Abstract
Escherichia coli has five genes encoding l,d-transpeptidases (Ldt) with varied functions. Three of these enzymes (YbiS, ErfK, YcfS) have been shown to cross-link Braun’s lipoprotein to the peptidoglycan (PG), while the other two (YnhG, YcbB) form direct meso-diaminopimelate (DAP-DAP, or 3-3) cross-links within the PG. In addition, Ldt enzymes can also incorporate non-canonical d-amino acids, such as d-methionine, into the PG. To further investigate the role of these enzymes and, in particular, 3-3 linkages in cell envelope physiology we constructed and phenotypically characterized a variety of multiple Ldt deletion mutants of E. coli. We report that a triple deletion mutant lacking ybiS, erfK and ycfS is hypersusceptible to the metal-chelating agent EDTA, leaks periplasmic proteins and is resistant to the toxic effect of d-methionine. A double ynhG ycbB mutant had no discernible phenotype; however, examination of the phenotypes of various Ldt mutants bearing an additional DAP auxotrophic mutation (dapA : : Cm) showed that a quintuple mutant strain lacking all Ldt genes was severely impaired for growth on media with limited DAP. These data demonstrate that loss of the E. coli Ldt enzymes involved with coupling the PG to Braun's lipoprotein resulted in the loss of outer membrane stability while loss of the Ldt enzymes involved with DAP-DAP linkages had no observable effect on the cell envelope. Loss of all Ldt enzymes proved detrimental to growth when cells were starved for DAP, indicating a combined role for both 3-3 and Braun’s lipoprotein cross-links in cell viability only under a specific PG stress.
Introduction
Peptidoglycan (PG) is a highly adaptable and tightly regulated component of bacterial cell envelopes that serves as a structural exoskeleton to the cell. Composed of a mesh-like network of linear glycan chains interconnected by peptide cross-bridges, PG withstands the turgor pressure of the cytoplasm (Vollmer et al., 2008; Weidel & Pelzer, 1964), thus maintaining cell shape, and has important roles in cell growth and division (Typas et al., 2012). The essential nature of the PG makes its synthesis a major target for antibiotics such as the widely used β-lactams and glycopeptide antibiotics, both of which inhibit transpeptidation of PG peptides (Barna & Williams, 1984; Goffin & Ghuysen, 2002).
The glycan strands of PG generally consist of alternating N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) residues linked by a β-1,4 glycosidic bond, with peptide subunits linked to the MurNAc moiety (Glauner & Höltje, 1990). The composition of the peptide subunits varies depending on the organism; however, the most commonly encountered peptide stem in Gram-negative organisms consists of l-alanyl-d-glutamyl-meso-diaminopimelyl-d-alanyl-d-alanine, which is found in the PG of Escherichia coli (Glauner & Höltje, 1990; Schleifer & Kandler, 1972).
In E. coli approximately 20–30 % of the stem peptide is cross-linked depending on growth conditions and phase (Glauner et al., 1988). There are two distinct types of cross-links, which are formed by two types of enzymic reactions. The majority of cross-links, formed by the d,d-transpeptidase activity of penicillin-binding proteins (PBPs), are of the 4-3 type and occur between the d-alanine residue at the fourth position of the peptide stem and a meso-diaminopimelic acid (DAP) at the third position of a neighbouring peptide stem. PBPs cleave the terminal d-alanine from the pentapeptide chain and attach the remaining d-alanine residue to the side chain of an adjacently located meso-DAP. PBPs are so termed because they are the target enzymes of penicillin-type β-lactam antibiotics that mimic the terminal d-alanyl-d-alanine moieties of the pentapeptide. Conversely, approximately 2–5 % of the peptide cross-links in E. coli are of the 3-3 type which occurs between two meso-DAP residues on adjacent peptide stems (Pisabarro et al., 1985; Glauner et al., 1988). These cross-links are formed by the concerted action of d,d-carboxypeptidases and l,d-transpeptidases (Ldt) in which the former recognizes the d-stereocentres of the terminal d-alanines of a pentapeptide chain and cleaves the last alanine creating a tetrapeptide. This tetrapeptide stem is the substrate of an Ldt which cleaves the bond between the l centre of the meso-DAP and the d-alanine and subsequently attaches the meso-DAP to a neighbouring meso-DAP of another peptide chain (Magnet et al., 2007a; Mainardi et al., 2000, 2002). Because Ldts recognize the l and d centres, they are insensitive to penicillin-type β-lactam antibiotics. The presence of 3-3 cross-links was first discovered in the PG of mycobacteria and has since been found to be widespread in bacterial PG (Vollmer et al., 2008; Wietzerbin et al., 1974). The reasons why bacteria produce both 4-3 and 3-3 linkages are unknown, but it has been proposed that the 3-3 linkages act as an inherent mechanism of resistance to β-lactam antibiotics and might reinforce the wall during times of stress and nonreplicating conditions (Goffin & Ghuysen, 2002).
The first enzyme found to possess Ldt activity, Ldtfm, was isolated from an ampicillin-resistant strain of Enterococcus faecium that exclusively made a 3-3 cross-linked PG in the presence of antibiotic. Orthologues have since been found in both Gram-positive and Gram-negative organisms such as Bacillus subtilis, Clostridium difficile, E. coli, Mycobacterium spp., and Vibrio cholerae (Bielnicki et al., 2006; Lavollay et al., 2011; Magnet et al., 2008; Peltier et al., 2011). These Ldts belong to the YkuD superfamily (alternatively the ErfK/YcfS/YnhG family), based on a highly conserved set of amino acids that constitute the catalytic domain, characterized by an active site cysteine residue, which differs from classical PBPs, which generally rely on a serine residue for catalysis. In addition to catalysing the formation of 3-3 cross-linkages in the PG, these enzymes have also been shown to form PG-lipoprotein cross-links in E. coli. Although the Ldts are insensitive to penicillin-type β-lactam antibiotics, they are susceptible to carbapenem-type antibiotics, such as imipenem, which act by acylation of the catalytic cysteine residue (Mainardi et al., 2007).
