Abstract
Aim
This study examined the long-term impact of a 24-month, empowerment-based diabetes self-management support (DSMS) intervention on sustaining health-gains achieved from previous diabetes self-management education (DSME).
Methods
This report is based on 60 African-American adults with type 2 diabetes (n=89 recruited at baseline) who completed the study. Prior to the intervention, all participants received 6 months of mailed DSME consisting of weekly educational newsletters coupled with clinical feedback. The intervention consisted of 88 weekly group-based sessions that participants were encouraged to attend as frequently as they needed. Sessions were guided by participants’ self-management questions and also emphasized experiential learning, coping, goal-setting, and problem-solving. Baseline, 6-month, and 30-month assessments measured A1C, weight, body mass index (BMI), blood pressure, lipids, self-care behaviors, and QOL.
Results
Post 6-month DSME, participants demonstrated significant improvements for diastolic BP (p<0.05), serum cholesterol (p<0.001), healthy diet (p<0.01), blood glucose monitoring (p<0.05) and foot exams (p<0.01). Post 24-month intervention, participants sustained the improvements achieved from the 6-month DSME and reported additional improvements for healthy diet (p<0.05), carbohydrate spacing (p<0.01), insulin use, (p<0.05), and quality of life (p<0.05).
Conclusions
Findings suggest that an empowerment-based DSMS model can sustain or improve diabetes-related health gains achieved from previous short-term DSME.
2. Introduction
Diabetes self-management is central to effective diabetes care [1–4]. Optimal diabetes self-management calls for initial diabetes self-management education (DSME) followed by ongoing diabetes self-management support (DSMS) [4]. According to the National Standards of Diabetes Education, DSME is defined as “the ongoing process of facilitating the knowledge, skill, and ability necessary for diabetes self-care,” and DSMS as “activities to assist the individual with diabetes to implement and sustain the ongoing behaviors needed to manage their illness” [4].
While compelling evidence demonstrates the positive impact of DSME interventions on diabetes-related health outcomes [5–9], without continued follow-up and support, these gains are not sustained over the long-term [2]. The short-lived benefits achieved from DSME programs may be attributed to characteristics of current models for DSME. First, DSME programs are typically time-limited. In fact, a meta-analysis of 31 RCTs examining the impact of DSME programs on glycemic control found 61% of studies (n=19) described interventions that lasted 6 months or less [5]. Not surprisingly, short-term DSME programs will most likely produce short-term improvements. For instance, in a pilot study of 44 adults with type 2 diabetes in a primary care setting, Bastiaens et al. [10] examined the impact of a 5-session DSME program with one follow-up contact (at 3-months) on body mass index (BMI), glycemic control and quality of life (QOL). At 12-month follow-up, the group showed significant improvements in BMI, glycemic control, and QOL. However, at 18-month follow-up, the only improvement that persisted was for BMI suggesting the need for continued selfmanagement support [10].
Current DSME models also tend to be structured and curriculum-driven [11–14], often overlooking the heterogeneity of self-management needs and priorities across patients. These standardized interventions consist of a set number of sessions delivered in a pre-determined sequence with each session devoted to a specific self-management topic [11–14]. While a standardized curriculum may be useful in the initial acquisition of core self-management concepts, this DSME model is not compatible with the complex and ever-changing challenges patients encounter over the long-term. In “real-world” settings, patients do not experience living with diabetes in a sequential order or in discrete categories.
To sustain diabetes-related health gains, effective models for long-term DSMS need to be ongoing and accessible to patients over the course of their lives in addition to being flexible to the unique and continually evolving needs of each patient. Thus, patient empowerment is an approach that is ideally suited for long-term DSMS [15–18]. The principles of patient empowerment underscore (1) the proactive role of the patient in making daily self-management decisions, (2) a collaborative patient-provider relationship which assumes the provider in an advisory role to the patient who exercises final control of self-management decisions, and (3) the importance of patient-selected self-management goals in initiating and sustaining meaningful behavior change [19]. An empowerment-based DSMS model is designed to accommodate individual differences in self-management needs and to be responsive to how these needs change over time.
To test this empowerment-based DSMS model, this study specifically recruited participants who had received previous DSME (inclusion criteria). To ensure that all participants had a similar level of baseline knowledge, we provided participants with 6 months of mailed DSME designed to reinforce and/or enhance DSME received in the past. The 6-month DSME enhancement period was followed by Lifelong Management (LM), a 24-month empowerment-based DSMS intervention delivered in a group setting. While we recognize that 24 months is still considered “time-limited,” theoretically, this model could be designed to be ongoing with no defined endpoint. The objectives of this study were two-fold:
To examine the short-term impact of a 6-month DSME enhancement period on clinical, self-care, and psychosocial outcomes.
