Abstract
Transcatheter patent foramen ovale (PFO) closure is an alternative to antiplatelet or anticoagulative therapy in patients with cryptogenic stroke, and it is associated with a small incidence of periprocedural sequelae. Because embolization of PFO closure devices is a very rare procedural complication, data on its frequency, causes, and management are sparse. We sought to review the medical literature and the cases of PFO closure-device embolization at our institution with the aim of identifying likely problems and reporting potential solutions. Out of 310 adult patients who underwent transcatheter PFO closure from June 2002 through April 2011, there were 2 cases (0.6%) of PFO closure-device embolization. In both patients, hypermobile septum primum and thick septum secundum were present. In one patient, failure to use a sizing balloon might have resulted in an underestimation of the PFO's size. In both patients, device embolization was identified in a timely fashion, the embolized device was safely retrieved, and the PFO was percutaneously closed with success.
The incidence of PFO closure-device embolization is very low. The cases described here underscore the importance of imaging in the identification of morphologic predispositions to closure-device malpositioning, in the recognition of impending embolization, and in the timely management of embolization.
Key words: Contrast media/diagnostic use; device removal/methods; echocardiography, transesophageal; fluoroscopy; foramen ovale, patent/radiography/therapy/ultrasonography; foreign body migration; ischemic attack, transient/prevention & control; prosthesis implantation/adverse effects; septal occluder device/adverse effects; stroke/prevention & control
Several studies1–3 have shown the feasibility of transcatheter patent foramen ovale (PFO) closure as an adjunct or alternative to antiplatelet or anticoagulative therapy in patients with cryptogenic stroke. Procedural sequelae are rare. In a meta-analysis of 10 studies consisting of more than 1,350 patients with transcatheter PFO closure,4 major sequelae (death, tamponade, hemorrhage requiring blood transfusion, fatal pulmonary embolism, and need for surgical intervention) occurred in 1.5% of patients, and minor sequelae (device embolization with percutaneous retrieval, arrhythmias, device fracture, asymptomatic device thrombosis, symptomatic air embolism, and groin sequelae) occurred in 7.9% of patients. Data exist on the predisposing factors for embolization of atrial-septal-defect closure devices (inadequate rim and undersized device)5–8; but PFO closure-device embolization is such a rare complication that data on its frequency, causes, and management are sparse.1,9,10
We sought to review the literature on PFO closure-device embolization and to describe such cases at our institution with the aim of identifying likely problems and reporting potential solutions. Table I shows the baseline characteristics of patients who underwent PFO closure from June 2002 through April 2011 at our institution, the indications for closure, and the different types of devices used. Out of 310 patients, 2 experienced device embolization (0.7%) during transcatheter PFO closure.
TABLE I. Baseline Characteristics of 310 Patients Who Underwent Transcatheter PFO Closure

Case Reports
Patient 1
The first patient to experience device embolization was an 83-year-old woman with recurrent transient ischemic attack. Cerebrovascular imaging, Holter monitoring for atrial arrhythmia, and panel testing for hypercoagulability yielded unremarkable results. She was not a candidate for anticoagulative therapy due to hematemesis while on therapeutic anticoagulation for lower-extremity deep-vein thrombosis 2 months earlier, for which an inferior vena cava filter had been placed. Transesophageal echocardiography (TEE) revealed a PFO with early right-to-left shunting. There was also an atrial septal aneurysm with a total septal excursion of approximately 18 mm. The septum secundum was 11 mm thick. The patient was referred for percutaneous transcatheter PFO closure.
