Abstract
Mitochondrial disease is a heterogeneous group of multisystemic diseases that develop consequent to mutations in nuclear or mitochondrial DNA. The prevalence of inherited mitochondrial disease has been estimated to be greater than 1 in 5,000 births; however, the diagnosis and treatment of this disease are not taught in most adult-cardiology curricula. Because mitochondrial diseases often occur as a syndrome with resultant multiorgan dysfunction, they might not immediately appear to be specific to the cardiovascular system. Mitochondrial cardiomyopathy can be described as a myocardial condition characterized by abnormal heart-muscle structure, function, or both, secondary to genetic defects involving the mitochondrial respiratory chain, in the absence of concomitant coronary artery disease, hypertension, valvular disease, or congenital heart disease. The typical cardiac manifestations of mitochondrial disease—hypertrophic and dilated cardiomyopathy, arrhythmias, left ventricular myocardial noncompaction, and heart failure—can worsen acutely during a metabolic crisis. The optimal management of mitochondrial disease necessitates the involvement of a multidisciplinary team, careful evaluations of patients, and the anticipation of iatrogenic and noniatrogenic complications.
In this review, we describe the complex pathophysiology of mitochondrial disease and its clinical features. We focus on current practice in the diagnosis and treatment of patients with mitochondrial cardiomyopathy, including optimal therapeutic management and long-term monitoring. We hope that this information will serve as a guide for practicing cardiologists who treat patients thus affected.
Key words: Cardiomyopathies/genetics/pathology/therapy; DNA, mitochondrial/analysis/genetics; energy metabolism/physiology; electron transport/physiology; genetic predisposition to disease; heart diseases/genetics; mitochondria/physiology; mitochondrial diseases/complications/diagnosis/genetics/physiopathology/drug therapy; risk factors; ventricular dysfunction, left/genetics
The myocardium depends on a high level of aerobic metabolism to supply blood and energy substrate to all organs of the body. The mitochondria have a key role in energy production and in the growth and regulation of cardiac bioenergetic arrangements. Specific mitochondrial diseases have been attributed to mitochondrial mutations, and cardiac involvement is frequent. However, these syndromes are generally not covered comprehensively in cardiology curricula and might not be widely recognized by practicing cardiologists who treat adults. Recent research has shown that derangements of energy metabolism are ultimately implicated in most forms of heart failure. In this review, we describe the biologic characteristics of the mitochondria and their role in cardiac bioenergetic arrangements, discuss the spectrum of mitochondrial disease, and provide a guide for practicing cardiologists to use when treating patients affected by mitochondrial crisis.
The Mitochondria: Energy Flow and Adaptation
Energy flow fuels growth, survival, and reproduction. Mitochondria are crucial to the flow of energy in cells. The 4 primary cellular functions of mitochondria are to supply energy to the cell in the form of adenosine triphosphate (ATP), generate and regulate reactive oxygen species, buffer cytosolic calcium ions, and regulate apoptosis through the mitochondrial permeability transition pore.1 In addition, mitochondria have a central role in protecting cellular metabolism against reactive oxygen species.2
Mitochondria presumably originated as parasites that formed a symbiotic relationship with eukaryotic cells more than 2 billion years ago, in response to an increase in atmospheric oxygen. As the symbiosis matured, the 2 organisms consolidated their metabolic pathways and exchanged genes to support multicellularity.3 The genes that regulate the mitochondrial genome were perhaps transferred to the nuclear DNA of eukaryotic cells during this genetic exchange. The transferred genes, including those that govern mitochondrial growth, regulation, and oxidative phosphorylation, were interspersed among the existing cellular bioenergetic genes in nuclear DNA (nDNA). As a result, the mitochondria retained 13 genes that encode subunits of the electron transport chain. In addition, mitochondrial DNA (mtDNA) encodes 2 ribosomal RNAs and 22 transfer RNAs that are involved in mitochondrial transcription and translation. Most mitochondrial proteins became encoded by the host nuclear genome, so cellular machinery evolved to import these proteins into the mitochondria and to target them to specific locations within the inner or outer membranes or the matrix of the mitochondria.3
The electron transport chain comprises 5 enzyme complexes embedded within the inner mitochondrial membrane. As electrons are transferred from one complex to the next, the released energy is used to pump protons across the mitochondrial membrane; this creates an electrochemical proton gradient. The potential energy generated by this gradient is used to produce heat, import proteins and calcium ions, and generate ATP from adenosine diphosphate. This electrochemical gradient is crucial to the energetic arrangements of cells, and mitochondrial enzyme complexes must work together to maintain it.
Disruptions in energy production have been implicated in a broad range of metabolic, neoplastic, degenerative, and age-related diseases. Mitochondrial disease is characterized by defects in the mitochondrial respiratory chain that result in deficient ATP production. Research conducted during the last 2 decades reveals that mitochondrial disease can result from mutations in either the mtDNA or the nDNA. Moreover, mitochondrial disease occurs far more frequently than was thought: 1 in 10,000 individuals has mitochondrial disease, and mtDNA mutations are found as frequently as in 1 of every 200 samples of cord blood.4,5 Mitochondrial disease usually affects tissues with high energy demands, such as the heart, brain, muscles, and endocrine system; however, any organ can be affected. In addition, mitochondrial disease has been implicated in aging and the development of diabetes mellitus, cardiovascular disease, and numerous other degenerative diseases.6 Therefore, understanding the complex biologic characteristics of the mitochondria is necessary for understanding this complicated group of diseases.
Cells contain hundreds of mitochondria, and each mitochondrion contains hundreds of copies of mtDNA. Accordingly, cells contain thousands of copies of mtDNA. When a mutation arises in mtDNA, it creates a mixed population of wild-type and mutant mtDNA within a single cell, which results in an unusual biologic state called heteroplasmy.7 As heteroplasmic cells divide, the mtDNA is distributed randomly to the daughter cells; curiously, this is known to result in cells with skewed populations of wild-type or mutant mtDNA. Therefore, the random mitotic segregation of mtDNA causes variations in heteroplasmy that result in varying proportions of mutant mtDNA in daughter cells.8–10 These variations in heteroplasmy are a key feature of mitochondrial disease. In addition, it is thought that the degree of heteroplasmy determines the clinical phenotype; that is, the clinical disease state becomes more severe as the proportion of mutant mtDNA increases. The absolute threshold that determines a disease state is usually 50% to 100% mutant mtDNA; however, clinical disease states have been reported when mutant mtDNA levels were as low as 10%.11 Furthermore, differences in the phenotypic expression of mitochondrial disease are influenced by the energy requirement of the specific tissue. Tissues that are metabolically active have a greater vulnerability to defects in mtDNA.9,12 Because mtDNA encodes only those genes that are involved in mitochondrial maintenance or oxidative phosphorylation, the unique system of mitochondrial inheritance might enable an ideal genetic system for rapid adaptation in response to changes in regional energy environments. Furthermore, this rapid adaptation might explain the higher mutation rate observed in mtDNA than in nDNA.13,14
Tissues with high aerobic metabolism demands, such as brain tissue, heart muscle, and skeletal muscle, are usually more severely affected in patients with mitochondrial disease. As a result, mitochondrial encephalomyopathy and cardiomyopathy are typically the most prominent manifestations. In a study of infants and children hospitalized with mitochondrial diseases, Holmgren and colleagues15 reported cardiomyopathy in 17% of the patients, and a mortality rate that was higher in the children with cardiomyopathy (71%) than in those without cardiac involvement (26%).