To examine the long-term impact of a 24-month empowerment-based DSMS intervention on sustaining the health-related gains achieved from previous DSME and a short-term, 6-month DSME enhancement period.
2. Methods
2.1 Participants and recruitment
This study received approval from the University of Michigan Institutional Review Board and recruited African-American (AA) adults with type 2 diabetes living in the greater Ypsilanti, Michigan area. Recruitment strategies included posting flyers in local organizations, community and health centers; taking out newspaper advertisements, and making invited presentations at local AA churches. Those interested in participating were instructed to call a toll-free number to undergo eligibility screening. Inclusion criteria for participation were: (1) being age 40 years or older, (2) being diagnosed with type 2 diabetes for at least one year, (3) having received some form of DSME in the past (e.g., attended a series of classes or a series of meetings with a diabetes educator or talked to a health care providers about some aspect of diabetes care), and (4) being under the care of a healthcare provider. It should be noted that we did not directly approach or call potential volunteers to participate. Instead, we invited (via flyers and oral presentations at local churches) individuals to contact us by telephone or approach us following presentations. Thus, we are unable to report a recruitment rate.
2.2 Study procedures
Eligible participants attended an enrollment orientation to learn about the study. Interested participants followed informed consent procedures and completed the baseline assessment which involved a blood sample, height, weight, and blood pressure measurements, and a self-report survey. Upon completion, participants received a $50 stipend for their time and effort.
2.3 Measures
Metabolic and cardiovascular measures were collected in person at assessment sessions and included A1C, lipid panel (serum cholesterol, HDL, LDL), systolic and diastolic blood pressures (SBP and DBP), weight, and body mass index (BMI). A1C and lipids were obtained via venous puncture.
Diabetes-specific quality of life was assessed using the Diabetes Distress Scale (DDS). The DDS is a 17-item instrument that measures emotional distress and functioning specific to living with diabetes [19]. Responses are scored on a 6-point Likert scale from “1” = no problem to “6” = serious problem. Scores can range from 17–102, with higher scores indicating poorer diabetes-specific quality of life.
Self-care behavior was assessed using items from the Summary of Diabetes Self-Care Activities Measure-revised (SDSCA-revised) [20]. Selected items assessed the frequency of following a healthy diet, spacing out carbohydrates evenly across the day, participating in physical activity, monitoring blood glucose, inspecting feet, and taking medication and/or insulin. Responses were based on a 7-day week and ranged from 0 days to 7 days, with greater number of days reflecting better self-management.
Diabetes empowerment was assessed using the Diabetes Empowerment Scale-Short Form (DES-SF). The DES-SF is an 8-item scale assessing perceived ability to manage the psychosocial demands and challenges associated with diabetes [21]. Items were scored on a 5-point Likert scale with a higher mean score indicating greater diabetes empowerment.
Demographic items included age, race, marital status, education, income, insurance coverage, years since diagnosis, type of diabetes treatment, and perceived health status.
This 30-month study consisted of two components: (1) a 6-month DSME enhancement period and (2) a 24-month, empowerment-based DSMS intervention. The 6-month DSME enhancement was a mailed intervention starting with clinical feedback (e.g., A1C, blood pressure, lipid panel) followed by weekly educational newsletters. Immediately following the baseline assessment, clinical results were sent to participants and their self-identified diabetes care providers. The results were presented in the form of a diabetes complications risk profile. The risk profile sent to patients included an explanation of the measure (e.g., A1C), the participant’s clinical values (e.g., A1C), the target range for clinical value (e.g., A1C ≤ 0.7% or ≤ 53 mmol/mol), and possible behavioral changes participants could make to bring their value into the target range.
In addition to providing clinical feedback we mailed participants a weekly newsletter focusing on different diabetes self-management topics. These newsletters were adapted and updated from a previous study [24] and addressed the critical content areas for diabetes education identified by the National Standards of Diabetes Education [4]. Following the 6-month DSME enhancement period participants completed a followup assessment. Data collected at the end of the 6-month DSME enhancement period also served as data for the start of the 24-month LM intervention.
2.4 Intervention
The Lifelong Management (LM) intervention
The LM intervention has been fully described in a previous publication [23]. The LM intervention is predicated on Anderson and Funnell’s [15–18] empowerment approach and consisted of 88 weekly sessions (75-minutes in length) over a period of 24-months. Sessions were offered in the morning and afternoon to accommodate participants’ differing schedules and participants were invited to attend sessions as frequently as they needed or could given competing life demands. Discussion was guided entirely by patients’ questions and concerns. While sessions were not based on a standardized curriculum they did involve a general process in which facilitators encouraged participants to (1) reflect on relevant self-management challenges or experiences, (2) recognize emotions associated with those experiences, (3) engage in group-based problem-solving, and (4) ask questions about diabetes and its care, and (5) set behavioral goals and make action plans to achieve those goals. Sessions were co-facilitated by a nurse certified diabetes educator and a clinical psychologist.
