Abstract
Chronic wasting disease (CWD) is a prion disease of captive and free-ranging deer (Odocoileus spp), elk (Cervus elaphus nelsonii) and moose (Alces alces shirasi). Unlike in most other prion diseases, in CWD prions are shed in urine and feces, which most likely contributes to the horizontal transmission within and between cervid species. To date, CWD ante-mortem diagnosis is only possible by immunohistochemical detection of protease resistant prion protein (PrPSc) in tonsil or recto-anal mucosa-associated lymphoid tissue (RAMALT) biopsies, which requires anesthesia of animals. We report on detection of CWD prions in urine collected from pre-symptomatic deer and in fecal extracts by using real time quaking-induced conversion (RT-QuIC). This assay can be useful for non-invasive pre-symptomatic diagnosis and surveillance of CWD.
Keywords: prion, chronic wasting disease, diagnosis, surveillance, RT-QuIC, urine, feces
Introduction
Chronic wasting disease (CWD) is to date the most contagious prion disease and affects captive and free-ranging elk, deer and moose in North America.1,2 The disease is caused by the accumulation of an abnormally folded isoform of the cellular prion protein PrPc, denominated PrPSc.3,4 CWD is the cervid equivalent of bovine spongiform encephalopathy (BSE), scrapie in sheep and goat5 or Creutzfeldt-Jakob disease (CJD) in humans.6 Although transmission studies of CWD prions to humanized transgenic mice or non-human primates suggest a strong species barrier,7-9 recent in vitro studies have demonstrated that human PrP can be converted by CWD prions into PrPSc upon adaptation.10 Therefore, a potential for zoonotic transmission, as exemplified by BSE,11 cannot be completely excluded.
A huge body of evidence suggests that CWD can be efficiently transmitted horizontally within and between cervid species,12 which may be the reason for geographical spread and increase in case numbers. Horizontal transmission is explained by the rather unusual peripheral distribution of prions in CWD affected animals and the high susceptibility to the disease by oral infection.13,14 Unlike in most other prion diseases, CWD prions can be found in a wide variety of tissues, such as skeletal and cardiac muscle15,16 or kidney,17 in addition to the lymphoreticular system and blood.18 Furthermore, they are shed in significant amounts in saliva,18,19 urine19 or feces,20 which enables oral infection of animals by foraging on contaminated pastures. In addition, it has been demonstrated that prions can persist in soil21 and that water in endemic areas can contain CWD-associated PrPSc 22.
Currently, disease surveillance is mainly based on testing hunter harvested animals. Since this testing is not obligatory, it depends on the compliance of hunters. CWD test systems are based on the detection of proteinase K resistant PrPSc, either by immunoblot, ELISA or immunohistochemistry.22 The main materials used for this are brain stem homogenates and tonsil or rectoanal mucosa-associated lymphoid tissue (RAMALT) biopsies.23,24 Therefore, intra vitam diagnosis is only possible by invasive methods that require anesthesia of animals. Ante-mortem and, ideally, pre-symptomatic detection of CWD prions in specimens that can be easily obtained without the necessity of anesthetizing animals is highly desirable in order to simplify diagnosis and surveillance. In vitro methods such as protein misfolding cyclic amplification (PMCA)25 or quaking-induced conversion (QuIC)26-28 assay have been proven very useful for sensitive detection of prions in various samples of different species. Using real time (RT-) QuIC, detection of prion amounts as low as 1 fg in cerebrospinal fluid is possible.26 The assay is based on monitoring the incorporation of the amyloid dye Thioflavin T into fibrils of newly converted recombinant PrP seeded by prions or PrPSc contained in the sample.
Since RT-QuIC offers advantages over PMCA, e.g., it does not require sonication, we have chosen this method for testing its usefulness in the detection of CWD prions in deer urine and feces. We demonstrate that CWD prions are detectable in urine of orally infected deer prior to the onset of clinical symptoms. Furthermore, we show that fecal extracts can be used as a seed in RT-QuIC assays. Thereby, we were able to detect CWD prions in fecal extracts collected at later stages of the disease. This study provides the first evidence that RT-QuIC can be successfully used for the preclinical diagnosis of CWD in specimens that are available by non-invasive methods.
