Abstract
Purpose
To estimate the long-term cumulative risk of late posterior chamber intraocular lens (IOL) dislocation after cataract extraction in a population-based cohort.
Design
Retrospective cohort study and nested case-control study
Methods
The records of all residents of Olmsted County, Minnesota who had cataract extraction from January 1, 1980 through May 31, 2009 (14,471 cases in 9,577 residents) and were diagnosed with late posterior chamber IOL dislocation in the same period were reviewed. Cases were identified through the Rochester Epidemiology Project. Three controls chosen from the cataract surgery cohort were matched to each IOL dislocation case by age, gender, and duration of follow-up. Records were reviewed to confirm case status and ascertain risk factor information. The cumulative risk of IOL dislocation was estimated by using the Kaplan-Meier method. Logistic regression models assessed differences between cases and controls.
Results
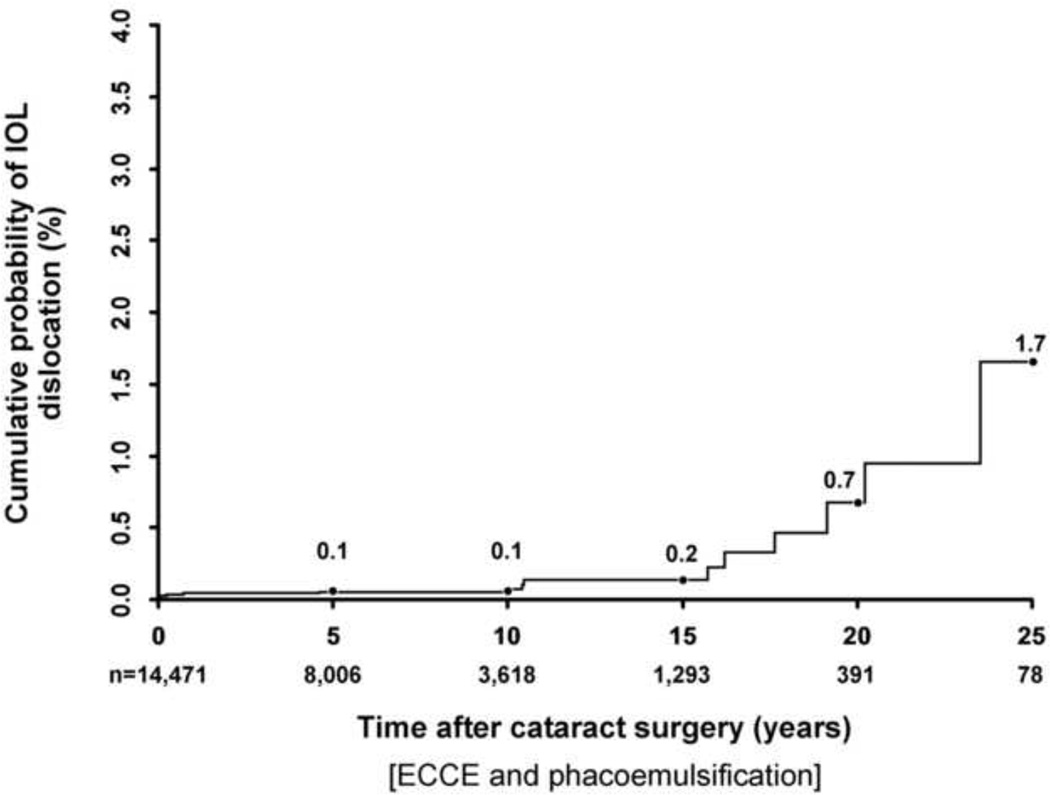
We identified 16 cases of late posterior chamber IOL dislocation, 9 with in-the-bag dislocations and 7 with out-of-the bag dislocations. At 5, 10, 15, 20, and 25 years after cataract extraction, the cumulative risk of IOL dislocation was 0.1%, 0.1%. 0.2%, 0.7%, and 1.7%, respectively. There was no significant difference in the risk of late IOL dislocation after extracapsular cataract extraction when compared to phacoemulsification (P=0.21), or between different decades of surgery (P=0.92). Pseudoexfoliation and zonular laxity at surgery were associated significantly with late IOL dislocation (P=0.01).
