The epithelial surfaces of the skin and intestinal, respiratory, and reproductive tracts are persistently exposed to the myriad of microorganisms present in the external environment. Because this constitutes a limited access border, the cells of these epithelia must be able to establish barriers against microbial intruders and to raise the alarm if the barriers are breached. The gastrointestinal epithelium, in particular, forms a critical interface between the internal milieu and the lumen. The barrier formed by the gastrointestinal tract is essential in preventing noxious luminal contents from accessing the interstitial tissues, and must also support digestion and active vectorial transport of nutrients, electrolytes, and water. Therefore, the barrier formed by gastrointestinal epithelium must be highly regulated and selectively permeable.
Because the intestinal epithelium must coexist with a high density of diverse micro-organisms, protection against these organisms occurs on several levels (ie, effectors of adaptive immunity, secretory immunoglobulin A, and mucins), and in addition requires specialized cell types. The specialized cells within the intestinal epithelium include the gut-associated lymphoid tissue, which is consists of the adaptive immune system components, such as the Peyer's patches that function to sample luminal antigens ensuring their uptake by antigen-presenting cells.1,2 Specialized epithelial cells, termed M (microfold) cells facilitate also the translocation of antigens to the dendritic cell (DC)-rich subepithelial areas of Peyer's patches. Therefore, during infection by enteric pathogens, the gut-associated lymphoid tissue plays a critical role in controlling infection by stimulating the production of pathogen-specific immunoglobulins or the expansion of cytotoxic and helper T lymphocytes.2 Paneth cells are another type of specialized epithelial cell and are recognized by their unusually large apical secretory granules that are released into the crypt. These cells reside at the base of the crypts and fulfill a crucial role in innate immunity since they produce several antimicrobial peptides and enzymes.3 In particular, Paneth cells are strategically located in proximity to the multipotent stem cells and are a major source of a-defensins, lysozyme, Reg3)', and group IIA phospholipase A2. These protective factors are produced constitutively to protect the crypts against invading microbes.2 In healthy individuals, Paneth cells are found only in the small intestine. However, under conditions of inflammatory bowel disease (Crohn's disease, ulcerative colitis), Paneth cells also develop in the colonic mucosa (metaplastic Paneth cells). These Paneth cells have many features in common with regular Paneth cells as they also produce á-defensin and lysozyme.4
In general, most of the host's antimicrobial molecules are cationic to ensure efficient binding to the anionic bacterial surface polymers and exhibit a broad-spectrum activity against Gram-positive and Gram-negative bacteria, yeast, fungi, and enveloped viruses; these include, but are not limited to, bactericidal peptides or proteins such as defensins, cathelicidins, and bactericidal/permeability- increasing protein.2 It is inferred that most of these nonenzymatically active antimicrobial peptides damage the integrity of bacterial membranes by pore formation.5,6 Lysozyme, however, is an antimicrobial enzyme. In addition to its presence in Paneth cells, this enzyme is found in neutrophils and macrophages.7 Lysozyme cleaves peptidoglycan, the bacterial cell wall polymer of both Gram-positive and Gram-negative bacteria, rendering bacteria susceptible to disruption by osmotic pressure. Peptidoglycan is also an important inflammatory mediator that activates the innate immune system via Toll-like receptor 2 and NOD receptors.8