Results
Detection of CWD in tissues or body fluids that are easily available and do not require invasive methods is highly desirable and would enable improved surveillance of the disease in free-ranging cervids. Therefore, our aim was to adapt the RT-QuIC assay for detection of CWD prions in deer urine and feces.
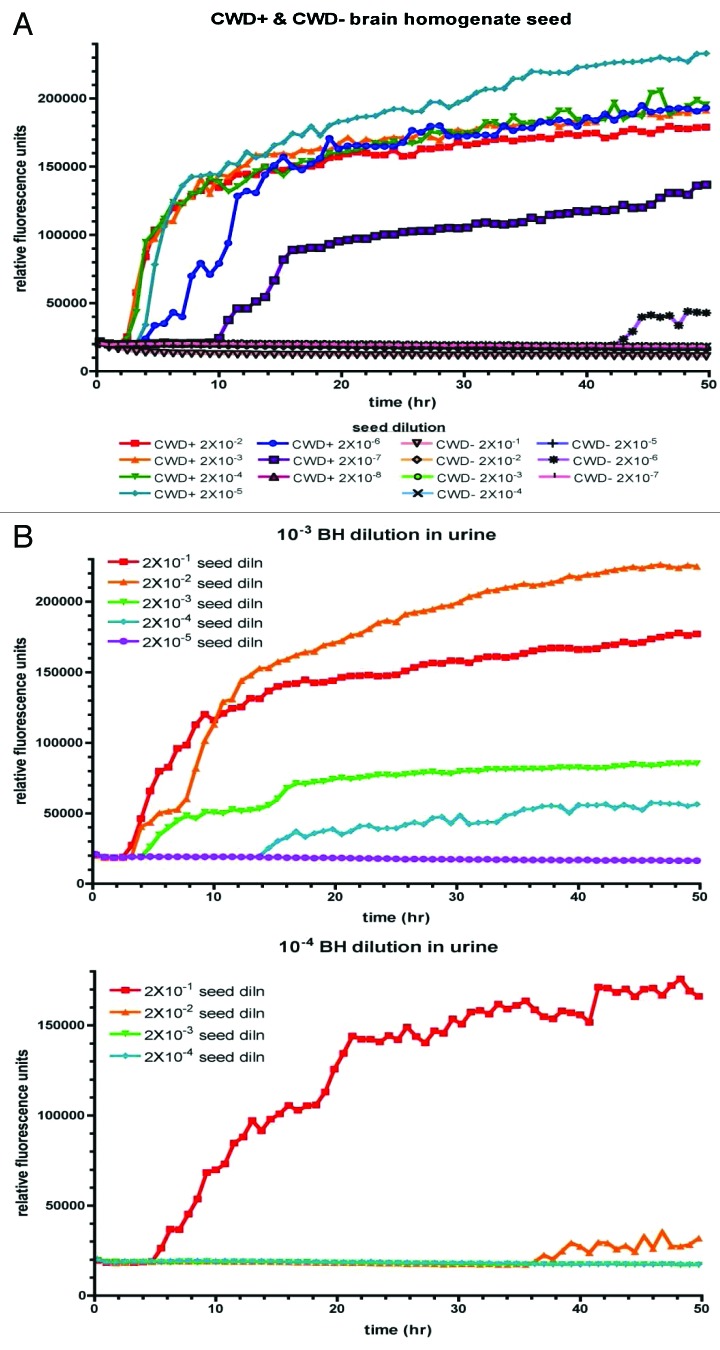
At first, we analyzed the sensitivity of detection of CWD prions using infected brain homogenate as a seed and recombinant cervid PrP as a substrate. Serial 10-fold dilutions of infected (CWD+) and non-infected (CWD-) brain homogenates (10%) were prepared and RT-QuIC reactions were seeded with these dilutions (Fig. 1A). For the lower dilutions, incorporation of Thioflavin T indicative for prion conversion was detected already after approximately 2 h. Conversion was detectable up to a brain homogenate dilution of 2 × 10−7, whereas the 2 × 10−8 dilution remained negative. RT-QuIC assays of serial dilutions of non-infected brain homogenates resulted in a base line signal, except for very little spontaneous conversion for the 10−6 dilution at a late reaction time point.