Conclusions
The long-term cumulative risk of late IOL dislocation after cataract extraction was low and did not significantly change over our nearly 30-year study period.
INTRODUCTION
Posterior chamber intraocular lens (IOL) dislocation as a late complication of cataract extraction has been reported with increasing frequency in recent years,1–7 leading to concerns of a pending large increase in IOL dislocations needing surgical intervention.1,3,7–9 A dislocated IOL often requires explantation or repositioning with subsequent serious potential complications, such as retinal tear, retinal detachment, and vitreous hemorrhage.3,10
It is not known if the observed increased number of late IOL dislocations is due to an increased rate of incidence of IOL dislocations or simply a larger community of at risk pseudophakic patients. Recently, two population-based studies in Sweden estimated that the incidence of late IOL dislocation is low after phacoemulsification, but the authors were unable to significantly demonstrate an increased rate of incidence.11,12 Similar population-based studies in the U.S. are not available.
The purpose of our population-based cohort study is to report the cumulative probability and the risk factors of late posterior chamber IOL dislocation after phacoemulsification and extracapsular cataract extraction (ECCE) in the stable, well-defined population of Olmsted County, Minnesota, U.S.A. during the nearly 30-year period 1980 to 2009.
METHODS
Data Source
Data were obtained by using the resources of the Rochester Epidemiology Project, a medical record linkage system established in 1966 to facilitate performance of population-based studies among residents of Rochester and surrounding Olmsted County, Minnesota.13 Virtually all medical care for this relatively isolated semi-urban county (2000 total county population 124,277) is provided by Mayo Clinic, Olmsted Medical Group, and their affiliated hospitals. The Rochester Epidemiology Project links Mayo Clinic’s medical record system with Olmsted Medical Group as well as other potential providers of medical care, including the University of Minnesota and Department of Veterans Affairs hospitals in Minneapolis 90 miles north of Rochester, other hospitals in surrounding counties, and the few independent medical practices in Olmsted County. Consequently, the Rochester Epidemiology Project provides a medical records linkage and retrieval system for virtually all sources of medical care utilized by the Olmsted County population. The usefulness and accuracy of the Rochester Epidemiology Project electronic databases for population- based studies of disease cause and outcomes have been well established.13,14
Rochester Epidemiology Project Cataract Surgery Cohort
Incident cases of cataract extraction performed on all Olmsted County residents between January 1, 1980 and May 31, 2009 were identified by using previously published 15 and updated resources of the Rochester Epidemilology Project. The Rochester Epidemiology Project cataract surgery cohort included 14,471 surgeries performed on 9,577 Olmsted County residents of all ages and predominantly Caucasian (96%). Cataract extraction performed by using phacoemulsification or ECCE as a primary procedure or a combined procedure with penetrating keratoplasty or trabeculectomy was included. Intracapsular cataract extraction, lensectomy combined with a planned pars plana vitrectomy, or cataract surgery in the surgical management of ocular trauma were excluded. Reliability tests on the data were performed and verified the accuracy of coded demographic and clinical data and estimated case over ascertainment of this cohort at < 1%.16
Rochester Epidemiology Project IOL Dislocation Cohort
We used Mayo Clinic modifications of International Classification of Diseases 9, Clinical Modification (ICD9-CM) diagnosis codes 379.32, 379.33, 379.34, 379.39, and 379.53 and ICD9-CM procedure codes 13.8 and 13.72 retrospectively to identify all potential IOL dislocation-related diagnoses made or procedures performed on residents in the REP cataract surgery cohort. Late IOL dislocation was defined as any posterior chamber IOL requiring IOL repositioning or exchange surgery that occurred more than 90 days after the primary cataract surgery in which the initial postoperative IOL position was noted as good in the operative report. We excluded early IOL dislocations that occurred at the time of surgery or in the immediate 90 day postoperative period. The medical records of all identified patients were reviewed to ensure the accuracy of the demographic and clinical data, that cataract surgery and IOL dislocation were matched to the same eye, and to verify residence by using previously validated procedures.13, 14
In the nested case-control study, we compared each incident case with approximately 3 control cases from the Rochester Epidemiology Project cataract surgery cohort who did not have an IOL dislocation. Controls were matched to cases by age, gender, and length of follow-up.
