Summary
TNF is a pleiotropic cytokine with intriguing biphasic pro-inflammatory and anti-inflammatory effects. Our previous studies demonstrated that TNF up-regulated FoxP3 expression and activated and expanded CD4+FoxP3+ regulatory T cells (Tregs) by utilizing TNFR2. Further, TNFR2-expressing Tregs exhibited maximal suppressive activity. In this study, we show that TNF, in concert with IL-2, preferentially up-regulated mRNA and surface expression of TNFR2, 4-1BB and OX40 on Tregs. Agonistic antibodies against 4-1BB and OX40 also induced the proliferation of suppressive Tregs. Thus, TNF amplifies its stimulatory effect on Tregs by inducing TNF receptor superfamily (TNFRSF) members. In addition, administration of neutralizing anti-TNF Ab blocked LPS-induced expansion of splenic Tregs and up-regulation of TNFR2, OX40 and 4-1BB receptors on Tregs in vivo, indicating that the expansion of Tregs expressing these co-stimulatory TNFRSF members in response to LPS is mediated by TNF. Taken together, our novel data indicate that TNF preferentially up-regulates TNFR2 on Tregs, and this is amplified by the stimulation of 4-1BB and OX40, resulting in the optimal activation of Tregs and augmented attenuation of excessive inflammatory responses.
Keywords: TNF, regulatory T cells, co-stimulation, immune regulation
Introduction
CD4+FoxP3+ regulatory T cells (Tregs) comprise only a minor fraction (~10%) of peripheral CD4 cells, but play a critical role in the establishment and maintenance of immunological tolerance to self antigens as well as to foreign antigens [1–2]. Certain cytokine receptors preferentially expressed by Tregs not only serve as surface markers for the identification of Tregs, but also promote the function of Tregs. CD25, the α chain of IL-2 receptor, is the prototype of such cytokine receptors [1–2]. Our previous studies indicate that TNFR2 is an important cytokine receptor preferentially expressed by the highly suppressive human and mouse Tregs [3–5].
TNFR2 is one of two receptors transducing the biological function of TNF, a pleiotropic cytokine which is a major participant in the initiation and orchestration of inflammation and immunity [6]. TNFR2 expression is restricted to certain T cell subpopulations [6], and acts as a co-stimulator for antigen-driven T cell responses [7]. We have found that TNF surprisingly was an activator of Tregs, resulting in their proliferative expansion, up-regulation of FoxP3 expression and increase of suppressive activity [3]. This data, albeit counterintuitive, is supported by the emerging evidence that TNF-TNFR2 interaction plays a critical role in the generation, expansion and function of human and mouse Tregs [8–12].
TNFR2 is constitutively expressed by human and mouse thymic Tregs [5, 13]. Normal human circulating Tregs expressed markedly higher levels of TNFR2 than CD4+FoxP3− effector T cells (Teffs) [4, 14–15]. Normally 30~40% of Tregs present in the peripheral lymphoid tissues of unstimulated Balb/c mice and C57BL/6 (B6) mice expressed a high level of TNFR2, while less than 10% of Teffs expressed a lower level of TNFR2 [3, 16]. Furthermore, TNFR2-expresssing Tregs exhibited the most potent suppressive activity, while TNFR2− Tregs even though CD25+ and FoxP3+ in normal C57BL/6 mice had only minimal or no suppressive activity [5, 16]. Intratumoral Tregs are maximally immunosuppressive, since the majority of tumor infiltrating Tregs were highly suppressive TNFR2+ cells [5, 16], and depletion of TNFR2+ Tregs was associated with tumor eradication after cyclophosphamide treatment [17]. When transferred to LPS-challenged recipient mice, Tregs from wild-type mice were able to inhibit inflammatory responses, while Tregs from TNFR2-deficient mice failed to do so [14]. In normal human peripheral blood (PB), TNFR2-expressing CD4+CD25+ cells comprised a high level of FoxP3+ cells and were functionally suppressive [4]. In malaria patients, proliferating TNFR2+ Tregs exhibited an enhanced suppressive activity [18]. These studies clearly demonstrate that TNFR2 not only serves as a marker, but also promotes Treg function.
We have investigated the effect of TNF on TNFR2 expression on Tregs. Since TNFR2 is a member of TNF receptor superfamily (TNFRSF) and other co-stimulatory TNFRSF members, such as 4-1BB [19] and OX40 [20] also have been reported to participate in Treg activity, we also investigated their response to TNF. We found that TNF preferentially up-regulates these TNFRSF on Tregs, which contribute to the optimal activation of Tregs and result in attenuation of excessive inflammatory responses.
