Abstract
A variety of stressors activate the hypothalamic-pituitary-adrenal axis, with secretion and compensatory enhanced synthesis of hypothalamic corticotropin-releasing hormone (CRH). Whether CRH is a major effector in the stress response of the neonatal rat and whether the peptide's gene expression is subsequently up-regulated are not fully understood. We studied the effect of cold-separation stress on plasma corticosterone (CORT) levels and CRH messenger RNA (CRH-mRNA) abundance in the paraventricular nucleus. Rats (4–16 days old) were subjected to maximal tolerated cold-separation. CORT and CRH-mRNA abundance were measured before and at several time points after stress. Cold-separation stress resulted in a significant plasma CORT increase in all age groups studied. This was abolished by the administration of an antiserum to CRH on both postnatal days 6 and 9. CRH-mRNA increased in rats aged 9 days or older, but not in 6-day-old rats, by 4 h after stress. These results suggest the presence of robust CRH-mediated adrenal responses to cold-separation stress in neonatal rats. Before postnatal day 9, however, the compensatory increase in CRH-mRNA abundance is minimal.
MECHANISMS of the hormonal stress response in the neonatal rat may differ from those in the adult (1-3). In the mature animal, a variety of stressors activate the hypothalamic-pituitary adrenal (HPA) system (4-7): Secretion of corticotropin-releasing hormone (CRH) from the paraventricular nucleus (PVN) results in ACTH release from the pituitary and, in turn, increased plasma corticosterone (CORT). This is followed by a compensatory increase in CRH messenger RNA (CRH-mRNA) abundance in the hypothalamic PVN (6-8). The responsiveness of the HPA axis to stress during the first 2 weeks of life has been the subject of intensive study (1-3, 9-19). Earlier studies found little increase in plasma CORT after cold, ether, or electroshock stress (2). More recently, stimulus-specific robust ACTH responses have been demonstrated in 8- and 10-day-old rats (13, 15, 17).
We have previously studied aspects of the regulation of CRH gene expression by glucocorticoids (GC) and chronic stress (16, 19). We found alterations in CRH-mRNA abundance in response to chronic stress and GC receptor blockade starting on the ninth postnatal day (19). The current study was undertaken to examine the regulation of CRH gene expression by acute cold-separation stress and the peptide’s role in this stressor-induced CORT secretion in the neonatal rat.
Materials and Methods
Animals
Timed pregnant Sprague-Dawley-derived rats were purchased from Zivic-Miller (Zelienople, PA) and transported by air on pregnancy days 15–18. Rats were maintained in a NIH-approved animal facility, kept on a 12-h light, 12-h dark cycle (lights on at 0700 h), and given access to unlimited lab chow and water. Delivery was verified at 12-h intervals, and the date of birth was considered day 0. Cages were undisturbed for 48 h before experiments. Experiments were approved by the institutional committee for animal care.
Cold-separation stress paradigm and time course of plasma CORT
Pups were separated from their mothers and placed individually in compartmentalized plastic cages (eight compartments per cage) in a cold room (4 C). Preliminary experiments defined maximally tolerated cold exposure as the development of rigor and ~10% mortality (core temperature averaged 9.8 C). Thus, cold exposure lasted an average of 30 min in 4- to 6-day-old animals and 60 min in 11- to 16-day-old pups. Stressed pups were rewarmed on a heating pad under a heat lamp as a group. Rats were killed by decapitation, and trunk blood was collected before the onset of stress and 0, 20, 40 (or 30), 60, 150, and 240 min after its termination. Control rats were left in home cages and killed within 45 sec of disturbance. All experiments were started at 0900 h. Plasma CORT levels were determined by RIA (ICN, Irvine, CA), as previously described (20). Assay sensitivity was 0.05 ng/ml. Interassay variability was determined by two dilutions of adult rat plasma and averaged 15%.
CRH antiserum (CRH-AS) effect on stress-induced plasma CORT elevation
CRH-AS (pool 238–293), produced in sheep, was a gracious gift from Dr. W. Vale. Normal sheep serum (NSS) was purchased from Calbiochem (La Jolla, CA). Experimental groups included undisturbed controls, injection controls, cold exposure, cold and NSS injection, and cold and CRH-AS injection. Rats were injected with CRH-AS or NSS (100 μl, ip) at 0930 h. One hour later, rats were exposed to cold for 25 min (6-day-old animals) or 40 min (9- to 10-day-old pups), rewarmed for 50 min and killed. Undisturbed controls were killed at 0900 h, and injection controls (not exposed to cold) were killed immediately before the corresponding cold-exposed groups.