2.5 Statistical Analyses
Descriptive statistics were used to assess the demographic and diabetes care-related characteristics of the sample. Bivariate correlations were conducted to examine the relationship between frequency of attendance and demographic variables. For the purposes of examining the relationship between frequency of attendance and demographic variables, we recoded martial status to 1 = “currently married” and 2 = “not currently married”; education was recoded to 1 = “high school graduate/GED or less” and 2 = “some college or more”; income was recoded to 1 = “$0 - $19,999” and 2 = “$20,000 - $59,999”, 3 = “$60,000 or more”; employment status was recoded to 1 = “currently employed” and 2 = “not currently employed.” Paired t-tests were performed to examine pre- post- changes associated with the 6-month DSME enhancement and the 24-month Lifelong Management intervention periods. Independent samples t-tests and Pearson chi-square or Fisher’s exact test (the latter for 2X2 distributions) were conducted to compare the demographic and clinical characteristics of participants who completed the study (i.e. completers) and participants who dropped out of the study before completion (i.e., dropouts).
3. Results
3.1 Characteristics of the sample
Table 1 presents the demographics of the sample. At baseline, we recruited 89 participants. At the end of the 6-month intervention, we retained 77 participants (attrition rate 13%); and at the end of the 24-month LM intervention, we retained 60 participants yielding an attrition rate of 33% (below the expected rate of 40% from baseline to 30-months). Participants were between the ages of 40 and 84 years with a mean of 62 years (SD = 10.2). Thirty percent (n=18) were men; 70% (n=42) were women and 45% were currently married (n=27). Thirty percent (n=18) had a high school degree or less; 80% (n=48) were not currently employed; 62% had Medicare (n=37).
Table 1.
Characteristics of Sample (N=60)
| Variable | Mean ± SD | N | % |
|---|---|---|---|
| Age | 62.4 ± 10.2 | ||
| Years since diagnosis | 12.2 ±11.3 | ||
| Gender | |||
| Female | 42 | 70 | |
| Male | 18 | 30 | |
| Martial Status | |||
| Currently married | 27 | 45 | |
| Separated/Divorced | 14 | 23 | |
| Widowed | 10 | 17 | |
| Other | 9 | 15 | |
| Race | |||
| African American | 60 | 100 | |
| Education | |||
| 8 grades or less | 2 | 3 | |
| Some high school | 4 | 7 | |
| High school graduate or GED | 12 | 20 | |
| Some college or technical school | 29 | 48 | |
| College graduate or higher | 13 | 22 | |
| Household income | |||
| $0 – $9,999 | 9 | 15 | |
| $10,000 – $19,999 | 12 | 20 | |
| $20,000 – $29,999 | 11 | 19 | |
| $30,000 – $59,999 | 14 | 24 | |
| $60,000 or more | 13 | 22 | |
| Employment Status | |||
| Currently working | 12 | 20 | |
| Not currently working | 48 | 80 | |
| Insurance Coverage | |||
| Individual Plan | 2 | 3 | |
| Employee Plan | 17 | 28 | |
| U.S. Governmental Health Plan | 2 | 3 | |
| Medicare | 37 | 62 | |
| Medicaid | 2 | 3 | |
| No health insurance | 0 | 0 | |
| Diabetes treatment | |||
| Using insulin | 16 | 27 | |
| Taking pills | 46 | 77 | |
| Using Byetta | 3 | 5 | |
| Using Symlin | 0 | 0 | |