Results
TNF, in the presence of IL-2, induces Tregs to express genes encoding co-stimulatory TNFRSF members
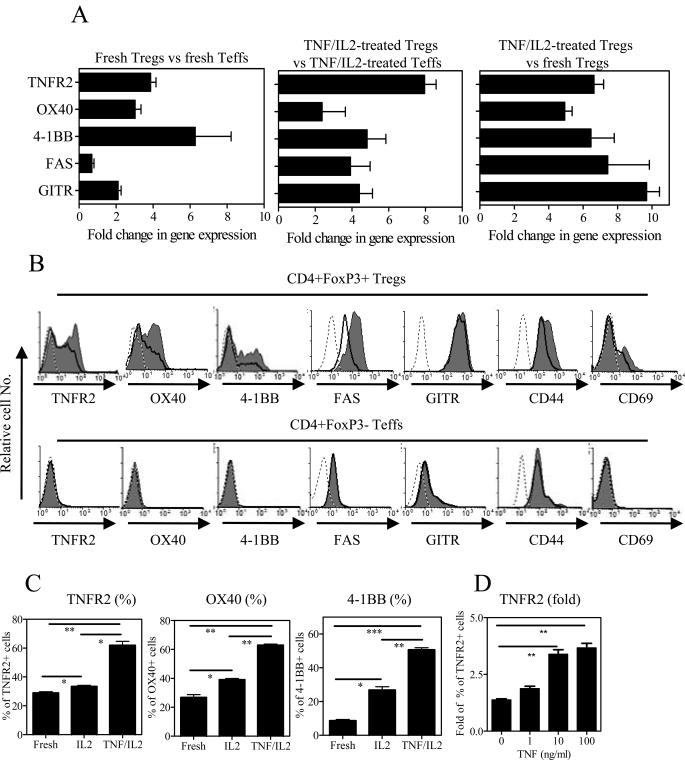
In order to test the effect of TNF on the expression of TNFR2 and other co-stimulatory TNFRSF members on Tregs, we performed gene profiling assay using the Mouse Tumor Necrosis Factor (TNF) Ligand and Receptor Signaling Pathways RT2 Profiler™ PCR Array (SABiosciences, Frederick, MD). This showed that, by comparison with freshly isolated Tregs or with TNF/IL-2-treated Teffs, Tregs treated with TNF/IL-2 for 12 hrs up-regulated their expression of genes encoding a number of TNFRSF members, including Tnfrsf1b (TNFR2), Tnfrsf4 (OX40), Tnfrsf6 (FAS), Tnfrsf9 (4-1BB), and Tnfrsf18 (GITR), by greater than 2-fold (data not shown). Our results are in agreement with a recent microarray study in human Tregs [15]. We next performed real time PCR assay to verify their changes in gene expression. As shown in Fig 1A, the expression of mRNA for TNFR2, OX40, 4-1BB and GITR was two-fold higher in freshly isolated Tregs than freshly isolated Teffs (left). After treatment with TNF/IL-2, the expression of mRNA for these TNFRSF members and FAS was at least two-fold higher in Tregs than in Teffs (middle). Treatment with TNF/IL-2 further up-regulated their mRNA expressions greater than 4-fold in Tregs, as compared with freshly isolated Tregs (right). Thus, in the presence of IL-2, TNF up-regulated the gene expression of TNFR2 and other co-stimulatory TNFRSF members in Tregs.
Figure 1.
Up-regulation of genes and surface expression of co-stimulatory TNFRSF members on Tregs by TNF. (A) Flow-sorted CD4+FoxP3/gfp+ Tregs and CD4+FoxP3/gfp− Teffs were cultured with TNF and IL-2 for 12 hours. RNA from freshly purified cells and TNF/IL-2-treated cells was isolated and gene expression of TNFRSF members was analyzed by real time PCR. Fold changes in gene expression between two indicated groups are shown. The data (means ± SEM, n=4) are summarized from four separate experiments with similar results. (B–D) CD4 cells were incubated with IL-2 or TNF/TNF for 3 days. The surface markers were analyzed with FACS by gating on FoxP3+ Tregs or FoxP3− Teffs. (B) Typical results of FACS analysis. Grey: TNF/IL-2; solid line: IL-2; dashed line: isotype control. (C) Summary of the percentage of TNFR2+, OX40+, and 4-1BB+ cells in Tregs. (D) In the presence of consistent concentration of IL-2, TNF up-regulated TNFR2 expression on FoxP3+ Tregs in a dose-dependent manner. Data shown are fold change in percentage of TNFR2+ Tregs over that of freshly isolated Tregs. Comparison of two indicated groups: *p<0.05, ** p<0.01, *** p<0.001. Data shown in C–D (means ± SEM, n=3) are representative of at least three separate experiments with similar results.
TNF, in concert with IL-2, up-regulates surface expression of co-stimulatory TNFRSF members on Tregs
Treatment with TNF/IL-2 for three days preferentially up-regulated the surface expression of TNFR2, OX40, 4-1BB and FAS on Tregs, but not on Teffs (Fig 1B). TNFR2, OX40 and 4-1BB expressed on IL-2/TNF-treated Tregs were increased by 2.1±0.2, 2.4±0.2 and 6.0±0.7 fold, respectively, over their expression on freshly isolated Tregs (p<0.05~0.001, Fig 1C). IL-2 alone also increased their surface expression (p<0.05), however, addition of TNF further increased their expression by up to ~2-fold over IL-2 alone (p<0.05~0.01, Fig 1C). TNF-induced up-regulation in the case of TNFR2 was dose-dependent (Fig 1D). TNF was also able to up-regulate surface expression of TNFR2, OX40 and 4-1BB on FACS-purified CD4+FoxP3/gfp+ Tregs (data not shown), indicating that TNF directly acts on Tregs.
The increased expression of these co-stimulatory TNFRSF members has been reported to be a consequence of the activation of CD4 cells [21]. Indeed, IL-2/TNF treatment markedly and preferentially enhanced the expression of the activation markers, CD44 and CD69, on Tregs (Fig 1B). Therefore, IL-2/TNF led to greater activation of Tregs.
TNF/IL-2 induces TNFR2 expression on TNFR2− Tregs
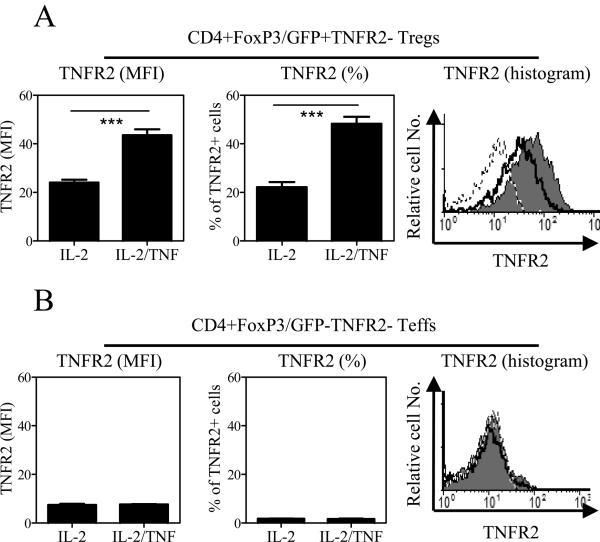
It is possible that TNF, in addition to expanding TNFR2+ Tregs, also converts TNFR2− Tregs into TNFR2+ Tregs. To test this, flow-sorted CD4+FoxP3/gfp+TNFR2− cells and CD4+FoxP3/gfp−TNFR2− cells were treated with IL-2 or TNF/IL-2. As shown in Fig 2A, IL-2 alone induced the expression of TNFR2 on FoxP3/gfp+TNFR2− Tregs. Presumably based on the initial induction of TNFR2 by IL-2, TNF further amplifies the expression levels of TNFR2 on FoxP3/gfp+TNFR2− Tregs (p<0.001). In contrast, neither IL-2 nor TNF/IL-2 was able to induce TNFR2 expression on FoxP3/gfp−TNFR2− Teffs (Fig 2B). Thus, TNF does have the capacity to induce nonfunctional TNFR2− Tregs into functional TNFR2+ Tregs.
