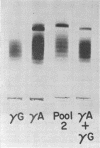
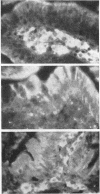
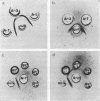
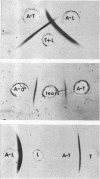
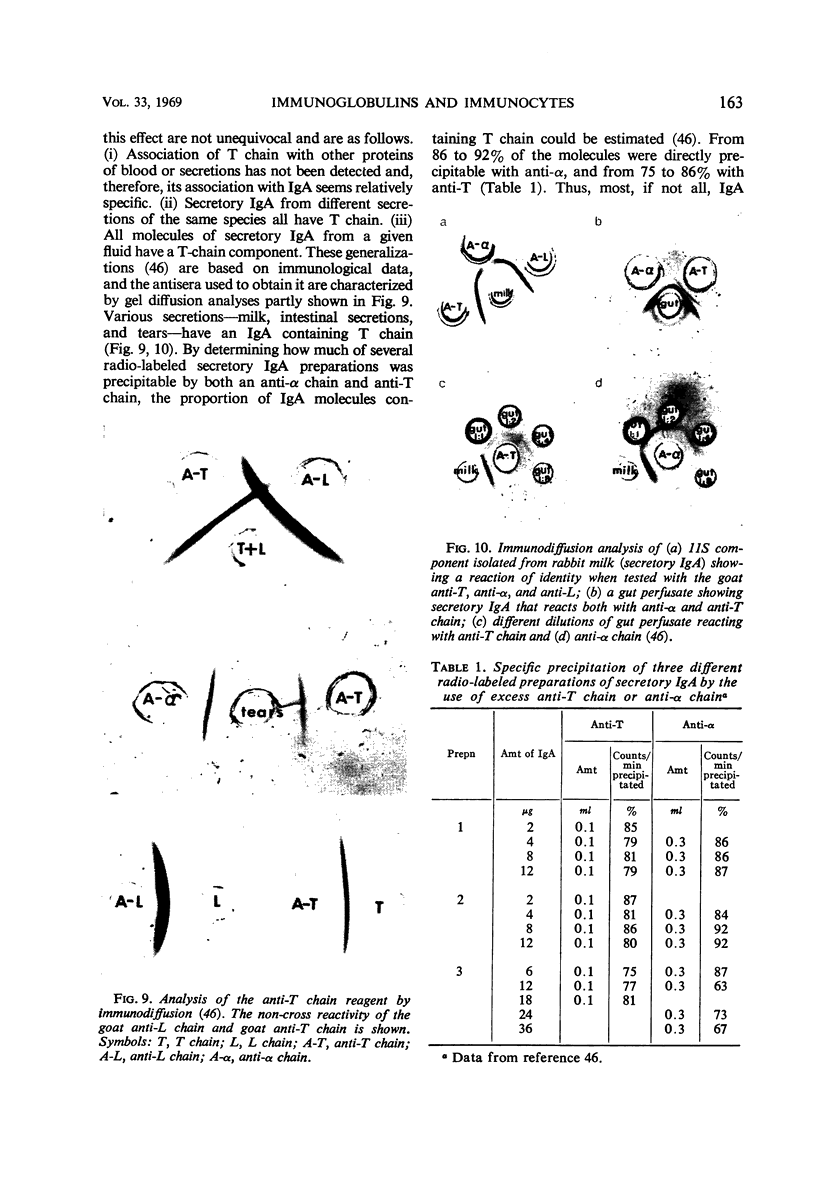
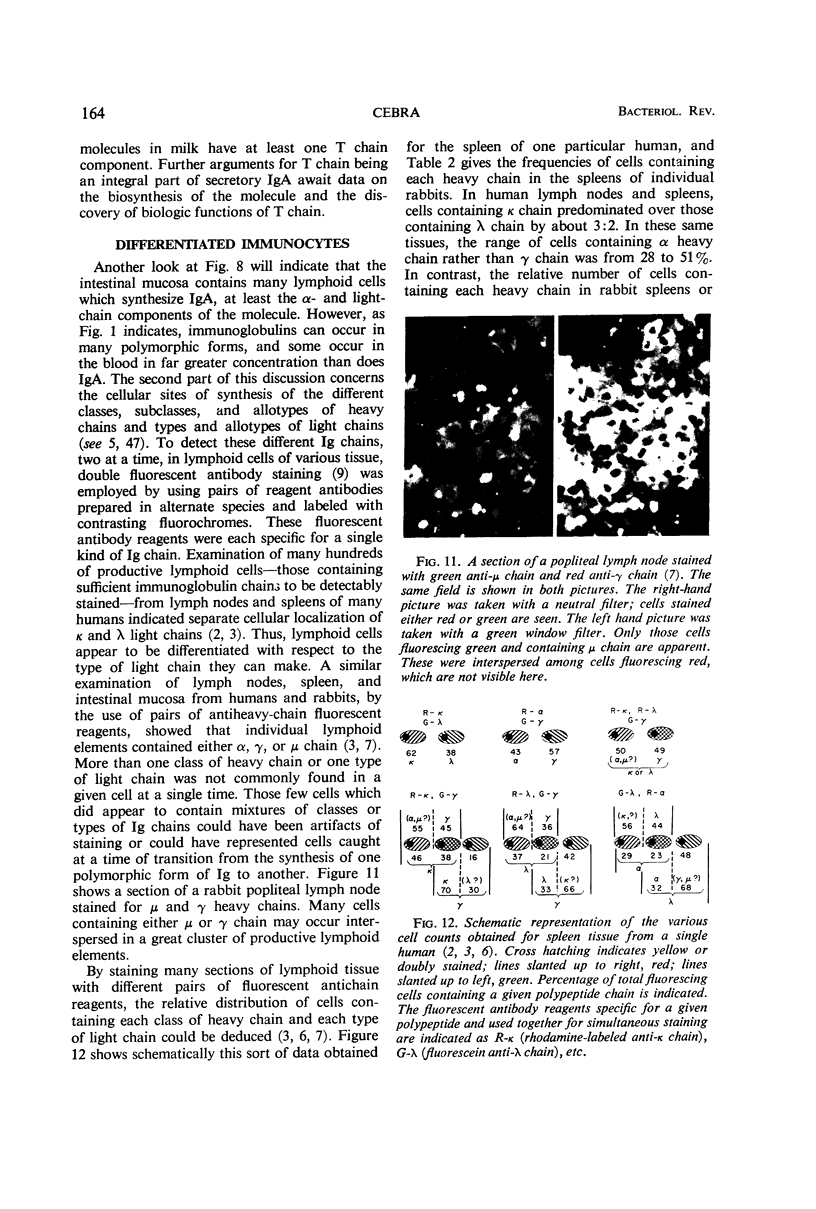
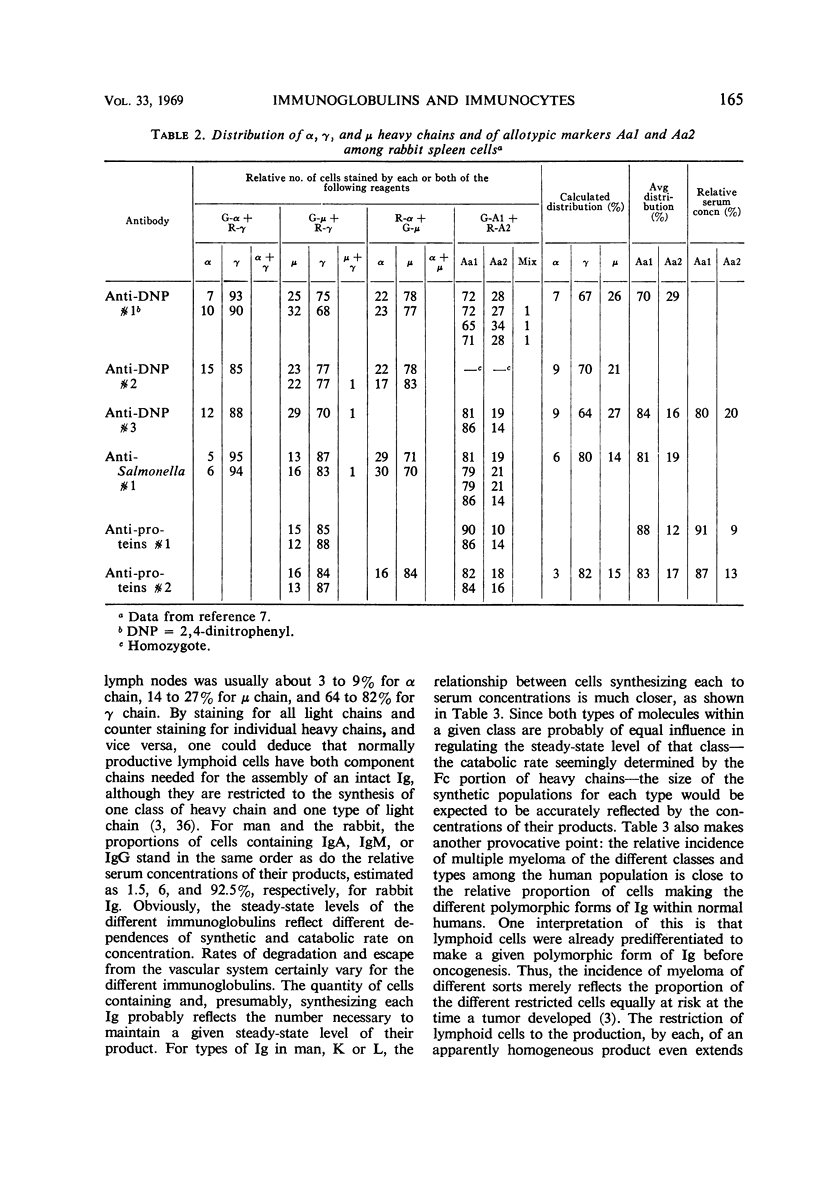
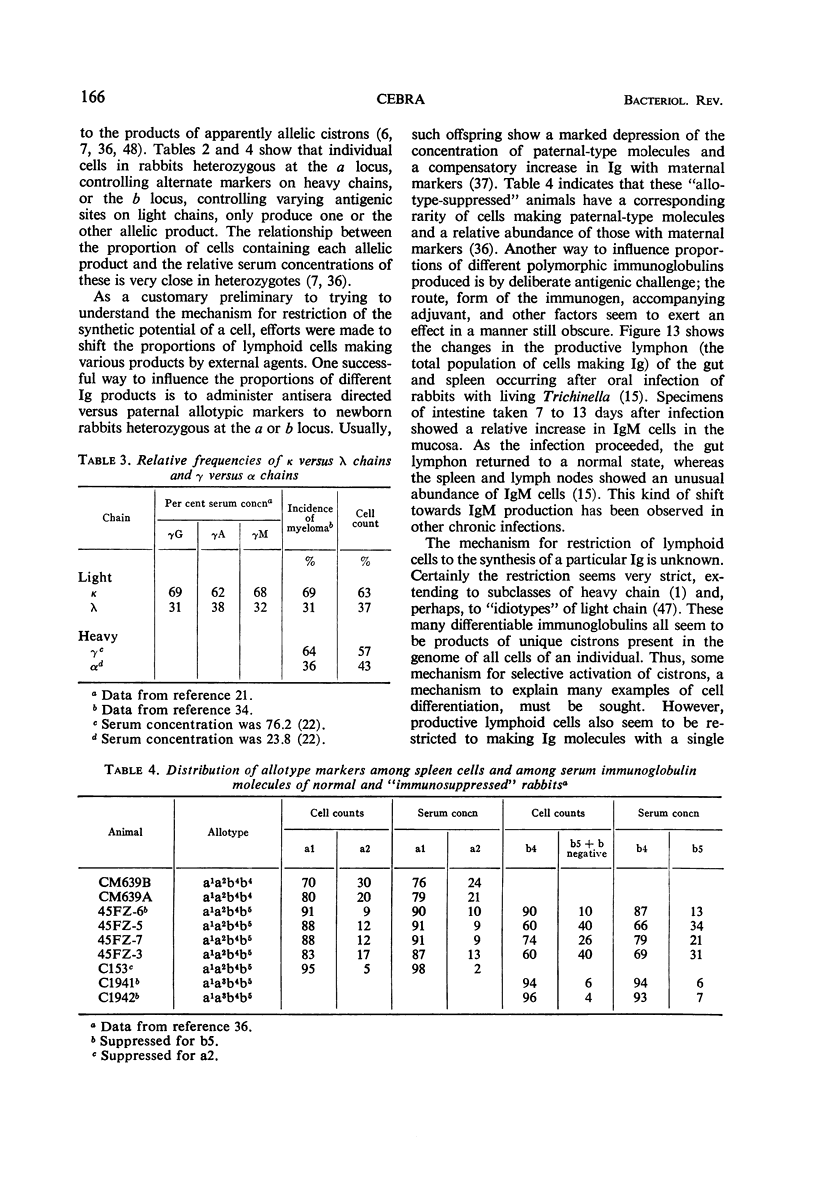
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Bruni C. B. Purification of an acid alpha-glucosidase by dextran-gel filtration. Biochem J. 1967 Oct;105(1):35–38. doi: 10.1042/bj1050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNIER G. M., CEBRA J. J. POLYPEPTIDE CHAINS OF HUMAN GAMMA-GLOBULIN: CELLULAR LOCALIZATION BY FLUORESCENT ANTIBODY. Science. 1964 Jun 26;144(3626):1590–1591. doi: 10.1126/science.144.3626.1590. [DOI] [PubMed] [Google Scholar]
- Bernier G. M., Ballieux R. E., Tominaga K. T., Putnam F. W. Heavy chain subclasses of human gamma G-globulin. Serum distribution and cellular localization. J Exp Med. 1967 Feb 1;125(2):303–316. doi: 10.1084/jem.125.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier G. M., Cebra J. J. Frequency distribution of alpha, gamma, kappa and lambda polypeptide chains in human lymphoid tissues. J Immunol. 1965 Aug;95(2):246–253. [PubMed] [Google Scholar]
- Cebra J. J., Colberg J. E., Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966 Mar 1;123(3):547–558. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Givol D., Porter R. R. Common peptides from the N-terminal half of heavy chain of immunoglobulin G from normal rabbit serum and a specific antibody. Biochem J. 1968 Mar;107(1):69–77. doi: 10.1042/bj1070069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Cebra J. J., Robbins J. B. Gamma-A-immunoglobulin from rabbit colostrum. J Immunol. 1966 Jul;97(1):12–24. [PubMed] [Google Scholar]