| Other medication | |||
| Taking cholesterol pills | 45 | 75 | |
| Taking blood pressure pills | 51 | 86 | |
| Perceived Health Status | |||
| Poor | 3 | 5 | |
| Fair | 20 | 33 | |
| Good | 31 | 52 | |
| Very good | 5 | 8 | |
| Excellent | 1 | 2 | |
3.2 Attendance patterns
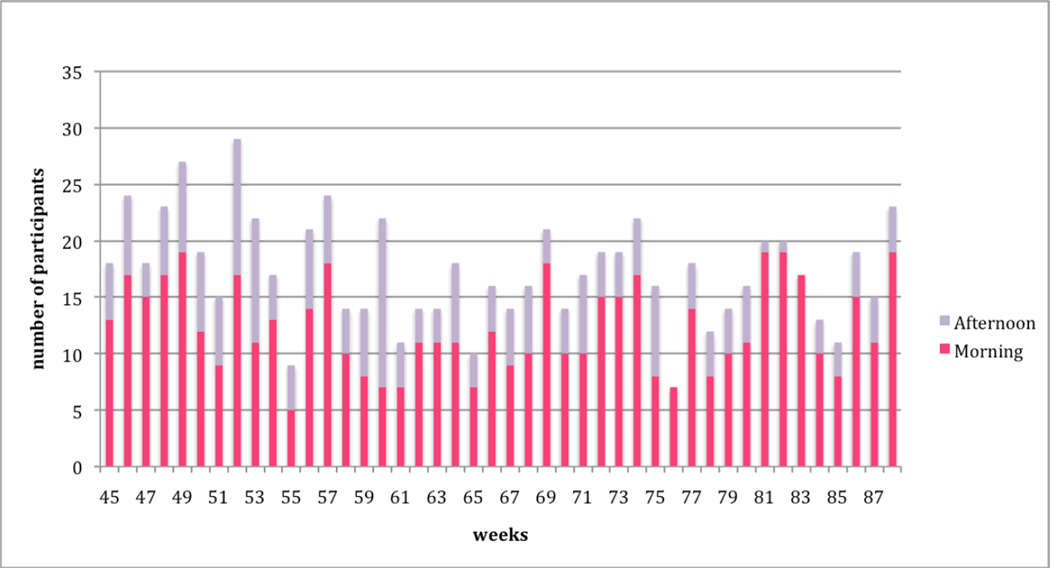
Figures 1 and 2 present the weekly attendance rate for Year 1 (weeks 1 thru 44) and Year 2 (weeks 45 thru 88), respectively. Two sessions (morning or afternoon) were offered each week over the 88-week period. Attendance level for the morning sessions versus the afternoon sessions are also delineated in Figures 1 and 2. Over the 88 weeks, 55% (n=33) attended 3 to 23 sessions, 20% (n=12) attended 24 to 44 sessions, and 25% (n=15) attended 35 to 83 sessions. Total attendance for any single week (2 sessions per week) ranged from 7 to 36 (m=19). Morning sessions attracted more participants with a mean attendance of 13 (SD = 4.0; range = 5 to 24); mean attendance for the afternoon session was 6 (SD= 2.6; range=1 to 15). On average, 32% (n=19) of the total sample attended each week.
Figure 1.
Lifelong Management Attendance: Year 1
Figure 2.
Lifelong Management Attendance: Year 2
There was a positive correlation between frequency of attendance and age (r=0.45, p<0.001) and a negative correlation between frequency attendance employment (r=-0.35, p<0.006). There was no relationship between frequency of attendance and other demographic variables.
3.3. 6-month DSME enhancement period
Table 2 presents participants’ clinical, self-care, and psychosocial measures prior to and immediately following the 6-month DSME enhancement period. Significant improvements were found for DBP (80.0 vs. 75.7 mm/Hg; p<0.05), and serum cholesterol (165.0 vs. 149.5 mg/dL; p<0.001). No significant changes were found for A1C, weight, BMI, HDL, LDL, or SBP.
Table 2.
Diabetes-related health outcomes for 6-month DSME enhancement period and 24-month DSMS intervention
| Variable | 6-month DSME enhancement | 24-month DSMS (Lifelong Management) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Pre | Posta | Post-PreΔ1 | n | Pre | Posta | Post−PreΔ2 | |
| Clinical indices | ||||||||
| A1C - % | 60 | 8.0±2.2 | 8.0±1.8 | −0.07±1.7 | 59 | 8.0±1.7 | 7.9±2.1 | −0.08±2.1 |
| A1C- mmol/mol | 60 | 64.4±23.8 | 63.6±19.2 | −0.79±18.5 | 59 | 64.0±19.1 | 63.1±22.5 | −0.87±23.2 |
| Weight | 60 | 207.2±43.8 | 207.7±44.4 | 0.42±8.0 | 60 | 207.7±44.4 | 203.8±39.5 | −3.8±23.1 |
| BMI | 60 | 34.5±7.3 | 34.6±7.4 | 0.08±1.3 | 60 | 34.6±7.4 | 33.9±6.5 | −0.65±4.0 |
| BP-systolic | 60 | 136.9±17.5 | 138.0±17.7 | 1.15±16.2 | 60 | 138.0±17.7 | 135.4±19.6 | −2.60±20.0 |
| BP-diastolic | 60 | 80.0±10.6 | 75.7±10.9 | −4.28±16.2* | 60 | 75.7±10.9 | 74.5±11.4 | −1.15±12.5 |
| Serum cholesterol | 60 | 165.0±41.3 | 149.5±35.9 | −15.5±30 7‡ | 60 | 149.5±35.9 | 158.1±50.0 | 8.6±48.6 |
| HDL | 60 | 51.8±13.5 | 51.7±12.8 | −0.04±7.7 | 60 | 51.7±12.8 | 50.5±14.6 | −1.3.±10.8 |
| LDL | 60 | 99.3±33.1 | 94.1±32.6 | −5.2±26.7 | 60 | 94.1±32.6 | 92.5±40.2 | −1.6±39.2 |
| Self-care behaviorsb | ||||||||
| Following a healthy diet | 59 | 3.4±2.2 | 4.2±2.2 | 0.85±2.1† | 60 | 4.2±2.2 | 4.8±2.0 | 0.55±2.0* |
