Abstract
In a prospective cohort of nondisabled adults aged 65 years or more in the Established Populations for Epidemiologic Studies of the Elderly (1981–1987 and 1985–1992), we used a competing risk approach to predict the 5-year risk of severe, persistent activities-of-daily-living (ADLs) disability, defined as dependence in ≥3 ADLs for 2 consecutive annual interviews or for 1 interview followed by death in the subsequent year. During 5 years, 6.8% developed severe, persistent ADL dependence, and 14.6% died without severe, persistent ADL dependence in the derivation cohort (n = 8,301); the corresponding percentages were 6.8% and 15.8% in the validation cohort (n = 4,177). A model based on age, current employment, visual impairment, self-rated health, diabetes mellitus, history of stroke or brain hemorrhage, cognitive function, and self-reported physical function showed good calibration. Discrimination, assessed by C statistics, for <70, 70–74, 75–79, and ≥80 years, was 0.75, 0.74, 0.65, and 0.66 in the derivation cohort and 0.70, 0.72, 0.70, and 0.65 in the validation cohort, respectively. In conclusion, a simple risk score based on routinely available clinical information can predict severe, persistent disability in 5 years. Future studies should examine whether physical performance measures can further improve prediction in the oldest old.
Keywords: activities of daily living, aged, prognosis
Despite primary prevention efforts and therapeutic advancements, recent data in the United States suggest an expansion of life with disability, rather than compression of morbidity (1). Over 20% of older Americans reported at least 1 activity-of-daily-living (ADL) disability, and long-term-care expenditure for disabled older adults reached $135 billion in 2004 (2). With the growing interest in quality of life and health-care cost reduction, ADL dependence is a meaningful, patient-oriented outcome because individual disease–oriented outcomes often fail to capture the overall impact of multiple comorbidities on a person's function. It also predicts institutionalization (3), home service use (4), hospitalization (5), and mortality (6).
Extensive research has identified a wide range of risk factors for ADL disability, including low socioeconomic status, sensory impairment, poor self-reported health, comorbidities, cognitive and physical functional impairment, and subclinical disease. However, the translation of research into clinical prediction has been slow. Although several prognostic tools are available (7–12), they are often resource intensive or require trained assessors (7–11). Some focused on mortality (12). Moreover, previous research did not consider the dynamic nature of disability or competing events that alter the risk of disability. In the era of health-care reform, identifying individuals who are more likely to lose their ability to live independently and utilize more resources is crucial to achieve more appropriate care, cost control, and meaningful use of resources.
This study aimed to develop and validate a practical tool that predicts 5-year risk of severe, persistent loss of independence in performing ADLs in a large representative cohort of community-dwelling older adults, using information that can be easily obtained in a general practice setting.
MATERIALS AND METHODS
Study population
This study used data from the Established Populations for Epidemiologic Studies of the Elderly (EPESE), a prospective cohort study of noninstitutionalized adults, aged 65 years or more, in 4 US communities (13). The details of study design and conduct were described elsewhere (14–16). Participants underwent baseline interviews in 1981–1982, except in the North Carolina cohort (in 1985–1986), and annual follow-up interviews for 6 years. Because additional interviews beyond 6 years were conducted in selected cohorts with varying intervals, we used data up to the sixth annual follow-up. After excluding 248 participants with missing data on more than 50% of potential predictors and 1,665 participants with ADL dependence at baseline, 12,478 participants were included in this analysis. The institutional review boards at Beth Israel Deaconess Medical Center and Hebrew SeniorLife exempted this study.
Definition of ADL dependence and other outcomes
Disability in 7 ADLs was self-reported at baseline and subsequent annual interviews, using the following question: “Other than when you have been in a hospital, was there any time in the past 12 months when you needed help from some person or equipment to do … (each of the following activities)?” for walking across a small room, bathing or shower, personal grooming, dressing, eating, getting from a bed to a chair, or using the toilet. Whether the help was from another person, equipment, or both was also asked: We considered requiring personal assistance to define ADL dependence. The event of interest was severe, persistent ADL dependence, defined as requiring personal help in ≥3 ADLs for at least 2 consecutive annual interviews or for 1 interview followed by death in the subsequent year to capture prolonged, severe disability status that is comparable to requiring a significant amount of personal assistance or nursing home care. Those who died during the follow-up without severe, persistent ADL dependence were considered as having experienced the competing event (refer to “Statistical analysis” below). As our outcome was defined on the basis of ADL status in 2 consecutive annual interviews, the fifth year was the last possible time in which the outcome could occur. Our definition of disability reduces misclassification from self-report and is more strongly associated with adverse outcomes and resource utilization than other definitions (e.g., self-reported difficulty, impairment in any ADLs, or disability at a single assessment) (17–21).