E. coli has five Ldt homologues: ErfK, YbiS, YcfS, YnhG and YcbB. PG analysis of Ldt mutant strains of E. coli identified that three of these enzymes (ErfK, YbiS, YcfS) function by covalently attaching Braun’s lipoprotein to the PG (Magnet et al., 2007b, 2008). This type of cross-link requires l,d-transpeptidation because the lipoprotein is attached through the ϵ-amino group of a lysine residue in the C terminus of the protein to the α-carbon of meso-DAP at the third position in the peptide stem (Braun & Sieglin, 1970; Braun & Wolff, 1970; Magnet et al., 2007b). These findings suggest a role for these Ldt enzymes in cell envelope stability, as Braun’s lipoprotein has previously been shown to be an important factor in the stabilization of the outer membrane (Cowles et al., 2011). The remaining two Ldt enzymes (YnhG, YcbB) have been shown to form 3-3 cross-links in E. coli, since a mutant lacking all five Ldt genes lacks these cross-links (Magnet et al., 2008). Although knowledge of the biochemical properties of the Ldt enzymes has dramatically increased since their discovery, our understanding of the biological significance of these enzymes remains unclear, particularly the importance of 3-3 linkages. In this study we used a genetic approach to study the contribution of Ldts in E. coli cell envelope physiology. We report that loss of the E. coli Ldt enzymes involved with coupling the PG to Braun's lipoprotein results in multiple phenotypes, while loss of the Ldt enzymes responsible for DAP-DAP linkages has no discernible effect on cell envelope physiology. However, a cell growth defect was observed for a mutant lacking all five Ldt genes and bearing a DAP auxotrophic mutation.
Methods
Bacterial strains, plasmids and growth conditions.
Bacterial strains used in this study are listed in Table 1. E. coli strain BW25113 was the parental strain, and all mutants discussed in this paper were constructed in this background (Baba et al., 2006; Datsenko & Wanner, 2000; Hoang et al., 1998). E. coli strains were grown at 37 °C in Luria–Bertani (LB) broth (Difco, BD Bioscience), LB agar, or M9 (Difco, BD Bioscience) minimal medium where indicated. When required, LB or M9 minimal medium was supplemented with meso-DAP at a concentration of 200 µg ml−1 and antibiotics were used at the following concentrations: chloramphenicol, 25 µg ml−1, and kanamycin, 50 µg ml−1. All antibiotics and additives were obtained from Sigma-Aldrich.
Table 1. Strains and plasmids.
| Strain | Genotype | Reference or Source |
| BW25113 | Δ(araD-araB)567 ΔlacZ4787( : : rrnB-3) λ− rph-1 Δ(rhaD-rhaB)568 hsdR514 | E. coli Genetic Stock Center; Baba et al. (2006) |
| JW0803-7 | BW25113 ΔybiS790 : : kan | E. coli Genetic Stock Center; Baba et al. (2006) |
| JW0908-1 | BW25113 ΔycbB742 : : kan | E. coli Genetic Stock Center; Baba et al. (2006) |
| JW1668-1 | BW25113 ΔynhG753 : : kan | E. coli Genetic Stock Center; Baba et al. (2006) |
| JW1968-1 | BW25113 ΔerfK761 : : kan | E. coli Genetic Stock Center; Baba et al. (2006) |
| JW5820-2 | BW25113 ΔycfS775 : : kan | E. coli Genetic Stock Center; Baba et al. (2006) |
| JW1667-5 | BW25113 Δlpp752 : : kan | E. coli Genetic Stock Center; Baba et al. (2006) |
| PM2180 | BW25113/pCA24N | This study |
| PM2214 | ΔybiS790 : : frt ΔycfS775 : : frt ΔerfK761 : : kan (Δldt3) | This study |
| PM2405 | ΔynhG753 : : frt ΔybiS790 : : frt ΔycfS775 : : frt ΔerfK761 : : frt ΔycbB742 : : kan (Δldt5) | This study |
| PM2406 | PM2405/pCA24N | This study |
| PM2478 | PM2214/pCA24N | This study |
| PM2482 | ΔynhG753 : : frt ΔycbB742 : : kan (Δldt2) | This study |
| PM2527 | PM2214/pCA24N ybiS | This study |
| PM2680 | BW25113 ΔdapA : : Cm | This study |
| PM2681 | PM2482 ΔdapA : : Cm | This study |
| PM2682 | PM2405 ΔdapA : : Cm | This study |
| PM2691 | PM2214 ΔdapA : : Cm | This study |
| PM2692 | Δlpp752 : : kan ΔdapA : : Cm | This study |
| Plasmids | ||
| pCA24N | lacI cm 6×His | This study |
| pCA24N.ybiS | yibS+ | Kitagawa et al. (2005) |
| pFLP2 | ori1600 rep sacB flp cI oriT bla | Hoang et al. (1998) |
Construction of multiple Ldt mutants.
All multiple mutants were constructed by P1vir bacteriophage transduction as previously described (Moore, 2011). Antibiotic cassettes were removed sequentially using the Flp recombinase (pFLP2), and mutations confirmed by PCR (Hoang et al., 1998). The DAP auxotroph PM2680 (BW25113 dapA : : Cm) and all other DAP auxotrophs were constructed by P1vir transduction with a lysate prepared from an E. coli dapA : : Cm strain originally obtained from C. Richaud (Institut Pasteur, Paris, France).
EDTA survival assay.
Bacterial cultures in stationary phase of growth were harvested by centrifugation and washed twice with 50 mM Tris/HCl pH 8.0. Cells were resuspended in 50 mM Tris/HCl pH 8.0 to a final optical density of 0.9 (600 nm). EDTA was added to cell suspensions at a final concentration of 1 mM and cultures were incubated at 37 °C for 4.5 h without shaking. Control suspensions received only buffer. Samples were taken at 90 min intervals and plated on LB agar for viable cell counts.
Precipitation of proteins from culture supernatants.
Bacterial strains were grown in M9 minimal medium supplemented with either 0.2 % glucose or 0.2 % maltose to stationary phase (OD600 1.0) and cells were harvested by centrifugation. Supernatants were collected and passed through a 0.45 µm filter to remove contaminating cells. Ice-cold trichloroacetic acid was added to the culture supernatants to a final concentration of 6 % (v/v), which were incubated for 1 h at 4 °C. The precipitated proteins were collected by centrifugation, washed twice with acetone and resuspended in SDS-PAGE sample buffer.
Immunoblotting.