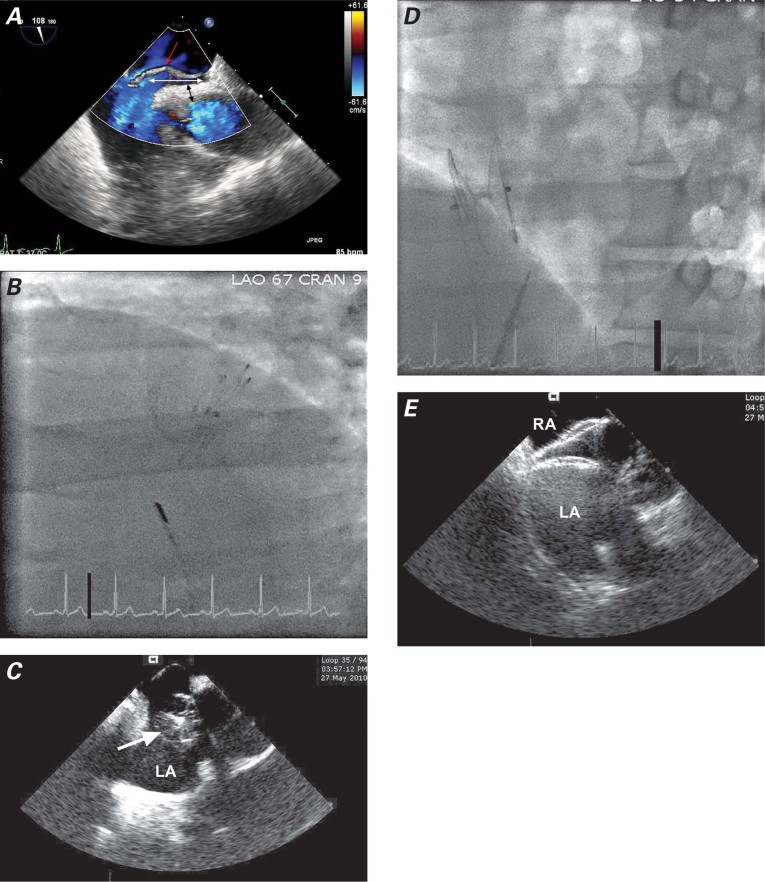
Two 11F femoral vein sheaths were placed, one on each side. After the PFO had been crossed with a Goodale-Lubin catheter, a 9F AMPLATZER® delivery sheath (St. Jude Medical, Inc.; St. Paul, Minn) was advanced into the left atrium. Balloon sizing was not performed because it was not routine at that time. A 25-mm AMPLATZER® PFO Occluder (St. Jude Medical) was delivered through the sheath and deployed across the atrial septum. The device initially seated well, and deployment was confirmed on “push and pull.” When the device was then released, it almost immediately slipped from the septum secundum. This was recognized on intracoronary echocardiographic (ICE) imaging and flouroscopy (Fig. 1A) and confirmed on angiography and TEE (Figs. 1B and 1C). An attempt at retrieval with a gooseneck snare dislodged the device into the left atrium. Subsequent embolization occurred through the left ventricle and the thoracic aorta into the proximal abdominal aorta. At this point, 12F sheaths were placed in the right and left femoral arteries. The device was initially immobilized with a snare from the right femoral artery. The right atrial disc button was then snared from the left femoral artery, and the device was successfully retrieved (Fig. 2A). Then a 35-mm AMPLATZER PFO Occluder