Abstract
Chronic graft-versus-host disease (GVHD) develops as a result of the immunologic response that donor T-lymphocytes generate against host tissue after allogeneic stem cell transplantation. We tried to elucidate the contribution of cardiac dysfunction to the high morbidity and mortality rates observed after GVHD.
Forty patients who had undergone bone marrow transplantation were enrolled in this prospective study: 14 patients who had been diagnosed with chronic GVHD (manifestations beyond day 100 after hemopoietic cell transplantation) and 26 patients who had not. All patients had undergone baseline echocardiography before bone marrow transplantation and were monitored. After the expected period of time had elapsed for GVHD after transplantation, these patients were divided into 2 groups in accordance with whether or not they developed chronic GVHD.
No significant differences were observed before bone marrow transplantation in the 2 groups' broad attributes or in their laboratory and echocardiographic findings (P >0.05). After transplantation, high-sensitivity C-reactive protein levels and erythrocyte sedimentation rates were significantly higher in the chronic GVHD group (P < 0.001 and P=0.01, respectively). Mean left ventricular mass was 227 ± 32.3 g in the GVHD group and 149.3 ± 27.4 g in the non-GVHD group (P < 0.001). The E/A flow rate was significantly higher in the non-GVHD group.
This study shows that chronic GVHD increases left ventricular mass and impairs left ventricular diastolic function in patients who have developed chronic GVHD. In addition, it shows that inflammatory markers increase to higher levels in these patients. Comprehensive studies with larger samples are needed to more fully elucidate the cardiac effects of this disease.
Key words: Bone marrow transplantation; C-reactive protein; diastole, left ventricular; graft vs host disease/prevention & control; hematopoietic stem cell transplantation/adverse effects
Stem cell transplantation is acknowledged as the basic treatment approach in many hematologic diseases. In patients who receive allogeneic bone marrow transplantation (BMT), graft-versus-host disease (GVHD) is the leading cause of morbidity and death. Graft-versus-host disease is a result of immunologic responses to the host's tissues by the donor's T-lymphocytes.1–5 Because the host's immune system is suppressed, it is less resistant to the donor's lymphocytes.6,7
The organs that are most commonly affected by GVHD are the liver, lungs, skin, gastrointestinal system, and lymphoid tissues. The risk of developing GVHD is higher in elderly hosts than in younger hosts and in older hosts whose donors are young. The incidence of GVHD after BMT is approximately 35% to 50%. The clinical manifestation of chronic GVHD often differs from that of acute GVHD. Mostly mesenchymal tissues are affected in chronic GVHD.8,9 The diagnostic and therapeutic approaches to diffuse organ involvement are well known for both acute and chronic GVHD, but the body of knowledge on cardiac involvement is more limited.
After the 2005 National Institutes of Health (NIH) Consensus Development Project on Chronic GVHD, major changes in the diagnosis, classification, and grading of chronic GVHD were proposed. Rather than focus on the customary 100 days post–bone marrow transplantation as the dividing line between acute and chronic GVHD in regard to the continued manifestation of symptoms, the proposed NIH Consensus Criteria invoke the diagnostic characteristics of the syndromes in order to classify them. Accordingly, those manifestations (dermal, oral, hepatic, ocular, respiratory, and gastrointestinal) occurring beyond day 100 are considered diagnostic of classic chronic GVHD. A revised scheme for grading the severity of GVHD assigns a score for each involved organ with attention to functional impairment and provides a global summary score (mild, moderate, or severe) that takes into account the number of organs involved and the severity of the condition of those organs.10
Yet we still define classic chronic GVHD as manifestations beyond day 100 after allogeneic hemopoietic cell transplantation. According to this older criterion, 14 patients were in our classic chronic GVHD group. The other 26 patients had neither chronic nor acute GVHD.
The purpose of this prospective study was to find out whether there are cardiac effects in patients who develop GVHD after receiving BMT and to determine their extent.
Patients and Methods
Patient Population. This study was performed at our institution from May 2008 through June 2010. We excluded from the study all patients with hypertension, diabetes mellitus, thyroid disease, coronary artery disease, congestive heart failure, cardiac valve disease, cardiomyopathy (restrictive, hypertrophic, or dilated), serious chronic obstructive pulmonary disease symptoms, or a history of cerebrovascular disease. Originally, 44 patients who were to receive allogeneic stem cell transplantation were enrolled. Four of these patients died during the follow-up period and therefore could not be included in the final evaluation. Ultimately, 40 patients who had undergone allogeneic hemopoietic BMT were enrolled: 14 patients (4 women and 10 men; mean age, 32 ± 9.4 yr) who had received BMT and had been diagnosed with chronic GVHD in the hematology department; and 26 patients (12 women and 14 men; mean age, 38 ± 13 yr) who had not developed GVHD after allogeneic BMT.
