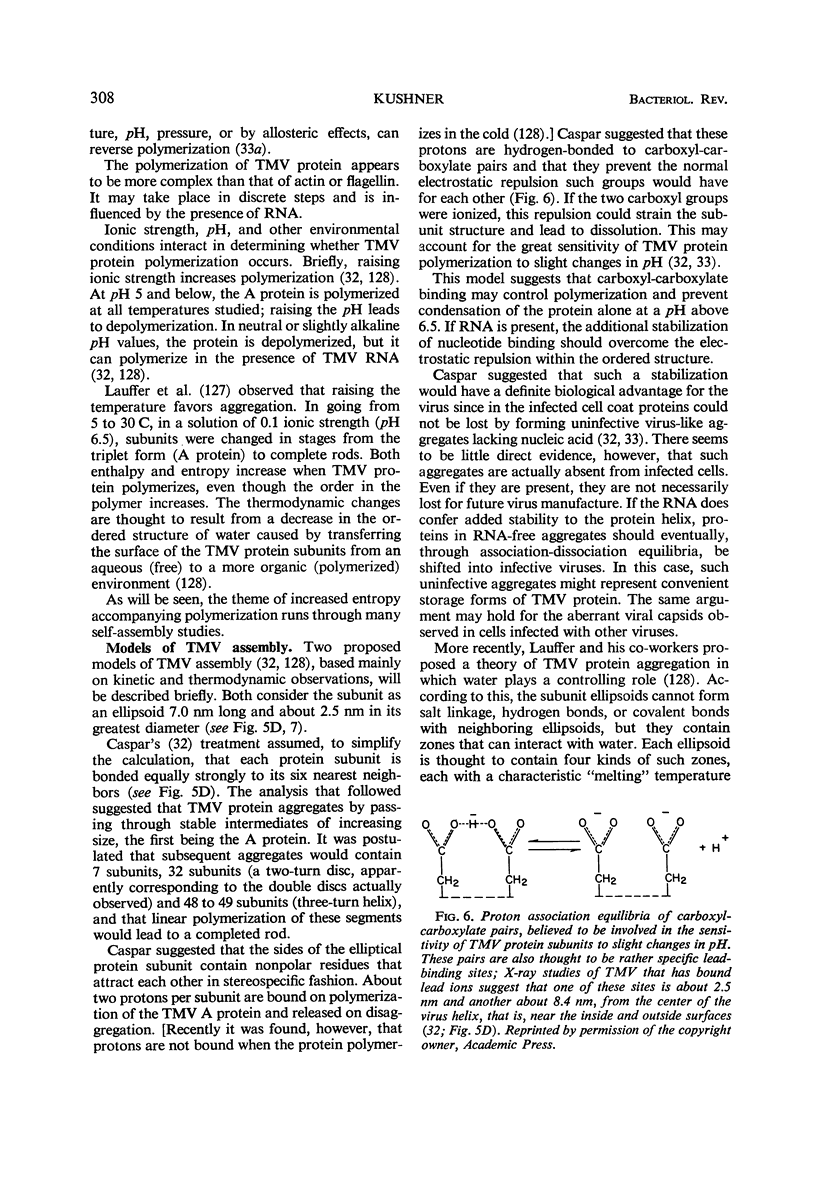
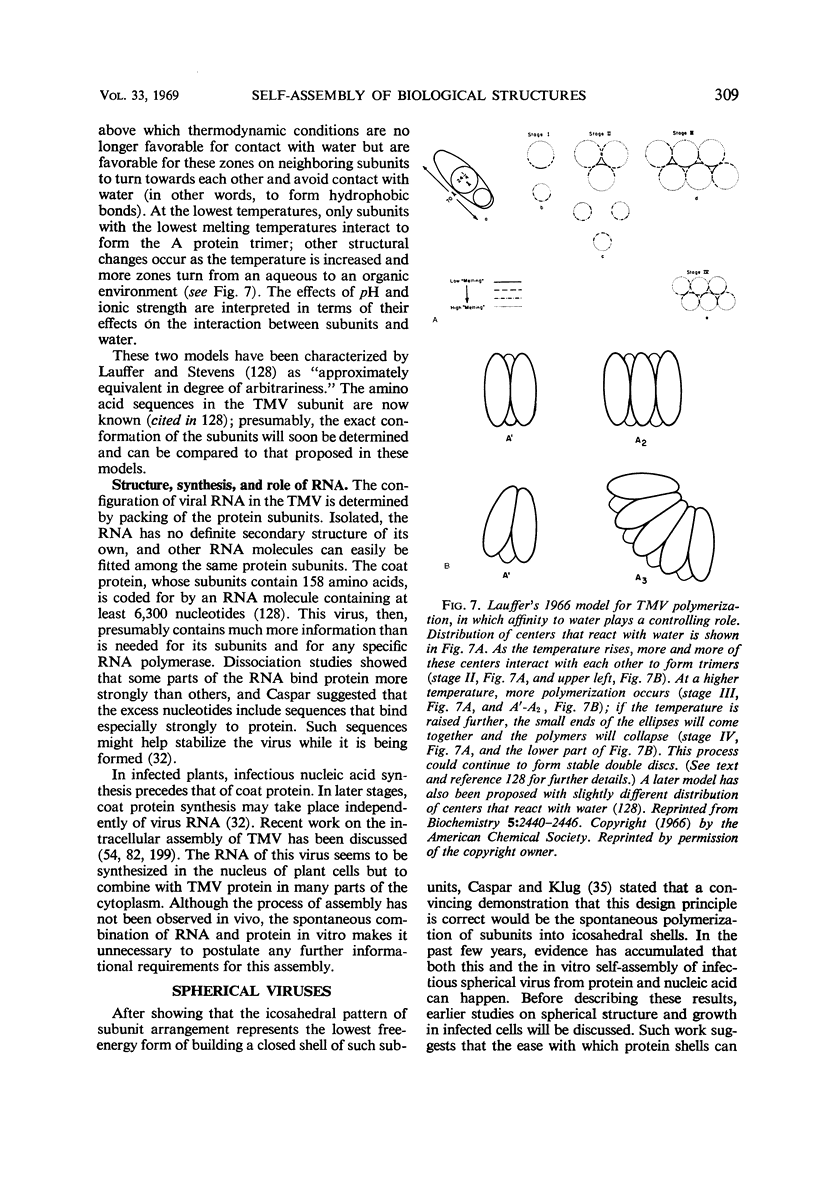
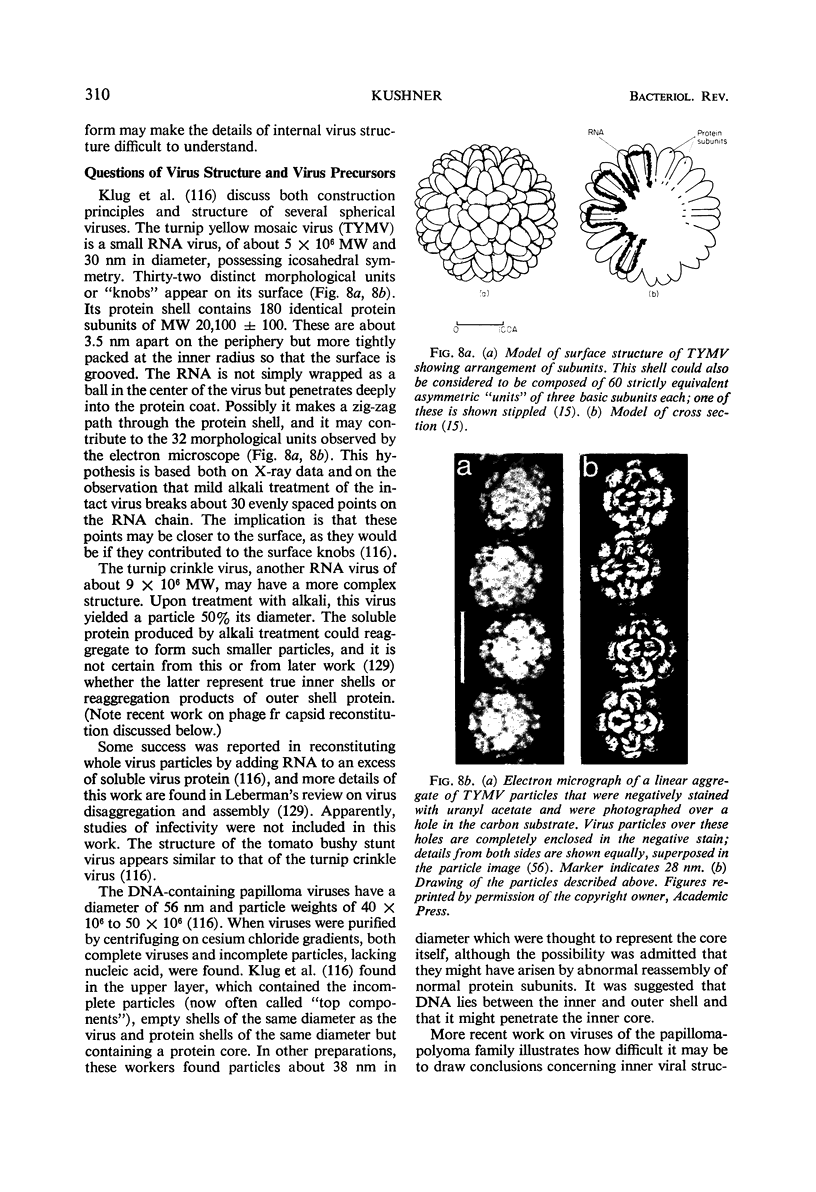
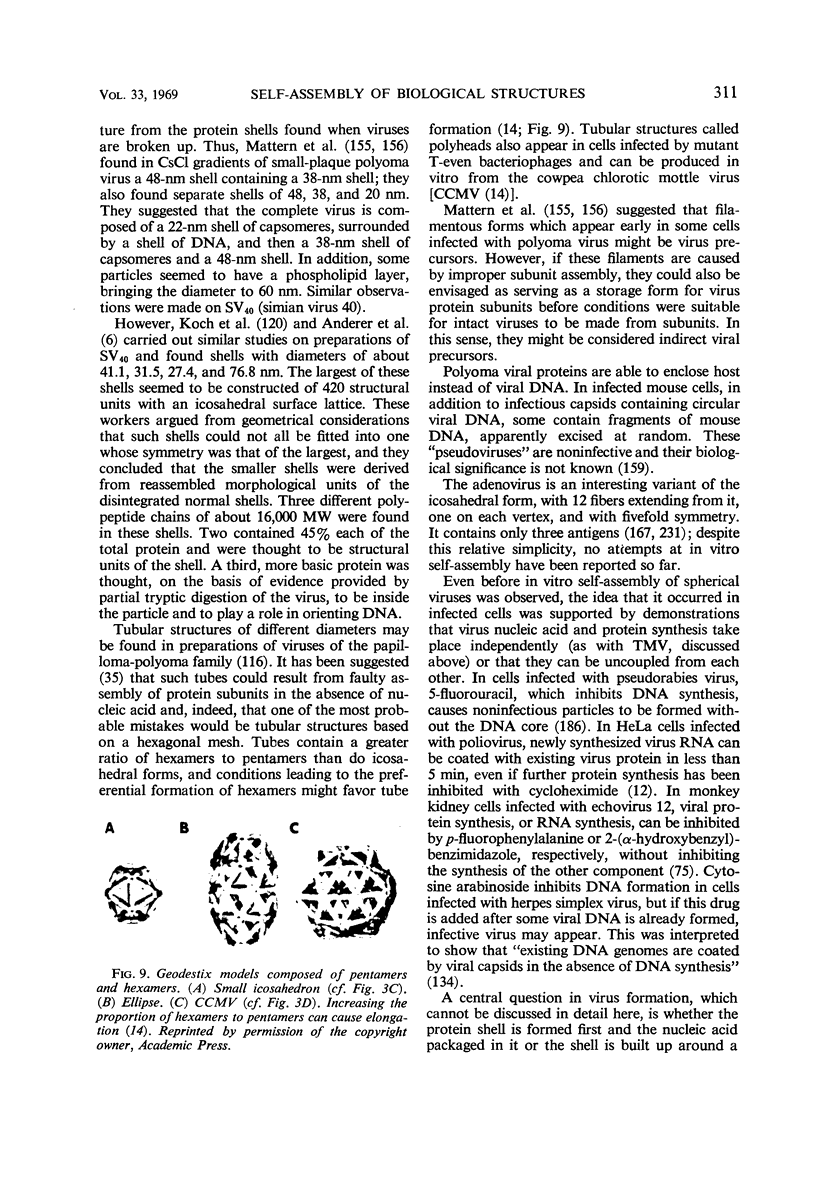
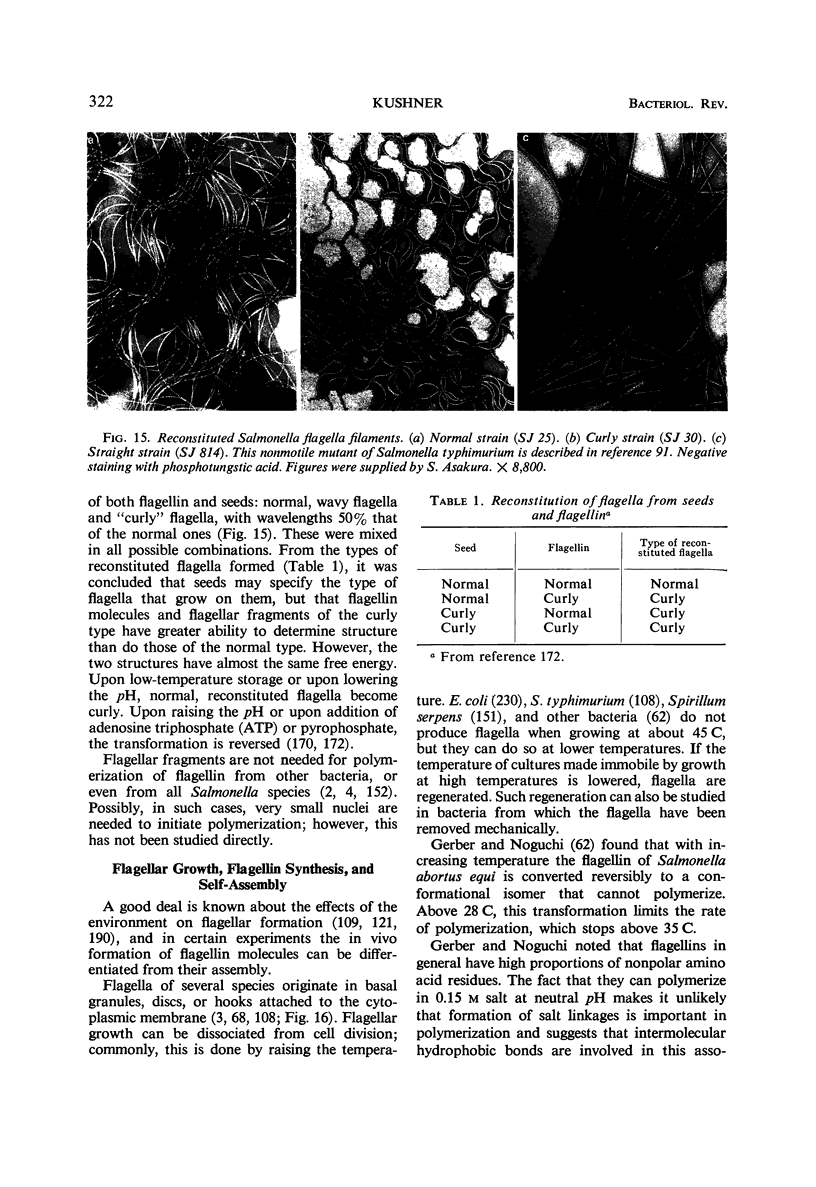
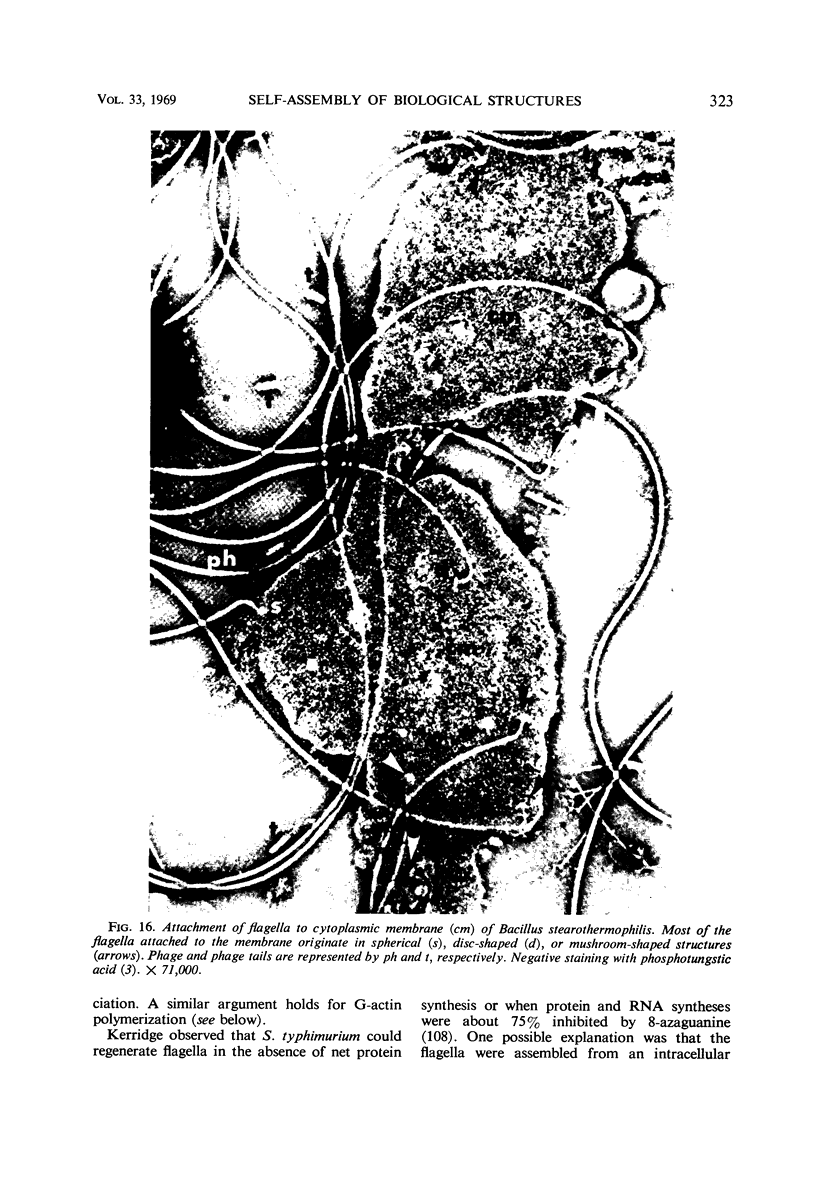
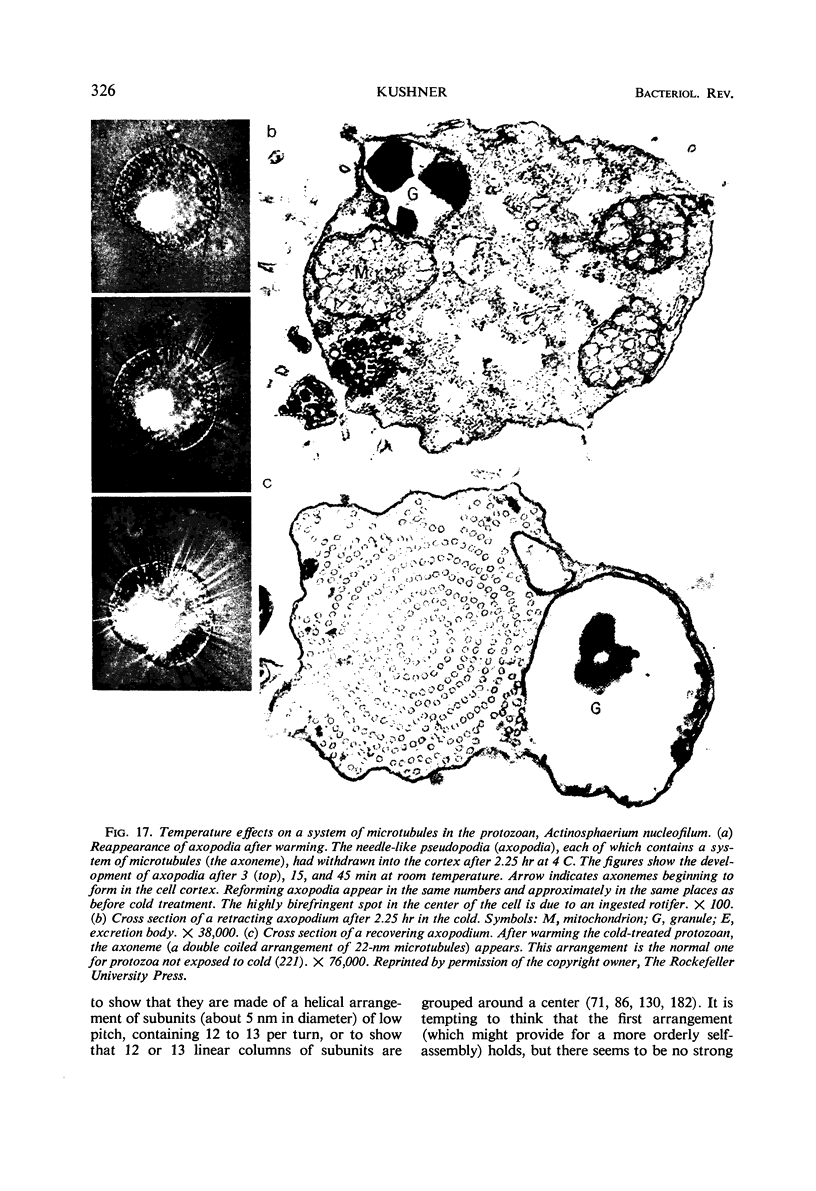
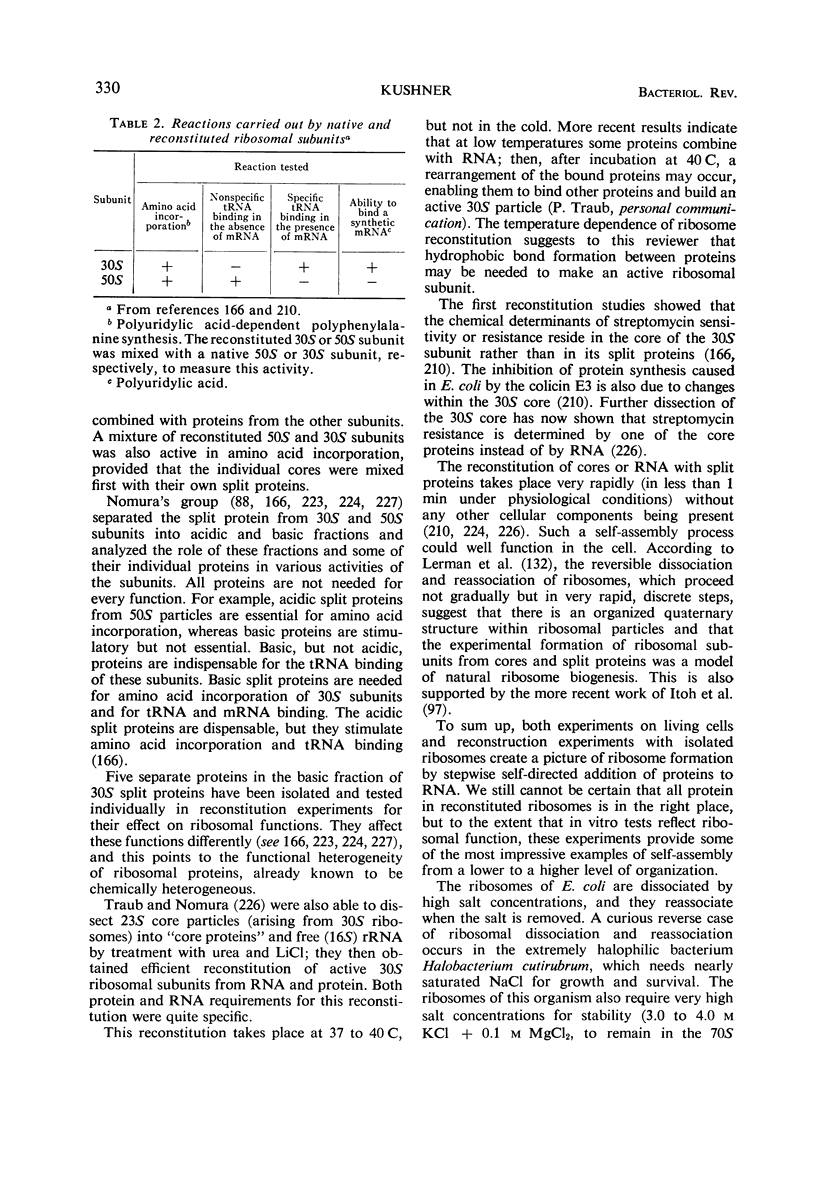
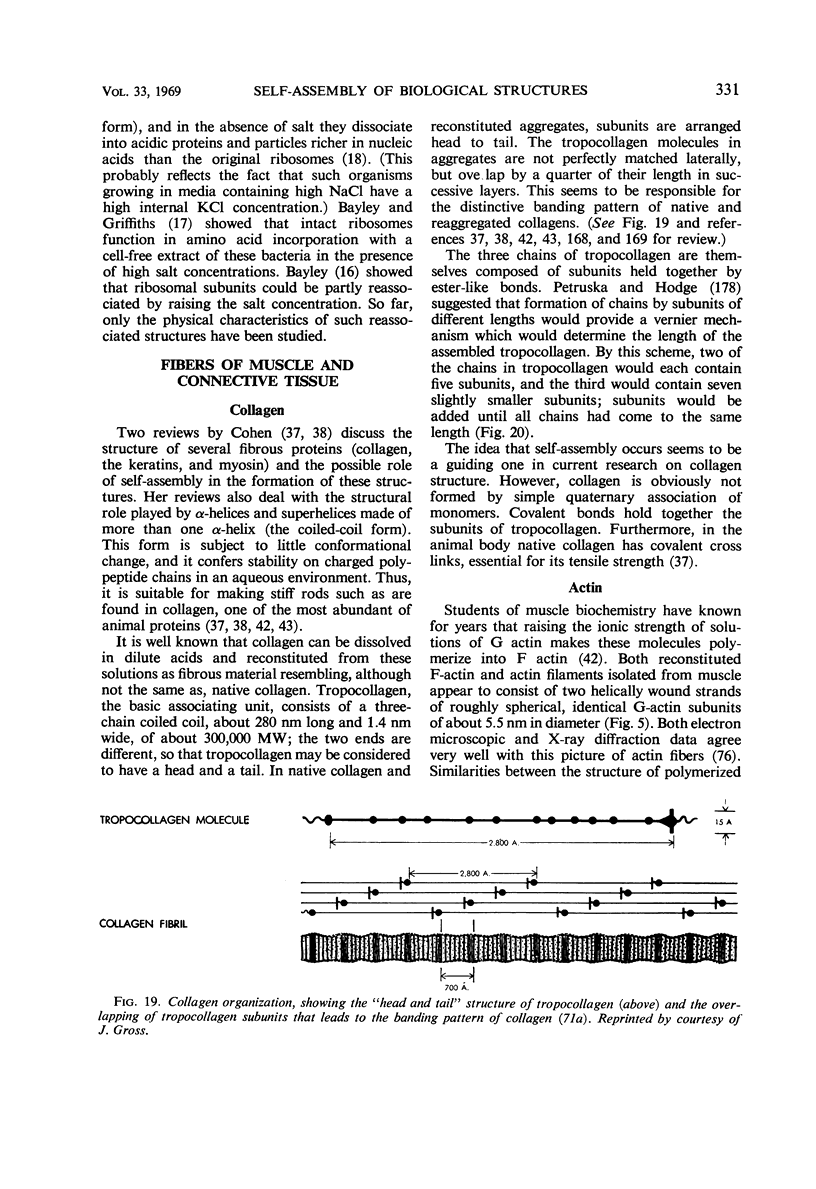
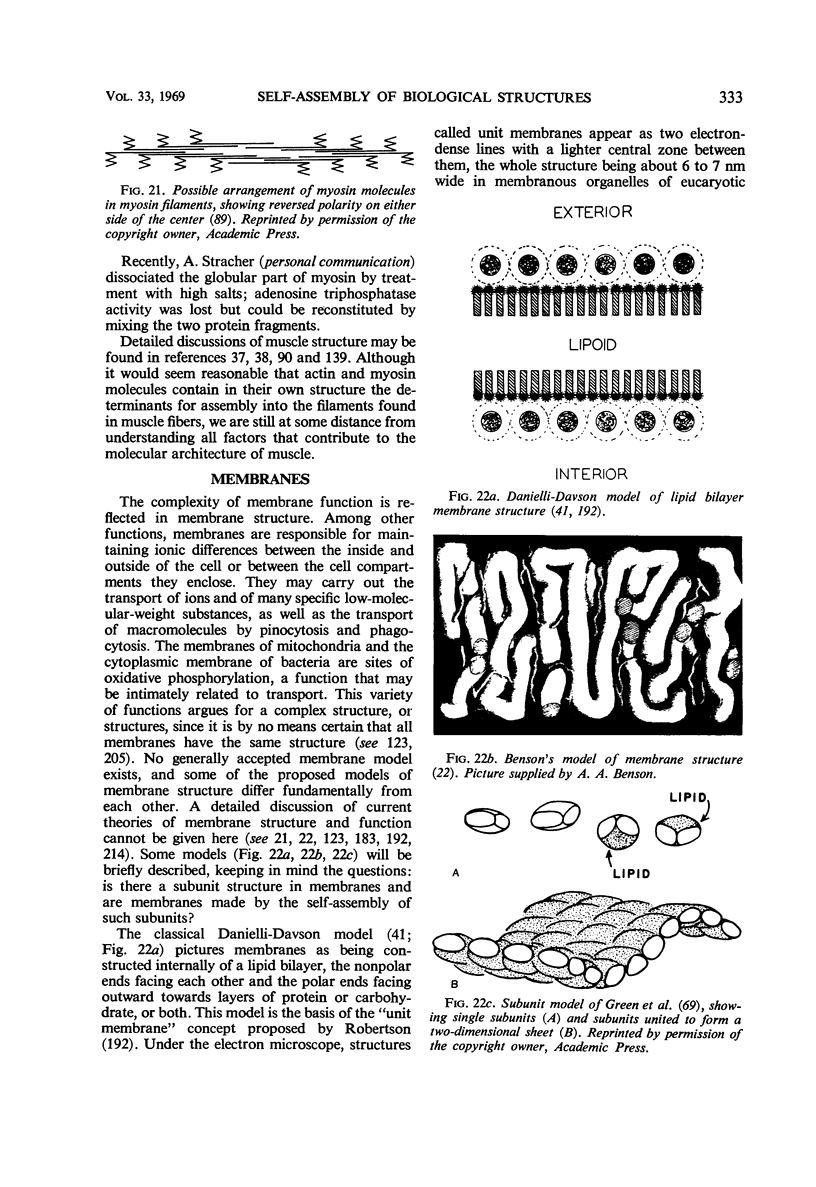
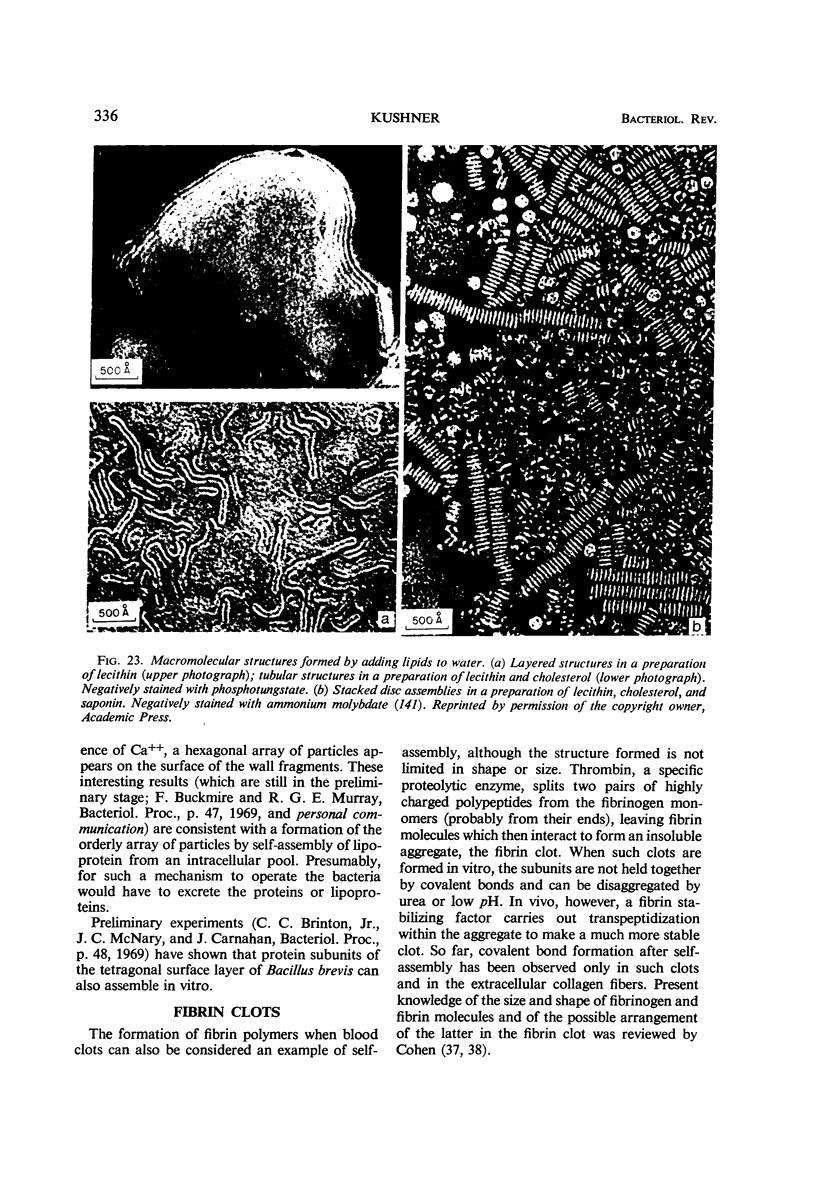
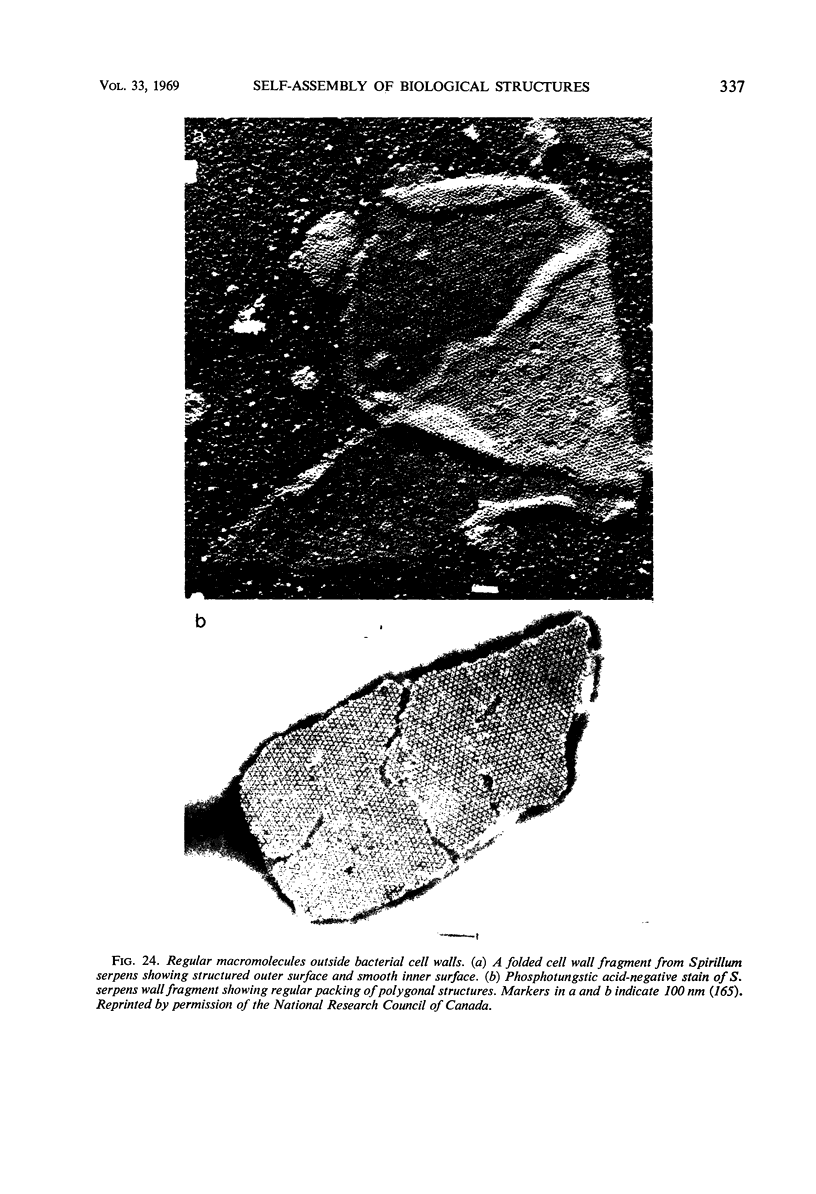
Full text
PDF


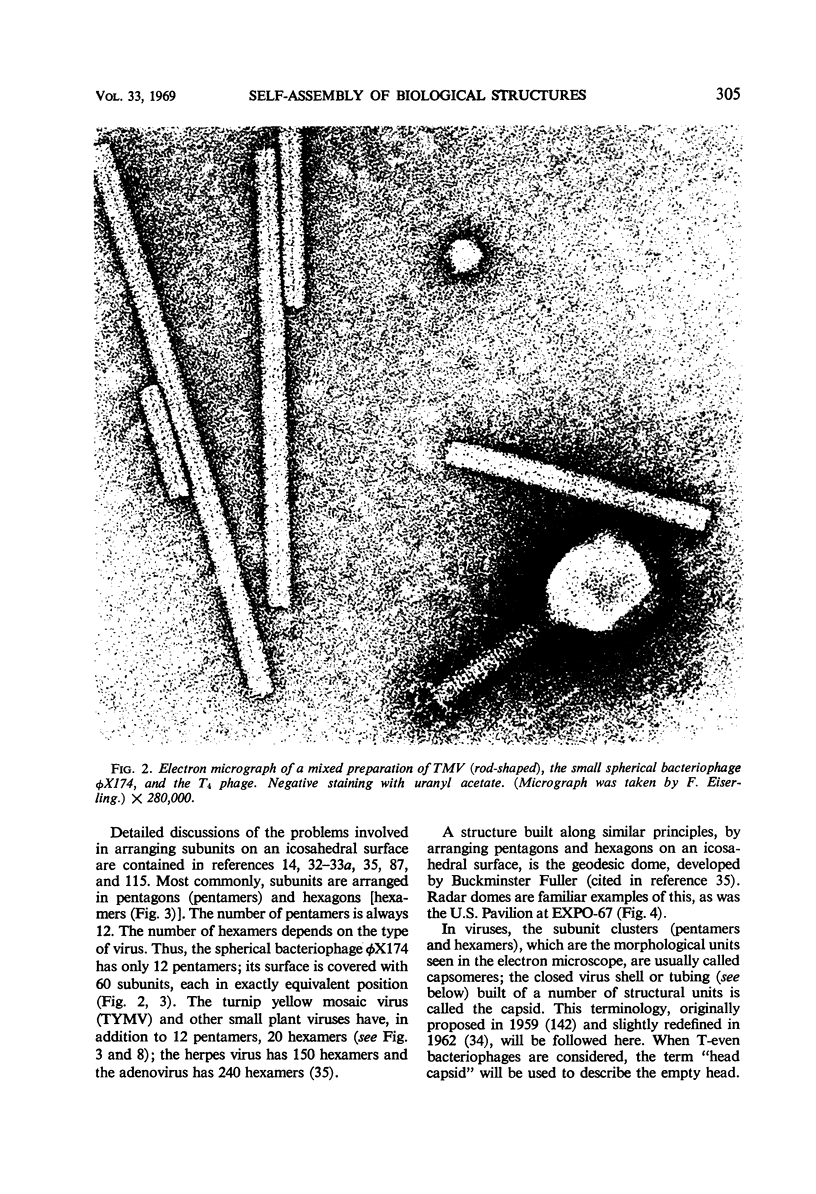
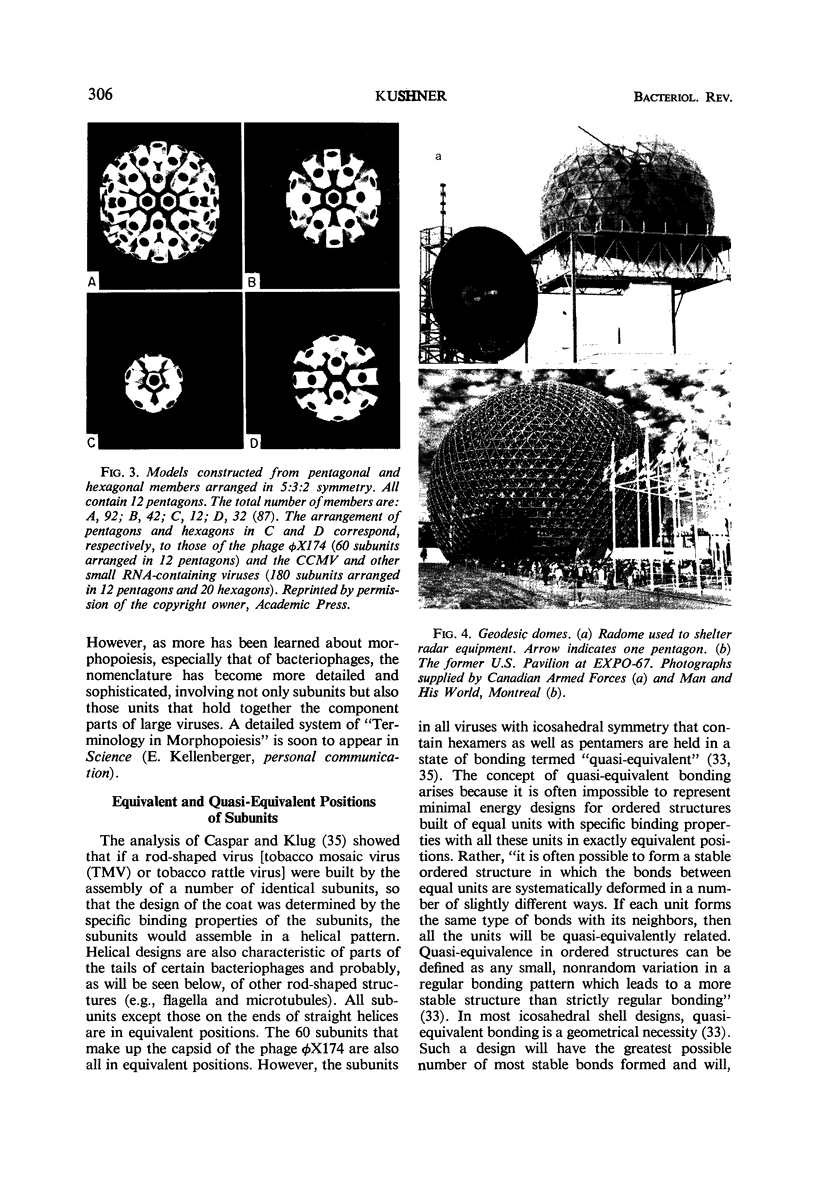















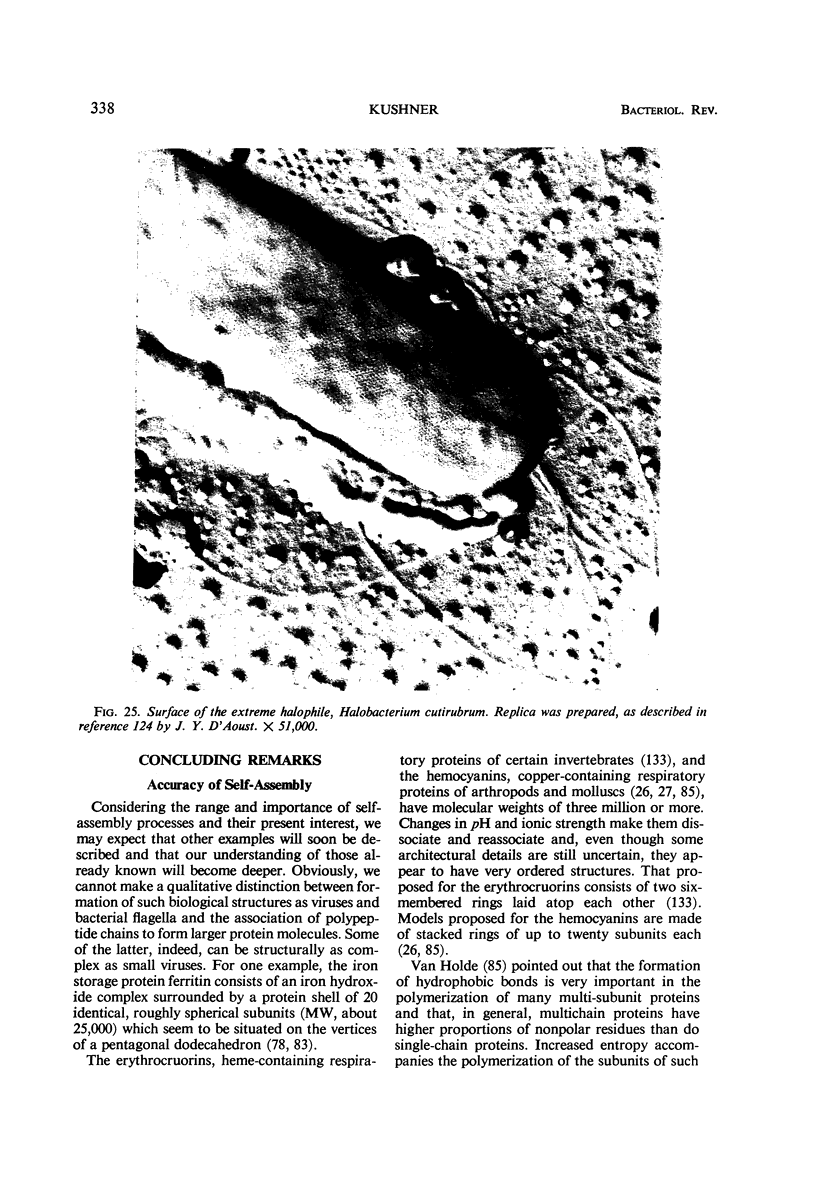







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., KOFFLER H. IN VITRO FORMATION OF FLAGELLA-LIKE FILAMENTS AND OTHER STRUCTURES FROM FLAGELLIN. J Mol Biol. 1964 Jul;9:168–185. doi: 10.1016/s0022-2836(64)80098-x. [DOI] [PubMed] [Google Scholar]
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. BEHAVIOUR OF ACTIVE BACTERIAL ANTIGENS DURING THE INDUCTION OF THE IMMUNE RESPONSE. I. PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA. Nature. 1963 Sep 28;199:1257–1259. doi: 10.1038/1991257a0. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P., REES M. W. Epsilon-N-Methyl-lysine in bacterial flagellar protein. Nature. 1959 Jul 4;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- Aamodt L. W., Eisenstadt J. M. Flagellar synthesis in Salmonella typhimurium: requirement for ribonucleic acid synthesis. J Bacteriol. 1968 Oct;96(4):1079–1088. doi: 10.1128/jb.96.4.1079-1088.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram D., Vatter A. E., Koffler H. Attachment and structural features of flagella of certain bacilli. J Bacteriol. 1966 May;91(5):2045–2068. doi: 10.1128/jb.91.5.2045-2068.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Anderson T. F., Stephens R. Decomposition of T6 bacteriophage in alkaline solutions. Virology. 1964 May;23(1):113–117. doi: 10.1016/s0042-6822(64)80017-9. [DOI] [PubMed] [Google Scholar]
- Asakura S. A kinetic study of in vitro polymerization of flagellin. J Mol Biol. 1968 Jul 14;35(1):237–239. doi: 10.1016/s0022-2836(68)80051-8. [DOI] [PubMed] [Google Scholar]
- Asakura S., Eguchi G., Iino T. Salmonella flagella: in vitro reconstruction and over-all shapes of flagellar filaments. J Mol Biol. 1966 Apr;16(2):302–316. doi: 10.1016/s0022-2836(66)80174-2. [DOI] [PubMed] [Google Scholar]
- Asakura S., Eguchi G., Iino T. Unidirectional growth of Salmonella flagella in vitro. J Mol Biol. 1968 Jul 14;35(1):227–236. doi: 10.1016/s0022-2836(68)80050-6. [DOI] [PubMed] [Google Scholar]
