Abstract
Response prediction is an important emerging concept in oncologic imaging, with tailored, individualized treatment regimens increasingly becoming the standard of care. This review aims to define tumour response and illustrate the ways in which imaging techniques can demonstrate tumour biological characteristics that provide information on the likely benefit to be received by treatment. Two imaging approaches are described: identification of therapeutic targets and depiction of the treatment-resistant phenotype. The former approach is exemplified by the use of radionuclide imaging to confirm target expression before radionuclide therapy but with angiogenesis imaging and imaging correlates for genetic response predictors also demonstrating potential utility. Techniques to assess the treatment-resistant phenotype include demonstration of hypoperfusion with dynamic contrast-enhanced computed tomography and magnetic resonance imaging (MRI), depiction of necrosis with diffusion-weighted MRI, imaging of hypoxia and tumour adaption to hypoxia, and 99mTc-MIBI imaging of P-glycoprotein mediated drug resistance. To date, introduction of these techniques into clinical practice has often been constrained by inadequate cross-validation of predictive criteria and lack of verification against appropriate response end points such as survival. With further refinement, imaging predictors of response could play an important role in oncology, contributing to individualization of therapy based on the specific tumour phenotype. This ability to predict tumour response will have implications for improving efficacy of treatment, cost-effectiveness and omission of futile therapy.
Keywords: Predict, response, tumour, cancer, imaging, biomarker
Principles of response prediction (what is meant by predicting response?)
Tumour response to therapy is a fundamental concept in oncology in which imaging plays an important role. An exact definition of response is difficult to clarify, but has generally been considered as any beneficial response of the tumour to a particular therapy[1,2]. However, the changes that are considered beneficial depend on the context in which treatment is given. For example, in an adjuvant context the intended benefit would be prolongation of survival, whereas in a palliative situation simple relief of symptoms might be considered beneficial. Table 1 summarizes the intended benefit for a range of treatment contexts.
Table 1.
Intended treatment benefit and associated imaging surrogate for a range of clinical contexts
| Clinical context | Intended benefit | Imaging surrogate |
|---|---|---|
| Phase 2 trial | Biological effect on tumour | CR/PR by RECIST 1.1 |
| Functional response (e.g. altered metabolism or perfusion) | ||
| Phase 3 trial | Prolongation in survival or TTP | TTP by RECIST 1.1 |
| CMR (e.g. lymphoma) | ||
| Neoadjuvant therapy | ||
| (a) Tumour reduction | Tumour shrinkage | Tumour size |
| (b) Potentially curative | Complete pathologic response | CR by RECIST 1.1 |
| Complete metabolic response | ||
| Adjuvant/curative therapy (induction/first line) | Prolongation in survival | TTP by RECIST 1.1 |
| Complete metabolic response | ||
| Palliative therapy | Improved quality of life |
CMR, complete metabolic response by FDG-PET; CR, complete response; PR, partial response; TTP, time to progression.
The definition of response is further complicated by the issue of surrogate response markers. These surrogates enable response to be assessed more readily (e.g. compared with pathology) or at an earlier time point than the definitive treatment benefit (e.g. as an alternative to survival).The appropriate surrogate also depends on the treatment context as outlined in Table 1. Thus, Partial Response by Response Evaluation Criteria In Solid Tumours (RECIST)[3] may be an appropriate surrogate for biological effect of treatment in a phase 2 trial but does not necessarily provide a surrogate for survival for which time to progression (TTP) by RECIST is a validated surrogate. More recently, fundamental limitations of anatomic response criteria such as RECIST have become apparent. For example, it is increasingly recognized that change in tumour size after treatment may not necessarily be linked to prolongation of survival for targeted therapies. Hence, functional imaging approaches are becoming increasingly incorporated into response evaluation[4,5].
The use of imaging to predict response encompasses the identification of tumour biological characteristics that provide information on the likely benefit from treatment. The above complexities around definitions of response are therefore equally pertinent to the use of imaging to predict response. The predicted benefit and/or its surrogate must be appropriate to the treatment context. For example, an imaging method that predicts response by RECIST might be appropriate for identifying populations enriched for response in phase 2 trials but, in an adjuvant setting, it would be necessary for the imaging technique to predict survival or an appropriate surrogate such as TTP. The use of imaging to identify patients who are more or less likely to respond to specific treatments would enable a more personalized approach to cancer care. Predictably ineffective treatments could be withheld or changed to more a more effective option. Deriving predictive markers from imaging studies performed as part of routine staging procedures has the benefit of avoiding the need for additional testing.
Imaging surrogates of response have most commonly been applied on completion of therapy and are therefore retrospective markers. More recently, imaging surrogates for response have been deployed during treatment. This application of imaging is sometimes referred to as response prediction in the sense that an early change in imaging signal may predict either the imaging status on completion of therapy or the ultimate treatment benefit. Patients for whom treatment is proving less effective can potentially be changed to an alternative and hopefully more beneficial therapeutic regime and avoid any morbidity associated with the current treatment. This situation can be complicated by uncertainty as to whether the patient will receive any benefit from completing the current treatment before additional therapy or whether an immediate change in treatment would be optimal. Randomized trials may be necessary to clarify such areas of uncertainty. Clearly, if treatment is to be completed in any case, then response prediction during treatment is pointless.
A range of imaging methods with the potential to provide earlier response surrogates have been proposed or are under evaluation. Due to its wide availability, [18F]fluorodeoxyglucose (FDG) has attracted greatest attention as a potential early response marker. Prospective studies have shown that early metabolic tumour response correlates with survival and/or histopathologic response for lymphoma[6–11] and oesophageal cancer[12–16]. Early changes in [18F]fluorothymidine (FLT) avidity on positron emission tomography (PET) imaging have been evaluated as a surrogate for response in head and neck[17], breast[18,19] and rectal cancer[20] with potential roles in phase 2 drug trials. By reflecting apoptosis, annexin V scintigraphy is potentially an early surrogate for response in patients with lung cancer treated with platinum-based chemotherapy[21] and in patients with breast cancer receiving chemotherapy[22]. Demonstration of reduced tumour choline content by magnetic resonance (MR) spectroscopy may also reflect apoptosis with the potential for providing an early marker of chemotherapy response in breast cancer[23,24]. Early signal changes on dynamic contrast-enhanced (DCE) MR and diffusion-weighted MR have also shown promise as a surrogates for response in a range of tumours[25–28]. However, these latter studies have not specifically demonstrated an association with overall patient survival. The above examples represent the use of imaging as an early surrogate end point rather than a predictive biomarker and therefore these applications are not considered further in this article.