Vital status was obtained through obituaries, hospitalization records, proxy interviews, and death certificates. Hospitalizations during the past 12 months were self-reported (yes/no) for selected conditions (myocardial infarction, stroke, cancer, and fracture). Assuming that each condition was associated with a distinct hospitalization, we summed the number of hospitalizations for 5 years.
Potential predictors
We chose 30 potential predictors of ADL disability that were identified from literature and collected via questionnaire and in-person assessment in the EPESE (Web Table 1 available at http://aje.oxfordjournals.org/). Multiple response categories for certain predictors were collapsed to ensure a sufficient number of events in each category, to improve interpretability, and to reduce model complexity without compromising model fit. Cognitive function was measured by using the Short Portable Mental Status Questionnaire (22), a validated instrument that comprises 10 questions on memory, orientation, current events, and a mathematical task. Cognitive function was classified as “normal” if the number of errors was ≤2; “mild” if 3 or 4; and “moderate to severe” if ≥5 (22). A cutpoint of 3 errors corresponds to the Mini-Mental Status Examination score of 23 (23). Because physical performance was measured at the sixth annual examination in the EPESE cohort, we used self-reported physical function obtained from questionnaires. These measures have been shown to be reproducible and validated against performance-based measures (24, 25).
Statistical analysis
All analyses were performed in Stata/SE 11.2 software (StataCorp LP, College Station, Texas). Two-sided P < 0.05 was considered statistically significant. The data set was randomly divided into a derivation cohort (2/3; n = 8,301) and a validation cohort (1/3; n = 4,177). Characteristics were compared between the 2 cohorts by using the t test or Wilcoxon rank-sum test for continuous variables and the Pearson χ2 test for categorical variables.
Missing data imputation
For those who had any interval missing on the number of ADL dependencies (7.4%), we imputed the missing values with the last nonmissing values for that individual carried forward. At baseline, 2.7% had missing data on more than 5 predictors. We implemented multivariable imputation that used all baseline variables, the time-to-event variable, and outcome status.
Model development
We used cause-specific proportional hazards regression to model the time to first occurrence of severe, persistent ADL dependence as a function of predictors, while accounting for death without severe, persistent ADL dependence as a competing event that prevents the occurrence of our outcome of interest (26–28). Because the exact event dates were not available, we assumed that they occurred at the midpoint between the 2 interviews.
In selecting predictors, we initially fitted a model with all 30 predictors in the derivation cohort, using severe, persistent ADL dependence as the outcome, and carried out backward elimination to find a model with the minimal Schwarz Bayesian Information Criterion (29). Because the proportional hazards assumption was violated for age categories, we fitted age-stratified Cox models. Categorical variables were modeled ordinally, if monotonic relationships existed and likelihood ratio tests favored a more parsimonious model over the model with indicator terms. Any significant interaction terms among age, sex, and other predictors that resulted in a lower Bayesian Information Criterion were retained.
Model performance
In the derivation and validation cohorts, calibration was assessed by calibration plots and the goodness-of-fit χ2 statistic (30). Discrimination was evaluated by C statistics that were modified to the context of competing risk such that individuals who failed from competing events remained in the risk set at all times (28).
Calculation of risk score
To facilitate clinical application, we developed a scoring system by assigning a score to each predictor in proportion to its regression coefficient in our final model. We assessed a potential loss in discrimination by comparing the C statistic from the risk score with that from the model. According to the distribution of the risk score in the derivation cohort, participants were classified into tertiles of risk score: low (from 0 to 7); moderate (from 8 to 15); and high (≥16). For each category, we calculated the observed absolute risk of severe, persistent ADL dependence (event of interest) and death without severe, persistent ADL dependence (competing event), as well as the risk of being free of both events in the derivation and validation cohorts. In secondary analyses, we estimated the 5-year risk of any or recurrent hospitalizations for selected conditions (myocardial infarction, stroke, cancer, and fracture), using Poisson regression with log follow-up time as the offset variable.
RESULTS
At the end of 5 years, 6.8% developed severe, persistent ADL dependence, and 14.6% died without severe, persistent ADL dependence in the derivation cohort (n = 8,301). The corresponding percentages were 6.8% and 15.8% in the validation cohort (n = 4,177). Loss to follow-up was 2.9% and 2.5%, respectively. Distributions of demographic characteristics and other predictors were similar in both cohorts (Table 1).
Table 1.
