Abstract
The relationship between cholesterol and coronary heart disease (CHD) is attenuated at older age. We analyzed cholesterol level as a predictor of CHD in 8,947 participants from the Atherosclerosis Risk in Communities (ARIC) Study, a large multicenter cohort study that enrolled participants in 1987–1989 at 4 field centers in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and Minneapolis, Minnesota. Participants in the present analysis had no history of CHD and were stratified by age (<65 or ≥65 years) and high-sensitivity C-reactive protein (hs-CRP) level (<2 or ≥2 mg/L). Visit 4 (1996–1997) was the baseline for this analysis, with follow-up through 2008. Cholesterol level was significantly associated with CHD among younger participants, and cholesterol level was similarly predictive of CHD among older participants with an hs-CRP level of <2 mg/L. In contrast, among older participants with an hs-CRP level of 2 mg/L or higher, the association of CHD with total cholesterol level was borderline significant (hazard ratio = 1.14, 95% confidence interval: 1.00, 1.29), and the association of CHD with low-density lipoprotein cholesterol level was nonsignificant (hazard ratio = 1.10; 95% confidence interval: 0.96, 1.26). Among older persons with an elevated hs-CRP level, cholesterol level was significantly less predictive of CHD (P < 0.05), whereas for those with an hs-CRP level of <2 mg/L, there was no significant difference compared with younger participants. In conclusion, we found that among the young-old, the association of cholesterol level with CHD was strong when hs-CRP level was not elevated and weak when hs-CRP level was elevated. Therefore, hs-CRP level could be useful for stratifying the young-old to assess the strength of cholesterol level in CHD risk prediction.
Keywords: C-reactive protein, elderly persons, serum cholesterol
Elevated serum cholesterol level is a well-established risk factor for coronary heart disease (CHD), and the use of cholesterol-lowering medications has been shown to be effective in the primary prevention of cardiovascular disease at all ages (1–3). However, the relationship between elevated serum cholesterol level and adverse cardiac events is attenuated at older age (4–7). Although the reason for cholesterol's decreased contribution to CHD risk in older age is unclear, it is possible that comorbid conditions that become more common with age could compete with traditional risk factors for CHD risk prediction and in some circumstances could lower the serum cholesterol level through inflammation or other processes (8). Therefore, methods for distinguishing which subset of older adults retains the strong association of cholesterol level with CHD and which subset does not would be useful in making progress toward improved risk prediction.
High-sensitivity C-reactive protein (hs-CRP) is a nonspecific inflammatory marker, elevated levels of which have been strongly associated with adverse cardiovascular events (9–12). Additionally, in the Justification for the Use of Statins in Primary Prevention trial (13), participants with an elevated hs-CRP level and a normal serum cholesterol level had a lower rate of major cardiovascular events after treatment with rosuvastatin. An elevated hs-CRP level has also been associated with many conditions beyond vascular inflammation, including malignancy (14–17), infection (18), heart failure (19), chronic kidney disease (20), airway disease (21), and general physical decline (22). Overall, these pathological states are more common in the elderly and could effectively compete with traditional risk factors for CHD risk prediction. It is also possible that the systemic inflammation associated with these conditions results in a lower serum cholesterol level because of associated poor nutrition or increased catabolism. Increased systemic inflammation also could exacerbate preexisting coronary artery disease, leading to a greater risk of clinical events. In extreme cases, such as end-stage renal disease, this can lead to a reversal of the expected relationship between serum cholesterol level and CHD (23).
We hypothesize that an elevated hs-CRP level will be a nonspecific but useful predictive marker that can integrate many pathological processes and can distinguish the elderly population into subgroups: 1) older adults with an elevated hs-CRP level in whom an elevated serum cholesterol level is weakly associated with or not associated with an increased risk of CHD, and 2) older adults with a normal hs-CRP level in whom the cholesterol level–CHD association is largely unchanged from that at younger ages.
MATERIALS AND METHODS
Study design and population
The study population for this analysis was derived from the Atherosclerosis Risk in Communities (ARIC) Study. ARIC is a large multicenter cohort study of persons who were between 45 and 64 years of age at the time of enrollment in 1987–1989. The ARIC Study is conducted at 4 field centers in Washington County, Maryland; Forsyth County, North Carolina; Jackson, Mississippi; and Minneapolis, Minnesota. Details of the design of the ARIC Study have been described previously (24). All participants who completed the visit 4 screening with a serum draw for measurement of hs-CRP and cholesterol were eligible for inclusion in this analysis (n = 11,148). Participants were excluded if they 1) had known coronary artery disease, defined as a history of myocardial infarction, coronary bypass or angioplasty, or electrocardiogram-diagnosed myocardial infarction (n = 922); 2) were taking a cholesterol-lowering medication (n = 1,168); or 3) were missing other important covariates (n = 111). The study population in this analysis after exclusions consisted of 8,947 participants.