- BAYLEY S. T., KUSHNER D. J. THE RIBOSOMES OF THE EXTREMELY HALOPHILIC BACTERIUM, HALOBACTERIUM CUTIRUBRUM. J Mol Biol. 1964 Sep;9:654–669. doi: 10.1016/s0022-2836(64)80173-x. [DOI] [PubMed] [Google Scholar]
- BENNETT H. S. The concepts of membrane flow and membrane vesiculation as mechanisms for active transport and ion pumping. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):99–103. doi: 10.1083/jcb.2.4.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B., Hiebert E. Formation of an infectious nucleoprotein from protein and nucleic acid isolated from a small spherical virus. Virology. 1967 Jun;32(2):354–356. doi: 10.1016/0042-6822(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B., Hills G. J., Markham R. A study of the self-assembly process in a small spherical virus. Formation of organized structures from protein subunits in vitro. Virology. 1967 Feb;31(2):354–379. doi: 10.1016/0042-6822(67)90180-8. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B., Wagner G. W., Bracker C. E. The self-assembly of a nucleic-acid free pseudo-top component for a small spherical virus. Virology. 1968 Sep;36(1):146–149. doi: 10.1016/0042-6822(68)90126-8. [DOI] [PubMed] [Google Scholar]
- Bayley S. T., Griffiths E. A cell-free amino acid incorporating system from an extremely halophilic bacterium. Biochemistry. 1968 Jun;7(6):2249–2256. doi: 10.1021/bi00846a030. [DOI] [PubMed] [Google Scholar]
- Bayley S. T. Reassociation of dissociated structural protein with ribosomal particles of an extremely halophilic bacterium. J Mol Biol. 1966 Jul;18(2):330–338. doi: 10.1016/s0022-2836(66)80250-4. [DOI] [PubMed] [Google Scholar]
- Behnke O. Incomplete microtubules observed in mammalian blood platelets during microtubule polymerization. J Cell Biol. 1967 Aug;34(2):697–701. doi: 10.1083/jcb.34.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 18. The masking of adenosine triphosphatase in submitochondrial particles and its reactivation by phospholipids. J Biol Chem. 1968 Jul 25;243(14):3901–3905. [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XVII. Further resolution of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1968 Jul 25;243(14):3891–3900. [PubMed] [Google Scholar]
- Butler T. F., Smith G. L., Grula E. A. Bacterial cell membranes. I. Reaggregation of membrane subunits from Micrococcus lysodeikticus. Can J Microbiol. 1967 Nov;13(11):1471–1479. doi: 10.1139/m67-195. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L. ASSEMBLY AND STABILITY OF THE TOBACCO MOSAIC VIRUS PARTICLE. Adv Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., DULBECCO R., KLUG A., LWOFF A., STOKER M. G., TOURNIER P., WILDY P. Proposals. Cold Spring Harb Symp Quant Biol. 1962;27:49–50. doi: 10.1101/sqb.1962.027.001.007. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L. Structure of bushy stunt virus. Nature. 