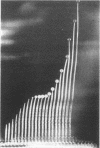
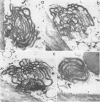
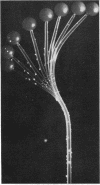
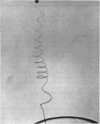
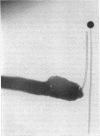
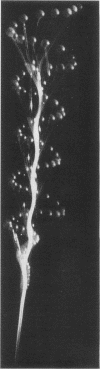
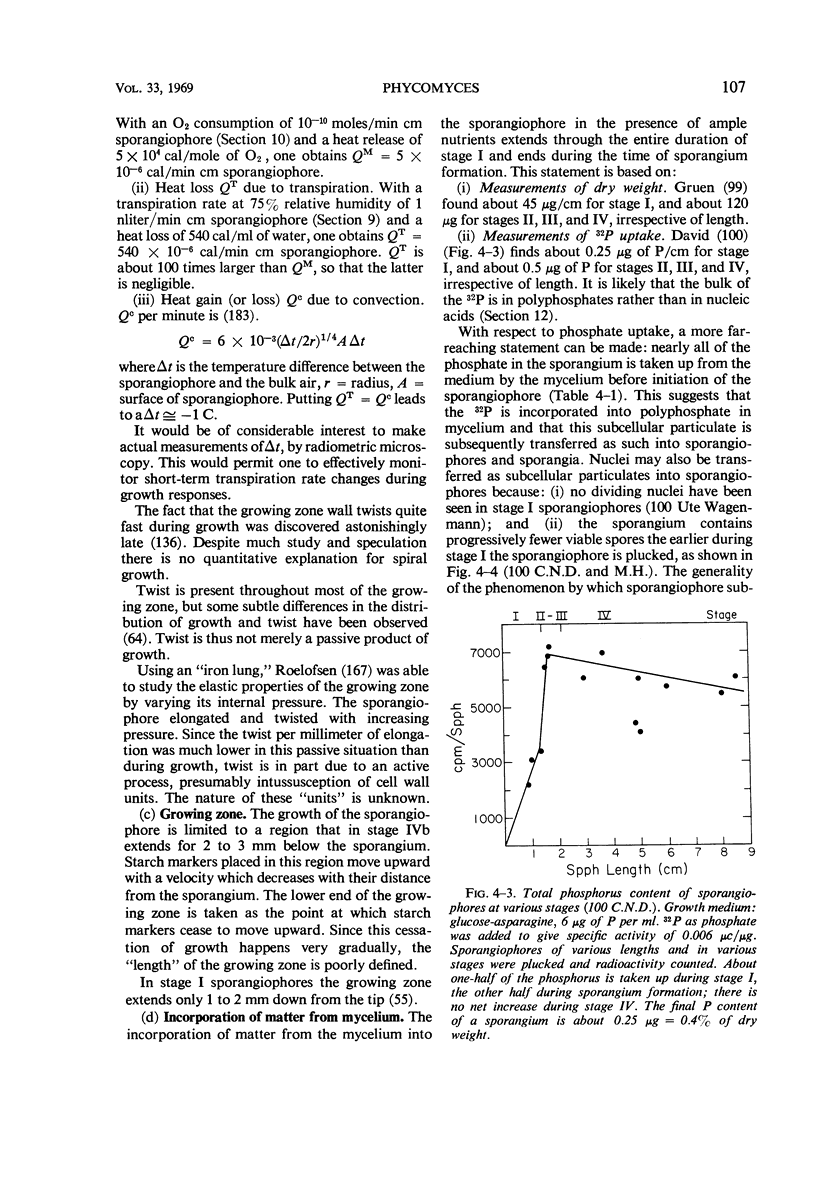
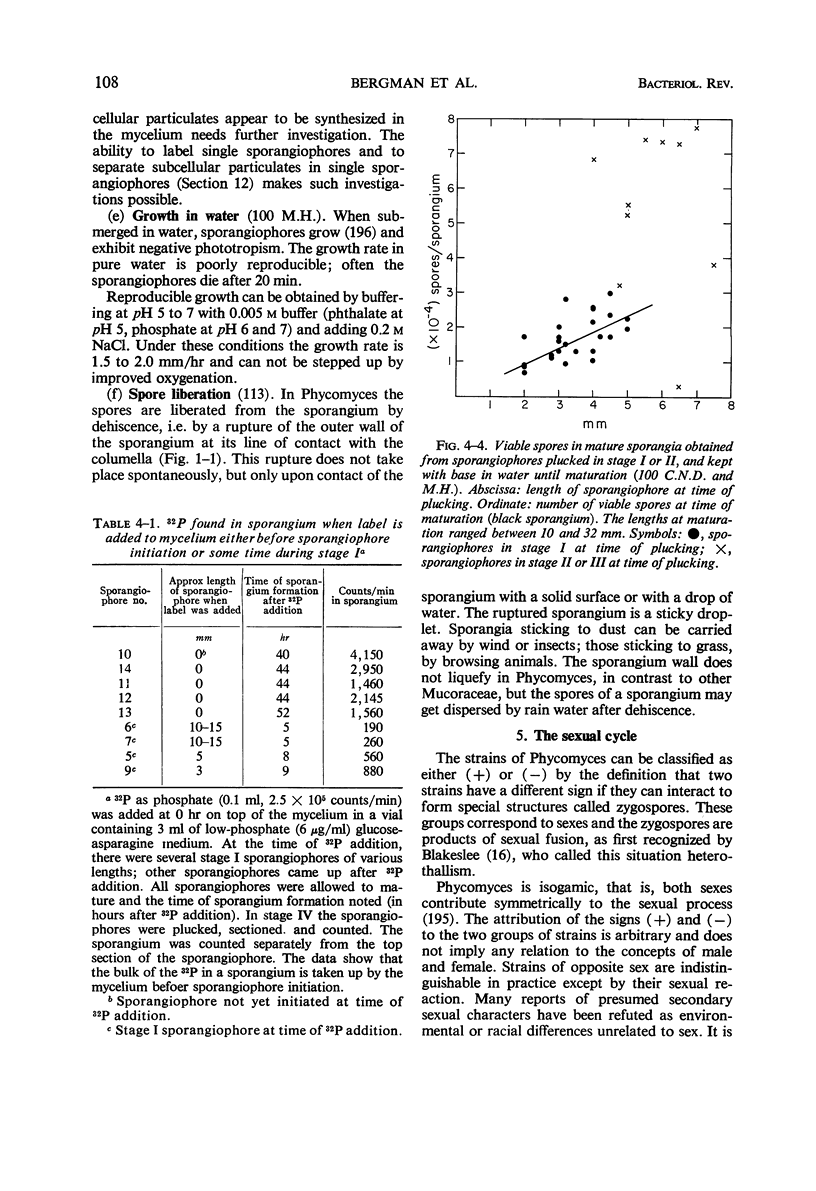
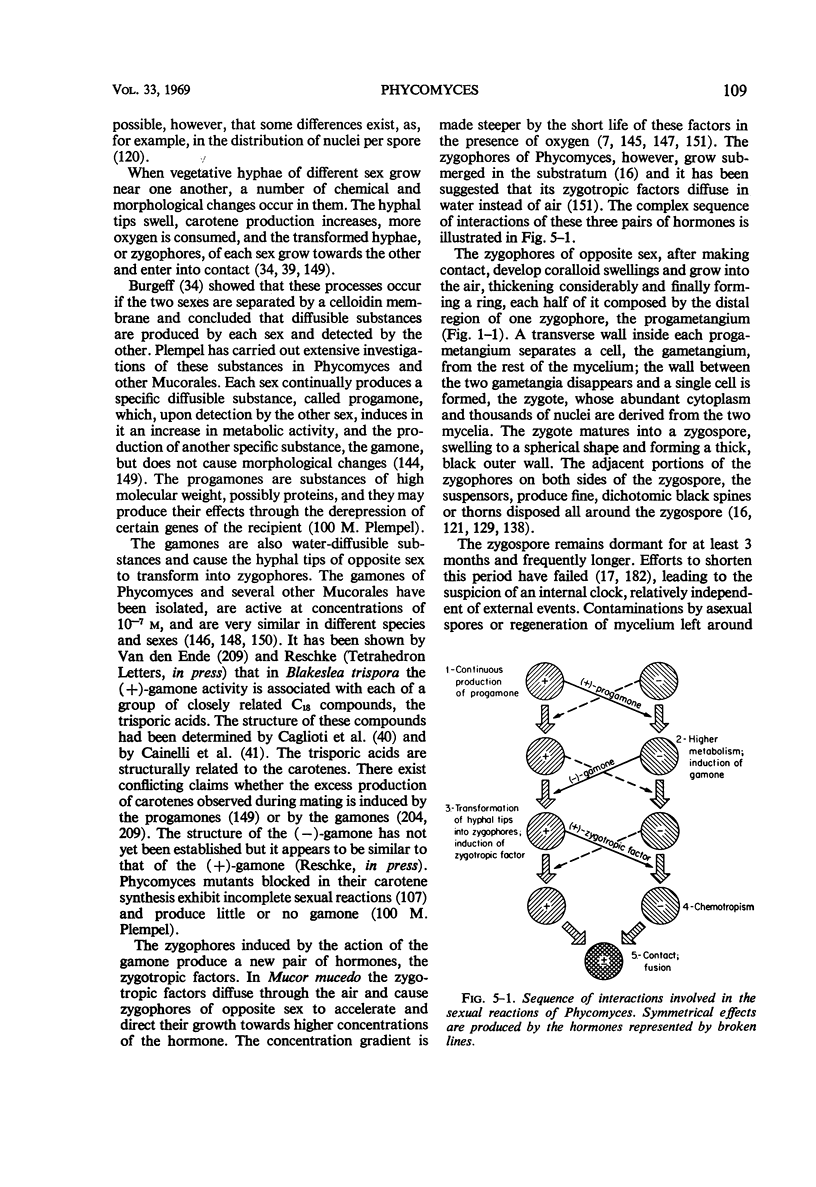
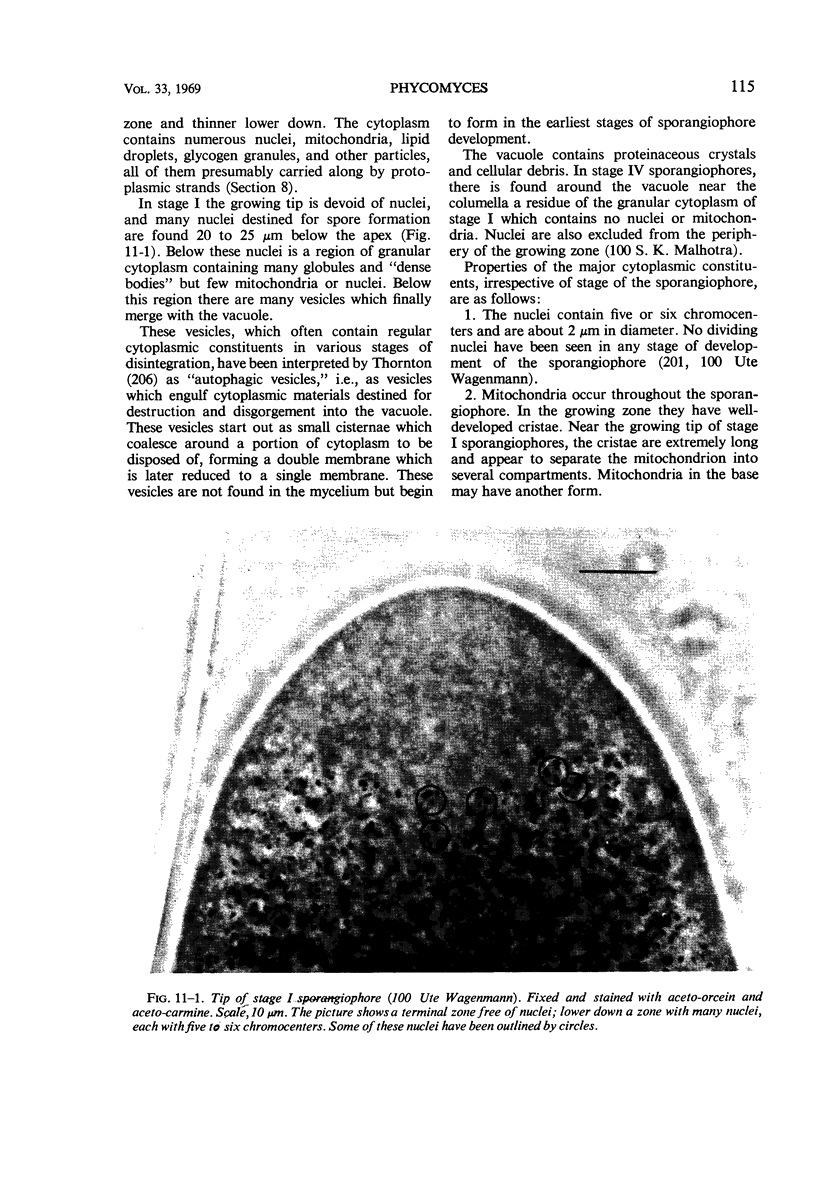
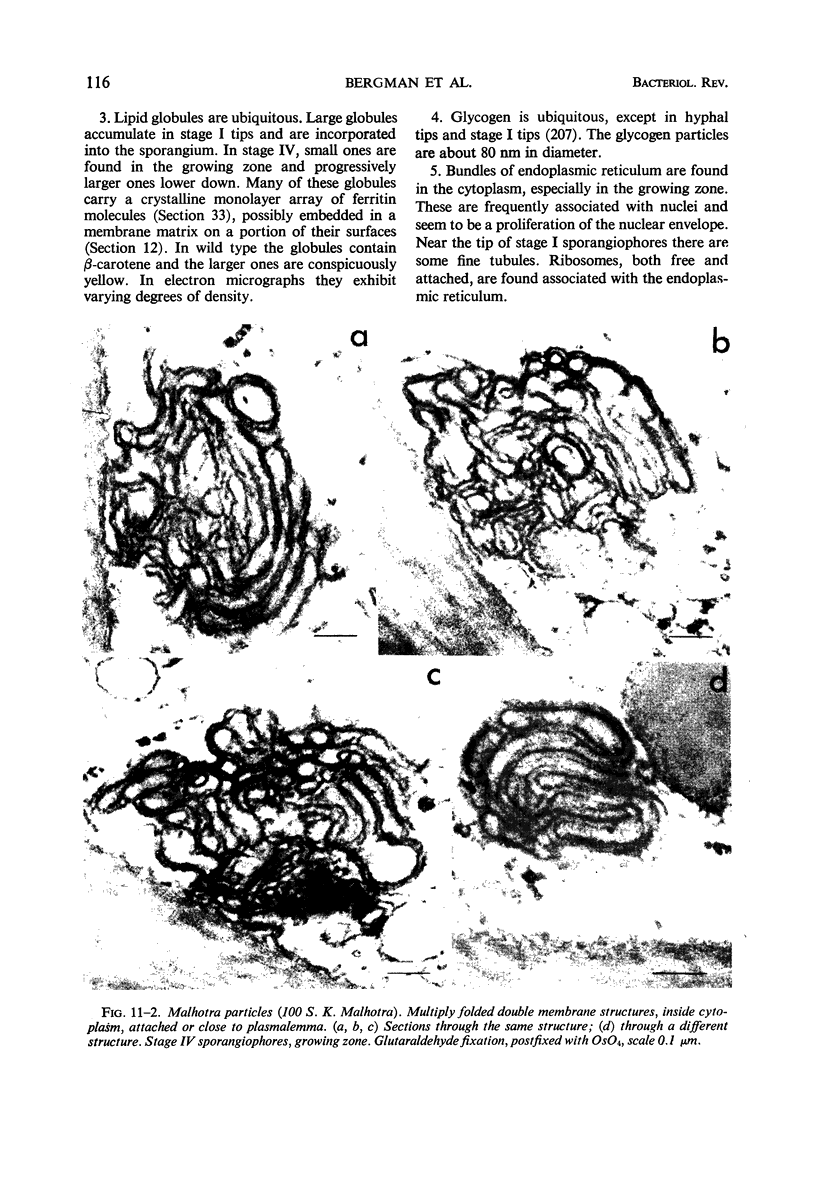
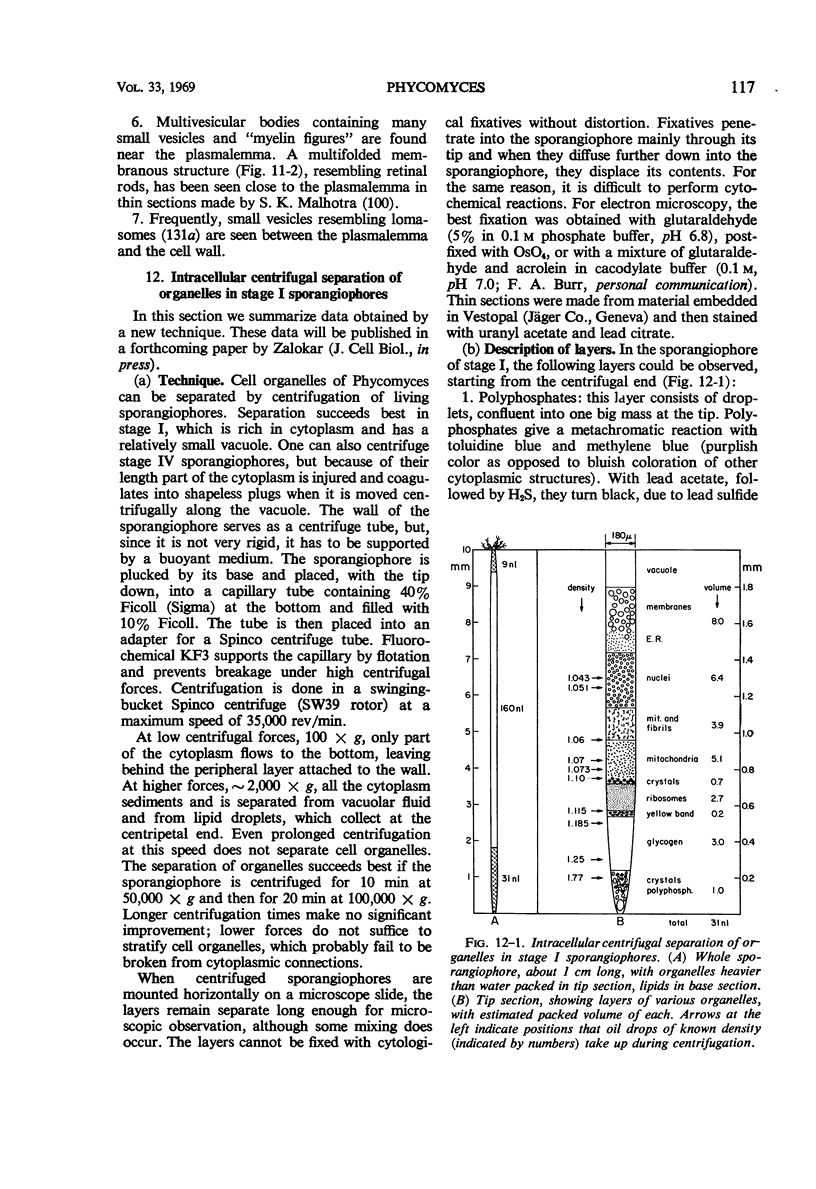
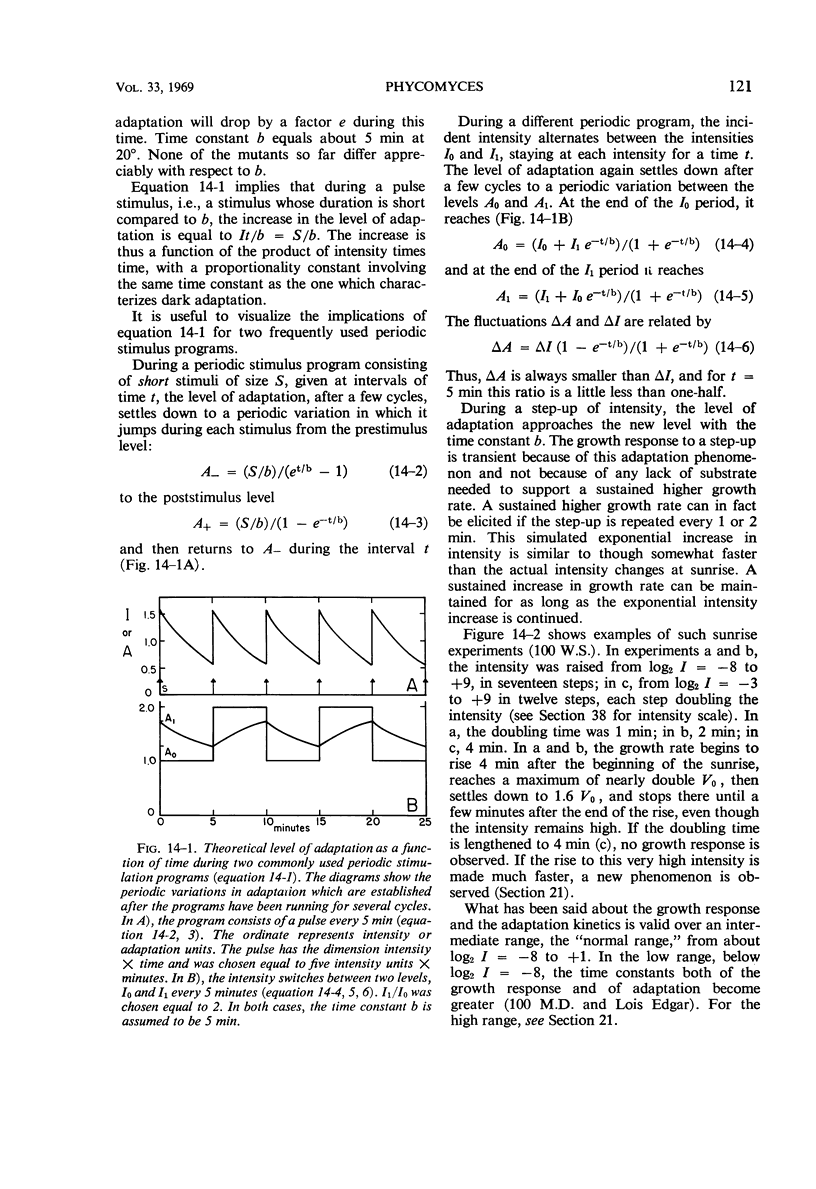
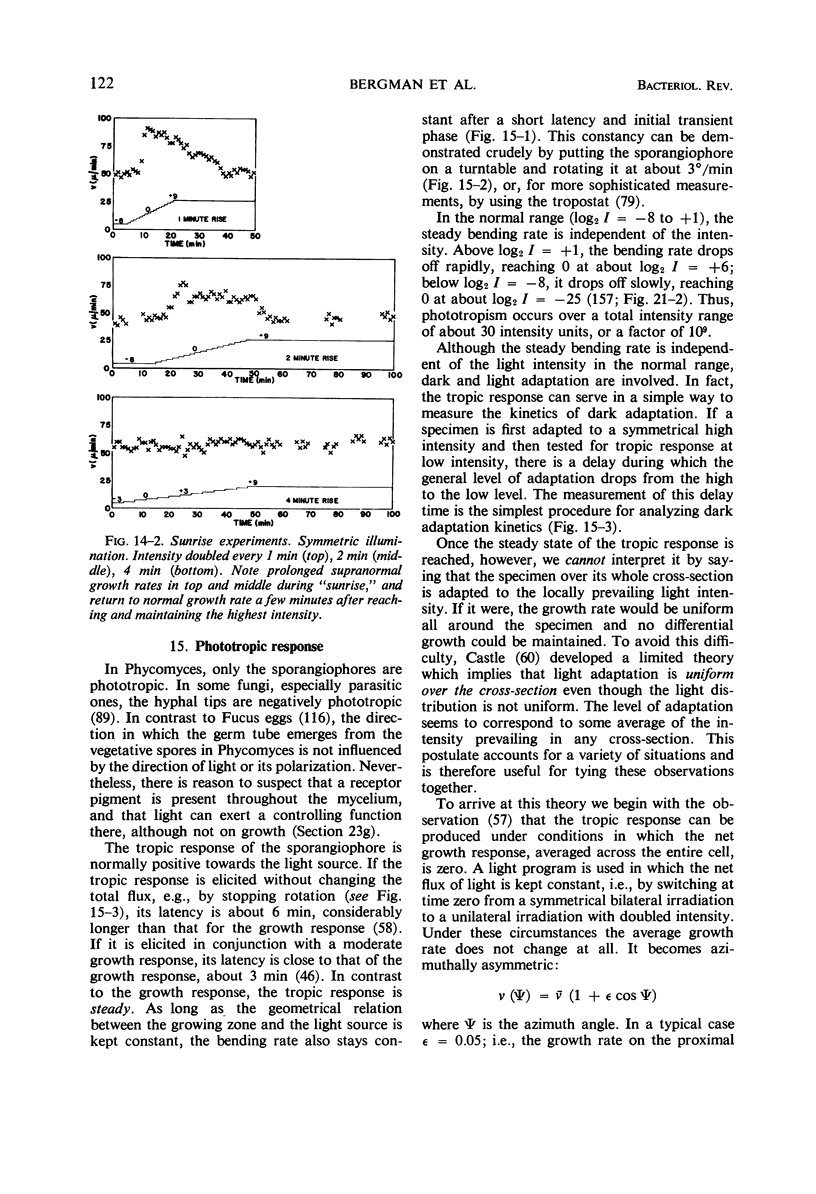
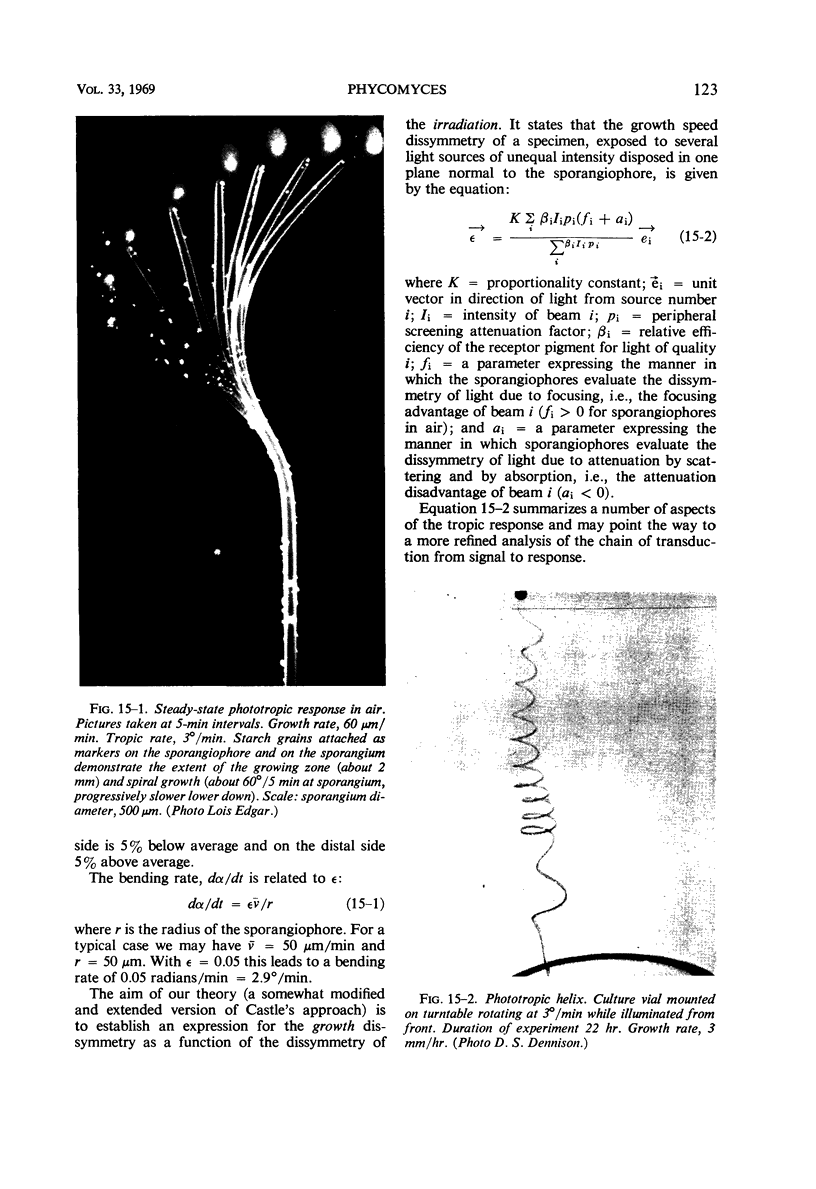
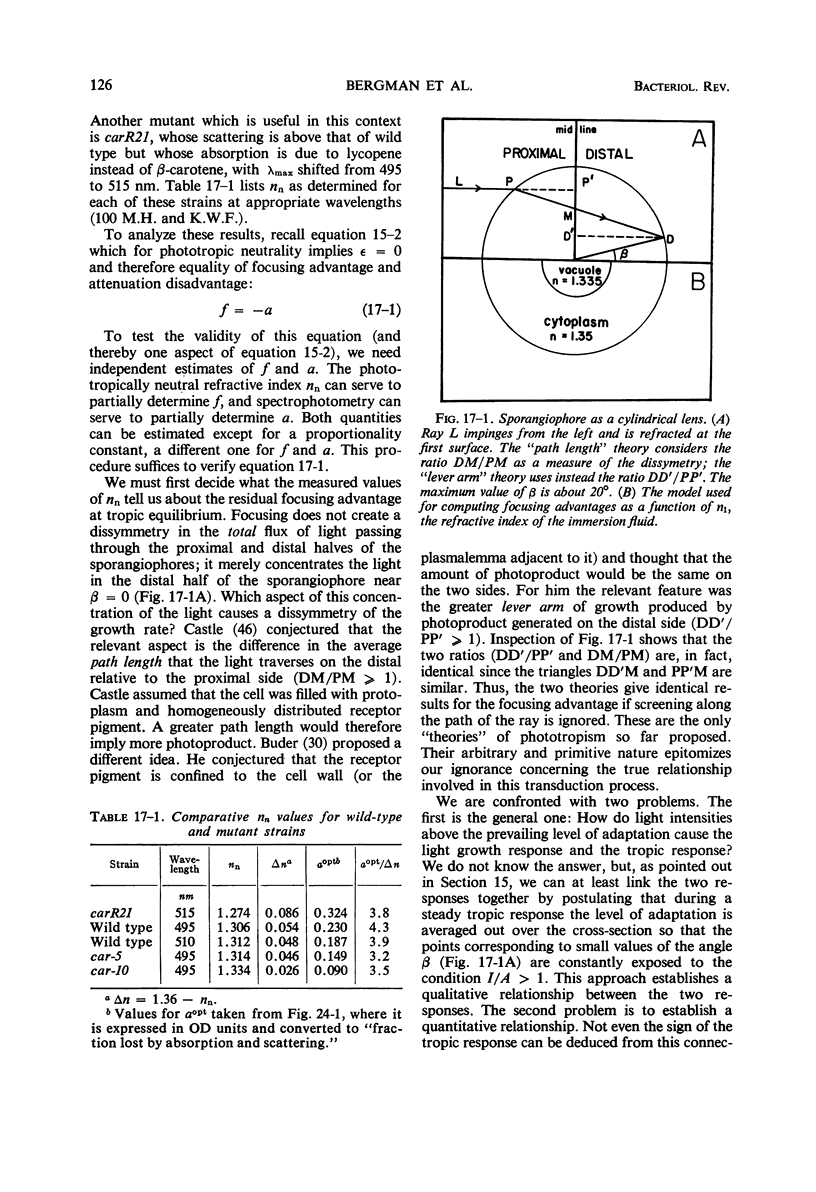
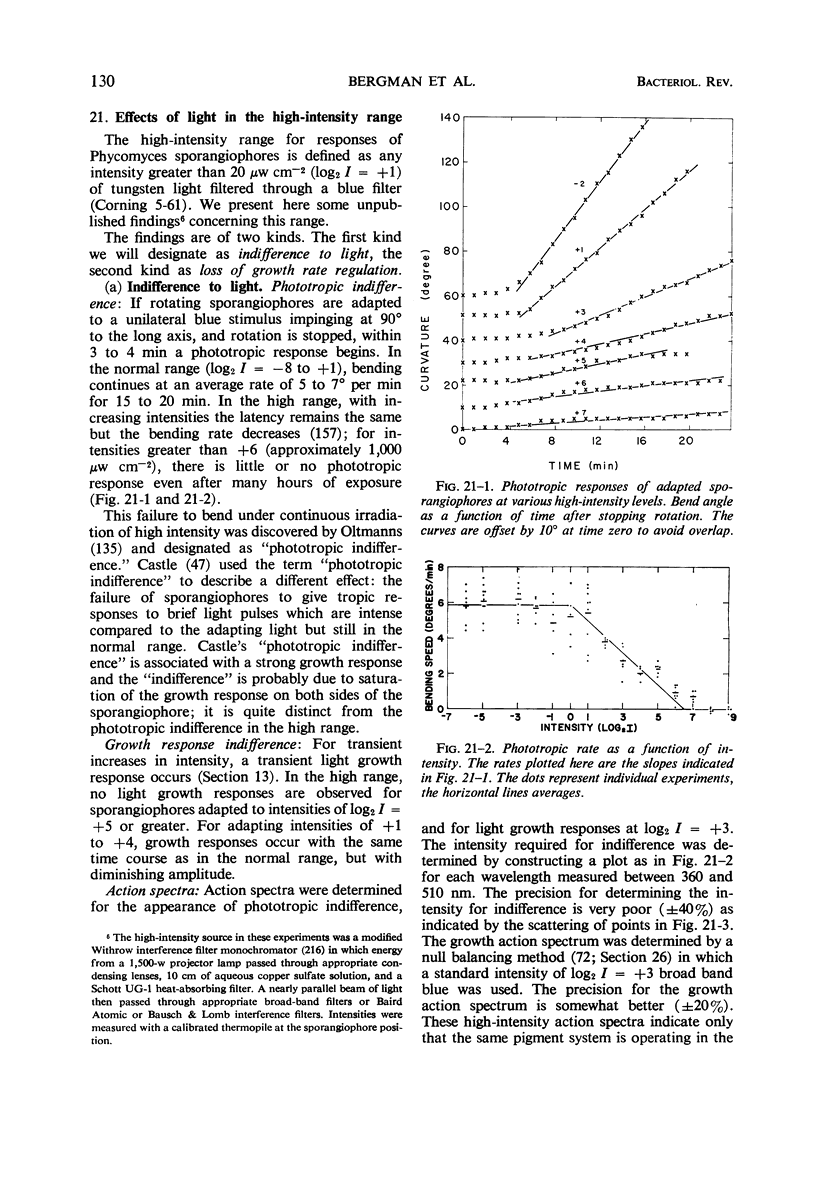
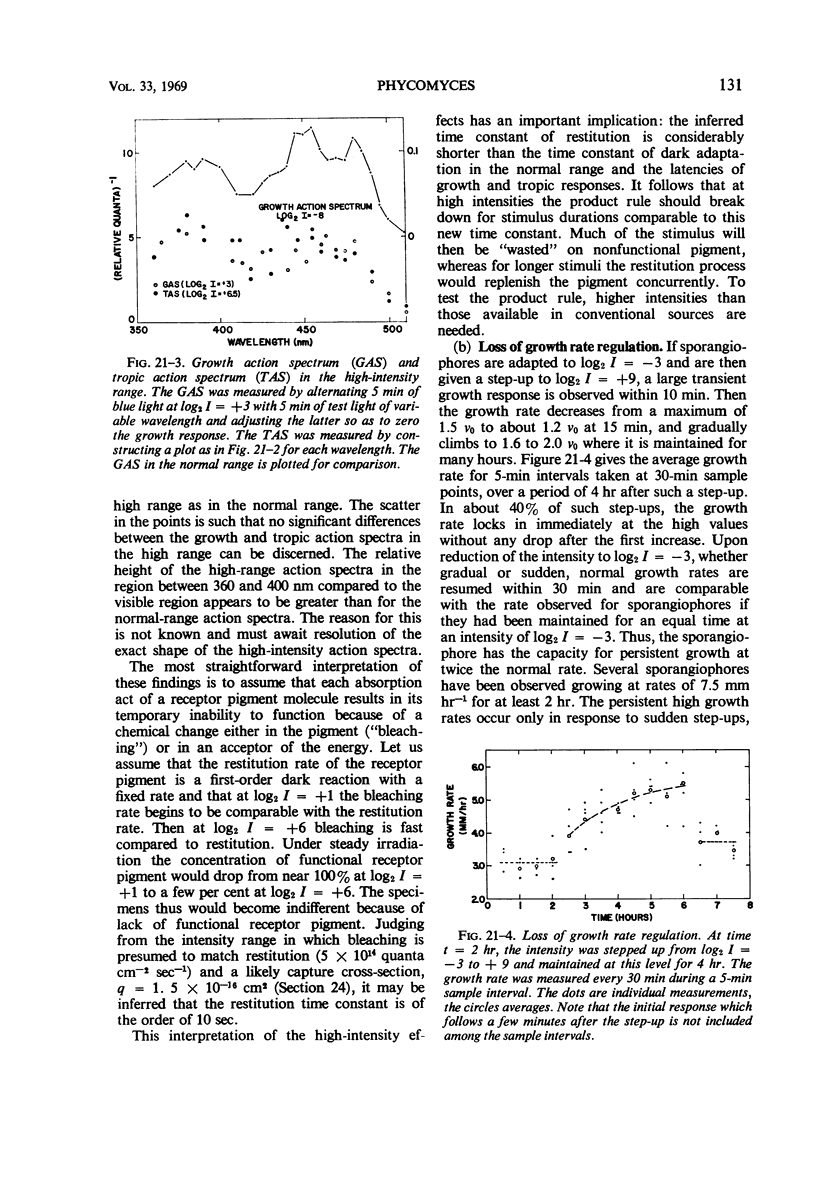
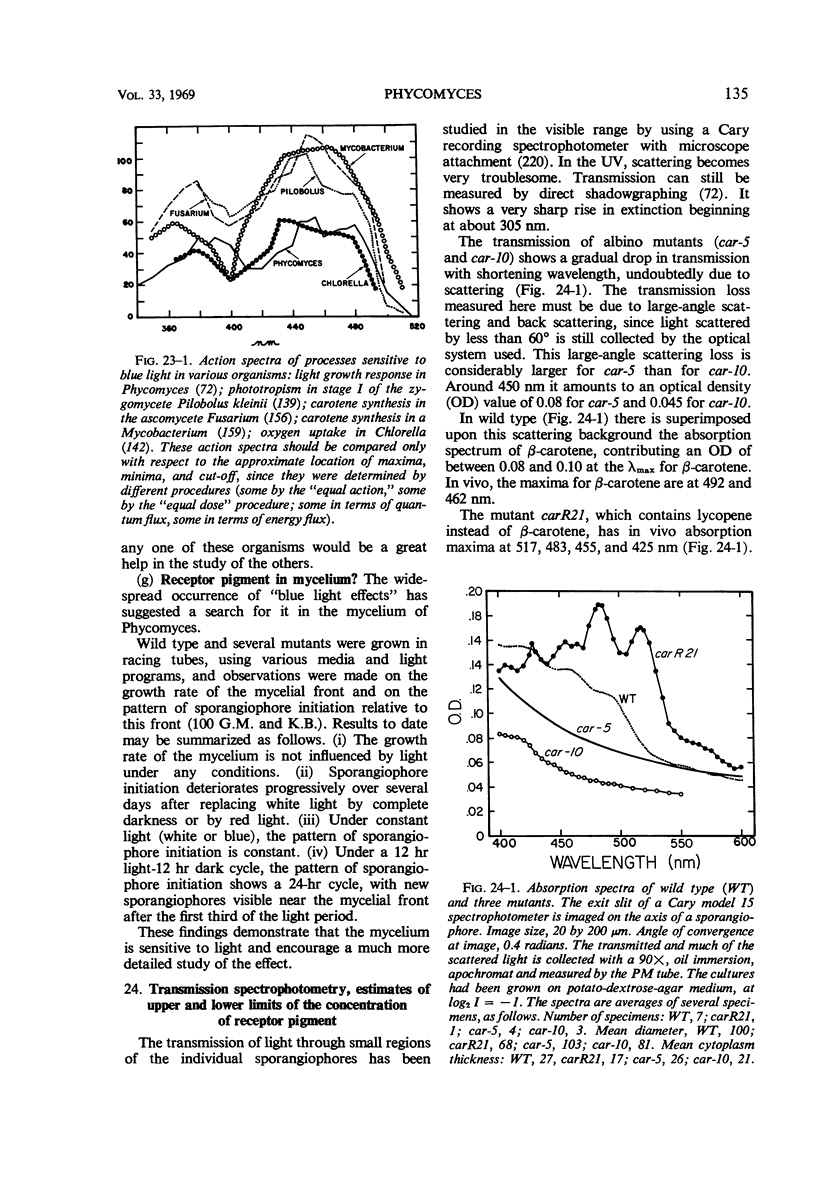
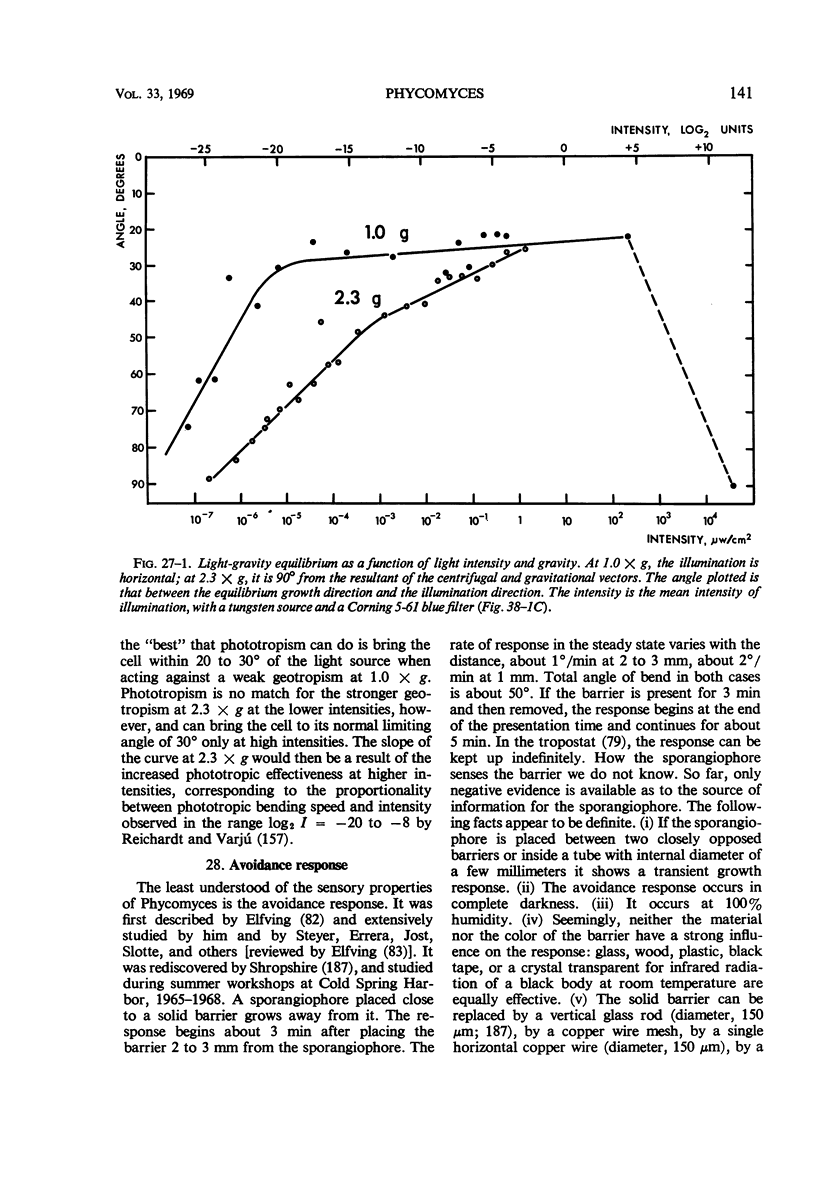
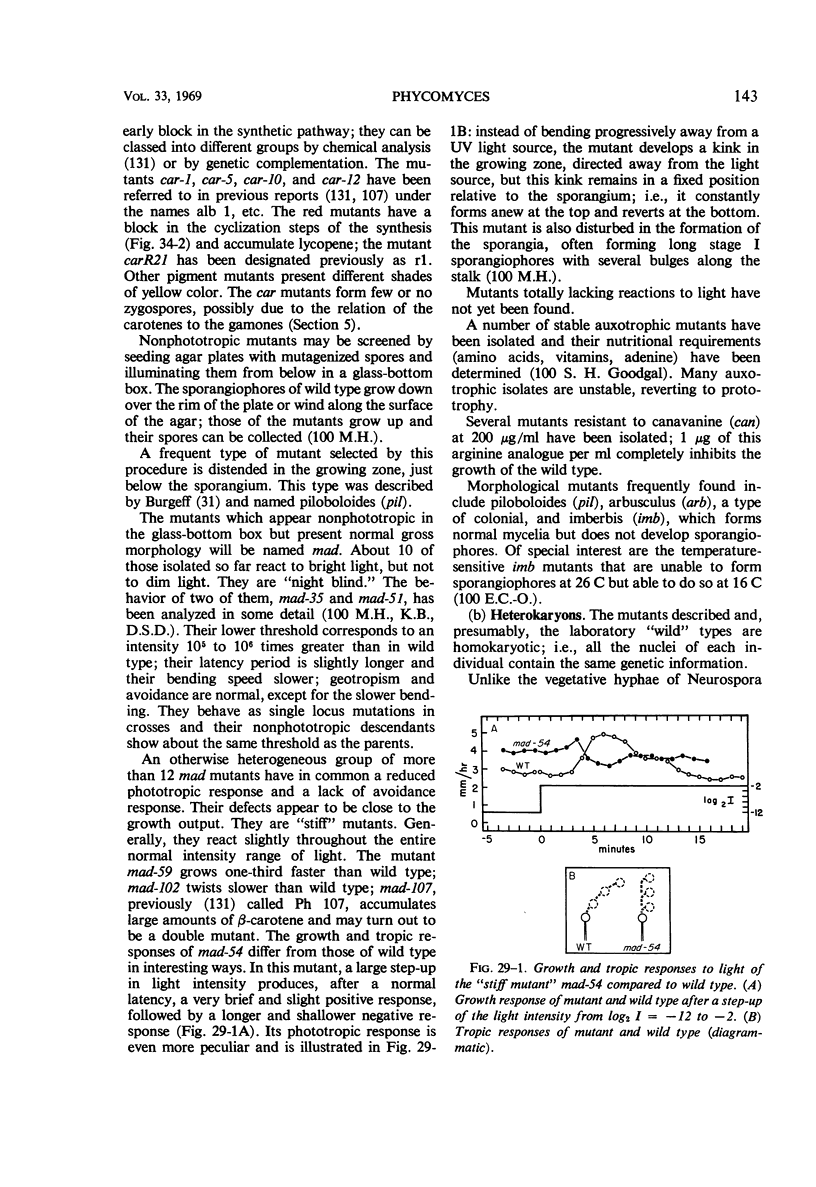
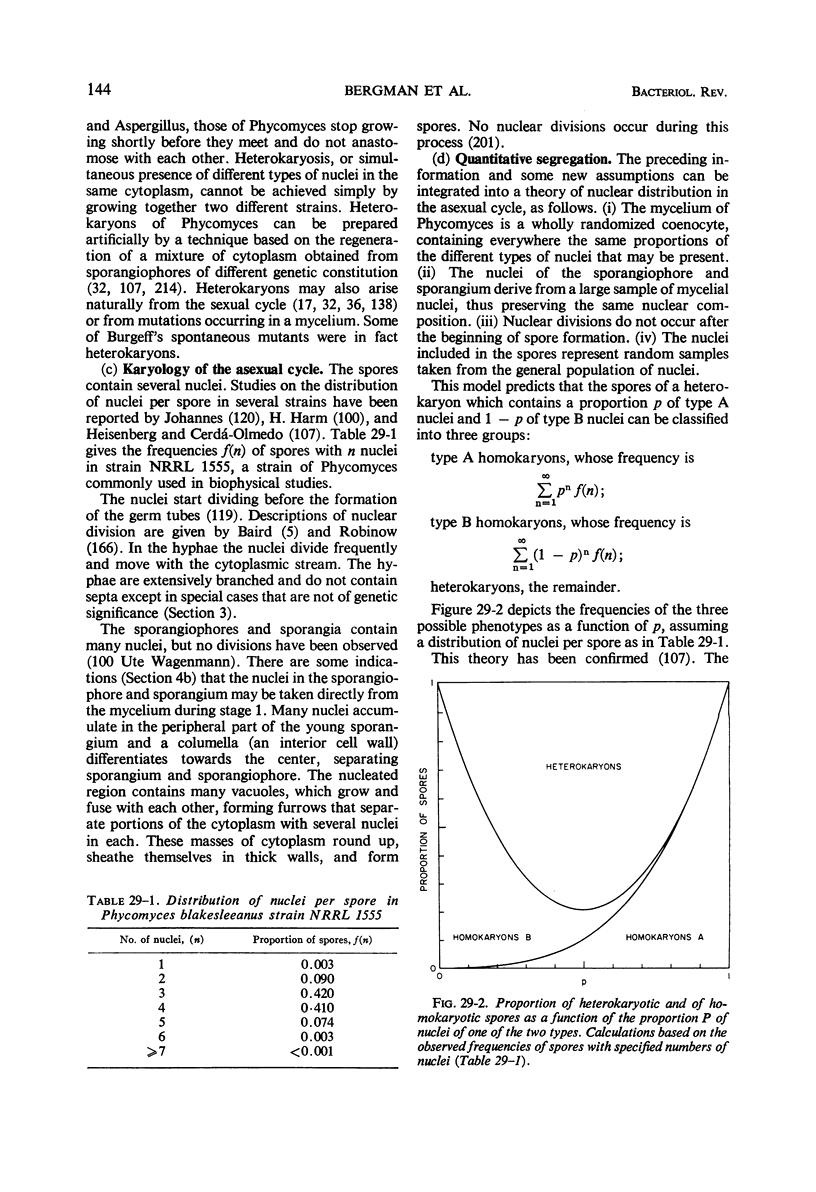
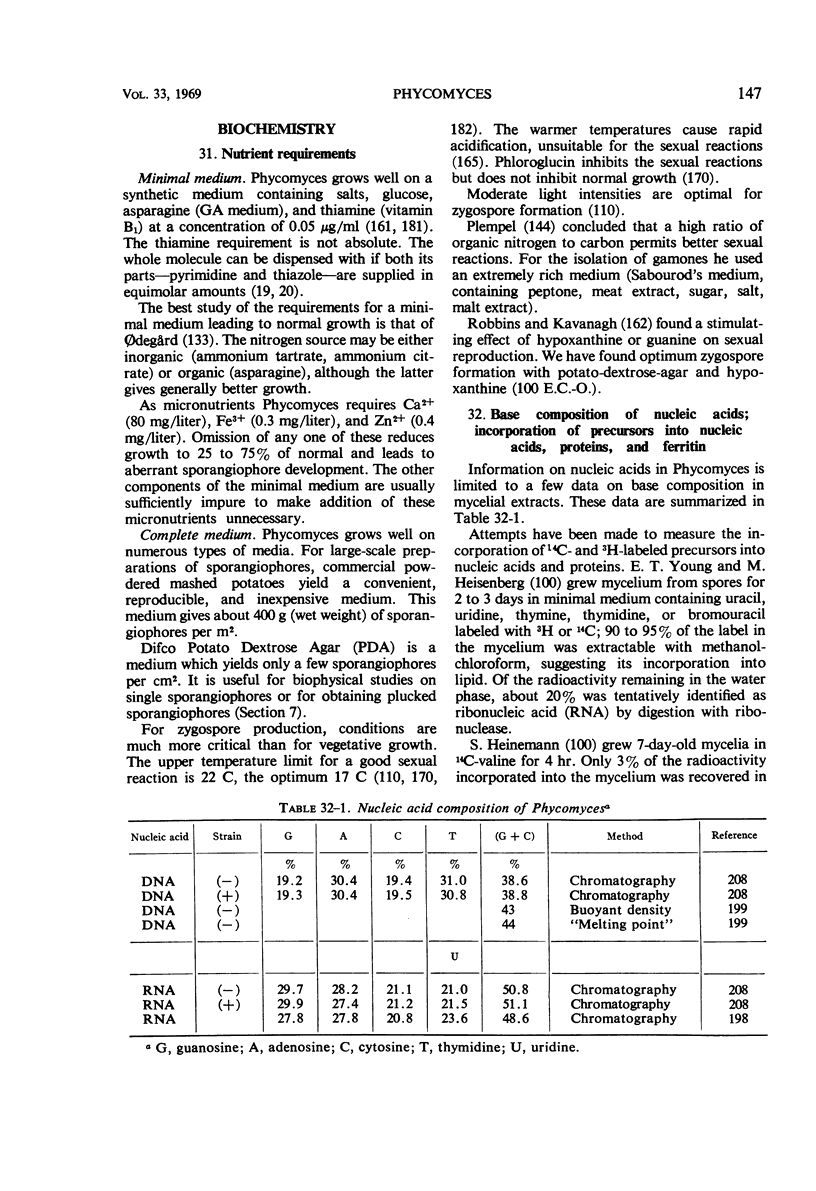
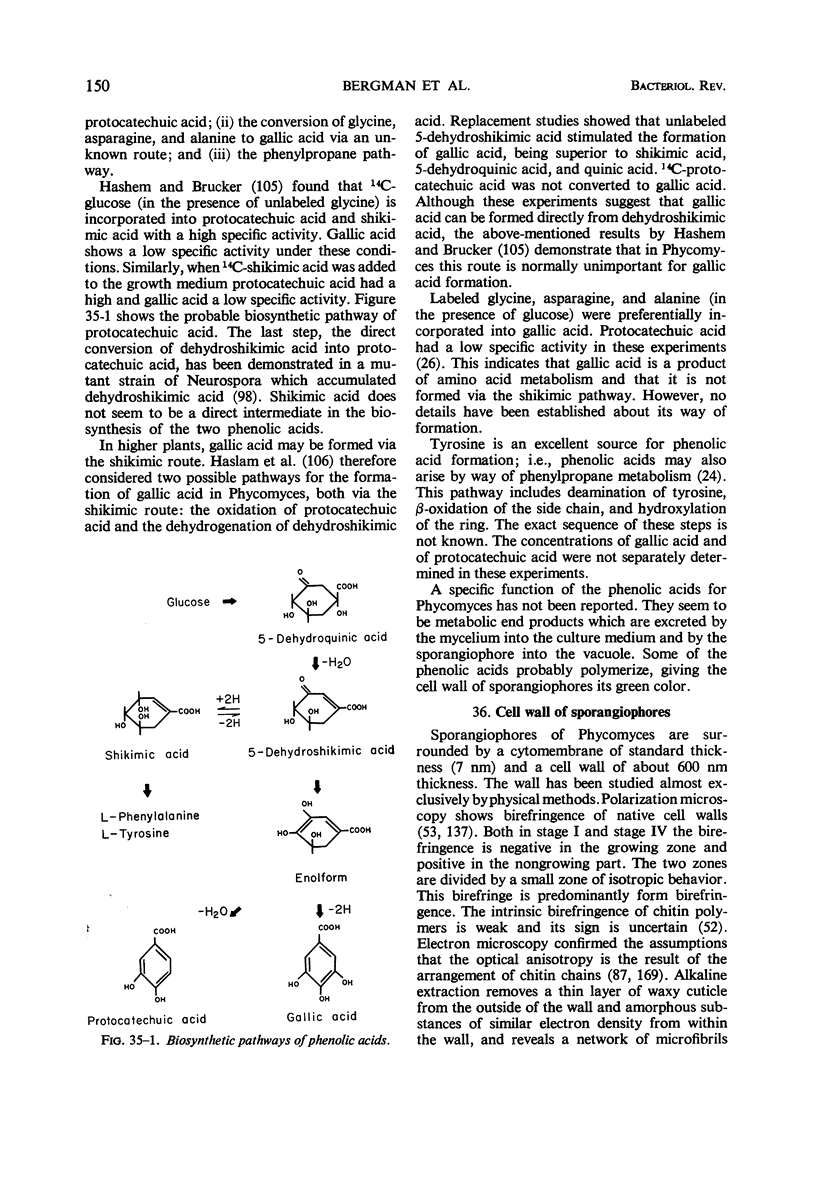
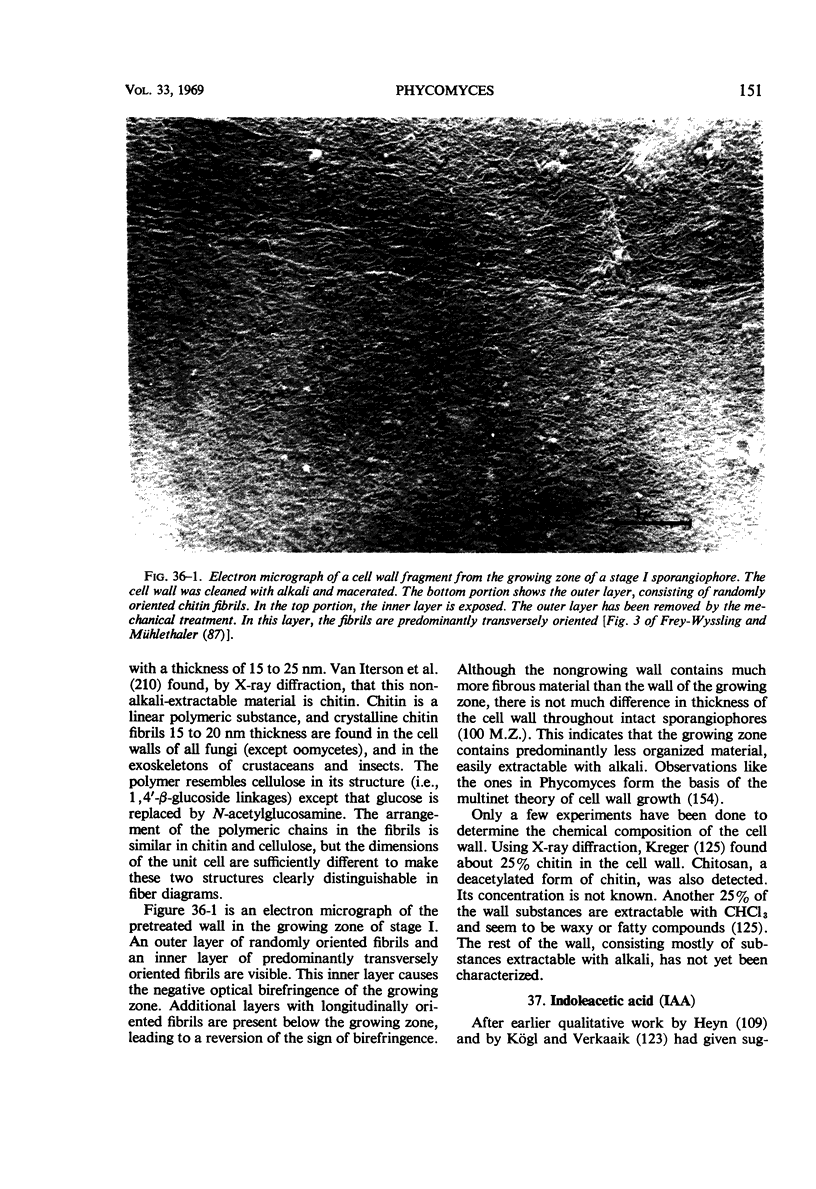
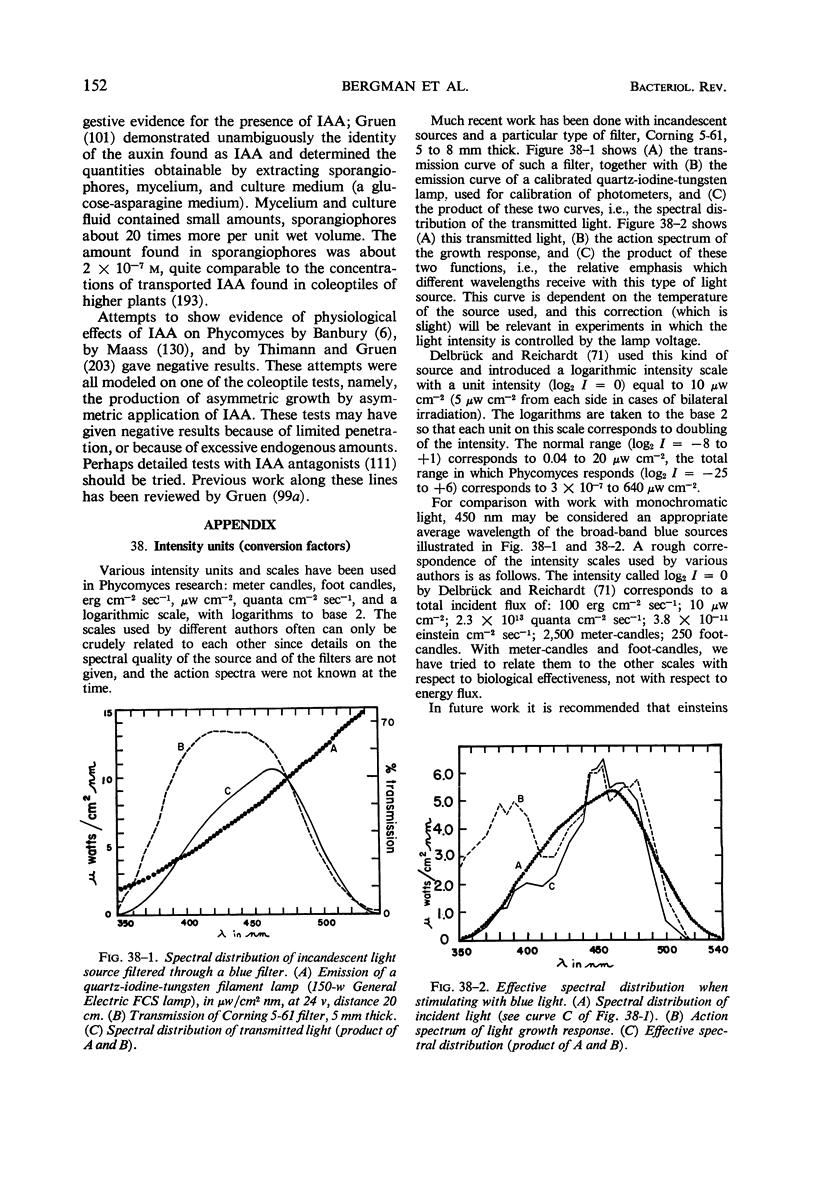
Full text
PDF


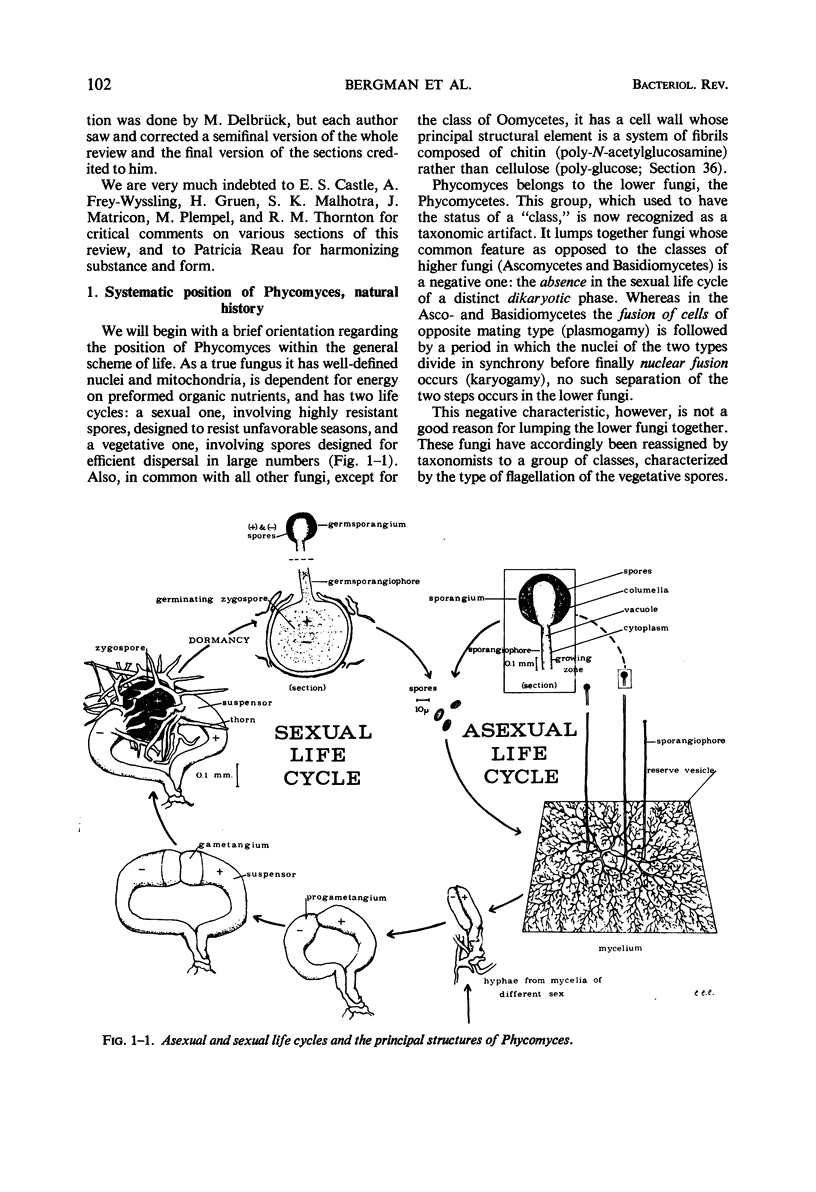



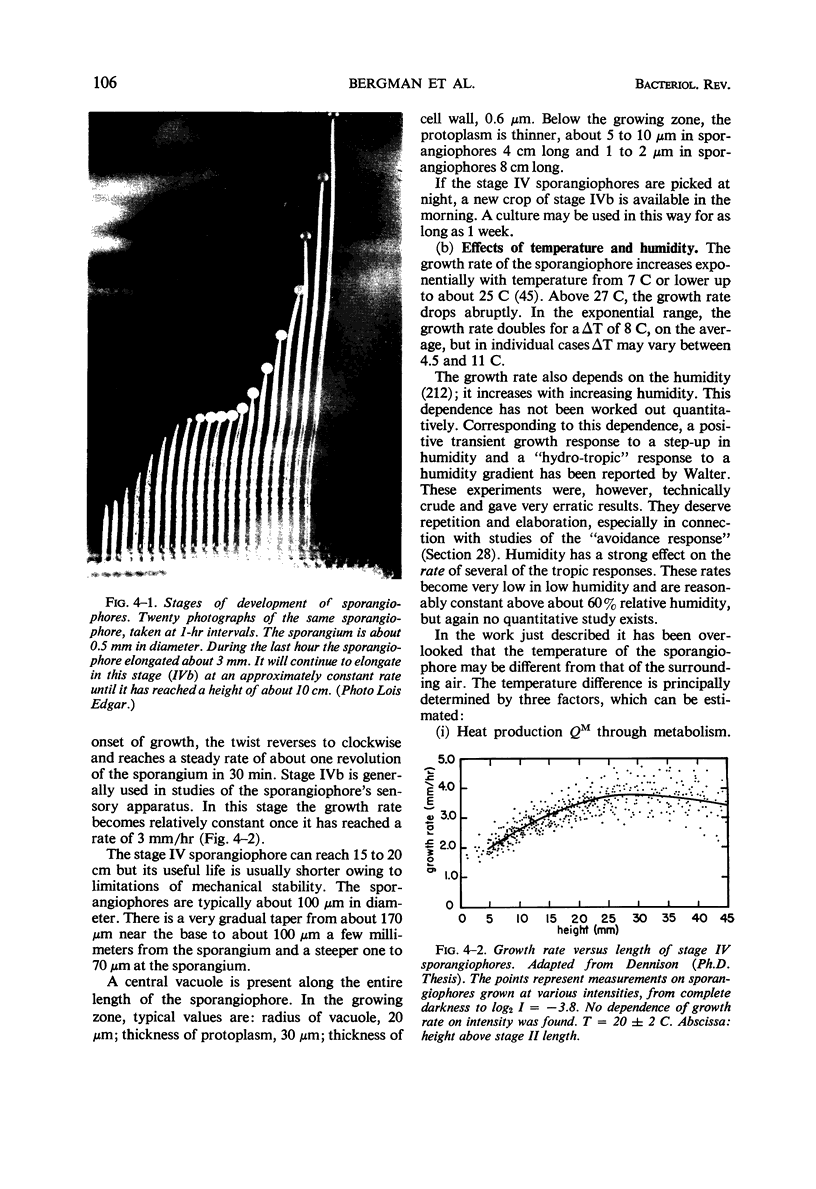































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- BERNHARD K., ALBRECHT H. Die Lipide aus Phycomyces Blakesleeanus. Helv Chim Acta. 1948 Jun 15;31(4):977–988. doi: 10.1002/hlca.19480310402. [DOI] [PubMed] [Google Scholar]
- BRUCKER W., DREHMANN U. Zur Phenolcarbonsäurebildung von Phycomyces aus 14C-markierten Substraten. Arch Mikrobiol. 1958;30(4):396–408. [PubMed] [Google Scholar]
- BRUCKER W. Hemmungsanalytische Studien zur Phenolcarbonsäurebildung durch Phycomyces blakesleeanus. Arch Mikrobiol. 1957;26(3):302–306. [PubMed] [Google Scholar]
- Bonner J., Buchman E. R. The Synthesis and Destruction of Vitamin B(1) by Phycomyces. Proc Natl Acad Sci U S A. 1939 Apr;25(4):164–171. doi: 10.1073/pnas.25.4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTLE E. S. DIFFERENTIAL GROWTH AND PHOTOTROPIC BENDING IN PHYCOMYCES. J Gen Physiol. 