Abstract
The development of an immunotherapeutic strategy targeting CD138 antigen could potentially represent a new treatment option for multiple myeloma (MM). This study evaluated the immune function of CD138 peptide-specific cytotoxic T lymphocytes (CTL), generated ex vivo using an HLA-A2-specific CD138 epitope against MM cells. A novel immunogenic HLA-A2-specific CD138260-268 (GLVGLIFAV) peptide was identified from the full-length protein sequence of the CD138 antigen, which induced CTL specific to primary CD138+ MM cells. The peptide-induced CD138-CTL contained a high percentage of CD8+ activated/memory T cells with a low percentage of CD4+ T cell and naive CD8+ T cell subsets. The CTL displayed HLA-A2-restricted and CD138 antigen-specific cytotoxicity against MM cell lines. In addition, CD138-CTL demonstrated increased degranulation, proliferation and γ–interferon secretion to HLA-A2+/CD138+ myeloma cells, but not HLA-A2−/CD138+ or HLA-A2+/CD138− cells. The immune functional properties of the CD138-CTL were also demonstrated using primary HLA-A2+/CD138+ cells isolated from myeloma patients. In conclusion, a novel immunogenic CD138260-268 (GLVGLIFAV) peptide can induce antigen-specific CTL, which might be useful for the treatment of MM patients with peptide-based vaccine or cellular immunotherapy strategies.
Keywords: CD138 peptide, multiple myeloma, peptide vaccine, cytotoxic T lymphocytes
INTRODUCTION
B cell malignancies, including multiple myeloma (MM), are among the most immune-responsive of human cancers, which has lead to a great deal of interest in the development of novel immunotherapeutic treatment options. Most immunotherapy protocols in development for MM have used tumour-specific idiotypic protein or whole tumour cell lysates to generate patient-specific vaccines (Lee, et al 2007, Yi, et al 2010). However, these protocols are very labour-intensive and are not cost-effective, making their general applicability difficult. Thus, development of an off-the-shelf based immunotherapy is necessary for treating patients more efficiently. Among several potential immunotherapeutic approaches, peptide-based vaccines can successfully induce anti-tumour immune responses and may prolong survival in cancer patients. Synthetic peptides also offer several advantages over individualized vaccines with regards to safety, cost of production, and monitoring for specific immune response in patients. Moreover, peptides have the ability to induce “epitope spreading”, whereby lysed target cancer cells release new antigenic epitopes which are then taken up, processed, and presented by antigen-presenting cells to a new repertoire of CTL, and thereby further tumour lysis (Disis, et al 2009, Parvanova, et al 2011). In addition, well-characterized adjuvants like Alum, granulocyte-macrophage colony-stimulating factor (GM-CSF), CD40L, IL-12, Montanide (incomplete Freund’s adjuvant) or Freund’s adjuvant can increase the efficacy of peptide vaccines (Benavides, et al 2011, Morse, et al 2011).
CD138, also known as Syndecan-1, offers a potential target for developing an immunotherapy targeting MM. This antigen is a heparan sulfate proteoglycan, which was first detected on human myeloma cells by Western Blot analysis and later on the surface of myeloma cells by flow cytometry (Ridley, et al 1993, Wijdenes, et al 1996). CD138 is expressed on epithelia, pre-B plasma cells, and other malignant cells including carcinomas, lymphoid malignancies, Hodgkin lymphoma, non-Hodgkin lymphoma and acquired immunodeficiency syndrome/human immunodeficiency virus-related lymphoma (Carbone, et al 1998, Carbone, et al 1999, Chilosi, et al 1999, Inki and Jalkanen 1996, Sanderson, et al 1989, Sebestyen, et al 1997). The syndecan-1 core protein is composed of a cytoplasmic, transmembrane, and extracellular domain. The cytoplasmic domain can be linked to cytoskeletal elements to potentiate anchorage of the cells and stabilize cell morphology, while the extracellular domain has up to three heparan sulfate chains that bind to numerous soluble and insoluble molecules. These associations include interactions with heparan-binding molecules on adjacent cells to mediate cell-cell adhesion, binding to molecules, such as collagens and fibronectin, to mediate cell adhesion to the extracellular matrix, as well as binding to growth factors (Carey, et al 1996, Liu, et al 1998). CD138 is also known to regulate the activity of many different growth factors and cytokines that can influence tumour growth (Bharti, et al 2004, Wolowiec, et al 2006). Therefore, we hypothesized that development of an immunotherapeutic strategy targeting CD138 antigen on malignant plasma cells could represent a novel treatment option for MM.
Here we report the identification of a novel immunogenic HLA-A2-specific peptide, CD138260-268 (GLVGLIFAV), which induces antigen-specific CTL. The CD138-CTL displayed HLA-A2-restricted cytotoxic activity against both primary MM cells and MM cell lines expressing CD138 antigen. In addition, HLA-A2+/CD138+ MM cells induced CTL degranulation, proliferation and γ-interferon (IFN-γ secretion, thereby confirming the functional activity of these tumour-specific CTL. Therefore, the immunogenic CD138260-268 (GLVGLIFAV) peptide is a potential candidate for development of a vaccine or cell-based immunotherapy for CD138-positive plasma cell disorders.
MATERIALS AND METHODS
Cell lines
The MM cell lines, McCAR (HLA-A2+/CD138+), MM1S (HLA-A2+/CD138−), and U226 (HLA-A2+/CD138+), and the chronic myeloid leukemia (CML) cell line K562 were obtained from the American Type Culture Collection (ATCC; Manassas, VA). The acute myeloid leukemia (AML) cell line, ML-2 (HLA-A2+/CD138−), was kindly provided by Dr. Y. Matsuo, Fujisaki Cell Centre, Okayama, Japan. The T2 cell line, a human B- and T-cell hybrid expressing HLA-A2 molecules (Zweerink, et al 1993), was provided by Dr. J. Molldrem (University of Texas M. D. Anderson Cancer Center, Houston, TX) and was utilized as antigen-presenting cells (APC) and target cells in the cytotoxicity assays. All cell lines were cultured in RPMI-1640 medium (Gibco-BRL Life Technologies, Rockville, MD) supplemented with 10% fetal calf serum (FCS) (BioWhittaker, Walkersville, MD), 100 iu/ml penicillin, and 100 μg/ml streptomycin (Gibco-BRL Life Technologies).
Reagents
Recombinant human interleukin (IL)-2, IL-4, IFN-α and tumour necrosis factor-α (TNF-α were purchased from R&D Systems (Minneapolis, MN) while GM-CSF was obtained from Immunex (Seattle, WA). Mouse anti-human CD80 or CD83 monoclonal antibodies (mAbs) conjugated with phycoerythrin (PE) were purchased from Immunotech (Hialeah, FL). Mouse anti-human CD3, CD4, CD8, CD14, CD40, CD45RA, CD45RO, CD69, CD86, CD107a, CD107b, CCR7, IFN-γ, HLA-A2 and HLA-DR mAbs conjugated with fluorescein isothiocyanate (FITC), PE, peridinin chlorophyll (PerCP), allo-phycocyanin, PacBlue, allo-phycocyanin-H7 or PE-Cy7 were purchased from Becton Dickinson (BD)/Pharmingen or BD/Biosciences (San Diego, CA).
Synthetic peptides
The full-length protein sequence of the CD138 antigen was analysed using the search software SYFPEITHI to predict HLA-A2-specific peptides, followed by the BIMAS program to select peptides with extended disassociation rates. Based on the results obtained from the software prediction programs, the following CD138 nanomers were selected and synthesized for evaluation as potential HLA-A2-specific peptides: CD138256-264 (VIAGGLVGL), CD138260-268 (GLVGLIFAV), CD1385-13 (ALWLWLCAL) and CD1387-15 (WLWLCALAL). Influenza virus matrix protein58-66 [IVMP58-66] (GILGFVFTL) and MAGE-3271-279 (FLWGPRALV) peptides were synthesized for use as HLA-A2-specific peptide controls. All peptides were synthesized by standard fmoc (9-fluorenylmethyl-oxycarbonyl) chemistry, purified to >95% using reverse-phase chromatography, and validated by mass-spectrometry for molecular weight (Biosynthesis, Lewisville, TX). Lyophilized peptides were dissolved in dimethyl sulfoxide (Sigma, St. Louis, MO), diluted in AIM-V medium (Gibco-BRL Life Technologies) containing human AB serum (BioWhittaker) to 1 mg/ml, aliquotted, and stored at −140°C until needed.
Examination of HLA-A2-specificity of peptides
T2 cells, a TAP-deficient human B- × T-lymphoblastoid hybrid cell line, were used to evaluate HLA-A2-specificity of the synthetic CD138 peptides. For the assay, T2 cells were washed, counted, and resuspended (1×106 cells/ml) in serum-free AIM-V media. T2 cells were pulsed with respective CD138 peptide (50 μg/ml) or influenza virus matrix protein58-66 peptide (IVMP58-66; 30 μg/ml) plus 3 μg/ml human β2-microglobulin (Sigma, St. Louis, MO) and incubated at 37°C, 5% CO2 in humidified air. Following overnight incubation, the cells were washed three times in phosphate-buffered saline (PBS: Gibco-BRL Life Technologies) containing 5% FCS and stained with mouse anti-human HLA A2-FITC mAb for 15 min at 4°C. After washing, the cells were analysed using a FACSort™ flow cytometer with CellQuest™ v2.1 software (Becton Dickinson, San Jose, CA). The peptide binding affinity to HLA-A2 was examined by the up-regulation of HLA-A2 on the T2 cells caused by specific peptide binding. Results, expressed as the Fluorescence Index (FI: [mean channel fluorescence of T2 cells pulsed with the peptide plus β2-microglobulin ÷ mean channel fluorescence of T2 cells pulsed with β2–microglobulin]) are shown as the mean FI ± standard error (SE) for three separate experiments.