Exposure variables
At each visit, standardized and validated interviewer-administered questionnaires were used to collect demographic information; smoking and alcohol consumption status; and history of cancer, diabetes, and hypertension. Smoking and alcohol consumption were categorized into never, former, and current groups. Height, body weight, and blood pressure were measured at each visit. Weight change in this analysis was defined as the difference in body weight between visits 3 and 4. Prevalent diabetes was defined as a fasting glucose level of 126 mg/dL or higher. Carotid intimal-medial thickness was measured by ultrasound at either visit 1 or visit 2 as described previously (25, 26). Twelve-hour fasting plasma total cholesterol, low-density lipoprotein (LDL) cholesterol, triglyceride, and high-density lipoprotein cholesterol levels were measured in a centralized laboratory at each visit. The assays and their performance have been reported (27). LDL cholesterol was calculated with the Friedewald formula. All measures followed a common protocol to maximize comparability across persons and visits. At visit 4, hs-CRP was measured in a central laboratory on plasma frozen at −80°C with an immunonephelometric assay on a BNII analyzer (Siemens Healthcare Diagnostics, Deerfield, Illinois) according to the manufacturer's protocol. The reliability coefficient for the hs-CRP assay was 0.99 and was based on 421 blinded replicates (28). In the present analysis, we used the Justification for the Use of Statins in Primary Prevention cutpoint of 2 mg/L or higher to classify participants with an elevated hs-CRP level (13).
Outcomes
The primary outcome of this analysis was incident CHD, defined as one of the following: silent infarction diagnosed by electrocardiogram, myocardial infarction, coronary artery bypass or angioplasty, or death from CHD before January 2009. Incident cases were verified by 2 reviewers from the ARIC Morbidity and Mortality Classification Committee, and any differences between reviewers were adjudicated by the committee chairperson.
Statistical methods
Statistical analyses were performed in Stata, version 11 (StataCorp LP, College Station, Texas). Analyses were conducted separately for those less than 65 and those 65 years of age or older. Within each age category, participants were grouped according to level of hs-CRP. An extension of the Wilcoxon rank-sum test was used to compare continuous variables, and the χ2 test was used to compare categorical variables. Crude incident rates were calculated per 1,000 person-years and compared by Poisson regression. Mean change in cholesterol level was calculated by taking the average difference between serum cholesterol level at visit 4 and at each previous visit. Cox proportional-hazard models were used to calculate hazard ratios and confidence intervals for the primary outcome for each age group by hs-CRP category. The proportional-hazards assumption was not violated on the basis of Schoenfeld residual testing (29). The interaction between total cholesterol level and hs-CRP category was assessed within each age group and between age groups. We examined the continuous interaction between age, hs-CRP level, and cholesterol level (total and LDL) by using a fully adjusted hazards model and also by using an additive model (30). The same models were used for LDL cholesterol. Covariates adjusted for in model 1 were age, sex, and race. Model 2 adjusted for age, sex, race, current smoking, diabetes, hypertension, and body mass index. Model 3 adjusted for all covariates in model 2, plus high-density lipoprotein cholesterol level. Sensitivity analyses were conducted with fully adjusted Cox proportional-hazards models stratified by sex, race, diabetes, and years of follow-up, and with exclusion of participants with an hs-CRP level greater than 10 mg/L.
RESULTS