There are two broad approaches by which imaging can provide information on the likely benefit from treatment of cancer: (a) identification of specific therapeutic targets and (b) identification of a treatment-resistant phenotype. The former approach predicts response to specific treatments, whereas the latter is likely to predict resistance to a broad range of treatment types (Table 2).
Table 2.
Summary of imaging identification of treatment targets
| Target | Imaging modality | Treatment |
|---|---|---|
| Radioisotope therapy | ||
| Na/I symporter | 123I | 131I |
| Somatostatin receptor | 111In-octreotide | PRRNT (e.g. 177Lu-DOTATATE) |
| 68Ga-DOTA PET | ||
| Noradrenaline transporter | [123I]MIBG | [131I]MIBG |
| Angiogenesis | ||
| Integrins | 18F-galacto-RGD PET, [18F]fluciclatide | Bevacizumab |
| PRRNT may be developed | ||
| VEGF | Radiolabelled VEGF | Bevacizumab |
| Imaging correlates for genetic markers | ||
| KRAS | 68Ga-labelled oligonucleotides | Anti-EGFR agents (e.g. Cetuximab, Panitumumab) |
| Multiparametric imaging | ||
PRRNT, peptide receptor radionuclide therapy.
Imaging identification of specific therapeutic targets
Radioisotope therapy
Treatment of cancer with radioisotopes has for a long time exploited specific tumour targets to increase tumour uptake of the radioactive compound. However, these targets may not be consistently expressed on all tumours. Pretreatment imaging using a diagnostic equivalent of the therapeutic agent can predict response by confirming sufficient expression of the target. If target expression is insufficient, an alternative treatment can be sought.
The most established example of this approach is the use of diagnostic iodine 123 scintigraphy before radioiodine therapy using iodine 131 for inoperable advanced differentiated thyroid carcinoma[29]. Uptake of iodine in thyroid cancer across the membrane of follicular cells is predicated on expression of the sodium/iodide symporter[30,31]. In order to increase expression levels of the sodium/iodide symporter, withholding of thyroxine replacement medication and/or thyroid-stimulating hormone (TSH) stimulation with recombinant human TSH are used before administration of radioiodine[31]. Diagnostic pretreatment 123I scintigraphy can confirm sufficient sodium/iodide symporter expression for effective therapy[32].
Similarly, overexpression of somatostatin receptors on the cell surface of many neuroendocrine tumours (NETs) make these tumours a potential target for peptide receptor radionuclide therapy (PRRNT). Functional imaging using 111In-pentetreotide (Octreoscan) (Fig. 1) or 68Ga-labelled somatostatin analogues such as DOTATATE (Fig. 2) or DOTATOC with PET/computed tomography (CT) is used to assess the somatostatin receptor status of NETs, in addition to staging the disease[33]. Patients presenting with high tumour somatostatin receptor expression have significantly better responses to PRRNTs such as 177Lu-DOTATATE or 90Y-DOTA-TOC[33]. The same approach is used for [131I]meta-iodobenzylguanidine (MIBG) therapy of inoperable or metastatic phaeochromocytomas and paragangliomas, for which previous diagnostic imaging with [123I]MIBG can be used to predict response[34].
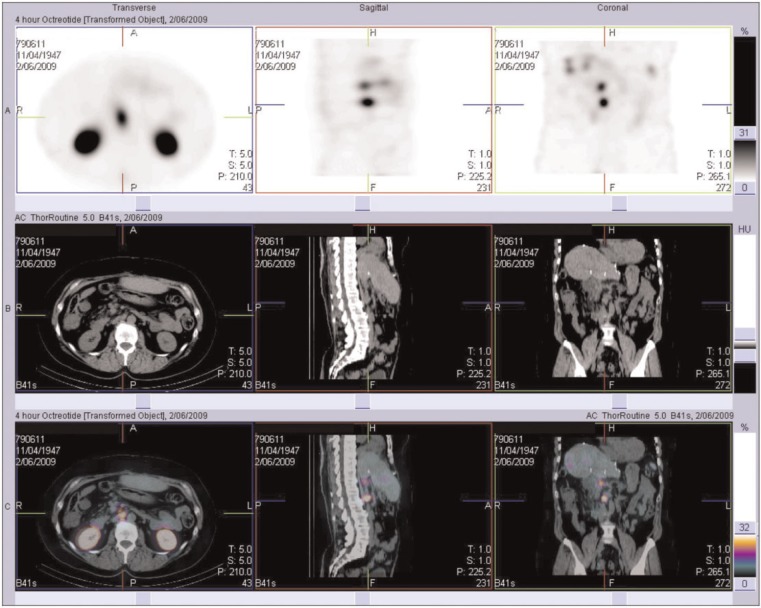
Figure 1.
111In-Octreotide SPECT/CT of the abdomen reveals octreotide-avid retroperitoneal lymphadenopathy in a patient with metastatic carcinoid disease.