1965 Jan;48:409–423. doi: 10.1085/jgp.48.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTLE E. S. Growth distribution in the light-growth response of Phycomyces. J Gen Physiol. 1959 Mar 20;42(4):697–702. doi: 10.1085/jgp.42.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTLE E. S. Phototropic curvature in Phycomyces. J Gen Physiol. 1962 Mar;45:743–756. doi: 10.1085/jgp.45.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTLE E. S. Phototropism, adaptation, and the light-growth response of Phycomyces. J Gen Physiol. 1961 Sep;45:39–46. doi: 10.1085/jgp.45.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTLE E. S. The topography of tip growth in a plant cell. J Gen Physiol. 1958 May 20;41(5):913–926. doi: 10.1085/jgp.41.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN R., DELBRUCK M. Distribution of stretch and twist along the growing zone of the sporangiophore of Phycomyces and the distribution of response to a periodic illumination program. J Cell Physiol. 1958 Dec;52(3):361–388. doi: 10.1002/jcp.1030520303. [DOI] [PubMed] [Google Scholar]
- COHEN R., DELBRUCK M. Photo-reactions in Phycomyces; growth and tropic responses to the stimulation of narrow test areas. J Gen Physiol. 1959 Mar 20;42(4):677–695. doi: 10.1085/jgp.42.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. A kinetic model for adaptation and the light responses of Phycomyces. J Gen Physiol. 1966 May;49(5):925–935. doi: 10.1085/jgp.49.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. DARK ADAPTATION AND THE DARK GROWTH RESPONSE OF PHYCOMYCES. J Gen Physiol. 1932 Sep 20;16(1):75–88. doi: 10.1085/jgp.16.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. ON "REVERSAL" OF PHOTOTROPISM IN PHYCOMYCES. J Gen Physiol. 1932 May 20;15(5):487–489. doi: 10.1085/jgp.15.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. PHOTOTROPISM AND THE LIGHT-SENSITIVE SYSTEM OF PHYCOMYCES. J Gen Physiol. 1930 Mar 20;13(4):421–435. doi: 10.1085/jgp.13.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. Phototropic Inversion in Phycomyces. Science. 1961 May 5;133(3462):1424–1425. doi: 10.1126/science.133.3462.1424. [DOI] [PubMed] [Google Scholar]
