Summary
Several groups have shown that detection of microbial components by Toll-like receptors (TLRs) on hematopoietic stem and progenitor cells (HSPCs) instructs myeloid cell generation, raising interest in the possibility of targeting TLRs on HSPCs to boost myelopoiesis. However, although “TLR-derived” cells exhibit myeloid cell characteristics (phagocytosis, cytokine production, antigen presentation), it isn’t clear whether they are functionally equivalent to macrophages derived in the absence of TLR activation. Our in vitro and in vivo studies show that macrophages derived from mouse and human HSPC subsets (including stem cells) exposed to a TLR2 agonist prior to or during macrophage differentiation produce lower levels of inflammatory cytokines (TNF-α, IL-6 and IL-1β) and reactive oxygen species (ROS). This is in contrast to prior exposure of differentiated macrophages to the TLR2 agonist (“tolerance”), which suppresses inflammatory cytokine production, but elevates ROS. Soluble factors produced following exposure of HSPCs to a TLR2 agonist can also act in a paracrine manner to influence the function of macrophages derived from unexposed HSPCs. Our data demonstrate that macrophage function can be influenced by TLR signaling in the HSPCs from which they are derived, and that this may impact the clinical utility of targeting TLRs on HSPCs to boost myelopoiesis.
Keywords: Monocytes/Macrophages, Hematopoietic Stem Cells, Hematopoietic Progenitor Cells, Myelopoiesis, Toll-Like Receptors
Introduction
Myeloid phagocytes – neutrophils, macrophages and dendritic cells – play key roles in inflammation and anti-microbial defense. They are responsible for the detection and removal of dead cells and infectious organisms, microbial killing, antigen processing and presentation to initiate adaptive immune responses, and the production of inflammatory mediators to recruit and activate other cells. These functions are controlled by integrating a variety of signals they receive from their environment. During infection they detect microbes and microbial components using pattern recognition receptors, including Toll-like receptors (TLRs), but their responses are also controlled by cell-cell contact and soluble factors (such as cytokines and chemokines) produced by other host cells.
Although myeloid cells are often classified according to functional characteristics, for example as M1 or M2 macrophages, their function is in fact highly dynamic. A macrophage’s response to a particular stimulus is not just determined by the signal transduction pathways that stimulus induces, but is also influenced by the effects of previous stimuli. For example, IFN-γ has long been known to prime macrophage responses, while TLR agonists such as lipopolysaccharide (LPS) can tolerize macrophage cytokine responses to subsequent stimulation. Moreover, studies demonstrating cross-protective anti-microbial immune responses that don’t appear to require adaptive immunity have led to the concept of “innate memory” or “trained immunity”[1]. Indeed, Netea and colleagues recently reported that prior exposure of monocytes/macrophages to BCG, Candida albicans or β-glucan particles enhances their subsequent response to challenge with a range of bacterial and fungal stimuli due to methylation of histones associated with inflammatory genes[2, 3]. In this study we investigated the possibility that macrophage function can additionally be influenced by prior microbial exposure of the precursor cells from which the macrophages are produced.
The supply of myeloid phagocytes, both in the steady state as well as during an “emergency” response to infection or tissue damage, is primarily maintained by the production of new cells from hematopoietic stem and progenitor cells (HSPCs) in the bone marrow and spleen by myelopoiesis. Upon infection, production of these cells must be accelerated to rapidly generate and mobilize sufficient myeloid cells to mount an effective immune response against the invading organism. Under such conditions hematopoietic stem cells (HSCs) are induced to proliferate and differentiate by two mechanisms: mature cells and committed progenitors are depleted as they differentiate and are mobilized to combat the infection, thereby exerting a “pull” signal on HSCs to replenish these populations; meanwhile cytokines and colony stimulating factors (CSFs) produced in response to the infection provide a “push” signal to stimulate myelopoiesis (reviewed by [4]).
Until recently, it was thought that enhanced myelopoiesis during infection was coordinated exclusively by cytokines and growth factors produced by differentiated cells in response to the infection. Recently however, several reports have demonstrated that a “push” signal can also be provided by direct interaction of HSPCs with microbes and microbial components. Several reports have now shown that HSPCs themselves express TLRs: Nagai et al. detected TLR2 and TLR4 on the surface of murine HSCs, common lymphoid progenitors (CLPs), common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs)[5], while Sioud et al. showed that freshly isolated human HSPCs (which are CD34+) express TLR4, TLR7 and TLR8[6].
There is no indication from TLR knockout mouse studies that mammalian TLRs are required for normal hematopoiesis, but their expression on HSPCs sparked interest in the possibility that microbial sensing by HSPCs during infection may influence cell division and fate determination, and ultimately the efficacy of host defense against infection. Indeed, several groups have demonstrated in vitro and in vivo that TLR stimulation can instruct HSPCs to produce myeloid cells[5–14]. For example, Kincade and colleagues showed that TLR2, TLR4 and TLR9 agonists can drive the in vitro differentiation of myeloid cells from mouse bone marrow progenitors in the absence of the growth factors normally required for differentiation of these cells; TLR stimulation drives HSCs, CMPs and GMPs towards the monocyte/macrophage lineage, while CLPs give rise to dendritic cells[5, 10]. Similarly, Sioud and Floisand showed that treatment with TLR2 or TLR7 agonists for 7 days promotes the differentiation of dendritic cells from human CD34+ HSPCs in vitro[6, 9]. Furthermore, Massberg et al. showed that high dose LPS exposure amplifies the myeloid differentiation of tissue HSCs[7]. Similar TLR-mediated effects have been shown by exposing HSPCs to whole microbes, including fungi[11–13].
Importantly, Megías et al. recently demonstrated that wild type mouse HSPCs transplanted into TLR2-, TLR4- or MyD88-deficient recipient mice produced myeloid cells upon injection of Pam3CSK4, LPS or CpG, respectively[8]. Since the recipient mice were unable to detect/respond to the injected TLR agonists, the responses must have been due to detection by the transplanted HSPCs. In a subsequent paper, Megías et al. showed that wild type but not TLR2-deficient donor HSPCs produced macrophages in vivo following infection of wild type recipient mice with C. albicans [14].
The concept that microbes can directly stimulate HSPCs to induce myelopoiesis (in addition to inducing myelopoiesis indirectly via detection by other cells) is attractive since it provides a mechanism for the rapid generation of myeloid cells to combat infection, as well as a potential therapeutic target to boost myelopoiesis. Since the bone marrow is vascularized, it is possible for cells in the bone marrow niche to be exposed to factors from the circulation, including circulating microbial components as well as inflammatory mediators produced in peripheral tissues {Clarke, 2010 #33; Shi, 2011 #34}. Intact microbes may also invade the bone marrow during systemic infection. Moreover, HSPCs have been detected in peripheral tissues and demonstrated to circulate to enable them to respond to localized infections in specific tissues {Massberg, 2007 #9; Mazo, 2011 #35}.