Time course and age dependence of stress-induced upregulation of CRH-mRNA abundance
In Exp I, 16-day-old rats were divided into six groups (n = 4). Groups 1–3 were subjected to cold-separation stress as described above, and killed 1, 4, or 28 h after termination of stress. Groups 4–6 served as controls for both separation-cold stress and diurnal variability in CRH-mRNA (21). Controls were undisturbed in home cages until death at the corresponding time.
In Exp II, 6-, 9-, and 16-day-old rats were subjected to cold-separation stress as described above, and decapitated 4 or 28 h after the onset of cold stress (28 h group was returned to home cages at 4 h and killed 24 h later).
In all experiments, trunk blood was collected and brains were rapidly removed onto powdered dry ice and stored at −80 C. Brains were cut into 20-μm coronal sections in a cryostat and mounted on gelatin-coated slides (22, 23).
In situ hybridization (ISH) for CRH-mRNA
Preparation of oligonucleotides and details of ISH and image analysis have been described previously (16, 19, 23). Briefly, before ISH, slides were brought to room temperature, air dried, and fixed in buffered paraformaldehyde. After a graded ethanol treatment, sections were exposed to acetic anhydride-triethanolamine, then dehydrated through 100% ethanol. Sections were prehybridized for 1 h, then hybridized for 20 h at 40 C in a humidity chamber. Serial washes (2 × SSC for 15 min, four times, at 40 C; 1 × and 0.3 × SSC (22) for 30 min each at room temperature) were followed by dehydration and apposition to film (Hyperfilm B-Max, Amersham, Arlington Heights, IL). Selected sections were subsequently dipped in emulsion (NTB-2, Eastman Kodak, Rochester, NY) and developed as previously described (19, 22, 23), except that the emulsion was not diluted.
Sections with the maximal area of PVN, based on cresyl violet staining, were used for quantitative image analysis. Quantitation and statistical analysis were described previously (19, 22, 23). Briefly, optical density (OD) was determined over PVN and parietal cortex, as background, using the MCID software image analysis system (Imaging Research, St. Catherine, Ontario, Canada). Each point was derived from 6–12 sections from a minimum of 4 individual rats. Ratios of PVN to parietal cortex were determined as a measure of the abundance of CRH-mRNA, thus eliminating background variability with age. Statistical significance between groups was determined using two-way analysis of variance, followed by Duncan's multiple range tests.
Results
Effect of cold-separation stress on plasma CORT: time course and age dependence
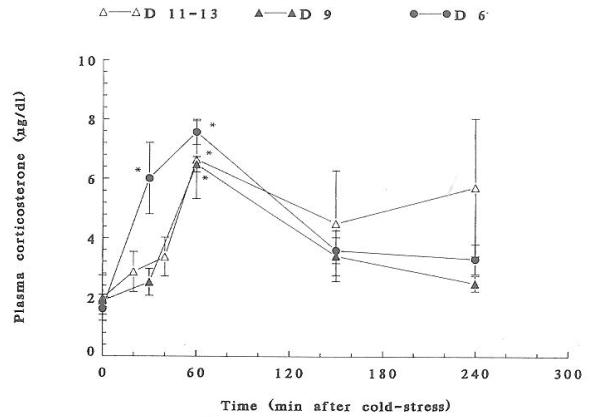
Exposure to cold-separation stress resulted in significant increases in plasma CORT in all age groups studied (Fig. 1). The time course and magnitude of cold-induced CORT elevation varied by age. On postnatal days (PND) 4–6, plasma CORT increased significantly at 40 and 60 min (P < 0.05) and returned to baseline by 2.5 h. On PND 9, a slightly delayed response (60 min peak) was observed. At 11–13 days, CORT peaked 60 min after stress termination and remained elevated for 4 h, with a significant interanimal variability (Fig. 1). Maximal cold stress-induced plasma CORT values were 7.57 ± 0.86, 6.48 ± 0.26, and 6.64 ± 1.31 μg/dl on PND 6, 9, and 11–13, respectively.
Fig. 1.
