Abstract
Acute uterine bleeding unrelated to pregnancy has been defined as bleeding “sufficient in volume as to, in the opinion of the treating clinician, require urgent or emergent intervention.” The Southern California Permanente Medical Group updated its guidelines for the management of this condition on the basis of the best available evidence, as identified in a systematic review of the available literature. Given the paucity of studies evaluating this condition, the guidelines, by necessity, include recommendations largely based on opinion or other sources such as case series that are, in general, categorized as low-quality evidence. Medical interventions with single or combined gonadal steroidal agents administered parenterally or orally show promise, but more high-quality studies are needed to better define the appropriate drugs, dose, and administrative scheduling. There is also some evidence that intrauterine tamponade may be useful in at least selected cases. Special attention must be paid to both diagnosing and treating inherited disorders of hemostasis, such as von Willebrand disease, that may otherwise be underdiagnosed in both adolescent and adult women.
Guideline History and Scope
The Abnormal Uterine Bleeding Working Group (AUBWG) was created by the Southern California Permanente Medical Group (SCPMG) (see Sidebar: Members of the Abnormal Uterine Bleeding Working Group) in 2004 to develop evidence-based guidelines, augmented with consensus where evidence was limited or conflicting, for the management of abnormal uterine bleeding for women in the Kaiser Permanente Southern California (KPSC) Region. The group determined that the scope of these guidelines included the management of women with acute uterine bleeding unrelated to pregnancy in the reproductive years. The first version of these guidelines, based on evidence published in December 2004, was completed in 2005 and published internally in 2006. In the same year, the guideline was published in an abbreviated format on the Guideline Clearinghouse of the Agency for Healthcare Research and Quality (AHRQ). The guideline presented here is an update of the 2004 guideline and represents the result of a rigorous systematic review of the evidence on acute uterine bleeding published since January 2005. The other guidelines developed by the group apply to women with chronic abnormal uterine bleeding in the reproductive years and to postmenopausal bleeding, and these guidelines are reported elsewhere.
Members of the Abnormal Uterine Bleeding Working Group.
| Malcolm G Munro, MD | Michael S Amann, MD |
| Hector E Anguiano, MD | Rosoalie A Bauman, MD |
| Mon-Lai Cheung, MD | Seth Kivnick, MD |
| Murali H Kamath, MD | Beatriz R Lauria, MD |
| Nakia T Mainor, MD | Paula D Richter, MD |
| Hazim K Shams, MD | Saad Z Solh, MD |
| Janet J Zhang, MD |
Acute uterine bleeding unrelated to pregnancy was defined in the first version of these guidelines (2005) as “that which is sufficient in volume as to, in the opinion of the treating clinician, require urgent or emergent intervention.” This definition is now officially accepted by the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO).1,2 It is a relatively common clinical condition that is a source of distress for patients, a challenge for clinicians, and a substantial drain on health care resources, as many of these women are managed with inpatient surgical procedures. Recommendations have been developed considering a diverse spectrum of practitioners and disciplines.
Methods
The chair of the AUBWG was selected by the Regional Chiefs of Obstetrics and Gynecology for the SCPMG, the only Medical Group contracted to provide care for the more than 3 million members of Kaiser Foundation Health Plan in the KPSC Region. The remaining members of the AUBWG were selected by the chairs of the Obstetrics and Gynecology Departments of each of the 13 Medical Centers.
After an introductory discussion on the general and SCPMG-specific issues involved in the general problem of acute uterine bleeding, the committee met as a whole to review methods of guideline development, to agree on terms for evidence classification, and to come to consensus on the scope of the guidelines to be developed (see the sections “Guideline History and Scope” and “Problem Formulation”). The Working Group Chair prepared a shell template to aid the guideline development process.
For the review and development of the initial version of this guideline in 2005, face-to-face meetings of the AUBWG were held monthly, with ad hoc face-to-face or teleconference-based meetings held as necessary. Subsequently there were two reviews of the guidelines performed at two-year intervals by the chair, but the review and revisions for the current version, performed in 2012, were undertaken with both face-to-face and teleconference meetings of the working group to consider evidence and propose evidence-based revisions or, where evidence was conflicting or insufficient, consensus revisions to the guidelines.
For the initial version of the guidelines, a subgroup of three individuals was charged with leading the investigation and developing draft documents for meetings of the whole group. Drafts were electronically distributed to the whole group and reviewed at the monthly face-to-face sessions. For the current version, the review was based on a systematic evidence search performed by the chair that resulted in the creation of summary evidence tables of studies potentially relevant to the review. These summary evidence tables were distributed to the AUBWG for comment. The working group then created a revised summary evidence list by adding or subtracting studies on the basis of inclusion and exclusion criteria developed in consensus by the group. The final list served as the foundation for developing the evidence tables used for the current version of the guidelines (available at: www.thepermanentejournal.org/files/Summer2013/SCPMG.pdf). The final recommendations and manuscript were approved first by the AUBWG and then the SCPMG Regional Chiefs of Obstetrics and Gynecology and subsequently reviewed by the chiefs of other disciplines, including Emergency Medicine, Family Practice, Internal Medicine, and Pediatrics.
Literature searches were performed in MEDLINE using PubMed, The Cochrane Database of Systematic Reviews, and The Cochrane Register of Controlled Trials Combinations of the following terms were used: uterine, acute, menorrhagia, treatment, management, heavy uterine bleeding, and von Willebrand. Also searched were the Web sites of the American College of Obstetricians and Gynecologists (www.acog.org/Resources_And_Publications), the Society of Obstetricians and Gynaecologists of Canada (www.sogc.org/clinical-practice-guidelines/), the New Zealand Guidelines Group (www.health.govt.nz/about-ministry/ministry-health-websites/new-zealand-guidelines-group), the Royal College of Obstetricians and Gynaecologists (www.rcog.org.uk/guidelines), the National Institute for Health and Care Excellence (www.nice.org.uk), and the Geneva Foundation for Medical Education and Research (www.gfmer.ch/000_Homepage_En.htm), each a repository of Web-published guidelines. The date of the last search of these sources was October 31, 2012.
The guideline was developed using the best available evidence and, where such evidence was insufficient or absent, a consensus process. Evidence was originally classified using a modification of the system of the US Preventive Services Task Force of the AHRQ. However, during the development of the original guideline, the Kaiser Foundation Guideline Development Group published another system (Third Edition, September 1, 2004) that was ultimately used for this guideline (Table 1). The AUBWG added a “Class 7” to allow for the inclusion of evidence generated from expert opinion, including that from guidelines or other consensus documents from national or international organizations or from the collective opinion of the members of the working group itself. The recommendations were created and classified according to the strength of the evidence system developed by the Guidelines Development Team (Tables 2 and 3).
Table 1.
Hierarchy of evidencea
| Class | Type of evidence |
|---|---|
| 1a | Meta-analysis of randomized clinical trials |
| 1b | Randomized clinical trial |
| 2 | Meta-analysis of studies that are not randomized Nonrandomized, but internally controlled trials. Controls are considered to be “internal” if they are included in the original design of the study. Post hoc or historical comparisons are not considered internal controls Comparisons of otherwise uncontrolled clinical series are not considered internal controls |
| 3 | Nonrandomized, but internally controlled trials Controls are considered to be “internal” if they are included in the original design of the study. Post hoc or historical comparisons are not considered internal controls Comparisons of otherwise uncontrolled clinical series are not considered internal controls. |
| 4 | Case-control studies |
| 5 | Cohort studies |
| 6 | Clinical series, without internal comparison |
| 7b | Expert opinion without available clinical studies |
Source: Kaiser Foundation Guideline Development Group, Edition 3, September 1, 2004.
Level of evidence added by the Abnormal Uterine Bleeding Working Group.
Table 2.
Summary of recommendationsa
| No. | Recommendation | Method | Strengthb |
|---|---|---|---|
| 1 | It is important to exclude pregnancy in all patients with acute abnormal uterine bleeding in the reproductive years | C | N/A |
| 2 | Perimenarcheal patients with acute abnormal uterine bleeding may be at increased risk of an inherited coagulopathy and should be screened accordingly with a structured history (see Sidebar: Screening for an underlying disorder of hemostasis in the patient with excessive menstrual bleeding) | E | A |
| 3 | There is fair evidence that for hemodynamically stable patients, oral multidose progestins or oral multidose, monophasic, combination oral contraceptives are equally effective (see Table 4) | E | B |
| 4 | There is fair evidence supporting the efficacy of intravenous conjugated equine estrogens for hemodynamically stable patients | E | B |
| 5 | Dilation and curettage should be reserved for patients who, in the opinion of the clinician, are inappropriate for, unresponsive to, or contraindicated from the use of medical therapy | C | N/A |
| 6 | When performed in the evaluation or treatment of patients with acute abnormal uterine bleeding, dilation and curettage should be accompanied by hysteroscopy | E | A |
| 7 | Antifibrinolytic agents such as parenteral and oral tranexamic acid may have a role in the management of patients with acute abnormal uterine bleeding who otherwise would be candidates for more invasive surgical procedures, including those that remove fertility, such as hysterectomy | C | N/A |
| 8 | Patients with acute uterine bleeding desiring uterine preservation, and who are unresponsive to or inappropriate for medical therapy, may consider uterine artery occlusion or embolization. The impact of uterine artery embolization or occlusion on fertility has not been adequately established | E | C |
| 9 | Patients with acute uterine bleeding desiring preservation of fertility and who are unresponsive to or inappropriate for medical therapy may be offered treatment with an intrauterine Foley catheter balloon | E | B |
| 10 | Endometrial ablation and hysterectomy may or will effectively treat acute uterine bleeding, but even endometrial ablation will generally preclude future embryo implantation by removing or destroying the endometrium | E | B |
| 11 | Many patients with acute uterine bleeding have an underlying chronic disorder that requires systematic evaluation and, in many instances, chronic therapy, following the arrest of the acute phase of the process | E | A |
| 12 | Endometrial sampling is not considered mandatory in all instances of acute uterine bleeding; however, it should be considered when risk factors are present such as chronic anovulation, obesity, prolonged exposure to unopposed estrogens or tamoxifen, or a family history that places the patient at increased risk of endometrial neoplasia. There is no consensus regarding a specific age at which sampling is considered mandatory | E | B |
Recommendations are evidence based (E) unless sufficient evidence is not available and consensus-based (C) recommendations are provided.
See Table 3; strength of evidence is not applicable (N/A) to consensus-based recommendations.
Table 3.
Support for recommendationsa
| Recommendation | Language | Evidence |
|---|---|---|
| A | Guideline Development Team (GDT) strongly recommends that clinicians routinely provide the intervention to eligible patients | Intervention improves important health outcomes, based on good evidence, and the GDT concludes that benefits substantially outweigh harms and costs |
| B | GDT recommends that clinicians routinely provide the intervention to eligible patients | Intervention improves important health outcomes, based on 1) good evidence that benefits outweigh harms and costs or 2) fair evidence that benefits substantially outweigh harms and costs |
| C | GDT makes no recommendation for or against routine provision of the intervention. At the discretion of the GDT, the recommendation may use the language “option” but must list all the equivalent options | Evidence is sufficient to determine the benefits, harms, and costs of an intervention, and there is at least fair evidence that the intervention improves important health outcomes. However, the GDT concludes that the balance of the benefits, harms, and costs is too close to justify a general recommendation |
| D | GDT recommends against routinely providing the intervention to eligible patients | GDT found at least fair evidence that the intervention is ineffective, or that harms or costs outweigh benefits |
| I | GDT concludes that the evidence is insufficient to recommend for or against routinely providing the intervention. At the discretion of the GDT, the recommendation may use the language “option” but must list all the equivalent options | Evidence that the intervention is effective is lacking, of poor quality, or conflicting, and the balance of benefits, harms, and costs cannot be determined |
Source: Kaiser Foundation Guideline Development Group, Edition 3, September 1, 2004.
The process was designed to be continuous, allowing for ongoing modifications and revisions as new, higher-quality or otherwise clarifying evidence becomes available.
The evidence-based document was created, approved by the members of the AUBWG and, on December 21, 2012, approved by the Chiefs of Obstetrics and Gynecology of the 13 SCPMG Service Areas (see Sidebars: Problem Formulation and Evidence Search).
