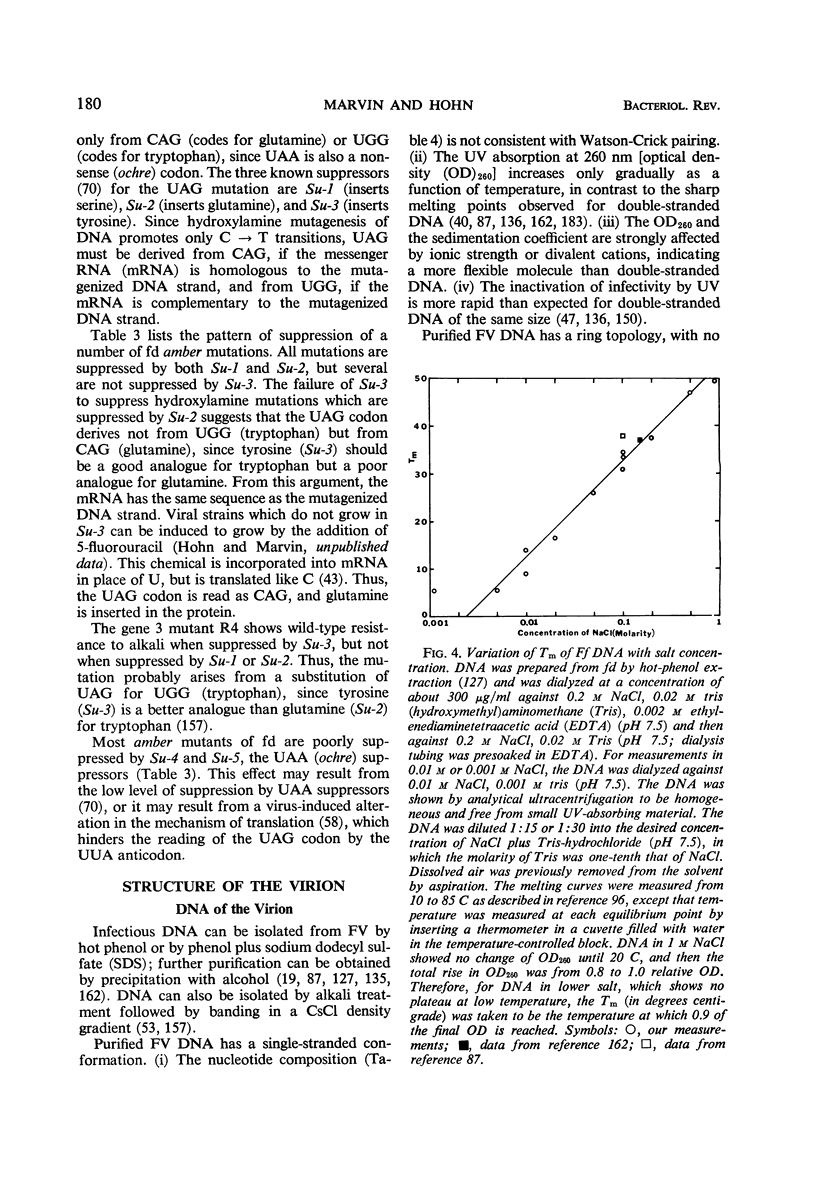
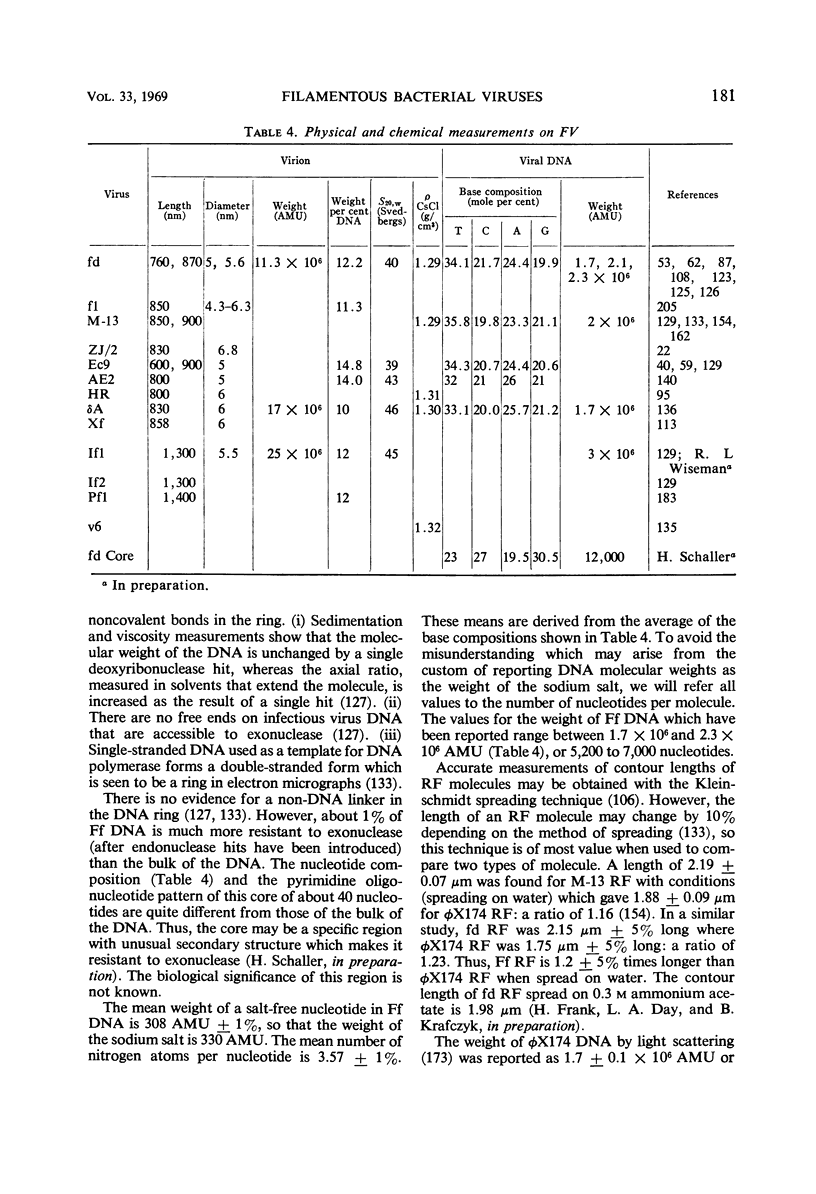
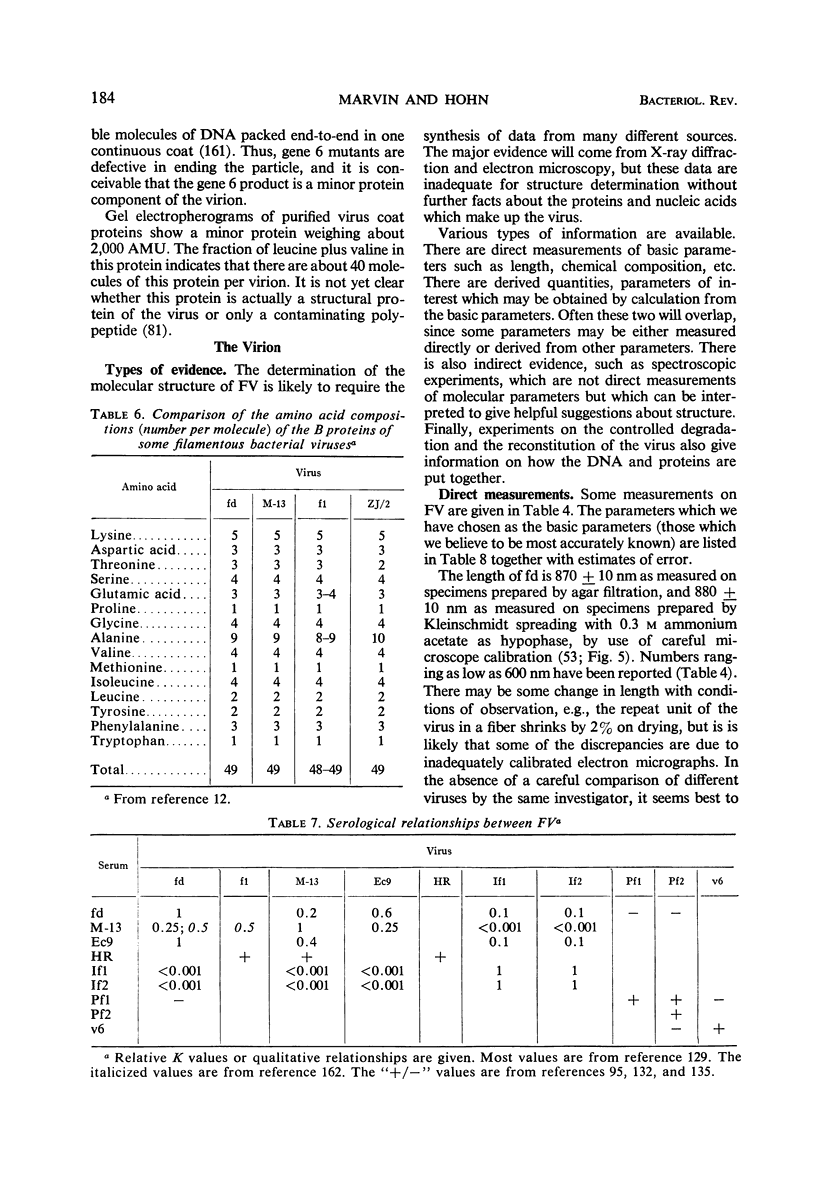
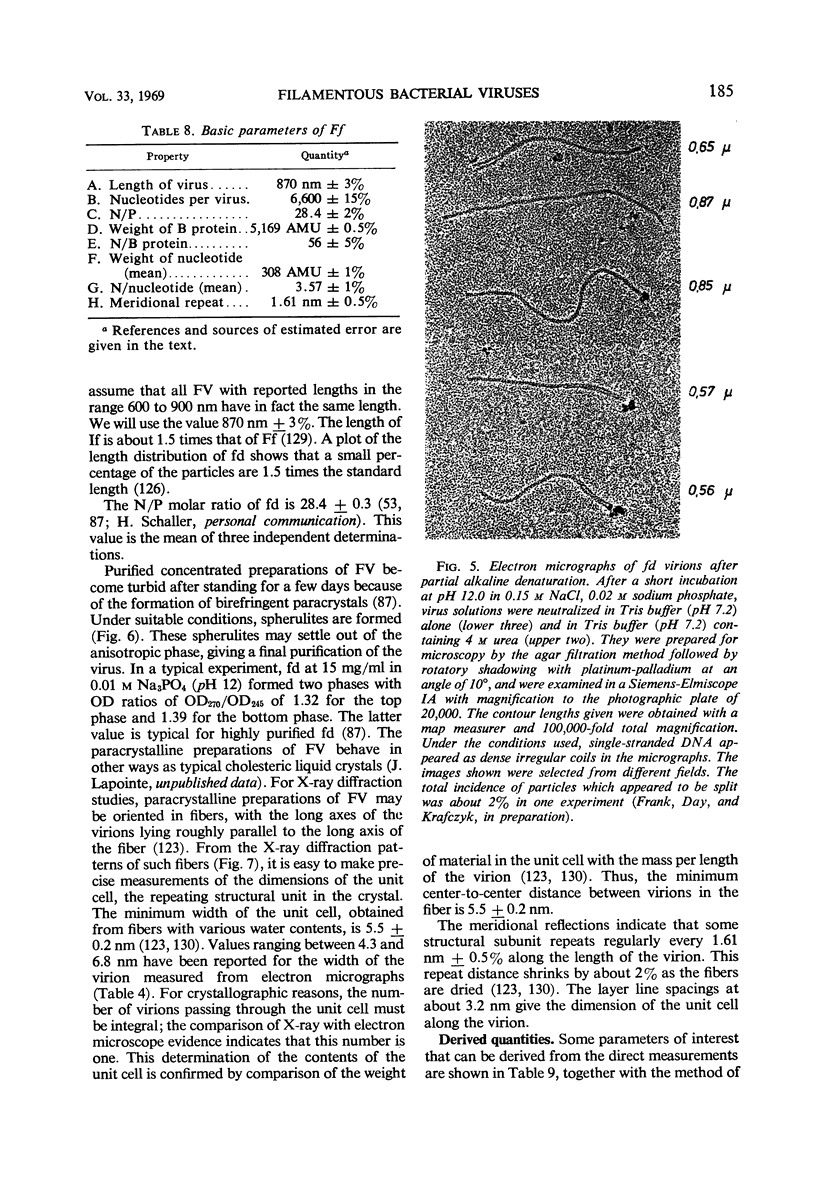
Full text
PDF

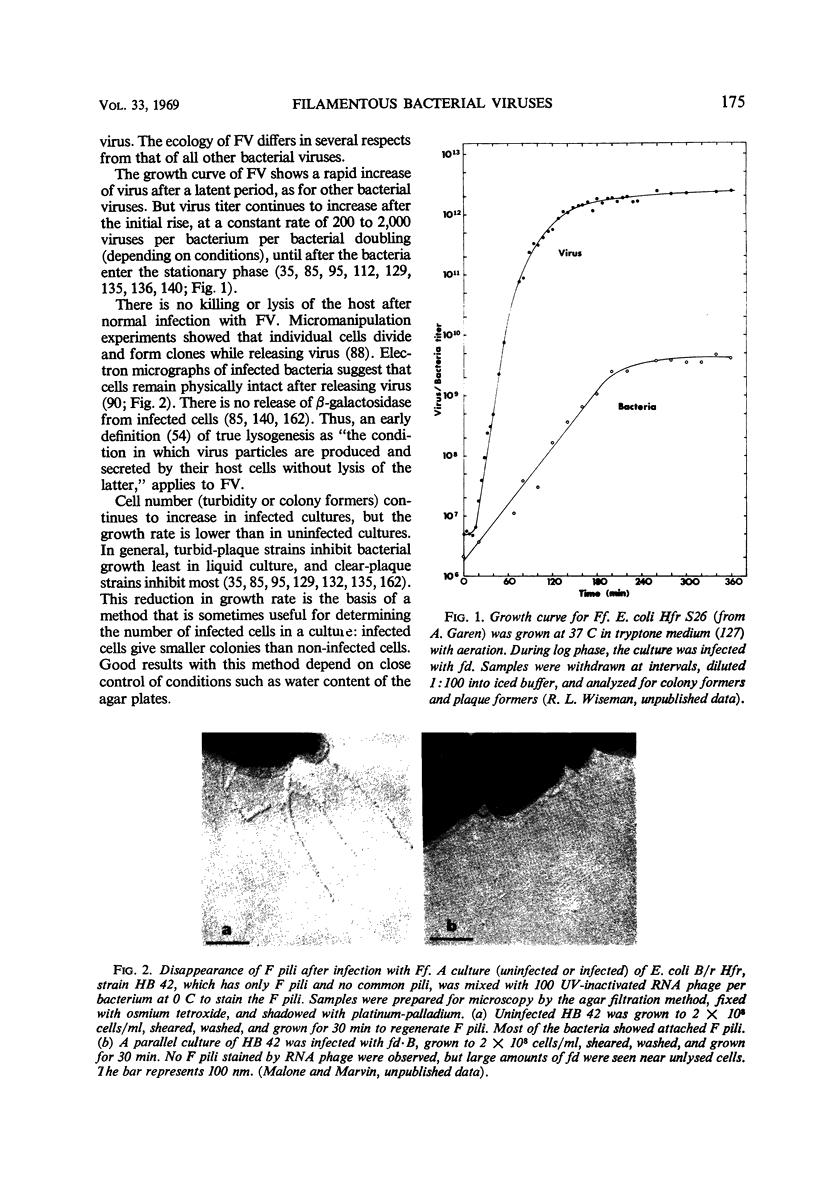


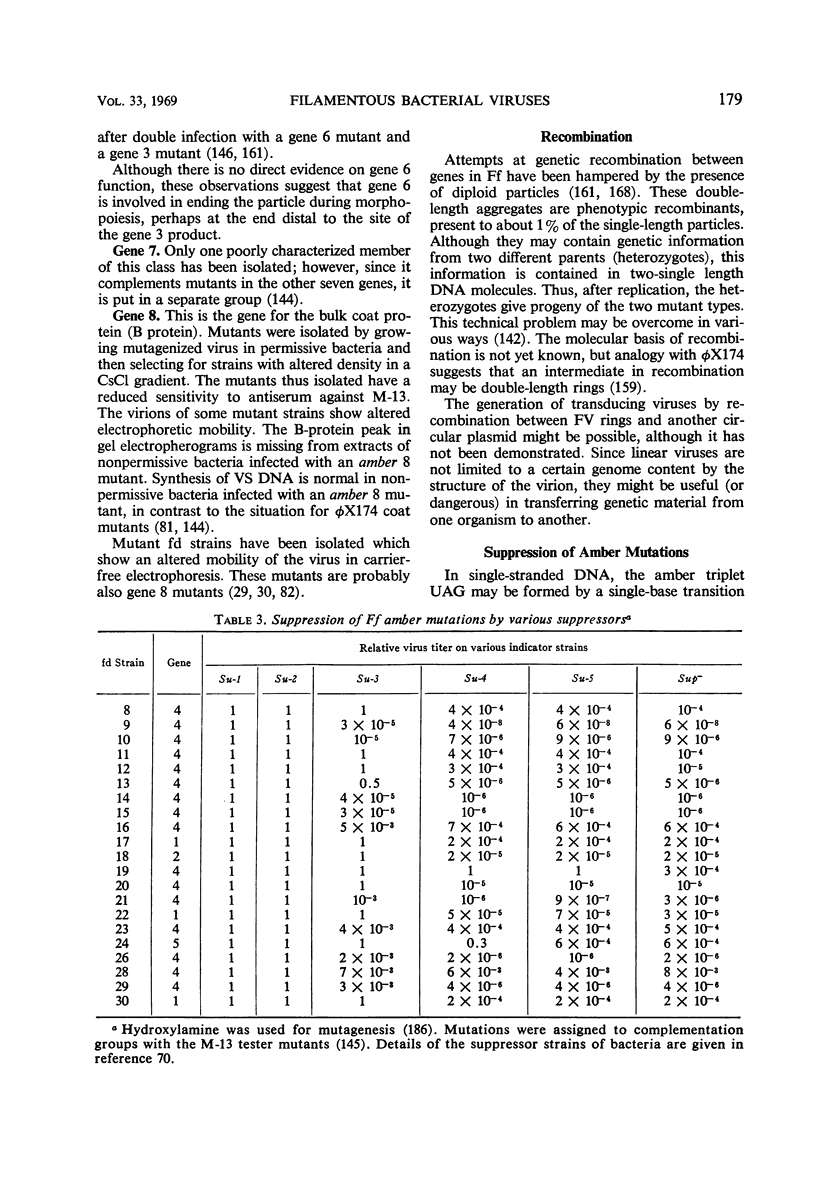
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. Recombination and segregation in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1958;23:47–58. doi: 10.1101/sqb.1958.023.01.007. [DOI] [PubMed] [Google Scholar]
- ANDERSON T. F., WOLLMAN E. L., JACOB F. Sur les processus de conjugaison et de recombinaison chez Escherichia coli. III. Aspects morphologiques en microscopie électronique. Ann Inst Pasteur (Paris) 1957 Oct;93(4):450–455. [PubMed] [Google Scholar]
- ANDREWES C. H. VIRUSES AND NOAH'S ARK. Bacteriol Rev. 1965 Mar;29:1–8. doi: 10.1128/br.29.1.1-8.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W. Host specificity of DNA produced by Escherichia coli. 9. Host-controlled modification of bacteriophage fd. J Mol Biol. 1966 Oct;20(3):483–496. doi: 10.1016/0022-2836(66)90004-0. [DOI] [PubMed] [Google Scholar]
- Arber W. Host-controlled modification of bacteriophage. Annu Rev Microbiol. 1965;19:365–378. doi: 10.1146/annurev.mi.19.100165.002053. [DOI] [PubMed] [Google Scholar]
- Arber W., Kühnlein U. Mutationeller Verlust B-spezifischer Restriktion des Bakteriophagen fd. Pathol Microbiol (Basel) 1967;30(6):946–952. [PubMed] [Google Scholar]
- Arnott S., Fuller W., Hodgson A., Prutton I. Molecular conformations and structure transitions of RNA complementary helices and their possible biological significance. Nature. 1968 Nov 9;220(5167):561–564. doi: 10.1038/220561a0. [DOI] [PubMed] [Google Scholar]
- BRADLEY D. E. SOME PRELIMINARY OBSERVATIONS ON FILAMENTOUS AND RNA BACTERIOPHAGES. J Ultrastruct Res. 1964 Jun;10:385–389. doi: 10.1016/s0022-5320(64)80017-4. [DOI] [PubMed] [Google Scholar]
- BRADLEY D. E. THE STRUCTURE OF SOME BACTERIOPHAGES ASSOCIATED WITH MALE STRAINS OF ESCHERICHIA COLI. J Gen Microbiol. 1964 Jun;35:471–482. doi: 10.1099/00221287-35-3-471. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., HOBOM G., HANNIG K. EIN EINFACHES VERFAHREN ZUR SELEKTIONIERUNG VON MUTANTEN. Hoppe Seylers Z Physiol Chem. 1964;338:278–280. doi: 10.1515/bchm2.1964.338.1-2.278. [DOI] [PubMed] [Google Scholar]
- BRAUNITZER G., HOBOM G. ZUR EINHEITLICHKEIT DER PROTEINSTRUKTUR. Hoppe Seylers Z Physiol Chem. 1964;338:276–277. doi: 10.1515/bchm2.1964.338.1-2.276. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Beierle J. W. Cell proliferation: enhancement by extracts from cell surfaces of polyoma-virus-transformed cells. Science. 1968 Aug 23;161(3843):798–799. doi: 10.1126/science.161.3843.798. [DOI] [PubMed] [Google Scholar]