Detection of maltose-binding protein (MBP) and aminoglycoside phosphotransferase (NPT) in cell culture supernatants and lysates was performed by Western blotting. Briefly, equivalent volumes of cell culture supernatants and cell lysates were separated on 10–12 % Bistris SDS-PAGE denaturing gels (Invitrogen). Proteins were electrotransferred to a polyvinylidene difluoride (PVDF) membrane and probed with either mouse IgG anti-MBP (Invitrogen) or rabbit IgG anti-NPT (Invitrogen). The membranes were then incubated with either rabbit anti-mouse IgG coupled to alkaline phosphatase or goat anti-rabbit IgG conjugated to horseradish peroxidase and the presence of MBP or NPT was detected by chemiluminescence using either the WesternBreeze or Novex ECL systems, respectively, (GE Healthcare) according to the manufacturer’s recommendations.
DAP survival assay.
Bacterial strains were grown to stationary phase in LB broth supplemented with 200 µg DAP ml−1 and subsequently harvested by centrifugation. Cultures were washed twice with PBS and resuspended to a final optical density of 0.9 (600 nm) in LB broth. Cultures were then plated for viable cell counts on LB agar supplemented with 0, 25 or 200 µg DAP ml−1.
d-Methionine sensitivity assay.
E. coli strains were grown to stationary phase (OD600 0.9) in LB liquid medium. Cultures were plated for viable cell counts on LB medium or LB supplemented with d-methionine to a final concentration of 25 mM (MP Biochemicals). Colony forming units (c.f.u.) were recorded after 24 h of incubation at 37 °C.
Results
The five Ldt genes in E. coli, ybiS, erfK, ycfS, ynhG and ycbB, produce secreted periplasmic proteins with the signature catalytic domain as shown in Fig. 1 (Magnet et al., 2007b, 2008). YcfS and YnhG both contain the non-covalent PG-binding module lysin motif domain (LysM), found in many cell wall hydrolases and secreted proteins in prokaryotes and eukaryotes (Buist et al., 2008). Clustal W alignments of the protein sequences show that YbiS, YcfS, ErfK and YnhG are all of similar size (~300 amino acids) and share 42–62 % sequence similarity. However, YcbB is twice the size of the other four proteins (610 amino acids) and shares only 11–17 % sequence similarity with them (Fig. 1). In addition, YcbB has a divergent active site sequence located near the C terminus.
Fig. 1.
Clustal W (Larkin et al., 2007) protein sequence alignment of the five YkuD family Ldts from E. coli. The signature residues composing the catalytic domain found in all YkuD family proteins are boxed with the essential active site cysteine underlined. YcfS and YnhG both contain an N-terminal PG-binding LysM motif (bold). Lpp, lipoprotein. Percentage sequence similarity between the five proteins is shown in the inset.
The enzymes can be grouped into two distinct functional categories based on the PG analysis of mutant strains. YbiS, YcfS and EfrK catalyse the linkage of Braun’s lipoprotein to the PG, and YnhG and YcbB form direct 3-3 cross-links in the PG (Magnet et al., 2008). To investigate the greater physiological role of the Ldt enzymes, mutant strains were constructed that were deleted for the three Braun’s lipoprotein cross-linking enzymes (Δldt3), the two 3-3 cross-linking enzymes (Δldt2), or all five Ldt enzymes (Δldt5). Growth rates of the mutants were comparable in rich and minimal media (data not shown). The mutant strains were tested under a variety of conditions that have been shown to affect the cell envelope (Schleifer & Kandler, 1972). EDTA has been shown to destabilize the outer membrane of Gram-negative organisms by chelation of magnesium ions that shield the negative charge of the LPS molecules in the outer membrane (Vaara, 1992). Braun’s lipoprotein is essential to the stability of the outer membrane in the presence of EDTA (Hirota et al., 1977). Therefore, we assayed the survival of E. coli Ldt mutant strains in the presence of 1 mM EDTA. While the parental strain showed no loss of viability during EDTA exposure, the triple mutant and a control Braun’s lipoprotein null mutant displayed a 3.0- and 4.0-log10 reduction in survival, respectively, after a 4 h incubation (Fig. 2a). EDTA had a more severe effect on the Δlpp strain, showing a 1.0-log10 decrease in survival over the Δldt3 strain (P = 0.06). The Δldt3 strain was the only Ldt mutant to display EDTA sensitivity as neither single nor double Braun's lipoprotein cross-linking mutants were affected (data not shown). In contrast to the Δlpp and Δldt3 mutants, the Δldt2 strain was not affected by the presence of EDTA. Simultaneous addition of 8 mM MgSO4 to the EDTA-treated cultures was able to abrogate the effects of EDTA on the triple mutant, showing that the process is specific to chelation of Mg2+ (Fig. 2b). To confirm that the EDTA sensitivity was due to the loss of Braun’s lipoprotein cross-linking enzymes, the Δldt3 strain was complemented in trans by each Ldt enzyme. Overexpression of either ybiS, ycfS or erfK in the triple mutant strain restored survival in the presence of EDTA (Fig. 2c and data not shown).
Fig. 2.