Problem Formulation.
|
Intended use of the guideline To assist physicians and other health care professionals in the evaluation and management of nongravid women with acute uterine bleeding requiring urgent or immediate medical intervention |
|
Health problem Acute uterine bleeding in the nongravid woman |
|
Health interventions Clinical investigation of, as well as medical and surgical interventions for, acute uterine bleeding for nongravid women |
|
Population Nongravid women of all ages |
|
Practitioners Physicians, physician assistants, nurse practitioners, and other health care professionals in the Departments of Emergency Medicine, Family Medicine, Internal Medicine, Obstetrics and Gynecology, and Pediatrics |
|
Medical setting Offices, clinics, Emergency Departments, and hospitals |
|
Most important health outcomes Outcomes of condition:
Outcomes of intervention:
|
Evidence Search.
Problem formulation
Investigation and treatment of acute uterine bleeding in the nongravid female.
Search methodology summary
Each of the databases listed were searched using the search terms listed in the text. For this revision, the searches started from the first day following the last date of the reviews for the last version (January 1, 2005, until October 31, 2012). Citations were listed and reviewed for appropriateness for this guideline on the basis of subject matter. Since the term acute uterine bleeding or acute heavy menstrual bleeding, or any of the other similar terms, was and is new, the articles were searched to determine if the subject population had bleeding that required urgent or emergent care. Those articles that likely or certainly met these criteria were included in the overall evidence table for review by the committee.
Databases last searched on October 31, 2012
The Cochrane Database of Systematic Reviews, 2012, Issue 10
The Cochrane Controlled Trials Register, 2012, Issue 10
MEDLINE RCTs (via PubMed)
MEDLINE (via PubMed)
American College of Obstetricians and Gynecologists (Practice) Bulletins and Committee Opinions
New Zealand Guidelines Group Guidelines
Society of Obstetricians and Gynaecologists of Canada Guidelines
Royal College of Obstetricians and Gynaecologists Menorrhagia Guidelines
Geneva Foundation for Medical Information and Research: listings of guidelines for the treatment of menorrhagia
United Kingdom National Institute for Health and Clinical Excellence
Search strategy and results
| The Cochrane Database of Systematic Reviews, 2012, Issue 10 | |
|---|---|
| Strategy | Search terms: Acute plus various combinations of Bleeding, Menstrual Bleeding, Menorrhagia, Uterine, Dysfunctional, Heavy, Coagulopathy, von Willebrand |
| Time period: Not applied | |
| Publication types: Systematic reviews | |
| Other limits: None | |
| Results | Total number of citations obtained: 0 |
| Number included in evidence tables: 0 | |
| The Cochrane Controlled Trials Register, 2012, Issue 10 | |
|---|---|
| Strategy | Search terms: Acute plus various combinations of Bleeding, Menorrhagia, Uterine, Dysfunctional, Heavy, Coagulopathy, von Willebrand |
| Time period: Not applied | |
| Publication types: Randomized controlled trials | |
| Other limits: None | |
| Results | Total number of citations obtained: 0 |
| Number included in evidence tables: 0 | |
| MEDLINE RCTs (via PubMed) | |
|---|---|
| Strategy | Search terms: Acute, Bleeding, Menorrhagia, Uterine, Dysfunctional, Heavy, Coagulopathy, von Willebrand(s) |
| Time period: 1966 to October 31, 2012 | |
| Publication types: Randomized controlled trials | |
| Other limits: English, female | |
| Results | Total number of citations obtained: 198 |
| Number included in evidence tables: 2 | |
| MEDLINE (via PubMed) | |
|---|---|
| Strategy | Search terms: Acute, Bleeding, Menorrhagia, Uterine, Dysfunctional, Heavy, von Willebrand(s) |
| Time period: 1966 to October 31, 2012 | |
| Publication types: All types | |
| Other limits: English, female | |
| Results | Total number of citations obtained: 502 (not excluding duplicates) |
| Number included in evidence tables: 10 | |
| American College of Obstetricians and Gynecologists Practice Bulletins and Committee Opinions | |
|---|---|
| Strategy | Search terms: Acute, Bleeding, Menorrhagia, Uterine, Dysfunctional, Heavy, von Willebrand(s) |
| Time period: No restrictions | |
| Publication types: Committee Opinions, Educational/Technical & Practice Bulletins, Technology Assessments | |
| Other limits: None | |
| Results | Total number of citations obtained: 4 |
| Number included in evidence tables: 0 | |
| New Zealand Guidelines Group Guidelines | |
|---|---|
| Strategy | Search terms: Bleeding |
| Time period: No restrictions | |
| Publication types: Published guidelines | |
| Other limits: None | |
| Results | Total number of citations obtained: 4 |
| Number included in evidence tables: 0 | |
| Society of Obstetricians and Gynaecologists of Canada Guidelines | |
|---|---|
| Strategy | Search terms: N/A |
| Time period: No restrictions | |
| Publication types: Guidelines | |
| Other limits: None | |
| Results | Total number of citations obtained: 10 |
| Number included in evidence tables: 0 | |
| Royal College of Obstetricians and Gynaecologists National Evidence-Based Guidelines | |
|---|---|
| Strategy | Search terms: N/A |
| Time period: No restrictions | |
| Publication types: Guidelines | |
| Other limits: None | |
| Results | Total number of citations obtained: 0 |
| Number included in evidence tables: 0 | |
| Geneva Foundation for Medical Information and Research: listings of guidelines for the treatment of menorrhagia | |
|---|---|
| Strategy | Search site: Obstetrics, Gynecology: Guidelines; Menstruation Disturbances |
| Time period: No restrictions | |
| Publication types: Practice guidelines | |
| Other limits: None | |
| Results | Total number of citations obtained: 53 |
| Number included in evidence tables: 0 | |
| United Kingdom National Institute for Health and Clinical Excellence | |
|---|---|
| Strategy | Search site: Acute; Heavy Menstrual Bleeding; Menorrhagia |
| Time period: No restrictions | |
| Publication types: Practice guidelines | |
| Other limits: None | |
| Results | Total number of citations obtained: 45 |
| Number included in evidence tables: 0 | |
NA = not available; RCT = randomized clinical trial.
Background and Rationale
Introduction
Frequently, uterine hemorrhage occurs secondary to pregnancy, but women may also experience acute bleeding in the reproductive years when they are not pregnant, a circumstance that has received relatively little attention in the literature. Consequently, for this guideline, acute uterine bleeding was defined as abnormal uterine bleeding in a nonpregnant woman that requires urgent or emergent medical intervention.2 Many such women, and particularly those who are perimenarcheal, have a disorder of systemic hemostasis, generally inherited in nature.3,4 Others often have underlying chronic abnormal uterine bleeding that is related to structural pathology of the genital tract, or to ovulatory disorders (abnormal uterine bleeding—ovulatory disorders [AUB-O]) or primary disorders that have an impact on endometrial hemostasis (abnormal uterine bleeding—endometrial hemostasis [AUB-E]).2 The clinical condition results in frequent utilization of urgent care, emergency, and operating room resources. Knowledge regarding the pathogenesis and guidance for the management of this clinical problem has been lacking, with relatively little basic or clinical research done in the area. It is apparent that the first version of this guideline, created in 2005 and published in summary form by the AHRQ, was the first to deal with the clinical situation directly, but since that time, a single “consensus” publication appeared in 2011,5 and the American College of Obstetricians and Gynecologists published a “Committee Opinion” in April of 2013.6
Evolving Concepts of Pathogenesis of Acute Uterine Bleeding in the Reproductive Years
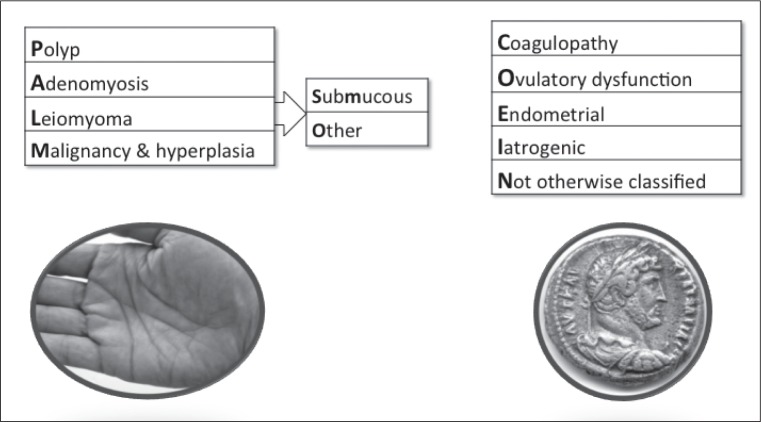
The details of the interplay of factors that results in acute heavy uterine bleeding are not entirely clear. In some instances, a localized lesion such as a prolapsed submucous leiomyoma may be the inciting factor. However, the problem often occurs in the context of chronic abnormal uterine bleeding that includes FIGO categorizations (Figure 1) such as AUB-E, which occurs in women who are ovulatory; in women with disorders of ovulation (AUB-O); in women with inherited, acquired, or iatrogenic disorders of systemic hemostasis or coagulopathy (abnormal uterine bleeding—coagulopathy [AUB-C]) processes that are unrelated to structural abnormalities of the genital tract; and in women with uterine arteriovenous malformations (AVMs), which are a cause of abnormal uterine bleeding not otherwise classified (AUB-N) in the FIGO system.2 It is the general clinical impression that acute heavy uterine bleeding occurs more commonly in women with ovulatory disorders, perhaps because, in anovulatory women at least, there is a lack of progesterone-dependent endometrial biosynthesis of factors important for endometrial hemostasis such as prostaglandin F2α and endothelin-1.7–10 A full discussion of the genesis of AUB-E, AUB-O, and AUB-N (not otherwise classified) is found elsewhere.2,11,12
Figure 1.
Basic FIGO classification system for causes of abnormal uterine bleeding in the reproductive years.
Basic system includes four categories that are defined by objective macro- or microstructural criteria (PALM: polyp; adenomyosis; leiomyoma; and malignancy and hyperplasia), four that are unrelated to structural anomalies (COEI: coagulopathy; ovulatory dysfunction; endometrial; iatrogenic), and one reserved for entities that are not otherwise classified (N). Leiomyoma category (L) is subdivided into patients with at least one submucous myoma (LSM) and “others” that do not distort the endometrial cavity (LO).
FIGO = Fédération Internationale de Gynécologie et d’Obstétrique.
Reprinted from International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 113(1), Munro MG, Critchley HO, Broder MS, Frazzer IS; FIGO Working Group on Menstrual Disorders, FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age, 3–13, ©2011 Apr, with permission from Elsevier.
The full spectrum of mechanisms involved in the development of AUB-O is not totally understood, but for many are systemic in nature. However, as suggested, local hemostatic mechanisms may be rendered deficient secondary to the absence of cyclical production of progesterone and the related biosynthesis of prostaglandins and other substances necessary to control blood loss. The etiology of anovulation in any given female patient may range from immaturity of the hypothalamic-pituitary-ovarian axis frequently seen in perimenarcheal girls to a number of entities that are known or suspected to affect the normal function of the hypothalamic-pituitary-ovarian axis. Polycystic ovarian syndrome is perhaps the most common entity but one that lacks a specific test. Hyperprolactinemia and hypothyroidism are endocrinopathies that can be diagnosed using suitable serum measurements.13 Pharmacologic agents that affect dopamine metabolism are known to affect ovulatory function in some women. Other causes of anovulation are thought to include psychological stress, rapid changes in weight, and excessive exercise. Another less well-known ovulatory disturbance is the luteal out-of-phase cycle found with increasing frequency in the later reproductive years, where follicular development in the luteal phase occurs secondary to sustained levels of follicle-stimulating hormone despite the presence of a corpus luteum.14 Such patients may even have heavier bleeding than women who are anovulatory despite the production of progesterone and progesterone-related vasoactive substances.15
Acute heavy uterine bleeding, particularly in perimenarcheal girls, may be a presentation of an inherited, acquired, or iatrogenic systemic disorder of hemostasis, also known as a coagulopathy (AUB-C). Inherited coagulopathies seem to be far more common than generally recognized. They were found in 10.7% of women with “menorrhagia” (a now discarded term) in a case-control study by the US Centers for Disease Control and Prevention16 (Evidence Class 5), a number much lower than the 17% and 34% recently reported from the United Kingdom and Sweden, respectively, but nonetheless higher than previously perceived17,18 (Class 5). In each instance, nearly all cases were von Willebrand disease, a diagnosis that may be increasing at least in part because of a greater understanding of the requirements for diagnosis. A systematic review of the literature has determined that 13% of women of all ages with heavy menstrual bleeding have von Willebrand disease19 (Class 2). Despite the reduced role of platelets in endometrial hemostasis, a number of platelet disorders exist that have been associated with acute heavy uterine bleeding, perhaps most commonly immune thrombocytopenia purpura3 (Class 5). How and whether these data apply to women with acute uterine bleeding is unclear.