Selected Characteristics in the Derivation and Validation Cohorts, the Established Populations for Epidemiologic Studies of the Elderly, United States, 1981–1987 and 1985–1992
| Characteristics | Derivation Cohort (n = 8,301), % | Mean (SD) | Validation Cohort (n = 4,177), % | Mean (SD) |
|---|---|---|---|---|
| Age, years | ||||
| <70 | 33.9 | 33.9 | ||
| 70–74 | 28.2 | 28.2 | ||
| 75–79 | 19.3 | 19.3 | ||
| ≥80 | 18.6 | 18.6 | ||
| Male sex | 38.3 | 38.4 | ||
| African-American race | 18.9 | 18.9 | ||
| Currently working at a paying job | 13.1 | 12.8 | ||
| Read ordinary newspaper print | 90.4 | 90.8 | ||
| Self-rated health | ||||
| Excellent or good | 63.8 | 63.3 | ||
| Fair | 29.0 | 29.4 | ||
| Poor | 7.2 | 7.3 | ||
| Weight loss more than 10 poundsa in the past year | 14.8 | 14.8 | ||
| Hypertension | 48.1 | 47.8 | ||
| Diabetes mellitus | 14.8 | 14.9 | ||
| Ever had myocardial infarction | 12.8 | 12.8 | ||
| Ever had stroke or brain hemorrhage | 5.1 | 5.3 | ||
| Ever had a cancer | 13.5 | 13.6 | ||
| Ever fractured a hip | 3.1 | 3.3 | ||
| Hospitalization in the past year | 16.8 | 16.0 | ||
| Ever stayed in a nursing home as a patient | 1.8 | 1.8 | ||
| Cognitive functionb | ||||
| Normal | 82.8 | 81.7 | ||
| Mild | 13.2 | 14.0 | ||
| Moderate to severe | 4.0 | 4.3 | ||
| Able to walk half a milec without help | 80.2 | 79.0 | ||
| Able to do heavy housework | 65.6 | 64.8 | ||
| No or a little difficulty in writing or handling small objects | 96.5 | 96.4 | ||
| Body mass indexd | 25.8 (4.3) | 25.7 (4.3) |
Abbreviation: SD, standard deviation.
a One pound = 0.45 kg.
b Cognitive function was classified as “normal” if the number of errors on the Short Portable Mental Status Questionnaire was ≤2; “mild” if 3 or 4; and “moderate to severe” if ≥5 (of 9) (22).
c Half a mile or about 8 ordinary blocks.
d Body mass index: weight (kg)/height (m)2.
Model development
In the derivation cohort, most predictors were statistically significantly associated with severe, persistent ADL dependence, as expected from our large sample size. However, the model that included 8 predictors achieved the smallest Bayesian Information Criterion (Table 2). Because the model was stratified by age because of violation of the proportional hazards assumption, the association with age was not directly estimable from the model. We found that self-rated health was a stronger predictor of severe, persistent ADL dependence in younger adults, but less so in older adults (Pinteraction < 0.001). Because this interaction term improved the overall model fit by reducing the Bayesian Information Criterion, it was included in our final model (Web Table 2). There was no statistically significant interaction between sex and other predictors (Pinteraction > 0.05).
Table 2.
Cox Proportional Hazards Model for Severe, Persistent Activity-of-Daily-Living Dependence in the Derivation Cohort, the Established Populations for Epidemiologic Studies of the Elderly, United States, 1981–1987 and 1985–1992
| Predictors | Response Categories | HRa | 95% CI |
|---|---|---|---|
| Currently working at a paying job | No vs. yes | 2.08 | 1.33, 3.23 |
| Able to read ordinary newspaper print | No vs. yes | 1.44 | 1.17, 1.77 |
| Self-rated health | Per each category increase (excellent/good, fair, or poor) | 1.22 | 1.07, 1.39 |
| Diabetes mellitus | Yes vs. no | 1.38 | 1.12, 1.71 |
| Ever had stroke or brain hemorrhage | Yes vs. no | 1.74 | 1.32, 2.29 |
| Cognitive functionb | Per each category increase (normal, mild, or moderate/severe) | 1.85 | 1.64, 2.08 |
| Able to walk half a milec | No vs. yes | 1.61 | 1.33, 1.95 |
| Able to do heavy housework | No vs. yes | 1.51 | 1.24, 1.83 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Age-stratified Cox model that included all predictors; indicators for study sites were fitted for severe, persistent activity-of-daily-living dependence. Interaction terms were not included in this model to better demonstrate the associations with the main-effect terms. Refer to Web Table 2 available at http://aje.oxfordjournals.org/ for the final model with interaction terms.
b Cognitive function was classified as “normal” if the number of errors on the Short Portable Mental Status Questionnaire was ≤2; “mild” if 3 or 4; and “moderate to severe” if ≥5 (of 9) (22).
c Half a mile or about 8 ordinary blocks.