Figure 1. Sensitive detection of CWD prions by RT-QuIC. (A) RT-QuIC reactions using cervid PrP as a substrate were seeded with serial dilutions of brain homogenate (10%) derived from a CWD-infected (CWD+) or non-infected (CWD-) mule deer. (B) Ten % CWD infected brain homogenate was diluted 10−3 or 10−4 with urine from a CWD-negative deer. Serial dilutions thereof were used to seed quadruplicate RT-QuIC reactions using cervid recombinant PrP as a substrate. The y-axis shows the mean ThT fluorescence. The x-axis depicts the reaction time.
In order to verify whether detection is inhibited when CWD prions are present in urine, we performed spiking experiments. CWD positive brain homogenate was serially diluted in deer urine derived from a CWD negative animal, with dilutions ranging between 10−1 and 10−4. Each sample was used as a seed in RT-QuIC and an endpoint titration was performed. In Figure 1B, the results for the 10−3 and 10−4 brain homogenate dilutions in urine are depicted. The sensitivity of detection is similar to that in brain homogenate, with an overall detection limit of 2 × 10−7 in the 10−3 brain homogenate dilution (upper panel) and 2 × 10−6 for the 10−4 brain homogenate dilution (lower panel), respectively.
Altogether, these data demonstrate that CWD prions can be detected in both brain homogenate and urine. The sensitivity does not differ significantly from that of detection in brain homogenate, indicating that urine does not or not significantly inhibit RT-QuIC reactions.
Next we were interested in whether it is possible to detect CWD prions in urine samples of orally infected mule deer or white-tailed deer.14 Depending on the genotype at codon 96, white-tailed deer in this study were tested positive for CWD prions in tonsil biopsies as early as 8.4 mo post infection (96GG and 96GS), whereas animals with the genotype 96SS remained negative until > 11.4 mo post infection. Clinical symptoms were observed at the earliest at 17.2 mo post infection in the 96GG animals, both 96GS and 96SS animals exhibited longer incubation times of the disease. Mule deer showed clinical signs between 16.2 and 25.9 mo post inoculation.14 Samples of two animals (W804; mule deer and W1004; white-tailed deer) collected 5, 13 and 16 mo post oral inoculation, respectively, were chosen for analysis. Notably, all samples were collected at a pre-symptomatic stage of the disease. A 2 × 10−1 dilution was used to seed RT-QuIC reactions, recombinant cervid PrP was employed as a substrate. Urine of three different non-infected deer served as a negative control, and these samples did not induce conversion (Fig. 2, #1 and #2 and #2 and #3, respectively). However, samples of both infected animals taken at 13 and 16 mo post inoculation were positive, whereas urine collected five months post inoculation did not contain detectable amounts of seed. In animal W804 (Fig. 2, upper panel), the conversion reaction started after approximately 18 h for the 16 mo sample and after approximately 30 h for the 13 mo sample, indicating that the seeding activity in the 16 mo sample was higher than in that collected 13 mo post inoculation. In the samples of animal W1004 (Fig. 2, lower panel), conversion started after 10 h in the 13 mo sample and after approximately 28 h in the 16 mo sample, respectively.