- Castle E. S. TEMPERATURE CHARACTERISTICS FOR THE GROWTH OF THE SPORANGIOPHORES OF PHYCOMYCES. J Gen Physiol. 1928 Mar 20;11(4):407–413. doi: 10.1085/jgp.11.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. THE DOUBLE REFRACTION OF CHITIN. J Gen Physiol. 1936 May 20;19(5):797–805. doi: 10.1085/jgp.19.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. THE PHOTOTROPIC EFFECT OF POLARIZED LIGHT. J Gen Physiol. 1934 Jul 20;17(6):751–762. doi: 10.1085/jgp.17.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E. S. THE REFRACTIVE INDICES OF WHOLE CELLS. J Gen Physiol. 1933 Sep 20;17(1):41–47. doi: 10.1085/jgp.17.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil R., Jack M. Lipid patterns in the major classes of fungi. J Bacteriol. 1966 May;91(5):2101–2102. doi: 10.1128/jb.91.5.2101-2102.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C., Guerola N. Mutagenesis of the replication point by nitrosoguanidine: map and pattern of replication of the Escherichia coli chromosome. J Mol Biol. 1968 May 14;33(3):705–719. doi: 10.1016/0022-2836(68)90315-x. [DOI] [PubMed] [Google Scholar]
- Chichester C. O., Wong P. S., Mackinney G. On the Biosynthesis of Carotenoids. Plant Physiol. 1954 May;29(3):238–241. doi: 10.1104/pp.29.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry G. M., Gruen H. E. ACTION SPECTRA FOR THE POSITIVE AND NEGATIVE PHOTOTROPISM OF PHYCOMYCES SPORANGIOPHORES. Proc Natl Acad Sci U S A. 1959 Jun;45(6):797–804. doi: 10.1073/pnas.45.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELBRUECK M., VARJU D. Photoreactions in Phycomyces. Responses to the stimulation of narrow test areas with ultraviolet light. J Gen Physiol. 1961 Jul;44:1177–1188. doi: 10.1085/jgp.44.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNISON D. S. STEADY-STATE PHOTOTROPISM IN PHYCOMYCES. J Gen Physiol. 1965 Jan;48:393–408. doi: 10.1085/jgp.48.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNISON D. S. THE EFFECT OF LIGHT ON THE GEOTROPIC RESPONSES OF PHYCOMYCES SPORANGIOPHORES. J Gen Physiol. 