Figure 2.
Induction of the expression of TNFR2 by IL-2 and TNF/IL-2 on FoxP3+TNFR2− Tregs. CD4+FoxP3/gfp+TNFR2− cells and CD4+FoxP3/gfp−TNFR2− cells were flow-sorted. The cells were cultured with IL-2 or TNF/IL-2 for 3 days. The surface expression of TNFR2 was determined with FACS. Data (means ± SEM, n=3) in the left panel show the MFI of TNFR2 expression, and in the middle panel show the percentage of TNFR2+ cells. The comparison of IL-2 and TNF/IL-2: *** p<0.001. Data in the right panel show results of a representative FACS analysis. Grey: TNF/IL-2; solid line: IL-2 alone; dashed line: isotype control. Data shown are representative of three separate experiments with similar results.
IL-2-independent effect of TNF on the up-regulation of FoxP3 and TNFR2 expression by Tregs
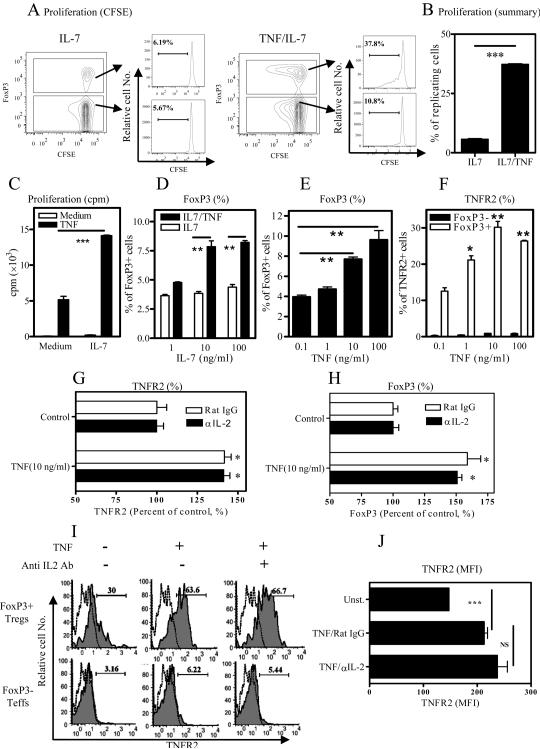
Treatment with TNF/IL-2 was previously shown to up-regulate the expression of CD25 on Tregs[3]. Thus the activating effects of TNF/IL-2 on Tregs and their stimulation of TNFR2 expression may depend entirely on the enhanced interaction of IL-2 with CD25. To test this hypothesis, we examined the effect of the combination of TNF and IL-7, another cytokine that uses common gamma chain and maintains the survival of Tregs in vitro [22]. Only 6% of Tregs, and approximately same proportion of Teffs, were induced to proliferate when CD4 cells were cultured with IL-7 alone (Fig 3A left panels). In the presence of IL-7, TNF stimulation resulted in 37.8% replicating cells in the FoxP3+ subset. In contrast, TNF treatment resulted in replication of only 10.8% of FoxP3− cells replicating (Fig 3A right panels). Thus, IL-7 also enabled TNF to preferentially stimulate the proliferation of Tregs (p<0.001, Fig 3B). We also investigated the effect of IL-7 with or without TNF on the proliferative responses of flow-sorted CD4+FoxP3/gfp+ Tregs to TCR stimulation. As shown in Fig 3C, although IL-7 by itself only had minimal effect, a combination of TNF and IL-7 synergistically promoted the proliferation of Tregs.
Figure 3.
Stimulation of Tregs in the presence of IL-7 by TNF. (A–B) CD4 cells were labeled with CFSE and cultured with IL-7, in the absence or presence of TNF. After 3 days, the cell replication, as shown by dilution of CFSE, on Tregs and Teffs was analyzed with FACS, by gating on FoxP3+ or FoxP3− cells respectively. Representative data from at least three separate experiments with similar results are shown in (A). The summary of replication of Tregs is shown in (B). (C) Flow-sorted CD4+FoxP3/gfp+ T cells were stimulated with APCs and anti-CD3, in the presence of medium, or IL-7, or TNF, or TNF/IL-7. After 72-hour incubation, cell proliferation was determined by [3]H thymidine incorporation assay. (D–E) CD4 cells were cultured with increasing concentrations of IL-7 alone or with consistent concentration of TNF (D), or cultured with consistent concentration of IL-7 and increasing concentrations of TNF (E), for 3 days. The proportion of FoxP3+ cells was analyzed by FACS. Open bar: IL-7 alone; black bar: TNF/IL-7 (D). (B–E): ** p<0.01, *** p<0.0001, as compared with indicated group. (F) CD4 cells were cultured same as (D). The proportion of TNFR2+ cells was analyzed with FACS by gating on FoxP3+ cells or FoxP3− cells. Black bar: FoxP3− cells; open bar: FoxP3+ cells. *p<0.05; ** p<0.01, as compared with lowest dose of TNF (0.1 ng/ml) (G–H) CD4 cells were cultured with IL-7 plus 0.1 ng/ml of TNF as control or 10 ng/ml of TNF, with Rat IgG (open bar) or neutralizing anti-IL-2 Ab (black bar). The proportion of TNFR2 (G) or FoxP3 (H) expression on Tregs was analyzed with FACS by gating on FoxP3+ cells. Data shown are percentage of control (%). Comparison with respective control, * p<0.05. (I–J) CD4 cells were cultured with TNF alone, with or without neutralizing anti-IL-2 Ab, for 24 hours. The expression of TNFR2 was analyzed with FACS, by gating on FoxP3+ cells or FoxP3− cells. A representative FACS analysis was shown in (I). Solid line: TNFR2; dashed line: isotype. Number in the histogram indicates percentage of TNFR2+ cells (%). MFI of TNFR2 expression on FoxP3+ cells was shown in (J). Comparison of indicated two groups, *** p<0.001. NS: no statistical significance. Data in (B–H, J) are means ± SEM (N=3). Data shown are representatives of at least three separate experiments with similar results.