- Bendet I. J., Mayfield J. E. Ultraviolet dichroism of fd bacteriophage. Biophys J. 2008 Dec 31;7(1):111–119. doi: 10.1016/S0006-3495(67)86578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzinger R., Delius H., Janenisch R., Hofschneider P. H. Infectious nucleic acids of Escherichia coli bacteriophages. 10. Preparation and properties of Escherichia coli competent for infectious DNA from bacteriophages phi X 174 and M 13 and RNA from bacteriophage M 12. Eur J Biochem. 1967 Nov;2(4):414–428. doi: 10.1111/j.1432-1033.1967.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Benzinger R. Restriction of infectious bacteriophage fd DNA's and an assay for in vitro host-controlled restriction and modification. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1294–1299. doi: 10.1073/pnas.59.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E., Dewar C. A. Intracellular changes in cells of Escherichia coli infected with a filamentous bacteriophage. J Gen Virol. 1967 Apr;1(2):179–188. doi: 10.1099/0022-1317-1-2-179. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton P. E., Sheinin R. Control of DNA synthesis in cells infected with polyoma virus. Virology. 1968 Dec;36(4):652–661. doi: 10.1016/0042-6822(68)90196-7. [DOI] [PubMed] [Google Scholar]
- Braunitzer G., Asbeck F., Beyreuther K., Kohler H., von Wettstein G. Die Konstitution des Hüllproteins des Bakteriophagen fd. Hoppe Seylers Z Physiol Chem. 1967 Dec;348(12):1689–1690. [PubMed] [Google Scholar]
- Bretscher M. S. Direct translation of a circular messenger DNA. Nature. 1968 Dec 14;220(5172):1088–1091. doi: 10.1038/2201088a0. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Dowell C. E. Replication of coliphage M-13. I. Effects on host cells after synchronized infection. J Virol. 1968 Nov;2(11):1290–1295. doi: 10.1128/jvi.2.11.1290-1295.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. R., Dowell C. E. Replication of coliphage M-13. II. Intracellular deoxyribonucleic acid forms associated with M-13 infection of mitomycin C-treated cells. J Virol. 1968 Nov;2(11):1296–1307. doi: 10.1128/jvi.2.11.1296-1307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Rall S. C., Stellwagen R. H., Cole R. D. Histone structure: asymmetric distribution of lysine residues in lysine-rich histone. Science. 1969 Jan 24;163(3865):391–393. doi: 10.1126/science.163.3865.391-a. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. Reversal of mutant phenotypes by 5-fluorouracil: an approach to nucleotide sequences in messenger-RNA. Proc Natl Acad Sci U S A. 1962 Apr 15;48:532–546. doi: 10.1073/pnas.48.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLVILL A. J., JORDAN D. O. THE INFLUENCE OF IONIC STRENGTH ON THE REVERSIBILITY OF THE DENATURATION OF DNA IN DILUTE SOLUTION. J Mol Biol. 1963 Dec;7:700–709. doi: 10.1016/s0022-2836(63)80117-5. [DOI] [PubMed] [Google Scholar]