During a median follow-up of 11 years, 967 incident CHD events occurred among the 8,947 participants. Baseline characteristics stratified by age and hs-CRP group are displayed in Table 1. For both age groups at baseline, there was a significantly higher prevalence of diabetes, hypertension, and smoking, as well as a higher mean body mass index, in the group with an elevated hs-CRP level. The unadjusted incidence rate of CHD per 1,000 person-years was higher in those with an elevated hs-CRP level for both age groups, with a higher overall rate observed in the elderly (14.4 per 1,000 person-years vs. 8.5 per 1,000 person-years; P < 0.001).
Table 1.
Characteristics of Persons Less Than 65 Years of Age and 65 Years of Age or Older by hs-CRP Level at Visit 4 (1996–1997), Atherosclerosis Risk in Communities Study, United States, 1987–2008
| hs-CRP Level |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age <65 Years |
Age ≥65 Years |
|||||||||||||||||
| <2 mg/L (n = 2,483) |
≥2 mg/L (n = 3,146) |
Overall (n = 5,629) |
<2 mg/L (n = 1,446) |
≥2 mg/L (n = 1,872) |
Overall (n = 3,318) |
|||||||||||||
| Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean | % | Median | Mean (SD) | % | Median (IQR) | Mean (SD) | % | Median (IQR) | Mean | % | Median | |
| Clinical characteristics | ||||||||||||||||||
| Age, years | 58.8 (3.18) | 58.8 (3.13) | 58.8 | 68.8 (2.55) | 68.7 (2.62) | 68.7 | ||||||||||||
| Male sex | 52.1 | 29.5* | 39.5 | 52.3 | 38.7* | 44.6 | ||||||||||||
| Black race | 20.6 | 30.5* | 26.1 | 15.1 | 22.6* | 19.3 | ||||||||||||
| Current smoker | 14.9 | 18.6* | 16.9 | 10.4 | 14.6* | 12.8 | ||||||||||||
| Current drinker | 58.3 | 48.6* | 52.9 | 48.4 | 42.1* | 44.8 | ||||||||||||
| Body mass indexa | 26.9 (4.42) | 30.7 (6.33)* | 29.0 | 26.4 (4.26) | 29.5 (5.68)* | 28.1 | ||||||||||||
| Weight change, kg | 0.5 (4.23) | 1.1 (5.10)* | 0.8 | −0.1 (4.04) | 0.4 (4.44)* | 0.2 | ||||||||||||
| Diabetes | 9.1 | 17.1* | 13.5 | 9.5 | 18.7* | 14.7 | ||||||||||||
| Hypertension | 30.6 | 46.4* | 39.4 | 45.7 | 56.0* | 51.5 | ||||||||||||
| Heart failure | 0.6 | 1.2* | 0.9 | 0.9 | 1.4 | 1.2 | ||||||||||||
| History of cancer | 7.4 | 8.5 | 8.0 | 14.4 | 15.1 | 14.8 | ||||||||||||
| Laboratory values | ||||||||||||||||||
| hs-CRP, mg/L | 1.0 (0.6, 1.4) | 5.1 (3.2, 8.5)* | 2.5 | 1.0 (0.6, 1.4) | 4.9 (3.2, 7.9)* | 2.4 | ||||||||||||
| Cystatin C, mg/L | 0.8 (0.26) | 0.8 (0.33)* | 0.8 | 0.9 (0.31) | 0.9 (0.37)* | 0.9 | ||||||||||||
| CIMT, mm | 0.7 (0.14) | 0.7 (0.14) | 0.7 | 0.8 (0.17) | 0.8 (0.20)* | 0.8 | ||||||||||||
| Total cholesterol, mmol/L | 5.2 (0.92) | 5.2 (0.94)* | 5.2 | 5.2 (0.93) | 5.3 (0.94) | 5.2 | ||||||||||||
| LDL cholesterol, mmol/L | 3.2 (0.86) | 3.2 (0.88) | 3.2 | 3.2 (0.84) | 3.2 (0.87) | 3.2 | ||||||||||||
| HDL cholesterol, mmol/L | 1.4 (0.45) | 1.3 (0.42)* | 1.3 | 1.4 (0.44) | 1.3 (0.43)* | 1.3 | ||||||||||||
| Outcome | ||||||||||||||||||
| CHD incidenceb | 7.8 | 9.1 | 8.5 | 11.7 | 16.5* | 14.4 | ||||||||||||
Abbreviations: CHD, coronary heart disease; CIMT, carotid intima-media thickness; hs-CRP, high-sensitivity C-reactive protein; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; SD, standard deviation.
* P < 0.05 (compared with low hs-CRP group).
a Weight (kg)/height (m)2.
b Per 1,000 person-years.
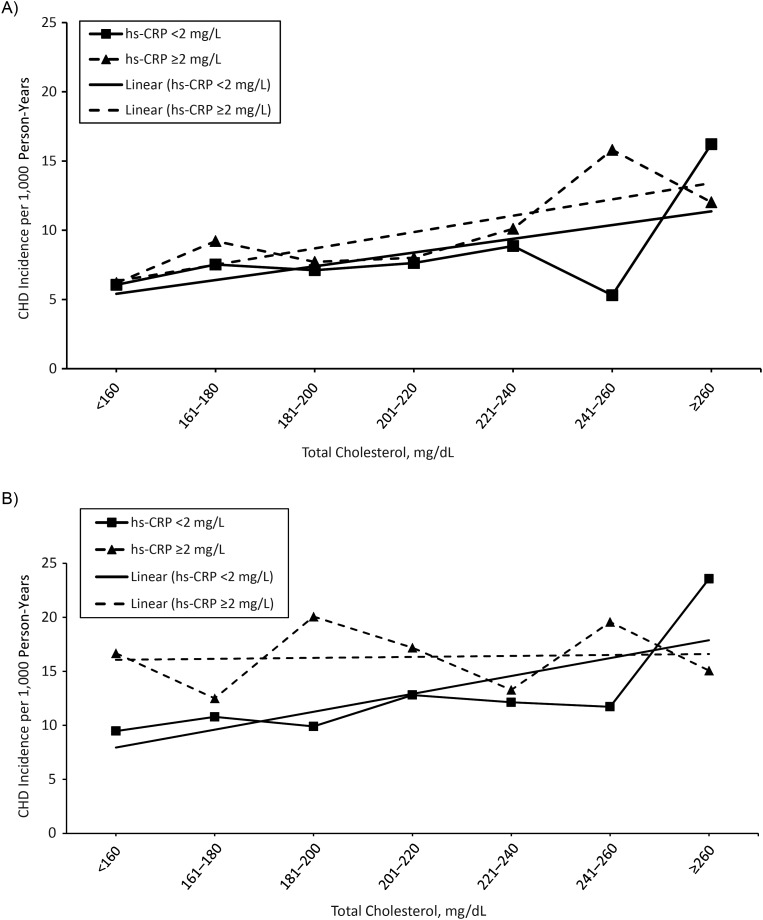
The incidence rate of CHD was linearly associated with total cholesterol levels among participants less than 65 years of age, regardless of hs-CRP level. In contrast, among participants 65–75 years of age at baseline, elevated hs-CRP level was associated with a higher CHD rate and a blunted, nearly flat association of cholesterol level with CHD risk (Figure 1). A spline model for the risk of CHD per 1.03-mmol/L increase in total cholesterol level stratified by hs-CRP group, with a knot at age 65, demonstrated similar results (see Web Figure 1, available at http://aje.oxfordjournals.org/).
Figure 1.
The association of crude coronary heart disease (CHD) incidence per 1,000 person-years of follow-up with total cholesterol category stratified by high-sensitivity C-reactive protein (hs-CRP) group, as measured at visit 4 (1996–1997) for participants less than 65 years of age (A) and 65–74 years of age (B) in the Atherosclerosis Risk in Communities Study, United States, 1987–2008. Lines without reference points represent the linear trend of the association between total cholesterol level and CHD incidence for each hs-CRP group.