Next, we examined the effects of TNF/IL-7 on the expression of FoxP3 and TNFR2 on Tregs. As shown in Fig 3D, after 3-day treatment with IL-7 alone, the proportion of FoxP3+ Tregs present in CD4 cells was only ~4%, which was lower than that in freshly isolated CD4 cells (~10%) or CD4 cells cultured with IL-2 (10 ng/ml, >10%) for 3 days. Even at the higher molar concentration of IL-7 was not as effective as IL-2 in the maintenance of survival of Tregs. Nevertheless, TNF in conjunction with IL-7 was able to increase the proportion of FoxP3+ cells (Fig 3D), in a dose-dependent manner (Fig 3E). Furthermore, in the presence of IL-7, TNF increased the proportion of TNFR2+ cells in the FoxP3+ subset, but not in FoxP3− cells (Fig 3F), indicating that IL-7 could also promulgate the Treg-activating effect of TNF. In order to eliminate a possible effect of IL-2 released by activated FoxP3− Teffs present in the unfractionated CD4 cells, neutralizing anti-IL-2 Ab was used. As shown in Fig 3G–H, in the presence of as high as 10 μg/ml of neutralizing anti-IL-2 Ab, TNF/IL-7 still up-regulated TNFR2 expression on Tregs and expanded FoxP3+ cells (p<0.05). Furthermore, treatment with TNF alone for 24 hrs also resulted in an increase of TNFR2 expression on Tregs, which was not blocked by the neutralizing anti-IL-2 Ab (Fig 3I–J). Thus, the effect of TNF on the proliferation of Tregs and up-regulation of TNFR2 on Tregs can occur independently of IL-2.
Effects of Activation of 4-1BB and OX40 on Tregs
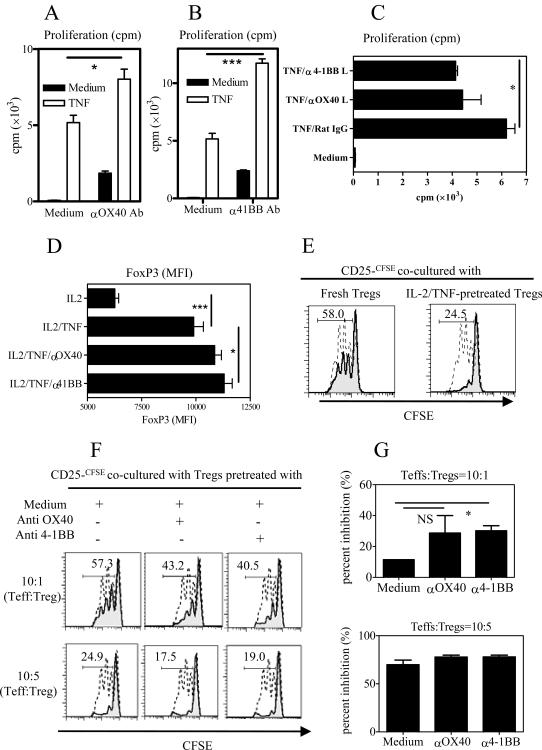
Next we examined whether 4-1BB and OX40 induced on Tregs by TNF were functional. As shown in Fig 4A–B, both agonistic anti-4-1BB and anti-OX40 Abs were able to partially overcome the anergic status of Tregs and induced proliferation of Tregs. Furthermore, the combination of TNF and anti-4-1BB Ab or anti-OX40 Ab synergistically stimulated the proliferation of Tregs (p<0.05~0.001. Fig 4A–B). In contrast, isotype control IgGs did not have any effect (data not shown). CD4-depleted splenocytes were used as APCs in this study and they expressed OX40 L and 4-1BB L (data not shown). We therefore examined the effect of blockade of OX40 L and 4-1BB L on the proliferation of Tregs. As shown in Fig 4C, TNF-induced the proliferative responses of CD4+FoxP3/gfp+ Tregs to APCs stimulation was partially abrogated by blocking antibodies to OX40 L and to a greater extent by anti-4-1BB L Ab (p<0.05). Thus, TNF-induced up-regulation of 4-1BB and OX40 appeared to augment the stimulatory signaling of agonistic Abs or their cognate ligands. In order to examine the effect of OX40 and 4-1BB activation on FoxP3 expression, CD4+FoxP3/gfp+ Treg cells were cultured in vitro with IL-2, or TNF/IL-2 with or without agonistic Abs for OX40 or 4-1BB. After 3-day culture, the levels of FoxP3 expression on a per cell basis (MFI) on Tregs was increased by ~2-fold after TNF/IL-2 treatment, as compared with IL-2 treatment alone (p<0.001, Fig 4D). Importantly, the TNF/IL-2-induced enhancement of FoxP3 expression in Tregs was preserved and even modestly increased by treatment with the 4-1BB agonistic Ab (p<0.05, Fig 4D). However, in our experimental system, the agonistic Abs for OX40 and 4-1BB did not further enhance TNFR2 expression on Tregs (data not shown), suggesting that the effect of TNF on the up-regulation of co-stimulatory TNFRSFs was unidirectional.
Figure 4.