A composite of plasma CORT time courses in response to cold-separation stress in postnatal (PND) rats of different ages. See text for experimental paradigm. Values are the mean ± SEM of six to eight rats per group. For all age groups, 60 min values are significantly different from prestress levels (P < 0.05). On PND 6, the 40 min value is significantly elevated as well (P < 0.05).
Effect of antiserum to CRH on stress-induced plasma CORT elevation
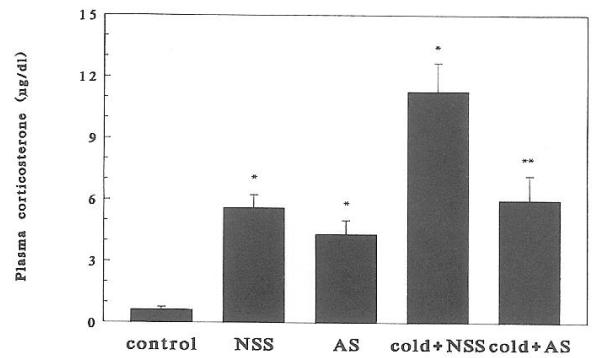
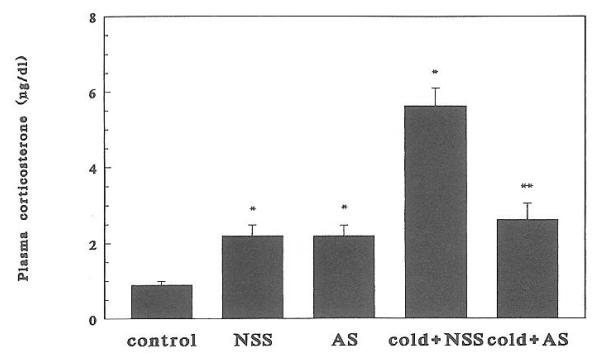
Injection of either AS or NSS resulted in increased plasma CORT, presumably because of handling and pain. Cold-separation induced a significant (P < 0.01) further increase in plasma CORT levels at both ages studied (Figs. 2 and 3). Administration of CRH-AS abolished cold-induced plasma CORT (P < 0.01, cold plus NSS vs. cold plus AS). This effect of CRH-AS was evident in both 6- and 9-day-old rats.
Fig. 2.
Effect of passive immunization against CRH on cold-separation stress-induced plasma corticosterone elevation in 6-day-old rats (n = 6/group). Bars indicate SEs. *, Significantly different from control (P < 0.05); **, significantly different from cold plus NSS stress (P < 0.05).
Fig. 3.
Effect of passive immunization against CRH on cold-separation stress-induced plasma CORT elevation in 9-day-old rats (n = 4/group). Bars indicate SEs. *, Significantly different from control (P < 0.05); **, significantly different from cold plus NSS (P < 0.05).
Stress-induced alteration in CRH-mRNA abundance
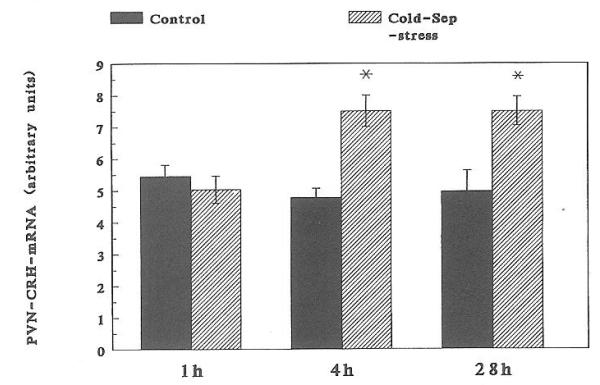
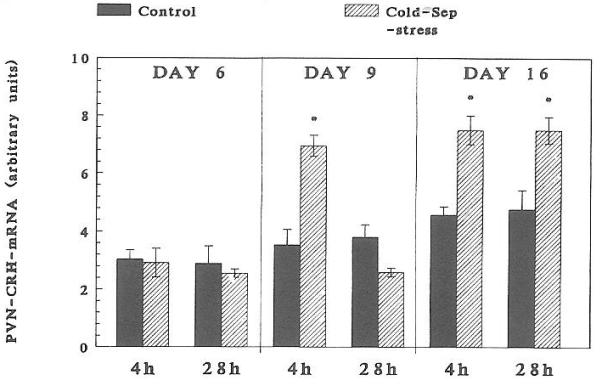
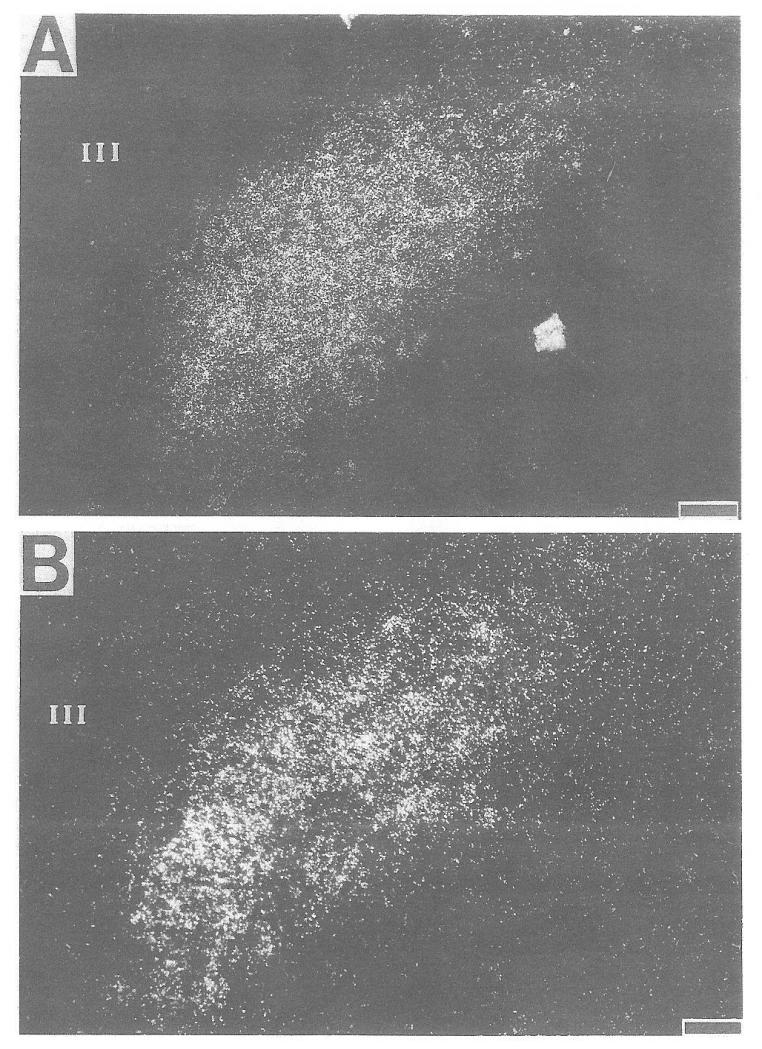