However, although some studies have demonstrated that TLR-induced cells exhibit myeloid functions (phagocytosis, cytokine production etc.), the functional characteristics of these cells have not been well defined. The idea that TLR activation of HSPCs is essentially a mechanism to generate more phagocytes to assist in immune defense assumes that the resulting cells function as effectively as pre-existing cells, but this has not previously been investigated.
In this study we found that macrophages derived in vitro and in vivo from mouse and human HSPCs exposed to the TLR2 agonist Pam3CSK4, either prior to or during macrophage development, exhibit reduced production of inflammatory cytokines and reactive oxygen species (ROS). This is in contrast to “tolerized” monocytes/macrophages previously exposed to Pam3CSK4 following differentiation, which produce lower levels of inflammatory cytokines but more ROS upon subsequent stimulation. The effects of exposure of HSPCs to the TLR2 agonist on the function of subsequently derived macrophages appear to be mediated by soluble factors acting by both autocrine and paracrine mechanisms.
Results
In vitro stimulation of murine HSPCs with a TLR2 agonist during myelopoiesis alters cytokine production by the macrophages they generate
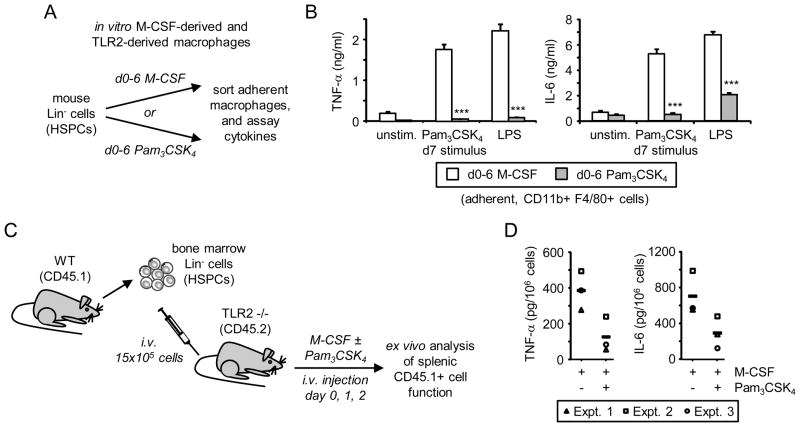
To investigate the functional consequences of exposure of HSPCs to a TLR2 agonist, we compared the function of macrophages derived in vitro using either M-CSF or Pam3CSK4. We cultured mouse Lin− cells, which comprise a mixture of stem cells and lineage-committed progenitors, with M-CSF or Pam3CSK4 for 6 days in media containing SCF and Flt3L, which support HSPC survival at the early stages of differentiation (Figure 1A). Culture of HSPCs with M-CSF (d0–6 M-CSF) predominantly produced adherent cells that were uniformly F4/80+ and CD11b+, while culture with Pam3CSK4 (d0–6 Pam3CSK4) yielded a mixture of non-adherent cells and adherent myeloid cells with more variable levels of F4/80 and CD11b (Supplementary Figure 1A and data not shown), consistent with previous reports[5, 12]. Since this heterogeneity complicates functional comparison, we sorted adherent F4/80+ CD11b+ cells (Supplementary Figure 1A, gated cells) from the Pam3CSK4 cultures (subsequently referred to as “TLR2-derived macrophages”) and compared them to adherent F4/80+ CD11b+ macrophages derived using M-CSF (“M-CSF-derived macrophages”).
Figure 1. Effect of continuous exposure to the TLR2 agonist Pam3CSK4 during in vitro and in vivo differentiation of murine HSPCs on macrophage inflammatory cytokine responses.
(A and B) Exposure to Pam3CSK4 during in vitro macrophage differentiation: (A) Lin− HSPCs from mouse bone marrow were cultured for 6 days in media containing serum plus SCF and Flt3L, and 50 ng/ml M-CSF or 1 μg/ml Pam3CSK4 (d0–6 M-CSF and d0–6 Pam3CSK4 respectively). (B) Day 6 F4/80+ CD11b+ adherent macrophages were sorted by FACS, plated at equal numbers, rested overnight and then stimulated with 100 ng/ml Pam3CSK4 or LPS on day 7. TNF-α and IL-6 levels in 24 h culture supernatants were assessed by ELISA. Data are presented as mean plus standard deviation of triplicate culture. *** p<0.001 Data are representative of at least 3 independent experiments. (C and D) Exposure to Pam3CSK4 during in vivo macrophage differentiation: (C) HSPCs from wild type CD45.1 donor mice were intravenously injected into TLR2 −/− CD45.2 recipient mice. Recipient mice then received an injection of 10 μg M-CSF plus/minus 100 μg Pam3CSK4 on days 0, 1 and 2. CD45.1+ CD11b+ F4/80+ macrophages were recovered from mouse spleens on day 3 for ex vivo stimulation on day 4. (D) Macrophages were stimulated with 100 ng/ml LPS, and TNF-α and IL-6 levels in 24 h culture supernatants were assessed by ELISA. Mean cytokine measurements (triplicate stimulation) from 3 mice/group are presented, and the group mean is indicated by a horizontal bar.
Upon stimulation, we found that while M-CSF-derived macrophages produced robust levels of the inflammatory cytokines TNF-α and IL-6, the TLR2-derived macrophages produced significantly less TNF-α and IL-6 upon stimulation with Pam3CSK4 or LPS (Figure 1B).
In vivo stimulation of murine HSPCs with a TLR2 agonist during myelopoiesis alters cytokine production by the macrophages they generate
Megías et al. recently showed that wild type (CD45.1+) HSPCs injected into TLR2-deficient (CD45.2+) recipients traffic rapidly to the bone marrow and the spleen, where they can be induced to produce macrophages and other myeloid cells upon injection of the TLR2 agonist Pam3CSK4[8]. Using this approach, we found that splenic macrophages derived from wild type donor HSPCs following Pam3CSK4 injection produced low levels of TNF-α upon ex vivo stimulation (~90 pg TNF-α/million cells). In order to further explore the effects of detection of the TLR2 agonist by HSPCs in vivo and permit functional comparison with other macrophage populations we modified the approach to derive macrophages by the injection of M-CSF with or without Pam3CSK4 (Figure 1C). As expected, donor HSPCs gave rise to CD11b+ F4/80+ macrophages in the spleen and, consistent with the previous report[8], Pam3CSK4 injection enhanced macrophage differentiation and yield (Supplementary Figure 1B and data not shown). Like the in vitro TLR2-derived macrophages (Supplementary Figure 1A), donor HSPC-derived macrophages produced in vivo in the presence of Pam3CSK4 expressed comparable levels of CD11b to control macrophages, but higher levels of F4/80 (Supplementary Figure 1B). Ex vivo stimulation of equal numbers of donor-derived macrophages with LPS revealed that the macrophages derived in the presence of Pam3CSK4 produced lower levels of the inflammatory cytokines TNF-α and IL-6 (Figure 1D). Since the recipient mice are unable to respond to Pam3CSK4, this effect must be due to detection of the TLR2 ligand by the donor HSPCs and possibly their progeny.