Activation of 4-1BB and OX40 expands potent suppressive Tregs. (A–B) Flow-sorted CD4+FoxP3/gfp+ Tregs were stimulated with APCs and anti-CD3, with or without TNF, or with agonistic anti-OX40 Ab, A) or with agonistic anti-4-1BB Ab (B). After 72-hr incubation, proliferation was determined by [3]H thymidine incorporation assay. (C) CD4+FoxP3/gfp+ Tregs were stimulated with APCs and anti-CD3, with medium, or with TNF, or TNF plus blocking anti-OX40 L Ab or anti-4-1BB L Ab. After 72-hr incubation, proliferation was determined by [3]H thymidine incorporation assay. (C) Flow-sorted CD4+FoxP3/gfp+ Tregs were cultured with IL-2 with or without TNF, or TNF plus agonistic anti-OX40 Ab, or anti-4-1BB Ab. After 3 days, MFI of FoxP3 expression was analyzed with FACS. Data (means ± SEM, n=3) shown in (A~D) are representative of three experiments with similar results. The comparison of two indicated groups, * p<0.05; *** p<0.001. (E) Flow-sorted CD4+CD25+ Tregs were treated with medium which contained TNF/IL-2 for 3 days. After washing, pre-treated Tregs or freshly FACS-purified CD4+CD25+ Tregs were added into the freshly flow-sorted, CFSE-labeled CD4+CD25− T cells at 10:5 ration (Teff:Treg). (F) Flow-sorted CD4+CD25+ Tregs were treated with medium which contained TNF/IL-2, with or without agonistic anti-4-1BB Ab or anti-OX40 Ab for 3 days. After washing, pre-treated Tregs were added into the freshly flow-sorted, CFSE-labeled CD4+CD25− Teffs at 10:1 and 10:5 ration (Teff:Treg). The cells were stimulated with APCs and anti-CD3 Ab. After 48-hr incubation, the proliferation was measured by CFSE dilution on Teffs with FACS. Dashed histogram: CFSE expression profile of Teffs alone; grey histogram: CFSE expression profile of Teffs co-cultured with Tregs. The number in the histogram stands for the proportion of CFSE-diluted cells in the presence of Tregs (%). Data shown are representatives of three separate experiments with similar results. (G) The summary of percent inhibition exerted by Tregs pretreated with medium alone (with TNF/IL-2) or with medium plus agonistic anti-OX40 Ab or anti-4-1BB Ab. Data shown (means ± SEM, n=6) were summarized from two separate experiments with similar results. Comparison with indicated two groups: *p<0.05. N.S., no statistical significance.
Next, the suppressive capability of Tregs expanded by the combination of TNF and anti-4-1BB Ab or anti-OX40 Ab was investigated. Consistent with our previous report [3], the suppressive activity of Tregs pre-treated with TNF/IL-2 on the proliferation by Teffs was markedly enhanced (Fig 6E). Moreover, Tregs pre-treated with TNF/IL-2 in combination with anti-4-1BB Ab or anti-OX40 Ab retained and in the case of anti-4-1BB Ab could enhance their potent suppressive potential, as compared with Tregs pre-treated with TNF/IL-2 (Medium) alone (p<0.05, Fig 4F–G). Our data therefore indicate that up-regulation of 4-1BB and OX40 by TNF/IL-2 on Tregs could further promote their proliferation, while preserving or even enhancing their potent suppressive activity.
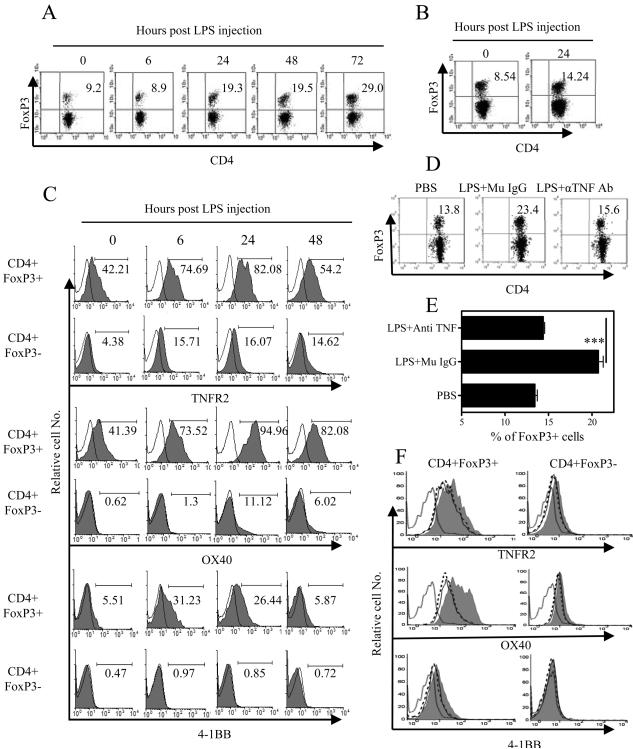
Neutralization of TNF blocks in vivo expansion of splenic Tregs after LPS challenge
It has been reported that LPS was able to activate and expand Tregs by interacting with TLR4 expressed on their surface [23]. Since LPS is a potent inducer of TNF[24], we hypothesized that TNF produced in response to LPS challenge may also contribute to the LPS-induced expansion of Tregs. The results showed that, in vivo injection of LPS resulted in ~2-fold and >3-fold increase in the proportion of FoxP3+ cells in the splenic CD4+ subsets by 24 and 72 hrs after injection, respectively (Fig 5A). Similarly, the proportion of FoxP3+ cells present in the draining mesenteric LN CD4 subset following intraperitoneal LPS injection was also increased from 8.54% in control mice to 14.24% (Fig 5B). The expansion of Tregs in CD4+ subset persisted until day 5 (data not shown). Moreover, the surface expression levels of TNFR2, 4-1BB and OX40 was markedly preferentially increased by 6 hrs on Tregs (Fig 5C). The up-regulation of these TNFRSF members on Tregs was transient, with a peak expression at 24 hrs for both TNFR2 and OX40, and 6 hrs for 4-1BB, respectively (Fig 5C). Thus, our data shows that in vivo administration of LPS also results in the activation and proliferation of Tregs. In order to confirm the role of TNF in the expansion of splenic Tregs, a neutralizing Ab against mouse TNF was injected 24 hrs and 1 hr before LPS challenge. The results showed that the neutralization of TNF markedly blocked the expansion of splenic Tregs (p<0.001, Fig 5D, E). Furthermore, expression of TNFR2, OX40 and 4-1BB on the splenic Tregs was also down-regulated by anti-TNF treatment (Fig 5F). Thus, TNF and TNFRSF contribute to the in vivo expansion of Tregs after LPS challenge.
Figure 5.