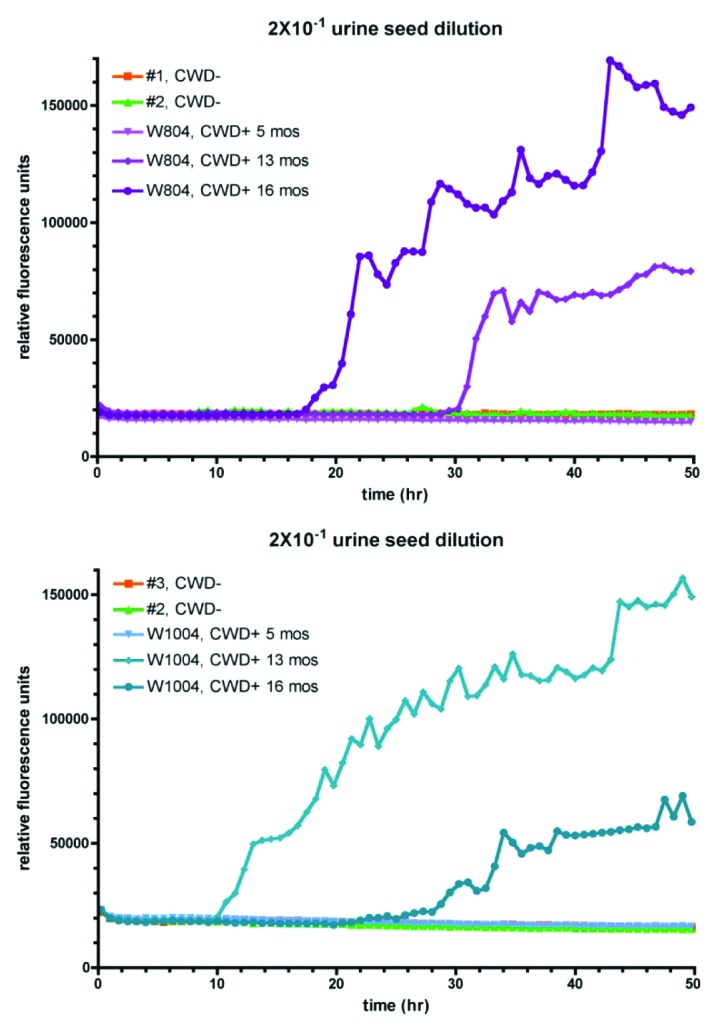
Figure 2. Detection of CWD prions in urine of pre-clinical deer. Urine of orally infected animals (W804 = mule deer; W1004 = white-tailed deer) collected 5, 13 and 16 mo post infection, respectively, was diluted 20-fold in RT-QuIC buffer and was then used to seed RT-QuIC reactions as described using cervid PrP as a substrate. Urine of non-infected animals served as a negative control.
In summary, we demonstrate that CWD prions can be detected by RT-QuIC in urine of orally infected white-tailed deer and mule deer at a pre-symptomatic stage of the disease.
Since urine might be difficult to collect from free-ranging animals, we were interested in whether it is possible to use feces extracts as a seed in RT-QuIC reactions. Fecal samples can be easily collected, both from captive and free-ranging animals, and testing of such samples, even if they cannot be assigned to a certain individual animal, might add valuable information about the distribution of CWD in certain areas or the occurrence of new cases in areas that were assumed to be free of CWD. To this end, fecal extracts of animal W1004, collected 20 or 30 mo post inoculation were prepared. RT-QuIC reactions were seeded with either undiluted extract or a 2 × 10−1 dilution thereof. Feces extracts of non-infected animals were used as a negative control (Fig. 3). Although no seeding activity was detectable in the 20 mo sample, both dilutions of the 30 mo sample were positive. However, conversion started late even in the undiluted extracts compared with the results of the urine or brain homogenate samples, which is indicative for low sensitivity.
