Abstract
The Human Immunodeficiency Virus (HIV) Nef protein is necessary for high viral loads and for timely progression to AIDS. Nef plays a number of roles but its effect on antigen presentation and immune evasion are among the best characterized. Cytotoxic T Lymphocytes (CTLs) recognize and lyse virally infected cells by detecting viral antigens in complex with host major histocompatibility complex class I molecules (MHC-I) on the infected cell surface. The HIV Nef protein disrupts antigen presentation at the cell surface by interfering with the normal trafficking pathway of MHC-I and thus reduces CTL recognition and lysis of infected cells. The molecular mechanism by which Nef causes MHC-I downmodulation is becoming more clear, but some questions remain. A better understanding of how Nef disrupts antigen presentation may lead to the development of drugs that enhance the ability of the anti-HIV CTLs to control HIV disease.
I. INTRODUCTION TO HIV-1 PATHOGENESIS
A. Summary of the HIV pandemic
Despite major advances in research and treatment, the human immunodeficiency virus (HIV) continues to persist as a pandemic. 33.4 million people are currently living with HIV, including 2.1 million children. In 2009 there were 2.7 million new infections and 2 million people died of acquired immunodeficiency syndrome (AIDS) (UNAIDS, 2009). While great progress has been made in drug therapies that dramatically decrease mortality and prevent mother to child transmission, a cure for the disease remains an elusive goal and an effective, prophylactic vaccine is not yet in hand.
B. Natural History of Untreated Disease
Following initial infection by HIV, there is evidence that a partially effective immune response reduces viral levels to a viral setpoint, the magnitude of which has prognostic significance with respect to how rapidly disease progression occurs (Mellors et al., 1996). HIV preferentially infects and destroys activated CD4+ T lymphocytes, including those that are HIV-specific, which eventually leads to a defective anti-HIV immune response (Douek et al., 2002). Once the total CD4+ T cell count reaches <200 cells per microliter of blood, the clinical definition of AIDS, the immune system is functionally impaired and HIV infected individuals become susceptible to opportunistic infections.
C. The Virus
Like all retroviruses, HIV-1 reverse transcribes its single-stranded RNA (ssRNA) genome into a DNA intermediate prior to integration into the host cell genomic DNA. HIV-1 causes a chronic infection with a long incubation period, characteristic of viruses categorized into the genus lentivirus,. Also typical of retroviruses, HIV encodes group antigen (gag), polymerase (pol), and envelope (env) (Figure 1). (for review see (Ganser-Pornillos, Yeager and Sundquist, 2008), (Hill, Tachedjian and Mak, 2005)). HIV also encodes tat and rev, which promote transcription of the viral genome and nuclear export of viral RNA, respectively (for review see (Nekhai and Jeang, 2006)).
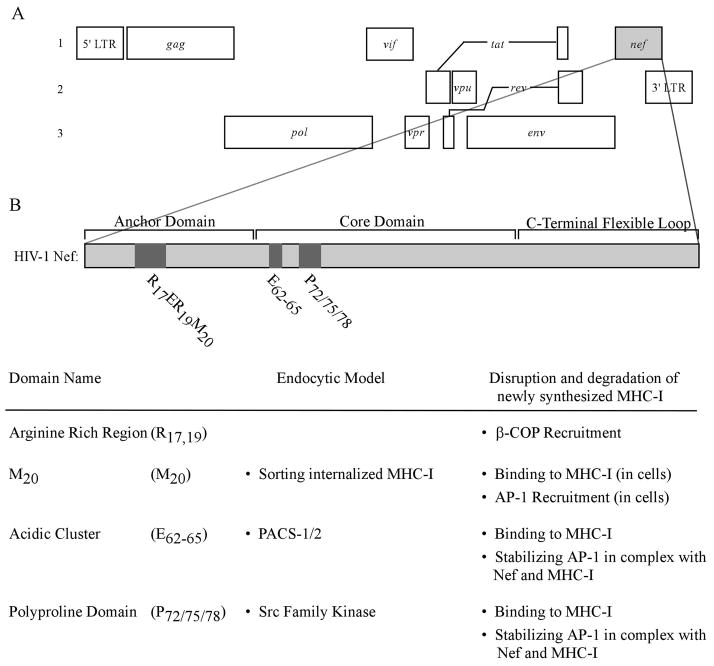
Figure 1.
The genome of HIV-1 and a detailed view of domains in Nef.
(A) Three reading frames are shown to reveal HIV-1 genes and their relative genome locations. Open reading frames are shown as rectangular boxes. The spliced reading frames, tat and rev, are shown as boxes connected by lines. (B) Domains in Nef that are pertinent to reducing the surface expression of MHC-I and their proposed functions in either of the two models of MHC-I downmodulation.
HIV is unique among retroviruses in that it has acquired accessory genes vif, vpr, vpu, and nef, which encode proteins that optimize viral fitness and spread in the host. The Vif protein counteracts intrinsic antiviral factor APOBEC3G by targeting it for degradation. Like Vif, Vpu also associates with a cellular ubiquitin ligase complex to degrade cellular targets, including the viral receptor CD4 and the intrinsic antiviral protein tetherin. Degradation of these targets allows more efficient budding of nascent virions. The role of Vpr is not entirely clear, but it is known that Vpr induces a G2 mitotic arrest in infected cells, a state which favors transcription from the HIV-1 LTR. Recent data indicates that Vpr associates with a cellular ubiquitin ligase complex to degrade cellular factors that may otherwise inhibit viral infection and/or spread in the host (for review see (Douglas et al., 2010; Kirchhoff, 2010))
Finally, the viral accessory protein Nef is a multifunctional protein that disrupts intracellular signaling and trafficking pathways to favor viral infection and spread. Nef has been reported to alter the intracellular trafficking of a variety of proteins, such as major histocompatibility complex proteins class I (MHC-I), CD4, CD28, and CD8 (Garcia and Miller, 1991; Schwartz et al., 1996; Stove et al., 2005; Swigut, Shohdy and Skowronski, 2001). This review will focus on the role of Nef in MHC-I downmodulation.
II. HIV-1 Nef
Non-human primate research has revealed that the simian immunodeficiency virus (SIV) Nef protein is required for eventual immune collapse. In fact, rhesus macaques infected with a Nef-deleted (Δnef) strain of SIV do not progress to AIDS (Kestler et al., 1991).
In addition there is a cohort of blood transfusion recipients exposed to an HIV-1 variant (Learmont et al., 1992) that contained a significant deletion in the viral genome including part of the nef gene and the long terminal repeat (LTR) (Deacon et al., 1995). Decades after infection and without anti-retroviral treatment (Dyer et al., 1997) none of these patients have been reported to progress to AIDS, though some do have reduced CD4+ T cell counts (Birch et al., 2001; Learmont et al., 1999). These patients are considered long term non-progressors (LTNPs). The combination of non-human primate research and longitudinal patient cohort studies has revealed the requirement of Nef for progression from HIV disease to AIDS.
A. Disruption of Antigen Presentation to Cytotoxic T Lymphocytes
CD8+ cytotoxic T lymphocytes (CTLs) are important for the control of chronic viral infections. CTLs bear receptors capable of distinguishing “self” from “non-self” peptide antigens presented by MHC-I on the cell surface (Figure 2). Normal cellular peptides typically do not activate a CTL response. However, in a virally infected cell, MHC-I molecules also present peptides derived from viral proteins (“non-self” peptides). Once the T cell receptor (TCR) on CD8+ CTLs recognize a “non-self” signal presented by MHC-I, the CTL releases perforins and granzymes which kill the virally infected cell preventing further spread of the virus (reviewed in (Berke, 1995)).