Methods. All patients received cyclosporine prophylaxis for the first 3 months after BMT. After diagnosis of classic chronic GVHD, only the chronic-GVHD group received cyclosporine and corticosteroid therapy.
Before BMT transplantation, detailed medical histories were obtained from each patient and physical examinations were performed. Patients' body mass indices were measured. Blood samples were collected to measure high-sensitivity C-reactive protein (hs-CRP) levels, erythrocyte sedimentation rates (ESR), and cyclosporine levels. Baseline echocardiography was performed, after which the patients were monitored. Once the expected period of time (150–180 d) for GVHD after transplantation had elapsed, these patients were divided into 2 groups on the basis of whether they had developed chronic GVHD, and they were again monitored. All GVHD patients were in the classic “chronic” group. There was neither acute GVHD nor any overlapping syndrome with features of both acute and chronic GVHD. All patients underwent physical examination, and we recorded the time periods from transplantation and from the beginning of medication use.
Conformity of the study to the Helsinki Declaration and to ethical principles was confirmed by the Ethics Committee of the Erciyes University Medical Faculty.
Echocardiography. We performed transthoracic echocardiography at baseline before transplantation and again after the expected time (150–180 d) had elapsed for GVHD development after transplantation. The echocardiographic examinations were performed with use of the Vivid 7® Dimension® cardiac ultrasonography system (GE VingMed Ultrasound AS; Horten, Norway) using a 2.5-MHz transducer. The patients were evaluated in the left lateral decubitus position, after a 5-minute rest. The standard window recommended by the American Society of Echocardiography—parasternal long-axis imaging by M-mode echocardiography—was used to measure left ventricular (LV) end-diastolic and end-systolic diameters and LV ejection fraction. The LV systolic functions, LV wall movements, and mitral, aortic, tricuspid, pulmonary valve structures and obstruction and insufficiencies of these valves were studied by means of 2-dimensional color-flow Doppler echocardiography. Left ventricular mass was calculated by the Penn convention.11
Left ventricular diastolic function was evaluated by means of transmitral and tissue-Doppler echocardiography. Pulsed-wave Doppler recordings of the mitral inflow were performed from the apical 4-chamber view with the sample volume placed between the tips of the mitral leaflets. The early (E) and late (A) transmitral diastolic flow rates, E-wave deceleration time (DT), and E/A ratio were measured. The isovolumetric relaxation time (IVRT) was also recorded from the apical 4-chamber view by a simultaneous recording of the aortic and mitral flows. Pulsed-wave tissue Doppler values were obtained from the apical 4-chamber view during end-expiration with a 5-mm sample size at the lateral wall, at the junction with the mitral annulus, and included peak systolic annular velocity (Sm), peak early diastolic annular velocity (Em), and peak late diastolic annular velocity (Am) waves.
Statistical Analysis
Statistical analysis was carried out with the aid of SPSS 15.0 software (IBM Corporation; Armonk, NY). Correspondence of quantifiable data to normal distribution was analyzed with the Kolmogorov-Smirnov test. Variables that fit normal distribution were expressed as mean ± SD. The independent-samples t test was used to compare variables with normal distribution, while the Mann-Whitney U test was used to compare values with non-normal distribution. Percentage data were compared by the χ2 test. P values less than 0.05 were considered statistically significant. Correlation analyses were performed using the Pearson coefficient of correlation. A probability value of P < 0.05 was considered significant, and 2-tailed P values were used for all statistics.
Results
The types of hematologic malignancy in the study patients were acute lymphoblastic leukemia (9, 22.5%), acute myeloid leukemia (29, 72.5%) and lymphoma (2, 5%). Eleven patients (78.6%) in the chronic GVHD group and 21 patients (80.8%) in the non-chronic GVHD group were related donors and recipients (P = 0.87). After the expected period of time had elapsed for the development of chronic GVHD after BMT, these 40 patients were invited to the clinic for examination. Chronic GVHD had developed in 14 of the 40 patients who were being monitored after baseline evaluation before BMT. A total of 40 patients were included in the study: 14 (10 men and 4 women) patients who did develop chronic GVHD and 26 (14 men and 12 women) patients who did not (P >0.05). The patients were monitored for 6 months after transplantation. In the follow-up period, there were no important differences in prognosis or outcome between the 2 groups of patients.