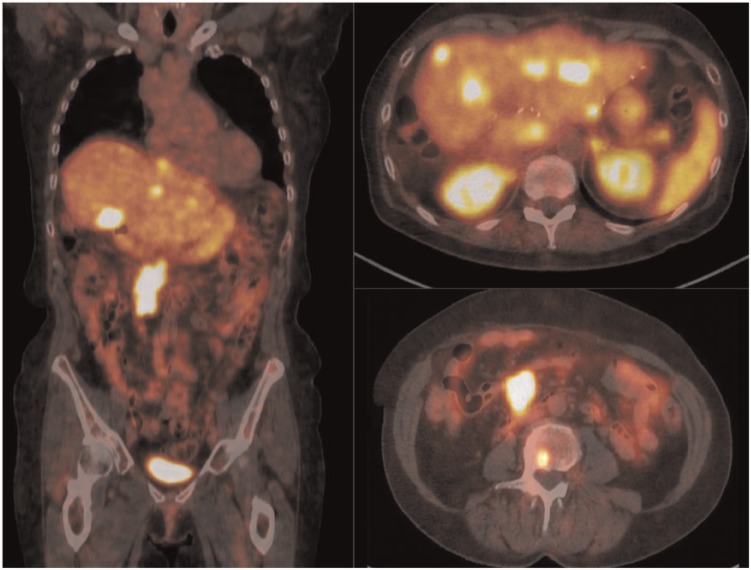
Figure 2.
68Ga-DOTATATE PET/CT in the same patient as Fig. 1 with metastatic carcinoid disease. Metastatic foci are present in the liver, retroperitoneal lymph nodes and lumbar vertebral body.
Anti-angiogenenic therapy
The tumour vasculature has become an important target for cancer treatment as illustrated by the increasing use of targeted antiangiogenic treatments such as bevacizumab. Imaging assessment of angiogenesis has the potential to identify patients most likely to benefit from such targeted agents.
Integrins, particularly the αvβ3 and αvβ5 subtypes, are strongly expressed in the activated endothelial cells of angiogenic tumour vessels, and bind proteins expressing arginine-glycine-aspartate (RGD)[35]. A number of RGD-based PET agents have been developed for imaging, including [18F]galacto-RGD and [18F]fluciclatide, and preclinical and clinical trials have been undertaken to assess their role in tumour response prediction and patient management[35].
Vascular endothelial growth factor (VEGF) acts on the VEGF receptor (VEGFR)-2 to stimulate endothelial cell migration and proliferation, and increases vascular permeability, an important pathway for angiogenesis in cancer[35]. Bevacizumab is a monoclonal antibody against VEGF used for antiangiogenic therapy. Radiolabelled VEGF with PET and single photon emission tomography (SPECT) imaging has been used in preclinical studies to assess angiogenesis, along with VEGFR-targeted microbubble ultrasonography contrast agents, although ongoing work is required for further development before clinical use[35].
Imaging correlates for genetic markers of response
Analysis of genetic material derived from tumour biopsies is increasingly used to identify likely response to specific cancer treatments. Most notable examples include the V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation in colorectal cancer, which predicts resistance to drugs that target the epidermal growth factor receptor (EGFR)[36], and EGFR-tyrosine kinase (TK) mutations in non-small cell lung cancer (NSCLC), which predict response to EGFR-TK targeting agents such as gefitinib[37]. However, histologic approaches to assessing tumour genetic status have limitations that create a need for more comprehensive approaches including correlative imaging techniques. One limitation of histologic evaluations of gene expression is heterogeneity of mutational status, which can be either intratumoural or between tumour sites[38,39]. Failure to determine gene mutational status due to insufficient biopsy material or poor DNA quality is a further limitation[40]. Imaging correlates for genetic markers would potentially provide an adjunct to histologic assessment by enabling simultaneous evaluation of the whole tumour and multiple tumour sites and by providing an indication of mutation status when tissue sampling has failed.
68Ga-labelled oligonucleotides for PET have been proposed as an imaging correlate for KRAS mutations but have not been evaluated in humans[41]. More recently, initial clinical studies have suggested potential for multiparametric imaging with FDG-PET, perfusion CT and CT texture analysis (CTTA) to identify KRAS mutations in human colorectal cancer[42].
Imaging the treatment-resistant phenotype
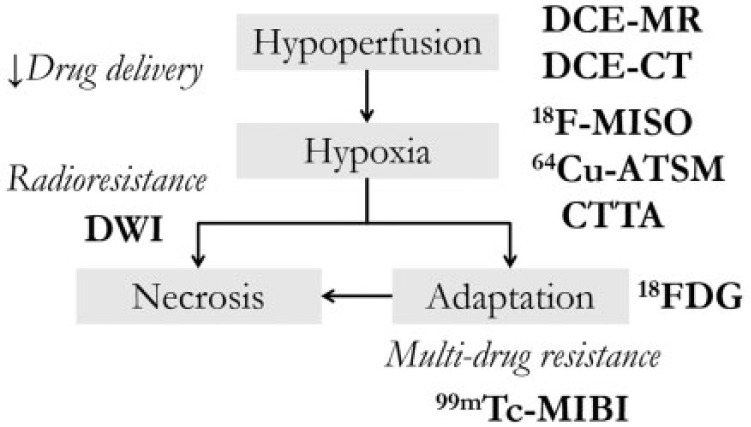
An important concept in predicting tumour response is determining when a neoplasm has pathophysiologic properties that may make it resistant to therapy. A number of these parameters can be assessed with modern imaging techniques, which therefore have the potential to guide appropriate treatment. The relationships between these imaging techniques and the biology of treatment resistance are shown in Fig. 3.
Figure 3.
Summary figure of the various contributors to the treatment-resistant phenotype and the modalities used to image and assess them.
Hypoperfusion
Tumour hypoperfusion results in reduced delivery of chemotherapeutic agents and predisposes to hypoxia. Two readily available techniques for assessing tumour perfusion are CT perfusion (CTP) and DCE MR.