Changes in serum cholesterol level and LDL cholesterol level between ARIC visits were stratified by age and hs-CRP groups (Table 2). The change in cholesterol level was greatest between visits 1 and 4 (9 years apart) for all groups. In participants less than 65 years of age, an elevated hs-CRP level was significantly associated with a larger drop in total and LDL cholesterol levels (P < 0.05). At older ages, the drop in serum cholesterol level was larger, at approximately 0.34 mmol/L (13 mg/dL) over the 9 years, but it did not differ significantly between hs-CRP groups.
Table 2.
Total Cholesterol and LDL Cholesterol Levels at Visit 4 (1996–1997) and Mean Difference When Compared With Previous Visits (1, 2, and 3), According to Age (<65 and ≥65 Years) and hs-CRP Level (<2 and ≥2 mg/L), Atherosclerosis Risk in Communities Study, United States, 1987–2008
| hs-CRP Level |
||||||
|---|---|---|---|---|---|---|
| Age <65 Years |
Age ≥65 Years |
|||||
| <2 mg/L | ≥2 mg/L | Overall | <2 mg/L | ≥2 mg/L | Overall | |
| Total cholesterol, mmol/L | ||||||
| Visit 4, mean (SD) | 5.17 (0.92) | 5.22 (0.94)* | 5.20 (0.94) | 5.20 (0.93) | 5.25 (0.94) | 5.23 (0.94) |
| Change to visit 4 | ||||||
| Visit 3, mean (SD) | 0.01 (0.64) | −0.10 (0.68)* | −0.06 (0.67) | −0.11 (0.64)** | −0.12 (0.67) | −0.12 (0.66)** |
| Visit 2, mean (SD) | −0.02 (0.75) | −0.08 (0.78)* | −0.06 (0.77) | −0.15 (0.73)** | −0.17 (0.78)** | −0.16 (0.76)** |
| Visit 1, mean (SD) | −0.09 (0.80) | −0.14 (0.82)* | −0.12 (0.81) | −0.34 (0.77)** | −0.34 (0.79)** | −0.34 (0.78)** |
| LDL cholesterol, mmol/L | ||||||
| Visit 4, mean (SD) | 3.19 (0.86) | 3.18 (0.88) | 3.19 (0.87) | 3.20 (0.84) | 3.22 (0.87) | 3.21 (0.86) |
| Change to visit 4 | ||||||
| Visit 3, mean (SD) | 0.04 (0.61) | −0.03 (0.64)* | −0.002 (0.63) | −0.06 (0.61)** | −0.06 (0.62) | −0.06 (0.62)** |
| Visit 2, mean (SD) | −0.07 (0.71) | −0.14 (0.75)* | −0.11 (0.74) | −0.20 (0.69)** | −0.20 (0.73)** | −0.20 (0.71)** |
| Visit 1, mean (SD) | −0.12 (0.73) | −0.18 (0.76)* | −0.15 (0.75) | −0.32 (0.73)** | −0.33 (0.75)** | −0.33 (0.74)** |
Abbreviations: hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; SD, standard deviation.
* P < 0.05 (compared with lower hs-CRP group); ** P < 0.05 (compared with lower age group).
Cox proportional-hazards models stratified by age and hs-CRP group were used to assess the adjusted association between serum cholesterol level and CHD risk (Table 3). In participants less than 65 years of age, serum total and LDL cholesterol levels remained significantly associated with CHD for both hs-CRP groups. Among older participants with an hs-CRP level of <2 mg/L, total and LDL cholesterol levels remained just as strongly associated with CHD as in younger participants in the same hs-CRP category. However, among older participants with an elevated hs-CRP level, CHD had only a borderline significant association with total cholesterol level (hazard ratio = 1.14, confidence interval: 1.00, 1.29) and a nonsignificant association with LDL cholesterol level (hazard ratio = 1.10, confidence interval: 0.96, 1.26). For persons less than 65 years of age, the association between CHD and serum cholesterol level did not differ significantly between hs-CRP groups. For participants 65 years of age or older with an hs-CRP level less than 2 mg/L, CHD was more strongly associated with total cholesterol level (P < 0.05) and LDL cholesterol level (P < 0.10) than in those with an elevated hs-CRP level. The association of total and LDL cholesterol levels with CHD was also significantly weaker in older participants with an elevated hs-CRP level than in younger participants with an elevated hs-CRP level (P < 0.05). The continuous 3-way interaction term between age, hs-CRP level, and cholesterol level in the fully adjusted hazards model (model 3) was significant for both total cholesterol (P = 0.003) and LDL cholesterol (P = 0.006). The results were similar when the interaction was tested on the additive scale, with P values of 0.01 for the total cholesterol model and 0.06 for the LDL cholesterol model. Consistent with previous ARIC publications, hs-CRP level was independently associated with CHD, with fully adjusted relative hazard ratios per natural log unit of 1.18 (95% confidence interval: 1.07, 1.29) at less than 65 years of age and 1.23 (95% confidence interval: 1.12, 1.35) at 65–75 years of age.
Table 3.