Pathophysiology
Although multiple biochemical pathways involve mitochondria, the term primary mitochondrial disease has typically been used to describe those diseases in which energy production is impaired through the alteration of oxidative phosphorylation. Mitochondrial diseases can be classified genetically, functionally, or biochemically. Genetic classification refers to the location of a mutation in either the mtDNA or the nDNA. Mitochondrial diseases arising from mtDNA are more prevalent in adults, whereas diseases arising from nDNA tend to be more prevalent in infants and children.16 Mitochondrial diseases can also be classified by the function of the proteins involved in the disease. Mutations can be found in genes that encode subunits of the electron transport chain complexes; in genes that encode ancillary proteins needed for the assembly, transport, and function of the electron transport chain complexes; or in genes that control activities of the mitochondria. In addition, mutations have been described in genes encoding proteins that synthesize cardiolipin, an integral part of the inner mitochondrial membrane.17,18 The most frequently identified biochemical abnormalities are deficiencies in NADH-coenzyme Q (CoQ) reductase (complex I) and cytochrome-c oxidase (complex IV)17,18 (Fig. 1).
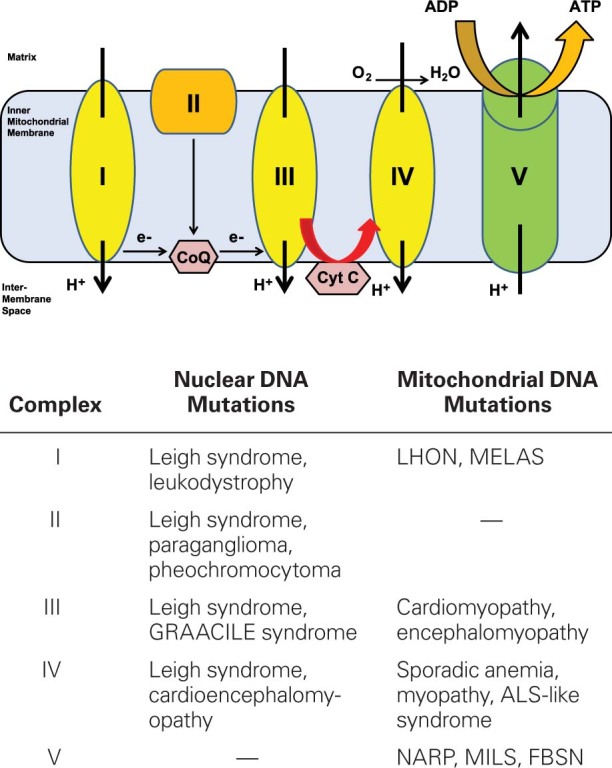
Fig. 1 Diagram shows the pathophysiology of mitochondrial disorders. The electron transport chain comprises complexes I to V, which are encoded by both nuclear and mitochondrial DNA. Mutations in either form of DNA can cause mitochondrial disorders.
ADP = adenosine diphosphate; ALS = amyotrophic lateral sclerosis; ATP = adenosine triphosphate; CoQ = coenzyme Q; Cyt C = cytochrome c; FBSN = familial bilateral striatal necrosis; GRAACILE = growth retardation, aminoaciduria, lactic acidosis, and early death; LHON = Leber hereditary optic neuropathy; MELAS = mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; MILS = maternally inherited Leigh syndrome; NARP = neuropathy, ataxia, and retinitis pigmentosa
Clinical Features
Because the heart is a muscle with high energy demands, most patients with mitochondrial disease are susceptible to cardiac involvement. Mitochondrial cardiomyopathy can be described as a myocardial disorder characterized by abnormal heart-muscle structure, function, or both, secondary to genetic defects involving the mitochondrial respiratory chain, in the absence of concomitant coronary artery disease, hypertension, valvular disease, and congenital heart disease. The presentation of mitochondrial cardiomyopathy includes hypertrophic, dilated, and left ventricular (LV) noncompaction,15,19 and the severity can range from no symptoms to devastating multisystemic disease. Severe cardiac manifestations include heart failure and ventricular tachyarrhythmia—which can worsen acutely during a metabolic crisis—and sudden cardiac death. Mitochondrial crisis is often precipitated by physiologic stressors such as febrile illness or surgery and can be accompanied by acute heart failure.
