity and as an intermediary processes bridging normal age-related changes and pathological aging processes. To further describe the involvement of inflammation in chronic diseases, dementia, atherosclerosis, cancer, and osteoporosis are presented. The final section describes CR and exercise as potential anti-inflammatory interventions that can regulate pro-inflammatory cytokines and adhesion molecules.
1. Age-related oxidative stress-induced redox imbalance
Aging is a biological process characterized by time-dependent, progressive, physiological declines accompanied by the increased incidence of age-related diseases. Over the past several decades, a number of theories have been proposed that have attempted to define the causality and the underlying mechanisms of aging (Dice, 1993). Currently widely accepted is the “oxidative stress hypothesis” (Yu and Yang, 1996a), that modified and advanced the free radical theory of aging (Harman, 1956). According to the oxidative stress hypothesis, oxidative damage is not only elicited by the uncontrolled production of reactive oxygen species (ROS) as proposed in the original free radical theory, but also by other oxidants, including reactive nitrogen species (RNS) and reactive lipid species. More importantly, the oxidative stress hypothesis emphasizes the essential role of anti-oxidant defenses as the crucial component of the overall redox balance of the organism, which was not considered in the original free radical theory.
Oxidative stress is reinforced by a number of RS, such as H2O2, ·O2− and singlet oxygen, and other radicals as well as non-radicals, which are formed continuously in the body as a consequence of aerobic metabolism, thereby potentially modifying cellular activity and basic structural components including nucleic acids, proteins, and lipids (Davies and Goldberg, 1987; Barry, 1993; Yu et al., 1996b). Biological sources of RS production vary widely depending on various cellular activities related to lipoxygenase, COX, plasma membrane-associated NADPH oxidase; mitochondrial electron transport system, ubiquinone, NADH dehydrogenase; cytochrome P450, cytochrome b5, microsomal electron transport; flavoproteins and oxidases in peroxisome; and xanthine oxidase (XOD) in cytosol (Bodamyali et al., 2006).
To protect itself against hostile oxidative environments, the organism has developed various antioxidant defenses that include the classical antioxidant enzymes, superoxide dismutase (SOD), glutathione (GSH), and catalase, as well as non-enzymatic ROS scavengers, vitamin E, vitamin C, β-carotene, and uric acid (Lykkesfeldt et al., 1998). Among these, GSH is the most abundant and effective biological anti-oxidative reductant (Cross et al., 1977). Although the levels of GSH and of GSH reductase decrease with age (Cho et al., 2003), CR (Roth et al., 2007) and proper physical exercise (Carter et al., 2007) blunt these age-related decreases.
In addition, another versatile member of the anti-oxidative defense system, thioredoxin (Trx) has been described (Holmgren, 1989). Trx, a small, globular, ubiquitous protein of 12 kDa with two redox-active half-cysteine residues in its catalytic active center, which contains the consensus amino acid sequence –Cys-Gly-Pro-Cys– (Holmgren, 1989). This protein exists either in a reduced form with a dithiol or in an oxidized form, in which the half-cysteine residues form an intramolecular disulfide bridge. Trx participates in redox reactions by the reversible oxidation of its active center dithiol to disulfide and catalyzes dithiol-disulfide exchange reactions (Holmgren, 1989) involving many thiol-dependent cellular processes including the gene expression and signal transduction of pro-inflammatory transcription factors, such as NF-κB, AP-1, and HIF-1 (Sur et al., 2005). Trx forms a physical interaction with Ref-1 in the nucleus, which activates pro-inflammatory transcription factors in a redox-dependent manner (Cho et al., 2003). Recent evidence indicates that age-related increases in redox-sensitive transcription factors, such as NF-κB, AP-1 and HIF-1, are related to increases in cellular oxidative stress and nuclear Trx and Ref-1 (Kim et al., 2002).
Trx and Trx reductase (TrxR) in cytoplasm decrease with age and CR is shown to prevent these decreases. In contrast, the nuclear translocation of redox regulators (Trx and Ref-1) increases with age but is also suppressed by CR (Cho et al., 2003). Therefore, Increased nuclear Trx and Ref-1 during aging may lead to the up-regulation of redox-sensitive transcription factors, such as NF-κB or AP-1, via the interaction of Ref-1 and Trx. Thus, a redox imbalance due to the change in the status of Trx and Ref-1 during aging may play a significant role in the age-related activation of key inflammatory mediators (Cho et al., 2003).
The interaction between oxidative stress and inflammation is closely related to the well-recognized biosynthetic pathway of prostaglandins (PGs) that produces various RS (Kim et al., 2005). PGs are arachidonic acid-derived lipid metabolites have potent pro-inflammatory and potentially pathogenic actions. Certain PG metabolites are active mediators of inflammation in their own right. However, as described below, RS production from PG metabolism exacerbates inflammatory conditions and promotes tissue damage (Baek et al., 1999; Lim et al., 2001). COX, a key enzyme in the PG synthetic pathway, converts arachidonic acid to prostaglandin H2 (PGH2) (Ikai et al., 1989). During the conversion of PGG2 to PGH2 by COX, RS are generated (Bazan et al., 1989). The RS produced by PG synthesis pathway can add significantly to the overall RS pool under both normal and pathological conditions, particularly during aging (Baek et al., 1999).
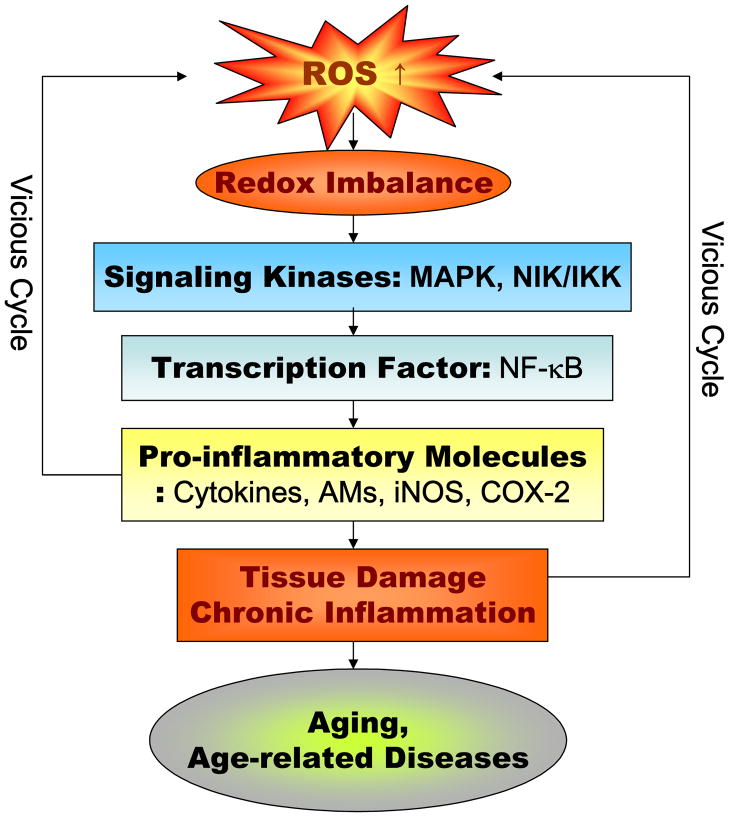
Maintaining a precise redox balance status is as important as the physiological acid-base buffer system of the body for the optimal operation of homeostatic cellular activities. Changes in redox balance are known to have a great impact on the cellular signaling pathways and transcriptional activities as most of their reactions and activation depend on reduction/oxidation processes. This review elaborates on an oxidative stress-induced redox imbalance as the patho-physiological basis of systemic inflammation, proposed as the converging link between normal aging and pathological processes (Fig. 1).
Fig. 1.
Major molecular pro-inflammatory pathways involved in aging and age-related diseases. ROS, reactive oxygen species; MAPK, mitogen-activated protein kinases, NIK, NFκ-B-induced kinase; IKK, IκB kinase; AMs, adhesion molecules; iNOS, inducible NO synthase; COX-2, cyclooxygenase.
2. The molecular activation of inflammation and pro-inflammatory mediators during aging
Recent evidence strongly supports the notion that the molecular inflammatory process plays a central role in the aging process and age-related diseases (Chung et al., 2006). COX-derived ROS generation as well as gene expressions of IL-1β, IL-6, TNF-α, COX-2 and iNOS are enhanced during aging (Kim et al., 2002; Chung et al., 2006). COX activity and the production of TXA2 and PGI2 are also increased during aging. Other pro-inflammatory proteins, such as AMs (VCAM-1, ICAM-1, P-, E-selectin), are all upregulated during the aging process (Zou et al., 2004).
The NF-κB transcription factor can be viewed as the master regulator of the inflammatory process and can be activated by oxidative stimuli. Indeed, the activation of NF-κB-dependent genes is a major culprit responsible for the wide-spread systemic inflammatory process (Makarov, 2000). Under activated conditions, pro-inflammatory genes encode pro-inflammatory proteins, such as cytokines, growth factors AMs or chemokines. As shown Fig. 1, NF-κB is also known to regulate the transcription of pro-inflammatory molecules, such as TNF-α and TNF-β, interleukins (IL-1β, IL-2, and IL-6), chemokines (IL-8 and RANTES), AMs, including ICAM-1, VCAM, E-selectin, and enzymes, including iNOS and COX-2 (Brand et al., 1996; Bohrer et al., 1997).