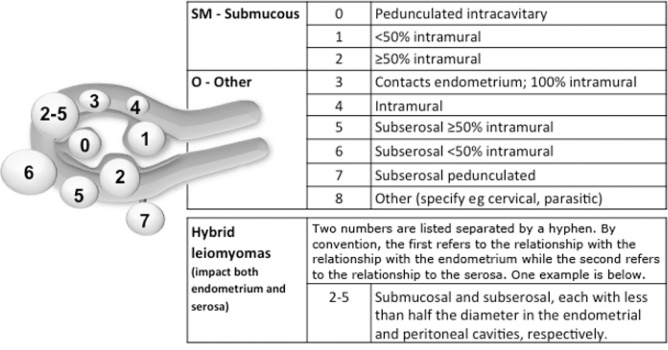
The cause of acute bleeding often is enigmatic, even when associated with leiomyomas, often called myomas or uterine fibroids (AUB-L) (Figure 2). It is clear that most myomas are asymptomatic, and it is generally accepted, but not proved, that myomas that cause bleeding are “submucous,” meaning that they are situated near or adjacent to the endometrium (AUB-LSM). Consequently, it can be postulated that many, if not most, women with leiomyomas who present with acute heavy uterine bleeding do so secondary to other mechanisms, such as ovulatory disorders (AUB-O). Available evidence regarding the potential mechanisms of leiomyoma-related chronic abnormal uterine bleeding is found in the SCPMG chronic abnormal uterine bleeding guideline (currently in preparation).
Figure 2.
FIGO Leiomyoma Subclassification System.
Submucous (SM) component is based on the system by Wamsteker et al1 and adds categorizations for the remaining “O” (Other) tumors. Intracavitary lesions are attached to the endometrium by a narrow stalk and are classified as Type 0, whereas Type 1 lesions are less than 50% and Type 2 at least 50% intramural. Type 3 lesions are totally extracavitary (do not distort the endometrial cavity) but abut the endometrium. Type 4 lesions are intramural leiomyomas that are entirely in the myometrium, with myometrium between the myoma and both the endometrium and myometrium and without distortion of the serosal layer. Subserous (Types 5–7) leiomyomas distort the serosal surface, with Type 5 being at least 50% intramural, Type 6 being less than 50% intramural, and Type 7 being attached to the serosa by a narrow stalk. Lesions that are transmural are categorized by their relationship to both the endometrial and serosal surfaces. The endometrial relationship is noted first, with the serosal relationship second (eg, Types 2–3). An additional category, Type 8, is reserved for leiomyomas that do not relate to the myometrium at all and would include cervical lesions, those that exist in the round or broad ligaments without direct attachment to the uterus, and other so-called parasitic lesions.
- Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol 1993 Nov;82(5):736–40.
Reprinted from International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 113(1), Munro MG, Critchley HO, Broder MS, Frazzer IS; FIGO Working Group on Menstrual Disorders, FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age, 3–13, ©2011 Apr, with permission from Elsevier.
Finally, although relatively rare, it is clear that AVMs can present with acute heavy uterine bleeding. Furthermore, it is apparent that this entity, although sometimes congenital, may occur secondary to previous curettage and may manifest following blind curettage when women are investigated for abnormal uterine bleeding20 (Class 6). Although precise delineation of the mechanisms involved in the development of acquired AVMs is unclear, it is apparent that prior curettage is frequently involved, typically for termination of pregnancy or treatment of postpregnancy bleeding.
Investigations
History and Physical Findings
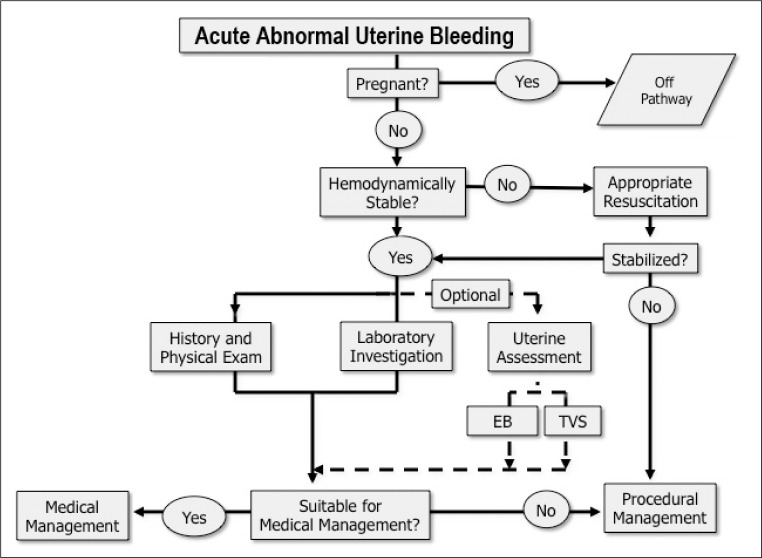
Acute abnormal uterine bleeding ranges essentially from modestly excessive menstrual bleeding at one end of the spectrum to acute heavy uterine bleeding that is associated with hypovolemic shock at the other. Evaluation starts with an assessment of the hemodynamic status of the patient (Figure 3), and if found to be hemodynamically unstable, all efforts should be directed at establishing appropriate intravenous lines and cross-matching of blood. These steps are followed by, or performed simultaneously with, obtaining enough history and performing a sufficient pelvic examination to confirm that the bleeding indeed is emanating from the uterus and to appropriately and safely manage the acute clinical situation.
Figure 3.
Evaluation pathway for acute abnormal uterine bleeding.
To qualify for this clinical pathway, the patient must not be pregnant. For those who are hemodynamically unstable, appropriate resuscitation is necessary and procedural management may be required. Once the patient is hemodynamically stable, the appropriate additional history, and laboratory evaluation and imaging may be obtained. At that point, the clinician should determine whether medical or procedural management is the most appropriate next step.
EB = endometrial biopsy; exam = examination; TVS = transvaginal sonography or transvaginal ultrasound.
Subsequently, the clinician implements appropriate medical or surgical approaches for stopping the hemorrhage, as described in Figures 4 and 5 and in subsequent sections of this guideline (see Medical Options for the Management of Acute Uterine Bleeding and Clinical Application of Therapeutic Options for Acute Abnormal Uterine Bleeding). If or when the patient becomes hemodynamically stable, it is prudent to spend time obtaining a complete menstrual history, seeking evidence of chronic abnormal uterine bleeding. Cyclical, predictable menses starting every 24 to 32 days are usually associated with ovulation21 (Class 3), even if heavy, whereas abnormal uterine bleeding associated with ovulatory disorders is less predictable. Both luteal out-of-phase events and anovulatory bleeding are typically unpredictably irregular in timing and flow, but the latter is often interspersed with episodes of amenorrhea14 (Class 3).
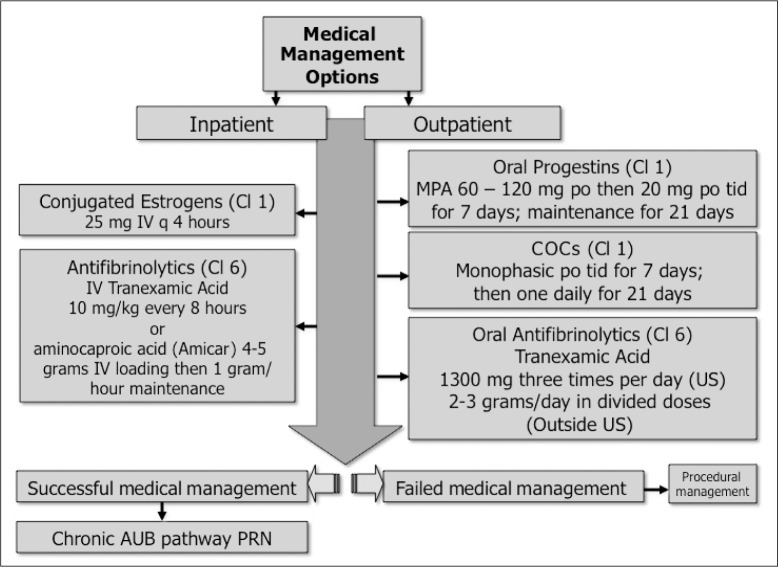
Figure 4.
Medical management of acute heavy uterine bleeding.
A variety of oral and parenteral interventions are available for women with acute abnormal uterine bleeding, with varying amounts and quality of evidence supporting their use. In general, women with low hemoglobin levels should be managed in the context of an Emergency Department, hospital, or other appropriate institutional environment with rapid-acting parenteral approaches. For those with relatively high hemoglobin levels at baseline, outpatient oral therapy can be considered. Failure to respond will typically lead to procedural management (see Figure 5).
AUB = abnormal uterine bleeding; Cl = Evidence Class; COCs = combination estrogen and progestin-containing oral contraceptives; IV = intravenous; MPA = medroxyprogesterone acetate; po = per os, or orally; PRN = as needed or if necessary; q = every; tid = three times per day.
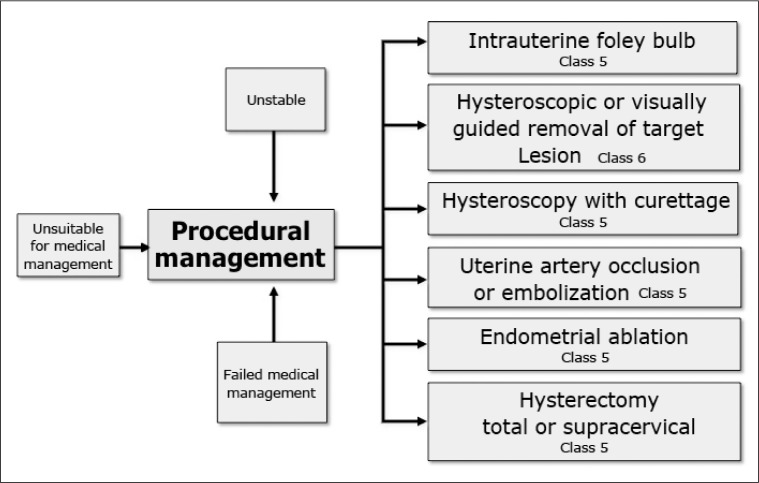
Figure 5.
Procedural management of acute heavy uterine bleeding.
None of these approaches has been systematically evaluated in the context of clinical trials. For those who wish to retain fertility, endometrial ablation and hysterectomy are inappropriate. Although the precise impact of uterine artery embolization on fertility and subsequent pregnancy is yet to be determined, it may be considered an acceptable alternative to hysterectomy in otherwise unresponsive or life-threatening conditions.
Anovulation frequently occurs without any evident cause, but may be a manifestation of any or a combination of a number of factors, including psychological stress, rapid weight change,22 obesity,23,24 excessive exercise,25,26 certain pharmacologic agents,27 and a number of endocrinopathies, most commonly polycystic ovarian syndrome,28,29 hyperprolactinemia,30,31 and hypothyroidism.32
The clinician should also consider a congenital or acquired coagulopathy by reviewing the personal, medical, and family history. It is evident that a history of lifelong heavy menstrual bleeding, postpartum bleeding, excessive bleeding with surgical or dental procedures, frequent bruising, and a family history can be used as a screening tool with 8% to 90% sensitivity to detect these relatively common disorders18,33 (Class 3). The screening by structured history is “positive,” with abnormal results necessitating additional laboratory evaluation, when any of the conditions in Sidebar: Screening tool for an underlying disorder of hemostasis in the patient with excessive menstrual bleeding are met.1
A history of curettage for the termination of pregnancy or for treatment of postpregnancy bleeding should alert the clinician to the possibility of a uterine AVM20 (Class 6).