1956 Mar 10;177(4506):475–476. doi: 10.1038/177475a0. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., WATSON J. D. Structure of small viruses. Nature. 1956 Mar 10;177(4506):473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- Campbell R. D. Desmosome formation: an hypothesis of membrane accumulation. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1422–1429. doi: 10.1073/pnas.58.4.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Cell-free protein synthesis programmed with R17 RNA: identification of two phage proteins. J Mol Biol. 1966 Oct 28;21(1):173–193. doi: 10.1016/0022-2836(66)90086-6. [DOI] [PubMed] [Google Scholar]
- Chapman D., Kamat V. B., de Gier J., Penkett S. A. Nuclear magnetic resonance studies of erythrocyte membranes. J Mol Biol. 1968 Jan 14;31(1):101–114. doi: 10.1016/0022-2836(68)90058-2. [DOI] [PubMed] [Google Scholar]
- Coetzee H. L., De Klerk H. C., Coetzee J. N., Smit J. A. Bacteriophage-tail-like particles associated with intra-species killing of Proteus vulgaris. J Gen Virol. 1968 Jan;2(1):29–36. doi: 10.1099/0022-1317-2-1-29. [DOI] [PubMed] [Google Scholar]
- Dimmitt K., Bradford S., Simon M. Synthesis of bacterial flagella. I. Requirement for protein and ribonucleic acid synthesis during flagellar regeneration in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):801–810. doi: 10.1128/jb.95.3.801-810.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. S., Lielausis I. Some steps in the assembly of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):263–276. doi: 10.1016/0022-2836(68)90008-9. [DOI] [PubMed] [Google Scholar]
- Edgar R. S., Wood W. B. Morphogenesis of bacteriophage T4 in extracts of mutant-infected cells. Proc Natl Acad Sci U S A. 1966 Mar;55(3):498–505. doi: 10.1073/pnas.55.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A., Bolle A., Epstein R. H. Electron microscopic study of the structure of mutants of bacteriophage T4D defective in tail fiber genes. Virology. 1967 Nov;33(3):405–412. doi: 10.1016/0042-6822(67)90116-x. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Terry T. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. I. Sodium dodecyl sulfate solubilization. Biochim Biophys Acta. 1967 Jul 3;135(3):381–390. doi: 10.1016/0005-2736(67)90028-4. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Kaesberg P. Comparative studies of the coat proteins of R-17 and M-12 bacteriophages. J Mol Biol. 1965 Aug;13(1):260–268. doi: 10.1016/s0022-2836(65)80095-x. [DOI] [PubMed] [Google Scholar]