was successfully deployed across the PFO, and placement was confirmed by TEE, ICE, and fluoroscopy (Figs. 2B and 2C). A transthoracic echocardiogram (TTE) performed on the following day showed that the device was well seated across the interatrial septum: neither color-flow Doppler nor agitated saline contrast solution revealed flow across the PFO. Similarly, 6 months later, a TTE showed the device to be well seated, with no flow evident by color Doppler or saline contrast.
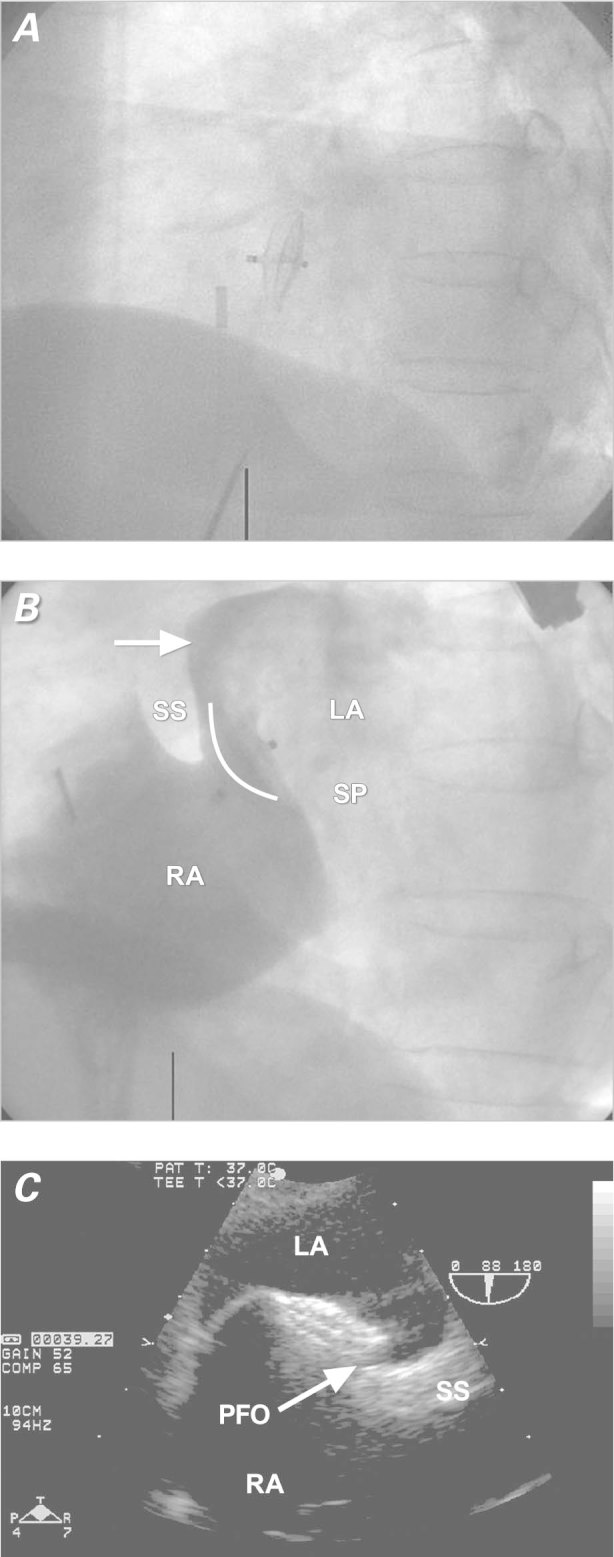
Fig. 1 Patient 1. A) Fluoroscopic image (lateral view) shows slippage of the device immediately after deployment. B) Right atrial angiogram after device deployment shows the right border of the device (curved white line) to be on the left side of the septum secundum (arrow) instead of the right side. C) Transesophageal echocardiogram shows the malpositioned device. Note also the PFO, still present after device embolization.
LA = left atrium; PFO = patent foramen ovale; RA = right atrium; SS = septum secundum; SP = septum primum
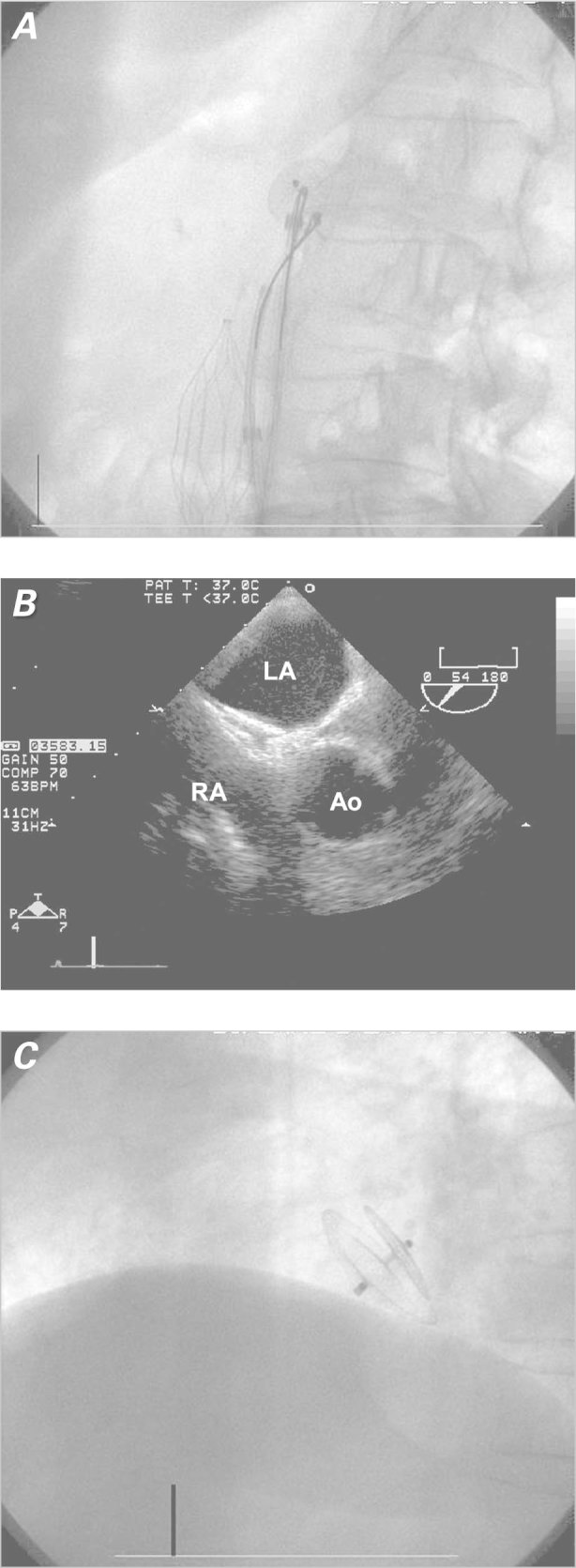
Fig 2. Patient 1. A) Fluoroscopic image shows the device being snared from the abdominal aorta. B) Transesophageal echocardiogram shows correct device placement. C) Fluoroscopic image (lateral view) shows correct device placement.