- Cairns J., Davern C. I. The mechanics of DNA replication in bacteria. J Cell Physiol. 1967 Oct;70(2 Suppl):65–76. doi: 10.1002/jcp.1040700406. [DOI] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. L., Dean A. C., Hinshelwood C. Factors affecting the growth of bacterial colonies on agar plates. Proc R Soc Lond B Biol Sci. 1968 Nov 5;171(1023):175–199. doi: 10.1098/rspb.1968.0063. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Stallions D. R. Energy requirements for specific pair formation during conjugation in Escherichia coli K-12. J Bacteriol. 1967 Aug;94(2):490–492. doi: 10.1128/jb.94.2.490-492.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day L. A. Conformations of single-stranded DNA and coat protein in fd bacteriophage as revealed by ultraviolet absorption spectroscopy. J Mol Biol. 1969 Jan;39(2):265–277. doi: 10.1016/0022-2836(69)90316-7. [DOI] [PubMed] [Google Scholar]
- Day L. A. Protein conformation in fd bacteriophage as investigated by optical rotatory dispersion. J Mol Biol. 1966 Jan;15(1):395–398. doi: 10.1016/s0022-2836(66)80239-5. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Marvin D. A. Altered coding in single stranded DNA viruses? Nature. 1969 Feb 22;221(5182):769–770. doi: 10.1038/221769a0. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- Ellis E. L., Delbrück M. THE GROWTH OF BACTERIOPHAGE. J Gen Physiol. 1939 Jan 20;22(3):365–384. doi: 10.1085/jgp.22.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaschi A., Kornberg A. A lipopolysaccharide inhibitor of a DNA methyl transferase. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1713–1720. doi: 10.1073/pnas.54.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G., Ippen K. A., Valentine R. C. Active fragments of a filamentous bacteriophage. Biochem Biophys Res Commun. 1966 Nov 11;25(3):275–284. doi: 10.1016/0006-291x(66)90772-8. [DOI] [PubMed] [Google Scholar]
- Fareed G., Valentine R. C. Fragments of filamentous phage f1: cellular fate of the DNA. Virology. 1968 Aug;35(4):512–518. doi: 10.1016/0042-6822(68)90281-x. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Miles H. T. The physical and chemical properties of nucleic acids. Annu Rev Biochem. 1967;36:407–448. doi: 10.1146/annurev.bi.36.070167.002203. [DOI] [PubMed] [Google Scholar]
- Garen A. Sense and nonsense in the genetic code. Three exceptional triplets can serve as both chain-terminating signals and amino acid codons. Science. 1968 Apr 12;160(3824):149–159. doi: 10.1126/science.160.3824.149. [DOI] [PubMed] [Google Scholar]
- Gibbs A. J., Harrison B. D., Watson D. H., Wildy P. What's in a virus name? Nature. 1966 Jan 29;209(5022):450–454. doi: 10.1038/209450a0. [DOI] [PubMed] [Google Scholar]