- Esau K., Cronshaw J. Relation of tobacco mosaic virus to the host cells. J Cell Biol. 1967 Jun;33(3):665–678. doi: 10.1083/jcb.33.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHER S., BRIERLEY G., KLOUWEN H., SLAUTTERBACK D. B. Studies of the electron transfer system. 47. The role of phospholipids in electron transfer. J Biol Chem. 1962 Oct;237:3264–3272. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B. Reconstitution of tobacco mosaic virus. III. Improved methods and the use of mixed nucleic acids. Biochim Biophys Acta. 1959 Jun;33(2):359–370. doi: 10.1016/0006-3002(59)90126-x. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Bancroft J. B. Structure of the reaggregted protein shells of 2 spherical viruses. Nature. 1968 Nov 23;220(5169):815–816. doi: 10.1038/220815a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Arrangement of protein subunits and the distribution of nucleic acid in turnip yellow mosaic virus. II. Electron microscopic studies. J Mol Biol. 1966 Jan;15(1):344–364. doi: 10.1016/s0022-2836(66)80231-0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Structure of broad bean mottle virus. I. Analysis of electron micrographs comparison with turnip yellow mosaic virus and its top component. J Mol Biol. 1967 Mar 14;24(2):289–302. doi: 10.1016/0022-2836(67)90333-6. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Evidence for long DNA strands in the replicating pool after T4 infection. Proc Natl Acad Sci U S A. 1968 Jan;59(1):131–138. doi: 10.1073/pnas.59.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., KERRIDGE D., HORNE R. W. THE FINE STRUCTURE AND MODE OF ATTACHMENT OF THE SHEATHED FLAGELLUM OF VIBRIO METCHNIKOVII. J Cell Biol. 1963 Aug;18:327–336. doi: 10.1083/jcb.18.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., FLEISCHER S. THE ROLE OF LIPIDS IN MITOCHONDRIAL ELECTRON TRANSFER AND OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1963 Oct 22;70:554–582. doi: 10.1016/0006-3002(63)90793-5. [DOI] [PubMed] [Google Scholar]
- GROSS J. Collagen. Sci Am. 1961 May;204:121–130. [PubMed] [Google Scholar]
- Gerber B. R., Noguchi H. Volume change associated with the G-F transformation of flagellin. J Mol Biol. 1967 Jun 14;26(2):197–210. doi: 10.1016/0022-2836(67)90291-4. [DOI] [PubMed] [Google Scholar]
- Gibbons I. R. Chemical dissection of cilia. Arch Biol (Liege) 1965;76(2):317–352. [PubMed] [Google Scholar]
- Gierer L., Gierer A. Synthesis of ribosomal proteins and formation of ribosomes in Escherichia coli. J Mol Biol. 1968 Jul 14;34(2):293–303. doi: 10.1016/0022-2836(68)90254-4. [DOI] [PubMed] [Google Scholar]
- Green D. E., Allmann D. W., Bachmann E., Baum H., Kopaczyk K., Korman E. F., Lipton S., MacLennan D. H., McConnell D. G., Perdue J. F. Formation of membranes by repeating units. Arch Biochem Biophys. 1967 Mar;119(1):312–335. doi: 10.1016/0003-9861(67)90461-4. [DOI] [PubMed] [Google Scholar]