Ao = aorta; LA = left atrium; RA = right atrium;
Patient 2
A 52-year-old man presented with sudden onset of a blind spot in his left eye from embolic occlusion of the left retinal artery. Further diagnostic evaluation—via computed tomography of the brain, carotid ultrasonography, magnetic resonance imaging and angiography of the brain, digital cerebral angiography, hypercoagulable panel, and 48-hour Holter monitoring—was unrevealing. A TTE showed a mobile interatrial septum and a large shunt consistent with PFO. A TEE showed a septum secundum 12 mm thick, an aneurysmal septum primum with a total septal excursion of about 29 mm, a tunnel 33 mm long (Fig. 3A) and a large PFO with significant passage of bubbles. The patient refused anticoagulative therapy and was referred for PFO closure.
Fig. 3 Patient 2. A) Transesophageal color-flow echocardiogram shows the patent foramen ovale with a tunnel 33 mm in length (white arrow), a septum secundum 12 mm thick (black arrow), and an atrial septal aneurysm (red arrow). B) Fluoroscopic image (lateral view) shows device embolization to the left atrium. C) Intracardiac echocardiogram shows device embolization (arrow) to the left atrium. D) Fluoroscopic image (lateral view) shows proper device deployment. E) Intracardiac echocardiogram shows proper device placement.
LA = left atrium; RA = right atrium
After the PFO had been crossed with a Goodale-Lubin catheter, a 25-mm NMT sizing balloon measured the stretched diameter of the PFO at 15 mm by both fluoroscopy and ICE. A 30-mm GORE® HELEX® Septal Occluder (W.L. Gore & Associates, Inc.; Flagstaff, Ariz) was delivered and deployed across the PFO. The device was noted to be in correct position after the locking loop was deployed, but almost immediately after release, the device slipped from the septum secundum (Fig. 3B) and settled mostly in the left atrium (Fig. 3C). The HELEX occluder was pulled through the PFO tunnel with a forceps retrieval device. By means of a gooseneck snare, it was pulled successfully through the delivery sheath and removed via the venous sheath. The PFO was closed with a 35-mm AMPLATZER “Cribriform” device (AGA Medical Corp.; Plymouth, Minn.) successfully deployed across the PFO without complication (Figs. 2D and 2E). A TEE performed on the next day showed the AMPLATZER device to be well seated, with no flow evident across the interatrial septum by color-flow Doppler or agitated saline contrast solution.
Discussion
The incidence of migration or embolization of PFO closure devices was low in our series, only 0.7%. In both cases, the migration and embolization was identified during the index procedure and successfully treated with percutaneous retrieval of the embolized device. These cases highlight the predisposing anatomy, imaging clues for device migration, and practical tips for the prevention and management of such sequelae.