CTP uses kinetic modelling to assess the temporal changes in tissue attenuation after the administration of a bolus of intravenous (IV) radiographic contrast medium[43]. This technique provides information regarding blood flow (BF), blood volume (BV) and permeability of tumour vessels[43]. Malignant tumours characteristically have increased perfusion and capillary permeability; a number of studies have suggested that tumours with a low baseline BF and BV are more likely to respond poorly to chemotherapy and radiotherapy[43]. Bellomi et al.[44] found that patients with rectal carcinoma who failed to be downstaged by neoadjuvant chemotherapy and radiotherapy had a significantly lower tumour BF and BV compared with responders. Hermans et al.[45] demonstrated that a low perfusion value in head and neck cancers correlated with a statistically significant poor clinical disease-free survival after radiotherapy. CTP has also been studied in patients with NSCLC; it has been shown that baseline BF is significantly lower in patients who fail to respond to chemoradiotherapy, assessed by anatomic imaging parameters as well as progression-free survival and overall survival. Bisdas et al.[46] found a similar pattern in patients with oropharyngeal squamous cell carcinoma, with high tumour perfusion favouring a tumour response to induction chemotherapy using RECIST criteria. This technique has also been evaluated for predicting response to chemoradiation in rectal cancer, as assessed by pre- and post-treatment imaging (Fig. 4B)[47]. CTP therefore has a potential role to play in response prediction, with low perfusion reflecting a treatment-resistant phenotype.
Figure 4.
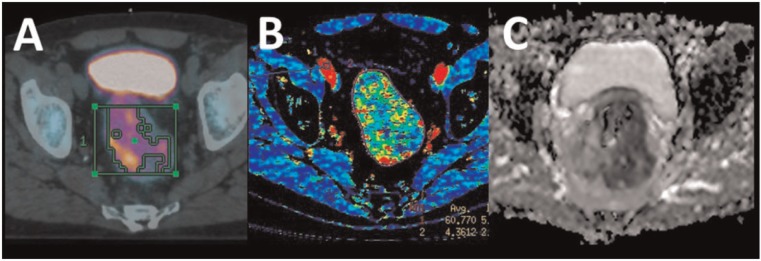
Rectal carcinoma as evaluated by FDG-PET (A), CT perfusion (B) and DWI using ADC mapping (C).
DCE MR involves assessment of changes in T1 signal intensity over time, using serial T1-weighted imaging after a bolus injection of a gadolinium-based contrast agent. As in CTP, these data can be used to assess tumour perfusion and permeability using quantitative and semiquantitative measures. Zahra et al.[48] found that cervical cancers that had relatively increased perfusion exhibited greater reductions in radiologic tumour volume after radiotherapy. These and a number of other studies suggest DCE MR may also play an important role in response prediction.
Hypoxia
Hypoxia is common in malignant tumours, and occurs due to various structural and functional abnormalities of tumour vasculature combined with altered oxygen diffusivity within the neoplasm[49]. The hypoxic environment within a tumour confers direct resistance to radiation therapy, but also causes adaptive responses with upregulation of hypoxia-inducible factor-1 (HIF-1), which ultimately causes greater degrees of tumour invasiveness and resistance to chemotherapy and radiotherapy[50]. A number of PET agents have recently been developed to enable non-invasive imaging of hypoxia, including [18F]fluoromixonidazole (18F-MISO) and 64Cu-diacetyl-bis(N4-methylthiosemicarbazone) (64Cu-ATSM). The ability of 18F-MISO PET imaging to predict response as assessed by RECIST and/or clinical and survival benefits from treatment has been demonstrated in a number of tumour types, including oesophageal cancer, NSCLC and renal cell carcinoma[51–53]. A number of trials have also shown the potential for 64Cu-ATSM PET hypoxia imaging to predict clinical tumour response to therapy and survival in patients with NSCLC, rectal cancer and head and neck carcinoma[54–56].
CTTA is an imaging technique that enables assessment of tumour heterogeneity. CTTA uses a number of mathematical models to evaluate grey-scale intensity and location of pixels within an image, producing texture maps that enable assessment of intratumoural heterogeneity[57]. Malignancies are heterogeneous due to spatial variation in tumour cellularity, neovascularization, extracellular matrix and necrosis[57]. Variations in neovascularity in particular are associated with poorly vascularized areas resulting in hypoxic voids. CTTA measurements have been shown to correlate with hypoxia in NSCLC[58]. Hence, tumours with heterogeneous internal contents are potentially associated with treatment resistance. A recent clinical study has shown that baseline CTTA measurements and changes in heterogeneity after two cycles of targeted therapy may predict TTP in metastatic renal cancer[59].
Necrosis
MRI diffusion-weighted imaging (DWI) has long been used as a biomarker for a number of conditions, including oncology imaging. It is well known that malignant tumours tend to have a low activated diffusion coefficient (ADC) value, which is attributed to the high cellularity of malignant tumours, and this fact is used in both the diagnosis and characterization of a variety of tumours such as prostate cancer (Fig. 5)[26]. However tumours with higher ADC values are more likely to have intratumoural necrosis, which, due to hypoxia-related radioresistance in adjacent tumour regions, predicts poor radiotherapy response[26]. A number of studies have also shown that ADC values can be used to predict early treatment response during therapy. Kim et al.[27] found that in patients with squamous cell carcinoma of the head and neck, those with lower pretreatment ADC values were more likely to respond to chemoradiation therapy, assessed by clinical, radiologic and pathologic parameters. In patients with rectal cancer, a negative correlation between pretreatment tumour ADC values and subsequent response to chemotherapy and chemoradiation therapy using radiologic WHO criteria has been demonstrated (see Fig. 4C)[60].
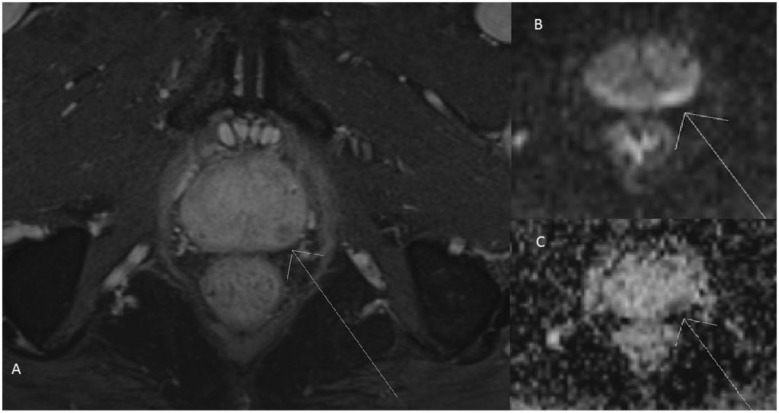
Figure 5.