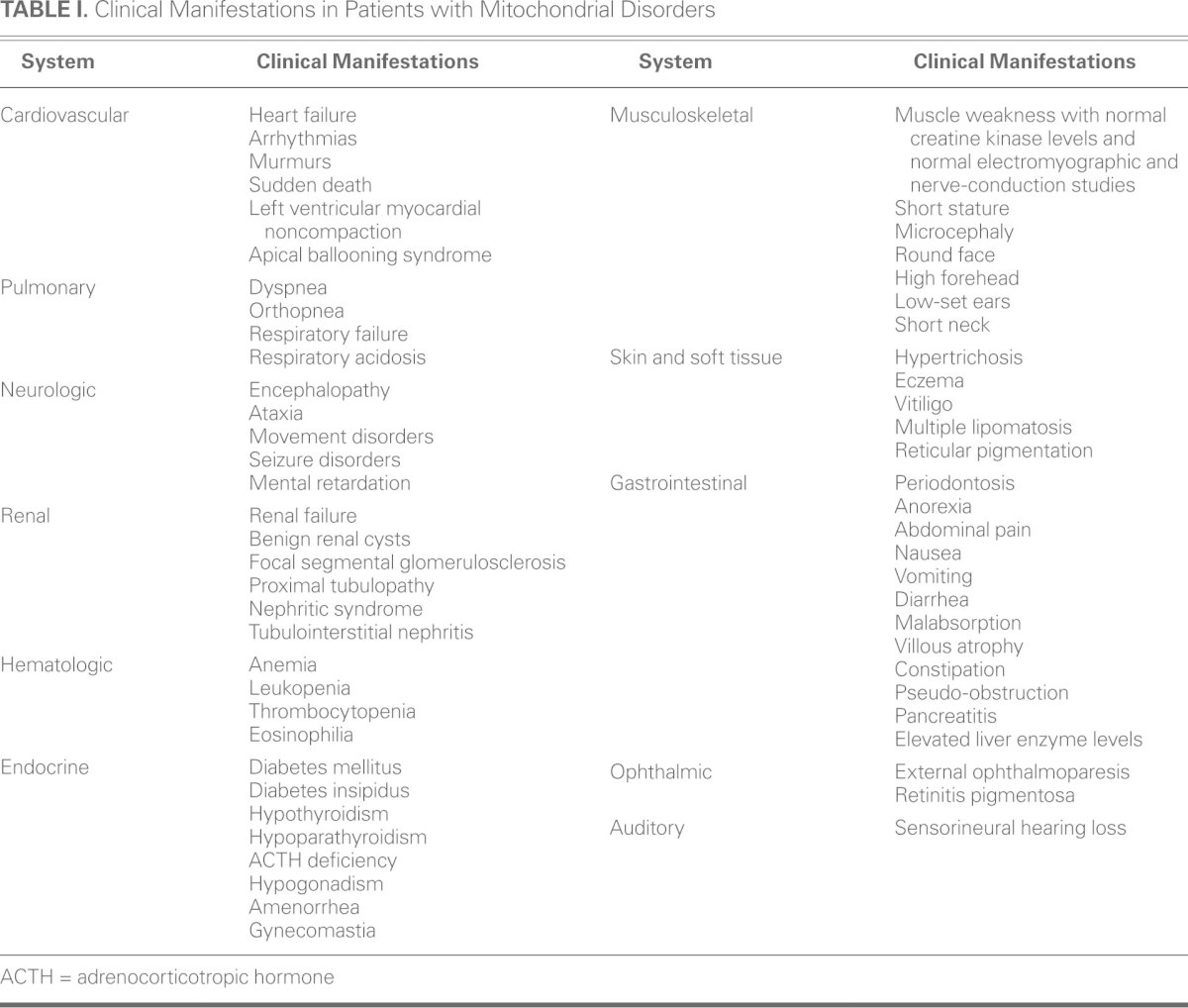
The clinical expression of mitochondrial cardiomyopathy is often accompanied by multisystemic disease (Table I). Most patients with neuromuscular symptoms present with normal or slightly elevated creatine kinase levels, a normal electromyogram, and normal results of nerve-conduction studies.20–23 Abnormal liver enzyme levels have been found in up to 10% of patients.16,21 Renal manifestations include proximal tubulopathy, nephritic syndrome, tubulointerstitial nephritis, and nonspecific renal failure.21,24 Pancytopenia is seen in 10% of patients.21 Endocrinopathies include diabetes mellitus, diabetes insipidus, hypothyroidism, hypoparathyroidism, adrenocorticotropic hormone deficiency, and hypogonadism.16 Short stature occurs in 20% of patients.20 Dysmorphic features are uncommon but can be similar to those seen in fetal alcohol syndrome: microcephaly, round face, high forehead, featureless philtrum, low-set ears, and short neck.21 Dermatologic features include hypertrichosis, eczema, vitiligo, multiple lipomatosis, and reticular pigmentation.15,25 Retinitis pigmentosa is the chief ophthalmologic abnormality.20 Sensorineural hearing loss occurs in 7% to 26% of patients, and its prevalence increases with age.20,26 Anorexia, abdominal pain, nausea, vomiting, diarrhea, constipation, and chronic intestinal pseudo-obstruction are the typical gastrointestinal symptoms.27,28
TABLE I. Clinical Manifestations in Patients with Mitochondrial Disorders
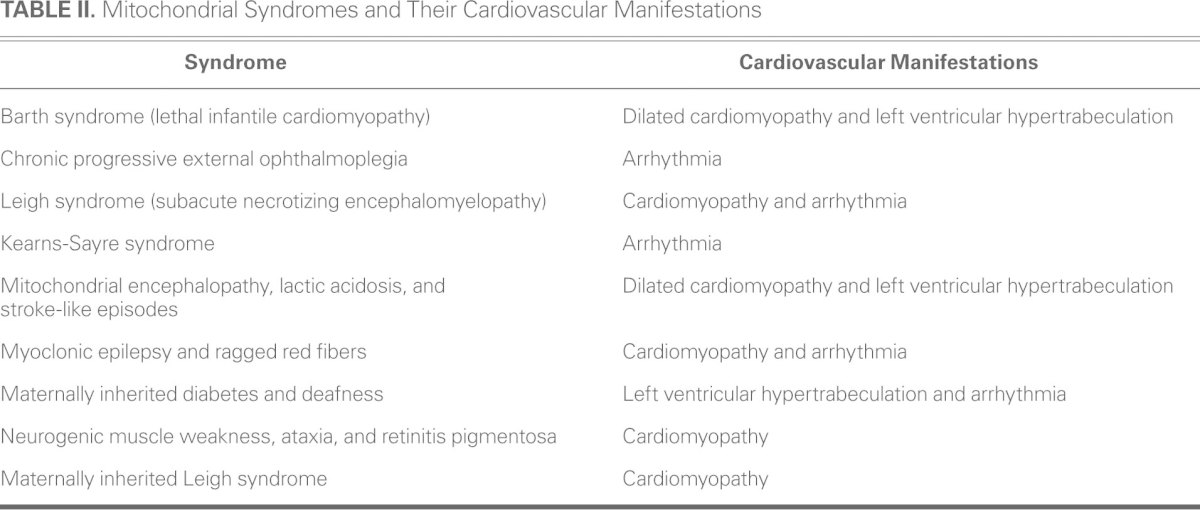
Several mitochondrial disorders with distinct clinical syndromes have been identified. Characteristic mitochondrial syndromes, which may be recognized by pediatric or adult neurologists, are listed with their associated cardiovascular manifestations in Table II. They include mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS); Leigh syndrome (subacute necrotizing encephalomyelopathy); myoclonic epilepsy with ragged red fibers (MERRF); maternally inherited deafness and diabetes (MIDD); neuropathy, ataxia, and retinitis pigmentosa (NARP); mitochondrial neurogastrointestinal encephalopathy (MNGIE); growth retardation, aminoaciduria, cholestasis, iron overload, lactic acidosis, and early death (GRAACILE); X-linked cardiomyopathy, mitochondrial myopathy, and cyclic neutropenia (Barth syndrome); Leber hereditary optic neuropathy (LHON); sideroblastic anemia and pancreatic dysfunction (Pearson syndrome); and Kearns-Sayre syndrome characterized by chronic progressive external ophthalmoplegia with pigmentary retinopathy, short stature, cerebellar ataxia, mental retardation, and cardiac conduction defects.