1964 Mar;47:651–665. doi: 10.1085/jgp.47.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNISON D. S. Tropic responses of Phycomyces sporangiophores to gravitational and centrifugal stimuli. J Gen Physiol. 1961 Sep;45:23–38. doi: 10.1085/jgp.45.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbrück M., Shropshire W. Action and Transmission Spectra of Phycomyces. Plant Physiol. 1960 Mar;35(2):194–204. doi: 10.1104/pp.35.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison D. S., Roth C. C. Phycomyces sporangiophores: fungal stretch receptors. Science. 1967 Jun 9;156(3780):1386–1388. doi: 10.1126/science.156.3780.1386. [DOI] [PubMed] [Google Scholar]
- ERWIN J., BLOCH K. BIOSYNTHESIS OF UNSATURATED FATTY ACIDS IN MICROORGANISMS. Science. 1964 Mar 6;143(3610):1006–1012. doi: 10.1126/science.143.3610.1006. [DOI] [PubMed] [Google Scholar]
- Ellis J. J., Roberson J. A. Viability of fungus cultures preserved by lyophilization. Mycologia. 1968 Mar-Apr;60(2):399–405. [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. III. Identification of the minor polyene components of the fungus Phycomyces blakesleeanus and a study of their synthesis under various cultural conditions. Biochem J. 1952 Feb;50(4):550–558. doi: 10.1042/bj0500550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R. The enzymatic conversion of 5-dehydroshikimic acid to protocatechuic acid. J Biol Chem. 1958 Nov;233(5):1146–1151. [PubMed] [Google Scholar]
- Gamow R. I., Goodell W. Local metabolic autonomy in phycomyces sporangiophores. Plant Physiol. 1969 Jan;44(1):15–20. doi: 10.1104/pp.44.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulston G., Goad L. J., Goodwin T. W. Sterol biosynthesis in fungi. Biochem J. 1967 Feb;102(2):15C–17C. doi: 10.1042/bj1020015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen H. E. Growth and Development of Isolated Phycomyces Sporangiophores. Plant Physiol. 1959 Mar;34(2):158–168. doi: 10.1104/pp.34.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen H. E. The production of indoleacetic acid by Phycomyces blakesleeanus. Mycologia. 1965 Sep-Oct;57(5):683–695. [PubMed] [Google Scholar]