Model performance
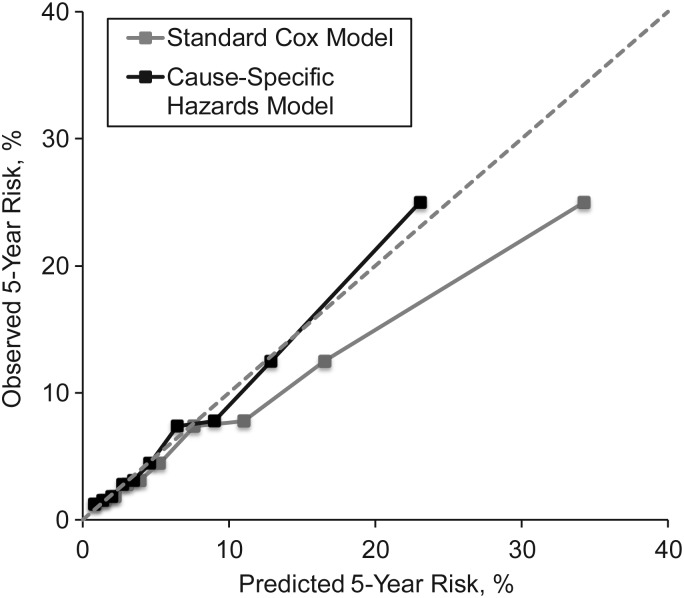
To estimate the absolute risk of severe, persistent ADL dependence, we fitted separate cause-specific Cox models (Web Table 2) and computed cumulative incidence function. There was a good agreement between the predicted risk estimated from the cause-specific hazards model and the observed risk (Figure 1 and Web Figure 1); the goodness-of-fit χ2 statistic = 5.50 (P = 0.79) in the derivation cohort, and the goodness-of-fit χ2 statistic = 3.56 (P = 0.94) in the validation cohort. However, it was evident that a standard Cox model that did not consider competing risk overestimated the risk (Figure 1).
Figure 1.
Calibration plot of a cause-specific hazards model and a standard Cox regression model for the prediction of severe, persistent activity-of-daily-living dependence in the validation cohort, the Established Populations for Epidemiologic Studies of the Elderly, United States, 1981–1987 and 1985–1992. The risk was estimated as the 5-year cumulative incidence of severe, persistent activity-of-daily-living dependence from the cause-specific hazards model that accounted for competing risk.
Discrimination was good in younger age and modest in older age: C statistics were 0.65–0.75 in the derivation cohort and 0.65–0.72 in the validation cohort (Table 3). By subgroups, the C statistic was particularly lower in those who were ≥80 years.
Table 3.
Discrimination in the Derivation and Validation Cohorts, the Established Populations for Epidemiologic Studies of the Elderly, United States, 1981–1987 and 1985–1992
| Subgroup | Derivation Cohort (n = 8,301) |
Validation Cohort (n = 4,177) |
||
|---|---|---|---|---|
| C Statistic | 95% CI | C Statistic | 95% CI | |
| By age, years | ||||
| <70 | 0.75 | 0.70, 0.81 | 0.70 | 0.61, 0.80 |
| 70–74 | 0.74 | 0.69, 0.78 | 0.72 | 0.66, 0.79 |
| 75–79 | 0.65 | 0.59, 0.70 | 0.70 | 0.62, 0.77 |
| ≥80 | 0.66 | 0.62, 0.69 | 0.65 | 0.60, 0.70 |
| By sex | ||||
| Male | 0.67 | 0.64, 0.71 | 0.71 | 0.66, 0.76 |
| Female | 0.73 | 0.71, 0.76 | 0.70 | 0.67, 0.74 |
| By race | ||||
| Non–African- American race | 0.72 | 0.69, 0.74 | 0.71 | 0.67, 0.74 |
| African- American race | 0.70 | 0.65, 0.74 | 0.70 | 0.64, 0.76 |
| By cardiovascular diseasea | ||||
| Yes | 0.67 | 0.63, 0.71 | 0.70 | 0.66, 0.75 |
| No | 0.73 | 0.71, 0.76 | 0.70 | 0.66, 0.74 |
Abbreviation: CI, confidence interval.
a Cardiovascular disease was defined as self-reported history of myocardial infarction, stroke, or diabetes mellitus.