- Gibbs A. Plant virus classification. Adv Virus Res. 1969;14:263–328. doi: 10.1016/s0065-3527(08)60562-x. [DOI] [PubMed] [Google Scholar]
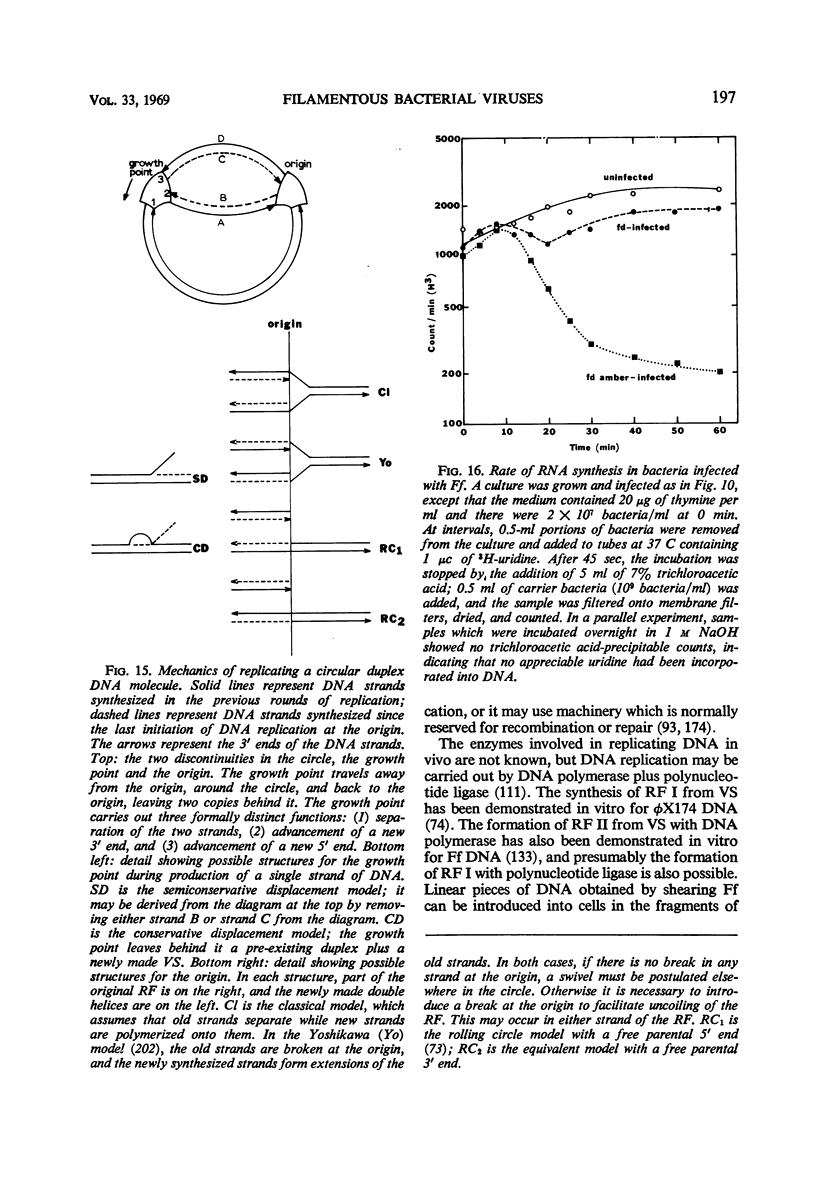
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Goulian M., Kornberg A. Enzymatic synthesis of DNA. 23. Synthesis of circular replicative form of phage phi-X174 DNA. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1723–1730. doi: 10.1073/pnas.58.4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI M., HAYASHI M. N., SPIEGELMAN S. RESTRICTION OF IN VIVO GENETIC TRANSCRIPTION TO ONE OF THE COMPLEMENTARY STRANDS OF DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:664–672. doi: 10.1073/pnas.50.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN BERLING H., MAZE R. RELEASE OF MALE-SPECIFIC BACTERIOPHAGES FROM SURVIVING HOST BACTERIA. Virology. 1964 Mar;22:305–313. doi: 10.1016/0042-6822(64)90021-2. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H., MARVIN D. A., DUERWALD H. EIN FAEDIGER DNS-PHAGE (FD) UND EIN SPHAERISCHER RNS-PHAGE (FR), WIRTSSPEZIFISCH FUER MAENNLICHE STAEMME VON E. COLI. 1. PRAEPARATION UND CHEMISCHE EIGENSCHAFTEN VON FD UND FR. Z Naturforsch B. 1963 Nov;18:876–883. [PubMed] [Google Scholar]
- HOFSCHNEIDER P. H., PREUSS A. M 13 BACTERIOPHAGE LIBERATION FROM INTACT BACTERIA AS REVEALED BY ELECTRON MICROSCOPY. J Mol Biol. 1963 Oct;7:450–451. doi: 10.1016/s0022-2836(63)80038-8. [DOI] [PubMed] [Google Scholar]
- Harpst J. A., Krasna A. I., Zimm B. H. Molecular weight of T7 and calf thymus DNA by low-angle light scattering. Biopolymers. 1968 Apr;6(4):595–603. doi: 10.1002/bip.1968.360060413. [DOI] [PubMed] [Google Scholar]
- Hayes W. Sex factors and viruses. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):230–245. doi: 10.1098/rspb.1966.0026. [DOI] [PubMed] [Google Scholar]
- Hobom G., Braunitzer G. Isolierung von Mutanten des Phagen fd mit proteinchemischen Methoden. Hoppe Seylers Z Physiol Chem. 1967 Jul;348(7):783–796. [PubMed] [Google Scholar]
- Hobom G., Braunitzer G. Untersuchung polycyclischer aromatischer Amine auf ihre mutagene Wirkung am Phagen fd. Hoppe Seylers Z Physiol Chem. 1967 Jul;348(7):797–803. [PubMed] [Google Scholar]
- Hobom G., Braunitzer G. Virusproteine, 3. Uber Mutator-Mutanten des Phagen fd. Hoppe Seylers Z Physiol Chem. 1967 Jul;348(7):804–807. [PubMed] [Google Scholar]