Assessment of the HLA-A2/peptide complex stability
T2 cells were pulsed with peptide and human β2-microglobulin as described above. After overnight incubation, the T2 cells were washed to remove unbound peptide and incubated with 10 μg/ml Brefeldin A (Sigma) at 37°C for 1 h to block cell surface expression of newly synthesized HLA-A2 molecules. The HLA-A2/peptide complex stability was then evaluated at 0, 2, 4, 6 and 14 h post-Brefeldin A treatment. Following each incubation period, the cells were stained with HLA-A2-FITC mAb and analysed by flow cytometry. The results are shown as the HLA-A2 FITC mean channel fluorescence (mean HLA-A2 MFI ± SE) at each time point for three separate experiments.
Enrichment of CD14+ monocytes and CD3+ T lymphocytes
Peripheral blood mononuclear cells (PBMC) were isolated by standard density gradient centrifugation over Ficoll-Paque™ Plus (Amersham Pharmacia Biotech AB, Uppsala Sweden) from leucopaks obtained from HLA-A2+ normal donors.
Monocytes
CD14+ monocytes were isolated from PBMC by positive selection using magnetic cell selection technology (Miltenyi Biotec, Auburn, CA). Highly enriched monocytes (>90%) were confirmed by flow cytometric analysis.
CD3+ T lymphocytes
CD3+ T lymphocytes were isolated from the monocyte-depleted cell fractions using the Pan T cell isolation kit (Miltenyi Biotec) by depletion of B cells, Natural Killer (NK) cells, monocytes, early erythroid cells, platelets and basophils. The effluent (negative cell fraction) was collected from the column as CD3+ T cells. Highly enriched T lymphocytes (>90%) were confirmed by flow cytometric analysis.
Generation of monocyte-derived dendritic cells (DC)
To obtain immature DC, enriched CD14+ monocytes were cultured for 7 days in the presence of 1,000 u/ml GM-CSF and 1,000 u/ml IL-4 in RPMI-1640 medium supplemented with 10% FCS. Fresh media plus GM-CSF and IL-4 was added to the cultures every other day. Mature DC (mDC) were obtained by adding 1,000 u/ml IFN-α plus 10 ng/ml TNF-α, along with fresh GM-CSF and IL-4 in RPMI-10% FCS, on day 7. After an additional three days of incubation, the mature DCs were harvested, washed, and pulsed with peptide for the stimulation of T lymphocytes.
Induction of peptide-specific CTL
The CD138-CTL were generated from six different HLA-A2+ normal donors for the evaluation of the functional activities targeting primary MM cells and MM cell lines. To generate CD138-CTL, mDC were resuspended in serum-free AIM-V media and pulsed with 50 μg/ml of the specific CD138 peptide overnight at 37°C, 5% CO2 in humidified air. Peptide-pulsed mDC were washed, counted, irradiated at 10 Gy and used to prime CD3+ T cells at a 1:20 APC/peptide-to-CD3+ T cell ratio in AIM-V media supplemented with 10% human AB serum. The cultures were restimulated every seven days with irradiated T2 cells pulsed with peptide (T2/APC) for a total of 4 cycles. To maintain the T cells ex vivo, IL-2 (50 u/ml) was added to the cultures two days after the second stimulation. Control T cell cultures were maintained under the same culture conditions in the presence of IL-2 (50 u/ml), but without APC/peptide stimulation.
Phenotypic analysis of CD138-CTL
CD138-CTL were stained with CD4 or CD8 mouse anti-human mAbs for flow cytometric analysis. In addition, the cells were stained with CD69 and CD45RO or CD45RA and CCR7 mouse anti-human mAbs to evaluate their activation/memory and memory/naïve status, respectively. After staining, the cells were washed and analysed by flow cytometry using a FACSort or FACSCanto (BD).
Calcein-release cytotoxicity assay
The cytotoxic activity of the CD138-CTL was measured using the calcein-release assay as described elsewhere (Roden, et al 1999). Briefly, target cells (U266, McCAR, ML-2, MM1S, K562 or primary MM cells) were incubated with 10 mM calcein-AM (Invitrogen, Carlsbad, CA) for 30 min at 37°C, washed three times in cold PBS with 5% FCS, and resuspended in PBS. The calcein-labelled target cells (5 × 103cells/well) were seeded with different concentrations of CD138-CTL in 96-well round-bottom plates to achieve the different effector/target cell ratios (triplicate wells per sample). Plates were incubated for 4 h at 37°C, 5% CO2 in humidified air. Following incubation, the plates were centrifuged at 1,000 rpm for 5 min to pellet the cells, and 100 μl of the cell-free supernatant was carefully transferred into wells of a flat bottom 96-well plate. Released calcein was measured in a fluorescence multi-well plate reader using VICTOR2 1420 multi-label counter (excitation wavelength 485 nm, emission wavelength 538 nm) (PerkinElmer, Wellesley, MA). The percentage of specific cell lysis was calculated as [(experimental release − spontaneous release) ÷ (maximum release − spontaneous release)]. Maximum release was obtained from the supernatants of detergent-treated calcein-AM labelled target cells, and spontaneous release from the supernatants of calcein-AM labelled target cells alone.
CD107 assay
The CD107 assay was set up as described by Betts et al. (2003), with minor modifications to evaluate the functional activity of the CD138-CTL. In the assay, primary HLA-A2+/CD38+ myeloma cells were co-incubated with CD138-CTL in 96-well round-bottom plates. At the same time, mouse anti-human CD107a-FITC and CD107b-FITC mAbs were added to each well. After 1 h incubation, Brefeldin A (BD) and Monensin (BD) were added and the cells were incubated for an additional 4 h. Following incubation, the cells were then harvested, stained with CD8-PE mAb for 30 min at 4°C, and analysed by flow cytometry. The expression of CD107ab by the CD8+ T cells was determined as a measure of degranulation of the CD138 peptide-specific CD8+ CTL in response to target cells.
Cell proliferation by Carboxy Fluorescein Succinimidyl Ester (CFSE) tracking
Specific cell proliferation of CD138-CTL was measured by flow cytometry using the CFSE tracking dye. In this assay, CD138-CTL were washed and resuspended to 1×106 cells/ml in PBS and labelled with CFSE (Invitrogen) at a final concentration of 5 μM. After incubation for 10 minutes at 37°C, five volumes of ice-cold PBS with 2% FCS (PBS-2% FCS) was added to quench the reaction. Cells were incubated for 5 minutes on ice, centrifuged, and washed an additional three times in cold PBS-2% FCS. After the final wash, the CFSE-labelled CD138-CTL were resuspended in RPMI-10% FCS at 2×106 cells/ml and co-incubated with stimulator cells (U266, McCAR, ML-2, MM1S, or primary MM cells; 2×105 cells/ml) at 37°C, 5% CO2 in humidified air. The stimulated CFSE-labelled CD138-CTL were examined on day 5 by flow cytometry for a decrease in CFSE expression, which is a direct measure of cell proliferation.
IFN-γ secretion by enzyme-linked immunosorbent assay (ELISA)
CD138-CTL were incubated with MM tumour cell lines (McCAR, MM1S) or the AML cell line (ML-2) for 24 h at 37°C, 5% CO2 in humidified air. Following incubation, cells were pelleted by centrifugation, and supernatants were collected for evaluation of IFN-γ release by the CTL using a human IFN-γ ELISA kit (BD Biosciences, San Diego, CA). Briefly, purified IFN-γ or supernatants from the CTL-tumour cell cultures were transferred into a 96-well plate pre-coated with a monoclonal anti-human IFN-γ capture antibody and incubated for 2 h at room temperature. After washing, the buffer containing detection antibody and avidin-horseradish peroxidase conjugate were added to each well and incubated for 1 h at room temperature. The wells were washed and developed by incubation with substrate solution for 30 min. Stop solution was added to each well, and absorbance was determined at 450 nm with a VICTOR2 1420 multi-label counter. The amount of IFN-γ (pg/ml) present in the CTL culture supernatants was calculated based on the standard curve (mean of triplicate wells). Results are shown as the mean IFN-γ secretion ± SE for three separate experiments.