Risk of Coronary Heart Disease per 1.03-mmol/L Increase in Total Cholesterol and LDL Cholesterol Levels, According to hs-CRP Category at Visit 4 (1996–1997), Atherosclerosis Risk in Communities Study, United States, 1987–2008
| hs-CRP Level |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age <65 Years |
Age ≥65 Years |
|||||||||||
| <2 mg/L (n = 2,483) |
≥2 mg/L (n = 3,146) |
Overall (n = 5,629) |
<2 mg/L (n = 1,446) |
≥2 mg/L (n = 1,872) |
Overall (n = 3,318) |
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Total cholesterol | ||||||||||||
| Model 1a | 1.33 | 1.15, 1.55 | 1.31 | 1.17, 1.46 | 1.32 | 1.20, 1.44 | 1.43 | 1.22, 1.68 | 1.11* | 0.98, 1.26 | 1.23 | 1.11, 1.35 |
| Model 2b | 1.35 | 1.16, 1.57 | 1.35 | 1.20, 1.51 | 1.35 | 1.24, 1.48 | 1.45 | 1.23, 1.70 | 1.11*,** | 0.98, 1.26 | 1.23 | 1.11, 1.36 |
| Model 3c | 1.39 | 1.20, 1.61 | 1.37 | 1.22, 1.53 | 1.38 | 1.26, 1.52 | 1.46 | 1.24, 1.71 | 1.14*,** | 1.00, 1.29 | 1.26 | 1.14, 1.39 |
| LDL cholesterol | ||||||||||||
| Model 1 | 1.42 | 1.21, 1.67 | 1.36 | 1.21, 1.53 | 1.39 | 1.26, 1.53 | 1.39 | 1.17, 1.64 | 1.17 | 1.02, 1.34 | 1.25 | 1.13, 1.39 |
| Model 2 | 1.45 | 1.24, 1.70 | 1.41 | 1.25, 1.59 | 1.43 | 1.30, 1.57 | 1.42 | 1.20, 1.68 | 1.16** | 1.02, 1.33 | 1.26 | 1.13, 1.40 |
| Model 3 | 1.43 | 1.21, 1.67 | 1.40 | 1.24, 1.58 | 1.41 | 1.28, 1.55 | 1.42 | 1.18, 1.67 | 1.10** | 0.96, 1.26 | 1.22 | 1.09, 1.36 |
Abbreviations: CI, confidence interval; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
* P < 0.05 (compared with lower hs-CRP group); ** P < 0.05 (compared with lower age group).
a Model 1 adjusted for age, sex, and race.
b Model 2 adjusted for age, sex, race, current smoking, diabetes, hypertension, and body mass index.
c Model 3 adjusted for age, sex, race, current smoking, diabetes, hypertension, body mass index, and high-density lipoprotein cholesterol.
We performed several sensitivity analyses to examine variation in subgroups. In general, the findings were similar when stratified by sex, race, diabetes, and length of follow-up, after the reduced power and precision resulting from further subdivision of the cohort were taken into account (Table 4). The attenuation with age and elevated hs-CRP level was present to some extent in all groups but was most marked among women and black participants. It is interesting that the attenuation was, if anything, more marked at follow-up of 5–10 years than in the first 5 years, despite the possible hypothesis that subclinical disease might manifest within the first 5 years of follow-up. We also performed an analysis that excluded participants with an hs-CRP level of 10 mg/L or higher because hs-CRP levels can be highly elevated if measured at the time of an active infection. Despite the smaller sample size, the findings were largely similar. The results were similar when we used the hs-CRP cutpoints of <1 mg/L, 1–<3 mg/L, and ≥3 mg/L to analyze the data. We also investigated models with adjustments for alcohol consumption, physical activity, and socioeconomic status and found no significant change in the estimates (not shown).
Table 4.
Sensitivity Analysis for Risk of Coronary Heart Disease per 1.03-mmol/L Increase in Total Cholesterol and LDL Cholesterol Levels, According to hs-CRP Category at Visit 4 (1996–1997), Atherosclerosis Risk in Communities Study, United States, 1987–2008a
| hs-CRP Level |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age <65 Years |
Age ≥65 Years |
|||||||||||
| <2 mg/L (n = 2,483) |
≥2 mg/L (n = 3,146) |
Overall (n = 5,629) |
<2 mg/L (n = 1,446) |
≥2 mg/L (n = 1,872) |
Overall (n = 3,318) |
|||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Total cholesterol | ||||||||||||
| Sex | ||||||||||||
| Male | 1.37 | 1.14, 1.63 | 1.39 | 1.17, 1.62 | 1.38 | 1.22, 1.56 | 1.40 | 1.14, 1.71 | 1.32 | 1.11, 1.57 | 1.36 | 1.20, 1.55 |