- HALPEREN S., EGGERS H. J., TAMM I. EVIDENCE FOR UNCOUPLED SYNTHESIS OF VIRAL RNA AND VIRAL CAPSIDS. Virology. 1964 Sep;24:36–46. doi: 10.1016/0042-6822(64)90145-x. [DOI] [PubMed] [Google Scholar]
- HARRISON P. M. The structure of apoferritin: molecular size, shape and symmetry from x-ray data. J Mol Biol. 1963 May;6:404–422. doi: 10.1016/s0022-2836(63)80052-2. [DOI] [PubMed] [Google Scholar]
- HOFMANN T., HARRISON P. M. The structure of apoferritin: degradation into and molecular weight of subunits. J Mol Biol. 1963 Apr;6:256–267. doi: 10.1016/s0022-2836(63)80087-x. [DOI] [PubMed] [Google Scholar]
- HORNE R. W., WILDY P. Symmetry in virus architecture. Virology. 1961 Nov;15:348–373. doi: 10.1016/0042-6822(61)90366-x. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Penniston J. T., Asai J., Green D. E. The conformational basis of energy conservation in membrane systems. II. Correlation between conformational change and functional states. Proc Natl Acad Sci U S A. 1968 Mar;59(3):830–837. doi: 10.1073/pnas.59.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R., Schubert D., Rudolph U. Self-assessment of protein subunits from bacteriophage fr. Biochem Biophys Res Commun. 1968 Mar 12;30(5):576–581. doi: 10.1016/0006-291x(68)90092-2. [DOI] [PubMed] [Google Scholar]
- Heysood S. M., Rich A. In vitro synthesis of native myosin, actin, and tropomyosin from embryonic chick polyribosomes. Proc Natl Acad Sci U S A. 1968 Feb;59(2):590–597. doi: 10.1073/pnas.59.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert E., Bancroft J. B., Bracker C. E. The assembly in vitro of some small spherical viruses, hybrid viruses, and other nucleoproteins. Virology. 1968 Mar;34(3):492–508. doi: 10.1016/0042-6822(68)90069-x. [DOI] [PubMed] [Google Scholar]
- Hirai A., Wildman S. G. Intracellular site of assembly of TMV-RNA and protein. Virology. 1967 Nov;33(3):467–473. doi: 10.1016/0042-6822(67)90122-5. [DOI] [PubMed] [Google Scholar]
- Hohn T. Selfassembly of defective particles of the bacteriophage fr. Eur J Biochem. 1967 Sep;2(2):152–155. doi: 10.1111/j.1432-1033.1967.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Fujimura R. K., Nomura M. Reconstitution of functionally active ribosomes from inactive subparticles and proteins. Proc Natl Acad Sci U S A. 1966 Jan;55(1):198–204. doi: 10.1073/pnas.55.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITANO H. A., ROBINSON E. A., GOTTLIEB A. J. DISSOCIATION AND ASSOCIATION OF HEMOGLOBIN SUBUNITS. Brookhaven Symp Biol. 1964 Dec;17:194–203. [PubMed] [Google Scholar]
- Iino T., Mitani M. A mutant of Salmonella possessing straight flagella. J Gen Microbiol. 1967 Oct;49(1):81–88. doi: 10.1099/00221287-49-1-81. [DOI] [PubMed] [Google Scholar]