| Spacing carbohydrates | 58 | 3.3 ±2.7 | 3.3±2.6 | −0.03 ±2.1 | 60 | 3.3±2.6 | 4.1±2.4 | 0.85±2.0† |
| Exercising | 60 | 1.9±2.3 | 2.2±2.2 | 0.27±2.8 | 60 | 2.2±2.2 | 2.2±2.3 | 0.08±2.8 |
| Monitoring BG | 60 | 4.9±2.6 | 5.6±2.3 | 0.65±2.1* | 60 | 5.6±2.3 | 5.3±2.6 | −0.27±2.2 |
| Inspecting feet | 60 | 4.8±2.8 | 5.6±2.3 | 0.78±2.1† | 60 | 5.6±2.3 | 5.7±2.2 | 0.15±2.2 |
| Taking medication | 50 | 5.9±2.4 | 5.9±2.4 | 0.06±2.5 | 48 | 6.4±1.8 | 6.1±2.1 | −0.31±2.3 |
| Using insulin | 25 | 4.1±3.5 | 3.9±3.3 | −0.20±2.1 | 29 | 3.8±3.3 | 5.2±2.9 | 1.41±3.1* |
| Psychosocial indices | ||||||||
| Quality of lifec | 60 | 31.6±15.7 | 29.8±15.0 | −1.77±10.2 | 60 | 29.8±15.0 | 26.7±13.2 | −3.12±10.6* |
| Empowermentd | 60 | 3.9±0.9 | 4.0±0.7 | 0.14±0.76 | 60 | 4.0±0.7 | 4.0±0.9 | 0.01±0.85 |
p < 0.05,
p < 0.01,
p < 0.001
Post 6-month DSME enhancement and pre 24-month DSMS means may be slightly different as Post-Pre differences are based on complete pairs
Responses range from 0 to 7 days;
Responses can range from 17 to 102 (ranging here from 17 to 78) with higher scores indicating poorer diabetes-specific quality of life
Responses scored on a 5-point likert scale with “1” = strongly disagree to “5” = strongly agree. Due to a technical error, 1 of the original 8-item scale was dropped. A recalculation of the Cronbach’s alpha indicates the reliability for the 7-items was excellent ranging from .85 to .90.
For self-care behaviors, significant improvements were found for following a healthy diet (3.4 vs. 4.2 days/wk; p<0.01), monitoring blood (4.9 vs 5.6 days/wk; p<0.05), and performing foot exams (4.8 vs 5.6 days/wk; p<0.01). No changes were found for physical activity, medication and/or insulin use.
No changes were found for diabetes-specific quality of life and diabetes empowerment.
3.4. 24-month Lifelong Management DSMS intervention
Table 2 presents participants’ clinical, self-care, and psychosocial measures prior to and immediately following the 24-month DSMS period. No significant changes were found for any clinical outcomes.
For self-care behaviors, significant improvements were found for healthy diet (4.2 vs. 4.8 days/wk; p<0.05), carbohydrate spacing (3.3 vs 4.1 days/wk; p<0.01), and insulin use (3.8 vs 5.2 days/wk; p<0.05). No changes were found for physical activity, blood sugar testing, foot care, and medication use.
For psychosocial outcomes, a significant improvement was found for diabetes-specific QOL (29.8 vs 26.7.2 days/wk; p<0.05). No changes were found for diabetes empowerment.
3.5. Completers versus Dropouts
Table 3 presents the comparison between participants who completed the 30-month study (6 months DSME plus 24 months DSMS) and participants who dropped out over the course of the study on demographic and clinical variables. With regard to demographic variables, compared to dropouts, completers were significantly older (M=62.4; SD=10.2 versus M=55.7; SD 10.0), had income distributed at higher levels, were more likely to have Medicare as a form of insurance coverage (62% versus 36%), and more likely to take cholesterol medications (75% versus 43%). No significant differences were found for clinical variables.
Table 3.