Lysozyme is active predominantly against Gram-positive bacteria whose peptidoglycan is easily accessible because of the absence of an outer membrane. The antimicrobial activity of lysozyme may also include activation of bacterial autolytic enzymes or membrane disruption.9 Lysozyme appears also to facilitate the inflammatory potential of peptidoglycan by increasing its solubility, clearance, and availability.2 For instance, lysozyme-deficient transgenic mice exhibit increased inflammation in Gram-positive infections and it is inferred that lysozyme production by intestinal Paneth cells also modulates peptidoglycan-induced inflammatory responses.10
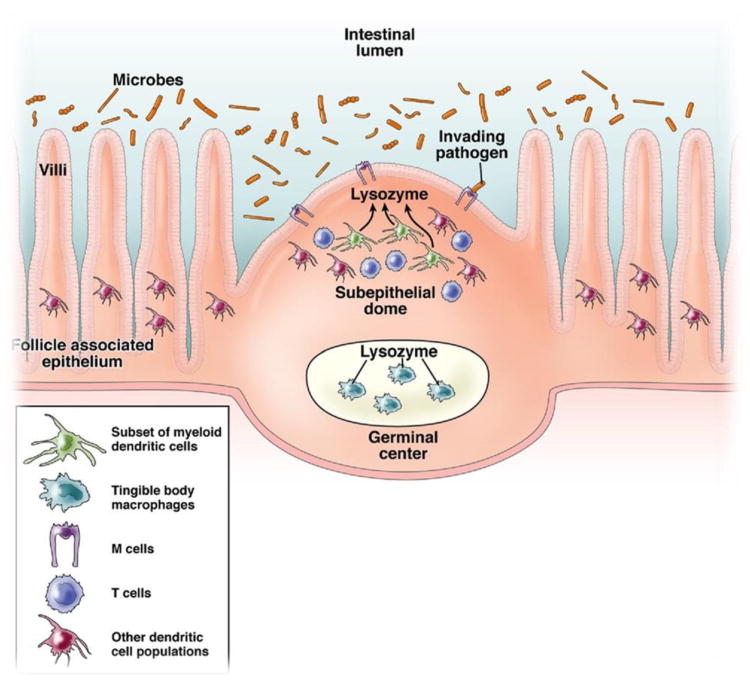
In this issue of Gastroenterology, Lelouard et al11 provide compelling new information to suggest that the Peyer's patches contains a unique population of intestinal DCs that secrete high levels of lysozyme. To understand the significance of this finding, it is instructive to consider the structural architecture of the Peyer's patches.12 Peyer's patches are secondary lymphoid tissues that are located along the wall of the small intestine and are essential for the generation of immunity to intestinal antigens. Foreign antigens in the gut are transported to the Peyer's patches by M cells, located at the follicle-associated epithelium (FAE) of the Peyer's patches. The incoming antigens are sampled by the DCs that reside just beneath the supepithelial dome (SED) region underlying the FAE (Figure 1). Analysis of the mouse Peyer's patches has revealed 3 distinct subsets of DCs based on their differential expression of specific cell-surface markers and their characteristic localization.13,14 All subsets express CD11c and major histocompatibility complex class II antigens, but differ in their expression of CD8a and CD11b. CD11c+CD8a+ “lymphoid-related” DCs are localized in the T-cell–rich interfollicular regions of the Peyer's patches. Myeloid DCs (CD11c+CD11b+) are found under the FAE in the SED of Peyer's patches and express interleukin-10. The third DC subset is described as “double-negative” (CD8a−CD11b−) and is found in the SED, the interfollicular region, and within the FAE. The CD8á+ and DN DCs share similar functional characteristics as they both secrete interleukin-12 p70 upon bacterial stimulation and both produce predominantly T helper-1 responses, whereas myeloid Peyer's patch DCs are better able to skew T-cell responses to T helper-2 differentiation.
Figure 1.
Distribution of lysozyme expressing cells in within Peyer's patches of the intestinal epithelium. Antigens within the intestinal mucosa are transferred to the Peyer's patches via M cells and are typically acquired by dendritic cells. Highlighted in this illustration are the roles played by LysoDCs in the subepithelial dome of the Peyer's patches and the tingible macrophage population located in the germinal center in their ability to secrete lysozyme. Release of lysozyme by these cells may afford a primary tier of protection within the Peyer's patches.