A significant increase in CRH-mRNA abundance in the PVN of 16-day-old rats was observed at 4 h (but not at 1 h) and persisted at 28 h subsequent to cold-separation stress (Fig. 4). Therefore, these time points were studied in younger rats. In 9-day-old rats, cold-separation stress enhanced CRH-mRNA abundance at 4 h, but not at 28 h. No change in CRH message was found in 6-day-old rats (Fig. 5). Darkfield photomicrographs of the PVN of control and cold-exposed 9-day-old rats after ISH for CRH-mRNA are shown in Fig. 6.
Fig. 4.
Time course of compensatory enhanced CRH-mRNA abundance in 16-day-old rats subjected to cold-separation (Cold-Sep) stress. See text for details of ISH and semiquantitative analysis. Shown are the mean and SE of at least four rats. *, Significantly different from control (P < 0.05).
Fig. 5.
Effect of cold-separation (Cold-Sep) stress on CRH mRNA in the PVN of 6-, 9-, and 16-day-old rats. Pups were subjected to age-appropriate maximal tolerated cold stress (see text). CRH mRNA was determined using ISH. Values (mean ± SEM) were derived as detailed in Materials and Methods. *, Significantly different from control (P < 0.05).
Fig. 6.
Darkfield photomicrograph of ISH for CRH mRNA in the PVN of 9-day-old rats. A, Control; B, cold-stressed. III, Third ventricle. Bar = 10 μm.
Discussion
In the current study, neonatal rats had a robust hormonal stress response to cold-separation stress throughout the first 2 postnatal weeks. As reported for a variety of stressors (2, 9, 14), basal plasma CORT was quite low on PNDs 4–13 (14). Still, the peak plasma CORT level in response to cold-separation stress represented an increase of 470% on PND 6, 345% on PND 9, and 564% in 11- to 13-day-old pups.
