Abstract
Calcified amorphous tumor of the heart is a very rare non-neoplastic intracavitary mass. The clinical presentation is similar to that of other cardiac masses. The precise cause and best approach to treatment remain unclear. We describe a case of cardiac calcified amorphous tumor presenting with refractory unilateral vision loss that was successfully treated by surgical excision. To our knowledge, this is only the 2nd reported case of retinal arterial embolism due to cardiac calcified amorphous tumor in the English-language literature.
Key words: Blindness/etiology; calcinosis/complications/radiography/surgery; diagnosis, differential; tumor, cardiac calcified amorphous; vision loss, sudden
Calcified amorphous tumor (CAT) of the heart is a very rare non-neoplastic cardiac intracavitary mass. It was first described as a distinct pathologic entity by Reynolds and colleagues in 1997.1 Since then, very few cases of cardiac CAT have been reported in the English-language medical literature2–19 (Table I). The clinical presentation is similar to that of other cardiac masses. The precise cause of the mass and the best means of treatment remain unclear.12
TABLE I. Clinicopathologic Findings of Published Cases of Cardiac Calcified Amorphous Tumor
TABLE I, continued. Clinicopathologic Findings of Published Cases of Cardiac Calcified Amorphous Tumor
In the present report, we describe a case in which cardiac CAT presented with refractory unilateral vision loss, which was successfully treated by surgical excision.
Case Report
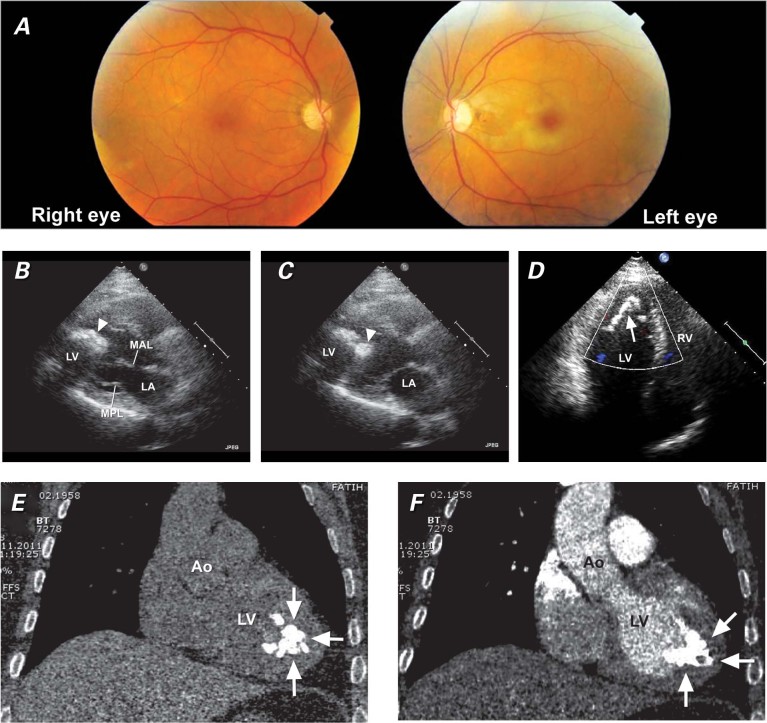
A 54-year-old woman presented to her local physician with unilateral vision loss (left eye), which had occurred suddenly 27 days before. The patient was referred to our hospital. Left central retinal arterial occlusion was detected upon fundus examination (Fig. 1A), and we set about to identify the source of the embolus. The results of her physical examination were otherwise normal. She had a history of hypothyroidism and was under treatment with oral levothyroxine. Laboratory tests, including tests for parathyroid hormone, thrombotic, and autoimmune irregularities, yielded results within normal limits. The electrocardiogram was also unremarkable. A plain chest film showed a localized dense calcified mass within the cardiac silhouette. Transthoracic echocardiography (TTE) then revealed a pedunculated mobile hyperechoic calcified mass 3.8 × 2.5 cm in size, which arose from the septoapical and anteroapical region of the left ventricle (Figs. 1B–D). No right-to-left shunt was detected by TTE. No laboratory data suggested infective endocarditis. Anticoagulative therapy was immediately started. A computed tomographic scan confirmed the presence of a heavily calcified, irregularly shaped mass 3.5 × 2.6 cm in size, which arose from the region of the left ventricular endomyocardium as indicated by TTE (Figs. 1E, 1F, and 2A). Duplex ultrasonographic imaging of the carotid and vertebral arteries yielded unremarkable results. Conventional coronary angiography showed normal coronary arteries. Brain magnetic resonance imaging also showed nothing of note.