- HALBSGUTH W., RUDOLPH H. Untersuchungen über die Wärmeaktivierung der Sporangiosporen von Phycomyces blakesleeanus. I. Arch Mikrobiol. 1959;32(3):296–308. [PubMed] [Google Scholar]
- HYDE B. B., HODGE A. J., KAHN A., BIRNSTIEL M. L. STUDIES ON PHYTOFERRITIN. I. IDENTIFICATION AND LOCALIZATION. J Ultrastruct Res. 1963 Oct;59:248–258. doi: 10.1016/s0022-5320(63)80005-2. [DOI] [PubMed] [Google Scholar]
- Hammel H. T. Freezing of xylem sap without cavitation. Plant Physiol. 1967 Jan;42(1):55–66. doi: 10.1104/pp.42.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M., Cerdá-Olmedo E. Segregation of heterokaryons in the asexual cycle of Phycomyces. Mol Gen Genet. 1968;102(3):187–195. doi: 10.1007/BF00385973. [DOI] [PubMed] [Google Scholar]
- JAFFE L. F. The effect of polarized light on the growth of a transparent cell. A theoretical analysis. J Gen Physiol. 1960 May;43:897–911. doi: 10.1085/jgp.43.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFE L. F. Tropistic responses of zygotes of the Fucaceae to polarized light. Exp Cell Res. 1958 Oct;15(2):282–299. doi: 10.1016/0014-4827(58)90031-4. [DOI] [PubMed] [Google Scholar]
- KREGER D. R. Observations on cell walls of yeasts and some other fungi by x-ray diffraction and solubility tests. Biochim Biophys Acta. 1954 Jan;13(1):1–9. doi: 10.1016/0006-3002(54)90264-4. [DOI] [PubMed] [Google Scholar]
- Kowallik W. Action spectrum for an enhancement of endogenous respiration by light in chlorella. Plant Physiol. 1967 May;42(5):672–676. doi: 10.1104/pp.42.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS D. Comparative incompatibility in angiosperms and fungi. Adv Genet. 1954;6:235–285. doi: 10.1016/s0065-2660(08)60131-5. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN R., DOUGLAS J. O., HUMPHREY W., Jr Ascites induced in mice by Staphylococcus. Science. 1959 Mar 20;129(3351):775–775. doi: 10.1126/science.129.3351.775. [DOI] [PubMed] [Google Scholar]