TABLE II. Mitochondrial Syndromes and Their Cardiovascular Manifestations
Diagnosis
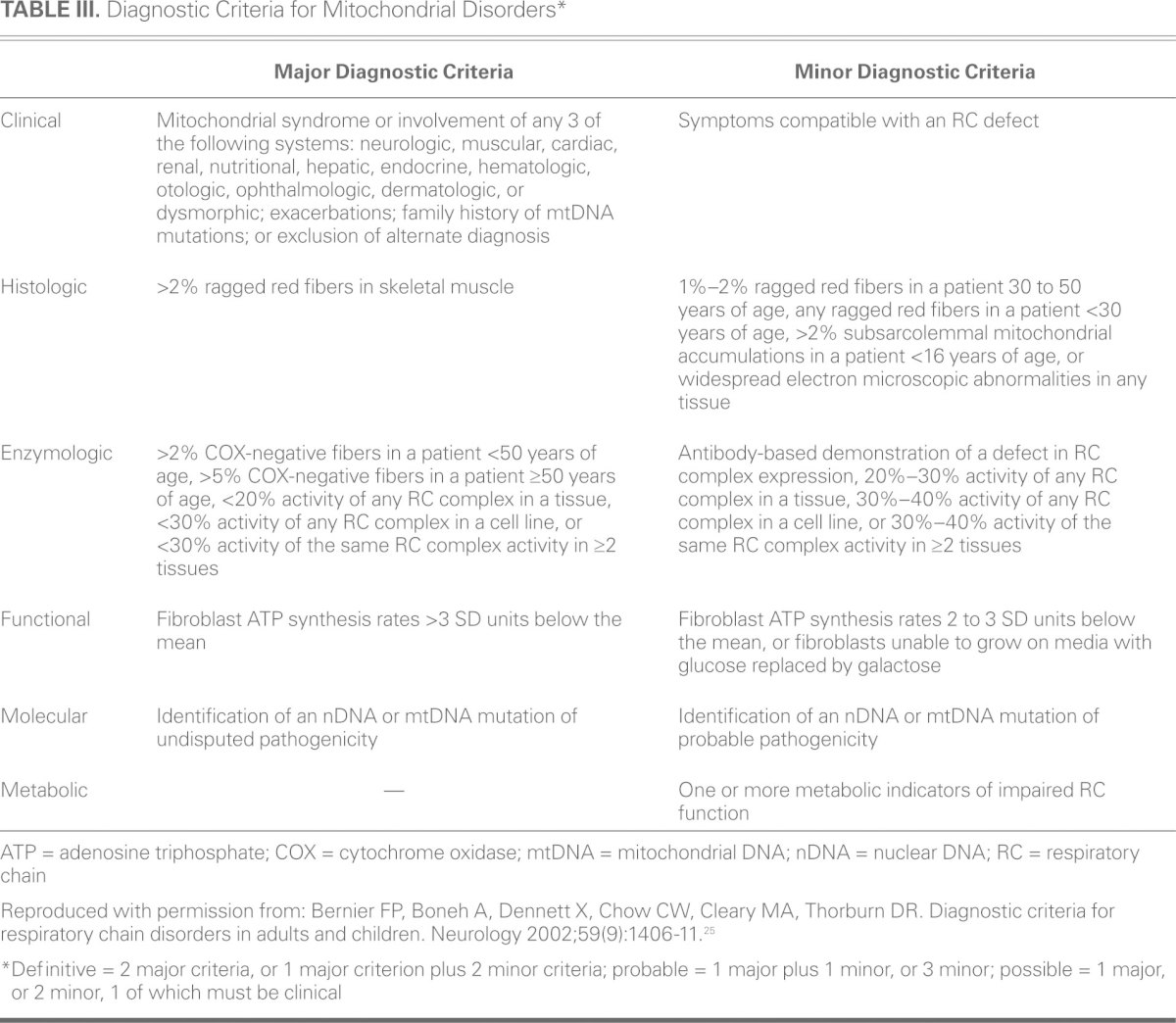
Primary mitochondrial diseases are clinically, biochemically, and genetically heterogeneous, and their diagnosis can be challenging. A family history of maternal inheritance or multisystemic disease should raise concern. A classic Mendelian inheritance pattern (autosomal dominant, autosomal recessive, or X-linked) usually indicates a nuclear mutation.18 Of note, because of the curious state of heteroplasmy, not all persons with mtDNA mutations will manifest symptomatic disease. Furthermore, if nDNA mutation is indeed present, multisystemic disease might not be present early in the disease course. Several proposed diagnostic criteria incorporate clinical, histologic, enzymologic, functional, and molecular factors. These criteria have improved the detection of mitochondrial disease (Table III)25; however, most are subject to interpretation, and standardization is not possible.
TABLE III. Diagnostic Criteria for Mitochondrial Disorders*
Muscle biopsy is considered to be the gold standard for the diagnosis of mitochondrial disease; however, its specificity and sensitivity are not 100%. Muscle biopsy specimens are routinely examined for structural changes with the use of light microscopy, histochemistry with specific enzymes, and ultrastructural examination by electron microscopy. Ragged red fibers arising from the subsarcolemmal accumulation of mitochondria as seen on a modified Gomori trichrome stain are considered to be the histologic hallmark of mitochondrial dysfunction (Fig. 2), but these fibers are typically absent in children and are seen only in late-stage disease in many adults. Cytochrome oxidase-negative fibers, the pathologic abnormality most frequently yielded by muscle biopsies, have not been reported in healthy individuals younger than 30 years of age. Other nonspecific findings in muscle biopsy specimens are internal nuclei, atrophic fibers, large quantities of lipid droplets, fiber-type grouping, type I or II fiber atrophy, fiber hypertrophy, glycogen, and inflammation.16,29 In some cases of mitochondrial disease, muscle biopsy findings can be completely normal histologically. Electron microscopy can sometimes reveal subsarcolemmal, enlarged, swollen mitochondria with irregular cristae and paracrystalline inclusions. Evaluation of electron transport chain function with use of polarographic and spectrophotometric assays is often performed in muscle tissue30,31; however, standards are not available for cardiac tissue analysis. Mitochondrial genome screening is typically performed on the muscle sample. Nuclear DNA abnormalities are often difficult to diagnose, because only a small proportion of these nDNA mutations have been identified. Despite the use of optimal diagnostic tools, results can be ambiguous and can pose a challenge for clinicians.