| Female | 1.45 | 1.11, 1.92 | 1.37 | 1.17, 1.60 | 1.40 | 1.22, 1.60 | 1.61 | 1.22, 2.12 | 0.98*,** | 0.81, 1.18 | 1.13** | 0.97, 1.32 |
| Race | ||||||||||||
| White | 1.38 | 1.06, 1.63 | 1.30 | 1.34, 1.82 | 1.33 | 1.20, 1.48 | 1.43 | 1.20, 1.71 | 1.21 | 1.05, 1.40 | 1.38 | 1.19, 1.60 |
| Black | 1.49 | 1.10, 2.02 | 1.70 | 1.33, 2.16 | 1.59 | 1.32, 1.92 | 1.55 | 1.04, 2.32 | 0.94*,** | 0.71, 1.24 | 1.11 | 0.89, 1.40 |
| Diabetes | ||||||||||||
| Yes | 1.43 | 1.08, 1.90 | 1.48 | 1.17, 1.88 | 1.48 | 1.24, 1.78 | 1.44 | 1.01, 2.06 | 1.03** | 0.80, 1.32 | 1.14 | 0.93, 1.39 |
| No | 1.36 | 1.14, 1.63 | 1.34 | 1.18, 1.53 | 1.36 | 1.22, 1.51 | 1.49 | 1.25, 1.79 | 1.18* | 1.02, 1.36 | 1.30 | 1.16, 1.46 |
| Years of follow-up | ||||||||||||
| 0–5 | 1.32 | 1.06, 1.63 | 1.56 | 1.34, 1.82 | 1.48 | 1.31, 1.69 | 1.52 | 1.19, 1.93 | 1.28 | 1.06, 1.54 | 1.38 | 1.19, 1.60 |
| >5–10 | 1.45 | 1.15, 1.82 | 1.09 | 0.89, 1.34 | 1.23 | 1.06, 1.43 | 1.29 | 0.99, 1.68 | 1.02 | 0.84, 1.24 | 1.11 | 0.95, 1.30 |
| hs-CRP level | ||||||||||||
| <10 mg/L | 1.39 | 1.20, 1.61 | 1.26 | 1.11, 1.44 | 1.32 | 1.20, 1.46 | 1.45 | 1.24, 1.71 | 1.17* | 1.02, 1.34 | 1.29 | 1.16, 1.43 |
| No rheumatoid arthritis or lupus | 1.43 | 1.22, 1.67 | 1.35 | 1.20, 1.52 | 1.39 | 1.27, 1.53 | 1.57 | 1.01, 1.32 | 1.27* | 1.15, 1.41 | 1.43 | 1.16, 1.77 |
| LDL cholesterol | ||||||||||||
| Sex | ||||||||||||
| Male | 1.39 | 1.12, 1.71 | 1.41 | 1.17, 1.71 | 1.43 | 1.25, 1.63 | 1.44 | 0.96, 2.15 | 1.32 | 1.09, 1.60 | 1.39 | 1.19, 1.57 |
| Female | 1.42 | 1.05, 1.92 | 1.40 | 1.19, 1.65 | 1.41 | 1.21, 1.63 | 1.42 | 1.17, 1.72 | 1.12*,** | 0.96, 1.32 | 1.00** | 0.88, 1.23 |
| Race | ||||||||||||
| White | 1.41 | 1.17, 1.70 | 1.32 | 1.14, 1.52 | 1.35 | 1.21, 1.52 | 1.39 | 1.15, 1.67 | 1.16 | 0.99, 1.37 | 1.27 | 1.12, 1.43 |
| Black | 1.53 | 1.11, 2.11 | 1.71 | 1.33, 2.21 | 1.62 | 1.33, 1.98 | 1.42 | 0.91, 2.20 | 0.94** | 0.70, 1.27 | 1.08** | 0.84, 1.38 |
| Diabetes | ||||||||||||
| Yes | 1.51 | 1.11, 2.04 | 1.46 | 1.13, 1.89 | 1.50 | 1.23, 1.83 | 1.44 | 1.96, 2.15 | 1.03 | 0.79, 1.35 | 1.13 | 0.91, 1.40 |
| No | 1.39 | 1.15, 1.68 | 1.38 | 1.20, 1.59 | 1.38 | 1.23, 1.54 | 1.42 | 1.17, 1.72 | 1.12 | 0.96, 1.32 | 1.25 | 1.10, 1.42 |
| Years of follow-up | ||||||||||||
| 0–5 | 1.34 | 1.05, 1.70 | 1.60 | 1.36, 1.89 | 1.51 | 1.31, 1.73 | 1.50 | 1.16, 1.96 | 1.20* | 0.98, 1.48 | 1.33 | 1.13, 1.56 |
| >5–10 | 1.50 | 1.18, 1.92 | 1.10 | 0.89, 1.37 | 1.25 | 1.07, 1.47 | 1.18 | 0.89, 1.57 | 1.03 | 0.84, 1.27 | 1.08 | 0.92, 1.28 |
| hs-CRP level | ||||||||||||
| <10 mg/L | 1.43 | 1.21, 1.67 | 1.27 | 1.10, 1.46 | 1.33 | 1.20, 1.48 | 1.41 | 1.18, 1.67 | 1.12 | 0.97, 1.31 | 1.25 | 1.11, 1.40 |
| No rheumatoid arthritis or lupus | 1.38 | 1.21, 1.56 | 1.42 | 1.28, 1.57 | 1.26 | 1.02, 1.55 | 1.11 | 0.96, 1.28 | 1.23 | 1.09, 1.38 | 1.45 | 1.17, 1.80 |
Abbreviations: ARIC, Atherosclerosis Risk in Communities Study; CI, confidence interval; HR, hazard ratio; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein.
* P < 0.05 (compared with lower hs-CRP group); ** P < 0.05 (compared with lower age group).
a Results were adjusted for age, sex, race, current smoking, diabetes, hypertension, body mass index, and high-density lipoprotein cholesterol.
DISCUSSION
The present study compares the association of serum cholesterol level and CHD among persons younger and older than 65 years, stratified by level of hs-CRP. In older participants 65–75 years of age with an hs-CRP level of <2 mg/L, serum total and LDL cholesterol levels were predictive of CHD, with a magnitude of association similar to that of participants 53–65 years of age. In contrast, in older participants with an elevated hs-CRP level, total cholesterol and LDL cholesterol levels were more weakly associated with CHD than in younger participants.