MRI of the pelvis in a patient with prostate cancer. (A) Delayed post-gadolinium image with fat saturation demonstrates early washout of the carcinoma in the left lateral aspect of the peripheral zone. (B) A DWI sequence demonstrating restricted diffusion. (C) An ADC map, with low ADC signal consistent with true diffusion restriction.
Adaption to hypoxia
Tumours may upregulate HIF-1 to adapt to their hypoxic environment. In turn, HIF-1 upregulates GLUT-1 glucose transporter and VEGF expression, resulting in increased tumour glucose uptake and metabolism, along with increased angiogenesis[61]. This adaptive response of tumours can be directly imaged with [18F]FDG-PET, a commonly used modality in oncology imaging. Several studies have suggested that tumours with high glucose metabolism (and therefore high standardized uptake values with FDG-PET imaging) respond poorly to treatment and are associated with poorer prognosis[62–64]. However, FDG uptake can also be increased in the absence of hypoxia and thus increased FDG uptake in the presence of hypoxia or low perfusion is more reflective of tumour adaption to hypoxia than measurements of FDG uptake alone[65]. Thorwarth et al.[53] reported that the combined use of [18F]FDG and [18F]fluoromisonidazole (FMISO) in patients with head and neck squamous cell cancer can be successfully used to predict response to radiation therapy, as defined by absence of detectable disease on follow-up clinical examination and endoscopy. Metabolic perfusion mismatch has been shown to be associated with treatment resistance in breast and pancreatic cancer[66–68].
99mTc-MIBI (sestamibi) has been shown in a number of tumours to be a non-invasive marker for the multidrug resistance (MDR)-related P-glycoprotein (P-gp)[69]. Overexpression of P-gp by tumours results in resistance to a broad spectrum of chemotherapeutic agents[70]. 99mTc-MIBI is a transport substance recognized by the MDR-related P-gp, with increased accumulation in MDR tumour cells caused by inhibition of the efflux transport function[70]. A recent systematic review by Mohan and Miles[69] found that 99mTc-MIBI SPECT imaging in patients with lung cancer identified chemotherapy responders with a sensitivity, specificity and accuracy of 94%, 90% and 92%, respectively. This review found that the use of 99mTc-MIBI to preselect non-resistant patients for chemotherapy could potentially result in significant health cost savings and modest increases in life expectancy[69]. The use of 99mTc-MIBI has also been studied to assess for the multidrug resistant phenotype, and thus to predict poor treatment response, in osteosarcoma and other musculoskeletal sarcomas, multiple myeloma, breast cancer and lymphoma[71–75].
Conclusion
Response prediction is becoming an important concept in oncology and radiology, particularly in the clinical setting of individualized cancer treatment regimens. Imaging has an emerging role to play in the prediction of tumour response, both by the assessment of specific therapy targets and the broad-scale assessment of the treatment-resistant phenotype. There are an increasing number of imaging techniques being developed to predict tumour response. However, to date, introduction of these techniques into clinical practice has often been constrained by inadequate cross-validation of predictive criteria and lack of verification against appropriate response end points such as survival. With further refinement, imaging predictors of response could play an important role in oncology, contributing to individualization of therapy based on the specific tumour phenotype. This ability to predict tumour response will have implications for improving the efficacy of treatment, cost-effectiveness and omission of futile therapy.
Conflict of interest
K.A. Miles is a director and shareholder of TexRAD Ltd, a supplier of texture analysis software for medical images.
Footnotes
This article was presented at the ICIS Society Meeting and 13th Annual Teaching Course, York, UK, 30 September to 2 October 2013.
References
- 1.Weber WA. Assessing tumor response to therapy. J Nucl Med. 2009;50(Suppl 1):1S–10S. doi: 10.2967/jnumed.108.057174. doi:10.2967/jnumed.108.057174. PMid:19380403. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan DC, Gatsonis C. Response to treatment series: part 1 and introduction, measuring tumor response–challenges in the era of molecular medicine. AJR Am J Roentgenol. 2011;197:15–17. doi: 10.2214/AJR.11.7083. doi:10.2214/AJR.11.7083. PMid:21701005. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. doi:10.1016/j.ejca.2008.10.026. PMid:19097774. [DOI] [PubMed] [Google Scholar]
- 4.Choi H. Critical issues in response evaluation on computed tomography: lessons from the gastrointestinal stromal tumor model. Curr Oncol Rep. 2005;7:307–311. doi: 10.1007/s11912-005-0055-4. PMid:15946591. [DOI] [PubMed] [Google Scholar]
- 5.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. doi:10.1200/jco.2006.09.2403. PMid:17242396. [DOI] [PubMed] [Google Scholar]
- 6.Mikhaeel NG, Hutchings M, Fields PA, O'Doherty MJ, Timothy AR. FDG-PET after two to three cycles of chemotherapy predicts progression-free and overall survival in high-grade non-Hodgkin lymphoma. Ann Oncol. 2005;16:1514–1523. doi: 10.1093/annonc/mdi272. doi:10.1093/annonc/mdi272. PMid:15980161. [DOI] [PubMed] [Google Scholar]
- 7.Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107:52–59. doi: 10.1182/blood-2005-06-2252. doi:10.1182/blood-2005-06-2252. PMid:16150944. [DOI] [PubMed] [Google Scholar]