ADL dependence, death, and hospitalization according to risk score
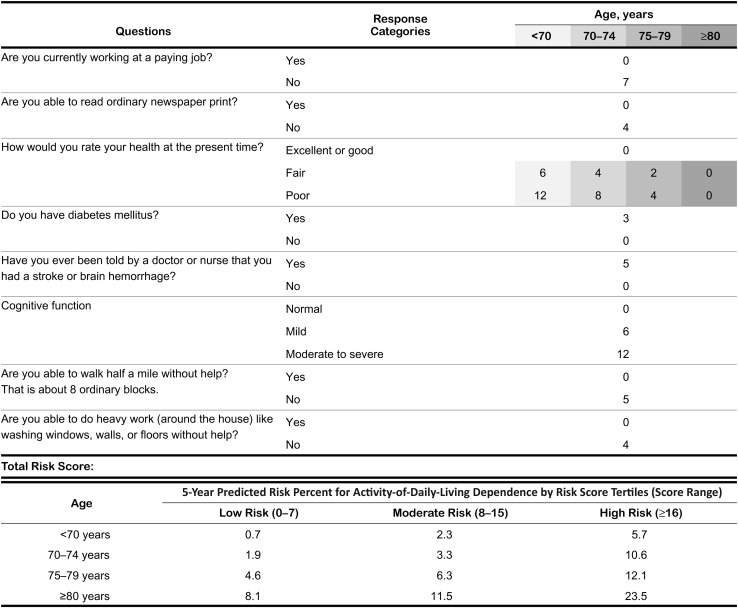
We generated an algorithm that assigned scores in proportion to regression coefficients, without a substantial loss in discrimination compared with the original model (C statistics were 0.64–0.76 in the derivation cohort and 0.66–0.71 in the validation cohort). The age-specific risk tertiles and corresponding risk of severe, persistent ADL dependence (Figure 2) are useful in assessing an individual's absolute risk as well as relative risk compared with others of similar age. For example, a man aged 73 years whose risk score is over 16 has 10.6% risk of developing severe, persistent ADL dependence in 5 years, which is approximately 3-fold higher than the risk of an average person of his age and is as high as that of an average person who is 10 years older.
Figure 2.
Score sheet to estimate 5-year risk of severe, persistent activity-of-daily-living dependence, the Established Populations for Epidemiologic Studies of the Elderly, United States, 1981–1987 and 1985–1992. The risk was estimated as the 5-year cumulative incidence of severe, persistent activity-of-daily-living dependence from the cause-specific hazards model that accounted for competing risk. Cognitive function was considered “normal” if the number of errors on the Short Portable Mental Status Questionnaire was ≤2; “mildly impaired” if 3 or 4; and “moderately to severely impaired” if ≥5 (22).
In order to provide comprehensive prognostic information, we summarized the 5-year risks of severe, persistent ADL dependence, death without severe persistent ADL dependence, and being alive without both events (Table 4). Those in the upper tertile had higher risks of severe, persistent ADL dependence, as well as death without severe, persistent ADL dependence. The risk tertiles also predicted hospitalizations for myocardial infarction, stroke, cancer, and fracture within 5 years in a dose-dependent manner, particularly among younger subgroups (Web Figure 2).
Table 4.
Risks of Severe, Persistent Activity-of-Daily-Living Dependence and Death by Age in the Derivation and Validation Cohorts, the Established Populations for Epidemiologic Studies of the Elderly, United States, 1981–1987 and 1985–1992a
| Risk Category (Risk Score) | Derivation Cohort (n = 8,301) |
Validation Cohort (n = 4,177) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. With ADL Dependence | Person-Years | Risk of ADL Dependence, % | Risk of Death Without ADL Dependence, % | Risk of Being Alive Without ADL Dependence, % | No. With ADL Dependence | Person-Years | Risk of ADL Dependence, % | Risk of Death Without ADL Dependence, % | Risk of Being Alive Without ADL Dependence, % | |
| Age <70 years | ||||||||||
| Low (0–7) | 9 | 6,453 | 0.7 | 6.0 | 93.3 | 7 | 3,163 | 1.0 | 6.8 | 92.2 |
| Moderate (8–15) | 15 | 2,971 | 2.3 | 10.2 | 87.5 | 9 | 1,644 | 2.5 | 12.9 | 84.6 |
| High (≥16) | 47 | 3,460 | 5.7 | 15.1 | 79.2 | 24 | 1,643 | 5.9 | 15.5 | 78.6 |
| Age 70–74 years | ||||||||||
| Low (0–7) | 19 | 4,527 | 1.9 | 10.1 | 88.0 | 9 | 2,291 | 1.8 | 10.3 | 87.9 |
| Moderate (8–15) | 25 | 3,328 | 3.3 | 12.6 | 84.1 | 18 | 1,612 | 4.8 | 14.5 | 80.7 |
| High (≥16) | 69 | 2,484 | 10.6 | 20.1 | 69.3 | 32 | 1,325 | 9.4 | 23.0 | 67.6 |
| Age 75–79 years | ||||||||||
| Low (0–7) | 25 | 2,456 | 4.6 | 10.8 | 84.6 | 7 | 1,239 | 2.5 | 12.4 | 85.1 |
| Moderate (8–15) | 39 | 2,622 | 6.3 | 15.7 | 78.0 | 16 | 1,249 | 5.2 | 19.5 | 75.3 |
| High (≥16) | 60 | 1,748 | 12.1 | 25.3 | 62.6 | 35 | 907 | 13.6 | 25.6 | 60.8 |
| Age ≥80 years | ||||||||||
| Low (0–7) | 31 | 1,526 | 8.1 | 17.9 | 74.0 | 16 | 837 | 8.0 | 17.1 | 74.9 |
| Moderate (8–15) | 55 | 1,812 | 11.5 | 24.4 | 64.1 | 24 | 923 | 9.6 | 28.8 | 61.6 |
| High (≥16) | 181 | 2,399 | 23.5 | 29.5 | 47.0 | 96 | 1,228 | 25.9 | 27.0 | 47.1 |
Abbreviation: ADL, activity of daily living.
a The risk was estimated as the 5-year cumulative incidence of severe, persistent activity-of-daily-living dependence and of death without severe, persistent activity-of-daily-living dependence from the cause-specific hazards model that accounted for competing risk.