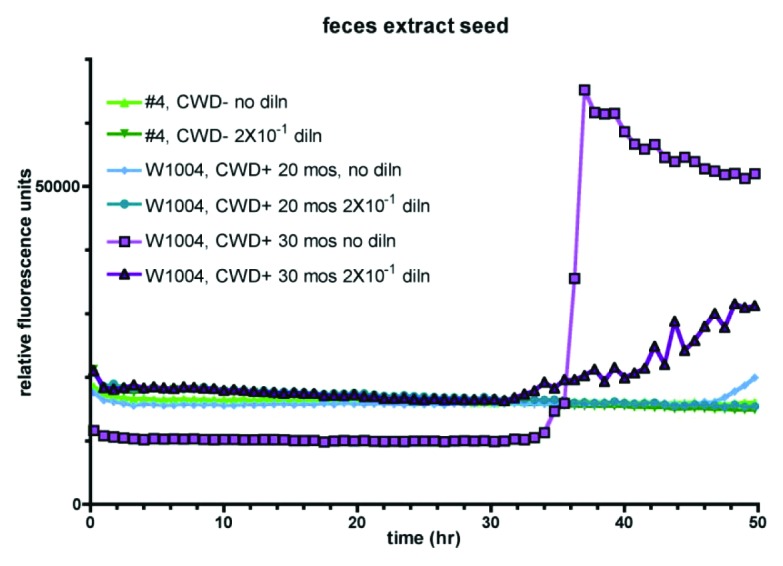
Figure 3. CWD prions are detectable in feces by RT-QuIC. Extracts of white-tailed deer (W1004) feces collected at 20 or 30 mo post oral inoculation were seeded either undiluted or in a 2 × 10−1 dilution into quadruplicate reactions. Fecal extracts from non-infected deer served as a negative control.
Nevertheless, we provide strong evidence that it is possible to detect CWD prions in feces of orally inoculated mule deer by RT-QuIC assay.
Discussion
With this study, we provide proof of concept that RT-QuIC is a highly useful method for the detection of CWD prions in urine and feces of orally infected deer. Of note, we were able to seed conversion reactions using urine collected at an early presymptomatic stage of the disease. Fecal extracts that were tested positive were taken at stages of the disease when clinical symptoms already might have occurred.
It has been shown previously that RT-QuIC can be used for the detection of CWD prions using full-length cervid PrP as a substrate.27 In this study, a 10-6.3 dilution of a 10% brain homogenate was used to optimize the NaCl concentration in the reaction buffer. In our endpoint titration experiment (Fig. 1A) the detection limit was reached at a dilution of 2 × 10−7 of a 10% brain homogenate, with a NaCl concentration of 300 mM. A similar sensitivity of detection was observed in our spiking experiments. Here, the detection limit was an overall dilution of 2 × 10−7, which is the combination of a 10−3 brain homogenate dilution in urine, and the further 2 × 10−4 dilution in RT-QuIC buffer, indicating that urine does not negatively influence the detection of seeding activity. Spontaneous conversion was only observed in the 2 × 10−6 dilution after approximately 42 h when reactions were seeded with negative brain homogenates and in the spiking experiment (Fig. 1B, lower panel) in an overall brain homogenate dilution of 2 × 10−6 upon a reaction time of approximately 36 h. These results indicate that at dilutions higher than 2 × 10−5 positive results have to be carefully evaluated since spontaneous conversion might occur.
Conversion efficiency of the cervid PrP substrate might be negatively influenced by differences in the primary structure of the seed due to polymorphisms. In order to confirm that differences between the primary structures of seed and substrate are tolerated we used recombinant mouse PrP as a substrate and obtained similar sensitivity and reaction kinetics as with cervid PrP (data not shown).
When using deer urine for seeding RT-QuIC, samples from both infected animals collected at 13 and 16 mo post oral inoculation resulted in positive signals, whereas the 5 mo sample and urine from non-infected deer were negative. However, collection time points of 13 and 16 mo are still in the pre-symptomatic stage of disease, since mule deer developed clinical signs between 16.2 and 25.9 mo post oral inoculation and white-tailed deer even later with the earliest signs 17.2 mo post inoculation.14 Until now, infectivity in urine of CWD infected animals has been determined by PMCA or bioassays using transgenic cervidized mice,19 however, in this study only urine from terminally sick white-tailed deer has been analyzed.19 When PMCA was combined with a highly sensitive immunoassay (surround optical fiber immunoassay; SOFIA), prion-disease associated seeding activity was found in white-tailed deer urine collected approximately 30 mo (891 d) post inoculation,29 which is still later in the course of the disease, and some of the samples used were derived from animals that already were clinically sick at this time point. Notably, the samples used by Rubenstein et al.29 were derived from the same experimental inoculation study as the samples we have used.14 However, our white-tailed deer sample was collected at a much earlier time point post inoculation, and even more important, seeding activity was detected in a single assay within 48 h.