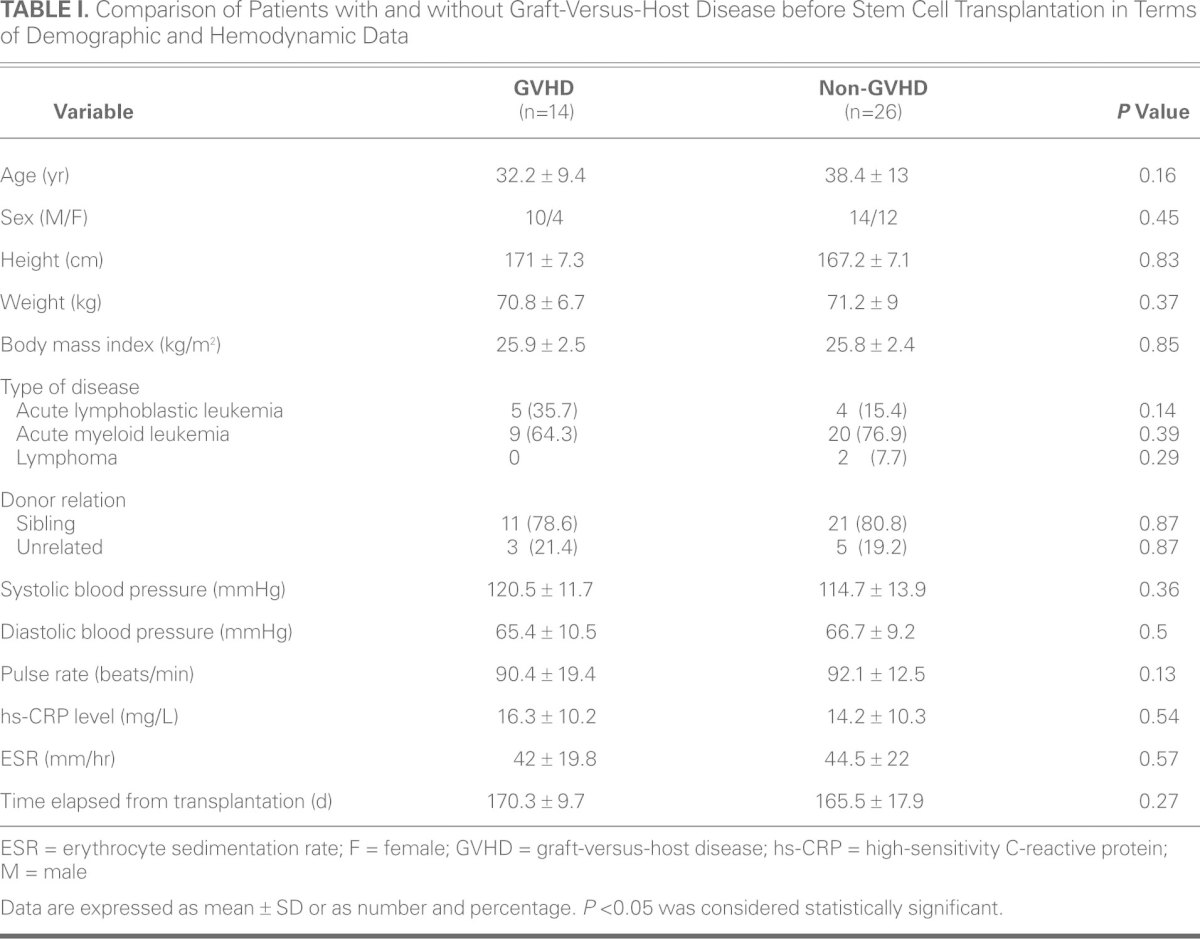
There were no significant differences between the 2 groups in mean age, sex, and body mass index before BMT (Table I). In addition, the 2 groups did not differ statistically in terms of systolic blood pressure, diastolic blood pressure, and heart rate. Nor was the time elapsed from BMT statistically different between the 2 groups. The pre-BMT hs-CRP level was 16.3 ± 10.2 mg/L in the GVHD group, compared to 14.2 ± 10.3 mg/L in the non-GVHD group, and the ESR was 42 ± 19.8 mm/hr in the GVHD group, compared to 44.5 ± 22 mm/hr in the non-GVHD group. The 2 groups did not differ significantly in terms of hs-CRP level and ESR before BMT (P = 0.54 and P = 0.57, respectively) (Table I).