Data Collection and Analysis
The records of all identified cohort and control cases were reviewed for precataract surgery data including, gender, date of birth, presence of pseudoexfoliation, glaucoma, macular degeneration, retina disease or surgery, uveitis, degree of myopia, axial length, keratometry, and trauma. Intra-operative data included date and technique of cataract surgery, IOL optic material, location of incision, anterior capsulotomy technique, capsular rupture, use of mechanical pupil dilation, or capsular tension ring. Data collected on IOL dislocation cases included type of IOL dislocation (in-the-bag versus out-of-the bag), date of dislocation, surgical management, and vision before and after surgical repositioning or exchange.
The cumulative risk of IOL dislocation after cataract surgery was estimated by using the Kaplan-Meier method. The duration of follow-up care after cataract surgery was based on the resident’s last computer documented re-registration date for any medical care within the REP or documented death. Differences between cases and controls were evaluated by using conditional logistic regression analysis. The Fisher exact test was used for comparisons with small numbers.
RESULTS
Medical record review identified 32 potential cases with a documented IOL dislocation after cataract surgery. Exclusion of 13 nonresidents and 3 anterior chamber IOL dislocations left 16 incident cases of late posterior chamber IOL dislocation in 16 residents. Table 1 shows the demographics and clinical data for the cataract surgery and IOL dislocation cohorts. Seven (44%) of the 16 IOL dislocations were out-of-the-bag cases in which the IOL migrated through a defect in the posterior capsule or zonule. Nine (56%) were in-the-bag cases in which the IOL remained within the capsular bag and the entire IOL-bag-complex dislocated. All IOL dislocations were in Caucasian residents. Fourteen of the 16 cases (88%) were managed by IOL exchange and 2 (12%) were re-positioned by using scleral fixation sutures. The median pre-operative Snellen visual acuity was 20/150 (25% to 75% Quartile, 20/60 – 20/250) and the median Snellen visual acuity after re-positioning or exchange was 20/50 (25% to 75% Quartile, 20/25 – 20/150), with 43% of eyes reaching a Snellen visual acuity of 20/40 or better.
Table 1.
Demographic Characteristics of the Rochester Epidemiology Project Primary Cataract Surgery and IOL Dislocation Cohort, 1980–2009
| Variable | Primary Cataract Surgery Cohort [n (%)] |
IOL Dislocation Cohort [n (%)] |
|---|---|---|
| Gender | ||
| Male | 5360 (37) | 8 (50) |
| Female | 9111 (63) | 8 (50) |
| Cataract Surgery Technique | ||
| Phacoemulsification | 10563 (73) | 10 (62) |