- Hoffmann-Berling H., Kaerner H. C., Knippers R. Small bacteriophages. Adv Virus Res. 1966;12:329–370. doi: 10.1016/s0065-3527(08)60852-0. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of microtubules in movement and alignment of nuclei in virus-induced syncytia. J Cell Biol. 1968 Dec;39(3):526–543. doi: 10.1083/jcb.39.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Hsu Y. C. Propagation or elimination of viral infection in carrier cells. Bacteriol Rev. 1968 Dec;32(4 Pt 1):387–399. [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. C. Regulation of the propagation of HR virus plasmid. Cold Spring Harb Symp Quant Biol. 1968;33:467–471. doi: 10.1101/sqb.1968.033.01.054. [DOI] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962 Aug;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- Inoué S., Sato H. Cell motility by labile association of molecules. The nature of mitotic spindle fibers and their role in chromosome movement. J Gen Physiol. 1967 Jul;50(6 Suppl):259–292. [PMC free article] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The nucleic acid pump of the male bacterium. Biochem Biophys Res Commun. 1966 Sep 22;24(6):880–887. doi: 10.1016/0006-291x(66)90331-7. [DOI] [PubMed] [Google Scholar]
- Ippen K. A., Valentine R. C. The sex hair of E. coli as sensory fiber, conjugation tube, or mating arm? Biochem Biophys Res Commun. 1967 Jun 23;27(6):674–680. doi: 10.1016/s0006-291x(67)80088-3. [DOI] [PubMed] [Google Scholar]
- Jehle H. Replication of double-strand nucleic acids. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1451–1455. doi: 10.1073/pnas.53.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse J., Eigner J. Physical properties of deoxyribonucleic acid. Annu Rev Biochem. 1966;35:789–834. doi: 10.1146/annurev.bi.35.070166.004041. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. 3. Specificity of protein-DNA association in vivo. J Mol Biol. 1966 Nov 14;21(2):305–312. doi: 10.1016/0022-2836(66)90101-x. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. I. Hydrodynamic measurements and biological characterization. J Mol Biol. 1966 Nov 14;21(2):281–292. doi: 10.1016/0022-2836(66)90099-4. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. II. Interaction of the protein with DNA in vitro. J Mol Biol. 1966 Nov 14;21(2):293–304. doi: 10.1016/0022-2836(66)90100-8. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- LOEB T. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science. 1960 Mar 25;131(3404):932–933. doi: 10.1126/science.131.3404.932. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Lawn A. M. Morphological features of the pili associated with Escherichia coli K 12 carrying R factors or the F factor. J Gen Microbiol. 1966 Nov;45(2):377–383. doi: 10.1099/00221287-45-2-377. [DOI] [PubMed] [Google Scholar]
- Liersch M., Hartmann G. Die Bindung von Proflavin und Actinomycin an Desoxyribonucleinsäure. II. Die Bindung an denaturierte und einsträngige DNA, Apyrimidinsäure und Apurinsäure. Biochem Z. 1965 Nov 5;343(1):16–28. [PubMed] [Google Scholar]