- 8.Terasawa T, Nihashi T, Hotta T, Nagai H. 18F-FDG PET for posttherapy assessment of Hodgkin's disease and aggressive non-Hodgkin's lymphoma: a systematic review. J Nucl Med. 2008;49:13–21. doi: 10.2967/jnumed.107.039867. doi:10.2967/jnumed.107.039867. PMid:18077527. [DOI] [PubMed] [Google Scholar]
- 9.Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, Hooft L, Riphagen II, Huijgens PC. 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica. 2006;91:522–529. PMid:16585017. [PubMed] [Google Scholar]
- 10.Juweid ME, Wiseman GA, Vose JM, et al. Response assessment of aggressive non-Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine-18-fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2005;23:4652–4661. doi: 10.1200/JCO.2005.01.891. doi:10.1200/jco.2005.01.891. PMid:15837965. [DOI] [PubMed] [Google Scholar]
- 11.Kasenda B, Haug V, Schorb E, et al. 18F-FDG PET is an independent outcome predictor in primary central nervous system lymphoma. J Nucl Med. 2013;54:184–191. doi: 10.2967/jnumed.112.108654. doi:10.2967/jnumed.112.108654. PMid:23249539. [DOI] [PubMed] [Google Scholar]
- 12.Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–3065. doi: 10.1200/JCO.2001.19.12.3058. PMid:11408502. [DOI] [PubMed] [Google Scholar]
- 13.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692–4698. doi: 10.1200/JCO.2006.06.7801. doi:10.1200/jco.2006.06.7801. PMid:16966684. [DOI] [PubMed] [Google Scholar]
- 14.Wieder HA, Brucher BL, Zimmermann F, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. doi: 10.1200/JCO.2004.07.122. doi:10.1200/jco.2004.07.122. PMid:14990646. [DOI] [PubMed] [Google Scholar]
- 15.Malik V, Lucey JA, Duffy GJ, et al. Early repeated 18F-FDG PET scans during neoadjuvant chemoradiation fail to predict histopathologic response or survival benefit in adenocarcinoma of the esophagus. J Nucl Med. 2010;51:1863–1869. doi: 10.2967/jnumed.110.079566. doi:10.2967/jnumed.110.079566. PMid:21078796. [DOI] [PubMed] [Google Scholar]
- 16.van Heijl M, Omloo JM, van Berge Henegouwen MI, et al. Fluorodeoxyglucose positron emission tomography for evaluating early response during neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer. Ann Surg. 2011;253:56–63. doi: 10.1097/SLA.0b013e3181f66596. doi:10.1097/SLA.0b013e3181f66596. PMid:21233607. [DOI] [PubMed] [Google Scholar]
- 17.de Langen AJ, Klabbers B, Lubberink M, et al. Reproducibility of quantitative 18F-3′-deoxy-3′-fluorothymidine measurements using positron emission tomography. Eur J Nucl Med Mol Imaging. 2009;36:389–395. doi: 10.1007/s00259-008-0960-5. doi:10.1007/s00259-008-0960-5. PMid:18931838. [DOI] [PubMed] [Google Scholar]
- 18.Contractor KB, Kenny LM, Stebbing J, et al. [18F]-3′Deoxy-3′-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin Cancer Res. 2011;17:7664–7672. doi: 10.1158/1078-0432.CCR-11-0783. doi:10.1158/1078-0432.ccr-11-0783. PMid:22028493. [DOI] [PubMed] [Google Scholar]
- 19.Kenny L, Coombes RC, Vigushin DM, Al-Nahhas A, Shousha S, Aboagye EO. Imaging early changes in proliferation at 1 week post chemotherapy: a pilot study in breast cancer patients with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Eur J Nucl Med Mol Imaging. 2007;34:1339–1347. doi: 10.1007/s00259-007-0379-4. doi:10.1007/s00259-007-0379-4. PMid:17333178. [DOI] [PubMed] [Google Scholar]
- 20.Dehdashti F, Grigsby PW, Myerson RJ, Nalbantoglu I, Ma C, Siegel BA. Positron emission tomography with [(18)F]-3′-deoxy-3′fluorothymidine (FLT) as a predictor of outcome in patients with locally advanced resectable rectal cancer: a pilot study. Mol Imaging Biol. 2013;15:106–113. doi: 10.1007/s11307-012-0566-y. doi:10.1007/s11307-012-0566-y. PMid:22684813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kartachova M, van Zandwijk N, Burgers S, van Tinteren H, Verheij M, Valdes Olmos RA. Prognostic significance of 99mTc Hynic-rh-annexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. J Clin Oncol. 2007;25:2534–2539. doi: 10.1200/JCO.2006.10.1337. doi:10.1200/jco.2006.10.1337. PMid:17577031. [DOI] [PubMed] [Google Scholar]
- 22.Rottey S, Slegers G, Van Belle S, Goethals I, Van de Wiele C. Sequential 99mTc-hydrazinonicotinamide-annexin V imaging for predicting response to chemotherapy. J Nucl Med. 2006;47:1813–1818. PMid:17079815. [PubMed] [Google Scholar]
- 23.Danishad KK, Sharma U, Sah RG, Seenu V, Parshad R, Jagannathan NR. Assessment of therapeutic response of locally advanced breast cancer (LABC) patients undergoing neoadjuvant chemotherapy (NACT) monitored using sequential magnetic resonance spectroscopic imaging (MRSI) NMR Biomed. 2010;23:233–241. doi: 10.1002/nbm.1436. doi:10.1002/nbm.1436. PMid:20175134. [DOI] [PubMed] [Google Scholar]
- 24.Sharma U, Baek HM, Su MY, Jagannathan NR. In vivo 1H MRS in the assessment of the therapeutic response of breast cancer patients. NMR Biomed. 2011;24:700–711. doi: 10.1002/nbm.1654. doi:10.1002/nbm.1654. PMid:21793075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ah-See ML, Makris A, Taylor NJ, et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14:6580–6589. doi: 10.1158/1078-0432.CCR-07-4310. doi:10.1158/1078-0432.CCR-07-4310. PMid:18927299. [DOI] [PubMed] [Google Scholar]
- 26.Padhani AR, Liu G, Koh D-M, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. doi:10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–994. doi: 10.1158/1078-0432.CCR-08-1287. doi:10.1158/1078-0432.CCR-08-1287. PMid:19188170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theilmann RJ, Borders R, Trouard TP, et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–837. doi: 10.1593/neo.03343. doi:10.1593/neo.03343. PMid:15720810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luster MF, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen WJ. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2008;35:1941–1959. doi: 10.1007/s00259-008-0883-1. [DOI] [PubMed] [Google Scholar]