Baseline characteristics for completers and dropouts
| Continuous variables | Completers (N=60) Mean ± SD |
Dropouts (N=29) Mean ± SD |
Independent- Samples t-tests |
|---|---|---|---|
| Age | 62.4 ± 10. 2 | 55.7±10. 0 | p=.004 |
| Years since diagnosis | 12.2 ±11. 3 | 9.4±8. 3 | no |
| A1C | 8.0±2. 2 | 7.7± 2.1 | no |
| BP-systolic | 136.9±17.5 | 139.6±22.5 | no |
| BP-diastolic | 80.0±10. 6 | 82.4±11.4 | no |
| Serum cholesterol | 165.0±41.3 | 178.3±45.5 | no |
| HDL | 51.8±13.5 | 54.7±13.8 | no |
| LDL | 99.3±33. 1 | 107.0±39.7 | no |
| Categorical Variables | Completers N (%) |
Dropouts N (%) |
Pearson Chi-Square† or Fisher’s Exact Test* |
|---|---|---|---|
| Gender | |||
| Female | 42 (70) | 18 (62) | |
| Male | 18 (30) | 11 (38) | no |
| Martial Status | |||
| Currently married | 27 (45) | 10 (34) | |
| Separated/Divorced | 14 (23) | 13 (45) | |
| Widowed | 10 (17) | 1 ( 3) | |
| Other | 9 (15) | 5 (17) | no |
| Race | |||
| African American | 60 (100) | 60 (100) | no |
| Education | |||
| 8 grades or less | 2 ( 3) | 0 ( 0) | |
| Some high school | 4 ( 7) | 4 (14) | |
| High school graduate or GED | 12 (20) | 6 (21) | |
| Some college or technical school | 29 (48) | 16 (55) | |
| College graduate or higher | 13 (22) | 3 (10) | no |
| Household income | |||
| $0 – $9,999 | 9 (15) | 13 (46) | |
| $10,000 – $19,999 | 12 (20) | 4 (14) | |
| $20,000 – $29,999 | 11 (19) | 4 (14) | |
| $30,000 – $59,999 | 14 (24) | 5 (18) | |
| $60,000 or more | 13 (22) | 2 ( 7) | p=.031† |
| Employment Status | |||
| Currently working | 12 (20) | 5 (17) | |
| Not currently working | 48 (80) | 24 (83) | no |
| Insurance Coverage | |||
| Individual Plan | 2 ( 3) | 0 ( 0) | |
| Employee Plan | 17 (28) | 8 (29) | |
| U.S. Governmental Health Plan | 2 ( 3) | 2 ( 7) | p=.031† |
| Medicare | 37 (62) | 10 (36) | |
| Medicaid | 2 ( 3) | 8 (29) | |
| No health insurance | 0 ( 0) | 0 ( 0) | p=.005† |
| Diabetes treatment | |||
| Using insulin | 16 (27) | 8 (29) | no |
| Taking pills | 46 (77) | 22 (79) | no |
| Using Byetta | 3 ( 5) | 0 ( 0) | no |
| Using Symlin | 0 ( 0) | 0 ( 0) | no |
| Other medication | |||
| Taking cholesterol pills | 45 (75) | 12 (43) | p=.004* |
| Taking BP pills | 51 (86) | 20 (71) | no |
| Perceived Health Status | |||
| Poor | 3 ( 5) | 2 ( 7) | |
| Fair | 20 (33) | 14 (48) | |
| Good | 31 (52) | 10 (34) | |
| Very good | 5 ( 8) | 3 (10) | |
| Excellent | 1 ( 2) | 0 ( 0) | no |
4. Discussion
Lifelong Management (LM) is an empowerment-based model ideally-suited for long-term DSMS because it is designed to be patient-driven and flexible to the unique needs, priorities, and life circumstances of each individual. This study examined the long-term impact of the 24-month LM intervention on sustaining the self-management gains achieved from previous DSME and a short-term, 6-month DSME enhancement period.
According to our findings, not only did the LM intervention sustain improvements achieved from the 6-month DSME enhancement period, but it also led to additional gains in self-care behaviors and psychosocial functioning. Considering that two key components of the LM intervention were (1) providing face-to-face group-based social support and (2) encouraging participants to set behavioral goals and make action plans, it is not surprising the DSMS intervention was associated with further enhancements in quality of life and three self-care practices (e.g., making healthy dietary decisions, spacing out carbohydrates, and using insulin as prescribed). These findings are consistent with those of previous studies that have found participation in self-management and goal-setting interventions to be associated with improvements in quality of life25 and self-care behaviors [25–26], respectively.
Participation in the 24-month LM intervention was not associated with any changes in glycemic control. According to the UKPDS study [27], without medical treatment, the natural progression of diabetes would result in approximately 0.2% increase in A1C each year (i.e., 12 months). Based on this information, we would expect to see a 0.4% increase in A1C at the end of 24 months. Because glycemic control remained relatively unchanged over the course of the 24-month LM intervention, it is possible that the intervention exerted a stabilizing effect, which is the goal of our DSMS model.