Serum cholesterol level strongly predicts adverse cardiac events in the general population (1). However, data conflict with regard to the precise nature of this relationship in elderly persons. Previous studies have reported U-shaped, J-shaped, and linear associations between serum cholesterol level and cardiac events in the elderly (8, 31–34). Furthermore, some studies have reported no relationship between the two in older adults (6, 35). Competing risk factors in the elderly that might be associated with both a decline in serum cholesterol level and an increase in cardiovascular events could confound the association and in part explain the nonlinear relationships described previously. The National Cholesterol Education Program, in its Adult Treatment Panel III report (36), acknowledges that even though serum cholesterol level is less predictive in the elderly, high-risk elderly persons still benefit from cholesterol-lowering therapies. The guidelines also state that the use of other risk-assessment tools can be helpful in identifying these high-risk persons. Our findings suggest that an elevated hs-CRP level can help to identify not only elevated CHD risk but also a subgroup of elderly persons in whom cholesterol level is not predictive of CHD risk.
The reason for the attenuated relationship between serum cholesterol level and CHD in the elderly remains unclear. One possible explanation is that competing risk factors emerge with older age and subsequently diminish the predictive capability of serum cholesterol level by elevating risk at all cholesterol levels. Additionally, some comorbid conditions that are more commonly found in the elderly (e.g., heart failure, malignancy) could lead to malnutrition and lower cholesterol levels. These persons are still at increased risk of CHD, despite their lowered cholesterol level attenuating the associations (8, 37). The impact of each of these and other known and unknown comorbidities and overall frailty on the relationship between serum cholesterol level and CHD is difficult to measure, which suggests that integrative markers such as hs-CRP might be useful alternative measures.
hs-CRP is an inflammatory marker that is predictive of adverse cardiac events—a relationship that likely is explained in part by the inflammatory nature of atherosclerosis (10–12, 38). However, an elevated hs-CRP level is not specific to CHD (9) and has been observed in several other conditions, such as heart failure (19), malignancy (14–17), renal insufficiency (20), and infectious disease (18). In turn, these conditions can confer an increased risk of adverse cardiac events independent of cholesterol levels. An extreme example in which inflammation and conditions that lower cholesterol alter the association with risk is that of dialysis patients, in whom a higher cholesterol level is associated with a lower risk of death (39). However, in an analysis restricted to dialysis patients without evidence of inflammation or malnutrition, serum cholesterol level was positively associated with all-cause mortality rate (23). We propose that the attenuated relationship between serum cholesterol level and CHD in elderly persons might be related to these competing comorbidities.
ARIC is a large, multicenter cohort study representing men, women, whites, and blacks in the US population at around the age of 65 years, the age at which the cholesterol relationship attenuates. Additionally, ARIC had high follow-up rates, and centralized laboratories were used to analyze serum biomarkers. The relatively narrow age range of 65–75 years among the elderly group is a strength because the study focused on a specific group (the young-old) but also a limitation because it did not address the older old. Elevated hs-CRP level is a nonspecific marker for inflammation, and therefore it does not precisely identify which comorbidities might be responsible for the attenuated relationship between cholesterol level and CHD risk in the elderly. Nevertheless, the nonspecific nature of hs-CRP means that it could integrate all of these comorbidities into a single group, precluding the necessity of identifying them individually.
Serum cholesterol level has a similar predictive risk of CHD in younger and older persons with an hs-CRP level of <2 mg/L. However, among older participants 65–75 years of age, an elevated hs-CRP level marked a subgroup in which total and LDL cholesterol levels were weak or nonsignificant risk factors for subsequent incidence of CHD. Thus, in addition to its use as a marker of elevated risk, hs-CRP level can be useful for stratifying older adults 65–75 years of age into subgroups: 1) a subgroup with low hs-CRP levels for whom the cholesterol level–CHD risk relationship is similar to those at younger ages and 2) a subgroup with elevated hs-CRP levels in whom the cholesterol level–CHD risk relationship is markedly attenuated.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Seamus P. Whelton, Probal Roy, Brad C. Astor, Josef Coresh); Welch Center for Prevention, Epidemiology, and Clinical Research, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Seamus P. Whelton, Probal Roy, Brad C. Astor, Josef Coresh); Department of Cardiology, The Australian School of Advanced Medicine, Macquarie University Hospital, Sydney, Australia (Probal Roy); Patient Safety, AstraZeneca, Wilmington, Delaware (Lin Zhang); Section of Atherosclerosis and Vascular Medicine, Baylor College of Medicine, Houston, Texas (Ron C. Hoogeveen); Center for Cardiovascular Disease Prevention, Methodist DeBakey Heart and Vascular Center, Houston, Texas (Ron C. Hoogeveen); and Department of Medicine, Baylor College of Medicine, Houston, Texas (Christie M. Ballantyne).
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022.
We thank the staff of the Atherosclerosis Risk in Communities Study for their important contributions.
Dr. Coresh has received institutional grant support from the National Kidney Foundation, the National Institutes of Health, and Amgen, and has been a consultant to Amgen and Merck.