Neutralizing anti-TNF Ab blocked LPS-induced the expansion of Tregs and up-regulation of TNFRSFs on Tregs. Normal B6 mice were injected with 200 μg of LPS (i.p.) or PBS. Mouse spleens and mesenteric LNs were harvested at indicated time after injection. The proportion of FoxP3+ cells present in the spleen (A) or mesenteric LNs (B) was analyzed with FACS, by gating on CD4+ T cells. (C) Expression of TNFR2, OX40 and 4-1BB on Tregs or Teffs present in the spleen was analyzed with FACS, by gating on CD4+FoxP3+ cells or CD4+FoxP3− cells. (D~F) Mice were i.p. injected with 200 μg of neutralizing anti-mouse TNF Ab (5E5) or control mouse IgG1 24 hrs and 1 hr before challenge of 200 μg of LPS or PBS (i.p.). On day 5 after LPS injection, the proportion of FoxP3+ cells was analyzed with FACS, by gating on CD4+ T cells. (D) Typical dot plot of FACS analysis. (E) The summary of percentage of FoxP3+ cells in splenic CD4 subset (means ± SEM, n=5). Comparison of indicated two groups, *** p<0.001. (F) Expression of TNFR2, OX40 (24 hrs after LPS injection) and 4-1BB (6 hrs after LPS injection) was analyzed with FACS, by gating on FoxP3+ and FoxP3− CD4 cells. Grey: LPS treatment; solid line: LPS and anti-TNF Ab treatment; dashed line, PBS control; dotted line: isotype control. The number in the dot plot indicates the percentage of cells in the respective quadrants (%). The number in the histogram indicates the percentage of positive cells (%). Data shown are representative of three separate experiments with similar results.
Discussion
In this study, we for the first time report that TNF, in the presence of common gamma chain interleukins, had the capacity to up-regulate the expression of a number of co-stimulatory TNFRSF members, including its own receptor, TNFR2, as well as 4-1BB and OX40, preferentially on Tregs. This provides a means of amplifying Treg numbers to optimally attenuate the harmful excessive inflammatory responses.
TNF is not sufficient to support the in vitro survival of Tregs and thus either IL-2 or IL-7 was used. TNF and IL-2 up-regulate both TNFR2 and CD25 on Tregs, resulting in a reciprocal-amplification loop in the activation of Tregs. Although Tregs express low levels of IL-7 receptor α chain (CD127) which could not be up-regulated by TNF (data not shown), IL-7 and TNF nevertheless synergistically promoted the proliferative response of Tregs to TCR stimulation. In addition, TNF, in combination with IL-15, also activated Tregs (data not shown). The relative potency in support of Treg-activating effect of TNF were IL-2>IL-7>IL-15. Further, the effect of TNF/IL-7 or TNF alone on Tregs was not blocked by neutralizing anti-IL-2 Abs. Thus, the activating effects of both TNF and TNF/IL-7 on Tregs were not mediated by IL-2. The synergistic effects of TNF with other Cγ chain cytokines and TCR stimulation also likely contribute to the expansion and activation of Tregs at inflammatory site. We favor the idea that TNF-TNFR2 signaling pathway plays an important role in the activation of Tregs. A greater understanding of these fundamental mechanisms is needed for the discovery of novel approach to up- or down-regulate Treg activity at signal transduction and molecular levels.
4-1BB and OX40 are members of TNFRSF whose genes are clustered on mouse chromosome 4 together with TNFR2 [25]. These molecules have some activities in common, such as regulating the expression of anti-apoptotic members of Bcl-2 family, promoting proliferation and survival of CD4 cells [21]. The effects of these two molecules, especially of OX40, on the function of Tregs remain controversial. It has been reported that the anti-tumor effect of OX86, an agonistic antibody for OX40, was associated with attenuation of the suppressive function of Tregs [26]. However, when used together with cyclophosphamide, OX86 actually induced the overactivation of tumor infiltrating Tregs, leading to selective apoptosis and eventual depletion of Tregs [27]. It has been proposed that, if the “cytokine milieu is right”, OX40 agonist could promote Treg activity [20]. Our data presented in this study indicate that TNF appear to be the “right cytokine” to provide activating effect of OX40 signaling on Tregs. Actually, OX40 signaling contributes to TNF-induced proliferative response of Tregs to APCs, since Treg proliferation was promoted by agonistic anti-OX40 Ab and partially abrogated by antagonistic anti-OX40 Ab (Fig 4A, C). This confirms a recent report of the contribution of OX40-OX40 ligand interaction to APC(DC)-mediated proliferation of Tregs [28]. The physiological relevance of our findings is supported by the emerging evidence showing the crucial role of OX40 in the expansion, accumulation and function of Tregs in control of TNF-enriched inflammation, such as EAE [20] and colitis [29–30]. In fact, the stimulatory effects of OX40 and 4-1BB on Tregs have been harnessed in protocols aimed at expanding Tregs for therapeutic purposes [19, 31] Thus, in addition to their known co-stimulatory effects on Teffs [21], OX40 and 4-1BB are also potent activators of Tregs.
Nagar and colleagues recently reported that stimulation with TNF up-regulated the transcription and surface expression of OX40 and 4-1BB in human Treg cells [15]. However, they concluded that TNF decreased the suppressive activity of Tregs, based on their evidence that TNF stimulated the proliferation and cytokine production in co-cultures of Tregs and Teffs [15]. Rather than decreasing Treg activity, their results can be attributed to the capacity of TNF to enhance the response of Teffs to TCR stimulation. Indeed, we have reported that TNF stimulated the activation of Teffs, which acquire the capacity to proliferate in spite of presence of Tregs in the early stage of co-culturing [3]. Furthermore, TCR-activated mouse Teffs up-regulated their TNFR2 expression and become relatively resistant to suppression by Tregs [16]. However, rather than impairing function of Tregs, TNF actually preferentially activated and expanded Tregs and eventually restored the suppression of co-cultures of mouse Tregs and Teffs [3]. This viewpoint is favored by their data showing that the levels of TNF-induced INFγ in their Treg-Teff co-cultures paralleled the levels in unstimulated co-cultures [15], indicating that the degree of suppression by Tregs was not diminished by TNF. Nevertheless, we do not exclude the possibility that differences in species, experimental methods and time frame of observation may also contribute to the discrepancy between our data ([3] and this study) and Nagar et al's data [15] regarding the impact of TNF on the inhibition of proliferation in co-cultures.