- 30.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. [PubMed] [Google Scholar]
- 31.Robbins RJ, Schlumberger MJ. The evolving role of 131-I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46(Suppl):28–47. [PubMed] [Google Scholar]
- 32.Silberstein EB, Alavi A, Balon HR, et al. The SNMMI practice guideline for therapy of thyroid disease with 131I 3.0. J Nucl Med. 2012;53:1633–1651. doi: 10.2967/jnumed.112.105148. doi:10.2967/jnumed.112.105148. PMid:22787108. [DOI] [PubMed] [Google Scholar]
- 33.Zaknun JJ, Bodei L, Mueller-Brand J, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–816. doi: 10.1007/s00259-012-2330-6. doi:10.1007/s00259-012-2330-6. PMid:23389427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bomanji JB, Papathanasiou ND. 111-In-DTPA-octreotide (Octreoscan), 131-I-MIBG and other agents for radionuclide therapy of NETs. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S113–125. doi: 10.1007/s00259-011-2013-8. doi:10.1007/s00259-011-2013-8. PMid:22388626. [DOI] [PubMed] [Google Scholar]
- 35.Patel N, Harris AL, Gleeson FV, Vallis KA. Clinical imaging of tumor angiogenesis. Future Oncol. 2012;8:1443–1459. doi: 10.2217/fon.12.136. [DOI] [PubMed] [Google Scholar]
- 36.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. doi:10.1158/0008-5472.can-06-0191. PMid:16618717. [DOI] [PubMed] [Google Scholar]
- 37.Mu XL, Li LY, Zhang XT, et al. Gefitinib-sensitive mutations of the epidermal growth factor receptor tyrosine kinase domain in chinese patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:4289–4294. doi: 10.1158/1078-0432.CCR-04-2506. doi:10.1158/1078-0432.ccr-04-2506. PMid:15958609. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe T, Kobunai T, Yamamoto Y, et al. Heterogeneity of KRAS status may explain the subset of discordant KRAS status between primary and metastatic colorectal cancer. Dis Colon Rectum. 2011;54:1170–1178. doi: 10.1097/DCR.0b013e31821d37a3. doi:10.1097/DCR.0b013e31821d37a3. PMid:21825899. [DOI] [PubMed] [Google Scholar]
- 39.Bossard C, Kury S, Jamet P, et al. Delineation of the infrequent mosaicism of KRAS mutational status in metastatic colorectal adenocarcinomas. J Clin Pathol. 2012;65:466–469. doi: 10.1136/jclinpath-2011-200608. doi:10.1136/jclinpath-2011-200608. PMid:22259183. [DOI] [PubMed] [Google Scholar]
- 40.Sundstrom M, Edlund K, Lindell M, et al. KRAS analysis in colorectal carcinoma: analytical aspects of Pyrosequencing and allele-specific PCR in clinical practice. BMC Cancer. 2010;10:660. doi: 10.1186/1471-2407-10-660. doi:10.1186/1471-2407-10-660. PMid:21122130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roivainen A, Tolvanen T, Salomaki S, et al. 68Ga-labeled oligonucleotides for in vivo imaging with PET. J Nucl Med. 2004;45:347–355. PMid:14960659. [PubMed] [Google Scholar]
- 42.Ganeshan B, Miles K, Ziauddin Z, Engledow A, Rodriguez-Justo M, Groves A. Identification of KRAS mutation in colorectal cancer by multi-parametric PET-CT. International Cancer Imaging Society. 2012 [Google Scholar]
- 43.Garcia-Figueiras R, Goh VJ, Padhani AR, et al. CT perfusion in oncologic imaging: a useful tool? AJR Am J Roentgenol. 2013;200:8–19. doi: 10.2214/AJR.11.8476. doi:10.2214/AJR.11.8476. PMid:23255736. [DOI] [PubMed] [Google Scholar]
- 44.Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244:486–493. doi: 10.1148/radiol.2442061189. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol. 2009;193:1090–1096. doi: 10.2214/AJR.08.1367. doi:10.2214/AJR.08.1367. PMid:19770333. [DOI] [PubMed] [Google Scholar]
- 46.Bisdas S, Rumboldt Z, Wagenblast J, et al. Response and progression-free survival in oropharynx squamous cell carcinoma assessed by pretreatment perfusion CT: comparison with tumor volume measurements. AJNR Am J Neuroradiol. 2009;30:793–799. doi: 10.3174/ajnr.A1449. doi:10.3174/ajnr.A1449. PMid:19351906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Zhu J, Cai G, et al. Prediction of tumor response with CT perfusion of neoadjuvant chemoradiation in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2010;78:S323. [Google Scholar]
- 48.Zahra MA, Tan LT, Priest AN, et al. Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int J Radiat Oncol Biol Phys. 2009;74:766–773. doi: 10.1016/j.ijrobp.2008.08.023. doi:10.1016/j.ijrobp.2008.08.023. PMid:19019563. [DOI] [PubMed] [Google Scholar]
- 49.Mees G, Dierckx R, Vangestel C, Van de Wiele C. Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging. 2009;36:1674–1686. doi: 10.1007/s00259-009-1195-9. doi:10.1007/s00259-009-1195-9. PMid:19565239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chitneni SK, Palmer GM, Zalutsky MR, Dewhirst MW. Molecular imaging of hypoxia. J Nucl Med. 2011;52:165–168. doi: 10.2967/jnumed.110.075663. doi:10.2967/jnumed.110.075663. PMid:21233176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eshmann S, Paulsen F, Matthias R, Dittman H. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46:253–260. [PubMed] [Google Scholar]
- 52.Hugonnet F, Fournier L, Medioni J, et al. Metastatic renal cell carcinoma: relationship between initial metastasis hypoxia, change after 1 month's sunitinib, and therapeutic response: an 18F-fluoromisonidazole PET/CT study. J Nucl Med. 2011;52:1048–1055. doi: 10.2967/jnumed.110.084517. doi:10.2967/jnumed.110.084517. PMid:21680694. [DOI] [PubMed] [Google Scholar]