| ECCE | 3589 (25) | 6 (38) |
| Other | 319 (2) | 0 (0) |
| Decade of Surgery | ||
| 1980 – 1989 | 2241 (15) | 6 (38) |
| 1990 – 1999 | 4624 (32) | 6 (38) |
| 2000 – 2009 | 7606 (53) | 4 (24) |
| Age | ||
| 0–9 | 27 (0.2) | 0 (0) |
| 10–19 | 24 (0.2) | 0 (0) |
| 20–29 | 38 (0.3) | 0 (0) |
| 30–39 | 91 (0.6) | 0 (0) |
| 40–49 | 396 (2.7) | 0 (0) |
| 50–59 | 1084 (7.5) | 2 (13) |
| 60–69 | 2823 (19.5) | 8 (50) |
| 70–79 | 5479 (37.9) | 5 (31) |
| 80–89 | 3997 (27.6) | 1 (6) |
| ≥ 90 | 512 (3.5) | 0 (0) |
| Total | 14471 (100) | 16 (100) |
IOL = intraocular lens
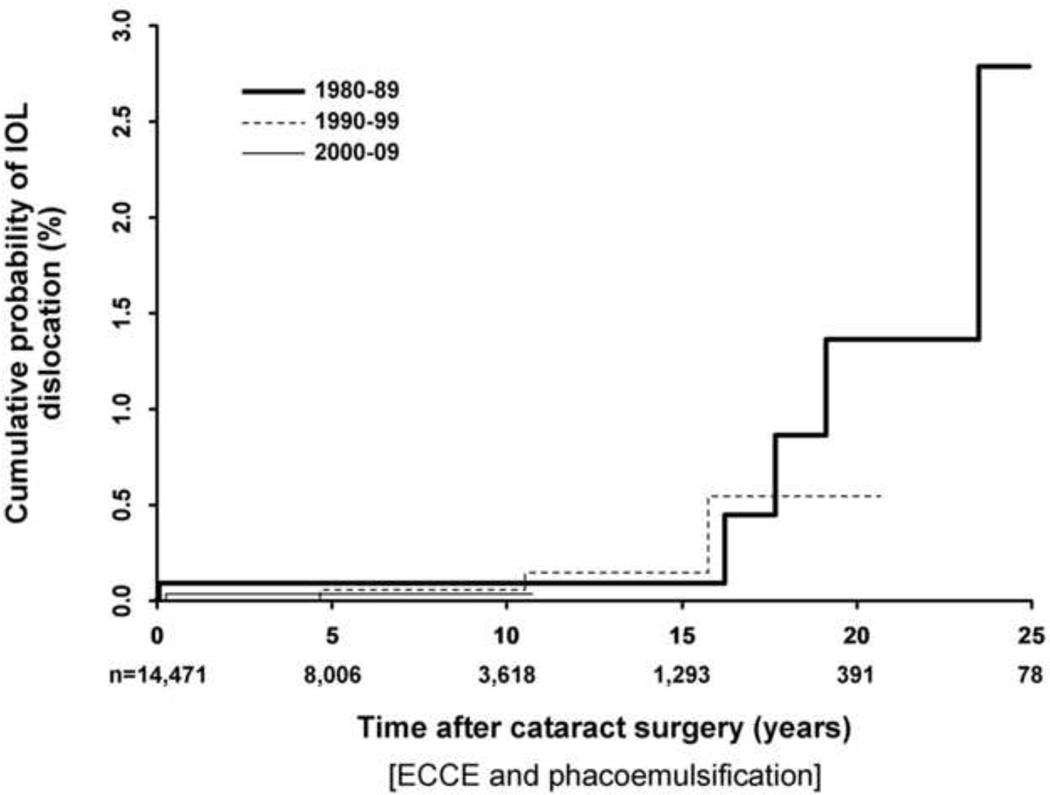
The cumulative risk of IOL dislocation up to 25 years after cataract surgery is shown in Figure 1. The mean interval between cataract surgery and IOL dislocation was 6.2 ± 7.7 years in the out-of-the-bag group compared to 12.4 ± 8.2 years in the in-the-bag group (P=0.14). There was no significant difference in late IOL dislocation rates when comparing cataract surgery performed in the 1980’s (0.1%), 1990’s (0.1%), and 2000’s (0.1%) and followed to 10 years (P=0.92; Figure 2). Our study had 90% power to detect at least a 0.5% difference in IOL dislocation rates between any of the 2 decade groups.
Figure 1.
Cumulative risk of late posterior chamber intraocular lens dislocation after cataract extraction (extracapsular cataract extraction and phacoemulsification) in all Olmsted County residents, 1980 – 2009; Kaplan-Meier analysis.
Figure 2.
Effect of decade of cataract surgery on the cumulative risk of late posterior chamber intraocular lens (IOL) dislocation after cataract extraction (extracapsular cataract extraction and phacoemulsification) in Olmsted County residents, 1980 – 2009. There was no significant difference in the risk of late IOL dislocation by decade of cataract surgery when tested to 10 years after surgery (P=0.92, log-rank test).
In the case-control portion of the study, 16 cases (8 men, 8 women) with IOL dislocation after cataract surgery were matched by age, gender, date of surgery, and length of follow-up with 47 controls (24 men, 23 women). The mean age at cataract extraction was 69 ± 9 years in cases and 70 ± 10 years in controls. Prior to surgery, the degree of myopia trended higher in IOL dislocation cases (−1.80 ± 3 diopters [D]) than in controls (−0.40 ± 2.0 D), but the difference was not significant (P = 0.07). We found no association between IOL dislocation and axial length in cases (23.89 ± 1.28 mm) versus controls (23.84 ± 1.21 mm; P=0.51) and in keratometry in cases (43.74 ± 1.65 D) versus controls (43.53 ± 1.47 D; P=0.83). Univariate associations between other selected variables and IOL dislocation are shown in Table 2.
Table 2.