Intracellular IFN-γ production
IFN-γ-producing effector memory (EM) CD8+ T cells were determined by intracellular cytokine and surface staining by flow cytometry. Briefly, 1 × 106 of peptide-specific CTL were stimulated with 1 × 106 HLA-A2+/CD138+ McCAR or U266 myeloma cell line. After 1 h incubation CD28/CD49d mAb (BD) and the protein transport inhibitors Brefeldin A (BD) and Monensin (BD) were added to the cells and were incubated for an additional 5 h. As a baseline control, CD138-CTL were cultured in media with CD28/CD49d mAb along with Brefeldin A and Monensin. After the incubation, cells were harvested, washed and surface stained with anti-CD3 PacBlue, anti-CD8 allo phycocyanin-H7, anti-CD45RO PE and anti-CCR7 PE-Cy7 mAbs for 30 min. Next, cells were permeabilized and fixed using Cytofix/Cytoperm (BD) as per manufacturer’s instructions. The cells were then stained with anti-IFN-γ FITC for 45 minutes to detect intracellular cytokine production. Lastly, cells were washed with 1× Perm/Wash solution (BD), fixed in 2% paraformaldehyde and acquired on a FACS Canto flow cytometer.
Statistical Analysis
Results are represented as mean ± SE. Groups were compared using unpaired Student’s t-test. Differences were considered significant when p < 0.05.
RESULTS
CD138260-268 (GLVGLIFAV) has high peptide binding affinity and stability to HLA-A2 molecules
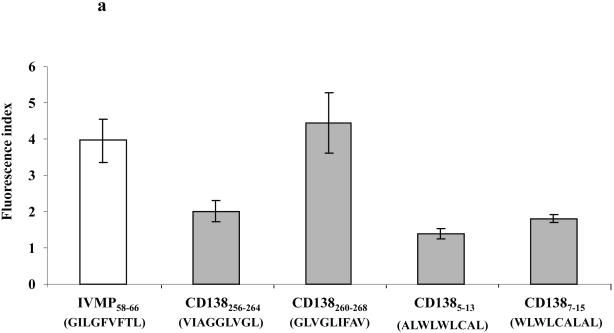
The HLA-A2 binding affinity and stability of the CD138256-264 (VIAGGLVGL), CD138260-268 (GLVGLIFAV), CD1385-13 (ALWLWLCAL) and CD1387-15 (WLWLCALAL) peptides were assessed using the T2 binding assay, as described previously (Bae, et al 2004). The affinity of CD138 peptides to HLA-A2 molecules is expressed as the Fluorescence Index (FI), which was calculated as [HLA-A2 MFI of T2 cells pulsed with the peptide plus β2-microglobulin ÷ HLA-A2 MFI of T2 cells alone plus β2-microglobulin]. A FI value >1 indicates upregulation of HLA-A2 molecules due to the specific binding of peptide to molecules on T2 cells. Among the four CD138 peptides evaluated (Figure 1a), the CD138260-268 peptide displayed the highest level of HLA-A2-specific binding (FI: 4.45), which was greater than the binding affinity of the control HLA-A2-specific influenza virus matrix protein58-66 (IVMP58-66) peptide (FI: 3.85). The CD138256-264, CD1385-13 and CD1387-15 peptides also displayed HLA-A2 specificity, but these peptides showed lower levels of HLA-A2 binding affinities, as shown by FI values of 2.01, 1.39 and 1.80, respectively (Figure 1a).
Figure 1. Affinity and stability of CD138260-268 (GLVGLIFAV) peptide binding to HLA-A2 molecules.
(a) The HLA-A2 binding affinity of CD138260-268 (GLVGLIFAV) peptide on T2 cells was analysed by flow cytometry. T2 cells were pulsed with CD138 peptide (50 μg/ml) or influenza virus matrix protein58-66 (30 μg/ml) in AIM-V serum-free media. After overnight incubation, the cells were washed to remove unbound peptides and stained with HLA-A2-FITC mAb. The binding affinity of peptides was determined as the Fluorescence Index (FI), which was calculated as [mean channel fluorescence of T2 cells pulsed with the peptide plus β2-microglobulin ÷ mean channel fluorescence of T2 cells pulsed with β2-microglobulin]. Among the peptides tested, the CD138260-268 (GLVGLIFAV) peptide displayed the highest HLA-A2 binding affinity. The values represent the mean ± SE of three separate experiments.
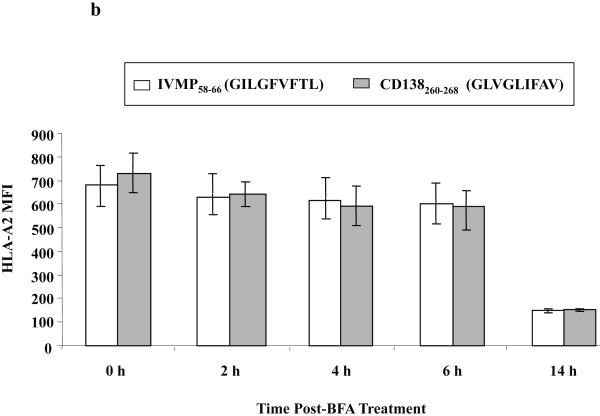
(b) The HLA-A2 binding stability of CD138260-268 (GLVGLIFAV) peptide on T2 cells was measured at different time points. After overnight peptide pulsing, the T2 cells were incubated with Brefeldin A, followed by staining with HLA-A2 FITC mAb at 0, 2, 4, 6 or 14 h incubation. The peptide binding stability was measured as HLA-A2 MFI. The stability of CD138260-268 (GLVGLIFAV) peptide was comparable to influenza virus matrix protein58-66 (GILGFVFTL) peptide, which was used as an HLA-A2-specific positive control. The values represent the mean ± SE of three separate experiments.
Next, we assessed the stability of CD138260-268 peptide binding to HLA-A2 molecules. Peptide-pulsed T2 cells were treated with Brefeldin A to block cell surface expression of newly synthesized HLA-A2 molecules, and then examined for HLA-A2 MFI at 0, 2, 4, 6 and 14 h by flow cytometry. CD138 peptide binding to HLA-A2 was observed for 6 h, which was similar to the control influenza virus matrix protein58-66 (IVMP58-66 HLA-A2 MFI = 603 vs. CD138260-268 HLA-A2 MFI = 597) (Figure 1b). Based on these results, the CD138260-268 peptide, which was shown to have high HLA-A2 specific binding affinity and stability, was further evaluated for its immunogenicity to evoke MM-specific CTL.
Repeated CD138 peptide stimulation induces CD138-CTL with distinct phenotypes
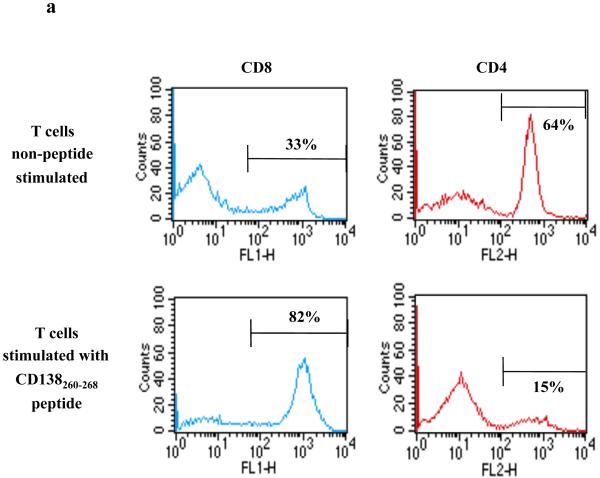
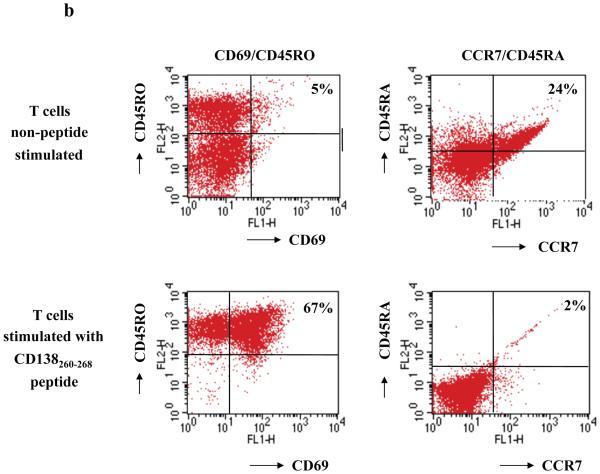
CD138-CTL were generated by repeated stimulation of normal donor HLA-A2+/CD3+ T lymphocytes with CD138260-268 (GLVGLIFAV) peptide-pulsed APC. The phenotype of the resulting CTL was evaluated one week after the fourth stimulation. Flow cytometric analysis showed a distinct change in the phenotype of the CD3+ T cell subsets stimulated with CD138260-268 peptide: CD138-CTL cultures contained a higher percentage of CD8+ T cells (82%) compared to non-peptide stimulated control CD3+ T cells (33%) with a corresponding lower percentage of CD4+ T cells (15%) compared to control CD3+ T cells (64%) (Figure 2a). We further examined the phenotype of the CD138-CTL as potential effector cells by analysing cell surface markers of activation (CD69) and memory (CD45RO). The proportion of CD69+/CD45RO+ activated/memory T cells was increased in CTL cultures stimulated with CD138260-268 peptide (67%) compared to control T cells (5%). In contrast, CD138-CTL contained a low percentage (2%) of CCR7+/CD45RA+ naïve T cells than control CD3+ T cells (24% of naïve T cells) (Figure 2b). These phenotypic changes demonstrate that repeated stimulation of CD3+ T cells with CD138260-268 peptide pulsed APC resulted in an expansion of CD8 CTL with an activated/memory cell phenotype.