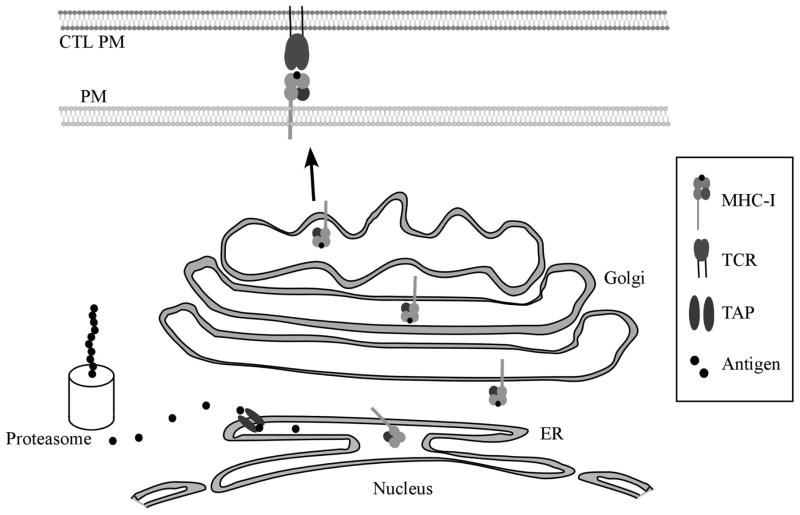
Figure 2.
Antigen presentation by class-I major histocompatibility complexes (MHC-I).
Intracellular peptides (antigens) are produced through protein synthesis and subsequent breakdown by proteasomes. The peptides are then transported into the endoplasmic reticulum through the transporter associated with antigen processing (TAP) and loaded onto MHC-I molecules. Complete MHC-I molecules are transported through the Golgi Network out to the plasma membrane (PM) where the antigen is recognized as “self” or “non-self” by the T Cell Receptor (TCR) on a CD8+ CTL. Cells expressing MHC-I in complex with “non-self” peptides are lysed to minimize spread of infection.
There is a great deal of evidence that CTLs play an important role in the control of HIV infection (for review see (Collins, 2004)) and recent evidence indicates that individuals mounting a Gag-specific CTL response have improved parameters with regard to controlling disease (Geldmacher et al., 2007; Kiepiela et al., 2007). Despite the efficacy with which CTLs control viral load early in infection, anti-HIV CTLs ultimately fail to prevent progression of disease. There is evidence that antigenic variation, viral effects on CTL differentiation, viability, proliferative capacity and function influence the ability of CTLs to control HIV infection. However, this review will focus on the effect of the HIV Nef protein on antigen presentation by the infected cell.
Studies performed in vitro have detected different degrees of susceptibility of HIV-infected T cells to anti-HIV CTL killing (Collins et al., 1998; Shankar, Xu and Lieberman, 1999; Yang et al., 1996; Yang et al., 1997). These differences are probably due to the use of viral strains that have variable expression of the HIV Nef protein. Under conditions where killing of HIV-infected cells was directly compared plus or minus Nef expression, it was clear that Nef protected infected cells from CTL-mediated lysis (Collins et al., 1998; Lewinsohn et al., 2002; Tomiyama et al., 2002; Yang et al., 2002). Nef has been shown to protect HIV-infected primary T cells from CTL lysis using flow cytometric killing assays (Collins et al., 1998; Lewinsohn et al., 2002), CTL co-culture assays (Yang et al., 2002) and chromium release assays (Tomiyama et al., 2002). Although Nef limits the ability of CTLs to recognize and kill infected cells, it does not appear to abrogate the capacity of CTLs to produce inhibitory cytokines in response to infected cells (Tomiyama et al., 2002). Recent in vivo evidence supports the hypothesis that CTLs may control HIV infection in vivo primarily by the elaboration of inhibitory cytokines, but fail to eradicate the infection because the CTLs cannot efficiently lyse the infected cell source of new virions (Wong et al., 2010).
B. In vivo Evidence for Nef-induced MHC-I Downmodulation
Based on in vivo studies, it is known that an intact nef gene is necessary for the timely development of AIDS in most humans and monkeys (Deacon et al., 1995; Kestler et al., 1991; Kirchoff et al., 1995). However, Nef has multiple functions, therefore these studies do not prove an important role for Nef-mediated MHC-I downmodulation in vivo. Several studies have used SIV systems to demonstrate that the capacity to downmodulate MHC-I is selected for in vivo (Carl et al., 2001; Munch et al., 2001; Swigut et al., 2004). In addition, it was recently demonstrated that the ability of in vivo-derived Nef to down-regulate MHC-I predicted the resistance of HIV-1 to suppression by CTL in vitro (Lewis et al., 2008). Taken together, these data demonstrate that the ability of Nef to down-regulate MHC-I in vivo is maintained by the need of HIV-1 to cope with the antiviral CTL response.
C. Natural killer cells
To counteract the effects of viral pathogens on MHC-I expression, natural killer (NK) cells monitor the overall surface levels of MHC-I. Low expression of MHC-I can activate NK cells to lyse target cells. There are three classical MHC-I genes expressed by all nucleated cells; HLA-A, HLA-B and HLA-C. These genes are highly polymorphic and hundreds of alleles of each have been identified. HLA-A and HLA-B are the primary allotypes that present antigens to CTLs, whereas HLA-C may function primarily to regulate NK cell function. In addition, a non-classical MHC-I called HLA-E, which does not commonly present antigens to CTLs also inhibits NK cell function (reviewed in (Natarajan et al., 2002)).
Nef has been shown to directly interact with an amino acid sequence (Y320SQAASS326) present in the cytoplasmic tail of HLA-A and HLA-B molecules (Williams et al., 2002). This region of the MHC-I cytoplasmic tail is also necessary for Nef-dependent downmodulation of MHC-I molecules (Cohen et al., 1999; Le Gall et al., 1998; Williams et al., 2002). In contrast, HLA-C and HLA-E have amino acid variations within this domain (Cohen et al., 1999; Le Gall et al., 1998; Williams et al., 2002) and thus remain unaffected by Nef. Therefore it has been proposed that Nef selectively downmodulates a subset of MHC-I molecules to evade CTL killing without activating NK cell lysis. However, recent evidence demonstrating that HLA-C is expressed at very low levels on primary T cells suggests that additional mechanisms may be necessary to fully explain HIV evasion of NK cells (Schaefer, 2007).
D. Functional Domains Required for Nef to Downmodulate MHC-I
Nef can be divided into the N-terminal anchor domain, the core domain, and the C-terminal flexible loop (Figure 1). Two sites in Nef are required for most of Nef’s functions. First, Nef is myristoylated at the glycine residue at position 2, which allows Nef to bind the inner leaflet of the plasma membrane (Fackler et al., 1999). In addition, an aspartic acid at position 123 (D123) is required to form homodimers of Nef (Liu et al., 2000). If either of these sites is mutated (G2A or D123G), Nef is inactive for almost all of its functions.
Three regions of Nef, an N-terminal α-helix (R17ERM20RRAEPA26 and specifically M20 ) (Akari et al., 2000; Mangasarian et al., 1999), an acidic cluster (E62–65), and a polyproline repeat (P72/75/78) are required for Nef to bind to the cytoplasmic tail of MHC-I (Williams et al., 2005) and for Nef to downmodulate MHC-I (Greenberg, Iafrate and Skowronski, 1998; Mangasarian et al., 1999) (Figure 1). The C-terminal loop of Nef contains a number of trafficking signals capable of binding adaptor proteins, a coatomer protein, and a vacuolar ATPase (for review see (Roeth and Collins, 2006)). However, for unclear reasons, the C-terminal loop of Nef is only active against other Nef targets, such as CD4 (Mangasarian et al., 1999) unless Nef is directly fused to the MHC-I cytoplasmic tail (Wonderlich, Williams and Collins, 2008). These observations suggest that there are structural constraints that limit the ability of the C-terminal loop to recruit trafficking factors when Nef is bound to MHC-I with its natural conformation.
III. Candidate host factors that partner with Nef
A. Adaptor protein complexes
Clathrin-coated vesicles transport cargo from the trans-Golgi network, plasma membrane, or endosomal network. Clathrin-associated adaptor proteins (APs) are composed of four subunits: two large subunits (β1 or β2 and AP-1γ, AP-2α, or AP-3δ), one medium subunit (μ), and one small subunit (σ) (Robinson, 2004; Robinson and Bonifacino, 2001; Traub, 2003). The four subunits combine to function as a heterotetrameric adaptor complex that recognizes Yxxφ (Y, tyrosine; φ, bulky hydrophobic amino acid; x, any amino acid) and [D/E]xxxLL (D, aspartic acid; E, glutamic acid; L, leucine) sorting signals and recruits clathrin coats. AP-1 transports proteins between the trans-Golgi network and endosomes (Doray et al., 2002; Klumperman et al., 1993; Waguri et al., 2003). AP-2 localizes to the plasma membrane and is necessary for internalization of some types of cargo into endosomes (Traub, 2003). AP-3 localizes to endosomes and is thought to transport proteins into acidic, degradative compartments (Peden et al., 2004).