Univariate Association of Selected Variables with Late IOL Dislocation after Cataract Surgery.
| Variable | Cases [n (%)] |
Control [n (%)] |
P Value |
|---|---|---|---|
| Pre-operative | |||
| Pseudoexfoliation | |||
| Yes | 3 (19) | 0 (0) | |
| No | 13 (81) | 47 (100) | 0.01a |
| Glaucoma – open angle | |||
| Yes | 3 (19) | 2 (4) | |
| No | 13 (81) | 45 (96) | 0.09b |
| Previous Anterior Vitrectomy | |||
| Yes | 0 (0) | 2 (4) | |
| No | 16 (100) | 45 (96) | 1.0a |
| Age-related Macular Degeneration | |||
| Yes | 3 (19) | 8 (17) | |
| No | 13 (81) | 39 (83) | 0.84b |
| Uveitis | |||
| Yes | 1 (6) | 0 (0) | |
| No | 15 (94) | 47 (100) | 0.25a |
| Previous Trauma | |||
| Yes | 1 (6) | 1 (2) | |
| No | 15 (94) | 46 (98) | 0.44b |
| Intra-operative | |||
| Zonular dehiscence or laxity | |||
| Yes | 3 (19) | 0 (0) | |
| No | 13 (81) | 47 (100) | 0.01a |
| Surgery Technique | |||
| Phacoemulsification | 10 (63) | 26 (55) | |
| ECCE | 6 (37) | 21 (45) | 0.24b |
| IOL Optic Material | |||
| Acrylic | 3 (19) | 16 (34) | |
| Silicone | 3 (19) | 5 (11) | |
| Polymethylmethacrylate | 10 (62) | 26 (55) | 0.45b |
| Incision | |||
| Temporal clear cornea | 4 (25) | 19 (40) | |
| Superior scleral tunnel | 12 (75) | 28 (60) | 0.12b |
| Anterior capsulotomy | |||
| CCC | 11 (69) | 26 (55) | |
| Can opener | 5 (31) | 21 (45) | 0.12b |
| Capsule rupture | |||
| Yes | 0 (0) | 1 (2) | |
| No | 16 (100) | 46 (98) | 1.0a |
IOL = intraocular lens
Fisher exact test
Conditional logistic regression analysis
DISCUSSION
The findings of our retrospective, population-based cohort study demonstrate that the risk of late posterior chamber IOL dislocation requiring surgical management after ECCE and phacoemulsification is low. We report a cumulative risk of late posterior chamber IOL dislocation that was 0.1% at 10 years after cataract extraction, increasing to 1.7% at 25 years.
Survey data since 1998 from the American and European Society of Cataract and Refractive Surgery indicate that IOL dislocation is the main reason for IOL explantation or secondary surgical intervention after cataract surgery.17–21 In the U.S., however, population-based data on the risk of late IOL dislocation after cataract surgery is lacking. Retrospective case series and surveys have estimated a cumulative probability of IOL dislocation of 0.2% to 3%.3,4,22,23 These dislocation estimates are higher than in our study as they included various types of IOL luxation, cases of very early dislocation, and clinically insignificant IOL subluxations. Additionally, the estimates were not calculated from a defined population and consequently are subject to patient inclusion bias.
More recently, Swedish population-based data estimate late posterior chamber IOL dislocation requiring surgical intervention to have an annual incidence of 0.05% in western Sweden 12 and a 10-year cumulative incidence of 1% in northern Sweden.11 By contrast, our 10-year cumulative risk of posterior chamber IOL dislocation was only 0.1%. This 10-fold lower IOL dislocation estimate in southern Minnesota compared to Sweden at 10 years after surgery may be due, in part, to a higher prevalence of PEX in the Swedish cohorts. PEX has consistently been shown to be a significant risk factor for late IOL dislocation in this study and by other investigators, especially for in-the-bag dislocations. 1–4,6,7,11,12 PEX is common in Sweden and Scandinavian populations, with a reported prevalence of 18% to 40% in patients presenting for cataract surgery or in a similar age group.24,25 In Swedish studies, PEX was present in 60% to 80% of late IOL dislocations. 11,12 In a recent U.S. retrospective study, PEX was present in 26% of IOL decentrations or dislocations.4 Similarly, PEX was present in 19% of late IOL dislocations in our population-base study. Minnesota residents have a strong Scandinavian and northern European heritage. Although the prevalence of PEX in our County is not known, it is likely lower than in Sweden as our estimated annual incidence of newly diagnosed PEX is 105 per 100,000 Olmsted County residents aged 60–69 years.26
As our study spanned nearly 30 years, our cataract extraction technique included both ECCE and phacoemulsification. In 1980, 91% of cataract surgeries were by ECCE and 0% by phacoemulsification. In 2000, <1% of surgeries were by ECCE and 99% were by phacoemulsification.15 Similar changes in cataract extraction technique were seen nationally during this same time period.27 In the Swedish population-based studies, the study time period was after 2000 and therefore phacoemulsification combined with continuous curvilinear capsulorrhexis (CCC) was the predominant cataract surgery technique. In certain eyes, phacoemulsification and CCC are believed to predispose to capsular contraction syndrome which is some cases precedes late IOL dislocation, especially in patients with PEX.3 In our cohort, we found no association between cataract extraction technique (ECCE and phacoemulsification) and increased risk for late IOL dislocation.