- Cebra J. J., Small P. A., Jr Polypeptide chain structure of rabbit immunoglobulins. 3. Secretory gamma-A-immunoglobulin from colostrum. Biochemistry. 1967 Feb;6(2):503–512. doi: 10.1021/bi00854a019. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Steiner L. A., Porter R. R. The partial sequence of two large peptides from the N-terminal half of heavy chains from normal rabbit immunoglobulin G. Biochem J. 1968 Mar;107(1):79–88. doi: 10.1042/bj1070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure and biological properties of immunoglobulins. Adv Immunol. 1967;7:1–89. doi: 10.1016/s0065-2776(08)60126-1. [DOI] [PubMed] [Google Scholar]
- Crandall R. B., Cebra J. J., Crandall C. A. The relative proportions of IgG-, IgAand IgM-containing cells in rabbit tissues during experimental trichinosis. Immunology. 1967 Feb;12(2):147–158. [PMC free article] [PubMed] [Google Scholar]
- DRAY S., YOUNG G. O., GERALD L. IMMUNOCHEMICAL IDENTIFICATION AND GENETICS OF RABBIT GAMMA-GLOBULIN ALLOTYPES. J Immunol. 1963 Sep;91:403–415. [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- FAHEY F. L., LAWRENCE M. E. QUANTITATIVE DETERMINATION OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND GAMMA-1-MACROGLOBULINS IN HUMAN SERUM. J Immunol. 1963 Nov;91:597–603. [PubMed] [Google Scholar]