- Linn S., Arber W. Host specificity of DNA produced by Escherichia coli, X. In vitro restriction of phage fd replicative form. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1300–1306. doi: 10.1073/pnas.59.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. W., Lovell F. M., Rudko A. D. Prediction of alpha-helical regions in proteins of known sequence. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1519–1526. doi: 10.1073/pnas.60.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwoff A. Principles of classification and nomenclature of viruses. Nature. 1967 Jul 1;215(5096):13–14. doi: 10.1038/215013a0. [DOI] [PubMed] [Google Scholar]
- Lwoff A., Tournier P. The classification of viruses. Annu Rev Microbiol. 1966;20:45–74. doi: 10.1146/annurev.mi.20.100166.000401. [DOI] [PubMed] [Google Scholar]
- MARVIN D. A., HOFFMANN-BERLING H. A FIBROUS DNA PHAGE (FD) AND A SPHERICAL RNA PHAGE (FR) SPECIFIC FOR MALE STRAINS OF E COLI. II. PHYSICAL CHARACTERISTICS. Z Naturforsch B. 1963 Nov;18:884–893. doi: 10.1515/znb-1963-1106. [DOI] [PubMed] [Google Scholar]
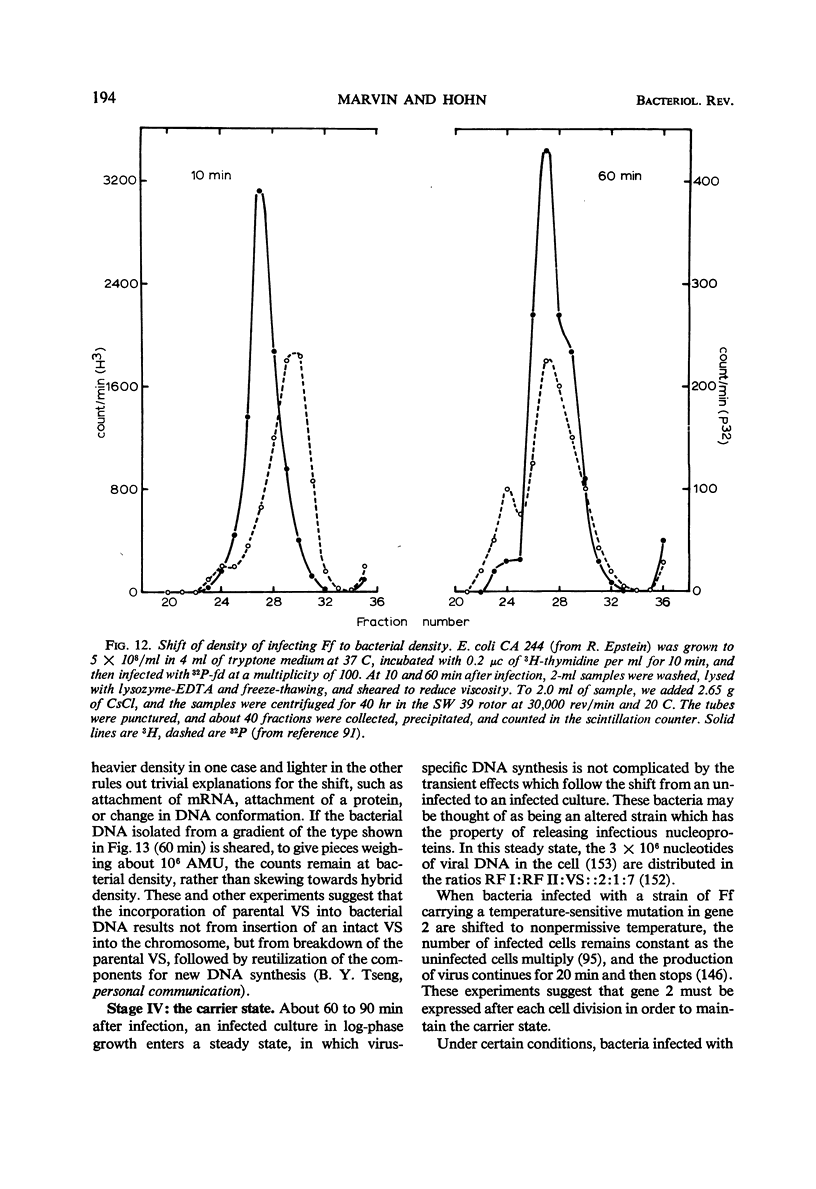
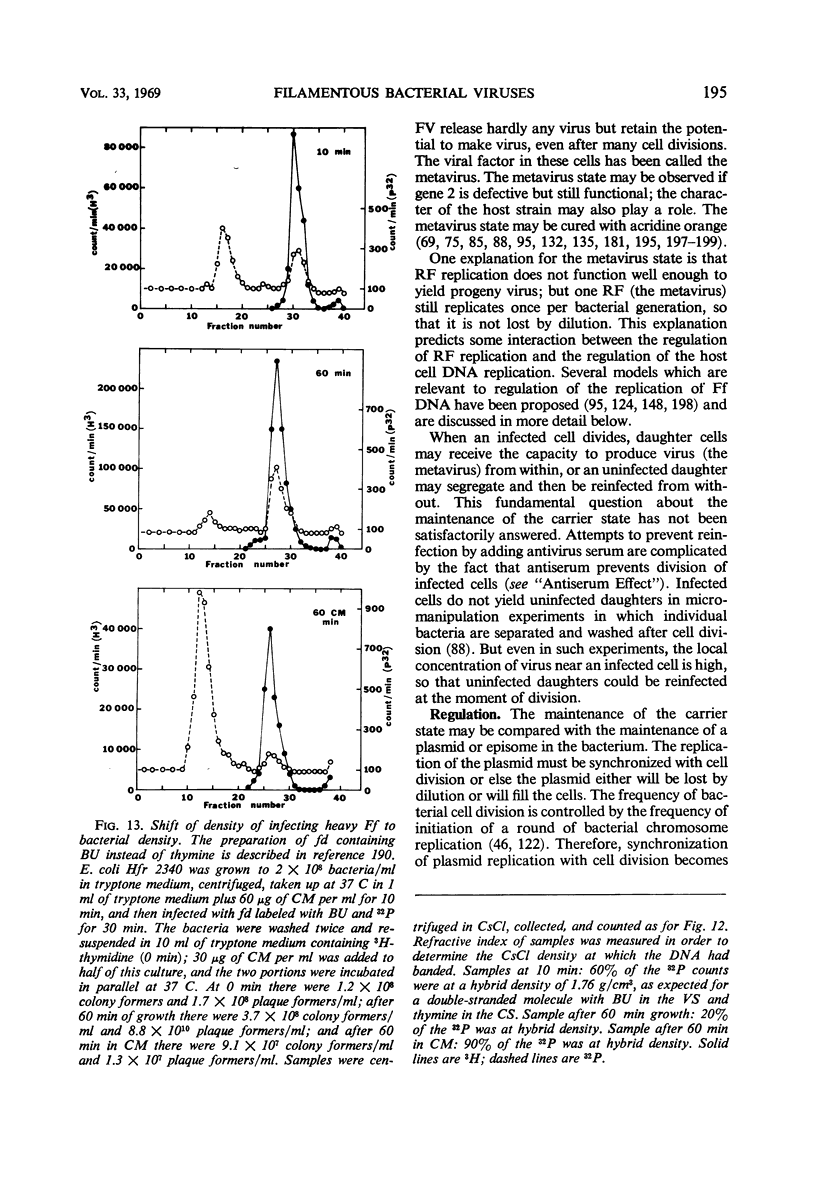
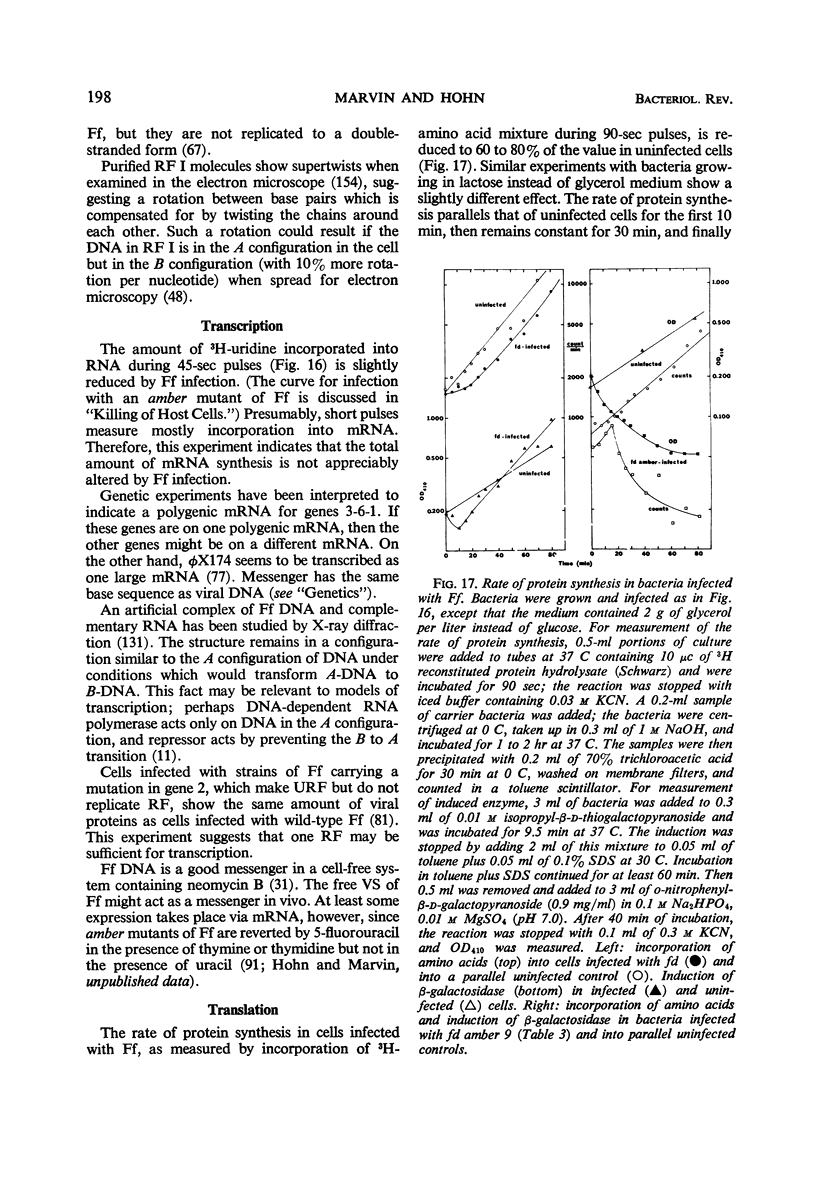
- Marvin D. A. Control of DNA replication by membrane. Nature. 1968 Aug 3;219(5153):485–486. doi: 10.1038/219485a0. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Schaller H. The topology of DNA from the small filamentous bacteriophage fd. J Mol Biol. 1966 Jan;15(1):1–7. doi: 10.1016/s0022-2836(66)80204-8. [DOI] [PubMed] [Google Scholar]
- Marvin D. A. X-ray diffraction and electron microscope studies on the structure of the small filamentous bacteriophage fd. J Mol Biol. 1966 Jan;15(1):8–17. doi: 10.1016/s0022-2836(66)80205-x. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Filamentous phages specific for the I sex factor. Nature. 1968 Mar 23;217(5134):1184–1186. doi: 10.1038/2171184a0. [DOI] [PubMed] [Google Scholar]
- Milman G., Langridge R., Chamberlin M. J. The structure of a DNA-RNA hybrid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1804–1810. doi: 10.1073/pnas.57.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamishima Y., Takeya K., Ohnishi Y., Amako K. Physicochemical and biological properties of fibrous Pseudomonas bacteriophages. J Virol. 1968 Mar;2(3):208–213. doi: 10.1128/jvi.2.3.208-213.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata C., Mårtensson O. On the mechanism of mutagenic action of hydroxylamine. J Theor Biol. 1968 May;19(2):133–146. doi: 10.1016/0022-5193(68)90109-4. [DOI] [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter R. A., Symons R. H. Isolation and properties of a DNA-containing rod-shaped bacteriophage. Aust J Biol Sci. 1966 Aug;19(4):565–573. doi: 10.1071/bi9660565. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Use of tetrazolium for improved resolution of bacteriophage plaques. J Bacteriol. 