A hormonal (ACTH and CORT) response to a variety of stressors (cold, histamine, ether, electroshock, and hypoglycemia) has been demonstrated in the neonatal rat (9, 13, 15, 17-19). The identity of the hypothalamic secretagogue(s) responsible for pituitary-adrenal activation in these paradigms, however, has not been fully established. A role for arginine vasopressin in hypoglycemia-induced CORT elevation was demonstrated by Muret et al. (15), who used passive immunization against arginine vasopressin to abolish ACTH secretion. Adrenergic blockade (17), but not CRH-AS altered HPA activation in this paradigm. CRH has been implicated as an ACTH secretagogue in response to urethane on days 3, 5, and 10 (9).
We chose cold-separation stress as an acute, powerful, age-gradable paradigm, combining both psychological and physical elements (6). In pilot experiments, the maximal tolerated period of cold exposure (complete rigor and 10% mortality) was determined for each age group of neonatal rats. Exposing infant rats to 4 C without the ability to huddle resulted in a predictable time- and age-dependent increase in plasma CORT. Although maternal separation per se alters the degree of HPA activation in the neonatal rat (10, 18), the effect was minimal with less than 8 h of deprivation (10). Nevertheless, an interaction between the cold and separation components of the current paradigm is plausible.
The CORT response to combined cold-separation stress is in accord with that reported by Walker et al. (13). The different time course of the hormonal response (peak CORT at 40–60 min vs. 4 h) is probably due to differences in technique; we warmed pups on a heating pad after cold stress, whereas Walker did not. Similar CORT elevations have been reported with ether, histamine injection (13), hypoglycemia (14, 15, 17), and prolonged maternal deprivation (10, 13, 19).
Cold is not a strong stressor in the adult (6-8): Harbuz and Lightman (6-8) found that in adult male rats, acute cold exposure did not enhance hypothalamic CRH mRNA abundance, in contrast to restraint stress. However, their experimental design permitted animals to huddle together, and plasma CORT was not reported. Immature thermoregulation (24) and the absence of fur clearly makes cold exposure a stronger stressor in the neonatal vs. the adult rat (13). Further, a rapid negative GC feedback on CRH gene expression may explain the absence of increase in CRH-mRNA in the adult (reviewed in Ref. 5).
Cold-separation-induced plasma CORT elevation was completely abolished by passive immunization against CRH in both 6- and 9-day-old rats. This suggests a significant role of CRH as the major effector in this paradigm of HPA activation. Interestingly, CRH-AS did not prevent the plasma CORT elevation induced by injection pain. This may suggest a different mechanism for pain-mediated HPA activation. Alternatively, CRH-mediated HPA activation initiated by separation, grasping, and needle insertion has progressed beyond the ability of CRH immunoneutralization to prevent it.
We found increased CRH-mRNA abundance in the PVN of 9- and 16-day-old pups 4 h after maximal cold-separation stress, a time defined by a time-course experiment in 16-day-old rats. This compensatory increase in CRH gene expression was not observed in 6-day-old rats. Passive immunization with CRH-AS strongly suggested that CRH secretion and resulting depletion of hypothalamic content (25) were required for CORT elevation. Thus, despite CRH participation in the cold-separation stress response in the 6-day-old rat, coordinated enhancement of the peptide's synthesis was not observed.
The absence of up-regulation of CRH-mRNA abundance in the 6-day-old rat may derive from immature afferent input (e.g. second messenger cascades) onto the CRH gene promoter. Grino et al. (11) found that adrenalectomy resulted in increased CRH-mRNA abundance in the PVN of 14-day-old and adult rats, but not in 7-day-old animals. We have previously demonstrated a distinct developmental profile of the regulation of CRH-mRNA abundance by GC and chronic stress. Pharmacological adrenalectomy did not increase CRH-mRNA abundance in the PVN of the fetal rat (16). Postnatally, chronic stress, with or without local blockade of GC receptors in the PVN, resulted in enhanced CRH-mRNA abundance only in rats older than 9 days despite the presence of abundant GC receptor mRNA in the PVN during the first postnatal week (19, 26). Although steady state mRNA abundance is not a direct measure of gene expression, maturation of CRH gene promoter activation by cellular signals induced by a variety of stressors may occur around the ninth postnatal day.