Many posterior chamber IOL models were used during our study period. Polymethymethacrylate IOL optic material is believed to induce less capsule fibrosis and shrinkage and provide less capsular bag contraction than silicone IOL optic material.28 We found no association between the IOL optic material and an increased risk for late IOL dislocation; this corroborates previous findings.7,12
Recent case series have noted an increase in the number of spontaneously dislocated IOLs.3,6,7 One objective of our population-based study was to compare the risk of late IOL dislocation between different decades to determine whether the risk of IOL dislocation has changed over time. In our study population, the incidence of cataract surgery increased 330% in the 2000’s compared to the 1980’s;15 consistent with changing patterns of cataract extraction worldwide.29–31 However, similar to a large Swedish population-base study, 12 we were unable to find a significantly increased risk of IOL dislocation during our nearly 30-year study period. In Olmsted County, Minnesota, at least, the rate of late IOL dislocation has not increased, and in other populations with demographics similar to ours, any observed increase in the number of late IOL dislocation may be simply due to a growing pseudophakic community.
Analysis of population-based studies of surgical outcome is useful as it avoids patient inclusion bias which is a common confounder of non-population based series. The usefulness of the Rochester Epidemiology Project in providing accurate population-based data has been realized previously.13,14 Any underascertainment of patients not coming to medical attention is unlikely, as the Rochester Epidemiology Project provides access to virtually all medical records of care provided to Olmsted County residents. A previous data review indicated that nearly all Olmsted County residents had cataract surgery at sites for which the Rochester Epidemiology Project would have virtually complete data capture.16 Similarly, we assume that Rochester Epidemioloy Project databases have a high capture rate for patients undergoing surgery for IOL dislocation.
It is important to note the limitations of the data. First, we studied a relatively racially homogenous population of a single geographic area. The Rochester Epidemiology Project cataract surgery cohort was 96% Caucasian and largely middle class. Although previous population-based studies from Olmsted County have demonstrated that the data from our present study should be applicable to the Caucasian population of the U.S.,13 we caution the validity of extrapolating our findings more broadly. Second, potential dislocation cases that either were miscoded as another diagnosis or had surgery at a site outside of the Rochester Epidemiology Project databases could result in our incidence estimates being low. Presumably, a review of records with related ICD9-CM diagnosis and procedure codes would decrease the likelihood of this occurrence. Third, cases that do not come to medical attention cannot be identified with the resources of the Rochester Epidemiology Project. The degree to which this might lower our estimated probability of dislocation after cataract surgery is unknown, although likely very low.
In summary, our findings highlight that late posterior chamber IOL dislocation is an uncommon complication of cataract surgery that involves both in-the-bag and out-of-the bag dislocations, and is more likely to occur in the higher-risk subgroup of PEX and zonular laxity at the time of surgery. Although an uncommon complication, late dislocated IOLs requiring surgical intervention may become more of a burden to society as the pseudophakic community continues to increase in size due to longer life spans and the large numbers of people undergoing cataract extraction.
ACKNOWLEDGMENT
This study was supported in part by Research to Prevent Blindness Inc., New York, New York; and Mayo Foundation, (Robert R. Waller Career Development Award) Rochester, Minnesota.
Design and conduct of study: (S.L.P., D.A.O., J.C.E.)