- MAASS W. Zur Frage einer Beteiligung von Indolylessigsäure beim Wachstum und beim Phototropismus von Phycomyces. Arch Mikrobiol. 1958;30(1):73–90. [PubMed] [Google Scholar]
- Meissner G., Delbruck M. Carotenes and retinal in Phycomyces mutants. Plant Physiol. 1968 Aug;43(8):1279–1283. doi: 10.1104/pp.43.8.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSON J. A., KNIZLEY H., Jr The effect of diphenylamine on carotenoid, sterol and fatty acid synthesis in Phycomyces blakesleeanus. Arch Biochem Biophys. 1962 Apr;97:138–145. doi: 10.1016/0003-9861(62)90055-3. [DOI] [PubMed] [Google Scholar]
- PLEMPEL M., BRAUNITZER G. Die Isolierung der Mucorineen-Sexualstoffe. I. Z Naturforsch B. 1958 May;13B(5):302–305. [PubMed] [Google Scholar]
- PLEMPEL M. Die Sexualstoffe der Mucoracea; Ihre Abtrennung und die Erklärung ihrer Funktion. Arch Mikrobiol. 1957;26(2):151–174. [PubMed] [Google Scholar]
- Pickett J. M., French C. S. The action spectrum for blue-light-stimulated oxygen uptake in Chlorella. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1587–1593. doi: 10.1073/pnas.57.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYES P., CHICHESTER C. O., NAKAYAMA T. O. THE MECHANISM OF BETA-IONONE STIMULATION OF CAROTENOID AND ERGOSTEROL BIOSYNTHESIS IN PHYCOMYCES BLAKESLEEANUS. Biochim Biophys Acta. 1964 Sep 4;90:578–592. doi: 10.1016/0304-4165(64)90237-5. [DOI] [PubMed] [Google Scholar]
- RILLING H. C. ON THE MECHANISM OF PHOTOINDUCTION OF CAROTENOID SYNTHESIS. Biochim Biophys Acta. 1964 May 25;79:464–475. doi: 10.1016/0926-6577(64)90212-8. [DOI] [PubMed] [Google Scholar]
- ROBINOW C. F. The structure and behavior of the nuclei in spores and growing hyphae of Mucorales. II. Phycomyces blakesleeanus. Can J Microbiol. 1957 Aug;3(5):791–798. doi: 10.1139/m57-088. [DOI] [PubMed] [Google Scholar]
- ROELOFSEN P. A. Cell-wall structure in the growth-zone of Phycomyces sporangiophores. II. Double refraction and electron microscopy. Biochim Biophys Acta. 1951 Jan;6(3):357–373. doi: 10.1016/0006-3002(50)90109-0. [DOI] [PubMed] [Google Scholar]