Our study also presented an ideal opportunity to observe natural patterns of attendance over a long period of time. Not surprisingly, the average attendance rate was lower for the last 12 months of the study (m=29%) compared to the first 12 months (m=34%). Notwithstanding, the attendance for the latter half of the intervention is higher than our attendance expectation (i.e., to attract 15–20% of total sample each week) and, therefore, suggests that participants remained engaged in the intervention
We found no relationship between frequency of attendance and improvements (i.e., no dosage effect). Contrary to our findings, a study by Thompson and colleagues [29] showed that participants who reported more weekly contacts with a community health worker (CHW) showed greater improvements in glycemic control. In Thompson et al’s study [28], there were several contexts in which a “contact” could occur in any given week (e.g., telephone counseling, face-to-face encounters, walking club, DSME classes, or depression support group etc.). In two of these contexts “contact” was initiated by CHWs rather than the patient. In contrast, in our study, a “contact” was defined as attending a weekly group session with attendance being initiated by the participant. In “real-world” settings, there is neither the time nor resources for healthcare providers to proactively initiate weekly contacts with every individual patient. Furthermore, patients may differ in the type and amount of support they need. Our finding that the frequency of attendance was not related to outcomes suggests that participants do have different support needs and, thereby, seek support (i.e., attend sessions) accordingly. In other words, participants who only need to come to sessions when in crisis may receive the same benefit as participants who need to come every week.
Given that this study was conducted over a period of 30 months, a retention rate of 67% is reasonable. It is not surprising that completers were significantly older than dropouts as older participants are more likely to be retired with more free-time and fewer competing demands (e.g., employed, managing a household) than younger participants. Furthermore, dropouts were more likely to fall into lower income brackets and subsequently, may not have the type of employment that is flexible enough to attend a group being conducted during work hours (10AM and 3PM groups). This finding underscores the challenge of designing an intervention that can accommodate the circumstances of all patients regardless of age, socioeconomic background, ethnicity, etc.
Our results suggest that this type of intervention can have a more expansive reach without additional cost. Typical group-based interventions recruit approximately 8 participants per group and each group is usually facilitated by one or two health care professionals. Based on this traditional model, we would need to conduct 7 or 8 groups to accommodate 60 participants. Alternatively, the LM intervention can accommodate 60 participants while conducting only 2 groups. Future investigations should include a formal cost-effectiveness evaluation.
There were several limitations to this study. Our inclusion criterion for previous DSME was loosely defined. Clearly, a participant who recently completed a 10-hour ADA accredited DSME program has received more intensive education than a participant who raised one or two self-management questions with his/her provider during a 15-minute health care visit. While we attempted to equalize these differences by employing a 6-month DSME enhancement period, it is unlikely that participants, as a whole, started the 24-month DSMS intervention with the maximum self-management benefits derived from participating in a comprehensive DSME program. Given that a high-intensity, face-to-face DSME intervention with active mechanisms for interaction and social support would likely yield greater improvements across more variables (e.g., clinical, behavioral, and psychosocial), subsequent investigations of this DSMS model should include a formal DSME component. While the LM intervention was associated with sustained and enhanced improvements, this study did not use a randomized controlled trial (RCT) design. Therefore, we cannot conclude with certainty that the diabetes-related health improvements were due to the intervention and not for other reasons such as study effects.
Given the chronic nature of diabetes, short-term DSME is not sufficient to produce enduring self-management improvements. To meet the long-term challenges of living with diabetes, newly-designed models for ongoing DSMS need to be flexible enough to accommodate different self-management needs across individuals and be responsive enough to adapt to the ever-changing needs within each individual.