Collection, management, and analysis of data: (S.L.P., D.A.O., J.C.E.)
Interpretation of data: (S.L.P., D.A.O., J.C.E.)
Preparation of manuscript: (S.L.P., D.A.O., J.C.E.)
Review and approval of manuscript: (S.L.P., D.A.O., J.C.E.)
The study protocol was reviewed and approved by the Institutional Review Board of the Mayo Clinic and adhered to the principles of the Declaration of Helsinki.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors indicate no financial conflict of interest.
REFERENCES
- 1.Jehan FS, Mamalis N, Crandall AS. Spontaneous late dislocation of intraocular lens within the capsular bag in pseudoexfoliation patients. Ophthalmology. 2001;108(10):1727–1731. doi: 10.1016/s0161-6420(01)00710-2. [DOI] [PubMed] [Google Scholar]
- 2.Gross JG, Kikame GT, Weinberg DV. In-the-bag intraocular lens dislocation: the dislocated in-the-bag lens study group. Am J Ophthalmol. 2004;137(4):630–635. doi: 10.1016/j.ajo.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Gimbal HV, Condon GP, Kohnen T, Olson RJ, Halkiadakis I. Late in-the-bag intraocular lens dislocation: incidence, prevention, and management. J Cataract Refract Surg. 2005;31(11):2193–2204. doi: 10.1016/j.jcrs.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 4.Jin GJ, Crandall AS, Jones JJ. Changing indications for and improving outcomes of intraocular lens exchange. Am J Ophthalmol. 2005;140($):688–694. doi: 10.1016/j.ajo.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Hirata A, Hayashi H. Possible predisposing factors for in-the-bag and out-of-the bag intraocular lens dislocation and outcomes of intraocular exchange surgery. Ophthalmology. 2007;114(5):969–975. doi: 10.1016/j.ophtha.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Smiddy WE, Feuer W, Shi W. Management of dislocated intraocular lenses. Ophthalmology. 2008;115(10):1699–1704. doi: 10.1016/j.ophtha.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Davis D, Brubaker J, Espandar L, et al. Late in-the-bag spontaneous intraocular lens dislocation: evaluation of 86 consecutive cases. Ophthalmology. 2009;116(4):664–670. doi: 10.1016/j.ophtha.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Shigeeda T, Nagahara M, Kato S, et al. Spontaneous posterior dislocation of intraocular lenses fixated in the capsular bag. J Cataract Refract Surg. 2002;28(9):1689–1693. doi: 10.1016/s0886-3350(01)01178-6. [DOI] [PubMed] [Google Scholar]
- 9.Drolsum L, Ringvold A, Nicolaissen B. Cataract and glaucoma surgery in pseudoexfoliation syndrome: a review. Acta Ophthalmol Scand. 2007;85(8):810–821. doi: 10.1111/j.1600-0420.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 10.Sarrafizadeh R, Ruby AJ, Hasaan TS, et al. A comparison of visual results and complications in eyes with posterior chamber intraocular lens dislocation treated with pars plana vitrectomy and lens repositioning or lens exchange. Ophthalmology. 2001;108(1):82–89. doi: 10.1016/s0161-6420(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 11.Mönestam EI. Incidence of dislocation of intraocular lenses and pseudophakodonesis 10 years after cataract surgery. Ophthalmology. 2009;116(12):2315–2320. doi: 10.1016/j.ophtha.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Jakobsson G, Zetterberg M, Lundström, et al. Late dislocation of in-the-bag and out-of-the-bag intraocular lenses: Ocular and surgical characteristics and time to lens repositioning. J Cataract Refrac Surg. 2010;36(10):1637–1644. doi: 10.1016/j.jcrs.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 15.Erie JC, Baratz KH, Hodge DO, Schleck CD, Burke JP. Incidence of cataract surgery from 1980–2004: 25-year population-based study. J Cataract Refract Surg. 2007;33(7):1273–1277. doi: 10.1016/j.jcrs.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Gray DT, Hodge DO, Ilstrup DM, et al. Concordance of Medicare data and population-based clinical data on cataract utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145(12):1123–1126. doi: 10.1093/oxfordjournals.aje.a009075. [DOI] [PubMed] [Google Scholar]