Exposure of murine HSPCs to a TLR2 agonist prior to differentiation alters cytokine production by macrophages subsequently derived from them
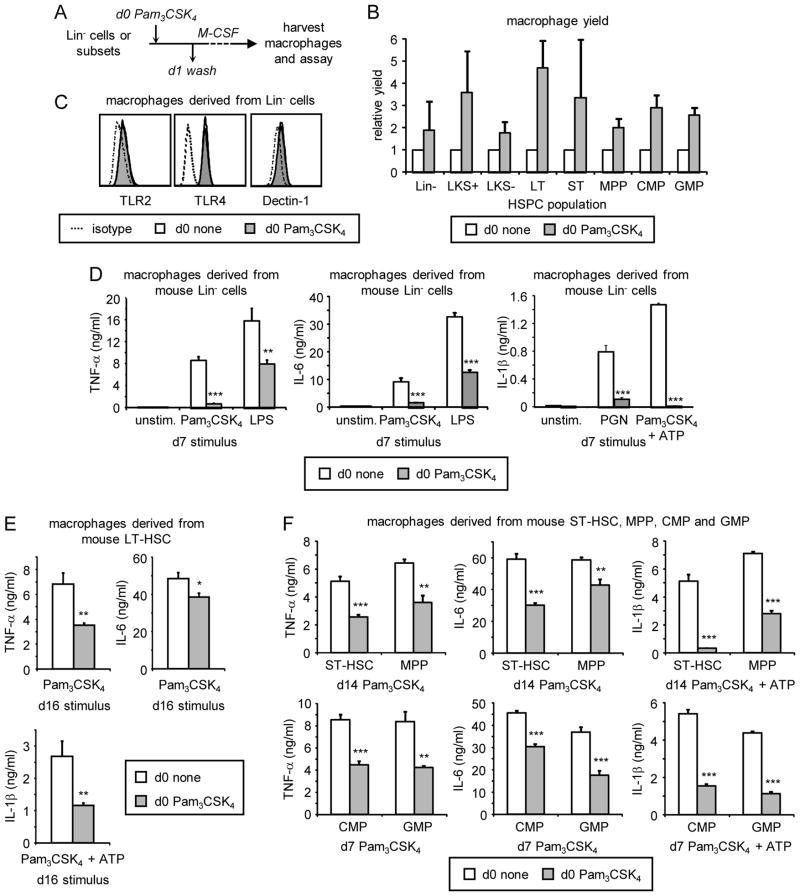
The above in vitro and in vivo data demonstrate that the function of macrophages can be influenced by exposure of progenitors to a TLR2 agonist during differentiation, but since the TLR2 agonist is present throughout differentiation, the functional alterations could be due to Pam3CSK4 detection after the cells have become monocytes/macrophages. To determine whether TLR2 ligation at the HSPC stage has functional consequences, we next investigated whether exposure of HSPCs to a TLR2 agonist prior to differentiation affects the function of macrophages subsequently derived from them in the absence of continued TLR2 stimulation. For these experiments, we used M-CSF to induce macrophage differentiation, and examined the functional consequences of exposing HSPCs to Pam3CSK4 for the first 24 hours of culture only. We treated mouse Lin− cells or sorted HSPC subsets (LT-HSC, ST-HSC, MPP, CMP and GMP; see Supplementary Figure 2) with Pam3CSK4 on day 0 (d0 Pam3CSK4), washed the cells thoroughly to remove the Pam3CSK4 on day 1, and then continued culture with M-CSF for a further 5–14 days to derive macrophages (Figure 2A). d0 Pam3CSK4 treatment of Lin− cells and all HSPC subsets boosted the yield of M-CSF-derived macrophages (Figure 2B), but did not alter the surface expression levels of F4/80 or CD11b (Supplementary Figure 3A and B), or the pattern recognition receptors used to activate the macrophages for subsequent functional analysis (TLR2, TLR4 and Dectin-1; Figure 2C and Supplementary Figure 3C). The enhanced yield appeared to be due to proliferation and/or survival of the progenitors during the first 24 hours of stimulation since Pam3CSK4-treated HSPCs yielded more myeloid colonies than untreated HSPCs when equal numbers of HSPCs were cultured in methylcellulose (Supplementary Figure 4).
Figure 2. Effects of transient exposure of murine HSPCs to the TLR2 agonist Pam3CSK4 prior to differentiation on macrophage yield and inflammatory cytokine production.
(A) HSPC subsets from mouse bone marrow were cultured on day 0 with M-CSF (plus SCF and Flt3L for LKS+, LKS−, LT-HSC, ST-HSC and MPP cultures) for 24 h in the presence or absence of 100 ng/ml Pam3CSK4. Cells were washed thoroughly on day 1 and culture was continued with M-CSF (plus SCF and Flt3L as above) for a further 5 days (Lin−, CMP and GMP cultures), 12 days (LKS+, LKS−, ST-HSC and MPP cultures) or 14 days (LT-HSC cultures) to derive macrophages. Adherent cells were harvested on day 6, 13 or 15, and macrophage yields were assessed by cell counting (B). Surface expression of pattern recognition receptors was assessed by flow cytometry (C). Macrophages were then plated at equal numbers and rested overnight prior to stimulation on day 7, 14 or 16 as indicated (D–F). Cytokine responses to 24 h stimulation with 100 ng/ml Pam3CSK4 or LPS (TNF-α and IL-6), and 20 μg/ml PGN or 100 ng/ml Pam3CSK4 plus 5 mM ATP for the final 2 h of stimulation (IL-1β), were assessed by ELISA. Data are presented as mean plus standard deviation of triplicate culture. * p<0.05, ** p<0.01, *** p<0.001 All data are representative of at least 3 independent experiments.
We next plated equal numbers of macrophages derived from unexposed and Pam3CSK4-exposed HSPCs and examined their function. TNF-α production by macrophages derived from transiently-exposed Lin−, LKS+ and LKS− cells as well as sorted stem cell and myeloid progenitor subsets (LT-HSC, ST-HSC, MPP, CMP, GMP) was suppressed (Figure 2D–F and data not shown). Macrophage responses to stimulation via both TLR2 and TLR4 were affected, but as noted above, surface expression of these receptors was not altered (Figure 2C and Supplementary Figure 3C). Production of IL-6 and IL-1β was also reduced (Figure 2D–F). There were no differences in cell viability or rates of macrophage proliferation (data not shown).
These data show that transient exposure of HSPCs to a TLR2 agonist prior to differentiation “programs” the function of macrophages subsequently derived from them.