Recent structural studies have provided confirmation that clathrin adaptor proteins have physically separate signal-recognition sites for Yxxφ and [D/E]xxxLL motifs. The μ subunit contains a tyrosine binding pocket (TBP) and a hydrophobic binding pocket, which recognize Yxxφ signals (Owen and Evans, 1998). In contrast, a hydrophobic pocket in the σ2 subunit plus a positively charged patch made from residues in both the σ2 and α subunits combine to form the recognition site for [D/E]xxxLL motifs (Kelly et al., 2008).
Yeast two-hybrid assays initially revealed that HIV Nef’s C-terminal dileucine motif (LL164,165) interacts with the μ subunit of AP-1 and AP-3 (Bresnahan et al., 1998; Craig, Pandori and Guatelli, 1998; Craig et al., 2000; Erdtmann et al., 2000; Greenberg et al., 1998; Janvier et al., 2003a; Janvier et al., 2003b; Le Gall et al., 1998; Piguet et al., 1998). However, consistent with the structural analysis described above, a much more robust interaction occurs between Nef’s dileucine motif and hemicomplexes composed of σ and γ or δ subunits (Janvier et al., 2003b). Recent data suggest that a robust interaction between the μ subunit of AP-1 and Nef also occurs, but only when Nef is bound to the MHC-I cytoplasmic tail. In this case, the MHC-I cytoplasmic tail provides the tyrosine residue necessary for binding to the AP-1 μ1 subunit tyrosine binding pocket (Noviello, Benichou and Guatelli, 2008; Roeth et al., 2004; Singh et al., 2009; Wonderlich, Williams and Collins, 2008).
B. β-COP
COP-I and COP-II coatomers are needed for normal protein trafficking within the Golgi and ER (Rothman, 1994; Schmid, 1997). More recently, COP-I coatomers have been found associated with low pH endosomes in an ARF-1-dependent manner (Aniento et al., 1996; Gu and Gruenberg, 2000). These COP-I coatomers are implicated in recycling endosome function (Daro et al., 1997) and in transport from the endolysosomal network into multivesicular bodies (Gu et al., 1997).
The COP-I subunit β-COP was first identified as a binding partner for Nef in a yeast two-hybrid screen (Benichou et al., 1994). A diacidic motif (EE155, 156) in the C-terminal loop domain of Nef mediates this interaction and targets internalized CD4 to degradative compartments. (Faure et al., 2004; Piguet et al., 1999; Schaefer et al., 2008). The interaction between Nef and βCOP requires ARF-1 but interestingly ARF-1 does not need to be in the activated, GTP bound state (Faure et al., 2004). β-COP binding to a separate region in the N-terminal alpha helical domain of Nef has also been implicated in targeting MHC-I for degradation (Schaefer et al., 2008).
C. PACS proteins
There is evidence that Nef interacts with phosphofurin acidic cluster sorting proteins (PACS-1 and PACS-2) through its acidic cluster E62–65 (Piguet et al., 2000). PACS 1 and 2 were originally discovered by studying proteins that bind to the phosphorylated cytoplasmic tail of furin (Wan et al., 1998). Models for PACS-1 and PACS-2 function propose that these proteins help recruit AP-1 or AP-3 to cargo with acidic clusters (Crump et al., 2001). While it has yet to be found in coated vesicles (cited as data not shown in (Youker et al., 2009)), PACS has been shown to recruit AP-1 to a protein important for vesicle-membrane fusion, the SNARE vesicle-associated membrane protein (VAMP)-4 (Hinners et al., 2003). Antisense to hPACS-1a increases steady state MHC-I surface expression in Nef-expressing cells by about 20% and redistributes the intracellular localization of MHC-I and a CD4-Nef fusion protein in A7 astrocytic cells. Based on these data a model was proposed in which Nef physically recruits MHC-I and links it to a PACS-1 based TGN retrieval pathway (Piguet et al., 2000). This model was later modified to indicate that PACS-2 was more important for localizing Nef to the TGN and that PACS-1 played a greater role in recycling in mouse embryonic fibroblasts and HeLa cells (Atkins et al., 2008). Another group confirmed that knockdown of PACS-1 inhibited Nef-induced MHC-I downmodulation in HeLa cells but not Jurkat T cells (Yi et al., 2010). Arguing against an important role for PACS proteins is data from other investigators who reported no effect of knocking down PACS-1 on Nef-induced downregulation of MHC-I HLA-A2 or on the localization of other proteins containing acidic cluster motifs in HeLa cells (Lubben et al., 2007). Additionally, Baugh et al was unable to demonstrate a significant interaction between the acidic cluster in Nef and the PACS-1 furin binding region (Baugh, Garcia and Foster, 2008). Moreover, mutating three of the four glutamates in the acidic cluster only decreased Nef’s effects on MHC-I by about 50% (Baugh, Garcia and Foster, 2008).
D. ADP-ribosylation factors
In addition to the clathrin-associated adaptor proteins, the small GTPases, ADP-ribosylation factors (ARFs), are important for cellular control of assembly and disassembly of various intracellular trafficking complexes (Balch, Kahn and Schwaninger, 1992; Kahn et al., 1992). ARFs are important for clathrin dependent (Donaldson et al., 1992; Orcl et al., 1993; Palmer et al., 1993; Robinson and Kreis, 1992) and clathrin independent (Taylor, Kahn and Melancon, 1992; Waters, Serafini and Rothman, 1991) trafficking pathways. ARF activation and recruitment to cellular membranes is cyclical and regulated by its GTP binding state. Guanine nucleotide exchange factors (GEFs) are required for the recruitment of GTP to ARF and are necessary for the maintenance of overall Golgi structure ((Helms and Rothman, 1992; Jackson and Casanova, 2000) and reviewed in (Donaldson and Klausner, 1994)). GTPase-activation proteins (GAPs) promote GTP hydrolysis, thus inactivating ARFs (Turner, West and Brown, 2001). ARF-6 localizes to the plasma membrane and is involved in clathrin-independent endocytosis and recycling (Radhakrishna and Donaldson, 1997).
E. ARF-6 and PI3-kinase
ARF-6 is regulated by ARNO, an ARF-6 GEF that is activated and recruited to the plasma membrane by PI3-kinase(Venkateswarlu and Cullen, 2000). There is evidence that overexpression of ARF-6 and ARNO mutants alters the intracellular localization of MHC-I in Nef-expressing HeLa cells and that overexpression of Nef and PACS-1 in A7 cells increases PI3-kinase-dependent GTP loading of ARF (Blagoveshchenskaya et al., 2002). A relatively small effect (approximately two fold) of a dominant negative ARF-6 mutant was noted in primary T cells when pan-MHC-I antibodies were used (Yi et al., 2010). These antibodies recognize all MHC-I allotypes, including those that are unaffected by Nef and thus relatively small effects of Nef are usually detected. Based on these data, the prior model of how PACS proteins were involved in Nef-dependent MHC-I trafficking was modified. Instead of proposing that Nef physically recruits MHC-I and links it to a PACS-1 based TGN retrieval pathway, it was instead proposed that PACS proteins were needed to localize Nef to the TGN and that this localization of Nef was important for PI3-kinase and ARF-6 activation (Blagoveshchenskaya et al., 2002) (Figure 3). Subsequent studies provided evidence that localization of Nef to the TGN was important for Nef to recruit a SRC family tyrosine kinase needed for activation of a kinase cascade that culminated in PI3-kinase activation (Hung et al., 2007). Arguing against this possible model is evidence that additional, more specific ARF-6 mutants had no effect on MHC-I downmodulation in Jurkat T cells (Larsen et al., 2004). Furthermore, inhibition of PI3-kinase, had no effect on the internalization step in U373mg astrocytoma cells (Larsen et al., 2004). Instead, other investigators provided evidence that PI3-kinase inhibitors affected localization of intracellular MHC-I to the TGN in Nef-expressing U373mg astrocytoma cells (Larsen et al., 2004; Swann et al., 2001).