- 17.Mamalis N. Complications of foldable intraocular lenses requiring explantation or secondary intervention – 1998 survey. J Cataract Refract Surg. 2000;26(5):766–772. doi: 10.1016/s0886-3350(00)00424-7. [DOI] [PubMed] [Google Scholar]
- 18.Mamalis N. Complications of foldable intraocular lenses requiring explantation or secondary intervention – 2000 survey update. J Cataract Refract Surg. 2001;27(8):1310–1317. doi: 10.1016/s0886-3350(01)01021-5. [DOI] [PubMed] [Google Scholar]
- 19.Mamalis N, Spencer TS. Complications of foldable intraocular lenses requiring explantation or secondary intervention – 2001 survey. J Cataract Refract Surg. 2002;28(12):2193–2201. doi: 10.1016/s0886-3350(02)01612-7. [DOI] [PubMed] [Google Scholar]
- 20.Mamalis N, Davis B, Nilson CD, Hickman MS, LeBoyer LM. Complications of foldable intraocular lenses requiring explantation or secondary intervention – 2003 survey update. J Cataract Refract Surg. 2004;30(10):2209–2218. doi: 10.1016/j.jcrs.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 21.Mamalis N, Brubaker J, Davis D, Espander L, Werner L. Complications of foldable intraocular lenses requiring explantation or secondary intervention 2007 survey update. J Cataract Refract Surg. 2008;34(9):1584–1591. doi: 10.1016/j.jcrs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 22.Stark MJ, Jr, Maumenee AE, Datiles M, et al. Intraocular lenses: complications and visual results. Trans Am Ophthalmol Soc. 1983;81:280–302. discussion, 302–309. [PMC free article] [PubMed] [Google Scholar]
- 23.Smith SG, Lindstrom RL. Malpositioned posterior chamber lenses; etiology, prevention, and management. Am Intra-Ocular Implant Soc J. 1985;11(6):584–591. doi: 10.1016/s0146-2776(85)80139-7. [DOI] [PubMed] [Google Scholar]
- 24.Ekström C, Alm A. Pseudoexfoliation as a risk factor for prevalent open-angle glaucoma. Acta Ophthalmol (Oxf) 2008;86(7):741–746. doi: 10.1111/j.1755-3768.2008.01248.x. [DOI] [PubMed] [Google Scholar]
- 25.Mönestam E, Kuusik M, Wachtmeister L. Topical anesthesia for cataract surgery: a population-based perspective. J Cataract Refract Surg. 2001;27(3):445–451. doi: 10.1016/s0886-3350(00)00637-4. [DOI] [PubMed] [Google Scholar]
- 26.Karger RA, Jeng SM, Johnson DA, Hodge DO, Good MS. Estimated incidence of pseudoexfoliation and pseudoexfoliative glaucoma in Olmsted County, Minnesota. J Glaucoma. 2003;12(3):193–197. doi: 10.1097/00061198-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ellwien LB, Urato CJ. Use of eye care and associated changes among the Medicare population: 1991–1998. Arch Ophthalmol. 2002;120(6):804–811. doi: 10.1001/archopht.120.6.804. [DOI] [PubMed] [Google Scholar]
- 28.Werner L, Pandey SK, Escobar-Gomez M, et al. Anterior capsule opacification: a histopathological study comparing different IOL styles. Ophthalmology. 2000;107(3):463–471. doi: 10.1016/s0161-6420(99)00088-3. [DOI] [PubMed] [Google Scholar]
- 29.Lundström M, Stenevi U, Thorburn W. The Swedish National Cataract Register: a 9-year review. Acta Ophthalmol Scand. 2002;80(3):248–257. doi: 10.1034/j.1600-0420.2002.800304.x. [DOI] [PubMed] [Google Scholar]
- 30.Lundström M, Albrecht S. Previous cataract surgery in a defined Swedish population. J Cataract Refract Surg. 2003;29(1):50–56. doi: 10.1016/s0886-3350(02)01505-5. [DOI] [PubMed] [Google Scholar]
- 31.Tan AG, Wang JJ, Rochtchina E, et al. Increase in cataract surgery prevalence from 1992–1994 to 1997–2000: analysis of two population cross-sections. Clin Exp Ophthalmol. 2004;32(3):284–288. doi: 10.1111/j.1442-9071.2004.00817.x. [DOI] [PubMed] [Google Scholar]