Prior exposure of HSPCs to a TLR2 agonist, unlike prior exposure of differentiated monocytes/macrophages to a TLR2 agonist (tolerance), results in reduced ROS production
Monocyte/macrophage “tolerance” is a widely described phenomenon whereby differentiated myeloid cells that previously responded to TLR agonists display a reduced ability to produce inflammatory cytokines upon subsequent stimulation with the same or another TLR agonist[15, 16]. Since the original description of tolerance however, investigators have shown that not all genes are tolerized to subsequent stimulation, and in fact that some responses are actually primed rather than tolerized by previous exposure to TLR agonists[17, 18]. We next broadened our investigation of the function of macrophages derived from Pam3CSK4-exposed HSPCs to determine whether they behave similarly to tolerized macrophages, which had been exposed to the TLR2 agonist after differentiation.
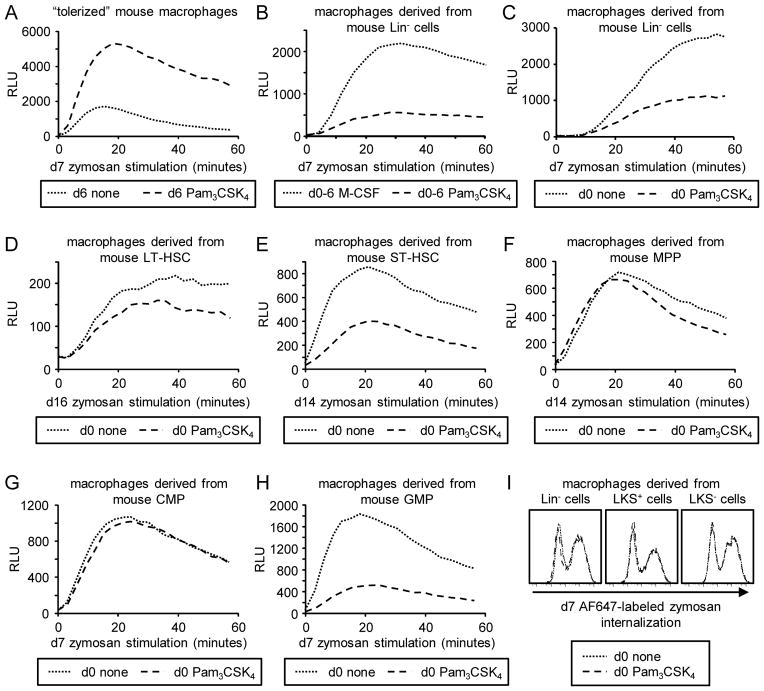
Treatment of differentiated macrophages with TLR agonists including Pam3CSK4 primes them to produce an elevated oxidative burst response upon activation of the β-glucan receptor Dectin-1[19]. We therefore examined the effects on macrophage reactive oxygen species (ROS) production of exposure to Pam3CSK4 prior to differentiation (d0 Pam3CSK4/“TLR2-programmed macrophages”, as in Figure 2), throughout differentiation (“TLR2-derived macrophages”, as in Figure 1), and following macrophage differentiation (“TLR2-tolerized macrophages”).
Consistent with previous reports[18, 19], TLR2-tolerized macrophages produced lower levels of inflammatory cytokines upon restimulation with TLR2/4 agonists (data not shown), but their ROS response to β-glucan particle (zymosan) stimulation was enhanced (Figure 3A). In contrast, compared to M-CSF-derived macrophages, Pam3CSK4-derived macrophages produced only very low levels of ROS (Figure 3B). Similarly, ROS production by macrophages derived from Lin−, LKS+ or LKS− cells as well as sorted LT-HSCs, ST-HSCs and GMPs following transient exposure to Pam3CSK4 prior to differentiation was also suppressed (Figure 3C–H and data not shown). Interestingly, ROS production by MPP- and CMP-derived macrophages did not seem to be affected (Figure 3F and G), although cytokine production was reduced (Figure 2F).
Figure 3. ROS production by macrophages derived from murine HSPCs exposed to the TLR2 agonist Pam3CSK4 prior to or during differentiation.
(A) Fully differentiated mouse macrophages (derived from Lin− cells) were “tolerized” by treatment with 100 ng/ml Pam3CSK4 for 24 h (d6 Pam3CSK4). Macrophages were then washed and stimulated with 100 μg/ml zymosan and ROS production was evaluated by luminol-ECL. (B) Macrophages were derived from Lin− cells using either M-CSF or 1 μg/ml Pam3CSK4 (plus SCF and FLt3L) for 6 days. Adherent CD11b+ F4/80+ cells were sorted, plated overnight and stimulated the following day with zymosan for ROS analysis. (C–I) HSPCs from mouse bone marrow were cultured on day 0 with M-CSF (plus SCF and Flt3L for LKS+, LKS−, LT-HSC, ST-HSC and MPP cultures) for 24 h in the presence or absence of 100 ng/ml Pam3CSK4. Cells were washed thoroughly on day 1 and culture was continued with M-CSF (plus SCF and Flt3L as above) for a further 5 days (Lin−, CMP and GMP cultures), 12 days (ST-HSC and MPP cultures) or 14 days (LT-HSC cultures) to derive macrophages. Adherent cells were harvested on day 6, 13 or 15, and plated for analysis of zymosan-induced ROS production (C–H) and phagocytic uptake of 20 μg/ml fluorescently labeled zymosan (I) the following day. ROS datapoints are means of triplicate culture. All data are representative of at least 3 independent experiments.
“Tolerized” macrophages are also more phagocytic: prior exposure of differentiated macrophages to Pam3CSK4 primes Dectin-1-mediated phagocytic uptake of β-glucan particles (Supplementary Figure 5A). In contrast, macrophage phagocytosis of β-glucan particles was unaffected by transient exposure of HSPCs to the TLR2 agonist (Figure 3I and Supplementary Figure 5B).