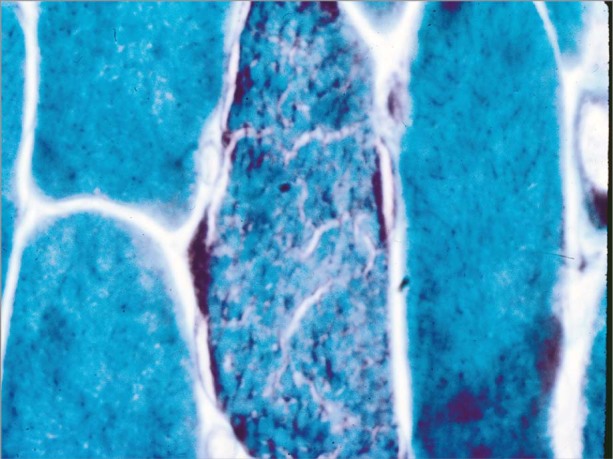
Fig. 2 Photomicrograph shows ragged red fibers in muscle tissue obtained from a patient with chronic progressive external ophthalmoplegia (Gomori trichrome stain, orig. ×400). Image courtesy of Ian J. Butler, Department of Neurology, University of Texas Health Science Center.
In cardiac specimens from patients with mitochondrial cardiomyopathy, the morphologic features are not specific, and the differential diagnosis encompasses a broad range of genetic, storage, metabolic, and environmental causes. In infants and children, mitochondrial cardiomyopathy frequently presents with a hypertrophic morphology that is usually associated with endocardial fibroelastosis and asymmetric septal hypertrophy.32 Microscopic features that suggest cardiomyopathy in mitochondrial disease include fusiform enlargement of affected myocytes around the perinuclear region with cytoplasmic clearing and replacement of cross striae by fine granules. Ultrastructural examination of endomyocardial biopsy samples from adults with mtDNA mutations has yielded more (48%) and larger (7%) mitochondria, higher glycogen concentrations (12%), greater lipid accumulation (8%), abnormal cristae, and more paracrystalline inclusions (3%) than in healthy tissue.33 Bernier and colleagues25 reported ultrastructural cardiac abnormalities in only 13% of patients who were known to have respiratory chain disease.
When mitochondrial disease is known or suspected, the cardiac examination should be directed toward eliciting signs of heart failure, including cardiac enlargement, elevated jugular venous pressure, auscultation of S3 and S4 gallop, bilateral lung crackles, pitting edema, hepatomegaly, and signs of hypoperfusion. The diagnostic laboratory evaluation should include a complete blood count and measurements of electrolyte levels, liver function, thyroid function, blood glucose, hemoglobin A1c, creatine kinase, lactate, and pyruvate. Echocardiography and 12-lead electrocardiography are necessary initially, and perhaps also repeatedly if a crisis state is suspected.
A high degree of suspicion is important when considering a diagnosis of mitochondrial disease. Clinicians should consider possible mitochondrial cardiomyopathy in any patient who presents with signs of acute heart failure and multisystemic involvement without a clear explanation, and the patient should be referred to a geneticist or other specialist who is familiar with diagnosing mitochondrial disease.
Cardiac Presentation of Mitochondrial Disease
Referrals for cardiac evaluation typically come from neurologists or geneticists who have diagnosed a mitochondrial disorder. Cardiologists who evaluate patients for hypertrophy, conduction abnormalities, and dilated cardiomyopathy should be aware of the spectrum of mitochondrial disease so that they can collaborate with neurologists, geneticists, and mitochondrial-disease specialists to make accurate diagnoses and arrange appropriate care for these patients. Certain syndromes predispose patients to specific abnormalities.34 For example, patients with Kearns-Sayre syndrome are predisposed to atrioventricular conduction defects that can present as syncope, Adams-Stokes syndrome, or sudden death. In these patients, retinopathy and ophthalmoplegia tend to occur before heart-block syndromes develop. Therefore, when ophthalmoplegia has been noted, affected patients should be monitored closely. Patients with MERRF and MELAS should be monitored for the development of cardiac hypertrophy and dilated cardiomyopathy. Patients with MERRF can have myoclonus, generalized convulsions, cerebellar ataxia, muscular atrophy, and elevated blood lactate and pyruvate levels, as well as ragged red fibers in muscle biopsy specimens. A case series of patients with MERRF and an m.8344A>G mutation of mtDNA revealed that early age of onset was the only factor associated with the occurrence of myocardial disease.35 The development of myocardial disease in this cohort was associated with a higher risk of sudden cardiac death. Patients with MELAS can also have ragged red fibers upon muscle biopsy; however, unlike MERRF patients, MELAS patients have normal early development and start to show symptoms only between 3 years of age and adulthood. Patients with MELAS tend to have short stature, seizures, hemiparesis, hemianopia, and blindness.34
Mitochondrial mutations are the typical cause of LV myocardial noncompaction, which is also known as LV hypertrabeculation (LVHT). Left ventricular hypertrabeculation is characterized by prominent ventricular trabeculations and deep recesses that extend from the LV cavity to the subendocardial surface of the ventricle, accompanied or not by LV dysfunction.36–38 Advances in diagnostic imaging and its widespread availability have led to more frequent diagnosis of LVHT; however, there is currently little agreement about the various diagnostic criteria that have been proposed.39,40 Left ventricular hypertrabeculation can be found in isolation or in association with other genetic neuromuscular conditions, cardiac abnormalities, or genetic syndromes. It has been clearly shown that LVHT is more often associated with genetic abnormalities than not.33 Patients who present with LVHT should be investigated for genetic disorders, particularly neuromuscular, and it is important to screen first-degree relatives.
Bioenergetic derangements are increasingly recognized as major culprits in the development of cardiac hypertrophy and in the progression to heart failure, in both acquired and inherited disease.41 The mitochondria are a crucial platform for energy transduction, signaling, and cell-death pathways that are broadly relevant to heart failure, even in the absence of an underlying mitochondrial myopathy. Oxidative stress and mitochondrial dysfunction are key factors in the development of most heart failure. The high energy demands of the heart change continually with variations in physiologic demand. The pathways that generate ATP must respond proportionally to myocardial demand, and this necessitates finely tuned metabolic responsiveness. These metabolic responses have short- and long-term components that function at multiple levels, including control of enzyme activity, signal transduction events, and the regulation of genes that encode rate-limiting enzymes.42
Studies have shown the importance of substrate flexibility in preserving normal cardiac function. In experimental models of pressure overload, failing human hearts have shifted from oxidizing fatty acids (the preferred substrate in the healthy heart) to oxidizing glucose for energy production.43–47 This metabolic switch is associated with the downregulation of genes involved in mitochondrial biogenesis and fatty-acid metabolism and is mediated by the deactivation of PPAR-α and its activator, PGC-α, which are members of a family of transcriptional coactivators involved in mitochondrial regulation and biogenesis. An increased reliance on glycolytic pathways could effectively reduce oxygen consumption in the short term; however, over time, reduced oxygen consumption might enable the progression of cardiac disease by creating an energy-deficient state.41,48 Identifying the mechanism by which alterations in substrate utilization cause cardiomyopathy is an area of intense research. Experimental evidence shows that elevated fatty-acid flux and fatty-acid oxidation (FAO)-deficient states can both be associated with cardiac dysfunction. Chronic increases in FAO, as seen in diabetes, and decreases in FAO, as seen in pressure-overload models of heart failure, can both lead to heart failure.42,47,49 Accordingly, energy deficiency can be broadly conceived as both a cause and an effect of heart failure.