The regulatory cascades of the CRH gene promoter has been studied. Majzoub et al. (27, 28) and Van et al. (29) provided evidence for a role for both protein kinase-A and -C. A consensus cAMP response element sequence is present up-stream from the CRH initiation site (30). An immature coupling of receptor-cAMP signal transduction has been demonstrated for CRH receptors in the neonatal rat (31).
In summary, graded cold-separation stress induced acute hormonal activation of the HPA axis in the neonatal rat. This activation was CRH dependent on both the sixth and ninth postnatal days. Regulatory mechanisms for coordinated up-regulation of CRH gene expression during acute stress in the first postnatal week may be immature. These findings are compatible with a dynamic, rapidly evolving developmental profile of HPA activation during the first 2 postnatal weeks in the rat.
Footnotes
This work was supported by Grants NS-01307 and NS-28912 (to T.Z.B.).
References
- 1.Rosenfeld P, Suchecki D, Levine S. Multifactorial regulation of the hypothalamic-pituitary-adrenal axis during development. Neurosci Biobehav Rev. 1992;16:553–568. doi: 10.1016/s0149-7634(05)80196-4. [DOI] [PubMed] [Google Scholar]
- 2.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 3.Widmaier EP. Glucose homeostasis and hypothalamic-pituitary-adreno-cortical axis during development in rats. Am J Physiol. 1990:601–613. doi: 10.1152/ajpendo.1990.259.5.E601. [DOI] [PubMed] [Google Scholar]
- 4.Vale W, Rivier C, Brown MR, Spiess J, Koob G, Swanson L, Bilezikjian L, Bloom F, Rivier J. Chemical and biological characterization of corticotropin releasing factor. Recent Prog Horm Res. 1983;3:339–375. doi: 10.1016/b978-0-12-571139-5.50010-0. [DOI] [PubMed] [Google Scholar]
- 5.Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH: variations on a theme of B. Recent Prog Horm Res. 1987;43:113–131. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 6.Lightman SL, Harbuz MS. Corticotropin-Releasing Factor. Wiley, Chichester: 1993. Expression of corticotropin releasing factor mRNA in response to stress; pp. 173–198. [DOI] [PubMed] [Google Scholar]
- 7.Lightman SL, Young WS., III Response of hypothalamic corticotropin releasing factor mRNA to stress, opiates and opiate withdrawal. J Physiol. 1988;403:511–519. doi: 10.1113/jphysiol.1988.sp017261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harbuz MS, Russell JA, Sumner BEH, Kawata M, Lightman SL. Rapid changes in the content of proenkephalin A and corticotrophin releasing hormone mRNAs in the paraventricular nucleus during morphine withdrawal in the urethane anaesthetized rats. Mol Brain Res. 1991;9:285–291. doi: 10.1016/0169-328x(91)90074-8. [DOI] [PubMed] [Google Scholar]
- 9.Walker C-D, Perrin M, Vale WW, Rivier CL. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 10.Stanton M, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 11.Grino M, Burgunder JM, Eskay RL, Eiden LE. Onset of glucocorticoid responsiveness of anterior pituitary corticotrophs during development is scheduled by corticotropin releasing factor. Endocrinology. 1989;124:2686–2692. doi: 10.1210/endo-124-6-2686. [DOI] [PubMed] [Google Scholar]
- 12.Walker C-D, Akana SF, Cascio CS, Dallman MF. Adrenalectomy in the neonate: adult-like adrenocortical system responses to both removal and replacement of corticosterone. Endocrinology. 1990;127:832–842. doi: 10.1210/endo-127-2-832. [DOI] [PubMed] [Google Scholar]
- 13.Walker C-D, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenal system of neonatal rats is responsive to stress throughout development in a time-dependent and stress-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 14.Arai M, Widmaier EP. Activation of the pituitary-adrenocortical axis in day old rats by insulin induced hypoglycemia. Endocrinology. 1991;129:1505–1512. doi: 10.1210/endo-129-3-1505. [DOI] [PubMed] [Google Scholar]