- FAHEY J. L. TWO TYPES OF 6.6 S GAMMA-GLOBULINS, BETA-2A-GLOBULINS AND 18 S GAMMA 1-MACROGLOBULINS IN NORMAL SERUM AND GAMMA-MICROGLOBULINS IN NORMAL URINE. J Immunol. 1963 Oct;91:438–447. [PubMed] [Google Scholar]
- Gershon H., Bauminger S., Sela M., Feldman M. Studies on the competence of single cells to produce antibodies of two specificities. J Exp Med. 1968 Jul 1;128(1):223–233. doi: 10.1084/jem.128.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givol D., De Lorenzo F. The position of various cleavages of rabbit immunoglobulin G. J Biol Chem. 1968 Apr 25;243(8):1886–1891. [PubMed] [Google Scholar]
- Green I., Vassalli P., Nussenzweig V., Benacerraf B. Specificity of the antibodies produced by single cells following immunization with antigens bearing two types of antigenic determinants. J Exp Med. 1967 Mar 1;125(3):511–526. doi: 10.1084/jem.125.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON L. A. Comparative immunological studies of the immune globulins of human milk and of blood serum. Int Arch Allergy Appl Immunol. 1961;18:241–267. doi: 10.1159/000229177. [DOI] [PubMed] [Google Scholar]
- HERZENBERG L. A. A CHROMOSOME REGION FOR GAMMA 2A AND BETA 2A GLOBULIN H CHAIN ISOANTIGENS IN THE MOUSE. Cold Spring Harb Symp Quant Biol. 1964;29:455–462. doi: 10.1101/sqb.1964.029.01.048. [DOI] [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilschmann N., Craig L. C. Amino acid sequence studies with Bence-Jones proteins. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1403–1409. doi: 10.1073/pnas.53.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNGOLD L. Abnormal plasma components and their significance in disease. Ann N Y Acad Sci. 1961 Aug 31;94:110–130. doi: 10.1111/j.1749-6632.1961.tb35536.x. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN R., DRAY S., POTTER M. LINKAGE IN CONTROL OF ALLOTYPIC SPECIFICITIES ON TWO DIFFERENT GAMMA-G-IMMUNOGLOBULINS. Science. 1965 Apr 30;148(3670):640–642. doi: 10.1126/science.148.3670.640. [DOI] [PubMed] [Google Scholar]
- Lummus Z., Cebra J. J., Mage R. Correspondence of the relative cellular distribution and serum concentration of allelic allotypic markers in normal and "allotype-suppressed" heterozygous rabbits. J Immunol. 1967 Oct;99(4):737–743. [PubMed] [Google Scholar]
- Mage R., Dray S. Persistent altered phenotypic expression of allelic gamma-G-immunoglobulin allotypes in heterozygous rabbits exposed to isoantibodies in fetal and neonatal life. J Immunol. 1965 Sep;95(3):525–535. [PubMed] [Google Scholar]
- Miller E. J., Osterland C. K., Davie J. M., Krause R. M. Electrophoretic analysis of polypeptide chains isolated from antibodies in the serum of immunized rabbits. J Immunol. 1967 Apr;98(4):710–715. [PubMed] [Google Scholar]
- Milstein C. Linked groups of residues in immunoglobulin k chains. Nature. 1967 Oct 28;216(5113):330–332. doi: 10.1038/216330a0. [DOI] [PubMed] [Google Scholar]
- Mäkelä O. Cellular heterogeneity in the production of an anti-hapten antibody. J Exp Med. 1967 Jul 1;126(1):159–170. doi: 10.1084/jem.126.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSSAL G. J., MAKELA O. Kinetic studies on the incidence of cells appearing to form two antibodies. J Immunol. 1962 May;88:604–612. [PubMed] [Google Scholar]