- Ikkai T., Ooi T. The effects of pressure on F-G transformation of actin. Biochemistry. 1966 May;5(5):1551–1560. doi: 10.1021/bi00869a015. [DOI] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V. The production of inactive phage P22 particles following induction. Virology. 1967 Oct;33(2):317–322. doi: 10.1016/0042-6822(67)90150-x. [DOI] [PubMed] [Google Scholar]
- Itoh T., Otaka E., Osawa S. Release of ribosomal proteins from Escherichia coli ribosomes with high concentrations of lithium chloride. J Mol Biol. 1968 Apr 14;33(1):109–122. doi: 10.1016/0022-2836(68)90284-2. [DOI] [PubMed] [Google Scholar]
- Iwabuchi M., Kono M., Oumi T., Osawa S. The RNA components in ribonucleoprotein particles occurring during the course of ribosome formation in Escherichia coli. Biochim Biophys Acta. 1965 Oct 11;108(2):211–219. doi: 10.1016/0005-2787(65)90005-5. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Benson A. A. Association of lipids and proteins in chloroplast lamellar membrane. Biochim Biophys Acta. 1968 Jun 11;150(4):686–693. doi: 10.1016/0005-2736(68)90058-8. [DOI] [PubMed] [Google Scholar]
- Ji T. H., Hess J. L., Benson A. A. Studies on chloroplast membrane structure. I. Association of pigments with chloroplast lamellar protein. Biochim Biophys Acta. 1968 Jun 11;150(4):676–685. doi: 10.1016/0005-2736(68)90057-6. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The cooperative nature of G-F transformation of actin. Biochim Biophys Acta. 1962 Feb 12;57:22–31. doi: 10.1016/0006-3002(62)91073-9. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. FLAGELLAR SYNTHESIS IN SALMONELLA TYPHIMURIUM: THE INCORPORATION OF ISOTOPICALLY-LABELLED AMINO ACIDS INTO FLAGELLIN. J Gen Microbiol. 1963 Oct;33:63–76. doi: 10.1099/00221287-33-1-63. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D., HORNE R. W., GLAUERT A. M. Structural components of flagella from Salmonella typhimurium. J Mol Biol. 1962 Apr;4:227–238. doi: 10.1016/s0022-2836(62)80001-1. [DOI] [PubMed] [Google Scholar]
- KERRIDGE D. The effect of inhibitors on the formation of flagella by Salmonella typhimurium. J Gen Microbiol. 1960 Dec;23:519–538. doi: 10.1099/00221287-23-3-519. [DOI] [PubMed] [Google Scholar]
- KOFFLER H. Protoplasmic differences between mesophiles and thermophiles. Bacteriol Rev. 1957 Dec;21(4):227–240. doi: 10.1128/br.21.4.227-240.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONO M., OSAWA S. INTERMEDIARY STEPS OF RIBOSOME FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1964 Jun 22;87:326–334. doi: 10.1016/0926-6550(64)90228-2. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Meselson M., Raskas H. J. Cyclic dissociation into stable subunits and re-formation of ribosomes during bacterial growth. J Mol Biol. 1968 Jan 28;31(2):277–289. doi: 10.1016/0022-2836(68)90444-0. [DOI] [PubMed] [Google Scholar]