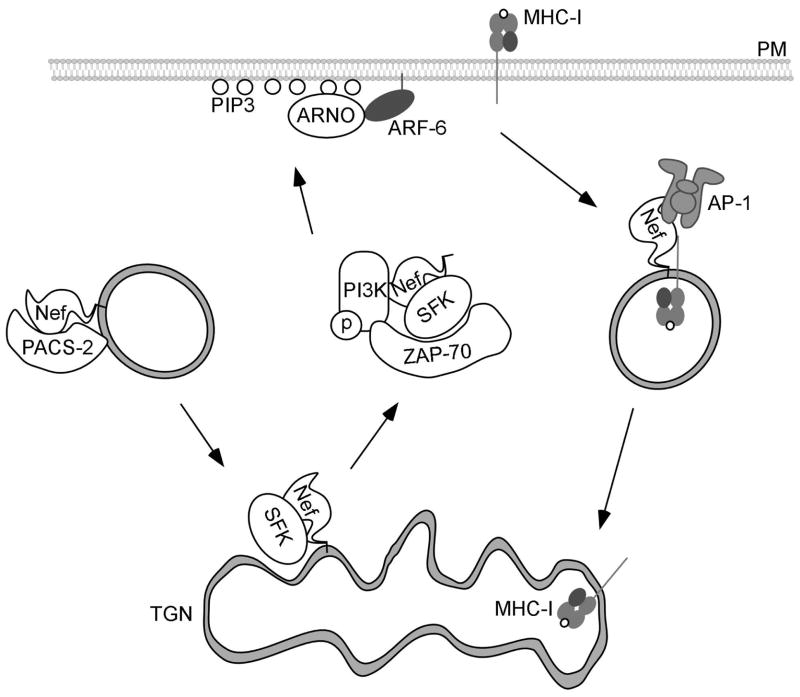
Figure 3.
Nef-induced endocytosis of MHC-I.
In this model, Nef is bound by the acidic cluster sorting protein, PACS-2, and localized to the trans-Golgi network (TGN). Nef binds to the Src Family kinase (SFK), Hck, which activates the tyrosine kinase ZAP-70. ZAP-70 then binds to and activates PI-3-Kinase. PI-3-Kinase creates PIP3 on the inner leaflet of the plasma membrane (PM), which recruits the ARF-6 GEF, ARNO, subsequently recruiting and activating ARF-6. MHC-I is then internalized by an ARF-6 – dependent mechanism into endosomes. Nef then recruits AP-1, which transports MHC-I to the TGN.
IV. Downmodulation of MHC-I: Endocytic Mechanism
Initial studies examining the effects of Nef on MHC-I trafficking in T cell lines revealed that the rate of MHC-I synthesis and trafficking through the ER and cis-Golgi is unaffected by Nef, but that MHC-I stability over time is decreased through lysosomal degradation (cited as data not shown (Schwartz et al., 1996). Furthermore, Nef causes an accumulation of MHC-I in juxtanuclear and endosomal compartments and enhances the rate of endocytosis in some cell types (for review see (Roeth and Collins, 2006)). T lymphocytes and macrophages spontaneously internalize and recycle MHC-I back to the plasma membrane at high rates in an AP-2 dependent manner (Machy et al., 1987; Tse and Pernis, 1984). However, in Nef-expressing cell lines, over-expression of a dominant negative dynamin (Le Gall et al., 2000; Swann et al., 2001) or a dominant negative mutant subunit of AP-2 (Blagoveshchenskaya et al., 2002) did not affect Nef-induced MHC-I endocytosis suggesting this process could be clathrin and AP-2 independent. More recently it was shown that a dominant negative dynamin reduced Nef-induced MHC-I downmodulation in primary T cells from about 50% in this assay system to approximately 25% (Yi et al., 2010). Furthermore, Greenberg et al determined that MHC-I co-localizes with AP-1, and not AP-2 in Nef-expressing cells (Greenberg, Iafrate and Skowronski, 1998) arguing against an AP-2-dependent internalization pathway. As discussed above, there is evidence for an ARF-6-dependent, clathrin-independent pathway by which Nef affects MHC-I, at least in some cell types (Figure 3).
V. Downmodulation of MHC-I: Evidence for targeting of newly synthesized protein in the secretory pathway
A. Disruption of MHC-I transport
A fairly dramatic effect of Nef on MHC-I is required for HIV-infected primary T cells to effectively evade anti-HIV CTLs (up to 300-fold reduction (cited as data not shown (Collins et al., 1998)). The degree of downmodulation of MHC-I in HeLa cells (2–4 fold reduction (Blagoveshchenskaya et al., 2002) is small relative to the effect of Nef on an endogenous MHC-I allotype (HLA-A2) in HIV-infected primary T lymphocytes (Collins et al., 1998). Thus, the internalization pathways described mainly in HeLa cells may not fully explain the intracellular trafficking required for the maximal effect of Nef necessary for HIV immune evasion in T cells. Indeed, direct comparison of Nef activity in HeLa versus T cell lines revealed striking differences in the degree of MHC-I downmodulation (Kasper and Collins, 2003).
Most viruses that disrupt antigen presentation target newly synthesized MHC-I rather than “old” MHC-I at the cell surface because the newly synthesized molecules harbor viral antigens present at the time of infection. For example, Herpes Simpex Virus, Human Cytomegalovirus, Epstein-Barr Virus, and Adenovirus all encode proteins that block peptide translocation into the ER, target nascent MHC-I for degradation, induce ER retention of peptide-loaded MHC-I, or prevent transport of MHC-I to the plasma membrane (for review see(Hansen and Bouvier, 2009)). Alternatively, older MHC-I are likely to be presenting cellular antigens, which are present prior to infection, and therefore would not be a threat to the virus. In fact, MHC-I loaded with cellular antigens would be protective against NK cell recognition.
In Nef-expressing cells, previous reports of MHC-I localizing to the trans-Golgi and AP-1-containing vesicles suggested that Nef could be directly disrupting MHC-I trafficking at the trans-Golgi network rather than only affecting MHC-I after it had reached the cell surface. The first experiment supporting this model examined the effect of Nef on an HLA-A2-GFP fusion protein in U373mg astrocytoma cells (Swann et al., 2001). In this series of experiments investigators utilized a temperature block (20°C ) to prevent TGN exit and to allow accumulation of MHC-I in the TGN. When cells were subsequently shifted to 37°C, MHC-I could be detected by microscopy at the cell surface within 15 minutes, whereas in Nef-expressing cells MHC-I remained within a juxta-nuclear compartment (Swann et al., 2001). These investigators were the first to report an effect of PI-3 kinase inhibitors on this pathway. However, long incubation times (overnight) with inhibitors were required to observe substantial re-accumulation of MHC-I at the cell surface in Nef-expressing cells. Biochemical experiments examining the transport of newly synthesized MHC-I to the cell surface in T cell lines confirmed that there was a dramatic effect of Nef on the transport of MHC-I to the cell surface. Moreover, the effect of Nef on transport of newly synthesized MHC-I was much greater than its effect on MHC-I internalization (Kasper and Collins, 2003). An effect of Nef on intracellular transport of endogenous MHC-I HLA-A2 was confirmed in HIV-infected primary T cells (Kasper and Collins, 2003). PI3-kinase inhibitors did not reduce the ability of Nef to disrupt MHC-I transport to the cell surface as measured by a one hour biochemical assay but the investigators could not rule out an effect of PI3-kinase on the intracellular localization of retained MHC-I molecules (Kasper and Collins, 2003) as was subsequently proposed (Larsen et al., 2004).