- 15.Muret L, Priou A, Oliver C, Grino M. Stimulation of adrenocortico-tropin secretion by insulin-induced hypoglycemia in the developing rat involves arginine vasopressin but not corticotropin-releasing factor. Endocrinology. 1992;130:2725–2732. doi: 10.1210/endo.130.5.1315256. [DOI] [PubMed] [Google Scholar]
- 16.Baram TZ, Schultz L. CRH gene expression in the fetal rat is not increased after pharmacological adrenalectomy. Neurosci Lett. 1992;142:215–218. doi: 10.1016/0304-3940(92)90376-i. [DOI] [PubMed] [Google Scholar]
- 17.Grino M, Oliver C. Ontogeny of insulin-induced hypoglycemia stimulation of adrenocorticotropin secretion in the rat: role of catecholamines. Endocrinology. 1992;131:2763–2768. doi: 10.1210/endo.131.6.1359963. [DOI] [PubMed] [Google Scholar]
- 18.Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- 19.Yi S-J, Masters JN, Baram TZ. Effects of a specific glucocorticoid receptor antagonist on corticotropin releasing hormone gene expression in the paraventricular nucleus of the neonatal rat. Dev Brain Res. 1993;73:253–259. doi: 10.1016/0165-3806(93)90145-z. [DOI] [PubMed] [Google Scholar]
- 20.Baram TZ, Schultz L. Fetal and maternal levels of corticosterone and ACTH after pharmacological adrenalectomy. Life Sci. 1990;47:485–489. doi: 10.1016/0024-3205(90)90607-s. [DOI] [PubMed] [Google Scholar]
- 21.Watts AG, Swanson LW. Diurnal variation in the content of prepro-corticotropin releasing hormone mRNA in the hypothalamic paraventricular nucleus of rats of both sexes as measured by in situ hybridization. Endocrinology. 1989;125:1734–1738. doi: 10.1210/endo-125-3-1734. [DOI] [PubMed] [Google Scholar]
- 22.Baram TZ, Lerner SP. Corticotropin releasing hormone: ontogeny of gene expression in rat hypothalamus. Int J Dev Neurosci. 1991;9:473–478. doi: 10.1016/0736-5748(91)90033-i. [DOI] [PubMed] [Google Scholar]
- 23.Baram TZ, Schultz L. Ontogeny of somatostatin gene expression in rat diencephalon: comparison with CRH. Dev Neurosci. 1991;13:176–180. doi: 10.1159/000112155. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt I, Kaul R, Heldmaier G. Thermoregulation and diurnal rhythms in 1-week-old rat pups. Can J Physiol Pharmacol. 1987;65:105–109. doi: 10.1139/y87-214. [DOI] [PubMed] [Google Scholar]
- 25.Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic CRF-mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 26.Yi SJ, Masters JN, Baram TZ. Glucocorticoid receptor-mRNA ontogeny in the fetal, postnatal rat brain. Mol Cell Neurosci. 1994;5:385–393. doi: 10.1006/mcne.1994.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler GK, Smas CM, Fiandaca M, Frim DM, Majzoub JA. Regulated expression of the human corticotropin releasing hormone mRNA by cyclic AMP. Mol Cell Endocrinol. 1990;70:165–174. doi: 10.1016/0303-7207(90)90156-3. [DOI] [PubMed] [Google Scholar]
- 28.Majzoub JA, Emanuel R, Adler G, Martinez C, Robinson B, Wittert G. Corticotropin Releasing Factor. Wiley, Chichester: 1993. Second messenger regulation of mRNA for corticotropin releasing factor; pp. 31–43. [DOI] [PubMed] [Google Scholar]
- 29.Van LP, Spengler DH, Holsboer F. Glucocorticoid repression of 3,5-cAMP-dependent human CRH gene promoter activity in a transfected mouse anterior pituitary cell line. Endocrinology. 1990;127:1412–1418. doi: 10.1210/endo-127-3-1412. [DOI] [PubMed] [Google Scholar]
- 30.Seasholtz AF, Thompson RC, Douglass IO. Identification of a cyclic AMP-responsive element in the rat corticotropin-releasing hormone gene. Mol Endocrinol. 1988;2:1311–1318. doi: 10.1210/mend-2-12-1311. [DOI] [PubMed] [Google Scholar]
- 31.Insel TR, Battaglia G, Fairbanks DW, De Souza EB. The ontogeny of brain receptors for corticotropin-releasing factor and the development of their functional association with adenylate cyclase. J Neurosci. 1988;8:4151–4158. doi: 10.1523/JNEUROSCI.08-11-04151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]