Figure 2. Generation of activated memory cells in CTL cultures stimulated with CD138260-268 (GLVGLIFAV) peptide.
HLA-A2+/CD3+ T cells were stimulated weekly with irradiated antigen-presenting cells pulsed with CD138260-268 peptide (GLVGLIFAV). One week after their fourth stimulation, the phenotype of the T cell cultures was analysed by flow cytometry.
(a) The percentage of CD8+ T cells was increased in the CD3+ T cell cultures stimulated with the CD138260-268 peptide as compared to the control (non-peptide stimulated) cells, which contained a lower percentage of CD8+ T cells and higher percentage of CD4+ T cells.
(b) The percentage of CD69+/CD45RO+ (activated memory) cells was increased with a corresponding decrease in the percentage of CCR7+/CD45RA+ (naive) cells in the cultures stimulated with the CD138260-268 peptide compared the control unstimulated cells.
CD138-CTL display HLA-A2-restricted and antigen-specific cytotoxicity, proliferation, and IFN-γ secretion in response to MM cell lines upon recognition of CD138256-264 peptide
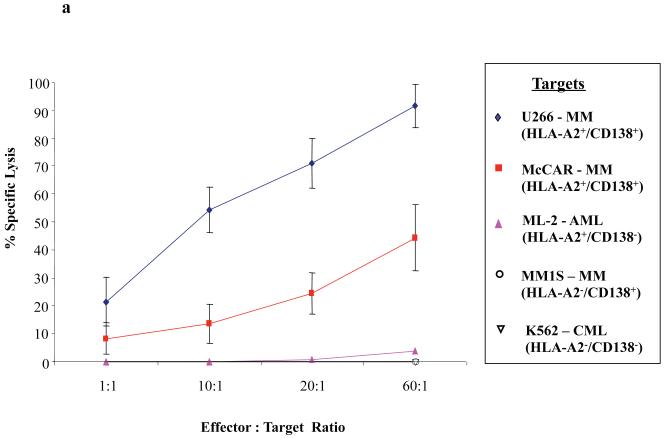
One week after the fourth stimulation with CD138260-268 peptide, CTL were analysed for their tumour-specific functional activities against various tumour cell lines. In calcein-release cytotoxicity assays, the CD138-CTL demonstrated effective killing of the HLA-A2+/CD138+ MM cell lines U266 (21–90%) and McCAR (9-41%) at different effector: target cell ratios (Figure 3a). CD138-CTL did not target and kill either the MM1S (HLA-A2−/CD138+) MM cell line or the ML-2 (HLA-A2+/CD138−) AML cell line. In addition, the CTL did not kill K562 cells (< 5% specific lysis), an NK-sensitive cell line, demonstrating that CD138-CTL cytotoxic activity was both HLA-A2-restricted and antigen-specific, and not mediated by non-specific NK cells.
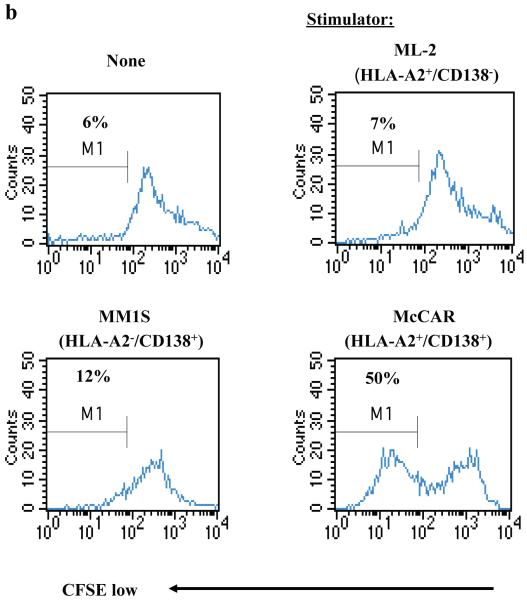
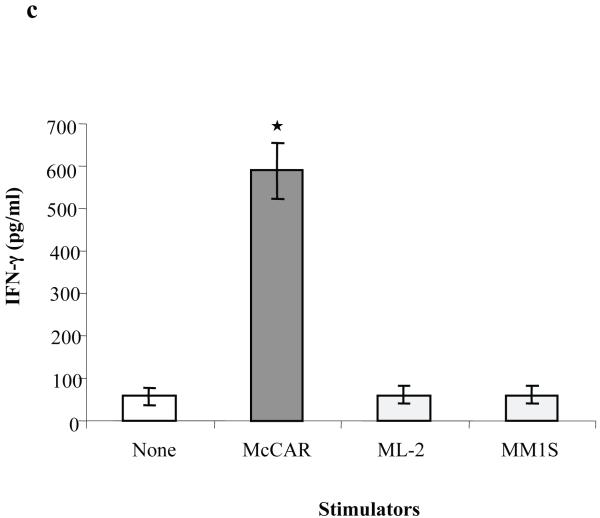
Figure 3. Functional activity of CD138-CTL in response to HLA-A2+/CD138+ MM cell lines.
(a) The tumour-specific cytotoxic activity of the CD138-CTL was tested in calcein-release cytotoxicity assays one week after the fourth peptide stimulation. CD138-CTL demonstrated HLA-A2 restricted lysis of U266 (CD138+/HLA-A2+) and McCAR (CD138+/HLA-A2+) cells, but not MM1S (CD138+/HLA-A2−) cells. The CD138-CTL did not show any significant lysis of antigen mismatched ML-2 (CD138−/HLA-A2+) AML cells or NK-sensitive K562 cells.
(b) Specific CD138-CTL proliferation was examined by flow cytometry after stimulating CFSE-labelled CTL with tumour cell lines for 5 days. The proliferating cell population was measured as the percent decrease in CFSE expression (M1-gated cells). The CTL proliferated in response to McCAR (CD138+/HLA-A2+) cells, but not in response to MHC-mismatched MM1S (CD138+/HLA-A2−) or antigen mismatched ML-2 (CD138−/HLA-A2+) cells. Background proliferation was determined using CFSE-labelled CD138-CTL cultured in media alone.
(c) IFN-γ secretion by CD138-CTL was measured by ELISA in the culture supernatants collected 1 day following stimulation with tumour cell lines. CD138-CTL showed a significantly higher level of IFN-γ secretion (*p < 0.05) in response to McCAR cells (CD138+/HLA-A2+) as compared to control CD138-CTL cultured in media alone. CD138-CTL IFN-γ secretion in response to MHC-mismatched MM1S (CD138+/HLA-A2−) or antigen-mismatched ML-2 (CD138−/HLA-A2+) tumour cell lines was similar to background IFN-γ production.
CD138-CTL proliferation in response to various tumour cell lines was measured on day 5 by flow cytometry using the CFSE assay (Figure 3b). The proliferating cell population was measured as the percent decrease in CFSE labelling, as defined by the M1-gated cells. A high level of CD138-CTL proliferation was observed in response to McCAR cell line (HLA-A2+/CD138+, 50%) compared to control CD138-CTL (unstimulated, 6%). In contrast, CD138-CTL did not proliferate when stimulated with either MM1S (HLA-A2−/CD138+, 12%) or ML-2 (HLA-A2+/CD138−, 7%) cells. These results demonstrate that CD138-CTL proliferation is both CD138 antigen-specific and HLA-A2-restricted.
Next, the CD138-CTL were tested for their ability to secrete IFN-γ which is associated with therapeutic efficacy in MM (Perez-Andres, et al 2006, Wang, et al 2007). The level of IFN-γ secreted by the CD138-CTL was measured upon stimulation with the McCAR, MM1S or ML-2 tumour cell lines by ELISA. A significant increase (p < 0.05) in CD138-CTL-induced IFN-γ secretion was observed following stimulation with the McCAR cell line (HLA-A2+/CD138+) compared to CD138-CTL cultured in media alone (Figure 3c). The level of IFN-γ secretion induced by the major histocompatibility complex (MHC) mismatched MM1S (HLA-A2−/CD138+) or antigen mismatched ML-2 (HLA-A2+/CD138−) cell lines was similar to CD138-CTL cultured in media alone. These results offer further evidence of the CD138 antigen-specific and HLA-A2-restricted responses by CD138-CTL.
CD138-CTL display anti-tumour activity against primary CD138+ cells isolated from HLA-A2+ myeloma patients
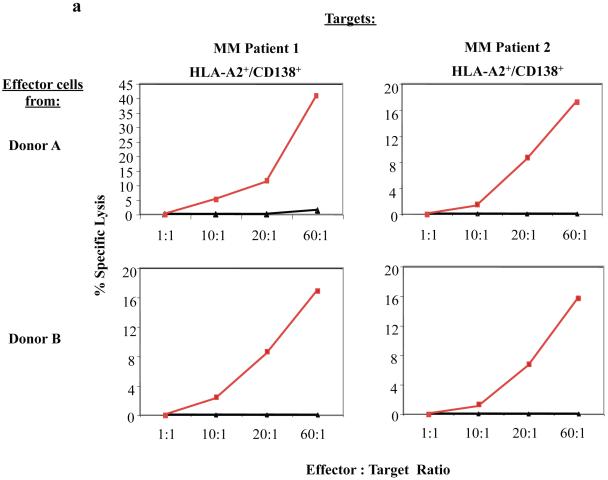
The myeloma-specific functional activities of CD138-CTL were evaluated against primary CD138+ cells isolated from HLA-A2+ myeloma patients. In calcein-release cytotoxicity assays, CD138-CTL generated from different donors demonstrated effective cytolysis of primary myeloma cells at the different effector: target cell ratios (Figure 4a). In contrast, unstimulated control T cells did not show cytotoxic activity against primary HLA-A2+/CD138+ patient MM cells.