A careful bimanual examination of the pelvis is performed seeking evidence of pregnancy, adenomyosis (usually symmetrically enlarged and frequently tender), and leiomyomas (typically irregularly enlarged) as well as findings suggestive of an adnexal mass or an ectopic gestation. The clinician should be cautioned that pelvic findings that suggest, for example, adenomyosis or leiomyomas (that are FIGO Types 4 through 7), frequently are not the cause of the bleeding (Class 7).
Laboratory Investigation
For women with acute heavy uterine bleeding, it is necessary to obtain a complete blood cell count (without differential white cell count), ABO blood type, and a urinary (or serum) assay for pregnancy. If there is suspicion about a systemic coagulation disorder, a number of assays should be considered, including fibrinogen, prothrombin time, and activated partial thromboplastin time. For those considered at risk of an inherited coagulopathy, including those with a “positive” structured history (see Sidebar: Screening tool for an underlying disorder of hemostasis in the patient with excessive menstrual bleeding),1 von Willebrand disease should be considered, including von Willebrand factor, factor VIII, and ristocetin cofactor34 (Class 2). Some assays, including von Willebrand factor, may be affected by the time in the menstrual cycle or the use of gonadal steroids and, if negative in the context of a suggestive history, may bear repeating.35 This issue is discussed in the SCPMG chronic abnormal uterine bleeding guideline currently in preparation. For those women using anticoagulants, international normalized ratio and other appropriate measures of coagulation function should be measured. If a disorder of systemic hemostasis is known or suspected (AUB-C), consultation with a specialist in hematology is generally appropriate (Class 7).
Screening tool for an underlying disorder of hemostasis in the patient with excessive menstrual bleeding1.
Initial screening for an underlying disorder of hemostasis in patients with excessive menstrual bleeding should be by a structured history:
|
| Additional laboratory evaluation necessitates a positive screen which consists of any of the following: 1) heavy bleeding since menarche, one from list B or 2) two or more from list C. |
Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet 1998 Feb 14;351(9101):485–9.
Uterine Assessment
Both histologic evaluation of the endometrium and sonographic and hysteroscopic evaluation of the structure of the endometrial cavity may be limited because of the influence of intrauterine blood and clot on obtaining a satisfactory specimen or imaging evaluation. In some instances, hemodynamic instability may dictate that they be deferred. Consequently, these steps may be performed as appropriate later in the clinical course (Class 7).
Histologic Assessment
It is not necessary to sample the endometrium in all women with acute heavy uterine bleeding. However, histologic evaluation of the endometrium with endometrial biopsy is generally recommended as an initial part of the investigation for selected women with abnormal uterine bleeding. Farquhar et al36 have determined that such women include those older than age 45 years or any age with any of a number of characteristics including chronic anovulation, infertility, diabetes, obesity (> 90 kg), a family history of endometrial cancer, or prolonged exposure to unopposed estrogen or tamoxifen (Class 3). However, not all investigators have found the same results as the Farquhar group. In a smaller scale study, Ash et al37 found that only irregular menstrual periods and older than age 40 years were associated with an increased risk of endometrial neoplasia (Class 3). Office endometrial sampling with narrow disposable catheters has been generally demonstrated to be equivalent to the so-called “formal” dilation and curettage for diagnosing endometrial carcinoma.38–40
Ultrasound Imaging of Endometrial Cavity
Blind instrumentation has been demonstrated to be inadequate for accurate depiction of the structure of the endometrial cavity.41–43 Consequently, accurate structural evaluation of the endometrial cavity requires imaging usually with ultrasonographic techniques and/or direct inspection with hysteroscopy.
Transvaginal Ultrasound
In the nonpregnant woman with abnormal bleeding, a thin endometrial echo complex (EEC) in combination with an absence of leiomyomas near to the EEC has been associated with normal results of hysteroscopic examination44 (Class 3). Nevertheless, transvaginal ultrasound is less sensitive than other techniques such as hysteroscopy and contrast sonography (typically saline infusion sonography) at detecting intrauterine lesions, especially polyps45 (Class 3). A Cochrane systematic review also suggests that transvaginal ultrasound is almost as accurate as saline infusion sonography and hysteroscopy, but the review did not include such an evaluation for women with acute abnormal uterine bleeding46 (Class 1a). The exception was a slightly lower sensitivity for the detection of submucous leiomyomas. Other investigators have found that even with relatively thin EEC measurements (5 mm or less) polyps may still be missed with transvaginal ultrasound45 (Class 1b). Consequently, although transvaginal ultrasound is a suitable screening test for primary evaluation of the endometrial cavity, the sensitivity is low enough that the clinician should consider techniques that are more sensitive for women with persisting symptoms. Furthermore, in the context of acute heavy uterine bleeding, the EEC may be difficult to interpret when thick because clot may be difficult to distinguish from endometrium or endometrial polyps (Class 7).
As a result, in the presence of an abnormally thick endometrium or when leiomyomas exist suspiciously close to the endometrium, additional evaluation with saline infusion sonography or hysteroscopy should be considered following the resolution or treatment of the acute symptoms.
Finally, when the history gives rise to suspicion for the presence of an AVM, ultrasonic evaluation of the uterus with color Doppler assessment is valuable. The presence of findings suggestive of AVM should alert the clinician to avoid blind curettage of the endometrial cavity and to consider interventions that are designed to occlude the vessels in or near to the endometrium20 (Class 6).
Contrast Sonography
Contrast sonography (also known as sonohysterography, hysterosonography, contrast uterine sonography, and saline infusion sonography) is the sonographic (usually transvaginal) evaluation of the endometrial cavity following the transcervical instillation of saline47 (Class 3). This approach is comparable to hysteroscopy for the detection of intrauterine lesions such as polyps and submucous leiomyomas48,49 (Class 3). However, in the context of acute uterine bleeding, the results of contrast sonography are frequently difficult to interpret because the clot and debris existing in the endometrial cavity preclude its use in the acute setting. Contrast sonography may be less accurate in the evaluation of the large uterus because of limited distensibility of the endometrial cavity45 (Class 1b). In such circumstances, hysteroscopy may be superior.
Hysteroscopy
The ability of diagnostic hysteroscopy to provide information not predictably obtainable by blind endometrial sampling has been adequately documented (Class 2)47,50 and (Class 3).51 In the setting of acute abnormal uterine bleeding, gaseous media are not likely to be productive, but fluid distention of the endometrial cavity with a continuous flow system may facilitate visualization of the endometrial cavity52–56 (Class 6). As with ultrasound imaging, hysteroscopy may be challenging in the face of acute uterine bleeding.
Myometrial Evaluation
Evaluation of the myometrium is rarely valuable in the investigation and management of acute abnormal uterine bleeding. Nonetheless, the clinician should remember to consider AVMs in those rare circumstances when bleeding does not respond to usual measures. If such suspicion exists, color Doppler or magnetic resonance imaging seems the most appropriate diagnostic technique57,58 (Class 5).
Medical Options for the Management of Acute Uterine Bleeding
General Considerations
Medical approaches to acute uterine bleeding will vary according to the clinical situation, including the hemodynamic status, baseline hemoglobin level, specifics of the patient’s history, and available clinical resources (Figure 4). There may be differences in the management of women known to have AUB-C and those not known or suspected to have a systemic disorder of hemostasis. Given the limited available evidence, in most instances, interventions with gonadal steroids (Table 4), procedures including endometrial tamponade, and even antifibrinolytic therapy will have potential efficacy regardless of the presence of AUB-C. However, in women with coagulopathies, a number of specific interventions may be useful, the details of which are outside the scope of this guideline.
Table 4.
Outpatient gonadal steroid therapy for acute abnormal uterine bleeding
| Regimen specifics | Combination oral contraceptives | Oral progestins |
|---|---|---|
| Formulation | Monophasic estrogen-progestin Estrogen: 30 μg to 35 μg ethinyl estradiol Progestin: Potent progestins such as norethindrone or norethindrone acetate (≥ 1 mg); or levonorgestrel (≥150 μg) Examples: Norinyl 1/35 (also sold as Ortho 1/35, Necon, and Norethin), Levlen, Nordette, and Lo/Ovral |
Medroxyprogesterone acetate (Provera) Megestrol acetate (Megace) Norethindrone acetate (Aygestin) |
| Dosing | Three or four times daily until bleeding stops for at least 2 days Then daily for 3 to 6 weeks (eliminating placebo pills) |
Medroxyprogesterone: 60 mg to 120 mg/day until bleeding stops for at least 2 days; 20 mg to 40 mg/day to follow for 3 to 6 weeks Megestrol: 80 mg to 160 mg/day until bleeding stops for at least 2 days; then 40 mg to 80 mg/day for 3 to 6 weeks Norethindrone: 5 mg to 15 mg daily until bleeding stops for at least 2 days; 5 mg to 10 mg daily to follow for 3 to 6 weeks |
| Precautions | Use with caution in those at high risk of thromboembolic disease May experience nausea; consider antiemetic Return for reassessment if bleeding is not adequately resolved in 48 to 72 hours Will require follow-up with gynecologist if underlying history of chronic abnormal uterine bleeding |
Medroxyprogesterone acetate alone is preferable to combined progestin and estrogen-containing compounds in those at higher risk of thromboembolic disease Return for reassessment if bleeding is not adequately resolved in 48 to 72 hours Will require follow-up with gynecologist if there is an underlying history of chronic abnormal uterine bleeding |
Estrogens
There exists only 1 published report, but of high quality, that demonstrates intravenous conjugated equine estrogens (Premarin; Phizer, Inc; New York, NY) as being effective in treating acute uterine bleeding thought, at the time, to be “dysfunctional” but ultimately without specified cause59 (Class 1b). In this randomized trial, 25 mg of intravenous conjugated equine estrogens administered every 4 hours resulted in cessation of bleeding by 5 hours in 72% of the treatment group vs 38% of the placebo group. The added benefit of continued dosing is uncertain. The mechanism of action of conjugated equine estrogens is unclear and may not be specific to the endometrium itself, as similar approaches have been successfully reported in the gastrointestinal and otolaryngology literature.60,61
Current practice suggests that continuation of this regimen for up to 24 hours may be necessary to obtain cessation of bleeding. In general, patients are converted to a regimen of monophasic multidose oral contraceptives (OCs) or a moderate- to high-dose progestin regimen, but no experimental data exist that support such an approach. Investigation into cause should be undertaken at a suitable time following treatment of the acute episode (see SCPMG chronic abnormal uterine bleeding guidelines).
Estrogens Plus Progestins
Combination OCs are frequently used for the treatment of acute abnormal uterine bleeding, an approach that, until 5 years ago, was supported only by a combination of textbook and other “expert” descriptions of approaches and protocols62 (Class 7). A KPSC-originated randomized controlled trial (RCT) demonstrated a multidose, monophasic OC-based regimen to be effective in this setting63 (Class 1b). This regimen used a formulation containing 35 μg of ethinyl estradiol and 1 mg of the progestin norethindrone administered 3 times per day for the first week, and then daily for 3 weeks. The regimen appeared to be successful and was associated with cessation of bleeding, on average, 3 days after the initiation of therapy. It was generally well tolerated, without the high incidence of nausea and vomiting that had been predicted. However, it was not compared with placebo, the sample size was relatively small (n = 40), and other regimens and formulations were not evaluated. Although other formulations could be successful as well, one would be concerned that the use of multiphasic or very-low-dose OCs may not be as efficacious (Class 7).
Progestins
There is evidence from 2 studies supporting the use of oral medroxyprogesterone acetate being effective in the treatment of acute heavy menstrual bleeding. The first study described a series of 24 adolescents who were hospitalized with excessive uterine bleeding and anemia64 (Class 6). Oral medroxyprogesterone acetate was administered with a total dose of 60 to 120 mg during the first day and 20 mg/day for the next 10 days. A reduction in blood loss was reported in all the individuals. Twenty-five percent stopped bleeding in the first 24 hours; bleeding ceased in 29% on the second day, in 21% by the third day, and in 25% on the fourth day. The investigators concluded that rapid saturation of the endometrium with progestins was a highly effective treatment modality for acute uterine bleeding in adolescents.