Exposure of human HSPCs to a TLR agonist prior to differentiation results in reduced inflammatory cytokine and ROS production
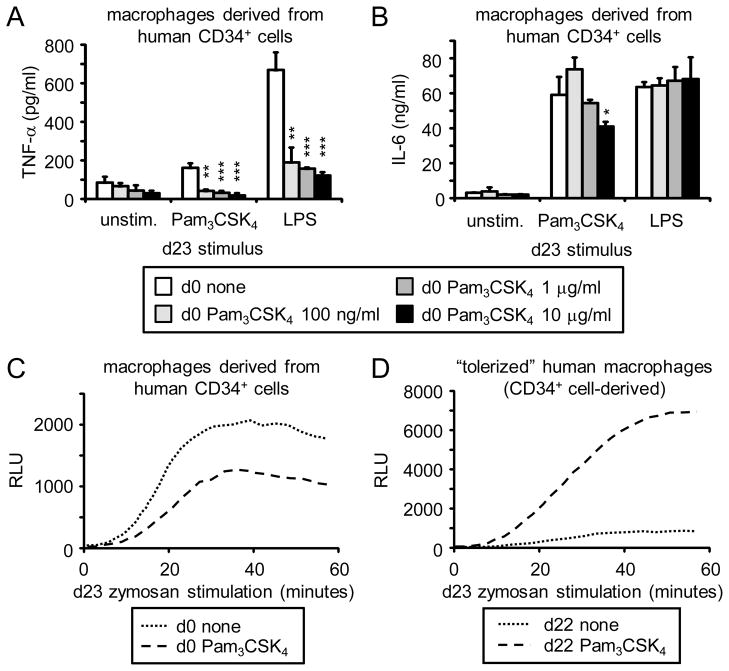
To determine whether TLR agonists have a similar functional programming effect on human HSPCs, we isolated CD34+ cells from cord blood, exposed them to Pam3CSK4 for 24 hours, and then washed the cells thoroughly prior to culture with M-CSF and a cocktail of other cytokines (see Materials and Methods) to derive macrophages. Consistent with the effects of exposure of mouse HSPCs to a TLR2 agonist, expression of human macrophage markers and TLRs was unaffected (Supplementary Figure 6), and macrophages derived from human HSPCs transiently exposed to Pam3CSK4 produced lower levels of TNF-α (but not IL-6) than macrophages derived from unexposed CD34+ cells (Figure 4A–B).
Figure 4. Inflammatory cytokine and ROS production by macrophages derived from human HSPCs transiently exposed to the TLR2 agonist Pam3CSK4 prior to differentiation.
(A–C) On day 0, CD34+ cells were sorted from human cord blood and cultured with macrophage-inducing cytokines (see Materials and Methods) for 24 h in the presence or absence of the indicated doses of Pam3CSK4 (A and B) or 100 ng/ml Pam3CSK4 (C). Cells were washed thoroughly on day 1 and culture was continued with macrophage-inducing cytokines for a further 3 weeks to derive macrophages. Adherent cells were then harvested, plated at equal numbers and stimulated the following day to measure TNF-α and IL-6 production in response to 500 ng/ml Pam3CSK4 or 1 μg/ml LPS for 24 h (A and B), and ROS production in response to 100 μg/ml zymosan (C). (D) Macrophages were derived from human CD34+ cord blood cells by 3-week culture with macrophage-inducing cytokines, treated on day 22 with 100 ng/ml Pam3CSK4, and washed on day 23 prior to assessment of zymosan-stimulated ROS production. Cytokine data are presented as mean plus standard deviation of triplicate culture. * p<0.05, ** p<0.01, ***p<0.001 ROS datapoints are means of triplicate culture. Data are representative of 3 independent experiments.
Transient exposure of human HSPCs to Pam3CSK4 also had a similar effect on ROS production by macrophages subsequently derived from them: CD34+ cord blood cells treated with Pam3CSK4 prior to differentiation gave rise to macrophages that produced less ROS than macrophages derived from unexposed CD34+ cells (Figure 4C), while Pam3CSK4 treatment of differentiated macrophages (“tolerance”) primed the cells for subsequent ROS induction (Figure 4D).
Taken together, the above mouse and human data demonstrate that while exposure to a TLR2 agonist prior to, during or after differentiation has similar implications for the production of inflammatory cytokines by macrophages, the oxidative burst response is differentially influenced depending on the stage of macrophage development at which the cells (progenitors versus differentiated monocytes/macrophages) are exposed.
Soluble factors produced by HSPCs exposed to a TLR2 agonist prior to macrophage development also act in a paracrine manner on unexposed HSPCs
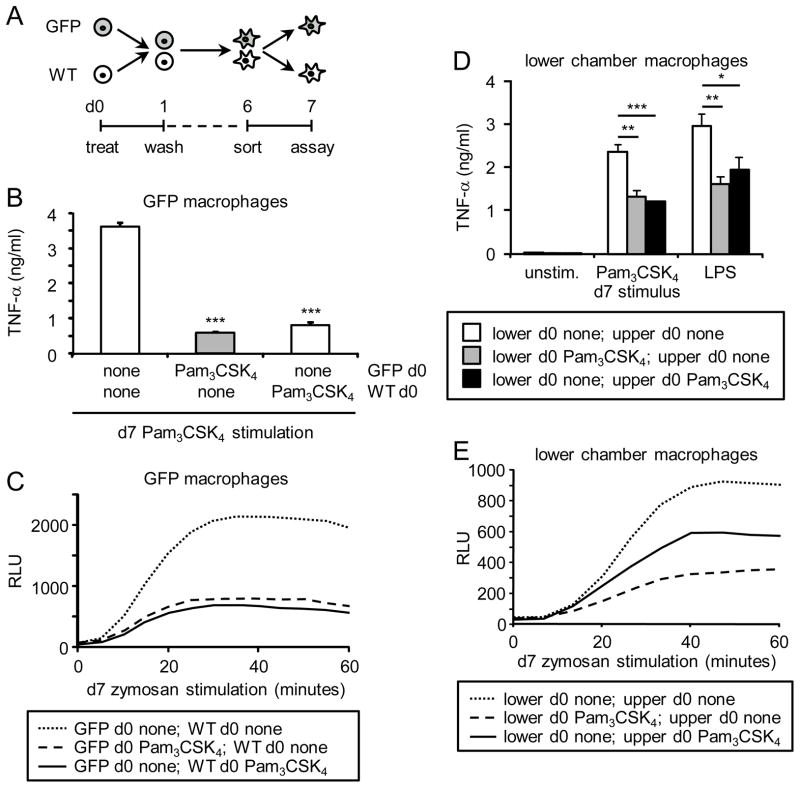
Finally, we wondered whether the effects of the TLR2 agonist on HSPCs are cell autonomous or can be conferred on unexposed HSPCs either via cell contact or by soluble factors. To address this, we used M-CSF to derive macrophages from unexposed GFP+ mouse HSPCs (Lin− cells) co-cultured with either unexposed GFP− HSPCs or d0 Pam3CSK4-exposed GFP− HSPCs (Figure 5A). We then sorted the adherent GFP+ macrophages and examined their ability to produce TNF-α and ROS. GFP+ macrophages derived in the presence of d0 Pam3CSK4-exposed GFP− cells produced reduced TNF-α and ROS, compared to GFP+ macrophages derived in the presence of control d0 untreated GFP− cells (Figure 5B and C). The same effect was observed when macrophages were co-derived from untreated GFP− HSPCs in the presence of d0 Pam3CSK4-exposed GFP+ HSPCs (data not shown).
Figure 5. Responsiveness of macrophages derived from unexposed HSPCs derived in the presence of TLR2 agonist-exposed HSPCs.