The evidence that inflammatory responses can actually drive the proliferative expansion as well as enhancing the suppressive activity of Tregs is compelling, and is compatible with our conclusion that the interaction of TNF and TNFR2 promote both proliferation and suppressive activities of Tregs [32]. Although counterintuitive and contradictory to most previous reports, our finding that TNF has the capacity to activate and expand Tregs has been supported by more recent studies. For example, TNF-TNFR2 interaction has been shown to be crucial for the generation and function of human CD4+ as well as CD8+ Tregs [8–9], as well as to promote survival of human Tregs in an inflammatory environment by inducing anti-oxidative thioredoxin-1 [11]. Interestingly, pathogenic Teff cell-derived TNF had the capacity to boost Treg activity in vivo and consequently suppressed autoimmunity in a mouse model [12].
Overall, our data indicate that, in concert with a common gamma chain cytokine (IL-2, IL-7 or IL-15), TNF preferentially up-regulates the expression of co-stimulatory members of TNFRSF such as TNFR2, 4-1BB and OX40 on Tregs, resulting in a positive feedback amplification of the stimulatory effect of TNF on Tregs. Thus, TNF enhances multiple TNFRSF pathways by up-regulating a number of receptors that can cooperate to curtail excessive inflammation and prevent self destructive tissue damage.
Materials and Method
Mice and reagents
Female wild type (wt) C57BL/6 mice were provided by the Animal Production Area of the National Cancer Institute (Frederick, MD). NCI-Frederick is accredited by American Association for the Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the Procedures outlined in the “Guide for Care and Use of Laboratory Animals” published by the National Research Council (National Research Council, National Academy of Sciences. (1996) Guide for Care and Use of Laboratory Animals. National Academy Press, Washington D.C. FoxP3/gfp KI mice were kindly provided by Dr. Yasmine Belkaid at Laboratory of Parasitic Diseases, NIAID, NIH, and maintained in the Animal Production Area of the NCI-Frederick.
Antibodies purchased from BD Pharmingen (San Diego, CA) consisted of PerCP anti-mouse CD3 (145-2C11), PE and APC and Pacific blue anti-mouse CD4 (RM4–5), FITC anti-mouse CD44 (IM7), PE anti-mouse CD120b/TNFR2 (TR75–89) and FITC anti-mouse CD90/FAS (Jo2). FITC and PerCP Cy5.5 anti-mouse CD4 (L3T4), FITC anti-mouse CD69 (H1.2F3), FITC anti-mouse GITR (DTA-1), PE anti-mouse CD134/OX40 (OX-86), PE anti-mouse CD137/4-1BB (17B5), PE and APC and eFuor 450 anti-mouse/rat FoxP3 staining set (FJK-16s), and functional grade purified anti-mouse IL-2 (JES6-1A12), CD137 (17B5) and CD134 (OX-86) were purchased from eBioscience (San Diego, CA). LEAF™ purified anti-mouse CD252 (OX40 ligand, RM134L) and LEAF™ purified anti-mouse CD137 ligand (4-1BB ligand, TKS-1) was purchased from Biolegend (San Diego, CA). Alexa 647 anti-mouse CD120b/TNFR2 (TR75–89) was purchased from Serotec (Raleigh, NC). Murine IL-2, IL-7 and TNF were purchased from PeproTech (Rocky Hill, NJ). A neutralizing anti-mouse TNF Ab (5E5) and murine IgG1 were generously provided by Drs. Teresa Born and John E. Sims (Amgen Inc., Seattle, WA).
Cell purification
Mouse lymphocytes were harvested from mouse spleens, axillary lymph nodes, inguinal lymph nodes and mesenteric lymph nodes. CD4+ cells were purified from lymphocytes with mouse CD4 (L3T4) MicroBeads and LS column (Miltenyi Biotec, Auburn, CA). CD4 subsets were purified using Cytomation MoFlo cytometer (Fort Collins, CO), yielding a purity of ~98% for each subsets. T-depleted spleen cells were used as APCs and were prepared by depletion of CD90+ cells with anti-mouse CD90 MicroBeads and LD column (Miltenyi Biotec). APCs were irradiated with 3,000 R.
In vitro cell culture and Treg function assay
In order to examine surface expression of TNFRSFs, CD4+ cells were cultured at 105 cells/well in a 96-well plate with medium [3] alone or IL-2 or IL-7 with or without TNF, or with neutralizing anti-IL-2 Ab, for desired time. Unless otherwise specified, the concentration of cytokines used in vitro cultures was 10 ng/ml, and the concentration of antibodies was 10 μg/ml. The surface expression of TNFRSFs and other markers on Tregs or Teffs was analyzed with FACS, by gating on FoxP3+ or FoxP3− cells. In some experiments, flow-sorted CD4+FoxP3/gfp+TNFR2− cells or CD4+FoxP3/gfp−TNFR2− cells from FoxP3/gfp KI mouse spleen and LNs were treated with IL-2 or IL-2 plus TNF. After 72-hr incubation, surface expression of TNFR2 was determined with FACS.
In some experiments, Flow-sorted CD4+FoxP3/gfp+ Tregs (2~5×104 cells/well) were cultured in a U-bottom 96-well plate with IL-7 or with IL-2, with or without TNF, or with agonistic Abs for OX40 or 4-1BB, or with antagonistic Abs for OX40 L or 4-1BB L. The cells were stimulated with 2×105 APCs/well plus 0.5 μg/ml of soluble anti-CD3 Ab. Cells were pulsed with 1 μCi [3H]thymidine (Perkin Elmer Life Sciences, Boston, MA) per well for the last 6 hr of the culture period.
In order to determine Treg function, CFSE-labelled responder Teffs (5×104 cells/well) were seeded in a U-bottom 96-well plate together with 2×105 cells/well of APCs and 0.5 μg/ml of anti-CD3 antibody. Flow-purified CD4+CD25+ cells were added to the wells at the desired ratio. After 48 hours, CFSE dilution was determined with FACS. In some experiments, flow-sorted Tregs were treated with TNF/IL-2, with or without agonistic anti-4-1BB Ab or agonist anti-OX40 Ab, for 72 hours. After thoroughly washing, pretreated Tregs were co-cultured with freshly isolated Teffs at the desired ratio to observe their suppressive potential.