- ROTHER W. Einfluss von beta-Indolylessigsäure und wasserlöslichen Wirkstoffen des Mais-Scutellums auf Keimung und Wachstum von Phycomyces Blakesleeanus. Arch Mikrobiol. 1954;20(1):89–108. [PubMed] [Google Scholar]
- Robbins W. J., Kavanagh F. Hypoxanthine, a Growth Substance for Phycomyces. Proc Natl Acad Sci U S A. 1942 Mar;28(3):65–69. doi: 10.1073/pnas.28.3.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins W. J., Kavanagh F. Intermediates of Vitamin B(1) and Growth of Phycomyces. Proc Natl Acad Sci U S A. 1937 Sep;23(9):499–502. doi: 10.1073/pnas.23.9.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW R. THE OCCURRENCE OF GAMMA-LINOLENIC ACID IN FUNGI. Biochim Biophys Acta. 1965 Apr 5;98:230–237. doi: 10.1016/0005-2760(65)90117-7. [DOI] [PubMed] [Google Scholar]
- SHROPSHIRE W., Jr Growth responses of Phycomyces to polarized light stimuli. Science. 1959 Aug 7;130(3371):336–336. doi: 10.1126/science.130.3371.336. [DOI] [PubMed] [Google Scholar]
- STOFFEL W., WIESE H. DIE BIOSYNTHESE DER OL-, LINOL- UND GAMMA-LINOLENSAEURE IN PHYCOMYCES BLAKESLEEANUS. Hoppe Seylers Z Physiol Chem. 1965;340:148–156. doi: 10.1515/bchm2.1965.340.1-2.148. [DOI] [PubMed] [Google Scholar]
- Shaw R. The polyunsaturated fatty acids of microorganisms. Adv Lipid Res. 1966;4:107–174. doi: 10.1016/b978-1-4831-9940-5.50011-9. [DOI] [PubMed] [Google Scholar]
- Shropshire W., Jr, Gettens R. H. Light induced concentration changes of adenosine-triphosphate in phycomyces sporangiophores. Plant Physiol. 1966 Feb;41(2):203–207. doi: 10.1104/pp.41.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W. Light Induced Concentration Changes of ATP From Phycomyces Sporangiophores: A Re-examination. Plant Physiol. 1968 Aug;43(8):1317–1318. doi: 10.1104/pp.43.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W. The Lens Effect and Phototropism of Phycomyces. J Gen Physiol. 1962 May 1;45(5):949–958. doi: 10.1085/jgp.45.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W., Withrow R. B. Action Spectrum of Phototropic Tip-Curvature of Avena. Plant Physiol. 1958 Sep;33(5):360–365. doi: 10.1104/pp.33.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifler R. B. Growth of Sporangiophores of Phycomyces Immersed in Water. Science. 1961 Mar 31;133(3457):1022–1022. doi: 10.1126/science.133.3457.1022. [DOI] [PubMed] [Google Scholar]