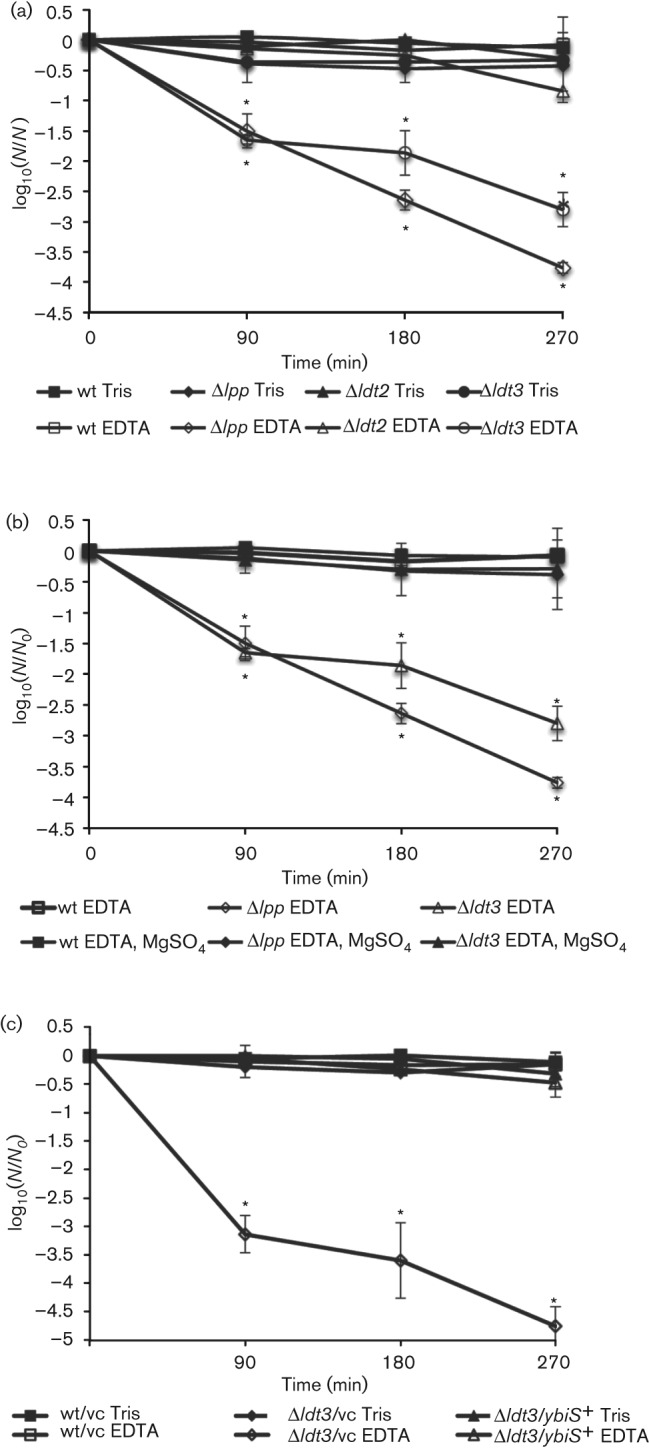
The E. coli Δldt3 strain is hypersensitive to EDTA. Wild-type and mutant E. coli strains grown to stationary phase in LB medium were pelleted and washed twice with 50 mM Tris pH 8.0. (a, c) Cells were resuspended in either 50 mM Tris pH 8.0 (Tris, control) or 50 mM Tris pH 8.0, 1 mM EDTA (EDTA). (b) Cells were resuspended in either 50 mM Tris pH 8.0, 1 mM EDTA (EDTA) or 50 mM Tris pH 8.0, 1 mM EDTA, 8 mM MgSO4 (EDTA, MgSO4). All cell suspensions were incubated at 37 °C for 4.5 h and plated onto LB agar for viability at 90 min intervals. (c) Wild-type and Δldt3 strains with the pCA24N plasmid expressing the ybiS (ybiS+) or the vector control (vc). Δlpp, Braun’s lipoprotein mutant; Δldt2, ΔynhBΔycbB; Δldt3, ΔerfKΔybiSΔycfS. Data are plotted as the viable counts at each time point divided by the viable counts at time zero on a log10 scale. Data were analysed using Student’s t-test. *P-values ≤0.05 were significant. Error bars, ±S.D.
The Δldt3 strain leaks periplasmic proteins
The EDTA sensitivity of the Δldt3 strain suggests a defect in outer membrane stability. Phenotypic analysis of a Braun’s lipoprotein mutant has shown that the strain leaks the periplasmic protein RNaseI (Yem & Wu, 1978). To determine if the Ldt mutant strains also release proteins into the extracellular milieu, we analysed filtrates of culture supernatants for the presence of the periplasmic MBP by Western blotting. MBP is the product of the gene malE whose expression is upregulated by the presence of maltose (Boos & Shuman, 1998). MBP was detected in the culture supernatants of the Δldt3 and Δlpp strains grown in the presence of 0.2 % maltose; however, it was not detected in the parental or Δldt2 strain, nor was it detected (or it was much reduced) in strains grown with 0.2 % glucose (Fig. 3a). To show that this effect is specific to the outer membrane and that the integrity of the cytoplasmic membrane remains uncompromised in these strains, culture supernatants were probed for NPT. NPT is encoded by the kanamycin antibiotic resistance cassette present as a marker of all of the mutant strains. NPT was detected in the cell lysates of Δldt3 and Δlpp as expected, but it was not detected in the culture supernatants (Fig. 3b).
Fig. 3.
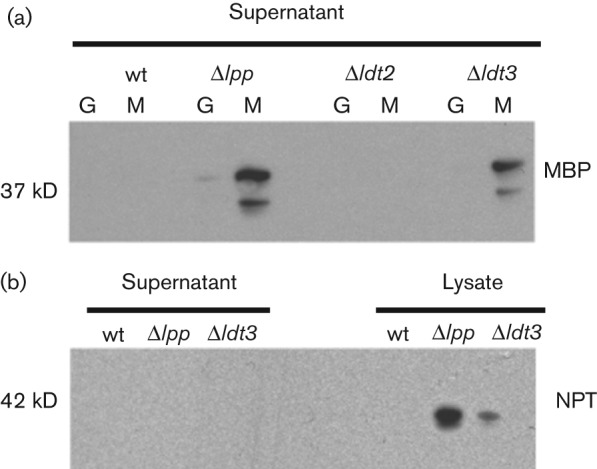
The E. coli Δldt3 strain leaks periplasmic enzymes. Western blot analysis of preparations from wild-type, Δlpp and Δldt3 mutant strains grown with maltose (M) or glucose (G) to induce or repress the expression of the MBP, respectively. (a) Precipitated culture supernatants probed with a monoclonal mouse anti-MBP for the presence of the 37 kDa, periplasmic MBP. (b) Whole cells lysates and their corresponding cell culture supernatants probed with a monoclonal rabbit anti-NPT for the expression of the 42 kDa, cytoplasmically located NPT encoded by mutant strains only. The presence of two bands in the MBP blot may be due to proteolysis after release of the protein.