The second study was the RCT by Munro et al63 described earlier that compared multidose combination OCs containing 35 μg of ethinyl estradiol with a regimen of medroxyprogesterone acetate in which 20 mg was administered 3 times per day (total 60 mg/day) for 1 week that was reduced to 20 mg once per day for 3 weeks (Class 1b). The group receiving medroxyprogesterone acetate had results nearly identical to those receiving the OC regimen, with no patients requiring surgical interventions and an average of 3 days until cessation of bleeding. The sample size was relatively small, and no comment can be made regarding the efficacy of other progestins such as megestrol acetate or norethindrone acetate.
Although theoretically attractive, the use of depot forms of medroxyprogesterone acetate for the treatment of acute abnormal uterine bleeding may be associated with a high frequency of irregular bleeding (AUB-I). Although there are no published data associated with acute uterine bleeding, the recognized high frequency of AUB-I encountered during initiation of contraceptive doses of medroxyprogesterone acetate should be considered and discussed with the patient (Class 7).
Antifibrinolytics
There are extensive high-quality trials demonstrating the efficacy of antifibrinolytics, particularly tranexamic acid, for the treatment of the symptom of cyclical heavy menstrual bleeding in an otherwise normal uterus, now called AUB-E.65 (Class 1a). However, there are no published studies specifically evaluating the use of such antifibrinolytic agents for the treatment of acute abnormal uterine bleeding. Nonetheless, the agents have been used, particularly in patients with systemic coagulopathies for treatment of generalized bleeding. The AUBWG members included those who have successfully used orally or intravenously administered tranexamic acid for the treatment of patients with acute uterine bleeding (Class 7). There is no evidence that tranexamic acid increases the incidence of thromboembolic disease, even when used in women at high risk66 (Class 5).
Gonadotropin-Releasing Hormone Agonists
There are no data evaluating the role of gonadotropin-releasing hormone (GnRH) agonists in the management of women with acute abnormal uterine bleeding. However, the agents may be considered for women who are planning surgical therapy and/or who wish to enhance blood indexes and iron stores. Clinicians should know that the estradiol “flare” that follows induction of a GnRH agonist lasts from approximately Day 5 to Day 14, and it is often accompanied by uterine bleeding, which may be heavy. Consequently, if GnRH agonists are used, they should be accompanied by 3 weeks of an orally administered progestin such as medroxyprogesterone acetate (10–20 mg twice daily) or a combination OC daily for the same time period (Class 7).
Clinical Application of Therapeutic Options for Acute Abnormal Uterine Bleeding
Resuscitation
Transfusion will be necessary in women with acute abnormal uterine bleeding who are hemodynamically unstable and who, in the judgment of the clinician, have compromised blood reserves (Class 7).
Medical Management
Medical management of acute abnormal uterine bleeding should be considered before surgical approaches unless bleeding is suspected to emanate from retained products of conception or from intrauterine lesions such as aborting submucous leiomyomas (Class 7).
Multidose Combination Oral Contraceptives
For less severe bleeding, or that associated with an acceptable hemoglobin and hematocrit, therapy may be initiated with a multidose, monophasic OC-based regimen. In an RCT the OC dose was tapered from a “high” dose (one tablet three times daily) to a daily dose after seven days63 (Class 1b). Whether or not the “taper” could be started earlier without compromise of the therapeutic effect has not been established. In the RCT, the combination OCs were continued daily for three weeks but, particularly in anemic women, continuation for a longer time may be of additional value. Combination OCs are generally considered inappropriate for smokers, in women with a previous history of deep venous thromboembolic disease or ischemic or thrombotic stroke, or in those who have thrombophilias such as protein C or S deficiency or Leiden factor V. The use of combination OCs for treatment of acute heavy uterine bleeding should be approached with caution in women with previous breast cancer.
Oral Progestins
An alternative to multidose combination OCs is the use of single-agent oral progestins. The only published data describe the use of medroxyprogesterone acetate. In the single-arm study of adolescents, 60 to 120 mg on Day 1 was followed by 10 to 20 mg/day for 7 to 10 days64 (Class 6). In the RCT performed by the SCPMG, a dose of 20 mg 3 times per day was administered for 7 days followed by 20 mg/day for 3 weeks63 (Class 1b). Potential, but unstudied, alternatives to medroxyprogesterone acetate include megestrol acetate, 40 to 80 mg twice daily for up to 7 days followed by reduced dosing, and norethindrone acetate (Aygestin, Teva Women’s Health Inc; Cincinnati, OH), 5 mg orally 3 times per day for up to 7 days followed by reduced dosing (eg, 5 mg/day) for up to 3 weeks (Class 7). Some committee members have had experience with much higher dosing in subsequent days—up to 120 mg/day, particularly in obese individuals—and continuing the dosing for a duration of at least 4 weeks depending on the clinical situation (Class 7). In such instances, megestrol acetate may provide a more convenient dose format.
There has been some confusion regarding the relationship of progestins in general, and particularly in relatively high doses, to the incidence of venous thromboembolic disease. Product labeling of progestins is similar to that for estrogens or estrogen-progestin combinations. A review of the literature conducted by the AUBWG failed to show any convincing impact of orally administered progestins on the clinical incidence of thromboembolic disease. In a large epidemiologic study of contraceptive use in Denmark evaluating more than 10 million women, and their years of use of orally administered progestin-only preparations, there was no increased risk of thromboembolic disease.67 On the other hand combination estrogen and progestin OCs were associated with a fourfold risk of thromboembolic in the first year, varying somewhat with the progestin but decreasing with increasing years of use67 (Class 4). Although there is published evidence linking megestrol acetate to an increased risk of thromboembolic disease in nursing home residents, the quality of this study was poor, with the possibility that selection bias influenced the results68 (Class 6).
There is conflicting evidence regarding the impact of injectable progestins on the risk of venous thromboembolic disease with some studies showing an increased risk as high as two69 or three times70 that of controls (Class 4). This finding is at odds with studies evaluating the absence of an impact of depot medroxyprogesterone acetate on measurable parameters of the risk of thromboembolic disease71 (Class 5).
Absent data to the contrary, it is our conclusion that short-term use of low- and moderate-dose progestins, at least those with 21 carbon atoms (eg, medroxyprogesterone acetate or megestrol acetate) should have little, if any, effect on thromboembolic potential and, consequently, may be a viable option for treatment of patients with acute heavy uterine bleeding who may not be good candidates for estrogen-containing compounds. Such women include those with a history of thromboembolic disease or those in whom the risk of thromboembolic disease is increased (Class 7). On the other hand, 19-carbon atom progestins, such as norethindrone and norethindrone acetate, may undergo conversion to ethinyl estradiol both in the liver and peripherally. The rate of conversion has been calculated in laboratory studies at between 1% and just over 2%.72,73 With 5 mg to 15 mg of norethindrone acetate, this could amount to as little as 5 μg of ethinyl estradiol, or as much as 30 μg, the latter equivalent to the amount in standard OC populations. As a result, and until well-designed studies prove otherwise, C19 progestins should be used with caution in women in whom exogenous estrogens are contraindicated (Class 7).
Conjugated Equine Estrogens
Acute heavy uterine bleeding may be treated with intravenous conjugated equine estrogens, 25 mg every 4 hours until the acute bleeding has subsided, usually occurring in 24 to 48 hours (Class 1b). Surgical intervention is indicated for those who fail to respond within this timeframe. Once the acute bleeding has subsided, the regimen of conjugated equine estrogens is typically discontinued and a progestin-based regimen is started, either alone (eg, medroxyprogesterone acetate, 20 mg twice daily; norethindrone, 5 mg twice daily) or in combination with an oral estrogen such a monophasic combined OC preparation as described later (Class 7). In selected instances, a GnRH agonist may be started concomitant with the start of conjugated equine estrogen therapy, but it may be important that the progestin-based regimen be continued for at least 2 to 3 weeks to prevent or reduce the severity of the GnRH “flare” bleed (Class 7). Subsequent therapy is dependent on a number of factors, including the cause of the bleeding and specific patient needs.
Similar to the case for combination OCs discussed earlier, estrogen therapy is generally inappropriate for smokers, those with a history of ischemic or thrombotic stroke, or for women with a previous history of deep venous thromboembolic disease or in those who have thrombophilias such as protein C or S deficiency or Leiden factor V.
Procedures for Acute Heavy Uterine Bleeding
Surgical therapy is generally considered second-line therapy for acute abnormal uterine bleeding but may be the first choice for patients with retained products of conception or known intracavitary lesions such as an aborting submucous leiomyoma (Figure 5).
Intracavitary Tamponade
Tamponade using a 30-mL Foley catheter placed in the endometrial cavity, which is then inflated, has been shown effective in a number of case reports, including one describing successful treatment in 2 women with coagulopathy74 (Class 7) and a series of 20 patients in which 17 were successfully treated75 (Class 6). The balloon is left inflated for 2 to 48 hours depending on a number of factors, including the perceived cause of the bleeding.
Dilation and Curettage (with Hysteroscopy)
The effectiveness of dilation and curettage for the treatment of acute uterine bleeding is generally accepted but little investigated. As discussed previously, when acute uterine bleeding occurs in temporal proximity to pregnancy, the possibility of an AVM should be considered, and appropriate imaging should be performed to minimize the risk of blind curettage resulting in even more profuse hemorrhage if the malformation is disrupted. It should be remembered that, for women with chronic abnormal uterine bleeding, the cycles that follow a successful dilation and curettage will resume, to be similar to those that were present before curettage76 (Class 6).
Because dilation and curettage frequently misses lesions that may contribute to or be the cause of the acute bleeding, concomitant hysteroscopy should be performed (Class 7).
Endometrial Ablation
Surgery that attempts selective destruction of the endometrium is commonly known as endometrial ablation. Endometrial ablation performed under direct vision with a resectoscope, also called resectoscopic endometrial ablation, has been introduced as a surgical option for women with chronic abnormal uterine bleeding (AUB-E and AUB-O), with the advantages of a short hospital stay, absence of surgical incisions, and subsequent rapid return to normal activity. For the patient with acute uterine bleeding, particularly if she may not be an ideal candidate for laparotomy approaches, resectoscopic endometrial ablation may be effective. Unfortunately, the literature contains only case reports or very short series describing the use of endometrial ablation in this clinical situation77–79 (Classes 6–7).
Nonresectoscopic endometrial ablation, a procedure initially introduced in the 1890s80 and published in the peer-reviewed literature in the 1930s as a series81 (Class 6), was refined and reintroduced to North America in the 1990s. Currently, 5 methods of nonresectoscopic endometrial ablation have been approved by the US Food and Drug Administration. To date, the only case reports evaluating the use of devices for nonresectoscopic endometrial ablation involved the use of the microwave device, no longer available82 (Class 7), and devices used to perform thermal balloon ablation83,84 (Class 7).
Uterine Artery Occlusion
Uterine artery occlusion using polyvinyl alcohol spheres, called uterine artery embolization, has been used frequently for treatment of obstetric hemorrhage, cervical ectopic pregnancies, and postoperative bleeding. Although there are very few reports in the literature using this approach for acute heavy uterine bleeding85 (Class 6), the approach should be considered, at least for women in whom medical management has failed and who may not tolerate surgical therapy, or who require or desire preservation of their uterus. There is evidence that uterine artery embolization may be the most appropriate intervention for women with acute and heavy uterine bleeding secondary to AVM20 (Class 6).
Hysterectomy
Hysterectomy is generally seen as a last resort in the management of women with acute uterine bleeding. The procedure can be performed via laparotomy, vaginally, or under laparoscopic direction. Subtotal or supracervical hysterectomy is a procedure that takes only the uterine corpus, thereby leaving the cervix. The advantages of abdominal supracervical hysterectomy are questionable, as recent randomized trials have demonstrated no differences in sexual and urinary functions outcomes in women treated with the two procedures86,87 (Class 1b). However, there may be less blood loss associated with supracervical hysterectomy86 (Class 1b), a feature that may justify considering this approach in women with acute uterine bleeding. The various techniques are discussed in more detail in the SCPMG chronic abnormal uterine bleeding guidelines currently in preparation.