Figure 4. CD138-CTL cytotoxic activity against primary CD138+ cells isolated from HLA-A2+ MM patients.
(a) The cytotoxic activity of CD138-CTL was evaluated in calcein-release assay against primary cells isolated from HLA-A2+ myeloma patients. Compared to unstimulated T cells (▲), CD138-CTL (■) generated from Donor A or Donor B showed effective lysis of the primary CD138+ cells isolated from bone marrow mononuclear cells of two HLA-A2+ MM patients.
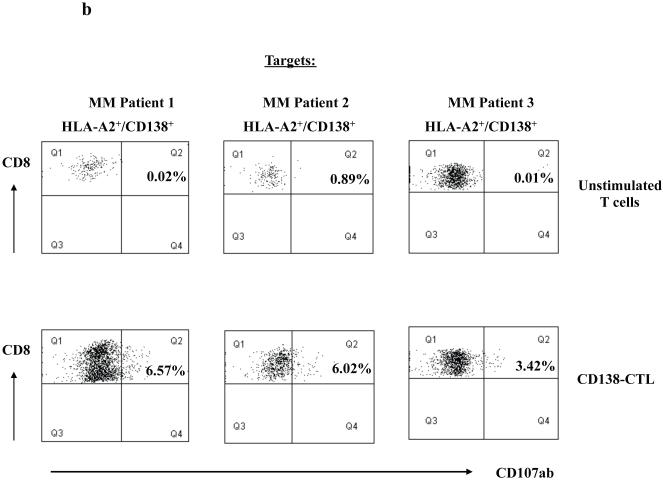
(b) Degranulation of CD138-CTL was evaluated by measuring CD107ab up-regulation on CD8+ CTL after stimulation with HLA-A2+/CD138+ primary MM cells using flow cytometry. CD138-CTL stimulated with primary cells from three HLA-A2+ MM patients showed higher level of CD107ab+/CD8+ cells as compared to unstimulated control cells.
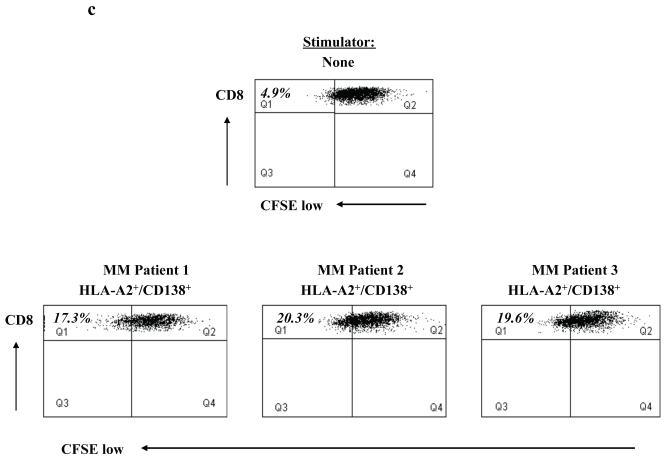
(c) Specific cell proliferation of CD138-CTL was evaluated in response to primary HLA-A2+/CD138+ cells isolated from MM patients by CFSE analysis. CD138-CTL showed a higher level of cell proliferation (Q1 gated cells) in response to the primary cells from three HLA-A2+ MM patients, compared to CD138-CTL cultured in media alone.
Degranulation of the CD138-CTL was demonstrated by measuring CD107ab expression in response to primary CD138+ myeloma cells from three different HLA-A2+ patients. Compared to unstimulated T cells (0.01% – 0.89%), the CD138-CTL (3.42% - 6.57%) showed increased expression of CD107ab against primary cells (Figure 4b), confirming a cytotoxic activity against myeloma cells.
Finally, we demonstrated CD138-CTL proliferation in response to HLA-A2+CD138+ primary myeloma cells in CFSE assays. The proliferating cell population was measured as the percent decrease in CFSE expression, as defined by cells found in Q1 (CD8+/CFSE-low). The CTL proliferated in response to the primary myeloma cells from three different HLA-A2+ patients (17.3% - 20.3%), as compared to CTL cultured in media alone without stimulation (4.9%) (Figure 4c). These results demonstrate the functional anti-tumour activity of the CD138-CTL, evidenced by their cytotoxicity, degranulation, and proliferation in response to primary myeloma cells.
CD138-CTL have a high level of intracellular IFN-γ production and cell proliferation in response to myeloma cell lines
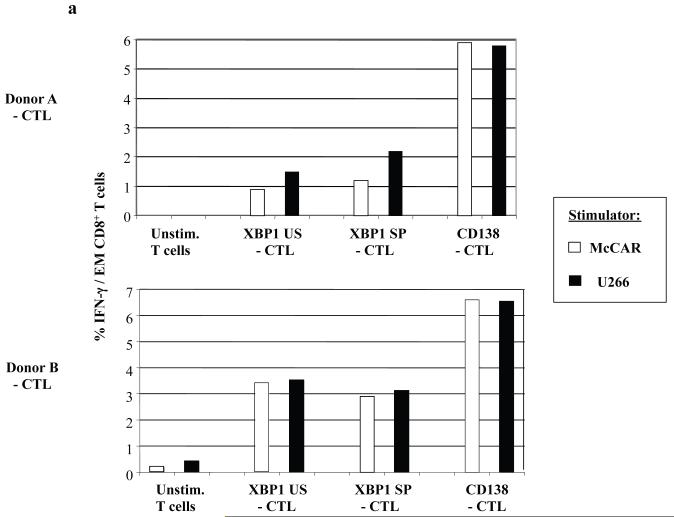
In previous studies, we defined the immunogenic properties of heteroclitic HLA-A2-specific XBP1 peptides (YISPWILAV, YLFPQLISV) from unspliced (US) or spliced (SP) proteins to induce myeloma specific CTL (Bae et al, 2011). The unspliced and spliced XBP1 proteins are implicated in myeloma pathobiology and over expressed in myeloma cells (Bagratuni, et al 2010, Patterson, et al 2008). In this study, we evaluated the CTL generated ex vivo from the same donors’ T cells using the heteroclitic XBP1 peptides and native CD138260-268 (GLVGLIFAV) peptides. Intracellular IFN-γ production was measured by flow cytometry in response to HLA-A2+ McCAR or U266 myeloma cell lines (Figure 5a). The highest level of intracellular IFN-γ production (> 5%: Donor A, > 6%: Donor B) was observed in the CD8+ effector memory (CD45RO+/CCR7−) cell subset of the CD138-CTL. The CD8+ effector memory cells of heteroclitic XBP1 US (YISPWILAV)-CTL and XBP1 SP (YLFPQLISV)-CTL also triggered IFN-γ in response to the myeloma cell lines, demonstrating their functional activity, but their response was lower than the response observed with the CD138-CTL.
Figure 5. Intracellular IFN-γ production and proliferation by CD138 or XBP1-peptide specific CTL in response to myeloma cell lines.
(a) Intracellular IFN-γ production was measured by flow cytometry after stimulation of the respective CD138-CTL, XBP1 US-CTL or XBP1-SP-CTL with HLA-A2+ McCAR or U266 myeloma cell line. Intracellular IFN-γ production was detected in the CTL effector memory (CD3+/CD8+/CD45RO+/CCR7−) subset from each of the respective CTL generated from two individual donors (Donor A, Donor B). A higher level of IFN-γ production was detected in response to McCAR or U266 cells in the CD138-CTL than the XBP1-CTLs. Control T cells did not produce IFN-γ in response to the myeloma cell lines.
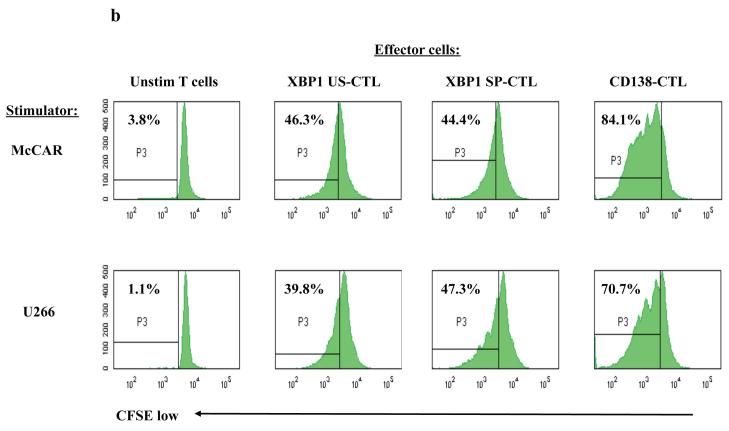
(b) Specific cell proliferation of each peptide-specific CTL was evaluated following stimulation with the HLA-A2+ myeloma cell lines on day 5 using a CFSE assay. The percentage of proliferating CTL is shown in the P3 gate (CFSE-low). A higher level of cell proliferation was detected in the CD138-CTL than the XBP1-CTLs in response to McCAR or U266 cells.