The Δldt3 mutant is resistant to d-methionine
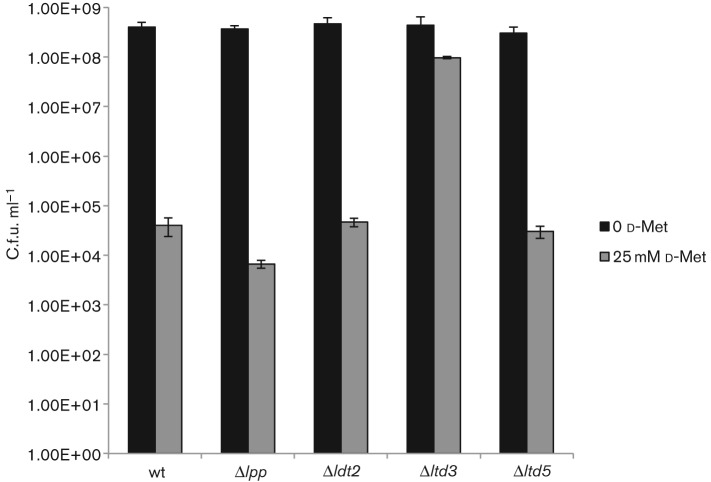
Another function for Ldts has emerged with the discovery that non-canonical d-amino acids (NCDAAs), are produced, secreted, and incorporated into the cell wall by many bacterial species (Horcajo et al., 2012). NCDAAs have been found at the fourth and fifth position of the peptide stem in PG and have an inhibitory effect on bacterial growth, presumably by preventing transpeptidation of stem peptides during PG synthesis (Cava et al., 2011; Grula, 1960). Ldts, l,d-carboxypeptidases, and d,d-transpeptidases have all been found to perform d-amino acid exchange reactions in vitro with PG substrates (Horcajo et al., 2012). However, Ldts have been shown to perform the d-amino acid exchange reaction in vivo as a V. cholerae strain lacking both of its Ldts (LdtA and LdtB) was unable to incorporate exogenously added NCDAAs into the fourth position of the stem peptide (Cava et al., 2011). Presumably, loss of Ldt activity, and therefore loss of NCDAA incorporation into the PG, may abrogate the inhibitory effects of elevated d-amino acid concentration on bacterial growth. In our analysis, growth of the wild-type, Δldt2 and Δldt5 strains was inhibited by 25 mM d-methionine (Fig. 4). However, no growth defect was detected in the Δldt3 strain (Fig. 4).
Fig. 4.
The Δldt3 strain is resistant to d-methionine. Wild-type and mutant strains were grown to OD600 0.9 in LB broth. Cultures were serially diluted 10-fold and plated on LB medium or LB medum supplemented with d-methionine to a final concentration of 25 mM for viable cell counts. Data were analysed using Student’s t-test. All values were significantly different when comparing d-methionine-treated and untreated samples for the same strain (P<0.001) except for strain Δldt3. The d-methionine-treated Δldt3 value was significantly different from the d-methionine-treated wild-type strain (P<0.001). Error bars, ±S.D.
The Δldt2 strain does not display cell envelope associated phenotypes
Many researchers have noted an increase in 3-3 cross-links in the PG of bacteria under certain growth conditions. Recent studies have shown that an elevated amount of 3-3 cross-links in the PG causes resistance to penicillin-type antibiotics in Ent. faecium, which is consistent with the idea that Ldts are mechanistically resistant to the activity of penicillins (Mainardi et al., 2005). Also, an increase in 3-3 cross-links has been demonstrated in E. coli under certain stress conditions: nutrient limitation, nitrogen limitation and elevated temperatures (Burman & Park, 1983; Glauner & Höltje, 1990; Pisabarro et al., 1985). In addition, studies have shown that 3-3 cross-links increase in number during stationary phase of many organisms (Lavollay et al., 2008; Quintela et al., 1997; Vollmer & Bertsche, 2008). However, the significance of these increases in E. coli physiology remains unclear. Thus, the Δldt2 strain was characterized under various conditions such as temperature and osmotic stress, stationary phase survival, nutrient deprivation, lysozyme, as well as antibiotic (ampicillin, imipenem, vancomycin, rifampicin) and chemical challenge (EDTA, SDS, bile salts, copper sulfate). The Δldt2 strain did not display any distinguishable differences from the wild-type (Fig. 2a, and data not shown).
The Δldt5 strain displays a growth defect when starved for meso-DAP
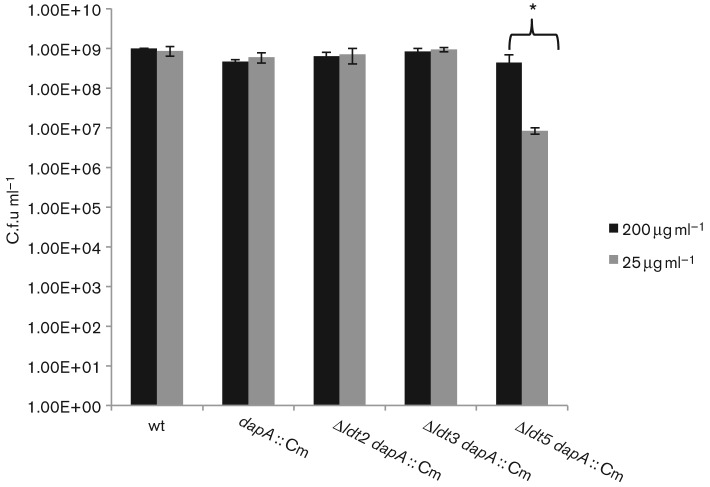
As an additional cell wall stressor, intracellular pools of PG precursors were depleted by introduction of a dapA : : Cm mutation into the ldt strains, rendering them DAP auxotrophs. Since meso-DAP is the third residue in the PG peptides, it is the essential residue involved in all known types of PG linkage in E. coli (4-3, 3-3 and Braun’s lipoprotein). The viability of the ldt meso-DAP auxotrophic strains was tested on media containing varying concentrations of meso-DAP. As shown in Fig. 5, the Δldt5 dapA strain was the only strain that displayed a growth defect on medium containing 25 µg DAP ml−1.
Fig. 5.
E. coli Δldt5 DAP auxotroph is impaired for growth on minimal DAP medium. DAP auxotrophic Ldt multiple mutant strains were grown to stationary phase in LB medium supplemented with 200 µg DAP ml−1. Cells were pelleted, washed twice with sterile PBS, and subsequently resuspended in LB medium without DAP. Cell suspensions were plated for viable cells on media containing either 200 µg or 25 µg DAP ml−1. Data were analysed using Student’s t-test. *P values ≤0.05 comparing 200 µg DAP ml−1-treated values with 25 µg DAP ml−1-treated values of the same strain were considered significant. Error bars, ±S.D.