Acknowledgments
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
Dr Munro is a consultant for Boston Scientific, Inc; Bayer HealthCare; Karl Storz Endoscopy Americas; Abbvie, Inc; and Ethicon Gynecare, Inc.
More Than the Mere Saving of Life
Modern gynecological surgery stands for much more than the mere saving of life. The day is gone by when the successful surgeon is the one who can report the greatest number of cases discharged from the hospital cured at the end of three or four weeks, to drag out as many years or it may be the remainder of their days in hopeless invalidism.
— Removal of the Uterus in bilateral diseases of the appendages; Chicago Medical Recorder1896 Jun;10:383–9. Lucy Waite, MD, Head Surgeon and Medical Superintendent of the Mary Thompson Hospital for Women and Children, Chicago, IL.
Evidence Tables
Presence of disorders of hemostasis in women with acute uterine bleedinga
| Meta-analysis author and year | Assessed quality? | Included studies | Excluded studies | Number of subjects with menorrhagia | Statistical model | Incidence of vWD in controls | Incidence of vWD in menorrhagia patients, % (95% confidence interval) |
|---|---|---|---|---|---|---|---|
| Shankar,1 2004 | Yes | 11 | 1 | 988 | Not specified | Not reported | 13 (11.0–15.6) |
This table includes meta-analyses of studies evaluating for the presence of disorders of vWD in women with “menorrhagia,” which is thought to include women with acute uterine bleeding. There were no identified studies specifically for acute uterine bleeding as described in this guideline.
vWD = von Willebrand disease
Shankar M, Lee CA, Sabin CA, Economides DL, Kadir RA. von Willebrand disease in women with menorrhagia: a systematic review. BJOG 2004 Jul;111(7):734–40. DOI: http://dx.doi.org/10.1111/j.1471-0528.2004.00176.x
Cross-sectional studies evaluating for the presence of multiple disorders of coagulation or platelet function in women with “menorrhagia” (heavy menstrual bleeding)
| Author and year | Number of subjects with menorrhagia | Controls | vWD, no. (%) | Factor deficiencies, no. (%) | Platelet abnormality, no. (%) | Combined/other, no. (%) | Overall incidence, no. (%) |
|---|---|---|---|---|---|---|---|
| Dilley,1 2001 | 121 | Yes | 8 (6.6) | 2 (1.6) | 3 (2.5) | Not reported | 13 (10.7) |
| Kadir,2 1998 | 150 | No | 18 (12.0) | 4 (12.0) | 1 (0.7) | 3 (2.0) | 26 (17) |
| James,3 2004 | 108 | Yes | 7 (6.0) | Not reported | 17 (15.7) | 4 (3.7) | 28 (25.9) |
| Philipp,4 2005 | 115 | No | 7 (6.1) | 5 (4.3) | 44 (3.3) | Not reported | 47 (40.9) |
vWD = von Willebrand disease
Dilley A, Drews C, Miller C, et al. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol 2001 Apr;97(4):630–6. DOI: http://dx.doi.org/10.1016/S0029-7844(00)01224-2
Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet 1998 Feb 14;351(9101):485–9.
James AH, Lukes AS, Brancazio LR, Thames E, Ortel TL. Use of a new platelet function analyzer to detect von Willebrand disease in women with menorrhagia. Am J Obstet Gynecol 2004 Aug;191(2):449–55. DOI: http://dx.doi.org/10.1016/j.ajog.2004.03.009
Philipp CS, Faiz A, Dowling N, et al. Age and the prevalence of bleeding disorders in women with menorrhagia. Obstet Gynecol 2005 Jan;105(1):61–6. DOI: http://dx.doi.org/10.1097/01.AOG.0000148889.15061.fb
Hysteroscopy for Evaluation of Patients with Acute Uterine Bleeding
| There were no identified studies specifically for acute uterinebleedingasdefined .guideline this |
Excluded Studies.
Nearly all results from the search strategy did not include articles that dealt with acute abnormal uterine bleeding, and these comprised all but a few of the excluded “studies” obtained in the evidence search. The systematic review of cross-sectional studies on the incidence of disorders of systemic hemostasis included all those studies that were found in the evidence search. Consequently, the individual studies were not listed separately.
Gonadal steroid therapy for acute uterine bleeding
| Systematic reviews/meta-analyses of randomized trials | ||||
|---|---|---|---|---|
| None were identified. | ||||
| Author and year | Intervention | Comparison | Study characteristics and results | Conclusions |
| Randomized Trials (Class 1b) | ||||
| DeVore,1 1982 | Intravenous CEE, 25 mg IV every 4 hours | Placebo | Double blinded N = 32 Primary outcome: Bleeding at 5 hours Bleeding stopped in 72% of CEE group and 38% of controls |
CEE is more effective than placebo in patients regardless of endometrial histology Relatively small sample size is a reason for caution in generalizing results |
| Munro,2 2006 | Oral MPA, 60 mg 3 times daily for a week, then 20 mg/day for 3 weeks | COC, 35 μg ethinyl estradiol + 1 mg norethindrone; 1 pill 3 times daily for 1 week, then once daily for 3 weeks | Open label N = 40; 33 evaluable Primary outcome: Avoidance of operative management Avoidance of surgery in 100% of MPA group and 95% of COC group Mean time to cessation of bleeding was 3 days in each group |
Each intervention is equally effective for the population selected Similar low incidence of side effects in each group Relatively small sample size |
| Case Series (Class 6) | ||||
| Aksu,3 1997 | Oral MPA, 60–120 mg on Day 1 and 20 mg/day for next 10 days | None | N = 24 adolescents Bleeding stopped in 25% in first 24 hours, and in 29.2%, 20.8%, and 25% in the second, third and fourth days, respectively |
Oral MPA may be effective for treatment of acute uterine bleeding Lack of a comparison group impairs ability to distinguish results from those of placebo or other interventions |
| Uterine Tamponade Case Reports and Series (Evidence Classes 6–7) | ||||
| Goldrath,4 1983 | Intrauterine placement of 30-mL Foley balloon for hours to 2 days | None | N = 20 adolescents “Successful” in 17 and “partially successful” in 2 patients Failure in 1 |
Intrauterine tamponade with a Foley balloon may be effective in selected patients with acute uterine bleeding Lack of a comparison group impairs ability to distinguish results from those of placebo or other interventions |
| Humani,5 2010 | Intrauterine balloon tamponade as a treatment of ITP-induced severe uterine bleeding | None | N = 2; aged 19 and 36 years Each with longstanding diagnosis of ITP Both successful short term |
Conclusions difficult with case reports Balloon tamponade a reasonable option in women with acute abnormal uterine bleeding |
| Nishino,6 2013 | Effective salvage of acute massive uterine bleeding using intrauterine balloon tamponade in a uterine adenomyosis patient receiving dienogest | None | N = 1 Patient with adenomyosis using a potent progestin |
Only a case report, but supports consideration of this approach in acute abnormal uterine bleeding |
| Hossain,7 2012 | Successful management of acute catastrophic juvenile vaginal bleeding in Glanzmann thromboasthenia by uterine tamponade | None | N = 1 | First report of balloon tamponade in a perimenarcheal patient with acute catastrophic bleeding |
CEE = conjugated equine estrogens; COC = combination oral contraceptives; ITP = immune thrombocytopenic purpura; IV = intravenous; MPA = medroxyprogesterone acetate.
DeVore GR, Owens O, Kase N. Use of intravenous Premarin in the treatment of dysfunctional uterine bleeding a double-blind randomized control study. Obstet Gynecol 1982 Mar;59(3):285–91.
Munro MG, Mainor N, Basu R, Brisinger M, Barreda L. Oral medroxyprogesterone acetate and combination oral contraceptives for acute uterine bleeding: a randomized controlled trial. Obstet Gynecol 2006 Oct;108(4):924–9. DOI: http://dx.doi.org/10.1097/01.AOG.0000238343.62063.22
Aksu F, Madazli R, Budak E, Cepni I, Benian A. High-dose medroxyprogesterone acetate for the treatment of dysfunctional uterine bleeding in 24 adolescents. Aust N Z J Obstet Gynaecol 1997 May;37(2):228–31. DOI: http://dx.doi.org/10.1111/j.1479-828X.1997.tb02260.x
Goldrath MH. Uterine tamponade for the control of acute uterine bleeding. Am J Obstet Gynecol 1983 Dec 15;147(8):869–72.