Management
The management of mitochondrial disease and cardiomyopathy is largely supportive. Physicians should be aware that patients can make a remarkable recovery from a severe crisis state. Pharmacologic strategies include the use of various dietary supplements. A typical “mitochondrial cocktail” would include coenzyme Q10 (CoQ10), creatine, L-carnitine, thiamine, riboflavin, folate, and other antioxidants such as vitamins C and E. Studies have suggested that the use of antioxidants partially improves clinical features30,31,48; in contrast, a systematic review by Chinnery and colleagues50 found no clear evidence to support the use of any supplement in patients with mitochondrial disease. In a randomized, double-blinded, crossover trial, 30 patients with mitochondrial cytopathy were given 1,200 mg/d of CoQ10 for 60 days.51 Monitoring was of blood lactate levels, urinary markers of oxidative stress, body composition, activities of daily living, quality of life, forearm handgrip strength, oxygen desaturation, cycle exercise, cardiorespiratory variables, and brain metabolites. The authors concluded that therapy with CoQ10 for 60 days had minor positive effects on cycle exercise, aerobic capacity, and post-exercise lactate levels, but not on grip strength, activities of daily living, and quality of life.51 The modest therapeutic benefits of CoQ10 are attributed to its function as an electron acceptor in the mitochondrial electron transport chain and its ability to scavenge free radicals. In a randomized controlled trial conducted in 7 patients with mitochondrial cytopathies, therapy with creatine monohydrate increased the patients' strength in high-intensity anaerobic- and aerobic-type activities but did not improve their performance of lower-intensity aerobic activities.52 L-carnitine supplementation is highly effective in patients who have dilated cardiomyopathy secondary to primary systemic carnitine deficiency; however, it has little effect on other types of mitochondrial cardiomyopathy. Dichloroacetate therapy to reduce lactic acid accumulation has not improved clinical or biochemical values and has been associated with severe, irreversible polyneuropathy,53 so this therapy should not be used. L-arginine infused in patients during the acute phase of MELAS improved their clinical symptoms within the first 24 hours, and an 18-month regimen of oral arginine reduced the frequency and severity of stroke symptoms.54
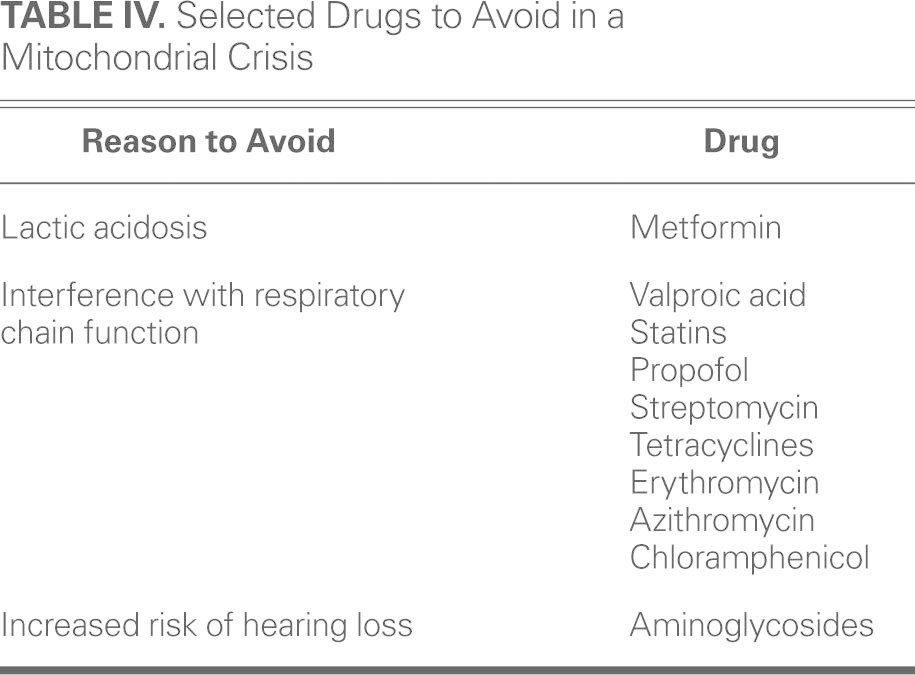
Patients with mitochondrial disease should avoid certain medications that interfere with mitochondrial function and can precipitate a crisis state (Table IV).
TABLE IV. Selected Drugs to Avoid in a Mitochondrial Crisis
Crisis Management
The mortality rate can be high in patients with mitochondrial disease that manifests into a crisis state, so urgent treatment is necessary. A mitochondrial crisis is defined as acute or subacute multiorgan failure secondary to mitochondrial respiratory chain function that is worsening because of fever, illness, stress, medications, or heat. Crises can be associated with striking elevations in lactate levels. Cardiac complications during a crisis include cardiogenic shock, atrial and ventricular arrhythmias, dilated cardiomyopathy, and sudden cardiac death. Management should be directed toward the underlying cause of the crisis and emphasize care that can optimize mitochondrial function. Table V shows a general management approach, including laboratory tests and recommended steps and precautions.
TABLE V. General Approach to Management of Acute Mitochondrial Crisis

Patients often have baseline acidemia, and the correction of acidosis should be gradual. Upon suspicion of infection, therapy with broad-spectrum antibiotics can be started. Oxygenation can worsen the crisis by increasing free-radical production, so the PaO2 should be maintained between 50 and 60 mmHg.55 To improve blood flow and correct hyperammonemia, a continuous infusion of 10% arginine should be started with a loading dose of 600 mg/kg and maintained at 600 mg/kg/d.56–58 Arginine is best administered through a central line, to prevent tissue necrosis and phlebitis; however, this therapy has been associated with hypotension, nausea, emesis, headache, and life-threatening hyperkalemia. In patients with worsening renal function, hemodialysis should be considered to correct lactic acidosis, hyperammonemia, or hyperkalemia.