- Kaiser A. D. On the internal structure of bacteriophage lambda. J Gen Physiol. 1966 Jul;49(6):171–178. doi: 10.1085/jgp.49.6.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
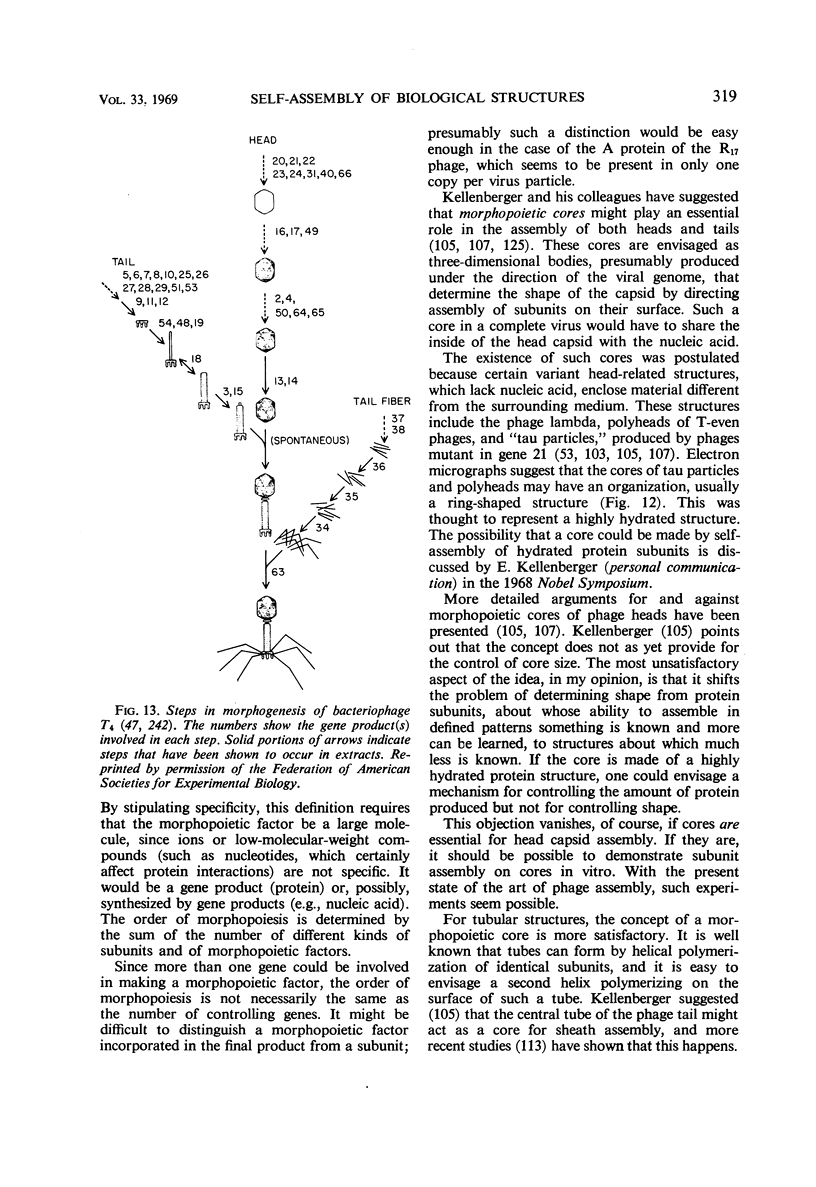
- Kellenberger E., Eiserling F. A., Boy de la Tour E. Studies on the morphopoiesis of the head of phage T-even. 3. The cores of head-related structures. J Ultrastruct Res. 1967 Dec 12;21(3):335–360. doi: 10.1016/s0022-5320(67)80099-6. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. Studies on the morphopoiesis of the head of phage T-even. V. The components of the T4 capsid and of other, capsid-related structures. Virology. 1968 Mar;34(3):549–561. doi: 10.1016/0042-6822(68)90074-3. [DOI] [PubMed] [Google Scholar]
- Kessel R. G. An electron microscope study of spermiogenesis in the grasshopper with particular reference to the development of microtubular systems during differentiation. J Ultrastruct Res. 1967 Jun;18(5):677–694. doi: 10.1016/s0022-5320(67)80213-2. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Klotz I. M. Protein subunits: a table. Science. 1967 Feb 10;155(3763):697–698. doi: 10.1126/science.155.3763.697. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. 3. Specificity of protein-DNA association in vivo. J Mol Biol. 1966 Nov 14;21(2):305–312. doi: 10.1016/0022-2836(66)90101-x. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. I. Hydrodynamic measurements and biological characterization. J Mol Biol. 1966 Nov 14;21(2):281–292. doi: 10.1016/0022-2836(66)90099-4. [DOI] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. II. Interaction of the protein with DNA in vitro. J Mol Biol. 1966 Nov 14;21(2):293–304. doi: 10.1016/0022-2836(66)90100-8. [DOI] [PubMed] [Google Scholar]
- Koch M. A., Eggers H. J., Anderer F. A., Schlumberger H. D., Frank H. Structure of simian virus 40. I. Purification and physical characterization of the virus particle. Virology. 1967 Jul;32(3):503–510. doi: 10.1016/0042-6822(67)90302-9. [DOI] [PubMed] [Google Scholar]
- Kopaczyk K., Asai J., Green D. E. Reconstitution of the repeating unit of the mitochondrial inner membrane. Arch Biochem Biophys. 1968 Jul;126(1):358–379. doi: 10.1016/0003-9861(68)90592-4. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- LAUFFER M. A., ANSEVIN A. T., CARTWRIGHT T. E., BRINTON C. C., Jr Polymerization-depolymerization of tobacco mosaic virus protein. Nature. 1958 May 10;181(4619):1338–1339. doi: 10.1038/1811338b0. [DOI] [PubMed] [Google Scholar]