TABLE I. Comparison of Patients with and without Graft-Versus-Host Disease before Stem Cell Transplantation in Terms of Demographic and Hemodynamic Data
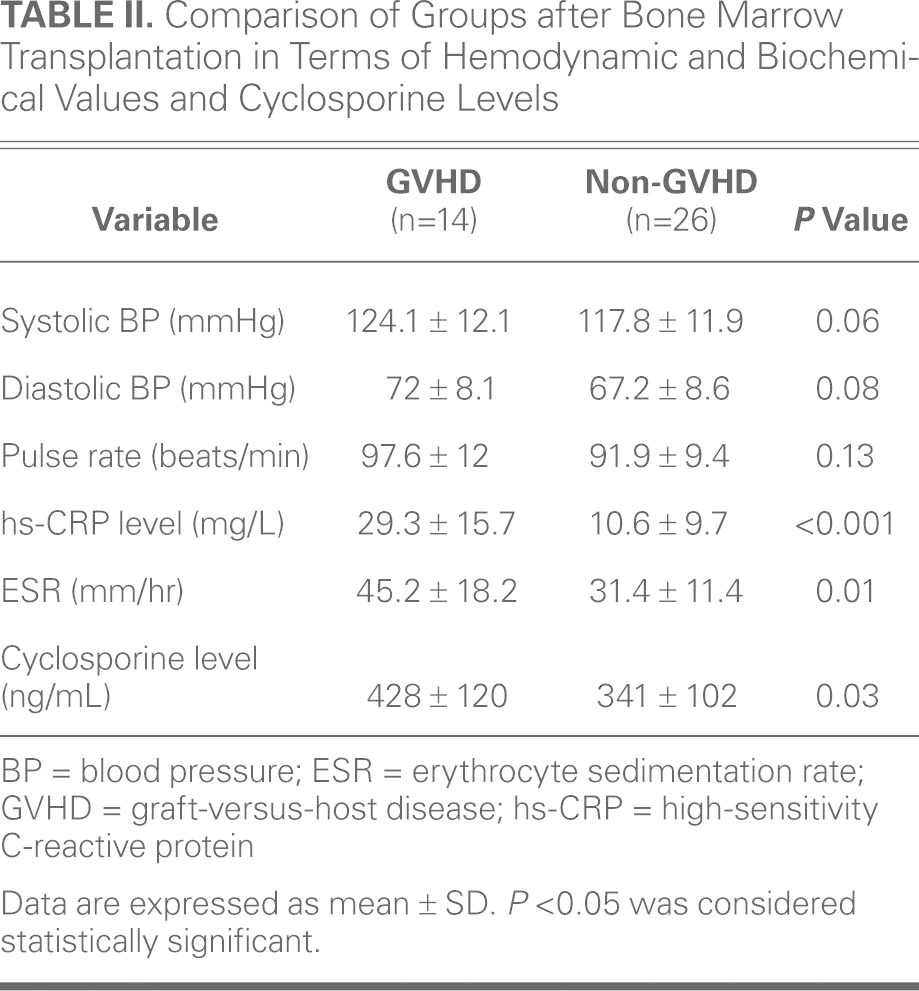
The post-BMT evaluation did not reveal significant differences between the 2 groups in terms of systolic and diastolic blood pressures and pulse rate (P >0.05) (Table II). The post-BMT hs-CRP level was measured as 29.3 ± 15.7 mg/L in the GVHD group and 10.6 ± 9.7 mg/L in the non-GVHD group. The ESR was 45.2 ± 18.2 mm/hr in the GVHD group and 31.4 ± 11.4 mm/hr in the non-GVHD group. The hs-CRP level and ESR were significantly higher in the group of patients who developed chronic GVHD, compared with those in the non-GVHD group (P < 0.001 and P = 0.01, respectively). Cyclosporine levels after BMT were 428 ± 120 ng/mL in the GVHD group and 341 ± 102 ng/mL in the non-GVHD group. The difference was statistically higher in the patients who developed GVHD than in the patients who did not (Table II).
TABLE II. Comparison of Groups after Bone Marrow Transplantation in Terms of Hemodynamic and Biochemical Values and Cyclosporine Levels
Echocardiographic Findings
Before Bone Marrow Transplantation. The pre-BMT Doppler values are presented in Table III. Left ventricular end-systolic diameter, end-diastolic diameter, septal and posterior-wall thickness, ejection fraction, and left atrial diameter measurements did not reveal a significant difference between groups. Valve functions and pulmonary artery pressure did not differ significantly between groups. There were no significant differences between the groups in terms of LV mass.
TABLE III. Comparison of Pre–Bone Marrow Transplantation Echocardiographic Data between the Groups
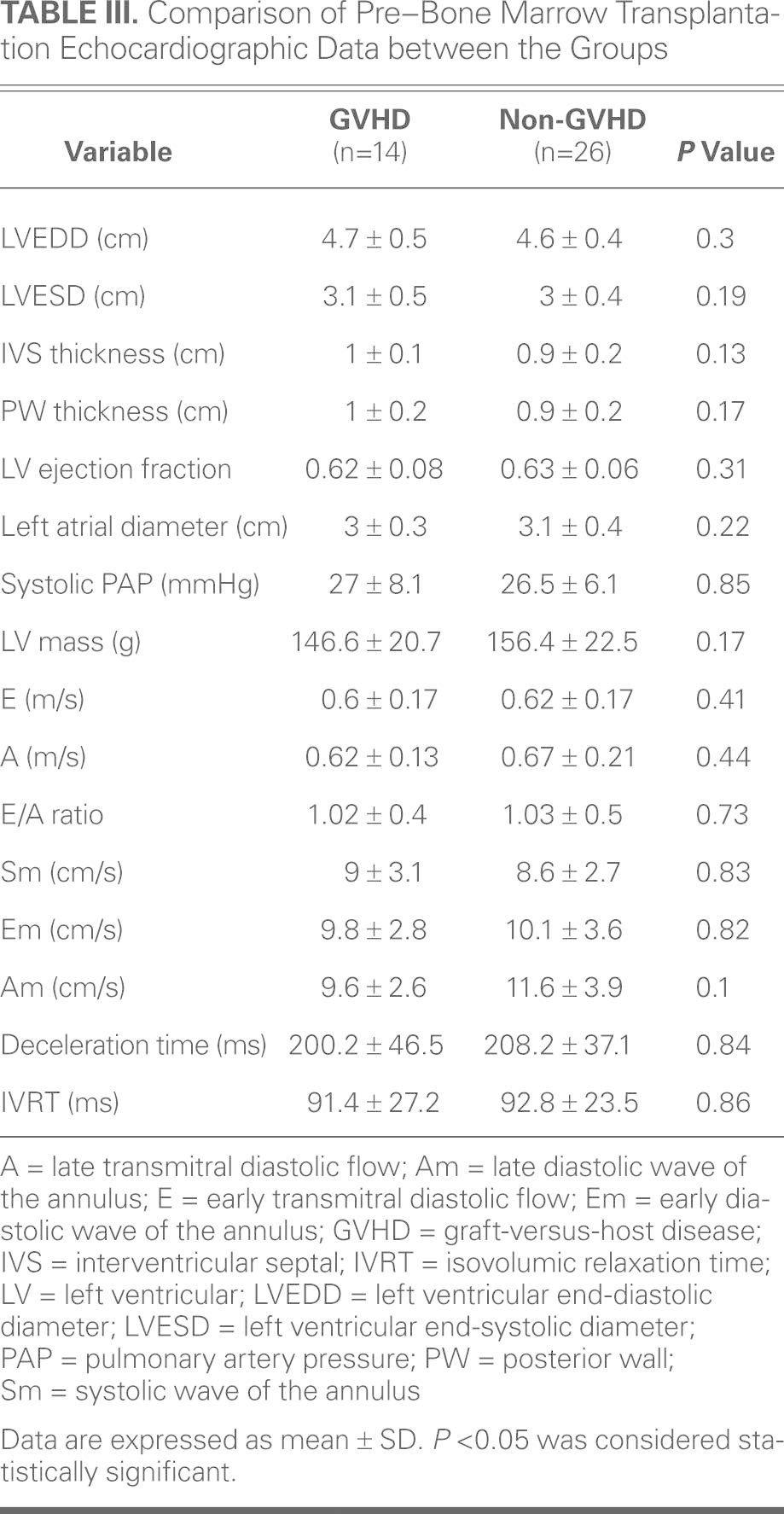
Mitral inflow Doppler analysis revealed similar results for mitral E, mitral A, E/A flow rate, and isovolumetric relaxation times between the groups. The LV lateral annulus systolic and diastolic rates were measured with tissue-Doppler echocardiography. There were no significant differences between the 2 groups in terms of Sm, Em, and Am rates.