Finally, we analyzed whether we are able to seed RT-QuIC reactions with fecal extracts. Unfortunately, we only had access to two samples of one white-tailed deer (W1004) which were taken 20 and 30 mo post inoculation, respectively. Only the 30 mo sample which was most likely collected already at a clinical stage of the disease gave a positive signal when used undiluted for seeding. Infectivity specific for CWD has been detected previously by bioassays using cervidized mice for intracerebral infection with γ-irradiated fecal homogenates derived from mule deer in a preclinical stage of disease.20 Furthermore, PMCA was successfully used to detect CWD prions in feces from free-ranging elk with high sensitivity.30 However, although sensitivity of detection certainly has to be improved, we provide first evidence that RT-QuIC assay can be used to detect seeding activity in fecal samples.
Overall, we demonstrate that RT-QuIC can be used for the pre-symptomatic diagnosis of CWD in urine. After improving sensitivity, e.g., by adaptation of the eQuIC protocol31,32 or by paramagnetic nanoparticle capture,33 the detection of seeding activity in fecal samples can be a versatile tool for simplifying CWD surveillance and diagnosis.
Materials and Methods
Brain homogenate preparation
A section of brain stem from a CWD+ or CWD- mule deer (Odocoileus hemionus) was excised, weighed, then transferred to a Dounce homogenizer. PBS (20 mM sodium phosphate [pH 7.4], 130 mM NaCl) was added to 10% (w/v) and the tissue was homogenized first with the loose, then the tight plunger. Homogenates were stored at -80°C.
Source of urine and feces
Deer urine and feces of known CWD status were collected from captive animals involved in a study in which white-tailed (Odocoileus virginianus) and mule deer (Odocoileus hemionus) were orally inoculated with CWD+ brain homogenate followed by monthly collection of blood, saliva, urine and feces (14). A herd of captive mule deer in Pullman, WA (a non-CWD endemic area) served as the source of CWD negative samples. After collection, samples were stored frozen.
Preparation of deer feces extracts
Single pellets of deer feces were weighed, then placed into 8 ml polypropylene bottles (Nalgene). Buffer (20 mM sodium phosphate, pH 7.1, 130 mM NaCl, 0.05% (v/v) Tween 20, 1 mM PMSF, 1X Roche Complete Protease Inhibitors [Cat# 11–697–498–001]) was added to 20% (w/v) final concentration. Bottles were placed onto a platform rotary shaker at ~30° angle and were shaken gently for one hour at room temperature. About 1.8 ml of solution was then transferred to a 2.0 ml screw cap microcentrifuge tube and insoluble debris was pelleted by centrifugation at 18,000Xg for 5 min at room temperature. Supernatants were transferred to fresh 2.0 ml screw cap microcentrifuge tubes and the samples were stored frozen.
Purification of recombinant prion protein
A plasmid containing a DNA sequence coding for a cervid prion protein (residues 24–234, accession AF156185; described in27) was a generous gift from Dr Byron Caughey (NINDS, NIH, Rocky Mountain Laboratories). Recombinant PrP was expressed in E. coli Rosetta DE3 and purified as described.27 In brief, cells were grown for 22–24 h in LB media supplemented with Overnight Express Autoinduction System 1 (EMD Cat# 71300–4). Harvested cells were subjected to 2 freeze/thaw cycles in liquid nitrogen and the cell paste was stored at -20°C. Inclusion bodies containing rPrP were prepared from the cell paste using BugBuster Master Mix (EMD Cat# 71456–3) to lyse the cells. Inclusion bodies were then washed twice with 0.1X BugBuster Master Mix, pelleted by centrifugation and stored at -20°C. Proteins from the inclusion bodies were denatured in 8 M guanidine-HCl, then batch-bound to Ni-NTA Superflow resin (Qiagen Cat# 30430) that had been pre-equilibrated in denaturing buffer (100 mM sodium phosphate, 10 mM Tris, 6 M guanidine-HCl [pH 8.0]). This was poured into an AKTA XK-16 column and attached to an AKTA Explorer chromatography system for protein purification at room temperature. Recombinant PrP was refolded on the column using a linear gradient of 100 mM sodium phosphate, 10 mM Tris (pH 8.0), then eluted using another linear gradient of 100 mM sodium phosphate, 10 mM Tris, 500 mM imidazole (pH 5.8). Eluted protein was collected in 2 ml fractions; 2 ml dialysis buffer (10 mM sodium phosphate [pH 5.8]) was previously added to the collection tubes to dilute the product. Fractions containing rPrP were pooled, filtered through 0.22 µm, then added to SnakeSkin dialysis tubing (7000 MWCO, Thermo Scientific Cat# 68700) for overnight dialysis at 4°C. Following dialysis, the sample was again filtered through 0.22 µm and protein concentration was determined using a Pierce BCA Protein Assay Kit (Thermo Scientific Cat# 23227). Aliquots of purified rPrP were stored at -80°C.