Using the CFSE assay, we compared the ability of the CD138 and XBP1 peptide specific CTL to proliferate in response to the myeloma cell lines. The CD138-CTL had a higher cell proliferation in response to both McCAR (84.1 %) and U266 (70.7%) cells, compared to heteroclitic XBP1 US-CTL (McCAR: 46.3%, U266: 39.8%) or heteroclitic XBP1 SP-CTL (McCAR: 44.4%, U266: 47.3%) measured on day 5 (Figure 5b). This pattern was also observed when the cells were measured on day 4 or day 6 (data not shown). Control unstimulated T cells did not proliferate in response to McCAR (3.8%) or U266 (1.1%). Taken together, these data demonstrate the functional anti-tumour activities of CTL induced by CD138 peptide as well as heteroclitic unspliced or spliced XBP1 peptides.
DISCUSSION
Despite improvement in therapeutic strategies for treating MM, novel therapies are urgently needed. Cancer vaccines or cell-based immunotherapy suggest promising treatment options to induce anti-tumour immunity, especially in B-cell malignancies, which represent the immune-responsive cancers (el-Shami and Smith 2008, Kannan and Neelapu 2009, Muzio, et al 2009). Active-specific immunotherapy has the distinct advantage of inducing highly effective T lymphocytes with anti-tumour activities (Slezak, et al 2010, Westers, et al 2011) although patient-specific immunotherapy requires individualized patient-specific products, which is labour intensive and costly. An attractive potential therapeutic option is to use immunogenic peptides, which would have broader applicability, low toxicity, specificity for tumour cell targets, and ease of production (Bae, et al 2005, Dudek, et al 2010). Although there is MHC restriction in this therapeutic approach, the use of cocktails of immunogenic peptides to different HLA molecules would broaden the induction of CTL specific to tumour cells of multiple MHC classifications. In addition, the efficacy of peptide-based immunotherapy could be further enhanced by targeting multiple antigens on tumour cells, thereby achieving epitope spreading in the patients. However, to date there are very few reports in MM on the application of peptide vaccines.
To develop a MM-specific immunotherapy, we investigated antigens that are over-expressed on MM cells as potential targets for inducing tumour-specific CTL. Previous studies by our group and others have revealed several unique expression patterns of antigens on myeloma cells: [1] markers associated with malignancy including aberrant expression of CD56 and CD28, but lack of CD27 (Dunphy, et al 2007, Kraj, et al 2008, Moreau, et al 2006, Pellat-Deceunynck, et al 1994); [2] markers of disease severity, such as over-expression of CD221 and XBP1 and lack of CD45 (Bataille, et al 2005, Heerema-McKenney, et al 2010, Papandreou, et al 2011); [3] markers of MM subsets defined by expression of CD19, CD20 or CD117 (Mateo, et al 2008, Robillard, et al 2003). Unlike these antigens, CD138 (syndecan-1) is expressed on the majority of MM cells within the BM, but not on other benign haematopoeitic elements or by endothelium (Bayer-Garner, et al 2001), making it a prime target for development of a MM-specific immunotherapy. In addition, a recent report from the European Myeloma Network demonstrated that a combination of CD138, CD38 and CD45 antibodies allows the most reproducible detection of neoplastic plasma cells (Rawstron, et al 2008). CD138 is expressed at the plasma cell stage of B-cell differentiation and over-expressed on the majority of myeloma cells (Bataille, et al 2006, Supiot, et al 2002). In addition, its expression on MM cells is independent of cytologic differentiation or intravascular invasion (Bayer-Garner, et al 2001). A member of the heparan sulfate family, CD138 (syndecan-1) mediates cell-cell adhesion through heparan-binding molecules expressed by adjacent cells and has a role as a co-receptor for numerous growth factors of myeloma cells; therefore, heparan sulfate or enzymes that regulate heparan sulfate could be viable targets for cancer therapy in MM (McKenzie 2007, Yang, et al 2007).
To identify CD138 peptides specific to HLA-A2 molecules, we screened the full-length CD138 protein sequence (a total of 310 amino acids) using the search software SYFPEITHI program to predict peptides having affinity to HLA-A2. Following this initial screening process, we utilized the BIMAS software to select peptides with extended half-time disassociation rates, and avoided the sequences of amino acid residues that potentially cause reduced binding to HLA-A2 molecules (Strothmeyer, et al 2010). Based on our bioinformatics search, we selected and synthesized four CD138 peptides for HLA-A2 binding. Among the peptides, CD138260-268 (GLVGLIFAV) was shown to have the highest binding affinity as well as stability to HLA-A2 molecules. Based on the high level of HLA-A2 affinity and specificity of the CD138260-268 peptide, we evaluated its immunogenicity without further modification of the epitope.
Previous studies have shown that tumour-reactive CTL have distinct phenotypes for cell activation and memory function, and also produce IFN-γ response to tumour cell recognition (Dobrzanski, et al 2008). These specific phenotypic characteristics are also displayed in CTL from MM patients (Barber, et al 2008, Svane, et al 2007) and provide a guide for evaluating the uniqueness of our CD138-specific CTL generated ex vivo. We demonstrated that the phenotype of CTL generated with the CD138260-268 peptide is distinguishable from the T cells cultured without peptide stimulation with a higher proportion of CD8+ and CD45RO+/CD69+ activated/memory T cells and a lower proportion of CD4+ T helper and CD45RA+/CCR7+ T cells. In addition, we demonstrated a significant level of tumour-specific cell proliferation and IFN-γ secretion in responsive CD138+/HLA-A2+ MM cell lines, thereby confirming the specific functional activity of the CD138-CTL. To date, there are several studies that have investigated the use of CD138-specific mAb to treat patients with MM (Ikeda, et al 2009, Sun, et al 2007, Tassone, et al 2004). However, CD138 mAb therapy did not induce CD138-specific effector cells with anti-tumour functional activities (tumour-specific proliferation, IFN-γ secretion), as were generated in our CD138260-268 peptide-stimulated cultures. This lack of evidence of a cellular response suggests a limitation in antibody therapy to induce long-term immune response in patients, and provides the rationale for development of active-specific immunotherapy against CD138 to treat MM patients.
A potential concern with CD138-based therapy is the targeting of normal cells. However, bone marrow sections from MM patients showed that CD138 expression is exclusively on myeloma cells, but not on endothelial cells, stromal cells or other haematopoietic elements including CD34+ haematopoietic stem cells (Bayer-Garner, et al 2001). Additional data demonstrated that treatment with CD138 mAb does not affect colony formation in haematopoietic stem cell cultures, nor decrease colony formation in haematopoietic precursor cells (Vooijs, et al 1996, Wijdenes, et al 1996). These previous studies also support the development and use of a CD138-specific immunotherapy to target neoplastic cells, without inducing damage to normal cells in MM patients.
The efficacy of peptide vaccines can be enhanced by stimulating immune effector cells with multiple immunogenic peptides, derived from different tumour-associated antigens. Induction of broad CTL responses targeting a variety of epitopes on myeloma cells may provide optimal immunotherapy. We previously identified two heteroclitic HLA-A2-specific immunogenic peptides, YISPWILAV and YLFPQLISV, from unspliced or spliced XBP1 antigens, which are over expressed on myeloma cells (Bae et al, 2011). These heteroclitic XBP1 peptides were able to induce myeloma-specific CTL. In this study, we compared the myeloma-specific activity of CTL specific to the heteroclitic unspliced XBP1184-192 peptide, heteroclitic spliced XBP1 SP367-375 peptide or native CD138260-268 peptide. Our results demonstrated that each of these peptide-specific CTL produce intracellular IFN-γ and proliferate in response to HLA-A2+/CD138+ McCAR or U266 myeloma cells (Figures 5a, 5b), suggesting that a multiepitope vaccine comprised of CD138, unspliced XBP1 and spliced XBP1 peptides may provide an effective broadly applicable immunotherapy by targeting different antigens on myeloma cells. We are also evaluating enhancing responses to peptide vaccination with an optimal delivery system, such as soluble polymers or conjugation with novel adjuvant or drug carrier to increase cell permeability, retention and bioavailability (Bolhassani, et al 2011, Shukla, et al 2011). In conclusion, we describe a novel HLA-A2-specific immunogenic nonamer CD138260-268 (GLVGLIFAV) peptide, which generates an effective MM-specific CTL ex vivo. This CD138-derived peptide may prove beneficial as a vaccine to generate CD138-specific CTL in patients with MM and other plasma cell disorders.
Acknowledgments
Research Support
This work is supported by: NIH grants; P50-100707, PO1-78378, (K.C.A. and N.C.M.), RO1-129295 (N.C.M.) and RO1-50947 (K.C.A.), Multiple Myeloma Research Foundation Awards, Leukemia and Lymphoma Society Scholar in Translational Research Award, and Department of Veteran’s Affairs merit review award.