(A–C) On day 0, Lin− cells from wild type mice (GFP−) and GFP transgenic mice (GFP+) were incubated separately with or without 100 ng/ml Pam3CSK4 as indicated for 24 h. The cells were washed thoroughly on day 1 to remove the Pam3CSK4 and then mixed and co-cultured for a further 5 days with M-CSF to derive macrophages. Adherent macrophages were harvested on day 6, and GFP+ cells were sorted by FACS. TNF-α production following stimulation with Pam3CSK4/LPS (100 ng/ml; 24 h) was assessed by ELISA (B), and ROS production in response to 100 μg/ml zymosan was assessed by luminol-ECL (C). (D–E) Day 0 untreated and Pam3CSK4-treated wild type Lin− cells were washed thoroughly on day 1 to remove the Pam3CSK4, and then cultured in the upper and lower chambers of 0.4 μm pore Transwell plates as indicated in the presence of M-CSF to derive macrophages. On day 6, adherent macrophages were harvested from the lower chamber and plated at equal numbers, and TNF-α (100 ng/ml Pam3CSK4/LPS, 24 h) and ROS production (100 μg/ml zymosan) was assessed on day 7. TNF-α data are presented as mean plus standard deviation of triplicate culture. * p<0.05, ** p<0.01, ***p<0.001 ROS datapoints are means of triplicate culture. Data are representative of at least 3 independent experiments.
We next performed Transwell assays to determine whether cell contact or soluble mediators are responsible for this effect. We found that when unexposed HSPCs were cultured with but physically separated from d0 Pam3CSK4-exposed HSPCs, the macrophages derived from the unexposed HSPCs still exhibited reduced TNF-α and ROS production (Figure 5D and E), indicating that soluble factors produced by the Pam3CSK4-exposed cells confer functional programming on unexposed cells at some point during their differentiation.
Thus TLR2 signaling in HSPCs induces the release of soluble mediators, which are likely responsible for the functional programming of HSPCs in an autocrine manner, and can additionally act in a paracrine manner to program unexposed HSPCs or their progeny during differentiation. Future studies will be required to identify the soluble factor(s) responsible for these effects and define at what stage of differentiation they influence the programming of macrophage function.
Discussion
Previous descriptions of cells derived from HSPCs by culture with microbial TLR agonists (“TLR-derived” cells) have shown that they express myeloid surface markers and exhibit myeloid functions (e.g. phagocytosis, cytokine production), but have not compared their function to myeloid cells derived using factors produced by the host. In this study we have shown that Pam3CSK4-derived macrophages are functionally quite distinct from M-CSF-derived macrophages. Interestingly, we also found that transient exposure of both mouse and human HSPCs to Pam3CSK4 prior to differentiation is sufficient to suppress the inflammatory cytokine response of macrophages subsequently derived from them using M-CSF. However, despite exhibiting reduced inflammatory responses to several microbial agonists, the phagocytic capacity of the macrophages was not affected, and they expressed normal or enhanced levels of CD11b and F4/80, which indicates that they are not simply immature or fully desensitized to subsequent stimulation. Rather, it seems that their specific behavior has been defined by the TLR2 signal the HSPCs received. Importantly, exposure to the TLR2 agonist prior to or during myelopoiesis did not have precisely the same effects on macrophage function as exposure after differentiation, which is widely described as “tolerance”; TLR2-tolerized macrophages produced more ROS, whereas macrophages derived from Pam3CSK4-exposed HSPCs produced lower levels of ROS.
Extensive profiling of macrophage gene induction will be important in future studies to fully characterize the macrophages derived from HSPCs exposed to the TLR2 agonists to determine whether for instance they resemble M1 versus M2 macrophages, or perhaps exhibit normal antimicrobial gene induction but suppressed transcription of inflammatory genes. Similarly, it will be important to assess their ability to combat infection, and whether or not these observations support the notion that TLR-induced myelopoiesis serves to boost anti-microbial immunity.
Nevertheless, these data demonstrate a novel mechanism whereby macrophage responses can be programmed by TLR signaling in HSPCs prior to and/or during differentiation. Future studies will be required to determine how long this effect persists, but since we observed effects on stem cells as well as lineage-restricted progenitors, the data also indicate that detection of microbes by HSPCs may alter macrophage function both during an infection and potentially for an extended period following microbial clearance. Interestingly, Esplin et al. reported that chronic LPS exposure results in a loss of HSC quiescence and myeloid skewing, effects also observed during normal ageing[20], and consistent with our observations, ageing is known to be associated with reduced inflammatory cytokine and reactive oxygen production by macrophages[21].
TLR activation in HSPCs could also alter the balance of inflammatory and anti-microbial responses in such a way that individuals are predisposed to or protected from subsequent infection or inflammatory disease. In fact, there is ample evidence that humans have altered susceptibility to specific secondary infections for an extended period following certain primary infections. For example, people infected with seasonal and pandemic influenza are significantly more susceptible to life-threatening bacterial pneumonia[22]. In mouse models, this susceptibility has been demonstrated to last weeks after clearance of the viral infection and has been associated with reduced macrophage function[23]. Conversely, prior BCG injection or infection with Candida albicans has been demonstrated to offer a degree of protection against subsequent infection with other microbes through mechanisms independent of adaptive immunity and recently attributed to macrophages[1–3]. Detection of microbial components by HSPCs may also contribute to these phenomena.
It will also be interesting to see whether activation of other pattern recognition receptors on HSPCs has the same effect as TLR2 ligation. Consistent with the current observations, we have previously shown that macrophages derived ex vivo from the bone marrow of mice exposed to ES-62, a TLR4 agonist secreted by a filarial nematode, produce lower levels of inflammatory cytokines than control macrophages[24]. ES-62 permits worm survival for long periods in an infected host by exerting immunosuppressive and immunomodulatory effects on a variety of cell types[25] and its active moiety, phosphorylcholine, is a TLR4 ligand[26, 27]. Although the ES-62 study examined the function of macrophages derived from unsorted total bone marrow cells (lineage positive as well as lineage negative cells), our unpublished studies indicate sorted HSPCs exposed to purified TLR4 agonists also produce macrophages that similarly exhibit reduced inflammatory responsiveness. Since TLR2 and TLR4 both signal via the adaptor MyD88, it is likely that their effects on HSPCs are MyD88-mediated and also extend to other TLRs (except perhaps TLR3, which does not signal via MyD88).
It is possible that signals triggered by other classes of pattern recognition receptors on HSPCs also affect the function of subsequently derived macrophages, although perhaps in different ways. This certainly seems to be the case with previous exposure of differentiated monocytes/macrophages to microbial components (e.g. TLR2 and TLR4 agonists suppress, while BCG, C. albicans and β-glucans enhance subsequent inflammatory cytokine responses) and may apply to HSPCs too.