The report by Lelouard et al11 highlights a novel role played by the Peyer's patches in the development of innate immune responses as their data reveal that the subepithelial dome of the Peyer's patches contains a unique population of intestinal DCs that secrete high levels of lysozyme (Figure 1). This remarkable finding documents that Paneth cells are not solely responsible for the expression lysozyme in the small intestine. Specifically, Lelouard et al11 established a distribution map of the myelomonocytic lineage throughout the small intestine. In the villi, the major myeloid subset was identified as CD11c+CD11bLoCX3CR1+F4/80+. The CD11c expression in this myeloid subset was found to vary along the crypt-to-villis axis, presumably representing different maturation/differentiation stages. Although lysozyme was not detected in the villi of myelomonocytic cells, in the Peyer's patches lysozyme was highly expressed by tingible-body macrophages of the germinal center and by an intestinal dendritic subset, called LysoDC, that was specifically localized in the SED and FAE. This DC subset exhibits the highest surface expression level of molecules required for antigen presentation. Furthermore, LysoDC were also found to be the primary DC subset involved in Salmonella enterica serotype Typhimurium uptake and are distinct from CCR6+DC,15 which have previously been shown to be involved in the activation of Salmonella- specific T-cell responses, perhaps suggesting a partnership between these 2 subsets early after bacterial infection. Last, LysoDC were found to be involved in the removal of dead cells, including M cells, and may offer an explanation as to how M cells disappear near the top of the dome. Taken together, these findings indicate that at least for the Peyer's patches of the intestinal epithelium the subset of lysozyme- expressing DCs have the greatest ability to capture incoming antigens. In addition, these cells are involved in the uptake of pathogenic bacteria, as well as the engulfment of dead cells.
Because the Peyer's patches of the small intestine are a major site of antigen sampling, it is not surprising that the mucosal immune system has evolved multiple layers of protection. In the case of LysoDC, these cells are ideally positioned to acquire samples from the gut lumen because they possess all the necessary machinery for efficient capture and antigen presentation. It is tempting to hypothesize that the increased expression of lysozyme in the SED of the Peyer's patches may protect this important antigen sampling site, thus representing a first line of defense with a potential link to adaptive immunity. However, more studies are necessary before this hypothesis can be validated.
Because this is the first report to show that lysozyme is expressed in the Peyer's patches of the intestinal epithelium by a specialized subset of DCs (LysoDC), additional questions emerge. How is this subset of cells activated? What is the status of these cells during chronic inflammatory diseases, such as Crohn's disease or ulcerative colitis? Are the LysoDC subset of DCs active at other mucosal surfaces (ie, lung, reproductive tract)? Do LysoDCs secrete other types of antimicrobial? Although these and a host of other questions remain to be determined, this study overall, represents a significant advance for the study of intestinal mucosal immunity.
References
- 1.MacDonald TT. The mucosal immune system. Parasite Immunol. 2003;25:235–246. doi: 10.1046/j.1365-3024.2003.00632.x. [DOI] [PubMed] [Google Scholar]
- 2.Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62:1297–1307. doi: 10.1007/s00018-005-5034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouellette AJ. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am J Physiol. 1999;277:G257–2561. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 4.Fahlgren A, Hammarstrom S, Danielsson A, et al. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003;131:90–101. doi: 10.1046/j.1365-2249.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zasloff M. Antimicrobial peptides in health and disease. N Engl J Med. 2002;347:1199–1200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- 6.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 8.Dziarski R. Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci. 2003;60:1793–1804. doi: 10.1007/s00018-003-3019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masschalck B, Michiels CW. Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria. Crit Rev Microbiol. 2003;29:191–214. doi: 10.1080/713610448. [DOI] [PubMed] [Google Scholar]
- 10.Salzman NH, Chou MM, de Jong H, et al. Enteric salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun. 2003;71:1109–1115. doi: 10.1128/IAI.71.3.1109-1115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelouard H, Henri S, de Bovis B, et al. Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer's patch dendritic cells that express lysozyme. Gastroenterology. 2010;138:173–184. doi: 10.1053/j.gastro.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 12.Sato A, Iwasaki A. Peyer's patch dendritic cells as regulators of mucosal adaptive immunity. Cell Mol Life Sci. 2005;62:1333–1338. doi: 10.1007/s00018-005-5037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–1394. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–4890. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 15.Salazar-Gonzalez RM, Niess JH, Zammit DJ, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer's patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]