Patients with mitochondrial disease who present with fever or are unable to eat or drink should be given dextrose-containing intravenous fluids—preferably D10 with half-normal saline content—at a maintenance dose, regardless of blood glucose levels. Their metabolic and volume status should be evaluated periodically. The management of these patients' cardiac complications, including heart failure, bradyarrhythmias, and tachyarrhythmias, follows the same guidelines as those for the general population. Therapy with β-blockers and angiotensin-converting enzyme inhibitors should be considered in all patients who have cardiomyopathy at the time of discharge from the hospital, even though there are no randomized controlled trials to support the use of such therapies in this population. If cardiac dysfunction is noted during a crisis, patients should be closely monitored: serial echocardiography should be performed upon their hospital discharge and during their 1- and 3-month follow-up examinations. In selected patients who have advanced heart failure due to cardiomyopathy, cardiac transplantation can be considered. Three pediatric patients with mitochondrial cardiomyopathy who underwent cardiac transplantation reportedly had excellent early and late outcomes.59
Conclusion
Mitochondrial cardiomyopathy is a distinct clinical entity in patients who have underlying genetic defects that involve the mitochondrial respiratory chain. Because diagnosing mitochondrial disease can be challenging for clinicians, the first step is maintaining a high degree of suspicion. Patients should be referred to a geneticist or mitochondrial specialist whenever mitochondrial disease is suspected. Patients with mitochondrial cardiomyopathy should be counseled about cardiovascular complications and appropriate medications. Clinicians should be aware of the established supportive strategies for the short-term care of patients in crisis states. Current pharmacologic strategies are incompletely effective, and large randomized controlled trials are warranted to direct future therapy.
The chief difficulty in developing pharmacologic therapies is the heterogeneity of mitochondrial disease. Furthermore, research is needed to better understand the complex bioenergetic arrangements and redox networks of the mitochondrion and cell. Future research in this area could lead to therapies for the currently recognized mitochondrial disease states and for more complex metabolic defects that are associated with cancer and diabetes.
Acknowledgments
The authors thank Nicole Stancel, PhD, ELS, Rebecca Bartow, PhD, and Stephen N. Palmer, PhD, ELS, of the Texas Heart Institute, for editorial assistance in the preparation of this manuscript. The authors also thank Dr. Ian J. Butler at the Department of Neurology, The University of Texas Health Science Center at Houston, for permission to publish a muscle biopsy slide (Fig. 2) from his personal collection.
Footnotes
Address for reprints: Deborah E. Meyers, MD, Texas Heart Institute, C 355K, 6770 Bertner Ave., Houston, TX 77030
E-mail: DMeyers@texasheart.org
References
- 1.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annu Rev Pathol 2010;5:297–348. [DOI] [PMC free article] [PubMed]
- 2.Venditti P, Di Stefano L, Di Meo S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013;13(2):71–82. [DOI] [PubMed]
- 3.Wallace DC. The epigenome and the mitochondrion: bioenergetics and the environment [corrected] [published erratum appears in Genes Dev 2010;24(17):1961]. Genes Dev 2010; 24(15):1571–3. [DOI] [PMC free article] [PubMed]
- 4.Elliott HR, Samuels DC, Eden JA, Relton CL, Chinnery PF. Pathogenic mitochondrial DNA mutations are common in the general population. Am J Hum Genet 2008;83(2):254–60. [DOI] [PMC free article] [PubMed]
- 5.Chinnery PF, Elliott HR, Hudson G, Samuels DC, Relton CL. Epigenetics, epidemiology and mitochondrial DNA diseases. Int J Epidemiol 2012;41(1):177–87. [DOI] [PMC free article] [PubMed]
- 6.Lane N. Evolution. The costs of breathing. Science 2011;334 (6053):184–5. [DOI] [PubMed]
- 7.Wallace DC. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu Rev Biochem 2007;76:781–821. [DOI] [PubMed]
- 8.Wai T, Teoli D, Shoubridge EA. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 2008;40(12):1484–8. [DOI] [PubMed]
- 9.Limongelli G, Masarone D, D'Alessandro R, Elliott PM. Mitochondrial diseases and the heart: an overview of molecular basis, diagnosis, treatment and clinical course. Future Cardiol 2012;8(1):71–88. [DOI] [PubMed]
- 10.Freyer C, Cree LM, Mourier A, Stewart JB, Koolmeister C, Milenkovic D, et al. Variation in germline mtDNA heteroplasmy is determined prenatally but modified during subsequent transmission. Nat Genet 2012;44(11):1282–5. [DOI] [PMC free article] [PubMed]
- 11.Sacconi S, Salviati L, Nishigaki Y, Walker WF, Hernandez-Rosa E, Trevisson E, et al. A functionally dominant mitochondrial DNA mutation. Hum Mol Genet 2008;17(12):1814–20. [DOI] [PMC free article] [PubMed]
- 12.Hirano M, Davidson M, DiMauro S. Mitochondria and the heart. Curr Opin Cardiol 2001;16(3):201–10. [DOI] [PubMed]
- 13.Nachman MW, Brown WM, Stoneking M, Aquadro CF. Nonneutral mitochondrial DNA variation in humans and chimpanzees. Genetics 1996;142(3):953–63. [DOI] [PMC free article] [PubMed]
- 14.Schriner SE, Ogburn CE, Smith AC, Newcomb TG, Ladiges WC, Dolle ME, et al. Levels of DNA damage are unaltered in mice overexpressing human catalase in nuclei. Free Radic Biol Med 2000;29(7):664–73. [DOI] [PubMed]
- 15.Holmgren D, Wahlander H, Eriksson BO, Oldfors A, Holme E, Tulinius M. Cardiomyopathy in children with mitochondrial disease; clinical course and cardiological findings. Eur Heart J 2003;24(3):280–8. [DOI] [PubMed]
- 16.Koenig MK. Presentation and diagnosis of mitochondrial disorders in children. Pediatr Neurol 2008;38(5):305–13. [DOI] [PMC free article] [PubMed]
- 17.DiMauro S. Mitochondrial myopathies. Curr Opin Rheumatol 2006;18(6):636–41. [DOI] [PubMed]
- 18.DiMauro S, Hirano M. Mitochondrial encephalomyopathies: an update. Neuromuscul Disord 2005;15(4):276–86. [DOI] [PubMed]