NF-κB activity is modulated by upstream signaling pathways such as IκB kinase (IKK) and MAPKs. The activated IKK complexes phosphorylate the IκB subunits of NF-κB/IκB to trigger the degradation of IκB, which then leads to the activation of NF-κB (Zandi et al., 1997; Karin, 2006). IKK activity is upregulated during aging by NF-κB (Kim et al., 2002), and there is further involvement of the ERK, JNK, and p38 MAPK pathways that control NF-κB-dependent transcription during the inflammatory response (Fig. 1). Recently, the aging process was shown to strongly enhance all three ERK, JNK, and p38 MAPK activities that paralleled increases in ROS production (Kim et al., 2002).
Under normal conditions, NF-κB activation in response to oxidative stimuli is short-lived, and the reaction ceases with resolution. However, if the input signal is not well controlled as happens during aging, chronic pro-inflammatory conditions conducive to many chronic diseases ensues (Yu and Chung, 2006). Some of the NF-κB-induced proteins like TNF-α, IL-1, 6 and COX-2 themselves are potent NF-κB activators that form an auto-activating loop (Handel et al., 1995; Fisher et al., 1996). Many studies on changes in the redox sensitive transcription factor, NF-κB have consistently shown increased activity with age and in a variety of tissues, including heart, liver, kidney, and brain tissues, and high NF-κB binding activity when comparing old and young rodents (Helenous et al., 1996; Kim et al., 2002). Korhonen et al. (1997) also reported a significant upregulation of NF-κB in the rat brain. In human studies, circulating levels of pro-inflammatory cytokines that are well-recognized biomarkers increase during aging as shown by increased plasma levels of TNF-α, IL-6, and IL-1ra (Bruunsgaard, 2006). Furthermore, aging is associated with increased levels of C-reactive protein (CRP), as well as high inflammatory cell (neutrophil, monocytes) counts (Bruunsgaard et al., 1999). Several studies have demonstrated that high plasma levels of IL-6 are correlated with greater disability, morbidity, and mortality in the elderly (Ferrucci et al., 1999). In addition, high levels of IL-6, IL-1β and CRP are significantly associated with disease conditions in older individuals (Bruunsgaad et al., 2003). Moreover, plasma levels of TNF-α are positively correlated with elevated quantities of IL-6 and CRP, indicating an interrelated activation of the entire inflammatory cascade (Bruunsgaard et al., 1999). It is interesting to note that Japanese centenarians have lower levels of CRP and IL-6, thrombin, and increased levels of adiponectin, even compared to younger individuals (Hirose et al., 2004).
The interaction between obesity and insulin resistance has been a hotly debated topic and studied with extensive scrutiny, but is without definitive resolution to date. However, recent evidence indicates inflammation may be the possible underlying link between these two conditions. Increased adiposity due to excessive caloric consumption leads to macrophage infiltration into adipose tissues, resulting in local chronic inflammation that potentiates insulin resistance (Xu et al., 2003). Overexpression of Mcp1 (a chemokine) increases macrophage infiltration, inflammation, and insulin resistance (Kamei et al., 2006). Moreover, de Luca and Olefsky (2007) have demonstrated that the knockout of Mcp1 or its receptor (Ccr2) impairs migration of macrophages, thereby lowering inflammation and improving insulin sensitivity.
In addition, it was recently shown that jnk 1 removal from hematopoietic cells (chimeric jnk1 knockout mice) had no effect on adiposity but conferred protection against high fat diet-induced insulin resistance by decreasing inflammation, thus indicating that inflammation, not obesity per se, likely causes insulin resistance (Solinas G, 2007). Together, these data indicate a strong causal relationship between inflammation and insulin resistance.
Inflammation has also consistently been shown to play a crucial role in vascular aging and in the pathogenesis of atherosclerosis through the regulation of AMs, which are cell surface membrane proteins that can contribute to vascular inflammation through their mediation of cell-cell interaction. For instance, endothelial cells (EC) express various AMs in response to such pro-inflammatory mediators as TNF-α, IL-1β, IL–6, and ROS. Various AMs regulate different steps of leukocytes infiltration. As long as the signals from pro-inflammatory mediators arrive at the endothelium, P-selectin and E-selectin are expressed on the ECs surface first, and then this step initiates the action of leukocytes. When leukocytes approach ECs, they are attached by ICAM-1 and VCAM-1, changing shapes into flat, called a firm adhesion. Then, with the help of ICAM-1, VCAM-1 and platelet/endothelial cell adhesion molecule-1 (PECAM-1), leukocytes pass through the tight-junction between ECs into the vessel wall where they are changed into macrophages, releasing more inflammatory mediators including cytokines, chemokines and ROS, triggering further inflammation. Uncontrolled expression of AMs contributes to many diseases of the vasculature, such as atherosclerosis (Davies et al., 1993; Johnson-Tidey et al., 1994).
Many studies report alterations in AM levels in animal models of age-related vascular diseases (Ashcroft et al., 1998; Merat et al., 2000; Kyrkanides et al., 2001; Saito et al., 2001), which is regulated by pro-inflammatory NF-κB activity. Our previous study probed the molecular link between normal and altered aortic AMs (Zou et al., 2004) and concluded that oxidative stress-induced inflammation is a potent factor responsible for the upregulation of AMs during aging (Zou et al., 2006). The upregulation of AMs may be responsible for several vascular diseases that manifest with age. These findings along with others strongly indicate the inflammatory process is a major contributing factor to vascular aging.
3. The inflammatory process, a possible link between normal and pathological aging
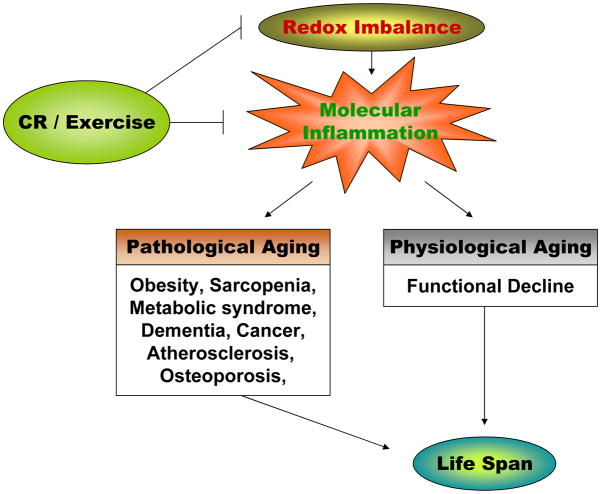
Although aging is the most well recognized risk factor for many chronic diseases, interactions between the aging process and age-related disease has not been seriously addressed or systematically explored. One of the most salient aspects of the molecular inflammation hypothesis of aging is that many age-related chronic diseases undergo a pathway common to the inflammatory process, which is a causative factor in or in part of disease progression (Chung et al., 2006). For example, a number of diseases including cardiovascular diseases, cancer, diabetes, metabolic syndrome, dementia, arthritis, and osteoporosis are recognized as inflammatory disorders, according to recent medical investigations (Yu and Chung, 2006). Sarcopenia and osteopenia are common conditions occurring with age and both are good illustrative examples of how inflammation is involved in the normal aging process that ultimately leads to pathogenesis (Fig. 2). Because a more expanded discussion on the interaction between metabolic syndrome and sarcopenia is presented in the later sections, a brief mention on the involvement of inflammation in obesity, insulin resistance, and sarcopenia is presented here.
Fig. 2.
The role of inflammation in pathophysiological process and the possible action of CR and exercise. CR, calorie restriction.
Inflammatory processes are linked to the potentially debilitating condition of obesity (Robinson et al., 2007). In this regard, age-related obesity is due to increased adiposity (particularly visceral fat deposits) during aging (Distefano et al., 2007; Weiss, 2007) through the redistribution of fat deposits with age (Yaffe, 2007). Most of pro-inflammatory cytokines are produced by adipocytes and resident macrophages in adipose tissues, causing systemic inflammation (Pou et al., 2007). This elevated pro-inflammatory status likely sets the stage for increased vulnerability for many age-related diseases. Sarcopenia and osteopenia, characteristic of normal aging process, are good illustrative examples for the involvement of inflammation in the normal aging process, to pathogenesis (Fig. 2).
Roth (2006) reporting the most recent findings regarding the potential role of inflammatory factors in the progression of age-related muscle wasting or sarcopenia, found that adiposity and lipid accumulation appear to play an important role in the inflammatory process and possibly the onset of sarcopenia and obesity. Future research will need to delve into the molecular interactions of chronic inflammation as the underlying link between muscle metabolism, obesity, and sarcopenia that develop with aging.
4. Implications of inflammation in aging and age-related diseases
Accumulating evidence indicates that unresolved, low-grade chronic systemic inflammation plays a significant role in modulating the aging process, and age-related diseases, such as metabolic syndrome, sarcopenia, dementia, atherosclerosis, cancer, and osteoporosis. The close involvement of inflammation in these diseases has led them to be named as “inflammatory diseases” (McGeer and McGeer 1999). Although the precise molecular inflammatory involvement of each disease may vary, the basic mechanisms of the inflammatory cytokines and other pro-inflammatory mediators are similar (see Fig. 2). In the discussion below, we highlight the role of chronic inflammation in these age-related inflammatory diseases.