The HLA-A2 cytoplasmic tail is phosphorylated at specific serines in vivo upon exiting the TGN (Eichholtz et al., 1992). Interestingly Nef preferentially binds immature, hypophosphorylated forms of HLA-A2 and inhibits phosphorylation of the MHC-I cytoplasmic tail (Kasper et al., 2005). Based on these data it was proposed that Nef binds MHC-I very early in the secretory pathway (Kasper et al., 2005). In support of this model, a recent study was able to observe a Nef-CFP fusion protein in complex with a subset of HLA-A2-Venus in the ER as well as in the Golgi and at the plasma membrane of HeLa cells using two photon two color fluorescence cross correlation spectroscopy (Yi et al., 2010). However, there was no detectable effect of Nef on MHC-I transport until MHC-I reached the trans-Golgi apparatus, thus binding to Nef was not sufficient for disruption of MHC-I trafficking (Kasper et al., 2005; Roeth et al., 2004)(Figure 4).
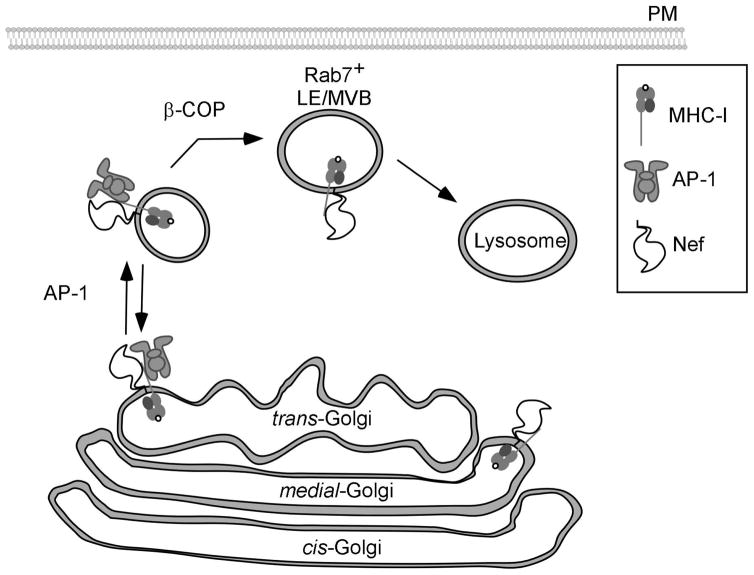
Figure 4.
Nef reroutes newly synthesized MHC-I into the endolysosomal network.
Nef binds to the cytoplasmic tail of MHC-I early in the secretory pathway. Nef blocks normal secretion of MHC-I to the plasma membrane by recruiting AP-1 to redirect MHC-I into the endosomal network. β-COP is then required to transport MHC-I bound to Nef from the endosomal network into late endosomal compartments for subsequent degradation in lysosomes.
B. AP-1 is a host factor that is required for disruption of antigen presentation by HIV Nef
Because AP-1 was a clathrin adaptor protein that acted at the TGN and because Nef had been reported to interact with AP-1, it was hypothesized that Nef might disrupt post-TGN transport of MHC-I by promoting an interaction between MHC-I and AP-1. Indeed, RNAi directed against AP-1 μ1 inhibited downmodulation of endogenous HLA-A2 in U373mg astrocytoma cells and exogenous HLA-A2 expressed in CEM-SS T cells (Roeth et al., 2004). Recently, siRNA directed against AP-1 μ1 also abolished Nef-induced downmodulation of MHC-I in HeLa and Jurkat cell lines, as well as in primary T Lymphocytes (Yi et al., 2010).
In addition, AP-1 co-precipitated with Nef and endogenous HLA-A2 from lysates made from HIV infected primary T cells (Roeth et al., 2004). In contrast, complexes of Nef-MHC-I and AP-1 were not detected in HeLa cells unless the cells were incubated at room temperature overnight. Further experiments revealed that temperature reduction decreased the rate of MHC-I trafficking sufficiently to allow the Nef-MHC-I-AP-1 complex to form. For unclear reasons, T cells naturally traffic MHC-I at slower rates and lower incubation temperatures do not change the ability of Nef to form this complex (Kasper et al., 2005). These data help explain why investigators that focused on non-T cell lines did not detect this pathway.
C. Nef stabilizes an interaction between the AP-1 tyrosine binding pocket and an extended domain on the MHC-I cytoplasmic tail that includes a tyrosine residue
Yeast two-hybrid interaction assays and microscopic analyses provided evidence that interactions between Nef and the adaptor proteins AP-1 and AP-3 depend on Nef’s dileucine motif (Bresnahan et al., 1998; Craig, Pandori and Guatelli, 1998; Craig et al., 2000; Erdtmann et al., 2000; Greenberg et al., 1998; Janvie ret al., 2003a; Janvier et al., 2003b; Piguet et al., 1998). In contrast, MHC-I downmodulation and AP-1 recruitment in T cell systems did not require these amino acids (Greenberg, Iafrate and Skowronski, 1998; Roeth et al., 2004; Williams et al., 2005). Thus, the complex between Nef-MHC-I and AP-1 most likely occurred independently of the dileucine motif and involved a separate AP-1 binding domain. Indeed, it was demonstrated that the MHC-I cytoplasmic tail mediated a key interaction between the Nef-MHC-I complex and AP-1 (Roeth et al., 2004). Remarkably, the tyrosine in the MHC-I cytoplasmic tail does not form a canonical Yxxφ AP-1 sorting signal and does not bind AP-1 in T cells in the absence of Nef. However, Nef binding to the cytoplasmic tail provides the necessary elements for this non-canonical tyrosine signal to function as a potent AP-1 binding motif (Roeth et al., 2004).
Additional mutational analysis of the MHC-I cytoplasmic tail revealed two other amino acids (A324 and D327) that were needed for co-precipitation of AP-1 but not Nef. Interestingly, all three MHC-I cytoplasmic tail amino acids necessary for formation of the Nef-MHC-I-AP-1 complex are only found in HLA-A and HLA-B allotypes but not in HLA-C or HLA-E. The fact that these three amino acids (Y320, A324, and D327) are important for co-precipitation of AP-1 suggests that this binding site may have a normal and as yet unidentified function in uninfected cells. Consistent with the fact that this site can be utilized by AP-1 in the absence of Nef, mutation of the cytoplasmic tail to create a somewhat more hydrophobic signal (Y320SQV323) allowed for AP-1 recruitment in the absence of Nef and significantly enhanced Nef’s ability to downmodulate HLA-A2 and recruit AP-1 (Wonderlich, Williams and Collins, 2008). Providing further support for the model that Nef stabilized an interaction between the AP-1 tyrosine-binding pocket (TBP) and the tyrosine residue in the MHC-I cytoplasmic tail, it was shown that a dominant negative mutant of AP-1 μ1 that had two amino acid substitutions in the tyrosine binding pocket (TBPM) dramatically and specifically inhibited Nef-mediated MHC-I downmodulation (Wonderlich, Williams and Collins, 2008).
D. Nef domains and AP-1-dependent MHC-I trafficking
All of the domains of Nef that are required for MHC-I downmodulation are also required for Nef to interact with the MHC-I cytoplasmic tail (Williams et al., 2005). To determine whether some of these domains might also be important for recruitment of AP-1, a fusion protein between MHC-I and Nef was examined (Roeth et al., 2004). These studies confirmed that the MHC-I cytoplasmic tail tyrosine was required for AP-1 recruitment and that Nef’s dileucine motif was dispensable for this interaction (Roeth et al., 2004; Wonderlich, Williams and Collins, 2008). In this system, the acidic cluster (E62–65) and polyproline helix (P72/75/78) of Nef were dispensable for AP-1 recruitment as long as a chemical crosslinker was used (Roeth et al., 2004). However, when a digitonin detergent based buffer that lacked crosslinker was substituted, a requirement for these domains to stabilize the interaction between AP-1 and MHC-I was noted (Wonderlich, Williams and Collins, 2008). In addition, the N-terminal α-helix and specifically M20, were required for AP-1 recruitment under all conditions tested (Roeth et al., 2004; Wonderlich, Williams and Collins, 2008). Therefore, at least three Nef domains are required for AP-1 recruitment and subsequent downmodulation of MHC-I in Nef expressing cells (Roeth et al., 2004; Wonderlich, Williams and Collins, 2008).