REFERENCES
- 1.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256(20):2823–2828. [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 4.Kronmal RA, Cain KC, Ye Z, et al. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153(9):1065–1073. [PubMed] [Google Scholar]
- 5.Zimetbaum P, Frishman WH, Ooi WL, et al. Plasma lipids and lipoproteins and the incidence of cardiovascular disease in the very elderly. The Bronx Aging Study. Arterioscler Thromb. 1992;12(4):416–423. doi: 10.1161/01.atv.12.4.416. [DOI] [PubMed] [Google Scholar]
- 6.Krumholz HM, Seeman TE, Merrill SS, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272(17):1335–1340. [PubMed] [Google Scholar]
- 7.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs D, Blackburn H, Higgins M, et al. Report of the conference on low blood cholesterol: mortality associations. Circulation. 1992;86(3):1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 9.Musunuru K, Kral BG, Blumenthal RS, et al. The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med. 2008;5(10):621–635. doi: 10.1038/ncpcardio1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 12.Tracy RP, Lemaitre RN, Psaty BM, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17(6):1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 14.Lundin E, Dossus L, Clendenen T, et al. C-reactive protein and ovarian cancer: a prospective study nested in three cohorts (Sweden, USA, Italy) Cancer Causes Control. 2009;20(7):1151–1159. doi: 10.1007/s10552-009-9330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu HM, Lin JT, Chen TH, et al. Elevation of C-reactive protein level is associated with synchronous and advanced colorectal neoplasm in men. Am J Gastroenterol. 2008;103(9):2317–2325. doi: 10.1111/j.1572-0241.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagaoka S, Yoshida T, Akiyoshi J, et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int. 2007;27(8):1091–1097. doi: 10.1111/j.1478-3231.2007.01550.x. [DOI] [PubMed] [Google Scholar]
- 17.Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24(33):5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz P, Christ-Crain M, Muller B. Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care. 2007;13(5):578–585. doi: 10.1097/MCC.0b013e3282c9ac2a. [DOI] [PubMed] [Google Scholar]
- 19.Araujo JP, Lourenco P, Azevedo A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Card Fail. 2009;15(3):256–266. doi: 10.1016/j.cardfail.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Rattazzi M, Puato M, Faggin E, et al. New markers of accelerated atherosclerosis in end-stage renal disease. J Nephrol. 2003;16(1):11–20. [PubMed] [Google Scholar]
- 21.van Durme YM, Verhamme KM, Aarnoudse AJ, et al. C-reactive protein levels, haplotypes, and the risk of incident chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179(5):375–382. doi: 10.1164/rccm.200810-1540OC. [DOI] [PubMed] [Google Scholar]
- 22.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64(4):455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 24.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 25.The ARIC Study Group. High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC) J Neuroimaging. 1991;1(2):68–73. [PubMed] [Google Scholar]
- 26.Chambless LE, Zhong MM, Arnett D, et al. Variability in B-mode ultrasound measurements in the Atherosclerosis Risk in Communities (ARIC) Study. Ultrasound Med Biol. 1996;22(5):545–554. doi: 10.1016/0301-5629(96)00039-7. [DOI] [PubMed] [Google Scholar]
- 27.Sharrett AR, Patsch W, Sorlie PD, et al. Associations of lipoprotein cholesterols, apolipoproteins A-I and B, and triglycerides with carotid atherosclerosis and coronary heart disease. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1994;14(7):1098–1104. doi: 10.1161/01.atv.14.7.1098. [DOI] [PubMed] [Google Scholar]
- 28.Roberts WL, Moulton L, Law TC, et al. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47(3):418–425. [PubMed] [Google Scholar]
- 29.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 30.Rod NH, Lange T, Andersen I, et al. Additive interaction in survival analysis: use of the additive hazards model. Epidemiology. 2012;23(5):733–737. doi: 10.1097/EDE.0b013e31825fa218. [DOI] [PubMed] [Google Scholar]
- 31.Corti MC, Guralnik JM, Salive ME, et al. Clarifying the direct relation between total cholesterol levels and death from coronary heart disease in older persons. Ann Intern Med. 1997;126(10):753–760. doi: 10.7326/0003-4819-126-10-199705150-00001. [DOI] [PubMed] [Google Scholar]
- 32.Benfante R, Reed D, Frank J. Do coronary heart disease risk factors measured in the elderly have the same predictive roles as in the middle aged? Comparisons of relative and attributable risks. Ann Epidemiol. 1992;2(3):273–282. doi: 10.1016/1047-2797(92)90060-4. [DOI] [PubMed] [Google Scholar]
- 33.Curb JD, Abbott RD, Rodriguez BL, et al. Prospective association between low and high total and low-density lipoprotein cholesterol and coronary heart disease in elderly men. J Am Geriatr Soc. 2004;52(12):1975–1980. doi: 10.1111/j.1532-5415.2004.52551.x. [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi H, Kita T, Mabuchi H, et al. Primary cardiovascular events and serum lipid levels in elderly Japanese with hypercholesterolemia undergoing 6-year simvastatin treatment: a subanalysis of the Japan Lipid Intervention Trial. J Am Geriatr Soc. 2004;52(12):1981–1987. doi: 10.1111/j.1532-5415.2004.52552.x. [DOI] [PubMed] [Google Scholar]
- 35.Corti MC, Guralnik JM, Salive ME, et al. HDL cholesterol predicts coronary heart disease mortality in older persons. JAMA. 1995;274(7):539–544. [PubMed] [Google Scholar]
- 36.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). [PubMed] [Google Scholar]
- 37.Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, et al. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350(9085):1119–1123. doi: 10.1016/s0140-6736(97)04430-9. [DOI] [PubMed] [Google Scholar]
- 38.Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baigent C, Landray MJ, Wheeler DC. Misleading associations between cholesterol and vascular outcomes in dialysis patients: the need for randomized trials. Semin Dial. 2007;20(6):498–503. doi: 10.1111/j.1525-139X.2007.00340.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.