1966 Sep;92(3):787–788. doi: 10.1128/jb.92.3.787-788.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Tzagoloff H., Erdahl W. S. Conditional lethal mutants of the small filamentous coliphage M13. I. Isolation, complementation, cell killing, time of cistron action. Virology. 1966 Nov;30(3):397–410. doi: 10.1016/0042-6822(66)90118-8. [DOI] [PubMed] [Google Scholar]
- Primrose S. B., Brown L. R., Dowell C. E. Host cell participation in small virus replication. I. Replication of M-13 in a strain of Escherichia coli with a temperature-sensitive lesion in deoxyribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1308–1314. doi: 10.1128/jvi.2.11.1308-1314.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prothero J. W. A model of alpha-helical distribution in proteins. Biophys J. 1968 Nov;8(11):1236–1255. doi: 10.1016/S0006-3495(68)86553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauth A. M. The Physical State of Viral Nucleic Acid and the Sensitivity of Viruses to Ultraviolet Light. Biophys J. 1965 May;5(3):257–273. doi: 10.1016/s0006-3495(65)86715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. I. Sedimentation analysis of crude lysates of M13-infected bacteria. Biochim Biophys Acta. 1969 Apr 22;179(2):398–407. [PubMed] [Google Scholar]
- Rossomando E. F., Zinder N. D. Studies on the bacteriophage fl. I. Alkali-induced disassembly of the phage into DNA and protein. J Mol Biol. 1968 Sep 28;36(3):387–399. doi: 10.1016/0022-2836(68)90163-0. [DOI] [PubMed] [Google Scholar]
- Rush M. G., Warner R. C. Multiple length rings of phi-X174 and S13 replicative forms. 3. A possible intermediate in recombination. J Biol Chem. 1968 Sep 25;243(18):4821–4826. [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- SANDER E. EVIDENCE OF THE SYNTHESIS OF A DNA PHAGE IN LEAVES OF TOBACCO PLANTS. Virology. 1964 Dec;24:545–551. doi: 10.1016/0042-6822(64)90206-5. [DOI] [PubMed] [Google Scholar]
- Salivar W. O., Henry T. J., Pratt D. Purification and properties of diploid particles of coliphage M13. Virology. 1967 May;32(1):41–51. doi: 10.1016/0042-6822(67)90250-4. [DOI] [PubMed] [Google Scholar]
- Sander E. Alteration of fd phage in tobacco leaves. Virology. 1967 Sep;33(1):121–130. doi: 10.1016/0042-6822(67)90100-6. [DOI] [PubMed] [Google Scholar]
- Schwartz F. M., Zinder N. D. Morphological changes in Escherichia coli infected with the DNA bacteriophage fl. Virology. 1968 Feb;34(2):352–355. doi: 10.1016/0042-6822(68)90246-8. [DOI] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Cation fluxes and permeability changes accompanying bacteriophage infection of Escherichia coli. J Virol. 1968 Aug;2(8):763–771. doi: 10.1128/jvi.2.8.763-771.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P. M., Rosenthal S., Mobach H., Valentine R. C. Two new classes of F-pili mutants of Escherichia coli resistant to infection by the male specific bacteriophage F2. Virology. 1968 Sep;36(1):142–146. doi: 10.1016/0042-6822(68)90125-6. [DOI] [PubMed] [Google Scholar]