Discussion
In this study we describe the physiological consequences of the loss of Ldts in E. coli. While many studies have characterized the specific biochemical properties of the Ldts in E. coli as well as various other species, the significance of the linkages catalysed by the Ldts has been underappreciated. We showed that the enzymes involved with linking Braun’s lipoprotein function in a redundant manner because mutant phenotypes of outer membrane instability are only evident when all three genes are deleted. Loss of the enzymes responsible for DAP-DAP linkages had no discernible effect on the cell envelope, unless DAP pools were reduced in the cells lacking all five ldt genes. Interestingly the 3-3 cross-linking enzyme, YnhG, is more closely related to the three Braun’s lipoprotein cross-linking enzymes in protein sequence. However, overexpression of ynhG in a Δldt3 or Δldt4 (ΔerfKΔybiSΔycfSΔynhG) background was unable to abrogate the phenotypes associated with the loss of Braun’s lipoprotein cross-links (data not shown). YcbB is unusual in that it only superficially resembles the other four Ldts in size and sequence. The protein is twice the size of the other Ldts in E. coli, which may indicate that this enzyme has multiple domains that have other functions in the cell. Also, its divergent active site sequence may suggest that this enzyme recognizes additional substrates such as NCDAAs or perhaps peptides that are already cross-linked (Typas et al., 2012).
Decades ago it was shown that Braun’s lipoprotein is covalently linked by the ϵ-amino group of a lysine residue in the C terminus of the protein to the carboxyl of the meso-DAP residue in the pentapeptide chain (Braun, 1975; Braun & Sieglin, 1970), but it was not until the recent work of Magnet et al. (2007b) that Ldts of the YkuD protein family were found to be responsible for this reaction. We have shown that treatment of the Δldt3 strain with 1 mM EDTA caused rapid cell death that can be abrogated by magnesium supplementation. EDTA has been shown to have severe effects on the outer membrane by the chelation of stabilizing divalent cations from their binding sites in LPS, causing the LPS to be shed (Vaara, 1992). This increases the permeability of the membrane by the recruitment of phospholipids into the outer leaflet, forming a channel for hydrophobic molecules to readily diffuse through (Vaara, 1992).
Braun’s lipoprotein has also been shown to play a role in the maintenance of the permeability barrier of the outer membrane as Braun’s lipoprotein mutants release periplasmic substances into the extracellular milieu (Hirota et al., 1977; Yem & Wu, 1977). Our data show that loss of Braun’s lipoprotein-PG cross-links also increases the permeability of the outer membrane, as the strain leaked the periplasmic MBP into the culture supernatant. Interestingly, the outer membrane of the Braun’s lipoprotein mutant appeared to be more unstable than that of the mutant lacking the ability to couple Braun’s lipoprotein to the PG, as comparably more MBP was detected in the cell culture supernatant and lysate. In addition, EDTA had a more profound effect on the Δlpp strain, showing a 4-log10 decrease in viability compared with the Δldt3 strain, which showed a 3-log10 reduction. These data can be attributed to the fact that the free form of Braun’s lipoprotein is still present in the Δldt3 strain and this form alone is likely able to aid in the stability of the outer membrane. These results are consistent with recent findings that the free and bound forms of Braun’s lipoprotein do not readily associate with each other and occupy distinct subcellular locations (Cowles et al., 2011). Our data support a dual role for Braun’s lipoprotein and suggest that the free and bound forms may have separate functions in the cell that ultimately contribute to cell envelope stability (Cowles et al., 2011).
Covalent attachment of proteins to PG is a process that is found in both Gram-positive and Gram-negative organisms (Dramsi et al., 2008). In Gram-positive organisms this reaction is carried out by sortases which use the cell wall as a fixture to display surface proteins involved in host–pathogen interaction (Dramsi et al., 2008). The discovery that Ldts are responsible for the attachment of Braun’s lipoprotein to the cell wall by a similar mechanism demonstrates an association between these two classes of enzymes. Indeed there is structural evidence that the Ldt active site residues closely resemble those of sortase enzymes, further linking the enzymic activity of these proteins (Bielnicki et al., 2006).
We have shown that the Δldt3 strain is resistant to d-methionine, even though it still has Ldt enzymes (YnhG, YcbB) which are known to be important for the incorporation of non-canonical d-amino acids into PG (Horcajo et al., 2012). Recent reports suggested that YnhG and YcbB were responsible for >75 % of the d-amino acid incorporation in E. coli and the PG of a strain lacking both genes was unable to be labelled with d-Cys (Cava et al., 2011). Interestingly, we found that a strain deleted for both ynhG and ycbB showed a similar level of sensitivity to d-methionine to the wild-type strain, suggesting that the toxicity may not be the result of incorporation by the DAP-DAP cross-linking enzymes. Conversely, while the Braun’s lipoprotein cross-linking enzymes were previously shown to play a minor role in d-amino acid incorporation (Cava et al., 2011), we showed that deletion of the genes encoding these enzymes eliminated the toxicity seen with the wild-type strain. These data suggest that the d-amino acid sensitivity is due to the Braun’s lipoprotein cross-linking enzymes; however, deleting ynhG and ycbB in the Δldt3 background (Δldt5) restored the toxic effects of d-methionine. Based on these findings we hypothesize that there are compound factors that contribute to d-amino acid sensitivity in E. coli and the d-methionine toxicity in each mutant may be the result of different mechanisms. For example, the Δldt5 mutant may be sensitive because d-methionine is incorporated only into the pentapeptides, which would inhibit standard 4-3 linkage formation. Resistance in the Δldt3 mutant could result from an increase in tetrapeptides from the action of carboxypeptidases, and if the remaining 3-3 linkage enzymes were particularly efficient in the exchange of d-methionine with d-Ala at the termini of the tetrapeptides, then these peptides could act as d-methionine sinks, thus preventing incorporation of d-methionine into the pentapeptides.
Multiple species that naturally produce NCDAAs are also able to incorporate d-amino acids into the fifth position of the pentapeptide chain by way of PBPs. When supplemented with 10 mM d-methionine in the growth medium, E. coli has not been shown to put NCDAA at the fifth position of the pentapeptide (Cava et al., 2011). In our studies, however, we found that d-methionine toxicity is only apparent in E. coli at a concentration >25 mM (MIC 50 mM); therefore at increased concentrations E. coli may be able to perform this exchange, suggesting that PBPs may also contribute to the sensitivity to d-methionine in wild-type as well as in strains lacking Ldts. Likewise, increased concentrations of d-methionine in the growth medium as well as in the PG itself may also affect recycling of PG material, specifically cytoplasmic PG precursors. In support of this, Ddl ligases in V. cholerae as well as other organisms have been shown to play a role in the cytoplasmic steps of NCDAA incorporation into PG (Cava et al., 2011). These data, together with our findings with d-methionine toxicity of the Ldt mutant strains, suggest a complex regulatory programme for NCDAA incorporation into PG that will require further investigation.