A. Obesity and aging
Metabolic syndrome was originally called Syndrome X, referring to a cluster of clinical factors, e.g., insulin resistance, hyperinsulinemia, high blood-glucose, hypertension, and dyslipidemia with elevated triglycerides and low HDL levels. However, decreased insulin sensitivity, associated with abdominal obesity, is the central feature of the syndrome.
The prevalence of obesity is increasing in virtually all populations and age groups worldwide. Obesity increases an individual’s risk for a large number of co-morbidities and increased mortality rates (World Health Organization, 2000). The epidemic rise of obesity has dramatically increased the interest of researchers and clinicians in the study of metabolic syndrome. Metabolic syndrome is usually defined by the presence of at least three of the following criteria: abdominal obesity, elevated triglycerides, reduced levels of HDL cholesterol, high blood pressure, insulin resistance, and high fasting glucose (Ford et al., 2002). Approximately one in four American adults been diagnosed with this condition (Hoffmann et al., 2007). Individuals with metabolic syndrome are at significant increased risk for heart attack, stroke or cardiovascular death compared to individuals without the syndrome (Isomaa et al., 2001).
Adipose tissue can be divided into 2 major types: white adipose tissue (WAT) and brown adipose tissue (BAT). WAT comprises the vast majority of adipose tissue in the organism and is the site of energy storage; whereas, the main role of BAT is for non-shivering thermogenesis, particularly in small mammals and human neonates (Fantuzzi, 2005). WAT is composed of many cell types, adipocytes being the most abundant. Recent studies have demonstrated that adipose tissue is characterized by increased infiltration of macrophages, suggesting the presence of inflammation in adipose tissue (Weisberg et al., 2003; Xu et al., 2003).
Current knowledge indicates that visceral adipose tissue secretes a large number of proteins termed, adipokines acting in an autocrine, paracrine, or endocrine fashion to regulate various metabolic functions (Greenberg and Obin, 2006). Furthermore, adipocytes can be the source of a large number of cytokines/chemokines, such as TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1) (Wang, 2005). Secretion of these inflammatory cytokines is remarkable within visceral adipose tissue. Metabolically reactive visceral fat is even more sensitive to endocrine actions than subcutaneous adipose tissue. Visceral adipose tissue and pro-inflammatory factors including IL-6, TNF-α, and interferon-γ (IFN-γ) increase with age (Greenberg and Obin, 2006) and, partly because these inflammatory factors are produced and regulated by fat tissue (Vick et al., 2007). The age-related inflammation is directly associated with visceral adipose tissue (Xu et al., 2003). Therefore, the age-related increase of visceral adipose tissue might be responsible for age-related systemic inflammation.
Increasing evidence also demonstrates that adipose tissue functions as an endocrine organ and is involved in regulation of various metabolic pathways (Wang et al., 2008; Bulcao et al., 2006). Adipose tissue has a homeostatic cycle that overlaps with the cycle of whole-body energy balance and storage. In this context, several substances having endocrine, paracrine and autocrine properties are secreted by adipose tissue (i.e., adipokines or adipocytokines) and act at different levels to maintain homeostasis (Staiger and Haring, 2005). The members of this group of cytokines, including leptin, adiponectin, IL-6, TNF-α, PAI-1, etc, are particularly interesting because they may be modifiable causes of morbidity in obese persons.
Interestingly, human CRP has been reported to be correlated with increased adiposity and leptin resistance due to CRP binding to leptin (Chen et al., 2006). These observations suggest that increased CRP and IL-6 and decreased adiponectin are associated with metabolic syndrome. Collectively, these findings indicate that adiponectin may be the link connecting obesity, insulin resistance, and inflammation.
Adipocytes constitutively express TNF-α, which in adipocytes is markedly increased with obesity. TNF-α stimulates the cellular kinase complex (i.e., IKK), which activates NF-κB, a transcription factor that, in turn, regulates the production of pro-inflammatory cytokines (including IL-1β and IL-6) (Chen and Kwak, 2003). It has been suggested that high-dose aspirin may improve insulin resistance in diabetic patients through the inhibition of this IKK/NF-κB pathway (Gao et al., 2003). Indeed, aspirin has been shown to suppress age-related NF-κB activation and upregulation of its dependent genes, COX-2, iNOS, VCAM-1 and ICAM-1, which are modulated by IκB degradation via the NIK/IKK pathway (Jung et al, 2006).
Interestingly, the inflammatory response occurring with obesity seems to be triggered and initially maintained by adipose tissue. Then, the systemic effects of inflammation take place in the complications related to the inflammatory process and obesity, by involvement of other metabolic sites (e.g., hepatic and skeletal muscle insulin resistance and atherosclerosis). It has been suggested that obesity may promote an increased infiltration of macrophages in the adipose tissue (accompanied by a consequent low-grade inflammatory status) (Harman-Boehm et al., 2007). The macrophages infiltration may cause a dysregulation in adipose tissue endocrine properties and enhance the production of inflammatory cytokines, consequently leading to endothelial dysfunction and insulin resistance.
B. Sarcopenia as muscular inflammation during the aging process
Sarcopenia (from the Greek, sarx for flesh and penia for loss) is a term coined to describe one of the most noticeable changes occurring in older individuals (Rosenberg, 1997). Sarcopenia is defined as the age-related loss of muscle mass, strength and function, and is common characteristic of frailty (Roubenoff, 2007). While an important correlate of impairment and physical disability in older persons (Villareal et al., 2004), it is associated with a decrease in muscular strength and endurance and a loss of autonomy in older persons (Janssen et al., 2002). The age-related decrease in muscle mass and strength is mainly caused by atrophy of muscle fibers, especially the type IIa fibers (Morley, 2001), which is associated with a decline in protein synthesis, particularly in the synthesis of myosin heavy chains (Morley et al., 2001). It has been suggested that this loss of muscle mass is not isolated but is strongly connected with a parallel increase in fat mass (Roubenoff, 2000).
Increased levels of inflammation have been shown to be detrimental to skeletal muscle in humans (Anker et al., 1999) as well as in animal models (Charters and Grimble, 1989; Hoshino et al., 1991; Garcia-Martinez et al., 1993). Roubenoff (2003) suggested that inflammation may negatively influence skeletal muscle through direct catabolic effects or through indirect mechanisms (i.e., decreases in GH and IGF-1 concentrations, induction of anorexia). It is worth noting that inflammation is inversely associated with IGF-1 (Leng, 2004) and that the reduction of IGF-1, a main messenger of growth hormone, is associated with sarcopenia, frailty, and mortality (Roth, 2006). Pro-inflammatory cytokines are commonly involved in cachexia and have been shown to be involved in the process responsible for the anorexia of aging (Bales and Ritchie, 2002). The anorectic effects of pro-inflammatory cytokines are particularly interesting because nutrition is a crucial factor in the prevention of sarcopenia.
Immune function also changes with aging, resulting in higher levels of catabolic cytokines. When considering potential gender differences as a basis for sarcopenia, it becomes evident that sex hormones (both estrogen and testosterone) influence the immune system in many ways.
Inflammation is strongly associated with apoptosis, a different biological pathway potentially leading to sarcopenia. Apoptosis is an evolutionary conserved program for cell suicide, which is important to the development of multi-cellular organisms, the elimination of superfluous tissues, and the maintenance of tissue homeostasis in adults. It has been shown that TNF-α is one of the prime signals inducing cellular apoptosis in muscle (Phillips and Leeuwenburgh, 2005) Apoptosis and inflammation are biological pathways that closely interact with a third mechanism involved in age-related reduced muscle mass and strength: oxidative damage. An ideal “golden triangle” of oxidative balance has been described, in which oxidants, antioxidants, and biomolecules are placed at each apex (Carmeli et al., 2002). Furthermore, TNF-α is shown to be responsible for muscle loss in aged rats through myocyte apoptosis. TNF-α is able to activate caspase-8, which subsequently activates caspase-3, thereby initiating the apoptosis cascade (Phillips et al., 2005). In a normal situation, equilibrium exists among these three elements. An excess generation of free radicals may overwhelm natural cellular antioxidant defenses leading to oxidation and further contributing to muscle damage (Cesari et al., 2005).
Skeletal muscle mass is maintained through a dynamic balance between protein synthesis and degradation, with proteolysis prevailing over synthesis in skeletal muscle atrophy, an important mechanism regulated by the ubiquitin-proteasome pathway. Interestingly, redox-sensitive NF-κB transcription factor, which induces many inflammatory mediator gene expressions, is activated by ubiquitin-proteasome system. Furthermore, inhibitors of NF-κB have been shown to attenuate body weight loss and protein degradation in skeletal muscle (Cai et al., 2004).
Based on the described observations, high levels of pro-inflammatory mediators, such as TNF-α and IL-6, are associated with reduced muscle strength and mass, poorer physical performance, and higher incidences of mobility disability in the elderly.
C. Dementia
A growing body of literature indicates that inflammatory processes are related with the development of dementia, both vascular and Alzheimer’s types. Several serum pro-inflammatory markers have been shown in relation to dementia and cognitive decline. Inflammatory responses are hypothesized to modulate the pathogenic processes associated with Alzheimer’s disease (AD) (Peila and Launer, 2006; Selkoe, 2006). Although the participation of the inflammatory process in aging brain functions has long been suspected, its direct implication in the brain aging process has been less appreciated until the proposal of the Inflammatory Hypothesis of Dementia (McGeer and McGeer, 1999). Now that the in vivo and in vitro properties of microglia have been more appropriately investigated (McGeer and McGeer, 1995; McGeer et al., 2000), it is well-known that their activation signifies a primary inflammatory state and that leukocyte invasion, when it occurs, is a secondary phenomenon. These investigations suggest that inflammation significantly contributes to AD pathogenesis.