- Niall H. D., Edman P. Two structurally distinct classes of kappa-chains in human immunoglobulins. Nature. 1967 Oct 21;216(5112):262–263. doi: 10.1038/216262a0. [DOI] [PubMed] [Google Scholar]
- Pernis B., Chiappino G., Kelus A. S., Gell P. G. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965 Nov 1;122(5):853–876. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R. The structure of the heavy chain of immunoglobulin and its relevance to the nature of the antibody-combining site. The Second CIBA Medal Lecture. Biochem J. 1967 Nov;105(2):417–426. doi: 10.1042/bj1050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W., Porter R. R. Allotype-related sequence variation of the heavy chain of rabbit immunoglobulin G. Biochem J. 1968 May;107(6):753–763. doi: 10.1042/bj1070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M. A., Cooper M. D., Wollheim F. A., Hong R., Good R. A. The IgA system. I. Studies of the transport and immunochemistry of IgA in the saliva. J Exp Med. 1966 Apr 1;123(4):615–627. doi: 10.1084/jem.123.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTSUMI S., KARUSH F. THE SUBUNITS OF PURIFIED RABBIT ANTIBODY. Biochemistry. 1964 Sep;3:1329–1338. doi: 10.1021/bi00897a024. [DOI] [PubMed] [Google Scholar]
- Weir R. C., Porter R. R. The antigen-binding capacity of the peptide chains of horse antibodies. Biochem J. 1966 Jul;100(1):69–72. doi: 10.1042/bj1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M., Press E. M., Porter R. R. The N-terminal sequence of the heavy chain of rabbit immunoglobulin IgG. Biochem J. 1966 Aug;100(2):303–308. doi: 10.1042/bj1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]