RT-QuIC assay
Real time QuIC was performed as described (27). Briefly, reactions were set up in a buffer containing 20 mM sodium phosphate (pH 6.9), 300 mM NaCl, 1 mM EDTA, 10 µM Thioflavin T, 0.1 mg/ml rPrP substrate. A reaction cocktail was prepared and 98 µl aliquots were added to the wells of a 96 well optical bottom plate (Nalge Nunc International Cat# 265301). Quadruplicate reactions were seeded with 2 µl of brain homogenate, urine or feces extract that were diluted in 20 mM sodium phosphate (pH 6.9), 130 mM NaCl, 0.1% (w/v) SDS, 1X N2 Supplement (Invitrogen Cat# 17502048); note that the final detergent concentration in each reaction was 0.002%. The plate was sealed with with Nunc Amplification Tape (Nalge Nunc International Cat# 232702) and placed in a BMG Labtech FLUOstar Omega fluorescence plate reader that was pre-heated to 42°C. A program of 1 min rest followed by 1 min shaking (700 rpm, double orbital) with fluorescence readings (450 nm excitation, 480 nm emission) every 15 min was run. This cycling was allowed to continue for 50 h (200 readings). Data was plotted as the average of quadruplicate reactions using every third time point (45 min) with GraphPad Prism software.
Acknowledgments
We are grateful to Dr Byron Caughey (NINDS, NIH, Rocky Mountain Laboratories, Hamilton, MT) for enabling training of TRJ and for providing the expression plasmid encoding cervid PrP. This work was supported by the Alberta Prion Research Institute (APRI) grant 20080238.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/prion/article/24430
References
- 1.Williams ES, Young S. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and elk (Cervus elaphus nelsoni) Vet Pathol. 1993;30:36–45. doi: 10.1177/030098589303000105. [DOI] [PubMed] [Google Scholar]
- 2.Gilch S, Chitoor N, Taguchi Y, Stuart M, Jewell JE, Schätzl HM. Chronic wasting disease. Top Curr Chem. 2011;305:51–77. doi: 10.1007/128_2011_159. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 4.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Windl O, Dawson M. Animal prion diseases. Subcell Biochem. 2012;65:497–516. doi: 10.1007/978-94-007-5416-4_18. [DOI] [PubMed] [Google Scholar]
- 6.Watts JC, Balachandran A, Westaway D. The expanding universe of prion diseases. PLoS Pathog. 2006;2:e26. doi: 10.1371/journal.ppat.0020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, et al. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci. 2005;25:7944–9. doi: 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamgüney G, Giles K, Bouzamondo-Bernstein E, Bosque PJ, Miller MW, Safar J, et al. Transmission of elk and deer prions to transgenic mice. J Virol. 2006;80:9104–14. doi: 10.1128/JVI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandberg MK, Al-Doujaily H, Sigurdson CJ, Glatzel M, O’Malley C, Powell C, et al. Chronic wasting disease prions are not transmissible to transgenic mice overexpressing human prion protein. J Gen Virol. 2010;91:2651–7. doi: 10.1099/vir.0.024380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barria MA, Telling GC, Gambetti P, Mastrianni JA, Soto C. Generation of a new form of human PrP(Sc) in vitro by interspecies transmission from cervid prions. J Biol Chem. 2011;286:7490–5. doi: 10.1074/jbc.M110.198465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasmézas CI, Deslys JP, Demaimay R, Adjou KT, Lamoury F, Dormont D, et al. BSE transmission to macaques. Nature. 1996;381:743–4. doi: 10.1038/381743a0. [DOI] [PubMed] [Google Scholar]
- 12.Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech. 2002;21:305–16. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–64. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 14.Miller MW, Wolfe LL, Sirochman TM, Sirochman MA, Jewell JE, Williams ES. Survival patterns in white-tailed and mule deer after oral inoculation with a standardized, conspecific prion dose. J Wildl Dis. 2012;48:526–9. doi: 10.7589/0090-3558-48.2.526. [DOI] [PubMed] [Google Scholar]