The involvement of mid-life, increased CRP levels in dementia has been examined in the Honolulu Asia Aging Study (Schmidt et al., 2002). In this study, elevated CRP levels were related to a significantly increased risk for vascular dementia (VaD) and AD, with or without cardiovascular disease (CVD) contributing to the dementia. The Rotterdam Study investigators also reported a relationship between elevated levels of α1 antichymotrypsin (ACT), CRP, IL-6, soluble ICAM, and soluble VCAM-1 and an increased risk for AD and VaD, whereas ACT was most strongly associated to the increased risk of VaD (Engelhart et al., 2004).
In both humans and in transgenic mouse models of AD, various inflammatory markers increase with the onset of AD pathology, including members of the complement pathway; cytokines and chemokines, such as IL-1β, IL-6, TNF-α and TGF-β; coagulation factors; acute-phase reactive proteins, such as α-2-macroglobulin and α1-ACT; reactive astrocytes; and activated microglia cells (Wyss-coray, 2006). Much research suggests that the immune response to Aβ can exacerbate ongoing pathological processes, but the role that inflammation plays in the disease process is complex (Wyss-Coray and Mucke, 2002).
In vivo studies show that long-term administration of NSAIDs can effectively suppress both inflammation and Aβ pathology in transgenic mouse models of AD. The first study to report this dual effect was a six-month preventative trial in the Tg2576 line of APP-transgenic mice, a line that shows many features of amyloidosis and inflammation in AD (Hsiao et al., 1996). A high dose of ibuprofen markedly suppressed amyloid plaque pathology and Aβ levels, reduced microglial activation and reactive astrocyte numbers, and decreased levels of the proinflammatory cytokine IL-1β (Lim et al., 2000).
Follow-up studies using ibuprofen and indomethacin treatments in transgenic mice reported similar decreases in inflammatory markers and Aβ (Townsend and Pratico, 2005). Experimental data suggest that NSAID-mediated COX inhibition might reduce both inflammation and Aβ accumulation in vivo by interrupting a putative feed-forward inflammatory mechanism (Hoozemans et al., 2003). COX-2 inhibitors or genetic ablation of COX-2 also attenuates the loss of neurons in rodent models of acute brain injury (Iadecola, 2005).
These observations suggest that dementia could be closely associated with chronic inflammation that might be an important contributor to the development of neurodegenerative diseases.
D. Atherosclerosis
Cardiovascular disease is the leading cause of death and illness in developed countries (Murray et al., 1997). It is estimated that by the year 2020 almost 40% of all deaths will be due to cardiovascular disease (Braunwald et al., 1997). The major contributor to cardiovascular disease is atherosclerosis, a progressive, multi-factorial process that focally affects large arteries and is characterized by the accumulation of lipids and fibrous elements in their walls. Inflammation is demonstrated to play a crucial role in all the phases of the atherosclerotic process, from the initial steps of leukocyte recruitment to the eventual rupture of a vulnerable plaque (Libby, 2002).
The early phase of atherogenesis is characterized by the attraction/adherence of monocytes to the vascular endothelium and their migration into the vessel wall. The expression of cellular AMs (e.g., selectins, ICAM-1, VCAM-1) (Johnson et al., 1997; Dong et al., 1998; Steffens et al., 2004) promotes the adhesion of leukocytes to the vascular endothelium, and is induced by inflammatory factors, including IL-1, TNF-α, and CRP (Willerson et al., 2004). In particular, VCAM-1 binds specifically to those classes of leukocytes found in nascent atheroma (i.e., monocytes and T lymphocytes). Both macrophages and endothelial cells produce ICAM-1 in response to inflammatory cytokines (e.g., IL-1, TNF-α, interferon-γ), whereas VCAM-1 expression is mainly restricted to endothelial cells. Furthermore, the progressive accumulation of macrophages and their uptake of oxidized LDL ultimately lead to the generation of foam cells and initiates fatty streaks.
The progression of lesions is characterized by the additional migration of smooth muscle cells from the medial layer of the artery wall into the subendothelial space. Smooth muscle cells proliferate and migrate under the control of several growth factors and cytokines produced by inflammatory and endothelial cells (Lefkowitz et al., 2001). However, smooth muscle cells themselves are a source of inflammatory mediators, such as MCP-1 (Nelken et al., 1991). The expression of such chemotactic molecules as MCP-1 has been reported also in response to the initiating insult produced by oxidized LDL (Navab et al., 1996). Mice deficient in MCP-1 or its chemokine receptor, CCR2 develop less atherosclerotic lesions (Han et al., 1999). Parallel to the upregulation of adhesion molecules and chemoattractant proteins, initiators of the atheroscletoric cascade (e.g., oxidized LDL) are shown to increase the production of growth factors, such as macrophage colony-stimulating factor (M-CSF) (Wang et al., 1997).
The proliferation of smooth muscle cells, the further recruitment of inflammatory cells, and the synthesis of extracellular matrix proteins lead to the formation of the atheroma, a mature atherosclerotic plaque composed by a fibrous cap separating the pro-thrombotic lipid pool from luminal blood flow. The activation of inflammatory cells (i.e., macrophages, T lymphocytes) and smooth muscle cells is associated with the release of additional mediators, such as adhesion molecules, cytokines, and growth factors (Lefkowitz et al., 2001; Libby, 2002). IL-6 is a major pro-coagulant (Libby et al., 200l) and pro-inflammatory cytokine (Mortensen, 2001). IL-6 increases plasma concentrations of fibrinogen, plasminogen activator inhibitor type 1, and CRP (which, in turn, amplify and enhance both inflammatory and pro-coagulant responses). These pro-inflammatory mediators are suggested to be involved in atherosclerosis pathogenesis.
E. Cancer
Carcinogenesis is a multi-step process that includes initiation, promotion, and progression as a consequence of the imbalance between cell proliferation and cell death. Progression from initiated cells to malignant tumors is modulated by genetic and epigenetic mechanisms.
NF-κB, which is activated by various stimuli including pro-inflammatory cytokines plays a crucial in carcinogenesis. The activation or expression of NF-κB is evident in several human cancers including breast cancer, non-small cell lung carcinoma, thyroid cancer, gastric cancer, colon cancer, hepatoma, and leukemia and in several virally induced tumors (Chen et al. 1999).
NF-κB also is found to have an anti-apoptotic effect in a variety of cell types by regulating apoptosis-related genes. Sumitomo et al. reported that apoptosis of multiple cytokine-producing bladder cancer cells can be modulated by the manipulation of NF-κB activity (Sumitomo et al., 1999). Aberrant nuclear expression of NF-κB is demonstrated to be related with breast cancer (Ricca, et al., 2000, Cogswell et al., 2000). Evidence shows a potential role for NF-κB in carcinogenesis by the observation that NF-κB activation is required in oncogenic Ras-induced transformation (Mayo et al., 1997). NF-κB also is known to be required for leukemogenesis initiated by the Bcr-Abl chimeric protein (Reuther et al., 1998).
Thus, chronic inflammation with its subsequent generation of free radicals and up-regulation of COX-2 can lead to a cascade of events that may eventually cause malignant transformation. Initiated cells can be further promoted by NF-κB proteins induced by up-regulated, pro-inflammatory cytokines. Because activated NF-κB can both inhibit apoptosis and stimulate production of pro-inflammatory cytokines, it further promotes proliferation of initiated cells. Hence, tissue exposed to chronic inflammation or to a pro-inflammatory milieu may be more susceptible to the carcinogenic process (Modugno et al., 2005).
Elsharkaway and Mann (2007) recently reported that cell-targeted perturbation of NF-κB activity has revealed complex and multi-cellular functions in hepatic inflammation, fibrosis, and the development of hepatocellular carcinoma through an inflammation-fibrosis-cancer axis. Further, activation of NF-κB by H. pylori is responsible for the generation of pro-inflammatory mediators, such as COX-2, TNF-α, IL-1, IL-6, metalloproteinases, endothelial growth factor (VEGF), and adhesion molecules that lead to gastric cancer (Smith et al., 2006). Colitis-associated cancer, as its name indicates, is a colorectal cancer that frequently occurs in patients suffering from ulcerative colitis, a chronic inflammatory bowel disease (Karin et al., 2006). Based on this evidence, activated NF-κB in tumor cells appears to play an important role in cancer development, progression, angiogenesis, and invasiveness of various tumors.
Several reviews suggest a direct relationship between chronic inflammation and cancer (Oshima et al., 2003; Moss et al., 2005; Aggarwal et al., 2006; Lu et al., 2006). Key molecular players that link inflammation to genetic alterations are prostaglandins, cytokines, nuclear factor NF-κB, chemokines, and angiogenic factors. The main chemical effectors are RS derived from inflammatory reactions, which may act as direct or indirect damaging agents through their reaction with other chemical or structural components in cells. The RS-sensitive transcription factor, NF-κB plays a critical role in mediating the vicious cycle between inflammation and RS. These observations suggest that age-related oxidative stress consequent to chronic inflammation contributes to the induction and progression of cancer via NF-κB signaling.