DISCUSSION
In a representative population of nondisabled, community-dwelling, older adults, we developed and validated a simple risk assessment model that predicted the 5-year risk of severe, persistent ADL dependence using age, 7 self-reported risk factors, and cognitive function that can be easily obtained in a general practice setting. This model provides the absolute risks of severe, persistent ADL dependence and death without ADL dependence that can be useful in personalized prognostication and care planning. Although we recognize that the age of the data set (ADL data collected 20–25 years ago) is a major limitation for clinical use of our model for contemporary older adults, several features of our approach deserve mention and enhance future research on prediction of disability in older adults.
Comparison with existing models that predict disability
Several frailty indices and validated risk assessment models predict ADL disability in community-dwelling older adults (Web Table 3). The amount and source of information needed for risk calculation vary across the models. The frailty index (31–33), Sarkisian et al. (34), and the Vulnerable Elder Survey-13 (35, 36) screened a wide range of potential predictors, whereas the Cardiovascular Health Study index (8), the Study of Osteoporotic Fracture index (37, 38), and the Short Physical Performance Battery (9, 39) mainly focused on physical performance with or without other predictors. Although variations in outcome definitions and health status of study populations do not allow a direct comparison among these models, C statistics ranged from 0.64 to 0.76.
Compared with existing models, our model has several strengths. We estimated the absolute risk of severe, persistent ADL dependence that is meaningful to clinicians, patients, and policy makers, in the presence of competing event. Although disability is a dynamic status, the severity and duration of ADL disability have rarely been considered in previous research. In a cohort of community-dwelling older adults, approximately 80% of the newly disabled recovered independence within 12 months, and the recovery rate was lower as the severity was higher (40). Self-reported dependence was more closely linked to hospitalizations and resource use than self-reported difficulty (19). Dependence in ≥3 ADLs was associated with an over 3-fold increase in institutionalization within 2–3 years (18). Thus, our model is likely to capture the most impaired subgroup of this older population with a greater likelihood of adverse health events that can affect quality of life and drive health-care expenditure.
In building our model, we considered multiple biological and psychosocial risk factors of ADL disability to represent heterogeneous disabling processes in community-dwelling older adults. Physical performance (e.g., gait speed) predicts ADL disability, but it was not measured at the EPESE baseline. Biomarkers and imaging studies may provide additional prognostic information with incremental costs. Our objective was to develop a model based on information that can be easily obtained without additional resources and costs in a busy practice setting (e.g., interview and routine physical examination in a clinic room) for a broader application. Our model showed C statistics across subgroups that were similar to other resource-intensive models (Table 3 and Web Table 3).
Potential implications for clinical care
Physicians often feel unsure about when and how to initiate a discussion about advance care planning, particularly for older adults with multicomorbidities who do not have a terminal diagnosis nor follow a predictable functional decline (41, 42). Routine use of a prognostic tool during a primary care visit can offer an opportunity to discuss prognosis and advance care planning, regardless of the levels of predicted risk (42). Unlike existing prognostic indices based on mortality (12), our model provides more comprehensive prognostic information on the risks of both severe disability and mortality within 5 years. At the individual level, clinicians can use this information to prioritize competing health issues and reset the goals of preventive care and chronic disease management. For high-risk adults, the focus should be minimizing avoidable harms and maintaining quality of life and function; for low-risk to moderate-risk adults, the focus should be increasing life expectancy and preventing disability. Importantly, almost one third of adults aged 80 years or more are in the low-risk group. They should not be excluded from preventive care and aggressive chronic disease management, based on age alone. At the health-care system level, practice-based interventions that target these individuals may reduce hospitalizations and health-care costs.
Limitations
Our study did not examine whether using a risk model leads to better clinical outcomes. Because our goal was to predict severe disability that was comparable to nursing home care, we did not examine individual patterns of ADL disability or study causal effects of risk factors. Thus, the effect estimates from our model (Table 2) cannot be interpreted as the expected reduction in disability risk with modification of predictors. Moreover, the discriminatory ability of our model was lower in older age. In older age, C statistics from the full model with all 30 predictors were almost the same as those from our final model, suggesting that additional self-reported information is unlikely to improve discrimination. Further improvement by physical performance or biomarkers remains possible. Future studies should examine an incremental value of physical performance tests or biomarkers, using our model as a referent model.