Direct 3-3 cross-links have been proposed to increase the rigidity of the cell wall and have been implicated in mediating adaptation to certain stress conditions as well as survival during periods of non-replication (Goffin & Ghuysen, 2002). An increase in 3-3 cross-links leads to resistance to penicillin-type antibiotics in Ent. faecium, and 3-3 cross-links have been shown to increase during temperature and osmotic stress and nutrient limitation in E. coli (Glauner et al., 1988; Mainardi et al., 2000). Various studies have also shown that that an increase of 3-3 cross-links occurs during stationary phase of many organisms, upon entry into the viable but non-culturable state of growth in E. coli, and during an in vitro model of persistence of Salmonella (Burman & Park, 1983; Lavollay et al., 2008; Quintela et al., 1997; Signoretto et al., 2002). A Mycobacterium tuberculosis strain with a single ldt gene inactivation, ldtmt2, showed altered colony morphology, was hypersusceptible to ampicillin, and attenuated in mice (Gupta et al., 2010). It was surprising that the Δldt2 E. coli strain lacking 3-3 cross-links remained phenotypically unchanged under the various conditions that we tested. This could be explained by the observation that E. coli produces a relatively thin layer of PG where only approximately 20–30 % of the peptide stems are cross-linked at a given time (Glauner et al., 1988). Comparatively, organisms such as M. tuberculosis or Staphylococcus aureus that have more complex cell walls cross-link up to 80–90 % of the peptides, respectively (Vollmer et al., 2008). Of the cross-bridges in E. coli, 3-3 linkages constitute approximately 2–5 % with the majority being of the 4-3 type (Vollmer & Bertsche, 2008; Vollmer et al., 2008). Therefore the small increases in 3-3 cross-links in E. coli under various conditions and growth states may have negligible effects on the stability of PG structure under laboratory conditions.
Despite the lack of observable phenotypes of the Δldt2 strain, it appears that loss of 3-3 cross-links contributed to the growth defect demonstrated by the Δldt5 strain when starved for meso-DAP. While neither the Δldt3 nor the Δldt2 strains displayed phenotypes when combined with a DAP auxotrophic mutation, the Δldt5 dapA strain displayed a decrease in viability when grown in suboptimal concentrations of DAP. During PG maturation, as E. coli enters stationary phase, the amount of peptide cross-linking increases as well as the amounts of covalently bound lipoprotein and 3-3 cross-links (Glauner et al., 1988; Glauner & Höltje, 1990); thus these modifications may be necessary for long-term survival under stress. Therefore, our data suggest a combined role for both types of Ldt enzymes in E. coli in the stability of the cell envelope.
Note added in proof
To help establish a genetic nomenclature for L,D-transpeptidases in E. coli that is consistent with that of other bacteria, the authors propose to redesignate the E. coli genes as follows: erfK (ldtA), ybiS (ldtB), ycfS (ldtC), ycbB (ldtD), and ynhG (ldtE).
Acknowledgements
This work was supported by NIH grants AI073772 (M. S. P.) and T32 AI007362 (A. N. S.). We thank G. Culver for pCA24N.ybiS, H. Schweizer for pFLP2, J. Clark-Curtiss for bacteriophage P1vir, and D. C. Crick, S. Mahapatra, and members of the Pavelka laboratory for reviewing this manuscript.
Abbreviations:
- DAP
diaminopimelic acid
- Ldt
l,d-transpeptidase
- MBP
maltose-binding protein
- NCDAA
non-canonical d-amino acid
- NPT
aminoglycoside phosphotransferase
- PBP
penicillin-binding protein
- PG
peptidoglycan
References
- Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006). Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2, 0008. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna J. C., Williams D. H. (1984). The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol 38, 339–357. 10.1146/annurev.mi.38.100184.002011 [DOI] [PubMed] [Google Scholar]
- Bielnicki J., Devedjiev Y., Derewenda U., Dauter Z., Joachimiak A., Derewenda Z. S. (2006). B. subtilis YkuD protein at 2.0 Å resolution: insights into the structure and function of a novel, ubiquitous family of bacterial enzymes. Proteins 62, 144–151. 10.1002/prot.20702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Shuman H. (1998). Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev 62, 204–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. (1975). Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta 415, 335–377. 10.1016/0304-4157(75)90013-1 [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. (1970). The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem 13, 336–346. 10.1111/j.1432-1033.1970.tb00936.x [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. (1970). The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem 14, 387–391. 10.1111/j.1432-1033.1970.tb00301.x [DOI] [PubMed] [Google Scholar]
- Buist G., Steen A., Kok J., Kuipers O. P. (2008). LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol Microbiol 68, 838–847. 10.1111/j.1365-2958.2008.06211.x [DOI] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. (1983). Changes in the composition of Escherichia coli murein as it ages during exponential growth. J Bacteriol 155, 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava F., de Pedro M. A., Lam H., Davis B. M., Waldor M. K. (2011). Distinct pathways for modification of the bacterial cell wall by non-canonical d-amino acids. EMBO J 30, 3442–3453. 10.1038/emboj.2011.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowles C. E., Li Y., Semmelhack M. F., Cristea I. M., Silhavy T. J. (2011). The free and bound forms of Lpp occupy distinct subcellular locations in Escherichia coli. Mol Microbiol 79, 1168–1181. 10.1111/j.1365-2958.2011.07539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97, 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramsi S., Magnet S., Davison S., Arthur M. (2008). Covalent attachment of proteins to peptidoglycan. FEMS Microbiol Rev 32, 307–320. 10.1111/j.1574-6976.2008.00102.x [DOI] [PubMed] [Google Scholar]
- Glauner B., Höltje J. V. (1990). Growth pattern of the murein sacculus of Escherichia coli. J Biol Chem 265, 18988–18996. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. (1988). The composition of the murein of Escherichia coli. J Biol Chem 263, 10088–10095. [PubMed] [Google Scholar]