- 53.Thorwarth D, Eschmann SM, Holzner F, Paulsen F, Alber M. Combined uptake of 18F-FDG and 18F-MISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol. 2006;80:151–156. doi: 10.1016/j.radonc.2006.07.033. doi:10.1016/j.radonc.2006.07.033. PMid:16920211. [DOI] [PubMed] [Google Scholar]
- 54.Dehdashti F, Mintun MA, Lewis JS, et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging. 2003;30:844–850. doi: 10.1007/s00259-003-1130-4. doi:10.1007/s00259-003-1130-4. PMid:12692685. [DOI] [PubMed] [Google Scholar]
- 55.Dietz DW, Dehdashti F, Grigsby PW, et al. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum. 2008;51:1641–1648. doi: 10.1007/s10350-008-9420-3. doi:10.1007/s10350-008-9420-3. PMid:18682881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minagawa Y, Shizukuishi K, Koike I, et al. Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: a pilot study. Ann Nucl Med. 2011;25:339–345. doi: 10.1007/s12149-011-0471-5. doi:10.1007/s12149-011-0471-5. PMid:21327756. [DOI] [PubMed] [Google Scholar]
- 57.Davnall F, Yip CS, Ljungqvist G, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573–589. doi: 10.1007/s13244-012-0196-6. doi:10.1007/s13244-012-0196-6. PMid:23093486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA. Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology. 2013;266:326–336. doi: 10.1148/radiol.12112428. doi:10.1148/radiol.12112428. PMid:23169792. [DOI] [PubMed] [Google Scholar]
- 59.Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–171. doi: 10.1148/radiol.11110264. doi:10.1148/radiol.11110264. PMid:21813743. [DOI] [PubMed] [Google Scholar]
- 60.Dzik-Jurasz A, Domenig C, George M, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307–308. doi: 10.1016/S0140-6736(02)09520-X. [DOI] [PubMed] [Google Scholar]
- 61.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 62.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–1300. doi: 10.1016/j.ijrobp.2003.12.039. doi:10.1016/j.ijrobp.2003.12.039. PMid:15275712. [DOI] [PubMed] [Google Scholar]
- 63.Kato H, Fukuchi M, Miyazaki T, et al. Prediction of response to definitive chemoradiotherapy in esophageal cancer using positron emission tomography. Anticancer Res. 2007;27:2627–2633. PMid:17695425. [PubMed] [Google Scholar]
- 64.Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol. 1999;17:3201–3206. doi: 10.1200/JCO.1999.17.10.3201. PMid:10506619. [DOI] [PubMed] [Google Scholar]
- 65.Miles KA, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging. 2008;8:81–86. doi: 10.1102/1470-7330.2008.0011. doi:10.1102/1470-7330.2008.0011. PMid:18390391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mankoff DA, Dunnwald LK, Partridge SC, Specht JM. Blood flow-metabolism mismatch: good for the tumor, bad for the patient. Clin Cancer Res. 2009;15:5294–5296. doi: 10.1158/1078-0432.CCR-09-1448. doi:10.1158/1078-0432.CCR-09-1448. PMid:19706819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komar G, Kauhanen S, Liukko K, et al. Decreased blood flow with increased metabolic activity: a novel sign of pancreatic tumor aggressiveness. Clin Cancer Res. 2009;15:5511–5517. doi: 10.1158/1078-0432.CCR-09-0414. doi:10.1158/1078-0432.CCR-09-0414. PMid:19706808. [DOI] [PubMed] [Google Scholar]
- 68.Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK. Blood flow and metabolism in locally advanced breast cancer: relationships to response to therapy. J Nucl Med. 2002;43:500–509. [PubMed] [Google Scholar]
- 69.Mohan HK, Miles KA. Cost-effectiveness of 99mTc-sestamibi in predicting response to chemotherapy in patients with lung cancer: systematic review and meta-analysis. J Nucl Med. 2009;50:376–381. doi: 10.2967/jnumed.108.055988. doi:10.2967/jnumed.108.055988. PMid:19223414. [DOI] [PubMed] [Google Scholar]
- 70.Piwnica-Worms D, Chiu ML, Budding M, Kronauge JF. Functional imaging of multidrug-resistant p-glycoprotein with an organotechnetium complex. Cancer Res. 1993;73:977–984. [PubMed] [Google Scholar]
- 71.Burak Z, Moretti J-L, Ersoy Ö, et al. 99mTc-MIBI imaging as a predictor of therapy response in osteosarcoma compared with multidrug resistance-associated protein and P-glycoprotein expression. J Nucl Med. 2003;44:1394–1401. [PubMed] [Google Scholar]
- 72.Fonti R, Del Vecchio S, Zannetti A, et al. Functional imaging of multidrug resistant phenotype by 99mTc-MIBI scan in patients with multiple myeloma. Cancer Biother Radiopharm. 2004;19:165–170. doi: 10.1089/108497804323071931. doi:10.1089/108497804323071931. PMid:15186596. [DOI] [PubMed] [Google Scholar]
- 73.Vecchio S, Salvatore M. 99mTc-MIBI in the evaluation of breast cancer biology. Eur J Nucl Med. 2004;31:S88–96. doi: 10.1007/s00259-004-1530-0. doi:10.1007/s00259-004-1530-0. [DOI] [PubMed] [Google Scholar]
- 74.Burak Z, Ersoy Ö, Moretti J-L, et al. The role of 99mTc-MIBI scintigraphy in the assessment of MDR1 overexpression in patients with musculoskeletal sarcomas: comparison with therapy response. Eur J Nucl Med. 2001;28:1341–1350. doi: 10.1007/s002590100588. doi:10.1007/s002590100588. [DOI] [PubMed] [Google Scholar]
- 75.Kao C-H, Tsai S-C, Wang J-J, Ho Y-J, Ho S-T, Changlai S-P. Technetium-99m-sestamethoxyisobutylisonitrile scan as a predictor of chemotherapy response in malignant lymphomas compared with P-glycoprotein expression, multidrug resistance-related protein expression and other prognosis factors. Br J Haematol. 2001;113:369–374. doi: 10.1046/j.1365-2141.2001.02763.x. doi:10.1046/j.1365-2141.2001.02763.x. [DOI] [PubMed] [Google Scholar]