The incidence of PFO device embolization reported in prior studies is 0.7% to 1.2%,1,9–11 similar to that of our series. Upon investigating the reasons for device embolization, we noted that the cases reported here have 2 morphologic features in common—an aneurysmal atrial septum and a thick septum secundum (>10 mm). In addition, Patient 2 had a very long tunnel (33 mm). In a previously published report,12 we found a mean tunnel length of 14 mm in a group of 58 patients who underwent transcatheter PFO closure for recurrent stroke or transient ischemic attack. Our Patient 1 had a mobile interatrial septum (maximum excursion, 18 mm) and a thick septum secundum (11 mm). A 25-mm AMPLATZER device—deployed in Patient 1 without balloon sizing—slipped from the septum secundum. At the time of this procedure, there was no consensus that balloon sizing should be applied to all patients. We have since routinely used balloon sizing to determine not only the size but the shape of the PFO.13,14 Balloon sizing poses no additional risk.15 Despite this, some operators still do not use balloon sizing routinely.16,17 In fact, balloon sizing was not recommended in the instructions-for-use document provided by AGA Medical, the manufacturer of the AMPLATZER PFO Occluder. In retrospect, lack of balloon sizing and the atrial septal aneurysm were felt to be risk factors for device embolization in Patient 1. Larger devices are usually recommended in cases of atrial septal aneurysm in order to cover the entire redundant and mobile septum.18 A larger device is less likely to slip from the septum secundum, yet it allows mobility of the septum primum between the disks of the device, which can lead to residual shunt. In order to minimize the risk of device erosion, caution must be exercised to ensure that a large device does not impinge on nearby structures, most notably the aortic root or the superior wall of the left atrium.19 Furthermore, our 2 cases of device embolization occurred early in our operators' experience with transcatheter PFO closure (among their first 25 cases). Although it is possible that more experience can mitigate the occurrence of such sequelae during transcatheter PFO closure, we cannot say this conclusively or objectively, given the lack of data.
Patient 2 also had a very mobile interatrial septum (total septal excursion, ca. 29 mm) and a thick septum secundum (12 mm). In addition, this patient had a long PFO tunnel (33 mm). On the basis of these measurements, together with fluoroscopic and ICE measurements that showed a PFO diameter of 15 mm, we selected a 30-mm HELEX device, which embolized soon after deployment. A randomized study (published in 2008) that compared 3 types of PFO closure devices found that device embolization occurred most often in patients who received the HELEX device.20 Two of the 3 patients in that study who experienced HELEX device embolization had an aneurysmal atrial septum. A long PFO tunnel can cause partial deployment (in the tunnel) of one of the discs. In fact, a less compliant device (such as the AMPLATZER PFO Occluder), when deployed across a PFO with a long tunnel, will cause that tunnel to retract or even to disappear, because the compliant septum primum is displaced inferiorly in such a manner that the 2 discs straddle the septum.21 Therefore, the AMPLATZER “Cribriform” device was subsequently chosen for Patient 2, and this resulted in successful closure of the PFO without complication. Other techniques for possible use in cases of a long PFO tunnel include transseptal puncture, balloon “detunnelization,”22 or other types of closure (with, for example, the Premere™ PFO closure device [St. Jude Medical]) that enable the operator to adjust the distance between the right and left arms in order to fit the PFO tunnel.23 It remains to be seen, in clinical trial, whether some specific closure devices are better suited than others to particular PFO anatomies.
Summary. The incidence of PFO closure-device embolization is very low. The cases described here underscore the importance of imaging in the identification of morphologic predispositions to closure-device malpositioning and in the recognition and timely management of embolization.
Footnotes
Address for reprints: Samir R. Kapadia, MD, FACC, Department of Cardiovascular Medicine, Cleveland Clinic Foundation, J2-3, 9500 Euclid Ave., Cleveland, OH 44195
E-mail: kapadis@ccf.org
References