REFERENCES
- Bae J, Martinson JA, Klingemann HG. Identification of novel CD33 antigen-specific peptides for the generation of cytotoxic T lymphocytes against acute myeloid leukemia. Cellular Immunology. 2004;227:38–50. doi: 10.1016/j.cellimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Bae J, Martinson JA, Klingemann HG. Identification of CD19 and CD20 peptides for induction of antigen-specific CTLs against B-cell malignancies. Clinical Cancer Research. 2005;11:1629–1638. doi: 10.1158/1078-0432.CCR-04-1612. [DOI] [PubMed] [Google Scholar]
- Bae J, Carrasco R, Lee AH, Prabhala R, Tai YT, Anderson KC, Munshi NC. Identification of novel myeloma-specific XBP1 peptides able to generate cytotoxic T lymphocytes: A potential therapeutic application in multiple myeloma. Leukemia. 2011 doi: 10.1038/leu.2011.120. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagratuni T, Wu P, Gonzalez de Castro D, Davenport EL, Dickens NJ, Walker BA, Boyd K, Johnson DC, Gregory W, Morgan GJ, Davies FE. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116:250–253. doi: 10.1182/blood-2010-01-263236. [DOI] [PubMed] [Google Scholar]
- Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Experimental Hematology. 2008;36:1318–1328. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille R, Robillard N, Avet-Loiseau H, Harousseau JL, Moreau P. CD221 (IGF-1R) is aberrantly expressed in multiple myeloma, in relation to disease severity. Haematologica. 2005;90:706–707. [PubMed] [Google Scholar]
- Bataille R, Jego G, Robillard N, Barille-Nion S, Harousseau JL, Moreau P, Amiot M, Pellat-Deceunynck C. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica. 2006;91:1234–1240. [PubMed] [Google Scholar]
- Bayer-Garner IB, Sanderson RD, Dhodapkar MV, Owens RB, Wilson CS. Syndecan-1 (CD138) immunoreactivity in bone marrow biopsies of multiple myeloma: shed syndecan-1 accumulates in fibrotic regions. Modern Pathology. 2001;14:1052–1058. doi: 10.1038/modpathol.3880435. [DOI] [PubMed] [Google Scholar]
- Benavides LC, Sears AK, Gates JD, Clifton GT, Clive KS, Carmichael MG, Holmes JP, Mittendorf EA, Ponniah S, Peoples GE. Comparison of different HER2/neu vaccines in adjuvant breast cancer trials: implications for dosing of peptide vaccines. Expert Review of Vaccines. 2011;10:201–210. doi: 10.1586/erv.10.167. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. Journal of Immunological Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, Raj-Vadhan S, Estrov Z, Talpaz M, Aggarwal BB. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- Bolhassani A, Safaiyan S, Rafati S. Improvement of different vaccine delivery systems for cancer therapy. Molecular Cancer. 2011;10:3. doi: 10.1186/1476-4598-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone A, Gaidano G, Gloghini A, Larocca LM, Capello D, Canzonieri V, Antinori A, Tirelli U, Falini B, Dalla-Favera R. Differential expression of BCL-6, CD138/syndecan-1, and Epstein-Barr virus-encoded latent membrane protein-1 identifies distinct histogenetic subsets of acquired immunodeficiency syndrome-related non-Hodgkin’s lymphomas. Blood. 1998;91:747–755. [PubMed] [Google Scholar]
- Carbone A, Gloghini A, Larocca LM, Antinori A, Falini B, Tirelli U, Dalla-Favera R, Gaidano G. Human immunodeficiency virus-associated Hodgkin’s disease derives from post-germinal center B cells. Blood. 1999;93:2319–2326. [PubMed] [Google Scholar]
- Carey DJ, Bendt KM, Stahl RC. The cytoplasmic domain of syndecan-1 is required for cytoskeleton association but not detergent insolubility. Identification of essential cytoplasmic domain residues. The Journal of Biological Chemistry. 1996;271:15253–15260. doi: 10.1074/jbc.271.25.15253. [DOI] [PubMed] [Google Scholar]
- Chilosi M, Adami F, Lestani M, Montagna L, Cimarosto L, Semenzato G, Pizzolo G, Menestrina F. CD138/syndecan-1: a useful immunohistochemical marker of normal and neoplastic plasma cells on routine trephine bone marrow biopsies. Modern Pathology. 1999;12:1101–1106. [PubMed] [Google Scholar]
- Disis ML, Wallace DR, Gooley TA, Dang Y, Slota M, Lu H, Coveler AL, Childs JS, Higgins DM, Fintak PA, dela Rosa C, Tietje K, Link J, Waisman J, Salazar LG. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. Journal of Clinical Oncology. 2009;27:4685–4692. doi: 10.1200/JCO.2008.20.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzanski MJ, Reome JB, Hylind JC, Rewers-Felkins KA, Abulsamad K, Adams SL. Ag-specific type 1 CD8 effector cells enhance methotrexate-mediated antitumor responses by modulating differentiated T cell localization, activation and chemokine production in established breast cancer. Clinical Immunology. 2008;128:205–218. doi: 10.1016/j.clim.2008.03.518. [DOI] [PubMed] [Google Scholar]
- Dudek NL, Perlmutter P, Aguilar MI, Croft NP, Purcell AW. Epitope discovery and their use in peptide based vaccines. Currebt Pharmaceutical Design. 2010;16:3149–3157. doi: 10.2174/138161210793292447. [DOI] [PubMed] [Google Scholar]
- Dunphy CH, Nies MK, Gabriel DA. Correlation of plasma cell percentages by CD138 immunohistochemistry, cyclin D1 status, and CD56 expression with clinical parameters and overall survival in plasma cell myeloma. Applied Immunohistochemistry and Molecular Morphology. 2007;15:248–254. doi: 10.1097/01.pai.0000213136.93912.84. [DOI] [PubMed] [Google Scholar]
- el-Shami K, Smith BD. Immunotherapy for myeloid leukemias: current status and future directions. Leukemia. 2008;22:1658–1664. doi: 10.1038/leu.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerema-McKenney A, Waldron J, Hughes S, Zhan F, Sawyer J, Barlogie B, Shaughnessy JD., Jr. Clinical, immunophenotypic, and genetic characterization of small lymphocyte-like plasma cell myeloma: a potential mimic of mature B-cell lymphoma. American Journal of Clinical Pathology. 2010;133:265–270. doi: 10.1309/AJCPUS3PRRT5ZXVS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, Kiziltepe T, Vallet S, Pozzi S, Santo L, Perrone G, Tai YT, Cirstea D, Raje NS, Uherek C, Dalken B, Aigner S, Osterroth F, Munshi N, Richardson P, Anderson KC. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clinical Cancer Research. 2009;15:4028–4037. doi: 10.1158/1078-0432.CCR-08-2867. [DOI] [PubMed] [Google Scholar]
- Inki P, Jalkanen M. The role of syndecan-1 in malignancies. Annals of Medicine. 1996;28:63–67. doi: 10.3109/07853899608999076. [DOI] [PubMed] [Google Scholar]
- Kannan S, Neelapu SS. Vaccination strategies in follicular lymphoma. Current Hematologic Malignancy Reports. 2009;4:189–195. doi: 10.1007/s11899-009-0025-2. [DOI] [PubMed] [Google Scholar]
- Kraj M, Sokolowska U, Kopec-Szlezak J, Poglod R, Kruk B, Wozniak J, Szpila T. Clinicopathological correlates of plasma cell CD56 (NCAM) expression in multiple myeloma. Leukemia & Lymphoma. 2008;49:298–305. doi: 10.1080/10428190701760532. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Choi BH, Kang HK, Park MS, Park JS, Kim SK, Pham TN, Cho D, Nam JH, Kim YJ, Rhee JH, Yang DH, Kim YK, Kim HJ, Chung IJ. Induction of multiple myeloma-specific cytotoxic T lymphocyte stimulation by dendritic cell pulsing with purified and optimized myeloma cell lysates. Leukemia & Lymphoma. 2007;48:2022–2031. doi: 10.1080/10428190701583975. [DOI] [PubMed] [Google Scholar]
- Liu W, Litwack ED, Stanley MJ, Langford JK, Lander AD, Sanderson RD. Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. The Journal of Biological Chemistry. 1998;273:22825–22832. doi: 10.1074/jbc.273.35.22825. [DOI] [PubMed] [Google Scholar]
- Mateo G, Montalban MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutierrez N, Rosinol L, Montejano L, Blade J, Martinez R, de la Rubia J, Diaz-Mediavilla J, Sureda A, Ribera JM, Ojanguren JM, de Arriba F, Palomera L, Terol MJ, Orfao A, San Miguel JF. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. Journal of Clinical Oncology. 2008;26:2737–2744. doi: 10.1200/JCO.2007.15.4120. [DOI] [PubMed] [Google Scholar]
- McKenzie EA. Heparanase: a target for drug discovery in cancer and inflammation. British Journal of Pharmacology. 2007;151:1–14. doi: 10.1038/sj.bjp.0707182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Robillard N, Jego G, Pellat C, Le Gouill S, Thoumi S, Avet-Loiseau H, Harousseau JL, Bataille R. Lack of CD27 in myeloma delineates different presentation and outcome. British Journal of Haematology. 2006;132:168–170. doi: 10.1111/j.1365-2141.2005.05849.x. [DOI] [PubMed] [Google Scholar]