In vivo administration of LPS and anti-mouse TNF Ab
Normal C57BL/6 mice were injected intraperitoneally with 200 μg of LPS (Sigma-Aldrich, St. Louis, MO, Cat#: L9764) in PBS. In some experiments, mice were injected (i.p.) with 200 μg of a neutralizing anti-mouse TNF Ab (5E5) or Mu IgG1 24 hours and 1 hour before injection of LPS. Mouse spleens and mesenteric LNs were harvested at 0, 6, 24, 48 and 72 hours after injection for the FACS analysis of phenotype.
Quantitative real time RT-PCR assay analysis of Tnfrsf genes
RNA samples were extracted from flow-sorted CD4+FoxP3/gfp+ or CD4+FoxP3/gfp− cells as described and reverse transcribed. Quantitative real time PCR was performed to determine relative mRNA expression using primers specific to Tnfrsf genes (SABiosciences RT2 qPCR Primer Assays). Ct values from each gene in each sample were normalized using the 2−ΔΔCt calculation method with Gapdh gene as housekeeping gene by following SABiosciences' instruction.
Flow Cytometry
After blocking FcR, cells were incubated with appropriately diluted antibodies. Acquisition was performed using a FACSort or a SLRII (BD Biosciences, Mountain View, CA) and data analysis was conducted using FlowJo software (Tree Star Inc., Ashland, OR).
Statistical analysis
Comparisons of two groups of data were analyzed by two-tailed Student's t test using GraphPad Prism 4.0. (GraphPad, San Diego, CA)
Acknowledgement
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. R.H. is supported by International Training Program of Japan Society for the Promotion of Science. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
We thank Drs. Teresa Born and John E Sims at Amgen Inc. for providing anti-mouse TNF antibody and isotype control Mu IgG, and Drs. O.M. Zack Howard, Hong Lou, Hongchuan Li and Gonzalo M. de la Rosa for help in this study.
Abbreviations
- MFI
mean fluorescence intensity
- Teffs
effector T cells
- Tregs
regulatory T cells
Footnotes
Conflict of interest The authors have no financial and commercial conflicts of interest.
References
- 1.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 7.Kim EY, Priatel JJ, Teh SJ, Teh HS. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol. 2006;176:1026–1035. doi: 10.4049/jimmunol.176.2.1026. [DOI] [PubMed] [Google Scholar]
- 8.Kleijwegt FS, Laban S, Duinkerken G, Joosten AM, Zaldumbide A, Nikolic T, Roep BO. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J Immunol. 2010;185:1412–1418. doi: 10.4049/jimmunol.1000560. [DOI] [PubMed] [Google Scholar]
- 9.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol. 2010;40:2891–2901. doi: 10.1002/eji.201040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma HL, Napierata L, Stedman N, Benoit S, Collins M, Nickerson-Nutter C, Young DA. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–440. doi: 10.1002/art.27203. [DOI] [PubMed] [Google Scholar]
- 11.Mougiakakos D, Johansson CC, Jitschin R, Bottcher M, Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- 12.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, Martin GH, Elhage R, Derian N, Carpentier W, Marodon G, Klatzmann D, Piaggio E, Salomon BL. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Mierlo GJ, Scherer HU, Hameetman M, Morgan ME, Flierman R, Huizinga TW, Toes RE. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol. 2008;180:2747–2751. doi: 10.4049/jimmunol.180.5.2747. [DOI] [PubMed] [Google Scholar]
- 15.Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, Bank I, Kloog Y, Rechavi G, Goldstein I. TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J Immunol. 2010;184:3570–3581. doi: 10.4049/jimmunol.0902070. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Hamano R, Subleski JJ, Hurwitz AA, Howard OM, Oppenheim JJ. Expression of costimulatory TNFR2 induces resistance of CD4(+)FoxP3(−) conventional T cells to suppression by CD4(+)FoxP3(+) regulatory T Cells. J Immunol. 2010;185:174–182. doi: 10.4049/jimmunol.0903548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Most RG, Currie AJ, Mahendran S, Prosser A, Darabi A, Robinson BW, Nowak AK, Lake RA. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: a role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol Immunother. 2009;58:1219–1228. doi: 10.1007/s00262-008-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 20.Ruby CE, Yates MA, Hirschhorn-Cymerman D, Chlebeck P, Wolchok JD, Houghton AN, Offner H, Weinberg AD. Cutting Edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J Immunol. 2009;183:4853–4857. doi: 10.4049/jimmunol.0901112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, −7, and −15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantuzzi G, Di Santo E, Sacco S, Benigni F, Ghezzi P. Role of the hypothalamus-pituitary-adrenal axis in the regulation of TNF production in mice. Effect of stress and inhibition of endogenous glucocorticoids. J Immunol. 1995;155:3552–3555. [PubMed] [Google Scholar]
- 25.Birkeland ML, Copeland NG, Gilbert DJ, Jenkins NA, Barclay AN. Gene structure and chromosomal localization of the mouse homologue of rat OX40 protein. Eur J Immunol. 1995;25:926–930. doi: 10.1002/eji.1830250410. [DOI] [PubMed] [Google Scholar]
- 26.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. GMCSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol. 2011;89:235–249. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piconese S, Pittoni P, Burocchi A, Gorzanelli A, Care A, Tripodo C, Colombo MP. A non-redundant role for OX40 in the competitive fitness of Treg in response to IL-2. Eur J Immunol. 2010;40:2902–2913. doi: 10.1002/eji.201040505. [DOI] [PubMed] [Google Scholar]
- 31.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, Wagner JE, June CH, Riley JL, Blazar BR. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Oppenheim JJ. TNF-alpha: an activator of CD4+FoxP3+TNFR2+ regulatory T cells. Curr Dir Autoimmun. 2010;11:119–134. doi: 10.1159/000289201. [DOI] [PMC free article] [PubMed] [Google Scholar]