Fig. 1 A) Fundus photography shows a cherry-red spot with retinal pallor typical of central retinal artery occlusion in the left eye and a normal right eye. In the B) diastolic phase and C) systolic phase, a transthoracic echocardiogram (parasternal long-axis view) shows a mobile calcified mass (arrowhead) in the left ventricle. D) A transthoracic echocardiogram (apical 4-chamber view) shows a hyperechoic calcified mass (arrow) originating from the anteroapical and septoapical region of the left ventricle. Computed tomograms (coronal oblique views) E) with and F) without contrast material show an echogenic intracavitary irregular mass (arrows) attached to the apex of the left ventricle.
Ao = aorta; LA = left atrium; LV = left ventricle; MAL = mitral anterior leaflet; MPL = mitral posterior leaflet; RV = right ventricle
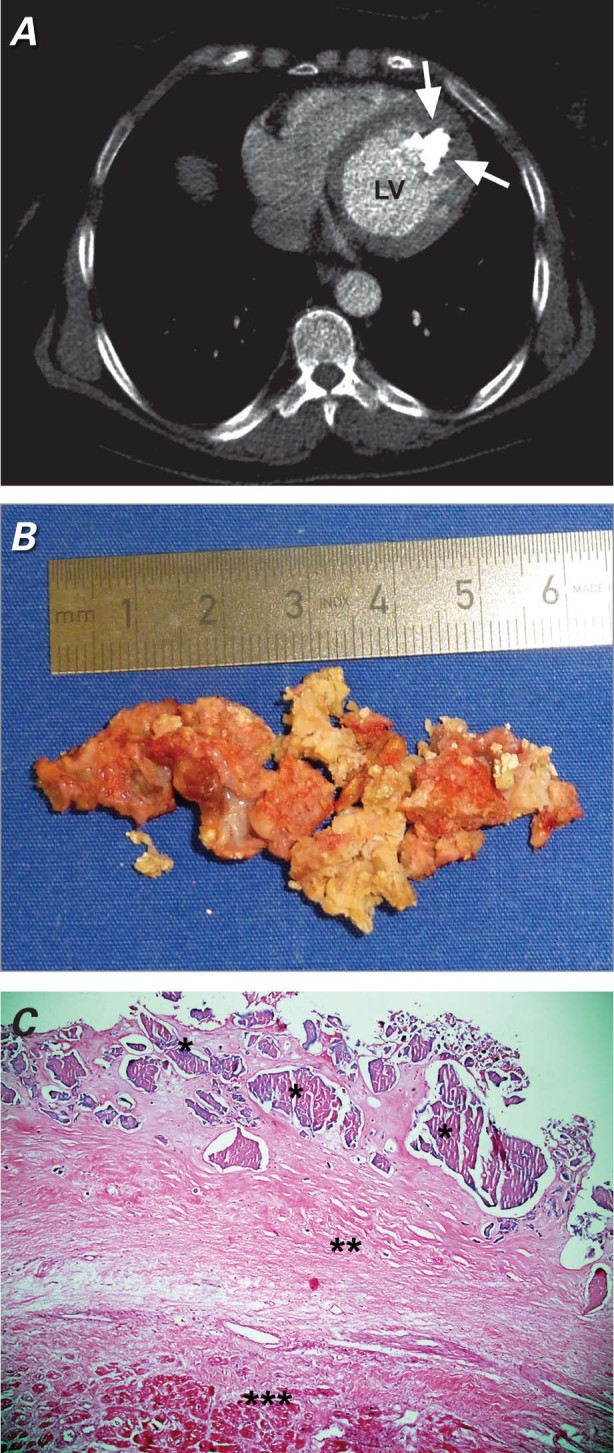
Fig. 2 A) Axial computed tomogram shows an echogenic intracavitary irregular mass (arrows) attached to the apex of the left ventricle (LV). B) View of the surgically excised calcified amorphous tumor as multiple pieces. C) Microscopic image of cardiac calcified amorphous tumor reveals amorphous eosinophilic hyalinized material (*) along with dense calcification (**). Myocardial muscle (***) is seen beneath the hyalinized material (H & E, orig. ×100).