Hamani Y, Ben-Shachar I, Kalish Y, Porat S. Intrauterine balloon tamponade as a treatment for immune thrombocytopenic purpura-induced severe uterine bleeding. Fertil Steril 2010 Dec;94(7):2769.e13–5. DOI: http://dx.doi.org/10.1016/j.fertnstert.2010.04.058
Nishino K, Hayashi K, Chaya J, Kato N, Yamamuro O. Effective salvage of acute massive uterine bleeding using intrauterine balloon tamponade in a uterine adenomyosis patient on dienogest. J Obstet Gynaecol Res 2013 Mar;93(3):738–41. DOI: http://dx.doi.org/10.1111/j.1447-0756.2012.02005.x
Hossain N, Shamsi TS, Feroz A. Successful management of acute catastrophic juvenile vaginal bleeding in Glanzmann’s thromboasthenia by uterine tamponade: A case report and review of the literature. Case Rep Hematol 2012;2012:530908. DOI: http://dx.doi.org/10.1155/2012/530908
References
- 1.Munro MG, Critchley HO, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding: Malcolm G Munro, Hilary O D Crithcley, Ian S Fraser, for the FIGO Working Group on Menstrual Disorders. Int J Gynaecol Obstet. 2011 Apr;113(1):1–2. doi: 10.1016/j.ijgo.2011.01.001. DOI: http://dx.doi.org/10.1016/j.ijgo.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011 Apr;113(1):3–13. doi: 10.1016/j.ijgo.2010.11.011. DOI: http://dx.doi.org/10.1016/j.ijgo.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Claessens EA, Cowell CA. Acute adolescent menorrhagia. Am J Obstet Gynecol. 1981 Feb 1;139(3):277–80. doi: 10.1016/0002-9378(81)90009-0. DOI: http://dx.doi.org/10.1097/00006254-198109000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Falcone T, Desjardins C, Bourque J, Granger L, Hemmings R, Quiros E. Dysfunctional uterine bleeding in adolescents. J Reprod Med. 1994 Oct;39(10):761–4. [PubMed] [Google Scholar]
- 5.James AH, Kouides PA, Abdul-Kadir R, et al. Evaluation and management of acute menorrhagia in women with and without underlying bleeding disorders: consensus from an international expert panel. Eur J Obstet Gynecol Reprod Biol. 2011 Oct;158(2):124–34. doi: 10.1016/j.ejogrb.2011.04.025. DOI: http://dx.doi.org/10.1016/j.ejogrb.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013 Apr;121(4):891–6. doi: 10.1097/01.AOG.0000428646.67925.9a. DOI: http://dx.doi.org/10.1097/01.AOG.0000428646.67925.9a. [DOI] [PubMed] [Google Scholar]
- 7.Abel MH, Baird DT. The effect of 17 beta-estradiol and progesterone on prostaglandin production by human endometrium maintained in organ culture. Endocrinology. 1980 May;106(5):1599–606. doi: 10.1210/endo-106-5-1599. [DOI] [PubMed] [Google Scholar]
- 8.Rees MC, Parry DM, Anderson AB, Turnbull AC. Immunohistochemical localisation of cyclooxygenase in the human uterus. Prostaglandins. 1982 Feb;23(2):207–14. doi: 10.1016/0090-6980(82)90047-8. DOI: http://dx.doi.org/10.1016/0090-6980(82)90047-8. [DOI] [PubMed] [Google Scholar]
- 9.Lumsden MA, Brown A, Baird DT. Prostaglandin production from homogenates of separated glandular epithelium and stroma from human endometrium. Prostaglandins. 1984 Oct;28(4):485–96. doi: 10.1016/0090-6980(84)90237-5. DOI: http://dx.doi.org/10.1016/0090-6980(84)90237-5. [DOI] [PubMed] [Google Scholar]
- 10.Word RA, Kamm KE, Casey ML. Contractile effects of prostaglandins, oxytocin, and endothelin-1 in human myometrium in vitro: refractoriness of myometrial tissue of pregnant women to prostaglandins E2 and F2 alpha. J Clin Endocrinol Metab. 1992 Oct;75(4):1027–32. doi: 10.1210/jcem.75.4.1400867. DOI: http://dx.doi.org/10.1210/jc.75.4.1027. [DOI] [PubMed] [Google Scholar]
- 11.Munro MG, Critchley HO, Fraser IS, FIGO Menstrual Disorders Working Group The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. 2011 Jun;95(7):2204–8. 2208.e1–3. doi: 10.1016/j.fertnstert.2011.03.079. DOI: http://dx.doi.org/10.1016/j.fertnstert.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 12.Munro MG, Critchley HO, Fraser IS. The FIGO systems for nomenclature and classification of causes of abnormal uterine bleeding in the reproductive years: who needs them? Am J Obstet Gynecol. 2012 Oct;207(4):259–65. doi: 10.1016/j.ajog.2012.01.046. DOI: http://dx.doi.org/10.1016/j.ajog.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Krassas GE, Pontikides N, Kaltsas T, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf) 1999 May;50(5):655–9. doi: 10.1046/j.1365-2265.1999.00719.x. DOI: http://dx.doi.org/10.1046/j.1365-2265.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 14.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS.Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition Menopause 2009January–Feb;16150–9.DOI: http://dx.doi.org/10.1097/gme.0b013e31817ee0c2 [DOI] [PubMed] [Google Scholar]
- 15.Van Voorhis BJ, Santoro N, Harlow S, Crawford SL, Randolph J. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol. 2008 Jul;112(1):101–8. doi: 10.1097/AOG.0b013e31817d452b. DOI: http://dx.doi.org/10.1097/AOG.0b013e31817d452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dilley A, Drews C, Miller C, et al. von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001 Apr;97(4):630–6. doi: 10.1016/s0029-7844(00)01224-2. DOI: http://dx.doi.org/10.1016/S0029-7844(00)01224-2. [DOI] [PubMed] [Google Scholar]
- 17.Edlund M, Blombäck M, von Schoultz B, Andersson O. On the value of menorrhagia as a predictor for coagulation disorders. Am J Hematol. 1996 Dec;53(4):234–8. doi: 10.1002/(SICI)1096-8652(199612)53:4<234::AID-AJH4>3.0.CO;2-Z. DOI: http://dx.doi.org/10.1002/(SICI)1096-8652(199612)53:4<234::AID-AJH4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998 Feb 14;351(9101):485–9. doi: 10.1016/S0140-6736(97)08248-2. [DOI] [PubMed] [Google Scholar]
- 19.Shankar M, Lee CA, Sabin CA, Economides DL, Kadir RA. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004 Jul;111(7):734–40. doi: 10.1111/j.1471-0528.2004.00176.x. DOI: http://dx.doi.org/10.1111/j.1471-0528.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 20.Peitsidis P, Manolakos E, Tsekoura V, Kreienberg R, Schwentner L. Uterine arteriovenous malformations induced after diagnostic curettage: a systematic review. Arch Gynecol Obstet. 2011 Nov;284(5):1137–51. doi: 10.1007/s00404-011-2067-7. DOI: http://dx.doi.org/10.1007/s00404-011-2067-7. [DOI] [PubMed] [Google Scholar]
- 21.Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003 Aug;102(2):317–8. doi: 10.1016/s0029-7844(03)00527-1. DOI: http://dx.doi.org/10.1016/S0029-7844(03)00527-1. [DOI] [PubMed] [Google Scholar]
- 22.Seidenfeld ME, Rickert VI. Impact of anorexia, bulimia and obesity on the gynecologic health of adolescents. Am Fam Physician. 2001 Aug 1;64(3):445–50. [PubMed] [Google Scholar]
- 23.Norman RJ. Obesity, polycystic ovary syndrome and anovulation—how are they interrelated? Curr Opin Obstet Gynecol. 2001 Jun;13(3):323–7. doi: 10.1097/00001703-200106000-00013. DOI: http://dx.doi.org/10.1097/00001703-200106000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A.Obesity and reproductive disorders in women Hum Reprod Update 2003July–Aug;94359–72.DOI: http://dx.doi.org/10.1093/humupd/dmg024 [DOI] [PubMed] [Google Scholar]
- 25.Abraham SF, Beumont PJ, Fraser IS, Llewellyn-Jones D. Body weight, exercise and menstrual status among ballet dancers in training. Br J Obstet Gynaecol. 1982 Jul;89(7):507–10. doi: 10.1111/j.1471-0528.1982.tb03649.x. DOI: http://dx.doi.org/10.1111/j.1471-0528.1982.tb03649.x. [DOI] [PubMed] [Google Scholar]
- 26.Warren MP, Perlroth NE. The effects of intense exercise on the female reproductive system. J Endocrinol. 2001 Jul;170(1):3–11. doi: 10.1677/joe.0.1700003. DOI: http://dx.doi.org/10.1677/joe.0.1700003. [DOI] [PubMed] [Google Scholar]
- 27.Morrell MJ, Giudice L, Flynn KL, et al. Predictors of ovulatory failure in women with epilepsy. Ann Neurol. 2002 Dec;52(6):704–11. doi: 10.1002/ana.10391. DOI: http://dx.doi.org/10.1002/ana.10391. [DOI] [PubMed] [Google Scholar]
- 28.Legro RS. Diagnostic criteria in polycystic ovary syndrome. Semin Reprod Med. 2003 Aug;21(3):267–75. doi: 10.1055/s-2003-43304. DOI: http://dx.doi.org/10.1055/s-2003-43304. [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetricians and Gynecologists ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists: number 41, December 2002. Obstet Gynecol. 2002 Dec;100(6):1389–402. doi: 10.1016/s0029-7844(02)02628-5. DOI: http://dx.doi.org/10.1016/S0029-7844(02)02628-5. [DOI] [PubMed] [Google Scholar]
- 30.Borenstein R, Katz Z, Lancet M, Caspi B, Ben-David M. Bromocriptine treatment of hyperprolactinemic infertility with ovulatory disturbances. Int J Gynaecol Obstet. 1980;18(3):195–9. doi: 10.1002/j.1879-3479.1980.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones EE. Hyperprolactinemia and female infertility. J Reprod Med. 1989 Feb;34(2):117–26. [PubMed] [Google Scholar]
- 32.Arojoki M, Jokimaa V, Juuti A, Koskinen P, Irjala K, Anttila L. Hypothyroidism among infertile women in Finland. Gynecol Endocrinol. 2000 Apr;14(2):127–31. doi: 10.3109/09513590009167671. DOI: http://dx.doi.org/10.3109/09513590009167671. [DOI] [PubMed] [Google Scholar]
- 33.Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008 Feb;198(2):163.e1–8. doi: 10.1016/j.ajog.2007.08.070. DOI: http://dx.doi.org/10.1016/j.ajog.2007.08.070. [DOI] [PubMed] [Google Scholar]
- 34.Lusher JM. Screening and diagnosis of coagulation disorders. Am J Obstet Gynecol. 1996 Sep;175(3 Pt 2):778–83. doi: 10.1016/s0002-9378(96)80084-6. DOI: http://dx.doi.org/10.1016/S0002-9378(96)80084-6. [DOI] [PubMed] [Google Scholar]
- 35.Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA. Variations in coagulation factors in women: effects of age, ethnicity, menstrual cycle and combined oral contraceptive. Thromb Haemost. 1999 Nov;82(5):1456–61. [PubMed] [Google Scholar]
- 36.Farquhar CM, Lethaby A, Sowter M, Verry J, Baranyai J. An evaluation of risk factors for endometrial hyperplasia in premenopausal women with abnormal menstrual bleeding. Am J Obstet Gynecol. 1999 Sep;181(3):525–9. doi: 10.1016/s0002-9378(99)70487-4. [DOI] [PubMed] [Google Scholar]
- 37.Ash SJ, Farrell SA, Flowerdew G. Endometrial biopsy in DUB. J Reprod Med. 1996 Dec;41(12):892–6. DOI: http://dx.doi.org/10.1097/00006254-199704000-00015. [PubMed] [Google Scholar]
- 38.Stovall TG, Solomon SK, Ling FW. Endometrial sampling prior to hysterectomy. Obstet Gynecol. 1989 Mar;73(3 Pt 1):405–9. [PubMed] [Google Scholar]
- 39.Stovall TG, Ling FW, Morgan PL. A prospective, randomized comparison of the Pipelle endometrial sampling device with the Novak curette. Am J Obstet Gynecol. 1991 Nov;165(5 Pt 1):1287–90. doi: 10.1016/0002-9378(91)90351-q. [DOI] [PubMed] [Google Scholar]
- 40.Tahir MM, Bigrigg MA, Browning JJ, Brookes ST, Smith PA. A randomised controlled trial comparing transvaginal ultrasound, outpatient hysteroscopy and endometrial biopsy with inpatient hysteroscopy and curettage. Br J Obstet Gynaecol. 1999 Dec;106(12):1259–64. doi: 10.1111/j.1471-0528.1999.tb08179.x. DOI: http://dx.doi.org/10.1111/j.1471-0528.1999.tb08179.x. [DOI] [PubMed] [Google Scholar]
- 41.Valle RF. Hysteroscopic evaluation of patients with abnormal uterine bleeding. Surg Gynecol Obstet. 1981 Oct;153(4):521–6. [PubMed] [Google Scholar]
- 42.Gimpelson RJ, Rappold HO. A comparative study between panoramic hysteroscopy with directed biopsies and dilatation and curettage. A review of 276 cases. Am J Obstet Gynecol. 1988 Mar;158(3 Pt 1):489–92. doi: 10.1016/0002-9378(88)90011-7. [DOI] [PubMed] [Google Scholar]