Acknowledgment
This study was supported by a K23 patient-oriented career development award from National Institutes of Health, K23 DK068375, National Institutes of Diabetes and Digestive and Kidney Diseases, and by a grant NIH P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. Am. J. Prev. Med. 2002;22(4S):15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 2.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes. A systematic review of randomized trials. Diabetes Care. 2001;24:561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 3.Mangione CM, Gerzoff RB, Williamson DF, Steers WN, Kerr EA, Brown AF, et al. The association between quality of care and the intensity of diabetes disease management programs. Ann. Intern. Med. 2006;145:107–116. doi: 10.7326/0003-4819-145-2-200607180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, et al. National standards for diabetes self-management education. Diabetes Care. 2009;32:S87–S94. doi: 10.2337/dc08-S087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: A meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 6.Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. Increasing diabetes self-management education in community settings. Am. J. Prev. Med. 2002;22(4S):39–66. doi: 10.1016/s0749-3797(02)00424-5. [DOI] [PubMed] [Google Scholar]
- 7.Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. J. Clin. Epidemiol. 1988;41:1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- 8.Deakin T, McShane CE, Cade JE, Williams RDRR. Group based training for self-management strategies in people with type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews. 2005;(Issue 2) doi: 10.1002/14651858.CD003417.pub2. Art. No:CD003417. Doi:10.1002/14651858. CD003417.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet. Med. 2006;23:544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 10.Bastiaens H, Sunaert P, Wens J, Sabbe B, Jenkins L, Nobels F, et al. Supporting diabetes self-management in primary care: Pilot-study of a group-based programme focusing on diet and exercise. Prim. Care Diabetes. 2009;3:103–109. doi: 10.1016/j.pcd.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Boegner C, Fontbonne A, Vidal MFG, Mouls P, Monnier L. Evaluation of a structured educational programme for type 2 diabetes patients seen in private practice. Diabetes Metab. 2008;34:243–249. doi: 10.1016/j.diabet.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Redmond EH, Burnett SM, Johnson MA, Park S, Fischer JG, Johnson T. Improvement in A1C levels and diabetes self-management activities following a nutrition and diabetes education program in older adults. J. Nutr. Elder. 2006;26:83–102. doi: 10.1300/J052v26n01_05. [DOI] [PubMed] [Google Scholar]
- 13.Speer EM, Reddy S, Lommel TS, Fischer JG, Park S, Johnson MA. Diabetes self-management behaviors and A1c improved following a community-based intervention in older adults in Georgia senior centers. J. Nutr. Elder. 2008;27:179–200. doi: 10.1080/01639360802060298. [DOI] [PubMed] [Google Scholar]
- 14.Philis-Tsimikas A, Walker C, Rivard L, Talavera G, Reimann JOF, Salmon M, et al. Improvement in diabetes care of underinsured patients enrolled in Project Dulce. Diabetes Care. 2004;27:110–115. doi: 10.2337/diacare.27.1.110. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RM, Funnell MM. Patient empowerment: reflections on the challenge of fostering the adoption of a new paradigm. Patient Educ. Couns. 2005;57:153–157. doi: 10.1016/j.pec.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Funnell MM, Anderson RM. Empowerment and self-management of diabetes. Clin. Diabetes. 2004;22:123–127. [Google Scholar]
- 17.Funnell MM, Anderson RM. Patient empowerment: A look back, a look ahead. Diabetes Educ. 2003;29:454–458. 460–462. doi: 10.1177/014572170302900310. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RM, Funnell MM, Arnold MS. Using the empowerment approach to help patients change behavior. In: Anderson B, Rubin R, editors. Practical Psychology for Diabetes Clinicians. 2nd edn. Alexandria, VA: American Diabetes Association; 2002. pp. 3–12. [Google Scholar]
- 19.Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: Development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 20.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care. 2000;23:943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 21.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF) (Letter) Diabetes Care. 2003;26:1641–1642. doi: 10.2337/diacare.26.5.1641-a. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RM, Fitzgerald JT, Funnell MM, Barr PA, Stepien CJ, Hiss RG, et al. Evaluation of an activated patient diabetes education newsletter. Diabetes Educ. 1994;20:29–34. doi: 10.1177/014572179402000106. [DOI] [PubMed] [Google Scholar]
- 23.Tang TS, Gillard ML, Funnell MM, Nwankwo R, Parker E, Spurlock D, et al. Developing a new generation of ongoing diabetes self-management support interventions: A preliminary report. Diabetes Educ. 2005;31:91–97. doi: 10.1177/0145721704273231. [DOI] [PubMed] [Google Scholar]
- 24.Cochran J, Conn VS. Meta-analysis of Quality of Life outcomes following diabetes self-management training. Diabetes Educ. 2008;34:815–823. doi: 10.1177/0145721708323640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeWalt DA, Davis TC, Wallace AS, Seligman HK, Byrant-Shilliday B, Arnold CL, et al. Goal setting in diabetes self-management: Take the baby steps to success. Patient Educ. Couns. 2009;77:218–223. doi: 10.1016/j.pec.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klug C, Toobert DJ, Fogerty M. Healthy changes for living with diabetes: An evidence-based community diabetes self-management program. Diabetes Educ. 2008;34:1053–1061. doi: 10.1177/0145721708325886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 28.Thompson JR, Horton C, Flores C. Advancing diabetes self-management in the Mexican American population. A community health worker model in a primary care setting. Diabetes Educ. 2007;33(S6):159S–154S. doi: 10.1177/0145721707304077. [DOI] [PubMed] [Google Scholar]