There is mixed evidence on how the disability patterns have changed in older Americans during the past 2 decades (43, 44). The relations of socioeconomical (e.g., currently working at a paying job) or biological risk factors (e.g., diabetes mellitus or stroke) with disability might have changed over time. Therefore, recalibration of absolute risk, as well as validation in a contemporary cohort, is necessary before widespread application of our model. In addition, hospitalizations were self-reported for selected conditions that accounted for 16% of total hospitalizations among older Americans in 2009 (45). Finally, the last-observation-carried-forward method for interval missing data on ADL dependence might have underestimated the incidence of ADL dependence and discrimination of our risk model, if those who did not respond to the interview had worsening disability.
Conclusions
A practical risk assessment tool that uses routinely available clinical information can predict severe, persistent disability that has important implications for quality of life, resource utilization, and health-care expenditure. Future research should validate our model in an independent cohort, examine the additional improvement in prediction by measurements of physical performance and biomarkers, and evaluate clinical outcomes of a comprehensive program that incorporates our risk model.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Gerontology, Department of Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts (Dae Hyun Kim, Lewis A. Lipsitz); Institute for Aging Research, Hebrew SeniorLife, Boston, Massachusetts (Dae Hyun Kim, Lewis A. Lipsitz); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Dae Hyun Kim); and Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania (Anne B. Newman).
This research was supported by a John A. Hartford Foundation Center of Excellence Award to D. H. K. at Harvard Medical School (Principal Investigator: Lewis Lipsitz). D. H. K. is also supported by the Charles A. King Trust Postdoctoral Fellowship from the Charles A. King Trust, Bank of America, N.A., Co-Trustee. L. A. L. is supported by National Institutes of Health grant AG025037 and holds the Irving and Edyth S. Usen and Family Chair in Geriatric Medicine at Hebrew SeniorLife. A. B. N. is supported by National Institutes of Health grants AG023629 and AG024827.
An earlier version of this work was presented at the Presidential Poster Session of the American Geriatrics Society Annual Meeting, Seattle, Washington, May 3, 2012.
The funding sources played no role in the study design or conduct, data management or analysis, or the decision to submit the manuscript for publication.
Conflict of interest: none declared.
REFERENCES
- 1.Crimmins EM, Beltran-Sanchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66(1):75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Congressional Budget Office. Washington, DC: Congressional Budget Office; 2004. Financing long-term care for the elderly http://www.cbo.gov/ftpdocs/54xx/doc5400/04-26-LongTermCare.pdf. (Accessed October 20, 2011) [Google Scholar]
- 3.Branch LG, Jette AM. A prospective study of long-term care institutionalization among the aged. Am J Public Health. 1982;72(12):1373–1379. doi: 10.2105/ajph.72.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soldo BJ, Manton KG. Health status and service needs of the oldest old: current patterns and future trends. Milbank Mem Fund Q Health Soc. 1985;63(2):286–319. [PubMed] [Google Scholar]
- 5.Branch L, Jette A, Evashwick C, et al. Toward understanding elders’ health service utilization. J Community Health. 1981;7(2):80–92. doi: 10.1007/BF01323227. [DOI] [PubMed] [Google Scholar]
- 6.Manton KG. A longitudinal study of functional change and mortality in the United States. J Gerontol. 1988;43(5):S153–S161. doi: 10.1093/geronj/43.5.s153. [DOI] [PubMed] [Google Scholar]
- 7.Tinetti ME, Inouye SK, Gill TM, et al. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 10.Balzi D, Lauretani F, Barchielli A, et al. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010;39(1):92–98. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempen GI, Ormel J. The impact of physical performance and cognitive status on subsequent ADL disability in low-functioning older adults. Int J Geriatr Psychiatry. 1998;13(7):480–483. doi: 10.1002/(sici)1099-1166(199807)13:7<480::aid-gps805>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 12.Yourman LC, Lee SJ, Schonberg MA, et al. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor JO, Wallace RB, Ostfeld AM, et al. Ann Arbor, MI: Inter-university Consortium for Political and Social Research (distributor); 1998. Established Populations for Epidemiologic Studies of the Elderly, 1981–1993: [East Boston, Massachusetts, Iowa and Washington counties, Iowa, New Haven, Connecticut, and north central North Carolina] [computer file]. ICPSR09915-v3 (doi:10.3886/ICPSR09915) [Google Scholar]