Future studies will be required to define the mechanisms underlying the effects of exposure of HSPCs to TLR agonists on macrophage function. It is likely that epigenetic modifications at specific gene loci are at least partly responsible for the transmission of functional programming through multiple cell divisions and myeloid differentiation, especially in the absence of continued exposure to the TLR agonists. Medzhitov and colleagues reported that LPS tolerance of inflammatory gene induction in differentiated macrophages is associated with the selective loss of positive histone modifications (H4 acetylation and H3K4 trimethylation)[17]. Conversely, Netea and colleagues reported that the “training” effects of BCG and β-glucans in differentiated macrophages are associated with increased H3K4 trimethylation[2, 3]. It will be interesting to determine whether these or other mechanisms also operate in HSPCs to permit functional information to be propagated through cell division and myeloid development. Alterations in the induction of transcription factors that control myelopoiesis and myeloid cell function (e.g. c/EBP family members) may also contribute.
Finally, our data indicate that TLR2-programmed HSPCs can transmit programming to unexposed HSPCs via the release of soluble factors into the culture supernatant, indicating that soluble factors produced by the Pam3CSK4-exposed HSPCs act in both an autocrine and a paracrine manner to program the function of exposed HSPCs as well as unexposed HSPCs in the vicinity. Further investigation will be necessary to determine at what stage of myeloid development these mediators are released (i.e. when the cells are still at the progenitor stage versus at a later stage of differentiation along the myeloid lineage) and to identify the specific factors responsible. Cytokines such as IFN-γ, TNF-α and IL-6 have been shown to influence myeloid differentiation[4] and a recent report showed that type I IFN mediates TLR7-induced emergency myelopoiesis[28], so these cytokines might be good candidates.
In conclusion, our data show that although TLRs represent an attractive target for the stimulation of myeloid cell production by HSPCs, thorough exploration of the functional consequences is critical to determine both the role of microbial detection by HSPCs during/following infection and the potential utility of the exploitation of this as a therapeutic strategy.
Materials and Methods
Mouse and human cells
Wild type C57BL/6, TLR2 −/− and GFP transgenic mice were cared for in accordance with the regulations of the Cedars-Sinai Medical Center and University of Valencia Institutional Animal Care and Use Committees. Bone marrow was collected from mouse femurs and tibias. Collection of human cord blood samples was approved by the Cedars-Sinai Institutional Review Board and samples were obtained from patients who gave informed consent.
Antibodies
The following antibodies were used in this study: Mouse – CD11b (M1/70), CD48 (HM48-1), CD68 (FA-11), CD117/c-Kit (2B8), CD150/SLAM (TC15-12F12.2), CD284/TLR4 (MTS510) and F4/80 (BM8) (BioLegend); CD16/CD32 (93) and CD282/TLR2 (6C2) (eBioscience); CD34 (RAM34) (BD Biosciences); Dectin-1/CLEC7A (218820) (R&D Systems). Human – CD11b (ICRF44), CD11c (3.9), CD14 (HCD14), CD34 (581), CD62L (DREG-56), CD68 (Y1/82A), CD282 (TL2.1), CD284 (HTA125) (BioLegend).
HSPC sorting and culture with M-CSF and TLR agonists
Mouse HSPCs were isolated from bone marrow by a combination of magnetic and fluorescence sorting. Lineage marker-negative cells (Lin−) and lineage-marker positive cells (Lin+) were first separated using a MACS lineage cell depletion kit (containing antibodies against CD5, CD45R (B220), CD11b, Gr-1, 7–4 and Ter-119) and an autoMACS Separator (both from Miltenyi). Lin− cells were then further fractionated to separate LKS+ cells (HSCs and lineage uncommitted progenitors; Lin− c-Kit+ Sca-1+), LKS− cells (lineage committed progenitors; Lin− c-Kit+ Sca-1−), LT-HSC (long-term repopulating HSC; LKS+ CD48− CD150+), ST-HSC (short-term repopulating HSC; LKS+ CD48− CD150−), MPP (multipotent progenitors: LKS+ CD48+ CD150−), CMP (common myeloid progenitors; LKS− CD34+ FcγRlo) and GMP (granulocyte-monocyte progenitors; LKS− CD34+ FcγRhi) by fluorescence sorting using a FACS Aria cell sorter (BD Biosciences). Macrophages were derived from HSPCs by culture in RPMI supplemented with 10% FCS plus Pam3CSK4 at the indicated doses and/or 50 ng/ml rhM-CSF. Where indicated, mouse HSPC cultures also contained 20 ng/ml SCF and 100 ng/ml Flt3L for the first week of culture to support the survival of HSCs. Macrophages were harvested from Lin−, CMP and GMP cultures on day 6, from LKS+, LKS−, ST-HSC and MPP cultures on day 13 and LT-HSC cultures on day 15. All macrophages were plated with M-CSF and rested overnight prior to stimulation.
Human CD34+ cells were obtained from cord blood by a combination of Ficoll density gradient fractionation and MACS sorting for CD34+ cells (Miltenyi). Human macrophages were derived from CD34+ cord blood cells by culture in IMDM supplemented with 10% FCS and cytokines as follows: IL-3 (20 ng/ml), SCF (50 ng/ml), M-CSF (20 ng/ml), GM-CSF (20 ng/ml), Flt3L (20 ng/ml) and IL-6 (20 ng/ml) for 2 weeks, followed by M-CSF for an additional week to enrich for adherent macrophages.
Mouse and human HSPCs were cultured with TLR ligands as specifically described in the Figure Legends.
Methylcellulose assays
To evaluate their hematopoietic potential, 1 × 103 untreated or Pam3CSK4-treated mouse Lin− cells were plated in quadruplicate in MethoCult GF M3434 (StemCell Technologies) methylcellulose-based medium and incubated for 2 weeks, after which the colonies were counted on the basis of their morphological characteristics in accordance with the manufacturer’s instructions.
Flow cytometry
Expression of mouse and human macrophage markers (F4/80, CD11b, CD11c, CD14, CD68) and pattern recognition receptors that detect the stimuli used for functional analysis (TLR2, TLR4, Dectin-1) was assessed by flow cytometry using commercially available antibodies and an LSR Fortessa analyzer (BD Biosciences). Data analysis was performed using FlowJo software.
ELISA, ROS and phagocytosis assays
Cytokine production (50,000 macrophages/well in a 96-well plate) was assessed using commercially available ELISA kits (Biolegend, eBioScience) according to the manufacturers’ instructions. Phagocytic uptake of 20 μg/ml fluorescently labeled zymosan particles (250,000 macrophages/well in a 24-well plate) was assessed by flow cytometry. Macrophages (50,000/well in a 96-well plate) were primed overnight with 25 U/ml IFN-γ prior to assessment of ROS production by luminol-enhanced chemiluminescence as described previously[29].