- 43.Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hystero-scopic view. Obstet Gynecol. 1989 Jan;73(1):16–20. DOI: http://dx.doi.org/10.1097/00006254-198905000-00026. [PubMed] [Google Scholar]
- 44.Emanuel MH, Verdel MJ, Wamsteker K, Lammes FB. A prospective comparison of transvaginal ultrasonography and diagnostic hysteroscopy in the evaluation of patients with abnormal uterine bleeding: clinical implications. Am J Obstet Gynecol. 1995 Feb;172(2 Pt 1):547–52. doi: 10.1016/0002-9378(95)90571-5. DOI: http://dx.doi.org/10.1016/0002-9378(95)90571-5. [DOI] [PubMed] [Google Scholar]
- 45.Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001 Aug;76(2):350–7. doi: 10.1016/s0015-0282(01)01900-8. DOI: http://dx.doi.org/10.1016/S0015-0282(01)01900-8. [DOI] [PubMed] [Google Scholar]
- 46.Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal women. Acta Obstet Gynecol Scand. 2003 Jun;82(6):493–504. doi: 10.1034/j.1600-0412.2003.00191.x. DOI: http://dx.doi.org/10.1034/j.1600-0412.2003.00191.x. [DOI] [PubMed] [Google Scholar]
- 47.Bonilla-Musoles F, Simón C, Serra V, Sampaio M, Pellicer A.An assessment of hysterosalpingosonography (HSSG) as a diagnostic tool for uterine cavity defects and tubal patency J Clin Ultrasound 1992March–Apr;203175–81.DOI: http://dx.doi.org/10.1002/jcu.1870200303 [DOI] [PubMed] [Google Scholar]
- 48.Widrich T, Bradley LD, Mitchinson AR, Collins RL. Comparison of saline infusion sonography with office hysteroscopy for the evaluation of the endometrium. Am J Obstet Gynecol. 1996 Apr;174(4):1327–34. doi: 10.1016/s0002-9378(96)70680-4. DOI: http://dx.doi.org/10.1016/S0002-9378(96)70680-4. [DOI] [PubMed] [Google Scholar]
- 49.Saidi MH, Sadler RK, Theis VD, Akright BD, Farhart SA, Villanueva GR. Comparison of sonography, sonohysterography, and hysteroscopy for evaluation of abnormal uterine bleeding. J Ultrasound Med. 1997 Sep;16(9):587–91. doi: 10.7863/jum.1997.16.9.587. [DOI] [PubMed] [Google Scholar]
- 50.van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007 Jun;114(6):664–75. doi: 10.1111/j.1471-0528.2007.01326.x. DOI: http://dx.doi.org/10.1111/j.1471-0528.2007.01326.x. [DOI] [PubMed] [Google Scholar]
- 51.Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB.Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy J Minim Invasive Gynecol 2008January–Feb;15187–91.DOI: http://dx.doi.org/10.1016/j.jmig.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 52.Brooks PG, Serden SP. Hysteroscopic findings after unsuccessful dilatation and curettage for abnormal uterine bleeding. Am J Obstet Gynecol. 1988 Jun;158(6 Pt 1):1354–7. doi: 10.1016/0002-9378(88)90367-5. [DOI] [PubMed] [Google Scholar]
- 53.Itzkowic DJ, Laverty CR. Office hysteroscopy and curettage—a safe diagnostic procedure. Aust N Z J Obstet Gynaecol. 1990 May;30(2):150–3. doi: 10.1111/j.1479-828x.1990.tb03250.x. DOI: http://dx.doi.org/10.1111/j.1479-828X.1990.tb03250.x. [DOI] [PubMed] [Google Scholar]
- 54.Iossa A, Cianferoni L, Ciatto S, Cecchini S, Campatelli C, Lo Stumbo F. Hysteroscopy and endometrial cancer diagnosis: a review of 2007 consecutive examinations in self-referred patients. Tumori. 1991 Dec 31;77(6):479–83. doi: 10.1177/030089169107700606. [DOI] [PubMed] [Google Scholar]
- 55.Gimpelson RJ. Office hysteroscopy. Clin Obstet Gynecol. 1992 Jun;35(2):270–81. DOI: http://dx.doi.org/10.1097/00003081-199235020-00009. [PubMed] [Google Scholar]
- 56.Crescini C, Artuso A, Repetti F, Reale D, Pezzica E. [Hysteroscopic diagnosis in patients with abnormal uterine hemorrhage and previous endometrial curettage]. [Article in Italian] Minerva Ginecol. 1992 May;44(5):233–5. [PubMed] [Google Scholar]
- 57.Huang MW, Muradali D, Thurston WA, Burns PN, Wilson SR. Uterine arteriovenous malformations: gray-scale and Doppler US features with MR imaging correlation. Radiology. 1998 Jan;206(1):115–23. doi: 10.1148/radiology.206.1.9423660. [DOI] [PubMed] [Google Scholar]
- 58.Timmerman D, Van den Bosch T, Peeraer K, et al. Vascular malformations in the uterus: ultrasonographic diagnosis and conservative management. Eur J Obstet Gynecol Reprod Biol. 2000 Sep;92(1):171–8. doi: 10.1016/s0301-2115(00)00443-7. DOI: http://dx.doi.org/10.1016/S0301-2115(00)00443-7. [DOI] [PubMed] [Google Scholar]
- 59.DeVore GR, Owens O, Kase N. Use of intravenous Premarin in the treatment of dysfunctional uterine bleeding—a double-blind randomized control study. Obstet Gynecol. 1982 Mar;59(3):285–91. [PubMed] [Google Scholar]
- 60.Marshall JK, Hunt RH. Hormonal therapy for bleeding gastrointestinal mucosal vascular abnormalities: a promising alternative. Eur J Gastroenterol Hepatol. 1997 May;9(5):521–5. doi: 10.1097/00042737-199705000-00020. DOI: http://dx.doi.org/10.1097/00042737-199705000-00020. [DOI] [PubMed] [Google Scholar]
- 61.Daniell HW. Estrogen prevention of recurrent epistaxis. Arch Otolaryngol Head Neck Surg. 1995 Mar;121(3):354. doi: 10.1001/archotol.1995.01890030080017. DOI: http://dx.doi.org/10.1001/archotol.1995.01890030080017. [DOI] [PubMed] [Google Scholar]
- 62.Chuong CJ, Brenner PF. Management of abnormal uterine bleeding. Am J Obstet Gynecol. 1996 Sep;175(3 Pt 2):787–92. doi: 10.1016/s0002-9378(96)80086-x. DOI: http://dx.doi.org/10.1016/S0002-9378(96)80086-X. [DOI] [PubMed] [Google Scholar]
- 63.Munro MG, Mainor N, Basu R, Brisinger M, Barreda L. Oral medroxyprogesterone acetate and combination oral contraceptives for acute uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2006 Oct;108(4):924–9. doi: 10.1097/01.AOG.0000238343.62063.22. DOI: http://dx.doi.org/10.1097/01.AOG.0000238343.62063.22. [DOI] [PubMed] [Google Scholar]
- 64.Aksu F, Madazli R, Budak E, Cepni I, Benian A. High-dose medroxyprogesterone acetate for the treatment of dysfunctional uterine bleeding in 24 adolescents. Aust N Z J Obstet Gynaecol. 1997 May;37(2):228–31. doi: 10.1111/j.1479-828x.1997.tb02260.x. DOI: http://dx.doi.org/10.1111/j.1479-828X.1997.tb02260.x. [DOI] [PubMed] [Google Scholar]
- 65.Cooke I, Lethaby A, Farquhar C. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(2):CD000249. doi: 10.1002/14651858.CD000249. DOI: http://dx.doi.org/10.1002/14651858.CD000249. [DOI] [PubMed] [Google Scholar]
- 66.Lindoff C, Rybo G, Astedt B. Treatment with tranexamic acid during pregnancy, and the risk of thrombo-embolic complications. Thromb Haemost. 1993 Aug 2;70(2):238–40. [PubMed] [Google Scholar]
- 67.Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009 Aug 13;339:b2890. doi: 10.1136/bmj.b2890. DOI: http://dx.doi.org/10.1136/bmj.b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kropsky B, Shi Y, Cherniack EP.Incidence of deep-venous thrombosis in nursing home residents using megestrol acetate J Am Med Dir Assoc 2003September–Oct;45255–6.DOI: http://dx.doi.org/10.1016/S1525-8610(04)70369-2 [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, case-control study. Contraception. 1998 May;57(5):315–24. DOI: http://dx.doi.org/10.1016/S0010-7824(98)00041-9. [PubMed] [Google Scholar]
- 70.van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscler Thromb Vasc Biol. 2010 Nov;30(11):2297–300. doi: 10.1161/ATVBAHA.110.211482. DOI: http://dx.doi.org/10.1161/ATVBAHA.110.211482. [DOI] [PubMed] [Google Scholar]
- 71.Goldstein J, Cushman M, Badger GJ, Johnson JV. Effect of depome-droxyprogesterone acetate on coagulation parameter: a pilot study. Fertil Steril. 2007 Jun;87(6):1267–70. doi: 10.1016/j.fertnstert.2006.11.040. DOI: http://dx.doi.org/10.1016/j.fertn-stert.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 72.Reed MJ, Ross MS, Lai LC, Ghilchik MW, James VH. In vivo conversion of norethisterone to ethynyloestradiol in perimenopausal women. J Steroid Biochem Mol Biol. 1990 Oct;37(2):301–3. doi: 10.1016/0960-0760(90)90342-i. DOI: http://dx.doi.org/10.1016/0960-0760(90)90342-I. [DOI] [PubMed] [Google Scholar]
- 73.Kuhnz W, Heuner A, Hümpel M, Seifert W, Michaelis K. In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in post-menopausal women. Contraception. 1997 Dec;56(6):379–85. doi: 10.1016/s0010-7824(97)00174-1. DOI: http://dx.doi.org/10.1016/S0010-7824(97)00174-1. [DOI] [PubMed] [Google Scholar]
- 74.Hamani Y, Ben-Shachar I, Kalish Y, Porat S. Intrauterine balloon tamponade as a treatment for immune thrombocytopenic purpura-induced severe uterine bleeding. Fertil Steril. 2010 Dec;94(7):2769.e13–5. doi: 10.1016/j.fertnstert.2010.04.058. DOI: http://dx.doi.org/10.1016/j.fertnstert.2010.04.058. [DOI] [PubMed] [Google Scholar]
- 75.Goldrath MH. Uterine tamponade for the control of acute uterine bleeding. Am J Obstet Gynecol. 1983 Dec 15;147(8):869–72. doi: 10.1016/0002-9378(83)90237-5. [DOI] [PubMed] [Google Scholar]
- 76.Nilsson L, Rybo G. Treatment of menorrhagia. Am J Obstet Gynecol. 1971 Jul 1;110(5):713–20. doi: 10.1016/0002-9378(71)90259-6. [DOI] [PubMed] [Google Scholar]
- 77.Richards SR. Endometrial ablation for life-threatening abnormal uterine bleeding. A report of two cases. J Reprod Med. 1994 Sep;39(9):741–2. [PubMed] [Google Scholar]
- 78.Milad MP, Valle RF. Emergency endometrial ablation for life-threatening uterine bleeding as a result of a coagulopathy. J Am Assoc Gynecol Laparosc. 1998 Aug;5(3):301–3. doi: 10.1016/s1074-3804(98)80037-6. DOI: http://dx.doi.org/10.1016/S1074-3804(98)80037-6. [DOI] [PubMed] [Google Scholar]
- 79.Osuga Y, Okagaki R, Ozaki S, et al. Successful emergency endometrial ablation for intractable uterine bleeding in a postmenopausal woman complicated with liver cirrhosis and morbid obesity. Surg Endosc. 2001 Aug;15(8):898. doi: 10.1007/s004640040042. DOI: http://dx.doi.org/10.1007/s004640040042. [DOI] [PubMed] [Google Scholar]
- 80.Fritsch HI. Uterusvapokauterisation, tod durch septische peritonitis nach spontaner sekundarer perforation. Centralblatt fur Gynakologie. 1898;52:1409–18. [Google Scholar]
- 81.Bardenheuer F. Elektrokoagulation der Uterusschleimhaut zur Behandlung-klimakterischer Blutungen. Zentralblatt fur Gynakologie. 1937;4:209–11. [Google Scholar]
- 82.Yeasmin S, Nakayama K, Ishibashi M, et al. Microwave endometrial ablation as an alternative to hysterectomy for the emergent control of uterine bleeding in patients who are poor surgical candidates. Arch Gynecol Obstet. 2009 Aug;280(2):279–82. doi: 10.1007/s00404-008-0885-z. DOI: http://dx.doi.org/10.1007/s00404-008-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nichols CM, Gill EJ. Thermal balloon endometrial ablation for management of acute uterine hemorrhage. Obstet Gynecol. 2002 Nov;100(5 Pt 2):1092–4. doi: 10.1016/s0029-7844(02)02101-4. DOI: http://dx.doi.org/10.1016/S0029-7844(02)02101-4. [DOI] [PubMed] [Google Scholar]
- 84.Chapa HO, Antonetti AG, Sandate J, Silver L.Emergent thermal balloon ablation for acute uterine hemorrhage: a report of 2 cases J Reprod Med 2010November–Dec;55.11–12 511–3. [PubMed] [Google Scholar]
- 85.Phelan JT, 2nd, Broder J, Kouides PA. Near-fatal uterine hemorrhage during induction chemotherapy for acute myeloid leukemia: a case report of bilateral uterine artery embolization. Am J Hematol. 2004 Oct;77(2):151–5. doi: 10.1002/ajh.20113. DOI: http://dx.doi.org/10.1002/ajh.20113. [DOI] [PubMed] [Google Scholar]
- 86.Thakar R, Ayers S, Clarkson P, Stanton S, Manyonda I. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002 Oct 24;347(17):1318–25. doi: 10.1056/NEJMoa013336. DOI: http://dx.doi.org/10.1056/NEJMoa013336. [DOI] [PubMed] [Google Scholar]
- 87.Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003 Sep;102(3):453–62. doi: 10.1016/s0029-7844(03)00664-1. DOI: http://dx.doi.org/10.1016/S0029-7844(03)00664-1. [DOI] [PubMed] [Google Scholar]