Our patient underwent cardiac exploration and removal of the mass to prevent further systemic embolization. A median sternotomy was performed. The cannulations for cardiopulmonary bypass were done via the ascending aorta and the right atrial appendage. After cross-clamping the ascending aorta, we arrested the heart with cold-blood cardioplegic solution administered antegrade under mild hypothermia. An anteroapical left ventriculotomy was then performed. Intraoperatively, we found a calcified mass adjacent to the septoapical and anteroapical region of the left ventricle. The large, rough, fragile mass was excised in toto (but as multiple pieces) through a left ventriculotomy (Fig. 2B). Perioperative transesophageal echocardiography detected no remains of the extirpated tumorous mass. Histopathologic examination showed amorphous eosinophilic hyalinized material, along with dense calcification (Fig. 2C). No myxomatous tissue was seen; the final diagnosis was calcified amorphous tumor of the heart.
The patient, who had no perioperative complication and experienced no further embolic events, was discharged from the hospital on the 6th postoperative day. Oral warfarin therapy was started preoperatively and was continued for 3 months postoperatively. One year later, the patient remained asymptomatic.
Discussion
The cardiac CAT comprises calcium deposits in a matrix of amorphous degenerating fibrinous material.11
It has been suggested that cardiac CAT has its origin in organized mural thrombus, but the exact cause is unknown. Differential diagnosis of this lesion might include calcified myxomas or fibromas, calcified tuberculoma, thrombi, emboli, vegetations, and tophaceous pseudogout—as well as tumoral calcinosis, especially in patients with end-stage chronic renal failure due to abnormalities in calcium, parathyroid hormone, or vitamin D3 metabolism.15
Calcified amorphous tumors have been said to occur in the absence of clinical preconditions for thrombosis or hypercalcemia. However, current laboratory analysis is now able to rule out the infrequent causes of hypercoagulability, such as inherited thrombophilias or antiphospholipid antibody syndrome, apart from the more common causes (smoking, use of contraceptive pills, pregnancy, and malignancies).10 All of these causes were ruled out in our patient.
Cardiac CATs can occur in any chamber of the heart. Most cardiac CATs present in ventricular cavities; however, they have also been described in atria, in valves, and in valvular annuli.20,21 Cardiac CATs cause symptoms due to obstruction or to the embolization of calcific fragments. The clinical presentation, which depends on the location and size of the masses, includes dyspnea, chest pain, syncope, and pulmonary or systemic embolism.10 The first description of retinal arterial embolism in association with a cardiac CAT was published in 2011.15 Mobile CATs definitely indicate a greater risk of cerebrovascular accident or systemic embolism than do immobile amorphous tumors.12 To our knowledge, our case is only the 2nd report of retinal arterial embolism due to cardiac CAT in the English-language medical literature.
In the absence of distinctive clinical and imaging features, preoperative differentiation between neoplastic and non-neoplastic masses remains difficult.11 It is important to remember that current cardiac imaging techniques still do not specifically recognize cardiac CAT, although they can help to narrow down the differential diagnosis of calcified lesions.10 Hence, excision of the lesion and histopathologic examination is necessary for accurate diagnosis.11 Histopathologically, CAT is characterized by nodular calcium deposits over a matrix of fibrin or amorphous fibrin-like material and by hyaline formation and the presence of chronic inflammatory cells and degenerated hematologic elements without malignant cells.3,8
Surgical excision is recommended if the lesion is large or symptomatic. In most instances, surgery is curative, especially for pedicled lesions.10 Most cases reported thus far have run a benign course after surgical excision of the intracardiac mass, although some residual calcified tissue at the location of the original mass might be seen.1 Cardiac CAT can recur and enlarge after excision, especially if excision has been incomplete.15 Such patients should be monitored vigilantly with repeat cardiac imaging after excision.4
Again, CAT's precise cause and the best approach to treatment remain unclear.
Footnotes
Address for reprints: Yunus Nazli, MD, Alparslan Turkes Caddesi No:57, 06510 Emek/Ankara, Turkey
E-mail: yunusnazli@gmail.com
References
- 1.Reynolds C, Tazelaar HD, Edwards WD. Calcified amorphous tumor of the heart (cardiac CAT). Hum Pathol 1997; 28(5):601–6. [DOI] [PubMed]
- 2.Chaowalit N, Dearani JA, Edwards WD, Pellikka PA. Calcified right ventricular mass and pulmonary embolism in a previously healthy young woman. J Am Soc Echocardiogr 2005; 18(3):275–7. [DOI] [PubMed]
- 3.Lewin M, Nazarian S, Marine JE, Yuh DD, Argani P, Halushka MK. Fatal outcome of a calcified amorphous tumor of the heart (cardiac CAT). Cardiovasc Pathol 2006;15(5):299–302. [DOI] [PubMed]
- 4.Fealey ME, Edwards WD, Reynolds CA, Pellikka PA, Dearani JA. Recurrent cardiac calcific amorphous tumor: the CAT had a kitten. Cardiovasc Pathol 2007;16(2):115–8. [DOI] [PubMed]
- 5.Khulbey S, Ramana KV, Kumar S, Dikshit V. Amorphous cardiac tumor of right atrium late after atrial septal defect closure. Indian J Thorac Cardiovasc Surg 2008;24(2):129–31.