E. Binding studies with purified proteins
Experiments using purified Nef-MHC-I cytoplasmic tail fusion proteins and either whole AP-1 complexes from crude lysates or purified μ1 subunit support the conclusion that Nef stabilizes an interaction between the MHC-I cytoplasmic tail and the AP-1 μ-1 subunit. Moreover, these experiments using purified protein provide evidence that the polyproline helix and the acidic domain within Nef are needed for Nef to stabilize the interaction between the AP-1 μ-1 subunit and the MHC-I cytoplasmic tail domain. In the pure protein system formation of a complex between the Nef-MHC-I cytoplasmic tail fusion protein and the AP-1 μ1 subunit also required an intact tyrosine binding pocket in the AP-1 μ1 subunit. However, no role for Nef M20 was identified and thus this amino acid, which is required for Nef-induced MHC-I downmodulation may not be directly involved in protein-protein interactions but may serve another role in intact cells (Singh et al., 2009).
F. A role for β-COP in disruption of antigen presentation by Nef
Although Nef recruits AP-1 to reroute MHC-I into the endosomal network (Roeth et al., 2004) (Figure 4), it remained unclear how Nef promoted accelerated degradation of MHC-I (Roeth et al., 2004; Schwartz et al., 1996). Prior reports had determined that Nef accelerated the degradation of internalized CD4 through an interaction between Nef and β-COP, a component of COP-I coats. Interestingly, MHC-I and internalized CD4 co-localize in Rab7+ late endosomes (Schaefer et al., 2008; Yi et al., 2010) and RNAi against β-COP disrupts degradation of both MHC-I and CD4 (Schaefer et al., 2008). Recent studies have shown that two distinct domains in Nef recruit β-COP, thus clearing up discrepancies in binding data found in previously published literature (Faure et al., 2004; Janvier et al., 2001; Lindwasser et al., 2008; Piguet et al., 1999). An arginine rich domain in the N-terminal alpha helix of Nef (R17XR19) mediate β-COP binding and MHC-I degradation, whereas a diacidic motif (EE155, 156) in the C-terminal flexible loop of Nef mediates β-COP binding and CD4 degradation. (Piguet et al., 1999; Schaefer et al., 2008). The inability of Nef to utilize sequences within the C-terminal loop to affect MHC-I downmodulation and the inability of Nef to utilize sequences within the N-terminal alpha helix to affect CD4 downmodulation support the notion that there are important structural differences between Nef molecules bound to the MHC-I cytoplasmic tail versus Nef bound to the CD4 tail.
VI. Summary
In sum, a consensus is starting to emerge regarding which host factors are required for Nef to disrupt antigen presentation in HIV infected cells. There is broad agreement among investigators that the cellular clathrin adaptor protein AP-1 is necessary for Nef to disrupt MHC-I trafficking in a wide variety of cell types (Dikeakos et al., 2010; Lubben et al., 2007; Roeth et al., 2004; Schaefer et al., 2008; Wonderlich, Williams and Collins, 2008; Yi et al., 2010). Additionally, there is agreement that three Nef domains are clearly required (acidic, polyproline and N-terminal alpha helix, including M20A) for Nef-induced MHC-I downmodulation (Blagoveshchenskaya et al., 2002; Greenberg, Iafrate and Skowronski, 1998; Mangasarian et al., 1999; Noviello, Benichou and Guatelli, 2008; Roeth et al., 2004; Singh et al., 2009; Williams et al., 2005; Wonderlich, Williams and Collins, 2008). There are data from two separate groups indicating that a three-way complex forms, which contains Nef, MHC-I and AP-1 proteins and that this complex can be detected in lysates from HIV infected primary T cells and in purified protein reactions (Roeth et al., 2004; Singh et al., 2009). At least two of the three required Nef domains plus the MHC-I cytoplasmic tail, including the tyrosine at position 320, are directly needed for formation of the Nef-MHC-I-AP-1 complex (Noviello, Benichou and Guatelli, 2008; Roeth et al., 2004; Singh et al., 2009; Wonderlich, Williams and Collins, 2008). Moreover, there is a consensus that a functional tyrosine binding pocket in the AP-1 μ1 subunit is needed for formation of the Nef, AP-1, MHC-I complex and for Nef to disrupt MHC-I antigen presentation (Singh et al., 2009; Wonderlich, Williams and Collins, 2008). Finally, a number of groups have noted that PI3-kinase inhibitors reduce the effect of Nef on steady state surface levels of MHC-I, although the exact role of PI3-kinase is debated (Blagoveshchenskaya et al., 2002; Hung et al., 2007; Larsen et al., 2004; Swann et al., 2001).
In conclusion, data from a number of laboratories have contributed to our current understanding of the mechanism by which Nef downmodulates MHC-I and its role in immune evasion in vitro and in vivo. While questions about the detailed molecular mechanism remain, much has been learned. A clearer understanding may promote the development of a compound designed to specifically inhibit the effect of Nef on MHC-I antigen presentation. The capacity to rescue viral antigen presentation to CTLs and allow the host’s immune system to maintain low-level viremia may improve the treatments available for HIV infected people.
Acknowledgments
This work was funded by NIH AI046998 and NIH AI051192. E.W. was supported by the Cellular and Molecular Biology training grant from NIH to the University of Michigan and a University of Michigan Rackham Predoctoral Fellowship. J. L. was supported by the Cellular and Molecular Biology training grant and the Molecular Mechanisms in Microbial Pathogenesis training grant from NIH to the University of Michigan
VII. References
- Akari H, Arold S, Fukumori T, Okazaki T, Strebel K, Adachi A. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J Virol. 2000;74:2907. doi: 10.1128/jvi.74.6.2907-2912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins KM, Thomas L, Youker RT, Harriff MJ, Pissani F, You H, Thomas G. HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice. J Biol Chem. 2008;283:11772. doi: 10.1074/jbc.M707572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Kahn RA, Schwaninger R. ADP-ribosylation factor is required for vesicular trafficking between the endoplasmic reticulum and the cis-Golgi compartment. J Biol Chem. 1992;267:13053. [PubMed] [Google Scholar]
- Baugh LL, Garcia JV, Foster JL. Functional characterization of the human immunodeficiency virus type 1 Nef acidic domain. J Virol. 2008;82:9657. doi: 10.1128/JVI.00107-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benichou S, Bomsel M, Bodeus M, Durand H, doute M, Letourneur F, Camonis J, Benarous R. Physical interaction of the HIV-1 Nef protein wih β-cop, a component of non-clathrin coated vesicles essential for membrane traffic. J Biol Chem. 1994;269:30073. [PubMed] [Google Scholar]
- Berke G. The CTL’s kiss of death. Cell. 1995;81:9. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Birch MR, Learmont JC, Dyer WB, Deacon NJ, Zaunders JJ, Saksena N, Cunningham AL, Mills J, Sullivan JS. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC) J Clin Virol. 2001;22:263. doi: 10.1016/s1386-6532(01)00198-6. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111:853. doi: 10.1016/s0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- Bresnahan PA, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene WC. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- Carl S, Greenough TC, Krumbiegel M, Greenberg M, Skowronski J, Sullivan JL, Kirchhoff F. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J Virol. 2001;75:3657. doi: 10.1128/JVI.75.8.3657-3665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Collins K, Chen B, Kalams S, Walker B, Baltimore D. HIV-1 Nef protein protects infected primary human cells from killing by cytotoxic T lymphocytes. Nature. 1998;391:397. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- Collins KL. Resistance of HIV-infected cells to cytotoxic T lymphocytes. Microbes Infect. 2004;6:494. doi: 10.1016/j.micinf.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Craig H, Pandori M, Guatelli J. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci. 1998;95:11229. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig HM, Reddy TR, Riggs NL, Dao PP, Guatelli JC. Interactions of HIV-1 nef with the mu subunits of adaptor protein complexes 1, 2 and 3: role of the dileucine-based sorting motif. Virology. 2000;271:9. doi: 10.1006/viro.2000.0277. [DOI] [PubMed] [Google Scholar]
- Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. Embo J. 2001;20:2191. doi: 10.1093/emboj/20.9.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daro E, Sheff D, Gomez M, Kreis T, Mellman I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component epsilon-COP. J Cell Biol. 1997;139:1747. doi: 10.1083/jcb.139.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker DJ, McPhee DA, Greenway AL, Ellett A, Chatfield C, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- Dikeakos JD, Atkins KM, Thomas L, Emert-Sedlak L, Byeon IJ, Jung J, Ahn J, Wortman MD, Kukull B, Saito M, Koizumi H, Williamson DM, Hiyoshi M, Barklis E, Takiguchi M, Suzu S, Gronenborn AM, Smithgall TE, Thomas G. Small Molecule Inhibition of HIV-1-Induced MHC-I Downregulation Identifies a Temporally Regulated Switch in Nef Action. Mol Biol Cell. 2010 doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci U S A. 1992;89:6408. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Klausner RD. ARF: a key regulatory switch in membrane traffic and organelle structure. Curr Opin Cell Biol. 1994;6:527. doi: 10.1016/0955-0674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Douglas JL, Gustin JK, Viswanathan K, Mansouri M, Moses AV, Fruh K. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010;6:e1000913. doi: 10.1371/journal.ppat.1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer WB, Geczy AF, Kent SJ, McIntyre LB, Blasdall SA, Learmont JC, Sullivan JS. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS. 1997;11:1565. doi: 10.1097/00002030-199713000-00004. [DOI] [PubMed] [Google Scholar]
- Eichholtz T, Vossebeld P, van Overveld M, Ploegh H. Activation of protein kinase C accelerates internalization of transferrin receptor but not of major histocompatibility complex class I, independent of their phosphorylation status. J Biol Chem. 1992;267:22490. [PubMed] [Google Scholar]
- Erdtmann L, Janvier K, Raposo G, Craig HM, Benaroch P, Berlioz-Torrent C, Guatelli JC, Benarous R, Benichou S. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the mu 1 chain of AP1 complex. Traffic. 2000;1:871. doi: 10.1034/j.1600-0854.2000.011106.x. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Luo W, Geyer M, Alberts AS, Peterlin BM. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol Cell. 1999;3:729. doi: 10.1016/s1097-2765(01)80005-8. [DOI] [PubMed] [Google Scholar]
- Faure J, Stalder R, Borel C, Sobo K, Piguet V, Demaurex N, Gruenberg J, Trono D. ARF1 regulates Nef-induced CD4 degradation. Curr Biol. 2004;14:1056. doi: 10.1016/j.cub.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Ganser-Pornillos BK, Yeager M, Sundquist WI. The structural biology of HIV assembly. Curr Opin Struct Biol. 2008;18:203. doi: 10.1016/j.sbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Geldmacher C, Currier JR, Herrmann E, Haule A, Kuta E, McCutchan F, Njovu L, Geis S, Hoffmann O, Maboko L, Williamson C, Birx D, Meyerhans A, Cox J, Hoelscher M. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J Virol. 2007;81:2440. doi: 10.1128/JVI.01847-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- Greenberg M, Iafrate A, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Aniento F, Parton RG, Gruenberg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183. doi: 10.1083/jcb.139.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu F, Gruenberg J. ARF1 regulates pH-dependent COP functions in the early endocytic pathway. J Biol Chem. 2000;275:8154. doi: 10.1074/jbc.275.11.8154. [DOI] [PubMed] [Google Scholar]
- Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol. 2009;9:503. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Hill M, Tachedjian G, Mak J. The packaging and maturation of the HIV-1 Pol proteins. Curr HIV Res. 2005;3:73. doi: 10.2174/1570162052772942. [DOI] [PubMed] [Google Scholar]
- Hinners I, Wendler F, Fei H, Thomas L, Thomas G, Tooze SA. AP-1 recruitment to VAMP4 is modulated by phosphorylation-dependent binding of PACS-1. EMBO Rep. 2003;4:1182. doi: 10.1038/sj.embor.7400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CH, Thomas L, Ruby CE, Atkins KM, Morris NP, Knight ZA, Scholz I, Barklis E, Weinberg AD, Shokat KM, Thomas G. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host Microbe. 2007;1:121. doi: 10.1016/j.chom.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Jackson CL, Casanova JE. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 2000;10:60. doi: 10.1016/s0962-8924(99)01699-2. [DOI] [PubMed] [Google Scholar]
- Janvier K, Craig H, Hitchin D, Madrid R, Sol-Foulon N, Renault L, Cherfils J, Cassel D, Benichou S, Guatelli J. HIV-1 Nef stabilizes the association of adaptor protein complexes with membranes. J Biol Chem. 2003a;278:8725. doi: 10.1074/jbc.M210115200. [DOI] [PubMed] [Google Scholar]
- Janvier K, Craig H, Le Gall S, Benarous R, Guatelli J, Schwartz O, Benichou S. Nef-induced CD4 downregulation: a diacidic sequence in human immunodeficiency virus type 1 Nef does not function as a protein sorting motif through direct binding to beta-COP. J Virol. 2001;75:3971. doi: 10.1128/JVI.75.8.3971-3976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol. 2003b;163:1281. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Randazzo P, Serafini T, Weiss O, Rulka C, Clark J, Amherdt M, Roller P, Orci L, Rothman JE. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992;267:13039. [PubMed] [Google Scholar]
- Kasper MR, Collins KL. Nef-mediated disruption of HLA-A2 transport to the cell surface in T cells. J Virol. 2003;77:3041. doi: 10.1128/JVI.77.5.3041-3049.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper MR, Roeth JF, Williams M, Filzen TM, Fleis RI, Collins KL. HIV-1 Nef disrupts antigen presentation early in the secretory pathway. J Biol Chem. 2005;280:12840. doi: 10.1074/jbc.M413538200. [DOI] [PubMed] [Google Scholar]
- Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8:55. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kirchoff F, Greenough T, Brettler D, Sullivan J, Desrosiers R. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Hille A, Veenendaal T, Oorschot V, Stoorvogel W, von Figura K, Geuze HJ. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J Cell Biol. 1993;121:997. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JE, Massol RH, Nieland TJ, Kirchhausen T. HIV Nef-mediated major histocompatibility complex class I down-modulation is independent of Arf6 activity. Mol Biol Cell. 2004;15:323. doi: 10.1091/mbc.E03-08-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S, Buseyne F, Trocha A, Walker BD, Heard JM, Schwartz O. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J Virol. 2000;74:9256. doi: 10.1128/jvi.74.19.9256-9266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall S, Erdtmann L, Benichou S, Berlloz-Torrent C, Liu L, Benarous R, Heard J, Schwartz O. Nef interacts with mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity. 1998;8:483. doi: 10.1016/s1074-7613(00)80553-1. [DOI] [PubMed] [Google Scholar]
- Learmont J, Tindall B, Evans L, Cunningham A, Cunningham P, Wells J, Penny R, Kaldor J, Cooper DA. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor [see comments] Lancet. 1992;340:863. doi: 10.1016/0140-6736(92)93281-q. [DOI] [PubMed] [Google Scholar]
- Learmont JC, Geczy AF, Mills J, Ashton LJ, Raynes-Greenow CH, Garsia RJ, Dyer WB, McIntyre L, Oelrichs RB, Rhodes DI, Deacon NJ, Sullivan JS. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N Engl J Med. 1999;340:1715. doi: 10.1056/NEJM199906033402203. [DOI] [PubMed] [Google Scholar]
- Lewinsohn DA, Lines R, Lewinsohn DM, Riddell SR, Greenberg PD, Emerman M, Bartz SR. HIV-1 Vpr Does Not Inhibit CTL-Mediated Apoptosis of HIV-1 Infected Cells. Virology. 2002;294:13. doi: 10.1006/viro.2001.1294. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Balamurugan A, Ohno A, Kilpatrick S, Ng HL, Yang OO. Functional adaptation of Nef to the immune milieu of HIV-1 infection in vivo. J Immunol. 2008;180:4075. doi: 10.4049/jimmunol.180.6.4075. [DOI] [PubMed] [Google Scholar]
- Lindwasser OW, Smith WJ, Chaudhuri R, Yang P, Hurley JH, Bonifacino JS. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J Virol. 2008;82:1166. doi: 10.1128/JVI.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LX, Heveker N, Fackler OT, Arold S, Le Gall S, Janvier K, Peterlin BM, Dumas C, Schwartz O, Benichou S, Benarous R. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J Virol. 2000;74:5310. doi: 10.1128/jvi.74.11.5310-5319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben NB, Sahlender DA, Motley AM, Lehner PJ, Benaroch P, Robinson MS. HIV-1 Nef-induced Down-Regulation of MHC Class I Requires AP-1 and Clathrin but Not PACS-1 and Is Impeded by AP-2. Mol Biol Cell. 2007;18:3351. doi: 10.1091/mbc.E07-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machy P, Truneh A, Gennaro D, Hoffstein S. Major histocompatibility complex class I molecules internalized via coated pits in T lymphocytes. Nature. 1987;328:724. doi: 10.1038/328724a0. [DOI] [PubMed] [Google Scholar]