- 15.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, et al. Prions in skeletal muscles of deer with chronic wasting disease. Science. 2006;311:1117. doi: 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 16.Jewell JE, Brown J, Kreeger T, Williams ES. Prion protein in cardiac muscle of elk (Cervus elaphus nelsoni) and white-tailed deer (Odocoileus virginianus) infected with chronic wasting disease. J Gen Virol. 2006;87:3443–50. doi: 10.1099/vir.0.81777-0. [DOI] [PubMed] [Google Scholar]
- 17.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol. 2011;85:6309–18. doi: 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–6. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 19.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, et al. Asymptomatic deer excrete infectious prions in faeces. Nature. 2009;461:529–32. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, Pedersen JA. Prions adhere to soil minerals and remain infectious. PLoS Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, et al. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion. 2009;3:171–83. doi: 10.4161/pri.3.3.9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane DP, Barr DJ, Keller JE, Hall SM, Langenberg JA, Bochsler PN. Comparison of retropharyngeal lymph node and obex region of the brainstem in detection of chronic wasting disease in white-tailed deer (Odocoileus virginianus) J Vet Diagn Invest. 2008;20:58–60. doi: 10.1177/104063870802000110. [DOI] [PubMed] [Google Scholar]
- 24.Wild MA, Spraker TR, Sigurdson CJ, O’Rourke KI, Miller MW. Preclinical diagnosis of chronic wasting disease in captive mule deer (Odocoileus hemionus) and white-tailed deer (Odocoileus virginianus) using tonsillar biopsy. J Gen Virol. 2002;83:2629–34. doi: 10.1099/0022-1317-83-10-2629. [DOI] [PubMed] [Google Scholar]
- 25.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–3. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 26.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011;17:175–8. doi: 10.1038/nm.2294. [DOI] [PubMed] [Google Scholar]
- 27.Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, Race B, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, Johnson LM, et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods. 2008;5:211–2. doi: 10.1038/nmeth0308-211. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein R, Chang BG, Gray P, Piltch M, Bulgin MS, Sorensen-Melson S, et al. Prion disease detection, PMCA kinetics, and IgG in urine from sheep naturally/experimentally infected with scrapie and deer with preclinical/clinical chronic wasting disease. J Virol. 2011;85:9031–8. doi: 10.1128/JVI.05111-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pulford B, Spraker TR, Wyckoff AC, Meyerett C, Bender H, Ferguson A, et al. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J Wildl Dis. 2012;48:425–34. doi: 10.7589/0090-3558-48.2.425. [DOI] [PubMed] [Google Scholar]
- 31.Orru CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, et al. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. Mbio 2011;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orrù CD, Wilham JM, Vascellari S, Hughson AG, Caughey B. New generation QuIC assays for prion seeding activity. Prion. 2012;6:147–52. doi: 10.4161/pri.19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MB, Supattapone S. Superparamagnetic nanoparticle capture of prions for amplification. J Virol. 2011;85:2813–7. doi: 10.1128/JVI.02451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]