- Silverman P., Rosenthal S., Valentine R. Mutant male strains with an altered nucleic acid pump. Biochem Biophys Res Commun. 1967 Jun 23;27(6):668–673. doi: 10.1016/s0006-291x(67)80087-1. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- TESSMAN I., PODDAR R. K., KUMAR S. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. I. IN VITRO MUTAGENESIS BY HYDROXYLAMINE, ETHYL METHANESULFONATE AND NITROUS ACID. J Mol Biol. 1964 Aug;9:352–363. doi: 10.1016/s0022-2836(64)80212-6. [DOI] [PubMed] [Google Scholar]
- TZAGOLOFF H., PRATT D. THE INITIAL STEPS IN INFECTION WITH COLIPHAGE M13. Virology. 1964 Nov;24:372–380. doi: 10.1016/0042-6822(64)90174-6. [DOI] [PubMed] [Google Scholar]
- Takeya K., Amako K. A rod-shaped Pseudomonas phage. Virology. 1966 Jan;28(1):163–165. doi: 10.1016/0042-6822(66)90317-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. Mutagenic treatment of double- and single-stranded DNA phages T4 ans S13 with hydroxylamine. Virology. 1968 Jun;35(2):330–333. doi: 10.1016/0042-6822(68)90275-4. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr The rule of the ring. J Cell Physiol. 1967 Oct;70(2 Suppl):13–33. doi: 10.1002/jcp.1040700404. [DOI] [PubMed] [Google Scholar]
- Trenkner E., Bonhoeffer F., Gierer A. The fate of the protein component of bacteriophage fd during infection. Biochem Biophys Res Commun. 1967 Sep 27;28(6):932–939. doi: 10.1016/0006-291x(67)90069-1. [DOI] [PubMed] [Google Scholar]
- Wallach D. F. Cellular membranes and tumor behavior: a new hypothesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):868–874. doi: 10.1073/pnas.61.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. G., Fenwick M. L. Degradation of the filamentous phage ZJ-2 by sodium dodecylsulphate. Nature. 1967 May 13;214(5089):712–713. doi: 10.1038/214712a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Naito T. Inactivation by nitrogen mustard of single- and double-stranded DNA and RNA bacteriophages. Science. 1965 Dec 17;150(3703):1603–1604. doi: 10.1126/science.150.3703.1603. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. The initiation of DNA replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1967 Jul;58(1):312–319. doi: 10.1073/pnas.58.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D., VALENTINE R. C., ROGER M., STOECKENIUS W. F1, A ROD-SHAPED MALE-SPECIFIC BACTERIOPHAGE THAT CONTAINS DNA. Virology. 1963 Aug;20:638–640. doi: 10.1016/0042-6822(63)90290-3. [DOI] [PubMed] [Google Scholar]