- Goffin C., Ghuysen J. M. (2002). Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol Mol Biol Rev 66, 702–738. 10.1128/MMBR.66.4.702-738.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grula E. A. (1960). Cell division in a species of Erwinia. I. Inhibition of division by d-amino acids. J Bacteriol 80, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Lavollay M., Mainardi J. L., Arthur M., Bishai W. R., Lamichhane G. (2010). The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med 16, 466–469. 10.1038/nm.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. (1977). On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A 74, 1417–1420. 10.1073/pnas.74.4.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. (1998). A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212, 77–86. 10.1016/S0378-1119(98)00130-9 [DOI] [PubMed] [Google Scholar]
- Horcajo P., de Pedro M. A., Cava F. (2012). Peptidoglycan plasticity in bacteria: stress-induced peptidoglycan editing by noncanonical d-amino acids. Microb Drug Resist 18, 306–313. 10.1089/mdr.2012.0009 [DOI] [PubMed] [Google Scholar]
- Kitagawa M., Ara T., Arifuzzaman M., Ioka-Nakamichi T., Inamoto E., Toyonaga H., Mori H. (2006). Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12, 291–299. 10.1093/dnares/dsi012 [DOI] [PubMed] [Google Scholar]
- Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A. & other authors (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- Lavollay M., Arthur M., Fourgeaud M., Dubost L., Marie A., Veziris N., Blanot D., Gutmann L., Mainardi J. L. (2008). The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J Bacteriol 190, 4360–4366. 10.1128/JB.00239-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavollay M., Fourgeaud M., Herrmann J. L., Dubost L., Marie A., Gutmann L., Arthur M., Mainardi J. L. (2011). The peptidoglycan of Mycobacterium abscessus is predominantly cross-linked by l,d-transpeptidases. J Bacteriol 193, 778–782. 10.1128/JB.00606-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S., Arbeloa A., Mainardi J. L., Hugonnet J. E., Fourgeaud M., Dubost L., Marie A., Delfosse V., Mayer C. & other authors (2007a). Specificity of l,d-transpeptidases from Gram-positive bacteria producing different peptidoglycan chemotypes. J Biol Chem 282, 13151–13159. 10.1074/jbc.M610911200 [DOI] [PubMed] [Google Scholar]
- Magnet S., Bellais S., Dubost L., Fourgeaud M., Mainardi J. L., Petit-Frère S., Marie A., Mengin-Lecreulx D., Arthur M., Gutmann L. (2007b). Identification of the l,d-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J Bacteriol 189, 3927–3931. 10.1128/JB.00084-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S., Dubost L., Marie A., Arthur M., Gutmann L. (2008). Identification of the l,d-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J Bacteriol 190, 4782–4785. 10.1128/JB.00025-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainardi J. L., Legrand R., Arthur M., Schoot B., van Heijenoort J., Gutmann L. (2000). Novel mechanism of β-lactam resistance due to bypass of dd-transpeptidation in Enterococcus faecium. J Biol Chem 275, 16490–16496. 10.1074/jbc.M909877199 [DOI] [PubMed] [Google Scholar]
- Mainardi J. L., Morel V., Fourgeaud M., Cremniter J., Blanot D., Legrand R., Frehel C., Arthur M., Van Heijenoort J., Gutmann L. (2002). Balance between two transpeptidation mechanisms determines the expression of β-lactam resistance in Enterococcus faecium. J Biol Chem 277, 35801–35807. 10.1074/jbc.M204319200 [DOI] [PubMed] [Google Scholar]
- Mainardi J. L., Fourgeaud M., Hugonnet J. E., Dubost L., Brouard J. P., Ouazzani J., Rice L. B., Gutmann L., Arthur M. (2005). A novel peptidoglycan cross-linking enzyme for a β-lactam-resistant transpeptidation pathway. J Biol Chem 280, 38146–38152. 10.1074/jbc.M507384200 [DOI] [PubMed] [Google Scholar]
- Mainardi J. L., Hugonnet J. E., Rusconi F., Fourgeaud M., Dubost L., Moumi A. N., Delfosse V., Mayer C., Gutmann L. & other authors (2007). Unexpected inhibition of peptidoglycan ld-transpeptidase from Enterococcus faecium by the β-lactam imipenem. J Biol Chem 282, 30414–30422. 10.1074/jbc.M704286200 [DOI] [PubMed] [Google Scholar]
- Moore S. D. (2011). Assembling new Escherichia coli strains by transduction using phage P1. Methods Mol Biol 765, 155–169. 10.1007/978-1-61779-197-0_10 [DOI] [PubMed] [Google Scholar]
- Peltier J., Courtin P., El Meouche I., Lemée L., Chapot-Chartier M. P., Pons J. L. (2011). Clostridium difficile has an original peptidoglycan structure with a high level of N-acetylglucosamine deacetylation and mainly 3-3 cross-links. J Biol Chem 286, 29053–29062. 10.1074/jbc.M111.259150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisabarro A. G., de Pedro M. A., Vázquez D. (1985). Structural modifications in the peptidoglycan of Escherichia coli associated with changes in the state of growth of the culture. J Bacteriol 161, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintela J. C., de Pedro M. A., Zöllner P., Allmaier G., Garcia-del Portillo F. (1997). Peptidoglycan structure of Salmonella typhimurium growing within cultured mammalian cells. Mol Microbiol 23, 693–704. 10.1046/j.1365-2958.1997.2561621.x [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. (1972). Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36, 407–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretto C., Lleò M. M., Canepari P. (2002). Modification of the peptidoglycan of Escherichia coli in the viable but nonculturable state. Curr Microbiol 44, 125–131. 10.1007/s00284-001-0062-0 [DOI] [PubMed] [Google Scholar]
- Typas A., Banzhaf M., Gross C. A., Vollmer W. (2012). From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M. (1992). Agents that increase the permeability of the outer membrane. Microbiol Rev 56, 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer W., Bertsche U. (2008). Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim Biophys Acta 1778, 1714–1734. 10.1016/j.bbamem.2007.06.007 [DOI] [PubMed] [Google Scholar]
- Vollmer W., Blanot D., de Pedro M. A. (2008). Peptidoglycan structure and architecture. FEMS Microbiol Rev 32, 149–167. 10.1111/j.1574-6976.2007.00094.x [DOI] [PubMed] [Google Scholar]
- Weidel W., Pelzer H. (1964). Bagshaped macromolecules – a new outlook on bacterial cell walls. Adv Enzymol Relat Areas Mol Biol 26, 193–232. [DOI] [PubMed] [Google Scholar]
- Wietzerbin J., Das B. C., Petit J. F., Lederer E., Leyh-Bouille M., Ghuysen J. M. (1974). Occurrence of d-alanyl-(d)-meso-diaminopimelic acid and meso-diaminopimelyl-meso-diaminopimelic acid interpeptide linkages in the peptidoglycan of Mycobacteria. Biochemistry 13, 3471–3476. 10.1021/bi00714a008 [DOI] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. (1977). Genetic characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol 131, 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. (1978). Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol 133, 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]