F. Osteoporosis
Similar to the role of sarcopenia in muscular mass decline, osteopenia, an age-associated low bone mass condition, results in impaired mobility and lowers quality of life. Ostopenia is becoming a significant, world-wide social and financial health care problem (McCormick, 2007). The crucial role of the bone organ is to provide a rigid structural frame to support body shape and protect essential organs (e.g., heart and brain); thus, bone tissue is actively re-constructed and continuously remodeled by a lifelong turnover process.
The process of bone remodeling is mainly controlled by the well-balanced homeostatic activities of osteoblasts and osteoclasts. Osteoblasts play an important role in bone formation by producing the bone extracellular matrix (ECM) and mineralizing the ECM. In contrast to the key role of osteoblasts in osteogenesis, osteoclasts melt the mineralized bone ECM and remove bone tissue. Hence, bone integrity is maintained by the well-balanced activities of osteoblasts and osteoclasts. An imbalance in homeostatic activity of these cells can lead to various bone disorders, such as osteoporosis, due to impaired bone resorption, and to osteoporosis resulting from hyper-bone resorption (Del Fattore et al., 2008).
Recent epidemiological evidence indicates age-associated chronic inflammation disrupts bone homeostasis, and thus may increase incidences of osteoporosis with age (Chung et al., 2006). For instance, an age-associated shift of T cell immunity alters systemic cytokine profiles from T helper 1 (Th1) type to T helper 2 (Th2) type (e.g., IL-4, IL-5 and IL-6), and this Th2 bias is frequently observed in various abnormal bone loss conditions, including pregnancy, HIV infection, steroid therapy and immunosuppression therapy, as well as aging (Yun and Lee, 2004). In fact, IL-6 aggravates systemic inflammation with age and increases hepatic CRP production. This increased systemic level of CRP has been shown to lower bone-mineral density in healthy women (Koh et al., 2005). CRP has consistently been found to be related to increased fracture risk in elderly women (Pasco et al., 2006). Additionally, IL-6 is an important stimulator of osteoclast differentiation, maturation, and activation together with IL-1 and TNF-α. These acute phase pro-inflammatory cytokines can induce apoptosis of osteoblasts via iNOS activation and bone loss (Koh et al., 2005) indicating osteoclasts may favor the intra- and/or extra-cellular inflammatory environments relatively more than osteoblasts. Indeed, osteoclasts, unlike osteoblasts are classified into a bone-specific mesenchymal cell lineage. Ontologically osteoclasts stem cell from a fusion of myeloid-derived immune cells, such as monocytes (Teitelbaum. 2007).
This may explain the following: 1) Why osteoclasts are sensitively activated by pro-inflammatory cytokines; 2) Why NF-κB signaling triggered by RANK-RNAKL interaction is primarily required for osteoclast differentiation, maturation and activation; and 3) How the augmented intracellular molecular inflammation, defined as a chronic upregulation of redox-senstitive NF-κB signals, might over-activate osteoclasts and disturb the balanced bone turnover process at the molecular level of aging.
Recently, Yamashita et al. has shown that NF-κB (p50/p52) signal induced by RANKL and TNF is essential for early osteoclast differentiation, which was followed by activation of NFATc1 and c-Fos. Thus, inhibition of NF-κB may prevent RANKL and TNF-induced bone loss (Yamashita et al., 2007). The involvement of p38 MAPK in osteoporosis is reported in several studies (Kumar et al., 2001; Zwerina et al., 2006), and a recent work from Park et al (2007). has shown that a natural product from plant, called Stewartia koreana, inhibits osteoclastogenesis in an in vitro system and ameliorates bone loss in LPS- (a potent inflammation agent) treated mice by down-activating ERK and p38 MAPK signaling and suppressing NFATc1 and c-Fos (Chung et al., 2004). These findings strongly suggest that the inhibition of NF-κB signals via ERK and down regulation of p38 MAPK might prevent age-induced abnormal bone resorption.
Taken together, evidence from various molecular and clinical studies indicates that chronic inflammation plays an etiological role in the incidence of osteoporosis during normal bone aging. This suggests that therapies that reduce chronic inflammation, such as non-steroidal anti-inflammatory drugs (e.g., aspirin), various NF-κB signaling antagonists, CR, and physical exercise, can attenuate the bone loss process with age.
5. Regulation of pro-inflammatory cytokines and molecules
A. Role of CR in modulating molecular inflammation and aging
Many researchers have now accepted CR as the only established anti-aging experimental paradigm. CR’s anti-aging effects are thought to be due mainly to its powerful resistance against oxidative stress and ability to maintain a proper cellular redox status as evidenced by suppressed oxidative damage to lipids, DNA, and proteins (Yu, 1996). Thus, the effects of short- and long-term CR are believed to be due to its powerful anti-oxidative action (Yu, 1996; Forster, 2000; Chandrasekar, 2001; Merry, 2004; Chung et al., 2006; Spindler, 2005). Recent evidence has also documented CR’s anti-inflammatory action, as shown in its modulation of pro-inflammatory genes such as TNF-α, IL-1, 6, AMs, iNOS, and COX-2 through the NF-κB signaling pathway (Chung, 2002; Kalan, 2006).
Acute and chronic inflammatory responses to cellular injury or tissue destruction are physiological, protective mechanisms that limit insult and promote repair (Chung et al., 2001; Chung et al., 2002; Chung et al., 2004). However, self-maintaining, chronic inflammation plays a key role in numerous disease states as well as in the aging process (Jones, 2001; Libby, 2001; Coussens and Werb, 2002; Pearson et al., 2003). Animal studies have shown repeatedly that CR enhances metabolic efficiency and increases stress resistances over the lifespan, thereby delaying the onset of age-related pathological conditions. Recently, great attention has been focused on the anti-inflammatory potential of CR. Indeed, CR has been shown to attenuate the age-related increase of systemic levels of several pro-inflammatory mediators (Ershler and Keller, 2000; Krabbe et al., 2004), such as TNF-α, IL-1β, IL-6, CRP and various AMs (Phillips and Leeuwenburgh, 2005; Kalani et al., 2006). In particular, our group has recently shown in rats that the age-associated rise of plasma TNF-α is attenuated by life-long, moderate (40%) CR in rats (You et al, 2007). Furthermore, Kalani et al. (2006) have demonstrated the capacity of long-term 40% CR to attenuate the increase in circulating levels of CRP with age, reporting even greater reduction by combining mild (8%) CR with life-long, voluntary exercise. Furthermore, You et al. (2007) have shown that CR reduces IL-6 secretion from adipose tissue and CRP serum levels in rats. Expression of most of the genes encoding for pro-inflammatory cytokines is regulated by NF-κB. Of note, CR is shown to blunt the age-dependent increase of NF-κB through up-regulation of cytoplasmic IκBα and IκBβ (Seo et al., 2006; Kwon, 2001). Furthermore, our group provides evidence that CR blunts the age-associated increase of NF-κB signaling in rat kidney (Sung et al., 2004).
Moreover, CR is shown to attenuate age-related decreases in mRNA levels, nuclear protein contents, and DNA binding activity of peroxisome proliferator-activated receptors (PPARs) (Sung et al., 2004), thus boosting the inhibitory effect of PPARs on NF-κB activation. In addition, studies show that CR effectively counteracts lipopolysaccharide (LPS) and IFN-γ-stimulated production of several cytokines, including IL-1β, IL-6 and TNF-α (Eanes, 1976; Spaulding et al., 1997; Vega et al., 2004). Furthermore, a synergistic anti-inflammatory effect of CR and n-3 fatty acids has been recently reported (Tasuchiva et al., 2005).
CR is known to suppress COX-derived ROS generation in the presence of indomethacin (Kim et al., 2000). Of note, a reduction of COX activity and the production of thromboxane A2, prostacyclin and PGE2 was also reported (Fernbach, 1992), likely stemming from a favorable modulation of NF-κB activity by CR (Kim et al., 2000; Horgan et al., 2001).
Another transduction pathway that is intricately involved in the age-related chronic inflammation and which is modulated by CR is that controlled by MAPK (Kim et al., 2002). Noticeably, MAPK family members regulate the cellular inflammatory reaction in response to various stimuli, including oxidative stress (Gupta et al., 1999; Irani et al., 1997; Peters et al., 2000; Zhu et al., 2001). CR suppresses the age-related activation of the three subtypes of MAPKs, namely ERK, JNK, and p38 MAPK (Kim et al., 2002). In addition, a recent study has reported that CR significantly attenuates the age-related increase of myeloperoxidase (MPO) activity and subsequent oxidant production in rats (Son et al., 2005).
In conclusion, emerging evidence supports the molecular inflammation hypothesis of aging and identifies redox imbalance as the major causative factor leading to age-related degenerative diseases. CR is the only non-genetic intervention that has so far been shown to extend mean and maximum life-spans in a variety of species, which may be due, at least in part, to CR’s anti-inflammatory properties. Preliminary studies employing 8% CR suggest that this approach may still be effective in mitigating age-related inflammation, which may be of particular importance when considering the application of CR to humans, especially at old age.