- 6.Ho HH, Min JK, Lin F, Wong SC, Bergman G. Images in cardiovascular medicine. Calcified amorphous tumor of the heart. Circulation 2008;117(9):e171–2. [DOI] [PubMed]
- 7.Gutierrez-Barrios A, Muriel-Cueto P, Lancho-Novillo C, Sancho-Jaldon M. Calcified amorphous tumor of the heart [in English, Spanish]. Rev Esp Cardiol 2008;61(8):892–3. [PubMed]
- 8.Flynn A, Mukherjee G. Calcified amorphous tumor of the heart. Indian J Pathol Microbiol 2009;52(3):444–6. [DOI] [PubMed]
- 9.Habib A, Friedman PA, Cooper LT, Suleiman M, Asirvatham SJ. Cardiac calcified amorphous tumor in a patient presenting for ventricular tachycardia ablation: intracardiac echocardiogram diagnosis and management. J Interv Card Electrophysiol 2010;29(3):175–8. [DOI] [PubMed]
- 10.Vaideeswar P, Karunamurthy A, Patwardhan AM, Hira P, Raut AR. Cardiac calcified amorphous tumor. J Card Surg 2010;25(1):32–5. [DOI] [PubMed]
- 11.Gupta R, Hote M, Ray R. Calcified amorphous tumor of the heart in an adult female: a case report. J Med Case Rep 2010; 4:278. [DOI] [PMC free article] [PubMed]
- 12.Kubota H, Fujioka Y, Yoshino H, Koji H, Yoshihara K, Tonari K, et al. Cardiac swinging calcified amorphous tumors in end-stage renal failure patients. Ann Thorac Surg 2010;90(5):1692–4. [DOI] [PubMed]
- 13.Ananthakrishna R, Nanjappa MC, Kamalapurkar G, Bhat P, Panneerselvam A, Chander N, Chandrasekaran D. Cardiac tumour in a patient with rheumatic heart disease. BMJ Case Rep 2011;2011. [DOI] [PMC free article] [PubMed]
- 14.Greaney L, Chaubey S, Pomplun S, St. Joseph E, Monaghan M, Wendler O. Calcified amorphous tumour of the heart: presentation of a rare case operated using minimal access cardiac surgery. BMJ Case Rep 2011;2011. [DOI] [PMC free article] [PubMed]
- 15.Vlasseros I, Katsi V, Tousoulis D, Tsiachris D, Bousiotou A, Souretis G, et al. Visual loss due to cardiac calcified amorphous tumor: a case report and brief review of the literature. Int J Cardiol 2011;152(3):e56–7. [DOI] [PubMed]
- 16.Lin YC, Tsai YT, Tsai CS. Calcified amorphous tumor of left atrium. J Thorac Cardiovasc Surg 2011;142(6):1575–6. [DOI] [PubMed]
- 17.Sousa JS, Tanamati C, Marcial MB, Stolf NA. Calcified amorphous tumor of the heart: case report [in English, Portuguese]. Rev Bras Cir Cardiovasc 2011;26(3):500–3. [DOI] [PubMed]
- 18.Fujiwara M, Watanabe H, Iino T, Kobukai Y, Ishibashi K, Yamamoto H, et al. Two cases of calcified amorphous tumor mimicking mitral valve vegetation. Circulation 2012;125(10): e432–4. [DOI] [PubMed]
- 19.Nishigawa K, Takiuchi H, Kubo Y, Masaki H, Tanemoto K. Calcified amorphous tumor: three-dimensional transesophageal echocardiography. Asian Cardiovasc Thorac Ann 2012; 20(3):355. [DOI] [PubMed]
- 20.Silver MA, Bonow RO, Deglin SM, Maron BJ, Cannon RO 3rd, Roberts WC. Acquired left ventricular endocardial constriction from massive mural calcific deposits: a newly recognized cause of impairment to left ventricular filling. Am J Cardiol 1984;53(10):1468–70. [DOI] [PubMed]
- 21.Waller BF, Roberts WC. Systolic clicks caused by rocks in the right heart chambers. Am Heart J 1981;102(3 Pt 1):459–60. [DOI] [PubMed]