After Bone Marrow Transplantation. Post-BMT echocardiographic data and Doppler values are presented in Table IV. Left ventricular end-systolic diameter, end-diastolic diameter, ejection fraction, and left atrial diameter measurements did not reveal a significant difference between the groups. The LV septal and posterior wall thicknesses were significantly increased in the group of patients that developed GVHD after BMT, compared to the non-GVHD group. Valve function and pulmonary artery pressure did not differ significantly between groups. Left ventricular mass was significantly higher in the GVHD group (227 ± 32.3 g vs 149.3 ± 27.4 g in the non-GVHD group) (P < 0.001).
TABLE IV. Comparison of Post–Bone Marrow Transplantation Echocardiographic Data between the Groups
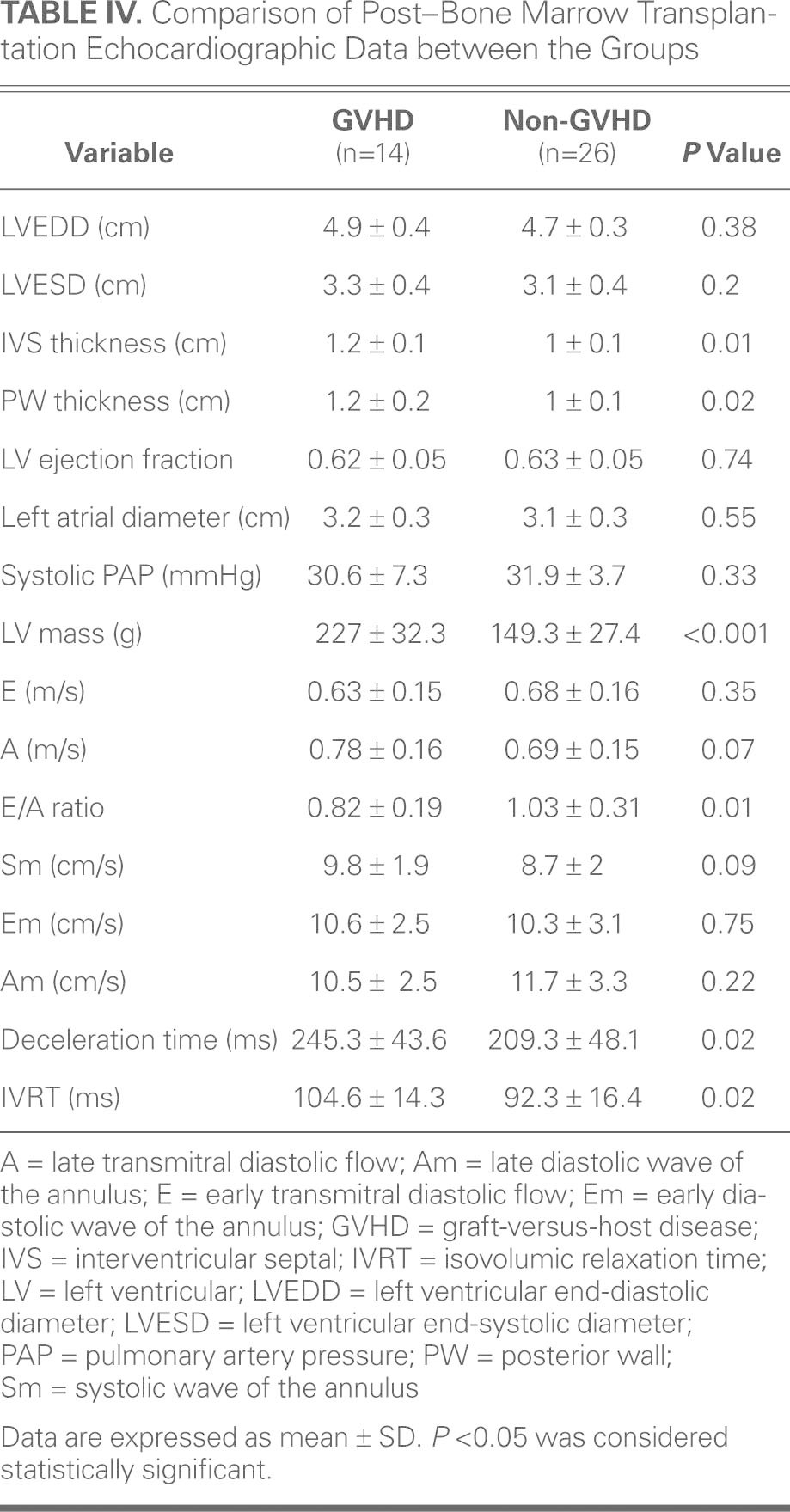
Mitral inflow Doppler echocardiographic analysis revealed that the groups had similar results for mitral E and mitral A. The E/A flow rate was significantly increased in the non-GVHD group, whereas isovolumetric relaxation times and deceleration times were significantly higher in the GVHD group. The inverse relationship between hs-CRP and E/A rate was statistically significant (r = −0.613, P = 0.02). The LV lateral annulus systolic and diastolic rates were measured by tissue-Doppler echocardiography. There were no significant differences between the 2 groups in terms of Sm, Em, and Am rates.
Discussion
Graft-versus-host disease, a result of the immunologic response that donor T-lymphocytes generate against host tissue after stem cell transplantation, is the leading cause of morbidity and death in such patients.1–5 However, the impact of diminished cardiac function on the high rates of morbidity and death observed after GVHD has not heretofore been elucidated. The present study investigated the cardiac functions of