B. Modulation of chronic inflammation by physical exercise
Chronic, low-grade inflammation, a common characteristic of aging, is reflected by increased systemic levels of several pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1β, and CRP, even in the absence of overt disease conditions (Bruunsgaard, 2001; Krabbe et al., 2004; Vasto et al., 2007). This age-related shift toward a pro-inflammatory state has been associated with several adverse outcomes, including loss of muscle mass (Roubenoff et al., 2003) and increased incidence of disability (Cesari et al., 2005) and mortality (Roubenoff et al., 2003).
Exercise training is well known to have beneficial effects across a broad spectrum of biological processes, including the favourable modulation of inflammatory signalling (Wannamethee et al., 2002). However, the response of the immune system to physical activity varies, depending on the frequency, intensity, volume of exercise, and on the subject’s endurance capacity. Indeed, strenuous exercise has been shown to increase local and systemic production of pro-inflammatory cytokines, possibly as a consequence of muscle damage and subsequent inflammation (Okolo et al., 2000).
On the other hand, moderate regular physical activity has been associated with reduced levels of TNF-α, IL-6, and CRP in a population of healthy older adults (Colbert et al., 2004). In addition, a recent study reported that aerobic exercise is associated with decrease serum levels of IL-6 and increased levels of IL-10, a potent anti-inflammatory cytokine, in healthy older men (Jankord and Jemiolo, 2004). Furthermore, our group recently demonstrated that life-long, voluntary wheel running reduces plasma levels of CRP, but not those of IL-6, in old rats (Kalani et al., 2006). We also have reported a reduced expression of TNF-R1 in the extensor digitorum longus muscle of aged rats subjected to 4 weeks of exercise training by treadmill running (Marzett et al., 2008).
The mechanisms underlying the anti-inflammatory effects of physical exercise are complex and not fully elucidated. However, it has been hypothesized that the reduced production of pro-inflammatory cytokines observed in response to regular physical exercise may stem at least partly from a reduction of adiposity (Colbert et al., 2004). Indeed, adipocytes are an important source of TNF-α and IL-6 (Coppack, 2001), this latter being the main stimulus for liver production and secretion of CRP (Du Clos, 2000). However, other mechanisms, independent of body composition, are thought to be involved in the modulation of inflammation by physical exercise. We recently reported that life-long wheel running combined with mild (8%) calorie restriction reduced the age-related activation of NF-κB in the liver of old rats (Seo et al., 2006). Importantly, NF-κB is considered to play a major role in orchestrating the inflammatory response, by promoting the expression of several pro-inflammatory mediators (Sarkar and Fisher, 2006).
Mechanisms underlying the anti-inflammatory action of exercise are complicated by the intricate interplay among cytokines. For a case in point, a recent study reported an anti-inflammatory effect exerted by muscle-derived IL-6 (Pedersen, 2007). Of note, plasma levels of IL-6 increase markedly during physical exercise, in proportion to its intensity and duration (Ostrowski et al., 2000), and to a higher extent in older individuals (Pedersen and Bruunsgaard, 2000). In skeletal muscle, gene expression and protein levels of IL-6 are elevated during contraction, paralleled by an increased IL-6 release into the blood stream (Hiscock et al., 2004). In this context, we have preliminary data indicating that mRNA abundance of IL-6 is increased in old rats following 4 weeks of treadmill running (unpublished). It has been hypothesized that muscle-derived IL-6 might induce several metabolic adaptations aimed at increasing substrate delivery to the contracting muscle, such as hepatic glycogenolysis and increased lipolysis (Petersen and Pedersen, 2006). Furthermore, muscle-derived IL-6 induces the release of cytokine inhibitors, such as IL-1ra and sTNFR, and anti-inflammatory cytokines, such as IL-10 (Pretolani, 1999). Interestingly, IL-10 has been shown to inhibit the synthesis of several pro-inflammatory cytokines, including TNF-α and IL-1β (Nielsen et al., 2007). It is interesting to note that muscle-derived cytokines appear to have important roles in metabolism, and exercise plays a role in modulating the interplay between cytokines and metabolism.
Physical exercise has also been shown to elicit the expression of IL-15 in skeletal muscle (Nielson et al., 2007). IL-15 is a recently discovered cytokine that exerts a potent anabolic effect on muscle by inhibiting protein degradation and stimulating protein synthesis (Quinn et al., 2002). Furthermore, IL-15 supplementation to rats bearing the Yoshida AH-130 ascites hepatoma resulted in a significant reduction of muscle wasting, sustained by an attenuation of apoptotic DNA fragmentation (Figueras, et al., 2004). In particular, the administration of IL-15 completely reversed the tumor-induced increased expression of TNF-R in skeletal muscle, suggesting that the anti-apoptotic effect of IL-15 might reside in the down regulation of the TNF-driven apoptotic pathway (Figueras et al., 2004). Interestingly, we have recently shown that TNF-triggered myocyte apoptosis was down-regulated in old rats subjected to 4 weeks of treadmill running (Marzetti et al., 2008).
In conclusion, several lines of evidence support an anti-inflammatory effect of regular physical exercise at old age. However, further research is warranted to better elucidate the molecular mechanisms of this phenomenon as well as to investigate whether physical exercise retains the ability of reducing systemic inflammation in frail older adults.
Conclusion
Putting together all available data, chronic molecular inflammation is a major biological mechanism underpinning the aging process and age-related diseases. The key mediators of inflammatory reactions (i.e., IL-1β, IL-6, TNF-α, COX-2, and iNOS) have all been shown to be up-regulated during the aging process. Moreover, the gene expression of these inflammatory markers is modulated by the redox-sensitive transcription factor, NF-κB, which is activated by the IKK/NIK and MAPK pathways. These signalling pathways appear to be modulated by both CR and exercise (Fig. 2). The activation of the network of NF-κB-dependent, pro-inflammatory molecules appears to be the molecular mechanism underlying numerous age-related diseases including dementia, cardiovascular disease, cancer, obesity, metabolic syndrome, and osteoporosis. Thus, accumulating evidence indicates molecular inflammation is a primary factor underlying age-related diseases and aging processes. A better understanding of the signalling pathways involved in chronic molecular inflammation may assist in the development of future interventions to treat the inflammatory diseases associated with aging.
Acknowledgments
This research was supported by grants from Pusan National University and National Institute of Health to CL (AG17994 and AG21042) and a University of Florida Claude D. Pepper Older Americans Independence Center NIH grant (1 P30 AG028740) and a fellowship grant from the American Heart Association to AYS (0615256B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann NY Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- Aigner T, Söder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3:391–399. doi: 10.1038/ncprheum0534. [DOI] [PubMed] [Google Scholar]
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36:182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683–693. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- Arvin B, Neville LF, Barone FC, Feuerstein GZ. The role of inflammation and cytokines in brain injury. Neurosci Biobehav Rev. 1996;20:445–452. doi: 10.1016/0149-7634(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78:47–58. [PubMed] [Google Scholar]
- Badley AD, Roumier T, Lum JJ, Kroemer G. Mitochondrion-mediated apoptosis in HIV-1 infection. Trends Pharmacol Sci. 2003;24:298–305. doi: 10.1016/S0165-6147(03)00125-1. [DOI] [PubMed] [Google Scholar]
- Baek BS, Kim JW, Lee JH, Kwon HJ, Kim ND, Kang HS, Yoo MA, Yu BP, Chung HY. Age-related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J Gerontol A Biol Sci Med Sci. 2001;56:B426–427. doi: 10.1093/gerona/56.10.b426. [DOI] [PubMed] [Google Scholar]
- Baek BS, Kwon HJ, Lee KH, Yoo MA, Kim KW, Ikeno Y, Yu BP, Chung HY. Regional difference of ROS generation, lipid peroxidation, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch Pharm Res. 1999;22:361–366. doi: 10.1007/BF02979058. [DOI] [PubMed] [Google Scholar]
- Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221–2222. [PubMed] [Google Scholar]
- Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R, Finch C, Schneider E, Cotman C, McClearn G. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodamyali T, Stevens CR, Blake DR, Winyard PG. Reactive oxygen/nitrogen species and acute inflammation: A physiological process. In: Winyard PG, Blake DR, Evans CH, editors. Free Radicals and Inflammation. Birkha user Verlag; Basel: 2000. pp. 11–16. [Google Scholar]
- Böhrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Männel D, Böttiger BW, Stern DM, Waldherr R, Saeger HD, Ziegler R, Bierhaus A, Martin E, Nawroth PP. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol. 1983;38:673–677. doi: 10.1093/geronj/38.6.673. [DOI] [PubMed] [Google Scholar]
- Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA, Neumeier D. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E. Shattuck Lecture - Cardiovascular medicine at the turn of the millenium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- Brod SA. Unregulated inflammation shortens human functional longevity. Inflamm Res. 2000;49:561–70. doi: 10.1007/s000110050632. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H. The clinical impact of systemic low-level inflammation in elderly populations. With special reference to cardiovascular disease, dementia and mortality. Dan Med Bull. 2006;53:285–309. [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Bulcao C, Ferreira SR, Giuffrida FM, Ribeiro-Filho FF. The new adipose tissue and adipocytokines. Curr Diabetes Rev. 2006;2:19–28. doi: 10.2174/157339906775473617. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–489. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- Carrington JL. Aging bone and cartilage: cross-cutting issues. Biochem Biophys Res Commun. 2005;328:700–708. doi: 10.1016/j.bbrc.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation--results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Leeuwenburgh C, Pahor M. Oxidative damage and platelet activation as new predictors of mobility disability and mortality in elders. Antioxid Redox Signal. 2005;8:609–619. doi: 10.1089/ars.2006.8.609. [DOI] [PubMed] [Google Scholar]
- Chae GN, Kwak SJ. NF-kappaB is involved in the TNF-alpha induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARgamma expression Exp. Mol Med. 2003;35:431–437. doi: 10.1038/emm.2003.56. [DOI] [PubMed] [Google Scholar]
- Charters Y, Grimble RF. Effects of recombinant human tumor necrosis factor alpha on protein synthesis in liver, skeletal muscle and skin of rats. Biochem J. 1989;258:493–497. doi: 10.1042/bj2580493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi D, Demens LM. New insights into the role of NF-κB, a ubiquitious transcription factor in the initiation of disease. Clin Chem. 1999;45:6–17. [PubMed] [Google Scholar]
- Chen K, Li F, Li J, Cai H, Strom S, Bisello A, Kelley DE, Friedman-Einat M, Skibinski GA, McCrory MA, Szalai AJ, Zhao AZ. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–432. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cheng KQ, Chung GJ. Molecular inflammation in aging process. Nippon Ronen Igakkai Zasshi. 2004;41:357–364. doi: 10.3143/geriatrics.41.357. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim JW, Yu BP. The inflammation hypothesis of aging: molecular modulation by calorie restriction Ann. N Y Acad Sci. 2001;928:327–335. [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Kim KW, Choi JS, Yu BP. Molecular inflammation hypothesis of aging based on the anti-aging mechanism of calorie restriction. Microsc Res Tech. 2002;59:264–272. doi: 10.1002/jemt.10203. [DOI] [PubMed] [Google Scholar]
- Chung HY, Kim HJ, Shim KH, Kim KW. Dietary modulation of prostanoid synthesis in the aging process: role of cyclooxygenase-2. Mech Ageing Dev. 1999;111:97–106. doi: 10.1016/s0047-6374(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CE, Hasegawa G, Reddy KA, Omaye ST. Enhanced lung toxicity of O2 in selenium-deficient rats. Res Commun Chem Pathol Pharmacol. 1977;16:695–706. [PubMed] [Google Scholar]
- Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223–229. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2007;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42:19–29. doi: 10.1016/j.bone.2007.08.029. [DOI] [PubMed] [Google Scholar]
- DiStefano PS, Curtis R, Geddes BJ. Insulin resistance, glycemic control and adiposity: key determinants of healthy lifespan. Curr Alzheimer Res. 2007;4:153–157. doi: 10.2174/156720507780362038. [DOI] [PubMed] [Google Scholar]
- Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- Eanes ED. The interaction of supersaturated calcium phosphate solutions with apatitic substrates. Calcif Tissue Res. 1976;20:75–89. doi: 10.1007/BF02546399. [DOI] [PubMed] [Google Scholar]
- Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Fernbach SK. Pediatric gastrointestinal imaging. Curr Opin Radiol. 1992;4:117–123. [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Figueras M, Busquets S, Carbó N, Barreiro E, Almendro V, Argilés JM, López-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett. 2004;569:201–206. doi: 10.1016/j.febslet.2004.05.066. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Gao Z, Zuberi A, Quon MJ, Dong Z, Ye J. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J Biol Chem. 2003;278:24944–24950. doi: 10.1074/jbc.M300423200. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez C, Lopez-Soriano FJ, Argiles JM. Acute treatment with tumor necrosis factor-alpha induces changes in protein metabolism in rat skeletal muscle. Mol Cell Biochem. 1993;125:11–18. doi: 10.1007/BF00926829. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- Gupta A, Rosenberger SF, Bowden GT. Increased ROS levels contribute to elevated transcription factor and MAP kinase activities in malignantly progressed mouse keratinocyte cell lines. Carcinogenesis. 1999;11:2063–73. doi: 10.1093/carcin/20.11.2063. [DOI] [PubMed] [Google Scholar]
- Han KH, Han KO, Green SR, Quehenberger O. Expression of the monocyte chemoattractant protein-1 receptor CCR2 is increased in hypercholesterolemia. Differential effects of plasma lipoproteins on monocyte function. J Lipid Res. 1999;40:1053–1063. [PubMed] [Google Scholar]
- Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Klöting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- Helenius M, Hänninen M, Lehtinen SK, Salminen A. Aging-induced up-regulation of nuclear binding activities of oxidative stress responsive NF-kB transcription factor in mouse cardiac muscle. J Mol Cell Cardiol. 1996;28:487–498. doi: 10.1006/jmcc.1996.0045. [DOI] [PubMed] [Google Scholar]
- Hirose N, Akai Y, Gondoh Y. Tokyo centenarians study: aging inflammation hypothesis. Geriatr Gerontol Int. 2004;14:S182–S185. [Google Scholar]
- Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;9:992–994. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Stellbrink E, Schroder J, Grawe A, Vogel G, Blindt R, Kelm M, Radke PW. Impact of the metabolic syndrome on angiographic and clinical events after coronary intervention using bare-metal or sirelimus-eluting stents. Am J Cardiol. 2007;100:1347–1352. doi: 10.1016/j.amjcard.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Rozemuller AJ, Eikelenboom P. Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer’s disease. Curr Drug Targets. 2003;4:461–468. doi: 10.2174/1389450033490902. [DOI] [PubMed] [Google Scholar]
- Hoshino E, Pichard C, Greenwood CE, Huo GC, Cameron RG, Kurian R. Body composition and metabolic rate in rat during a continuous infusion of cachectin. Am J Physiol. 1991;260:E27–E36. doi: 10.1152/ajpendo.1991.260.1.E27. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1567–1568. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- Jankord R, Jemiolo B. Influence of physical activity on serum IL-6 and IL-10 levels in healthy older men. Med Sci Sports Exerc. 2004;36:960–964. doi: 10.1249/01.mss.0000128186.09416.18. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Chapman SM, Dong ZM, Ordovas JM, Mayadas TN, Herz J, Hynes RO, Schaefer EJ, Wagner DD. Absence of P-selectin delays fatty streak formation in mice. J Clin Invest. 1997;99:1037–1043. doi: 10.1172/JCI119231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144:952–961. [PMC free article] [PubMed] [Google Scholar]
- Jones RW. Inflammation and Alzheimer’s disease. Lancet. 2001;358:436–437. doi: 10.1016/S0140-6736(01)05667-7. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Kim JY, Zou Y, Kim YJ, Yu BP, Chung HY. Effect of short-term, low dose aspirin supplementation on the activation of pro-inflammatory NF-kappaB in aged rats. Mech Ageing Dev. 2006;127:223–230. doi: 10.1016/j.mad.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J Gerontol A Biol Sci Med Sci. 2006;61:211–217. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- Karin M. Role for IKK2 in muscle: waste not, want not. J Clin Invest. 2006;116:2866–2868. doi: 10.1172/JCI30268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NK, Menolascino FJ. Physicians’ awareness and attitudes toward the retarded. Ment Retard. 1975;13:10–13. [PubMed] [Google Scholar]
- Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim KW, Yu BP, Chung HY. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radic Biol Med. 2000;28:683–692. doi: 10.1016/s0891-5849(99)00274-9. [DOI] [PubMed] [Google Scholar]
- Koh JM, Khang YH, Jung CH, Bae S, Kim DJ, Chung YE, Kim GS. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int. 2005;89:735–742. doi: 10.1007/s00198-005-1840-5. [DOI] [PubMed] [Google Scholar]
- Korhonen P, Helenius M, Salminen A. Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci Lett. 1997;225:61–64. doi: 10.1016/s0304-3940(97)00190-0. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kumar S, Votta BJ, Rieman DJ, Badger AM, Gowen M, Lee JC. IL-1- and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J Cell Physiol. 2001;187:294–303. doi: 10.1002/jcp.1082. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, O’Banion MK, Whiteley PE, Daeschner JC, Olschowka JA. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Willerson JT. Prospects for cardiovascular research. JAMA. 2001;285:581–587. doi: 10.1001/jama.285.5.581. [DOI] [PubMed] [Google Scholar]
- Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103:1718–1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Wu L, Zeng Y. The effect of nuclear factor-kappaB protein expression in spiral ganglion cells of cochlea by overdosage sodium salicylate injection in guinea pigs. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2006;20:891–893. [PubMed] [Google Scholar]
- Makarov SS. NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today. 2000;6:441–448. doi: 10.1016/s1357-4310(00)01814-1. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- Mayo MW, Wang C, Cogswell PC. Requirement of NFκB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med. 2008;44:160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- McCormick RK. Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev. 2007;12:113–145. [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation of the brain in Alzheimer’s disease: implications for therapy. J Leukoc Biol. 1999;65:409–415. doi: 10.1002/jlb.65.4.409. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Yasojima K. Alzheimer disease and neuroinflammation. J Neural Transm Suppl. 2000;59:53–57. doi: 10.1007/978-3-7091-6781-6_8. [DOI] [PubMed] [Google Scholar]
- Merat S, Fruebis J, Sutphin M, Silvestre M, Reaven PD. Effect of aging on aortic expression of the vascular cell adhesion molecule-1 and atherosclerosis in murine models of atherosclerosis. J Gerontol A Biol Sci Med Sci. 2000;55:B85–B94. doi: 10.1093/gerona/55.2.b85. [DOI] [PubMed] [Google Scholar]
- Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE. Anorexia, sarcopenia, and aging. Nutrition. 2001;17:660–663. doi: 10.1016/s0899-9007(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res. 2001;24:163–176. doi: 10.1385/IR:24:2:163. [DOI] [PubMed] [Google Scholar]
- Moss SF, Blaser MJ. Mechanisms of disease: Inflammation and the origins of cancer. Nat Clin Pract Oncol. 2005;2:90–97. doi: 10.1038/ncponc0081. [DOI] [PubMed] [Google Scholar]
- Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epimiol Biomark Prev. 2005;14:2840–2847. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- Navab M, Berliner JA, Watson AD, Hama SY, Territo MC, Lusis AJ, Shih DM, Van Lenten BJ, Frank JS, Demer LL, Edwards PA, Fogelman AM. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991;88:1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–12. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okolo SN, Onwuanaku C, Okonji M, VanderJagt DJ, Millson M, Churchwell C. Glew RH Concentration of eight trace minerals in milk and sera of mother-infant pairs in northern Nigeria. J Trop Pediatr. 2000;46:160–162. doi: 10.1093/tropej/46.3.160. [DOI] [PubMed] [Google Scholar]
- Oshima H, Hara M, Kono T, Shibamoto Y, Mishima A, Akita S. Cardiac hemangioma of the left atrial appendage: CT and MR findings. J Thorac Imaging. 2003;18:204–206. doi: 10.1097/00005382-200307000-00012. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans--effect of intensity of exercise. Eur J Appl Physiol. 2000;83:512–515. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim HJ, Kwak HB, Lee TH, Bang MH, Kim CM, Lee Y, Chung DK, Baek NI, Kim J, Lee ZH, Kim HH. Inhibitory effects of Stewartia koreana on osteoclast differentiation and bone resorption. Int Immunopharmacol. 2007;7:1507–1516. doi: 10.1016/j.intimp.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Kotowicz MA, Henry MJ, Nicholson GC, Spilsbury HJ, Box JD, Schneider HG. High-sensitivity C-reactive protein and fracture risk in elderly women. JAMA. 2006;296:1353–1355. doi: 10.1001/jama.296.11.1353. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Bruunsgaard H. Possible beneficial role of exercise in modulating low-grade inflammation in the elderly. Scand J Med Sci Sports. 2003;13:56–62. doi: 10.1034/j.1600-0838.2003.20218.x. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N, van Hall G, Plomgaard P, Febbraio MA. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- Pedersen BK. IL-6 signaling in exercise and disease. Biochem Soc Trans. 2007;35:1295–1297. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- Peila R, Launer LJ. Inflammation and dementia: epidemiologic evidence. Acta Neurol Scand Suppl. 2006;185:102–106. doi: 10.1111/j.1600-0404.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- Peters SL, Mathy MJ, Pfaffendorf M, van Zwieten PA. Reactive oxygen species-induced aortic vasoconstriction and deterioration of functional integrity. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:127–128. doi: 10.1007/s002109900148. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57:43–51. [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber-specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Jr, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O’Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- Pretolani M. Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy. 1999;29:1164–1171. doi: 10.1046/j.1365-2222.1999.00456.x. [DOI] [PubMed] [Google Scholar]
- Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argilés JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002;280:55–63. doi: 10.1006/excr.2002.5624. [DOI] [PubMed] [Google Scholar]
- Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS., Jr A requirement for NF-κB activation in Bcr-Abl-mediated transformation. Gene Dev. 1998;12:968–981. doi: 10.1101/gad.12.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca A, Biroccio A, Del Bufalo D, Mackay AR, Santoni A, Cippitelli M. bvl-2 over-expression enhances NF-kappa B activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86:188–196. doi: 10.1002/(sici)1097-0215(20000415)86:2<188::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Robinson LE, Buchholz AC, Mazurak VC. Inflammation, obesity, and fatty acid metabolism: influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:1008–1024. doi: 10.1139/H07-087. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:E790–E800. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Roth SM, Metter EJ, Ling S, Ferrucci L. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18:625–630. doi: 10.1097/01.bor.0000245722.10136.6d. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54:S40–47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. doi: 10.1093/gerona/58.11.m1012. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007;65:S208–S212. doi: 10.1111/j.1753-4887.2007.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Roubenoff R, Parise H, Payette HA, Abad LW, D’Agostino R, Jacques PF, Wilson PW, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham Heart Study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Saito T, Oda Y, Sugimachi K, Kawaguchi K, Tamiya S, Tanaka K, Matsuda S, Sakamoto A, Iwamoto Y, Tsuneyoshi M. E-cadherin gene mutations frequently occur in synovial sarcoma as a determinant of histological features. Am J Pathol. 2001;159:2117–224. doi: 10.1016/s0002-9440(10)63063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236:13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Amyloid beta-peptide is produced by cultured cells during normal metabolism: a reprise. J Alzheimers Dis. 2006;9:163–168. doi: 10.3233/jad-2006-9s319. [DOI] [PubMed] [Google Scholar]
- Seo AY, Hofer T, Sung B, Judge S, Chung HY, Leeuwenburgh C. Hepatic oxidative stress during aging: effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Son TG, Zou Y, Yu BP, Lee J, Chung HY. Aging effect on myeloperoxidase in rat kidney and its modulation by calorie restriction. Free Radic Res. 2005;39:283–289. doi: 10.1080/10715760500053461. [DOI] [PubMed] [Google Scholar]
- Spaulding CC, Walford RL, Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- Spindler SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of calorie restriction. Mech Ageing Dev. 2005;126:960–966. doi: 10.1016/j.mad.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Staiger H, Haring HU. Adipocytokines: fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes. 2005;113:69–79. doi: 10.1055/s-2004-830555. [DOI] [PubMed] [Google Scholar]
- Steffens S, Mach F. Inflammation and atherosclerosis. Herz. 2004;29:741–748. doi: 10.1007/s00059-004-2634-9. [DOI] [PubMed] [Google Scholar]
- Sumitomo M, Tachibana M, Ozu C. Induction of apoptosis of cytokine-producing bladder cancer cells by adenovirus-mediated I kappa B alpha overexpression. Hum Gene Ther. 1999;10:37–47. doi: 10.1089/10430349950019174. [DOI] [PubMed] [Google Scholar]
- Sung B, Park S, Yu BP, Chung HY. Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KP, Pratico D. Novel therapeutic opportunities for Alzheimer’s disease: focus on nonsteroidal anti-inflammatory drugs. FASEB J. 2005;19:1592–1601. doi: 10.1096/fj.04-3620rev. [DOI] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Vick MM, Adams AA, Murphy BA, Sessions DR, Horohov DW, Cook RF, Shelton BJ, Fitzgerald BP. Relationships among inflammatory cytokines, obesity, and insulin sensitivity in the horse. J Anim Sci. 2007;85:1144–1155. doi: 10.2527/jas.2006-673. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- Wang B, Jenkins JR, Trayhum P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E731–E740. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]
- Wang GP, Deng ZD, Ni J, Qu ZL. Oxidized low density lipoprotein and very low density lipoprotein enhance expression of monocyte chemoattractant protein-1 in rabbit peritoneal exudate macrophages. Atherosclerosis. 1997;133:31–36. doi: 10.1016/s0021-9150(97)00109-3. [DOI] [PubMed] [Google Scholar]
- Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008 doi: 10.1002/jcp.21386. (in press) [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel FL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. Fat distribution and storage: how much, where, and how? Eur J Endocrinol. 2007;157:S39–S45. doi: 10.1530/EJE-07-0125. [DOI] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II-2–II-10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficail response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease-a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K. Metabolic syndrome and cognitive disorders: is the sum greater than its parts? Alzheimer Dis Assoc Disord. 2007;21:167–171. doi: 10.1097/WAD.0b013e318065bfd6. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, Xing L, Boyce BF. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem. 2007;282:18245–18253. doi: 10.1074/jbc.M610701200. [DOI] [PubMed] [Google Scholar]
- You T, Sonntag WE, Leng X, Carter CS. Lifelong caloric restriction and interleukin-6 secretion from adipose tissue: effects on physical performance decline in aged rats. J Gerontol A Biol Sci Med Sci. 2007;62:1082–1087. doi: 10.1093/gerona/62.10.1082. [DOI] [PubMed] [Google Scholar]
- Yu BP, Chung HY. Adaptive mechanisms to oxidative stress during aging. Mech Ageing Dev. 2006;127:436–443. doi: 10.1016/j.mad.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Yun AJ, Lee PY. Maldaptation of the link between inflammation and bone turnover may be a key determinant of osteoporosis. Med Hypotheses. 2004;63:532–537. doi: 10.1016/S0306-9877(03)00326-8. [DOI] [PubMed] [Google Scholar]
- Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem. 2001;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- Zou Y, Yoon S, Jung KJ, Kim CH, Son TG, Kim MS, Kim YJ, Lee J, Yu BP, Chung HY. Upregulation of aortic adhesion molecules during aging. J Gerontol A Biol Sci Med Sci. 2006;61:232–244. doi: 10.1093/gerona/61.3.232. [DOI] [PubMed] [Google Scholar]
- Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS, Schett G. Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum. 2006;54:463–472. doi: 10.1002/art.21626. [DOI] [PubMed] [Google Scholar]