In vivo exposure of HSPCs to Pam3CSK4
Wild type CD45.1 Lin− cells (approximately 1.5 × 106 cells in 100 μl PBS) were intravenously injected into each CD45.2 TLR2 −/− recipient mouse, along with total bone marrow cells (minus erythrocytes; 10 × 106 cells) from a CD45.2 TLR2 −/− donor[8]. Recipient mice were then injected i.v. with 10 μg rmM-CSF (Miltenyi) or 10 μg rmM-CSF plus 100 μg Pam3CSK4 once daily for three days. Mice were then sacrificed and their spleens were removed. As previously described[8], total spleen cells were obtained by collagenase D treatment and erythrocytes were lysed using ammonium chloride buffer. Following Fc receptor blocking, CD45.2+ cells were depleted by MACS sorting (Miltenyi Biotec), and the remaining cells were stained for CD45.1, CD11b and F4/80. Triple positive cells were sorted by FACS (MoFlo, Beckman Coulter), plated in media containing 50 ng/ml rmM-CSF and stimulated the following day for analysis of cytokine production.
Transwell Assays
Transwell assays were performed using 6-well plates with 0.4 μm pore-containing polycarbonate membranes from Corning. Day 0 untreated or day 0 Pam3CSK4-treated wild type HSPCs (Lin− cells) were thoroughly washed on day 1 to remove the Pam3CSK4 and then plated in the upper and lower chambers as indicated, and macrophages were derived using M-CSF for a further 5 days. Adherent macrophages were harvested from the lower chamber on day 6, plated at equal numbers, and rested overnight prior to stimulation to assess cytokine production.
Statistical Analysis
All data are representative of at least 3 independent experiments. Statistical significance was determined using paired t-tests.
Supplementary Material
Acknowledgments
The authors thank Drs. Xiao (Gregory) Shen and Seigo Hatada (Cedars-Sinai Medical Center) for assistance breeding GFP transgenic mice and helpful discussions.
This study was funded by a fellowship from the Ministerio de Educación y Ciencia, Spain (to AY), a grant from the Ministerio de Economia y Competitividad, Spain (SAF2010-18256; to MLG), a Scientist Development Grant from the American Heart Association (to HSG), and an R21 (AI082379; to HSG), an R01 (AI071116; to DMU) and a CTSI grant (UL1TR000124; to UCLA) from the NIH.
Abbreviations
- HSPC
hematopoietic stem and progenitor cells
Footnotes
Conflict of Interest
The authors have no conflicts of interest.
References
- 1.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintin J, Saeed S, Martens JH, Giamarellos-Bourboulis EJ, Ifrim DC, Logie C, Jacobs L, Jansen T, Kullberg BJ, Wijmenga C, Joosten LA, Xavier RJ, van der Meer JW, Stunnenberg HG, Netea MG. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sioud M, Floisand Y, Forfang L, Lund-Johansen F. Signaling through toll-like receptor 7/8 induces the differentiation of human bone marrow CD34+ progenitor cells along the myeloid lineage. J Mol Biol. 2006;364:945–954. doi: 10.1016/j.jmb.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 7.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, von Andrian UH. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megias J, Yanez A, Moriano S, O’Connor JE, Gozalbo D, Gil ML. Direct Toll Like Receptor-Mediated Stimulation of Hematopoietic Stem and Progenitor Cells Occurs in vivo and Promotes Differentiation Towards Macrophages. Stem Cells. 2012;30:1486–1495. doi: 10.1002/stem.1110. [DOI] [PubMed] [Google Scholar]
- 9.Sioud M, Floisand Y. TLR agonists induce the differentiation of human bone marrow CD34+ progenitors into CD11c+ CD80/86+ DC capable of inducing a Th1-type response. Eur J Immunol. 2007;37:2834–2846. doi: 10.1002/eji.200737112. [DOI] [PubMed] [Google Scholar]
- 10.Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanez A, Flores A, Murciano C, O’Connor JE, Gozalbo D, Gil ML. Signalling through TLR2/MyD88 induces differentiation of murine bone marrow stem and progenitor cells to functional phagocytes in response to Candida albicans. Cell Microbiol. 2010;12:114–128. doi: 10.1111/j.1462-5822.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 12.Yanez A, Megias J, O’Connor JE, Gozalbo D, Gil ML. Candida albicans induces selective development of macrophages and monocyte derived dendritic cells by a TLR2 dependent signalling. PLoS One. 2011;6:e24761. doi: 10.1371/journal.pone.0024761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanez A, Murciano C, O’Connor JE, Gozalbo D, Gil ML. Candida albicans triggers proliferation and differentiation of hematopoietic stem and progenitor cells by a MyD88-dependent signaling. Microbes Infect. 2009;11:531–535. doi: 10.1016/j.micinf.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Megias J, Maneu V, Salvador P, Gozalbo D, Gil ML. Candida albicans stimulates in vivo differentiation of haematopoietic stem and progenitor cells towards macrophages by a TLR2-dependent signalling. Cell Microbiol. doi: 10.1111/cmi.12104. in press. [DOI] [PubMed] [Google Scholar]
- 15.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Medvedev AE, Sabroe I, Hasday JD, Vogel SN. Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J Endotoxin Res. 2006;12:133–150. doi: 10.1179/096805106X102255. [DOI] [PubMed] [Google Scholar]
- 17.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 18.Pabst MJ, Johnston RB., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980;151:101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esplin BL, Shimazu T, Welner RS, Garrett KP, Nie L, Zhang Q, Humphrey MB, Yang Q, Borghesi LA, Kincade PW. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J Immunol. 2011;186:5367–5375. doi: 10.4049/jimmunol.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Curr Immunol Rev. 2011;7:104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goulding J, Snelgrove R, Saldana J, Didierlaurent A, Cavanagh M, Gwyer E, Wales J, Wissinger EL, Hussell T. Respiratory infections: do we ever recover? Proc Am Thorac Soc. 2007;4:618–625. doi: 10.1513/pats.200706-066TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodridge HS, Marshall FA, Wilson EH, Houston KM, Liew FY, Harnett MM, Harnett W. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113:491–498. doi: 10.1111/j.1365-2567.2004.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harnett W, Harnett MM. Immunomodulatory activity and therapeutic potential of the filarial nematode secreted product, ES-62. Adv Exp Med Biol. 2009;666:88–94. doi: 10.1007/978-1-4419-1601-3_7. [DOI] [PubMed] [Google Scholar]
- 26.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, Liew FY, Harnett W, Harnett MM. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–293. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 27.Goodridge HS, McGuiness S, Houston KM, Egan CA, Al-Riyami L, Alcocer MJ, Harnett MM, Harnett W. Phosphorylcholine mimics the effects of ES-62 on macrophages and dendritic cells. Parasite Immunol. 2007;29:127–137. doi: 10.1111/j.1365-3024.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- 28.Buechler MB, Teal TH, Elkon KB, Hamerman JA. Cutting Edge: Type I IFN Drives Emergency Myelopoiesis and Peripheral Myeloid Expansion during Chronic TLR7 Signaling. J Immunol. 2013;190:886–891. doi: 10.4049/jimmunol.1202739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.