patients who did and did not develop GVHD before and after BMT, using echocardiographic methods. Left ventricular wall thickness and LV mass were significantly increased in the patient group that developed chronic GVHD, compared with the group that did not develop chronic GVHD. The LV diameters and ejection fractions were similar in the 2 groups.
Using echocardiography in 23 patients with GVHD who received cyclosporine before transplantation and for about 5 to 8 weeks thereafter, Espino and colleagues12 showed that LV mass and LV wall thicknesses increased significantly after transplantation. These authors associated the LV hypertrophy with cyclosporine use—its renal vasoconstrictive activity, systemic vasoconstriction, and prostaglandin release, together with its effects on the renin-angiotensin aldosterone system and sympathetic nerve system activation.12 Kupari and colleagues13 showed increased LV mass by performing echocardiographic analysis before and one year after transplantation on a group of 45 patients who underwent BMT and received cyclophosphamide. Our present study, which also used echocardiography to compare groups of patients who did and did not develop chronic GVHD after BMT, found higher levels of cyclosporine in patients who developed chronic GVHD. As in the studies described above, LV wall thickness and mass were significantly increased. The administration of immunosuppressive agents to patients who developed chronic GVHD after BMT—at higher doses and for longer durations than to patients who did not—might explain the significantly increased LV wall thicknesses and masses in those patients. Although the mean ages of the patients in our GVHD and non-GVHD groups were relatively younger than those of patients in the above-mentioned studies, we noted similar increases in LV wall thickness and mass. Our results indicate that increases in LV wall thickness and mass in patients who develop GVHD after BMT occur in a manner independent of age.