- Morse MA, Secord AA, Blackwell K, Hobeika AC, Sinnathamby G, Osada T, Hafner J, Philip M, Clay TM, Lyerly HK, Philip R. MHC Class I-Presented Tumor Antigens Identified in Ovarian Cancer by Immunoproteomic Analysis Are Targets for T-Cell Responses against Breast and Ovarian Cancer. Clinical Cancer Research. 2011;17:3408–3419. doi: 10.1158/1078-0432.CCR-10-2614. [DOI] [PubMed] [Google Scholar]
- Muzio M, Bertilaccio MT, Simonetti G, Frenquelli M, Caligaris-Cappio F. The role of toll-like receptors in chronic B-cell malignancies. Leukemia & Lymphoma. 2009;50:1573–1580. doi: 10.1080/10428190903115410. [DOI] [PubMed] [Google Scholar]
- Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvanova I, Rettig L, Knuth A, Pascolo S. The form of NY-ESO-1 antigen has an impact on the clinical efficacy of anti-tumor vaccination. Vaccine. 2011;29:3832–3836. doi: 10.1016/j.vaccine.2011.03.073. [DOI] [PubMed] [Google Scholar]
- Patterson J, Palombella VJ, Fritz C, Normant E. IPI-504, a novel and soluble HSP-90 inhibitor, blocks the unfolded protein response in multiple myeloma cells. Cancer Chemotherapy and Pharmacology. 2008;61:923–932. doi: 10.1007/s00280-007-0546-0. [DOI] [PubMed] [Google Scholar]
- Pellat-Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Juge-Morineau N, Wijdenes J, Amiot M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84:2597–2603. [PubMed] [Google Scholar]
- Perez-Andres M, Almeida J, Martin-Ayuso M, Moro MJ, Martin-Nunez G, Galende J, Hernandez J, Mateo G, San Miguel JF, Orfao A. Characterization of bone marrow T cells in monoclonal gammopathy of undetermined significance, multiple myeloma, and plasma cell leukemia demonstrates increased infiltration by cytotoxic/Th1 T cells demonstrating a squed TCR-Vbeta repertoire. Cancer. 2006;106:1296–1305. doi: 10.1002/cncr.21746. [DOI] [PubMed] [Google Scholar]
- Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, Dalva K, Fuhler G, Gratama J, Hose D, Kovarova L, Lioznov M, Mateo G, Morilla R, Mylin AK, Pellat-Omede P, Deceunynck C, Perez Andres M, Petrucci M, Ruggeri M, Rymkiewicz G, Schmitz A, Schreder M, Seynaeve C, Spacek M, de Tute RM, Van Valckenborgh E, Weston-Bell N, Owen RG, San Miguel JF, Sonneveld P, Johnsen HE. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008;93:431–438. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- Ridley RC, Xiao H, Hata H, Woodliff J, Epstein J, Sanderson RD. Expression of syndecan regulates human myeloma plasma cell adhesion to type I collagen. Blood. 1993;81:767–774. [PubMed] [Google Scholar]
- Robillard N, Avet-Loiseau H, Garand R, Moreau P, Pineau D, Rapp MJ, Harousseau JL, Bataille R. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood. 2003;102:1070–1071. doi: 10.1182/blood-2002-11-3333. [DOI] [PubMed] [Google Scholar]
- Roden MM, Lee KH, Panelli MC, Marincola FM. A novel cytolysis assay using fluorescent labeling and quantitative fluorescent scanning technology. Journal of Immunological Methods. 1999;226:29–41. doi: 10.1016/s0022-1759(99)00039-3. [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regulation. 1989;1:27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebestyen A, Mihalik R, Petak I, Kopper L. Modulation of apoptosis signaling in etoposide-treated lymphoma cells. Anticancer Research. 1997;17:2609–2614. [PubMed] [Google Scholar]
- Shukla NM, Lewis TC, Day TP, Mutz CA, Ukani R, Hamilton CD, Balakrishna R, David SA. Toward self-adjuvanting subunit vaccines: Model peptide and protein antigens incorporating covalently bound toll-like receptor-7 agonistic imidazoquinolines. Bioorganic & Medicinal Chemistry Letters. 2011;21:3232–3236. doi: 10.1016/j.bmcl.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak SL, Worschech A, Wang E, Stroncek DF, Marincola FM. Analysis of vaccine-induced T cells in humans with cancer. Advances in Experimental Medicine and Biology. 2010;684:178–188. doi: 10.1007/978-1-4419-6451-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strothmeyer AM, Papaioannou D, Duhren-von Minden M, Navarrete M, Zirlik K, Heining-Mikesch K, Veelken H. Comparative analysis of predicted HLA binding of immunoglobulin idiotype sequences indicates T cell-mediated immunosurveillance in follicular lymphoma. Blood. 2010;116:1734–1736. doi: 10.1182/blood-2010-02-270199. [DOI] [PubMed] [Google Scholar]
- Sun WP, Wang FM, Xie F, Wang GQ, Sun J, Yu GH, Qiu YH, Zhang XG. A novel anti-human syndecan-1 (CD138) monoclonal antibody 4B3: characterization and application. Cellular & Molecular Immunology. 2007;4:209–214. [PubMed] [Google Scholar]
- Supiot S, Faivre-Chauvet A, Couturier O, Heymann MF, Robillard N, Kraeber-Bodere F, Morandeau L, Mahe MA, Cherel M. Comparison of the biologic effects of MA5 and B-B4 monoclonal antibody labeled with iodine-131 and bismuth-213 on multiple myeloma. Cancer. 2002;94:1202–1209. doi: 10.1002/cncr.10286. [DOI] [PubMed] [Google Scholar]
- Svane IM, Nikolajsen K, Johnsen HE. Antigen-specific T-cell immunity in multiple myeloma patients is restored following high-dose therapy: implications for timing of vaccination. Scandinavian Journal of Immunology. 2007;66:465–475. doi: 10.1111/j.1365-3083.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- Tassone P, Goldmacher VS, Neri P, Gozzini A, Shammas MA, Whiteman KR, Hylander-Gans LL, Carrasco DR, Hideshima T, Shringarpure R, Shi J, Allam CK, Wijdenes J, Venuta S, Munshi NC, Anderson KC. Cytotoxic activity of the maytansinoid immunoconjugate B-B4-DM1 against CD138+ multiple myeloma cells. Blood. 2004;104:3688–3696. doi: 10.1182/blood-2004-03-0963. [DOI] [PubMed] [Google Scholar]
- Vooijs WC, Post J, Wijdenes J, Schuurman HJ, Bolognesi A, Polito L, Stirpe F, Bast EJ, de Gast GC. Efficacy and toxicity of plasma-cell-reactive monoclonal antibodies B-B2 and B-B4 and their immunotoxins. Cancer Immunology, Immunotherapy. 1996;42:319–328. doi: 10.1007/s002620050289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hong S, Wezeman M, Qian J, Yang J, Yi Q. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Frontiers in Bioscience. 2007;12:3566–3575. doi: 10.2741/2335. [DOI] [PubMed] [Google Scholar]
- Westers TM, van den Ancker W, Bontkes HJ, Janssen JJ, van de Loosdrecht AA, Ossenkoppele GJ. Chronic myeloid leukemia lysate-loaded dendritic cells induce T-cell responses towards leukemia progenitor cells. Immunotherapy. 2011;3:569–576. doi: 10.2217/imt.11.3. [DOI] [PubMed] [Google Scholar]
- Wijdenes J, Vooijs WC, Clement C, Post J, Morard F, Vita N, Laurent P, Sun RX, Klein B, Dore JM. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. British Journal of Haematolology. 1996;94:318–323. doi: 10.1046/j.1365-2141.1996.d01-1811.x. [DOI] [PubMed] [Google Scholar]
- Wolowiec D, Dybko J, Wrobel T, Urbaniak-Kujda D, Jazwiec B, Tomaszewska-Toporska B, Kapelko-Slowik K, Potoczek S, Kuliczkowski K. Circulating sCD138 and some angiogenesis-involved cytokines help to anticipate the disease progression of early-stage B-cell chronic lymphocytic leukemia. Mediators of Inflammation. 2006;2006:42394. doi: 10.1155/MI/2006/42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, Venkataraman G, Sasisekharan R, Naggi A, Torri G, Casu B, Vlodavsky I, Suva LJ, Epstein J, Yaccoby S, Shaughnessy JD, Jr., Barlogie B, Sanderson RD. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q, Szmania S, Freeman J, Qian J, Rosen NA, Viswamitra S, Cottler-Fox M, Barlogie B, Tricot G, van Rhee F. Optimizing dendritic cell-based immunotherapy in multiple myeloma: intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. British Journal of Haematology. 2010;150:554–564. doi: 10.1111/j.1365-2141.2010.08286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink HJ, Gammon MC, Utz U, Sauma SY, Harrer T, Hawkins JC, Johnson RP, Sirotina A, Hermes JD, Walker BD, Biddisont WE. Presentation of endogenous peptides to MHC class I-restricted cytotoxic T lymphocytes in transport deletion mutant T2 cells. Journal of Immunology. 1993;150:1763–1771. [PubMed] [Google Scholar]