- Mangasarian A, Piguet V, Wang JK, Chen YL, Trono D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J Virol. 1999;73:1964. doi: 10.1128/jvi.73.3.1964-1973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Rinaldo C, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Munch J, Stolte N, Fuchs D, Stahl-Hennig C, Kirchhoff F. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J Virol. 2001;75:10532. doi: 10.1128/JVI.75.21.10532-10536.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Dimasi N, Wang J, Mariuzza RA, Margulies DH. Structure and function of natural killer cell receptors: multiple molecular solutions to self, nonself discrimination. Annu Rev Immunol. 2002;20:853. doi: 10.1146/annurev.immunol.20.100301.064812. [DOI] [PubMed] [Google Scholar]
- Nekhai S, Jeang KT. Transcriptional and post-transcriptional regulation of HIV-1 gene expression: role of cellular factors for Tat and Rev. Future Microbiol. 2006;1:417. doi: 10.2217/17460913.1.4.417. [DOI] [PubMed] [Google Scholar]
- Noviello CM, Benichou S, Guatelli JC. Cooperative binding of the class I major histocompatibility complex cytoplasmic domain and human immunodeficiency virus type 1 Nef to the endosomal AP-1 complex via its mu subunit. J Virol. 2008;82:1249. doi: 10.1128/JVI.00660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcl L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364:732. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DJ, Helms JB, Beckers CJ, Orci L, Rothman JE. Binding of coatomer to Golgi membranes requires ADP-ribosylation factor. J Biol Chem. 1993;268:12083. [PubMed] [Google Scholar]
- Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol. 2004;164:1065. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Chen YL, Mangasarian A, Foti M, Carpentier JL, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. Embo J. 1998;17:2472. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier JL, Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol. 2000;2:163. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Roeth JF, Collins KL. Human immunodeficiency virus type 1 Nef: adapting to intracellular trafficking pathways. Microbiol Mol Biol Rev. 2006;70:548. doi: 10.1128/MMBR.00042-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeth JF, Kasper MR, Williams M, Filzen TM, Collins KL. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol. 2004;167:903. doi: 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schaefer MR, Wonderlich ER, Roeth JF, Leonard JA, Collins KL. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog. 2008;4:e1000131. doi: 10.1371/journal.ppat.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer MS, Williams M, Gonzalez PK, Collins KL. The HLA-C cytoplasmic tail contains trafficking signals that allow regulated expression with differentiation of macrophages. 2007. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nature Medicine. 1996;2:338. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Shankar P, Xu Z, Lieberman J. Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood. 1999;94:3084. [PubMed] [Google Scholar]
- Singh RK, Lau D, Noviello CM, Ghosh P, Guatelli JC. An MHC-I cytoplasmic domain/HIV-1 Nef fusion protein binds directly to the mu subunit of the AP-1 endosomal coat complex. PLoS One. 2009;4:e8364. doi: 10.1371/journal.pone.0008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stove V, Van de Walle I, Naessens E, Coene E, Stove C, Plum J, Verhasselt B. Human immunodeficiency virus Nef induces rapid internalization of the T-cell coreceptor CD8alphabeta. J Virol. 2005;79:11422. doi: 10.1128/JVI.79.17.11422-11433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann SA, Williams M, Story CM, Bobbitt KR, Fleis R, Collins KL. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology. 2001;282:267. doi: 10.1006/viro.2000.0816. [DOI] [PubMed] [Google Scholar]
- Swigut T, Alexander L, Morgan J, Lifson J, Mansfield KG, Lang S, Johnson RP, Skowronski J, Desrosiers R. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J Virol. 2004;78:13335. doi: 10.1128/JVI.78.23.13335-13344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigut T, Shohdy N, Skowronski J. Mechanism for down-regulation of CD28 by Nef. Embo J. 2001;20:1593. doi: 10.1093/emboj/20.7.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TC, Kahn RA, Melancon P. Two distinct members of the ADP-ribosylation factor family of GTP-binding proteins regulate cell-free intra-Golgi transport. Cell. 1992;70:69. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- Tomiyama H, Akari H, Adachi A, Takiguchi M. Different effects of Nef-mediated HLA class I down-regulation on human immunodeficiency virus type 1-specific CD8(+) T-cell cytolytic activity and cytokine production. J Virol. 2002;76:7535. doi: 10.1128/JVI.76.15.7535-7543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse DB, Pernis B. Spontaneous internalization of Class I major histocompatibility complex molecules in T lymphoid cells. J Exp Med. 1984;159:193. doi: 10.1084/jem.159.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, West KA, Brown MC. Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol. 2001;13:593. doi: 10.1016/s0955-0674(00)00256-8. [DOI] [PubMed] [Google Scholar]
- UNAIDS. AIDS epidemic update. 2009 http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2009/default.asp.
- Venkateswarlu K, Cullen PJ. Signalling via ADP-ribosylation factor 6 lies downstream of phosphatidylinositide 3-kinase. Biochem J. 2000;345(Pt 3):719. [PMC free article] [PubMed] [Google Scholar]
- Waguri S, Dewitte F, Le Borgne R, Rouille Y, Uchiyama Y, Dubremetz JF, Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell. 2003;14:142. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Molloy S, Thomas L, Liu G, Xiang Y, Rybak S, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Waters MG, Serafini T, Rothman JE. ‘Coatomer’: a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991;349:248. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]
- Williams M, Roeth JF, Kasper MR, Filzen T, Collins KL. Human Immunodeficiency Virus Type 1 Nef Domains Required for Disruption of Major Histocompatibility Complex Class I Trafficking Are Also Necessary for Coprecipitation of Nef with HLA-A2. Journal of Virology. 2005;79:632. doi: 10.1128/JVI.79.1.632-636.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Roeth JF, Kasper MR, Fleis RI, Przybycin CG, Collins KL. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J Virol. 2002;76:12173. doi: 10.1128/JVI.76.23.12173-12184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlich ER, Williams M, Collins KL. The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J Biol Chem. 2008;283:3011. doi: 10.1074/jbc.M707760200. [DOI] [PubMed] [Google Scholar]
- Wong JK, Strain MC, Porrata R, Reay E, Sankaran-Walters S, Ignacio CC, Russell T, Pillai SK, Looney DJ, Dandekar S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010;6:e1000748. doi: 10.1371/journal.ppat.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang O, Kalams S, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker B, Johnson RP. Efficient Lysis of Human Immunodeficiency Virus Type 1-Infected Cells by Cytotoxic T Lymphocytes. J Virol. 1996;70:5799. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Nguyen PT, Kalams SA, Dorfman T, Gottlinger HG, Stewart S, Chen IS, Threlkeld S, Walker BD. Nef-Mediated Resistance of Human Immunodeficiency Virus Type 1 to Antiviral Cytotoxic T Lymphocytes. J Virol. 2002;76:1626. doi: 10.1128/JVI.76.4.1626-1631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Tran AC, Kalams SA, Johnson RP, Roberts MR, Walker BD. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc Natl Acad Sci U S A. 1997;94:11478. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Rosales T, Rose JJ, Chaudhury B, Knutson JR, Venkatesan S. HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking. J Biol Chem. 2010;285:30884. doi: 10.1074/jbc.M110.135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youker RT, Shinde U, Day R, Thomas G. At the crossroads of homoeostasis and disease: roles of the PACS proteins in membrane traffic and apoptosis. Biochem J. 2009;421:1. doi: 10.1042/BJ20081016. [DOI] [PMC free article] [PubMed] [Google Scholar]