The adverse effect of cyclosporine on blood pressure is well known.14 The exact mechanism of cyclosporine-induced hypertension is unclear, but several hypotheses have been proposed, including increased synthesis of prostaglandin and decreased excretion of water, sodium, and potassium.15 In our study, systolic and diastolic blood pressures were higher in post-BMT than in pre-BMT patients, but these results were not statistically significant. Indirectly, cyclosporine might lead to increased LV mass and to diastolic dysfunction.
In 1976, Buja and associates16 identified cardiomegaly, cardiac atrophy, hemorrhage, and myocardial interstitial alterations in autopsies of 29 subjects who had received BMT. In the endocardial and myocardial tissues of 6 subjects, these authors found prevalent histiocytes, lymphoid cells, and plasma cells; they reported GVHD in 5 of those 6 subjects.16 In the present study, histopathologic analysis of the myocardium could not be performed because ethical considerations excluded myocardial biopsy.
There are a few studies on the effect of GVHD on LV systolic function. Impairments in systolic function are known to be caused by the administration of chemotherapeutic agents before BMT. Significant reductions in LV ejection fraction have been noted, particularly after the administration of cardiotoxic agents.17,18 In the present study, however, echocardiographic analysis revealed no significant differences in LV systolic diameter, diastolic diameter, or ejection fraction (compared to baseline values). This was true even though higher cyclosporine levels were found in patients who developed chronic GVHD after BMT. We believe that these findings should be confirmed by further studies with larger samples.
The number of studies investigating the effects of chronic GVHD on diastolic function is also very limited. To the best of our knowledge, only animal studies have examined the effect of chronic GVHD on diastolic values. In their study on mice, Gelpi and colleagues19 determined that treatment with cyclosporine prolonged IVRT and resulted in diastolic dysfunction. In our study, there were no significant differences in mitral E, lateral S, lateral E, and lateral A values measured after the development of GVHD when these values were compared with baseline in either of the groups. However, the E/A rate in the group that developed GVHD was significantly decreased when compared with the baseline E/A rate. The mean mitral A value was higher than the baseline mitral A value in the patients who developed chronic GVHD. Although there were no significant differences between the baseline E/A rates of the 2 groups, the E/A rate of the patients who developed GVHD was significantly lower than that of the comparative group. The IVRT and DT did not differ significantly in baseline and follow-up values among patients who did not develop GVHD, but the prolongation of IVRT and DT was statistically significant among patients who developed GVHD. We believe that these impairments in diastolic function among patients who developed GVHD after BMT are secondary to higher levels of cyclosporine and to the associated increases in LV wall thickness and mass among these patients.
Because CRP is an acute-phase reactant, its plasma levels increase during the process of inflammation. Higher levels of CRP in GVHD indicate the presence of an inflammatory process. Accordingly, higher levels of hs-CRP were observed in our patients who developed chronic GVHD after BMT. Among our patients who developed GVHD, those with higher hs-CRP levels had severe clinical symptoms.
In the present study, there were no significant differences between the 2 groups in terms of hs-CRP levels before BMT, although the levels were higher in the chronic-GVHD group than in the non-GVHD group after BMT. Mean hs-CRP levels increased significantly in patients after the development of GVHD (compared with pre-BMT levels), but no significant difference was noted in the group of patients who did not develop GVHD. Moreover, ESR, another indicator of systemic inflammation, was also increased in the group of patients who developed chronic GVHD after BMT. In the study by Fuji and co-authors,20 CRP levels were studied in 224 patients who underwent stem cell transplantation, and a significantly more advanced stage of GVHD developed in patients with higher levels of CRP. Schots and colleagues21 determined that CRP level was an independent factor for transplantation-associated complication and death in 96 patients with CRP >100 mg/L who had undergone stem cell transplantation. The higher ESR and hs-CRP levels found in the group of patients who developed GVHD in the present study support the conclusions of previous studies.
Limitations of the Study
The present study had a small number of subjects, but our results might inspire further studies with larger samples. Histopathologic analyses of biopsy specimens of the liver, skin, and gastrointestinal system are routinely performed to study the systemic effects of chronic GVHD. The most accurate way to evaluate the cardiac effects of GVHD would be to obtain cardiac biopsy samples and subject them to histopathologic analysis. We did not perform biopsies because the invasive nature of the procedure would have put patients at risk and raised ethical concerns.
In conclusion, our study revealed increased LV mass and impaired LV diastolic function in patients who developed chronic GVHD after BMT. Comprehensive studies with larger samples are needed to more fully understand the cardiac effects of this disease.