- 14.Cornoni-Huntley J, Ostfeld AM, Taylor JO, et al. Established populations for epidemiologic studies of the elderly: study design and methodology. Aging (Milano) 1993;5(1):27–37. doi: 10.1007/BF03324123. [DOI] [PubMed] [Google Scholar]
- 15.Cornoni-Huntley J, Brock DB, Ostfeld AM, et al. Established Populations for Epidemiologic Studies of the Elderly: Resource Data Book. Bethesda, MD: National Institute on Aging; 1986. [Google Scholar]
- 16.Cornoni-Huntley J, Brock DB, Ostfeld AM, et al. Established Populations for Epidemiologic Studies of the Elderly: Resource Data Book, 2. Bethesda, MD: National Institute on Aging; 1990. [Google Scholar]
- 17.Ferrucci L, Guralnik JM, Simonsick E, et al. Progressive versus catastrophic disability: a longitudinal view of the disablement process. J Gerontol A Biol Sci Med Sci. 1996;51(3):M123–M130. doi: 10.1093/gerona/51a.3.m123. [DOI] [PubMed] [Google Scholar]
- 18.Gaugler JE, Duval S, Anderson KA, et al. Predicting nursing home admission in the U.S: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill TM, Robison JT, Tinetti ME. Difficulty and dependence: two components of the disability continuum among community-living older persons. Ann Intern Med. 1998;128(2):96–101. doi: 10.7326/0003-4819-128-2-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Pearlman DN, Crown WH. Alternative sources of social support and their impacts on institutional risk. Gerontologist. 1992;32(4):527–535. doi: 10.1093/geront/32.4.527. [DOI] [PubMed] [Google Scholar]
- 21.Steinbach U. Social networks, institutionalization, and mortality among elderly people in the United States. J Gerontol. 1992;47(4):S183–S190. doi: 10.1093/geronj/47.4.s183. [DOI] [PubMed] [Google Scholar]
- 22.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 23.Hooijer C, Dinkgreve M, Jonker C, et al. Short screening tests for dementia in the elderly population. I. A comparison between AMTS, MMSE, MSQ and SPMSQ. Int J Geriatr Psychiatry. 1992;7(8):559–571. [Google Scholar]
- 24.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38(10):1105–1112. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21(4):556–559. doi: 10.1093/geronj/21.4.556. [DOI] [PubMed] [Google Scholar]
- 26.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 27.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6(2):461–464. [Google Scholar]
- 30.D'Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, Rao CR, editors. Handbook of Statistics. Vol 23. Amsterdam, the Netherlands: Elsevier BV; 2004. pp. 1–25. [Google Scholar]
- 31.Jones DM, Song X, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc. 2004;52(11):1929–1933. doi: 10.1111/j.1532-5415.2004.52521.x. [DOI] [PubMed] [Google Scholar]
- 32.Rockwood K, Mitnitski A, Song X, et al. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitnitski AB, Song X, Rockwood K. The estimation of relative fitness and frailty in community-dwelling older adults using self-report data. J Gerontol A Biol Sci Med Sci. 2004;59(6):M627–M632. doi: 10.1093/gerona/59.6.m627. [DOI] [PubMed] [Google Scholar]
- 34.Sarkisian CA, Liu H, Gutierrez PR, et al. Modifiable risk factors predict functional decline among older women: a prospectively validated clinical prediction tool. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48(2):170–178. doi: 10.1111/j.1532-5415.2000.tb03908.x. [DOI] [PubMed] [Google Scholar]
- 35.Min L, Yoon W, Mariano J, et al. The Vulnerable Elders-13 Survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 37.Ensrud KE, Ewing SK, Cawthon PM, et al. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;57(3):492–498. doi: 10.1111/j.1532-5415.2009.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 39.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 40.Hardy SE, Gill TM. Recovery from disability among community-dwelling older persons. JAMA. 2004;291(13):1596–1602. doi: 10.1001/jama.291.13.1596. [DOI] [PubMed] [Google Scholar]
- 41.Schonfeld TL, Stevens EA, Lampman MM, et al. Assessing challenges in end-of-life conversations with elderly patients with multiple morbidities. Am J Hosp Palliat Care. 2012;29(4):260–267. doi: 10.1177/1049909111418778. [DOI] [PubMed] [Google Scholar]
- 42.Smith AK, Williams BA, Lo B. Discussing overall prognosis with the very elderly. N Engl J Med. 2011;365(23):2149–2151. doi: 10.1056/NEJMp1109990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manton KG, Gu X, Lowrimore GR. Cohort changes in active life expectancy in the U.S. elderly population: experience from the 1982–2004 National Long-Term Care Survey. J Gerontol B Psychol Sci Soc Sci. 2008;63(5):S269–S281. doi: 10.1093/geronb/63.5.s269. [DOI] [PubMed] [Google Scholar]
- 44.Seeman TE, Merkin SS, Crimmins EM, et al. Disability trends among older Americans: National Health and Nutrition Examination Surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100(1):100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Center for Health Statistics, Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. Health data interactive http://www.cdc.gov/nchs/hdi.htm. (Accessed December 31, 2011) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.