- 1.Braun MU, Fassbender D, Schoen SP, Haass M, Schraeder R, Scholtz W, Strasser RH. Transcatheter closure of patent foramen ovale in patients with cerebral ischemia. J Am Coll Cardiol 2002;39(12):2019–25. [DOI] [PubMed]
- 2.Martin F, Sanchez PL, Doherty E, Colon-Hernandez PJ, Delgado G, Inglessis I, et al. Percutaneous transcatheter closure of patent foramen ovale in patients with paradoxical embolism. Circulation 2002;106(9):1121–6. [DOI] [PubMed]
- 3.Bruch L, Parsi A, Grad MO, Rux S, Burmeister T, Krebs H, Kleber FX. Transcatheter closure of interatrial communications for secondary prevention of paradoxical embolism: single-center experience. Circulation 2002;105(24):2845–8. [DOI] [PubMed]
- 4.Khairy P, O'Donnell CP, Landzberg MJ. Transcatheter closure versus medical therapy of patent foramen ovale and presumed paradoxical thromboemboli: a systematic review. Ann Intern Med 2003;139(9):753–60. [DOI] [PubMed]
- 5.Chan KT, Cheng BC. Retrieval of an embolized Amplatzer septal occluder. Catheter Cardiovasc Interv 2010;75(3):465–8. [DOI] [PubMed]
- 6.Levi DS, Moore JW. Embolization and retrieval of the Amplatzer septal occluder. Catheter Cardiovasc Interv 2004;61 (4):543–7. [DOI] [PubMed]
- 7.Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002;39(6):1061–5. [DOI] [PubMed]
- 8.Fischer G, Stieh J, Uebing A, Hoffmann U, Morf G, Kramer HH. Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart 2003;89(2):199–204. [DOI] [PMC free article] [PubMed]
- 9.Beitzke A, Schuchlenz H, Gamillscheg A, Stein JI, Wendelin G. Catheter closure of the persistent foramen ovale: mid-term results in 162 patients. J Interv Cardiol 2001;14(2):223–9. [DOI] [PubMed]
- 10.Sievert H, Horvath K, Zadan E, Krumsdorf U, Fach A, Merle H, et al. Patent foramen ovale closure in patients with transient ischemia attack/stroke. J Interv Cardiol 2001;14(2):261–6. [DOI] [PubMed]
- 11.Alameddine F, Block PC. Transcatheter patent foramen ovale closure for secondary prevention of paradoxical embolic events: acute results from the FORECAST registry. Catheter Cardiovasc Interv 2004;62(4):512–6. [DOI] [PubMed]
- 12.Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira EI, Borek PP, Krasuski RA, et al. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol 2009;103(1):124–9. [DOI] [PubMed]
- 13.Hildick-Smith D, Behan M, Haworth P, Rana B, Thomas M. Patent foramen ovale closure without echocardiographic control: use of “standby” intracardiac ultrasound. JACC Cardiovasc Interv 2008;1(4):387–91. [DOI] [PubMed]
- 14.Alibegovic J, Bonvini R, Sigwart U, Dorsaz P, Camenzind E, Verin V. The role of the sizing balloon in selection of the patent foramen ovale closure device size. Exp Clin Cardiol 2008; 13(1):42–6. [PMC free article] [PubMed]
- 15.Bonvini RF, Sigwart U, Verin V. Interatrial septum rupture during balloon measurement of a patent foramen ovale in a young patient presenting cryptogenic stroke. Catheter Cardiovasc Interv 2007;69(2):274–6. [DOI] [PubMed]
- 16.Kern MJ. Is PFO sizing necessary? Catheter Cardiovasc Interv 2007;70(3):469–71. [DOI] [PubMed]
- 17.Wahl A, Tai T, Praz F, Schwerzmann M, Seiler C, Nedeltchev K, et al. Late results after percutaneous closure of patent foramen ovale for secondary prevention of paradoxical embolism using the Amplatzer PFO occluder without intraprocedural echocardiography: effect of device size. JACC Cardiovasc Interv 2009;2(2):116–23. [DOI] [PubMed]
- 18.Schwerzmann M, Salehian O. Hazards of percutaneous PFO closure. Eur J Echocardiogr 2005;6(6):393–5. [DOI] [PubMed]
- 19.Yared K, Baggish AL, Solis J, Durst R, Passeri JJ, Palacios IF, Picard MH. Echocardiographic assessment of percutaneous patent foramen ovale and atrial septal defect closure complications. Circ Cardiovasc Imaging 2009;2(2):141–9. [DOI] [PubMed]
- 20.Taaffe M, Fischer E, Baranowski A, Majunke N, Heinisch C, Leetz M, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder). Am J Cardiol 2008;101(9):1353–8. [DOI] [PubMed]
- 21.Marshall AC, Lock JE. Structural and compliant anatomy of the patent foramen ovale in patients undergoing transcatheter closure. Am Heart J 2000;140(2):303–7. [DOI] [PubMed]
- 22.Spence MS, Khan AA, Mullen MJ. Balloon assessment of patent foramen ovale morphology and the modification of tunnels using a balloon detunnelisation technique. Catheter Cardiovasc Interv 2008;71(2):222–8. [DOI] [PubMed]
- 23.Donti A, Giardini A, Salomone L, Formigari R, Picchio FM. Transcatheter patent foramen ovale closure using the Premere PFO occlusion system. Catheter Cardiovasc Interv 2006;68 (5):736–40. [DOI] [PubMed]