- 19.Debray FG, Lambert M, Chevalier I, Robitaille Y, Decarie JC, Shoubridge EA, et al. Long-term outcome and clinical spectrum of 73 pediatric patients with mitochondrial diseases. Pediatrics 2007;119(4):722–33. [DOI] [PubMed]
- 20.Petty RK, Harding AE, Morgan-Hughes JA. The clinical features of mitochondrial myopathy. Brain 1986;109(Pt 5):915–38. [DOI] [PubMed]
- 21.Munnich A, Rotig A, Chretien D, Cormier V, Bourgeron T, Bonnefont JP, et al. Clinical presentation of mitochondrial disorders in childhood. J Inherit Metab Dis 1996;19(4):521–7. [DOI] [PubMed]
- 22.Jackson MJ, Schaefer JA, Johnson MA, Morris AA, Turnbull DM, Bindoff LA. Presentation and clinical investigation of mitochondrial respiratory chain disease. A study of 51 patients. Brain 1995;118(Pt 2):339–57. [DOI] [PubMed]
- 23.DiMauro S, Bonilla E, De Vivo DC. Does the patient have a mitochondrial encephalomyopathy? J Child Neurol 1999;14 Suppl 1:S23–35. [DOI] [PubMed]
- 24.Rotig A. Renal disease and mitochondrial genetics. J Nephrol 2003;16(2):286–92. [PubMed]
- 25.Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology 2002;59(9):1406–11. [DOI] [PubMed]
- 26.Hsu CH, Kwon H, Perng CL, Bai RK, Dai P, Wong LJ. Hearing loss in mitochondrial disorders. Ann N Y Acad Sci 2005;1042:36–47. [DOI] [PubMed]
- 27.Finsterer J. Central nervous system manifestations of mitochondrial disorders. Acta Neurol Scand 2006;114(4):217–38. [DOI] [PubMed]
- 28.Gillis LA, Sokol RJ. Gastrointestinal manifestations of mitochondrial disease. Gastroenterol Clin North Am 2003;32(3):789–817, v. [DOI] [PubMed]
- 29.Engel WK, Cunningham GG. Rapid examination of muscle tissue. An improved trichrome method for fresh-frozen biopsy sections. Neurology 1963;13:919–23. [DOI] [PubMed]
- 30.Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM, Munnich A. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 1994;228(1):35–51. [DOI] [PubMed]
- 31.Rustin P, Chretien D, Bourgeron T, Wucher A, Saudubray JM, Rotig A, Munnich A. Assessment of the mitochondrial respiratory chain. Lancet 1991;338(8758):60. [DOI] [PubMed]
- 32.Taylor GP. Neonatal mitochondrial cardiomyopathy. Pediatr Dev Pathol 2004;7(6):620–4. [DOI] [PubMed]
- 33.Finsterer J. Cardiogenetics, neurogenetics, and pathogenetics of left ventricular hypertrabeculation/noncompaction. Pediatr Cardiol 2009;30(5):659–81. [DOI] [PubMed]
- 34.Anan R, Nakagawa M, Miyata M, Higuchi I, Nakao S, Suehara M, et al. Cardiac involvement in mitochondrial diseases. A study on 17 patients with documented mitochondrial DNA defects. Circulation 1995;91(4):955–61. [DOI] [PubMed]
- 35.Wahbi K, Larue S, Jardel C, Meune C, Stojkovic T, Ziegler F, et al. Cardiac involvement is frequent in patients with the m.8344A>G mutation of mitochondrial DNA. Neurology 2010;74(8):674–7. [DOI] [PubMed]
- 36.Stollberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol 2002;90(8):899–902. [DOI] [PubMed]
- 37.Jenni R, Oechslin EN, van der Loo B. Isolated ventricular non-compaction of the myocardium in adults. Heart 2007;93 (1):11–5. [DOI] [PMC free article] [PubMed]
- 38.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001;86(6):666–71. [DOI] [PMC free article] [PubMed]
- 39.Thavendiranathan P, Dahiya A, Phelan D, Desai MY, Tang WH. Isolated left ventricular non-compaction controversies in diagnostic criteria, adverse outcomes and management. Heart 2013;99(10):681–9. [DOI] [PubMed]
- 40.Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J 2008; 29(1):89–95. [DOI] [PubMed]
- 41.Sebastiani M, Giordano C, Nediani C, Travaglini C, Borchi E, Zani M, et al. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol 2007;50(14):1362–9. [DOI] [PubMed]
- 42.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 2005;115(3):547–55. [DOI] [PMC free article] [PubMed]
- 43.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol 1994;267(2 Pt 2):H742–50. [DOI] [PubMed]
- 44.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol 1970;218(1):153–9. [DOI] [PubMed]
- 45.Christe ME, Rodgers RL. Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol 1994;26(10):1371–5. [DOI] [PubMed]
- 46.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev 2002;7(2):161–73. [DOI] [PubMed]
- 47.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension 1988;11(5):416–26. [DOI] [PubMed]
- 48.Arbustini E, Diegoli M, Fasani R, Grasso M, Morbini P, Banchieri N, et al. Mitochondrial DNA mutations and mitochondrial abnormalities in dilated cardiomyopathy. Am J Pathol 1998;153(5):1501–10. [DOI] [PMC free article] [PubMed]
- 49.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 2002;109(1):121–30. [DOI] [PMC free article] [PubMed]
- 50.Chinnery P, Majamaa K, Turnbull D, Thorburn D. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2006(1):CD004426. [DOI] [PubMed]
- 51.Glover EI, Martin J, Maher A, Thornhill RE, Moran GR, Tarnopolsky MA. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve 2010;42(5):739–48. [DOI] [PubMed]
- 52.Tarnopolsky MA, Roy BD, MacDonald JR. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 1997;20(12):1502–9. [DOI] [PubMed]
- 53.Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology 2006;66(3):324–30. [DOI] [PubMed]
- 54.Koga Y, Akita Y, Nishioka J, Yatsuga S, Povalko N, Tanabe Y, et al. L-arginine improves the symptoms of strokelike episodes in MELAS. Neurology 2005;64(4):710–2. [DOI] [PubMed]
- 55.Martin DS, Grocott MP. Oxygen therapy in critical illness: precise control of arterial oxygenation and permissive hypoxemia. Crit Care Med 2013;41(2):423–32. [DOI] [PubMed]
- 56.Koga Y, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T. MELAS and L-arginine therapy: pathophysiology of stroke-like episodes. Ann N Y Acad Sci 2010;1201:104–10. [DOI] [PubMed]
- 57.Flynn NE, Meininger CJ, Haynes TE, Wu G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother 2002;56(9):427–38. [DOI] [PubMed]
- 58.Haberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis 2012;7:32. [DOI] [PMC free article] [PubMed]
- 59.Golden AS, Law YM, Shurtleff H, Warner M, Saneto RP. Mitochondrial electron transport chain deficiency, cardiomyopathy, and long-term cardiac transplant outcome. Pediatr Transplant 2012;16(3):265–8. [DOI] [PubMed]