Footnotes
Address for reprints: Orhan Dogdu, MD, Department of Cardiology, Yozgat State Hospital, 66200 Yozgat, Turkey
E-mail: orhandogdu@yahoo.com
References
- 1.Antin JH. Clinical practice. Long-term care after hematopoietic-cell transplantation in adults. N Engl J Med 2002;347(1):36–42. [DOI] [PubMed]
- 2.Deeg HJ. Delayed complications after hematopoietic transplantation. In: Forman SJ, Blume KG, Donnall TE, editors. Hematopoietic cell transplantation. 2nd ed. Malden (MA): Blackwell Science; 1999. p. 776–88.
- 3.Kansu E, Sullivan KM. Late complications of stem cell transplantation. In: Ho A, Haas R, Champlin R, editors. Hematopoietic stem cell transplantation. New York: Marcel Dekker; 2000. p. 413–33.
- 4.Sale GE, Lerner KG, Barker EA, Shulman HM, Thomas ED. The skin biopsy in the diagnosis of acute graft-versus-host disease in man. Am J Pathol 1977;89(3):621–36. [PMC free article] [PubMed]
- 5.Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981;57(2):267–76. [PubMed]
- 6.Socie G, Mary JY, Esperou H, Robert DV, Aractingi S, Ribaud P, et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. Br J Haematol 2001;113(1):194–201. [DOI] [PubMed]
- 7.Curtis RE, Rowlings PA, Deeg HJ, Shriner DA, Socie G, Travis LB, et al. Solid cancers after bone marrow transplantation. N Engl J Med 1997;336(13):897–904. [DOI] [PubMed]
- 8.Miflin G, Russell NH, Hutchinson RM, Morgan G, Potter M, Pagliuca A, et al. Allogeneic peripheral blood stem cell transplantation for haematological malignancies–an analysis of kinetics of engraftment and GVHD risk. Bone Marrow Transplant 1997;19(1):9–13. [DOI] [PubMed]
- 9.Schlegel PG. The role of adhesion and costimulation molecules in graft-versus-host disease. Acta Haematol 1997;97(1–2):105–17. [DOI] [PubMed]
- 10.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11(12):945–56. [DOI] [PubMed]
- 11.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977;55(4):613–8. [DOI] [PubMed]
- 12.Espino G, Denney J, Furlong T, Fitzsimmons W, Nash RA. Assessment of myocardial hypertrophy by echocardiography in adult patients receiving tacrolimus or cyclosporine therapy for prevention of acute GVHD. Bone Marrow Transplant 2001;28(12):1097–103. [DOI] [PubMed]
- 13.Kupari M, Volin L, Suokas A, Hekali P, Ruutu T. Cardiac involvement in bone marrow transplantation: serial changes in left ventricular size, mass and performance. J Intern Med 1990;227(4):259–66. [DOI] [PubMed]
- 14.Vercauteren SB, Bosmans JL, Elseviers MM, Verpooten GA, De Broe ME. A meta-analysis and morphological review of cyclosporine-induced nephrotoxicity in auto-immune diseases. Kidney Int 1998;54(2):536–45. [DOI] [PubMed]
- 15.Kutkuhn B, Hollenbeck M, Heering P, Koch M, Voiculescu A, Reinhard T, Grabensee B. Development of insulin resistance and elevated blood pressure during therapy with cyclosporine A. Blood Press 1997;6(1):13–7. [DOI] [PubMed]
- 16.Buja LM, Ferrans VJ, Graw RG Jr. Cardiac pathologic findings in patients treated with bone marrow transplantation. Hum Pathol 1976;7(1):17–45. [DOI] [PubMed]
- 17.Kocak G, Erbil KM, Ozdemir I, Aydemir S, Sunar B, Tuncel M, Atalay S. The protective effect of melatonin on adriamycin-induced acute cardiac injury. Can J Cardiol 2003;19(5):535–41. [PubMed]
- 18.Oredipe OA, Furbert-Harris PM, Laniyan I, Green WR, Griffin WM, Sridhar R. Mice primed with swainsonine are protected against doxorubicin-induced lethality. Cell Mol Biol (Noisy-le-grand) 2003;49(7):1089–99. [PubMed]
- 19.Gelpi RJ, Gao S, Zhai P, Yan L, Hong C, Danridge LM, et al. Genetic inhibition of calcineurin induces diastolic dysfunction in mice with chronic pressure overload. Am J Physiol Heart Circ Physiol 2009;297(5):H1814–9. [DOI] [PMC free article] [PubMed]
- 20.Fuji S, Kim SW, Fukuda T, Mori S, Yamasaki S, Morita-Hoshi Y, et al. Preengraftment serum C-reactive protein (CRP) value may predict acute graft-versus-host disease and nonrelapse mortality after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2008;14(5):510–7. [DOI] [PubMed]
- 21.Schots R, Van Riet I, Othman TB, Trullemans F, De Waele M, Van Camp B, Kaufman L. An early increase in serum levels of C-reactive protein is an independent risk factor for the occurrence of major complications and 100-day transplant-related mortality after allogeneic bone marrow transplantation. Bone Marrow Transplant 2002;30(7):441–6. [DOI] [PubMed]