- Storck R. Nucleotide composition of nucleic acids of fungi. I. Ribonucleic acids. J Bacteriol. 1965 Nov;90(5):1260–1264. doi: 10.1128/jb.90.5.1260-1264.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck R. Nucleotide composition of nucleic acids of fungi. II. Deoxyribonucleic acids. J Bacteriol. 1966 Jan;91(1):227–230. doi: 10.1128/jb.91.1.227-230.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton R. M. The fine structure of Phycomyces. 1. Autophagic vesicles. J Ultrastruct Res. 1967 Dec 12;21(3):269–280. doi: 10.1016/s0022-5320(67)80096-0. [DOI] [PubMed] [Google Scholar]
- VARJU D., EDGAR L., DELBRUCK M. Interplay between the reactions to light and to gravity in Phycomyces. J Gen Physiol. 1961 Sep;45:47–58. doi: 10.1085/jgp.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSINK E. C., BOUMAN M. A. Can phototropism be initiated by a one-quantum-per-cell-process? Enzymologia. 1947;12(3):193–197. [PubMed] [Google Scholar]
- Withrow R. B. An Interference-Filter Monochromator System for the Irradiation of Biological Material. Plant Physiol. 1957 Jul;32(4):355–360. doi: 10.1104/pp.32.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALOKAR M. Biosynthesis of carotenoids in Neurospora; action spectrum of photoactivation. Arch Biochem Biophys. 1955 Jun;56(2):318–325. doi: 10.1016/0003-9861(55)90252-6. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Burke P. V., Delbrück M. Absorption and screening in Phycomyces. J Gen Physiol. 1967 Aug;50(7):1893–1906. doi: 10.1085/jgp.50.7.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ende H. Relationship between sexuality and carotene synthesis in Blakeslea trispora. J Bacteriol. 1968 Oct;96(4):1298–1303. doi: 10.1128/jb.96.4.1298-1303.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]