- LEVIN O. Electron microscope observations on some 60 s erythrocruorins and their split products. J Mol Biol. 1963 Jan;6:95–101. doi: 10.1016/s0022-2836(63)80084-4. [DOI] [PubMed] [Google Scholar]
- LODISH H. F., COOPER S., ZINDER N. D. HOST-DEPENDENT MUTANTS OF THE BACTERIOPHAGE F2. IV. ON THE BIOSYNTHESIS OF A VIRAL RNA POLYMERASE. Virology. 1964 Sep;24:60–70. doi: 10.1016/0042-6822(64)90148-5. [DOI] [PubMed] [Google Scholar]
- LOWY J., HANSON J. ELECTRON MICROSCOPE STUDIES OF BACTERIAL FLAGELLA. J Mol Biol. 1965 Feb;11:293–313. doi: 10.1016/s0022-2836(65)80059-6. [DOI] [PubMed] [Google Scholar]
- LOWY J., MCDONOUGH M. W. STRUCTURE OF FILAMENTS PRODUCED BY RE-AGGREGATION OF SALMONELLA FLAGELLIN. Nature. 1964 Oct 10;204:125–127. doi: 10.1038/204125a0. [DOI] [PubMed] [Google Scholar]
- LWOFF A., ANDERSON T. F., JACOB F. [Remarks on the characteristics of the infectious viral particle]. Ann Inst Pasteur (Paris) 1959 Sep;97:281–289. [PubMed] [Google Scholar]
- Laemmli U. K., Eiserling F. A. Studies on the morphopoiesis of the head of phage T-even. V. The formation of polyheads. Mol Gen Genet. 1968 May 17;101(4):333–345. doi: 10.1007/BF00436231. [DOI] [PubMed] [Google Scholar]
- Lauffer M. A., Stevens C. L. Structure of the tobacco mosaic virus particle; polymerization of tobacco mosaic virus protein. Adv Virus Res. 1968;13:1–63. doi: 10.1016/s0065-3527(08)60250-x. [DOI] [PubMed] [Google Scholar]
- Lerman M. I., Spirin A. S., Gavrilova L. P., Golov V. F. Studies on the structure of ribosomes. II. Stepwise dissociation of protein from ribosomes by caesium chloride and the re-assembly of ribosome-like particles. J Mol Biol. 1966 Jan;15(1):268–281. doi: 10.1016/s0022-2836(66)80226-7. [DOI] [PubMed] [Google Scholar]
- Levitt J., Becker Y. The effect of cytosine arabinoside on the replication of herpes simplex virus. Virology. 1967 Jan;31(1):129–134. doi: 10.1016/0042-6822(67)90016-5. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Horiuchi K., Zinder N. D. Mutants of the bacteriophage f2. V. On the production of noninfectious phage particles. Virology. 1965 Oct;27(2):139–155. doi: 10.1016/0042-6822(65)90154-6. [DOI] [PubMed] [Google Scholar]
- MCDONOUGH M. W. AMINO ACID COMPOSITION OF ANTIGENICALLY DISTINCT SALMONELLA FLAGELLAR PROTEINS. J Mol Biol. 1965 Jun;12:342–355. doi: 10.1016/s0022-2836(65)80258-3. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D., Silengo L. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry. 1968 Jan;7(1):456–472. doi: 10.1021/bi00841a058. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Brown D. M., Glazer A. N. The formation of bacterial flagella. 3. Characterization of the subunits of the flagella of Bacillus subtilis and Spirillum serpens. J Mol Biol. 1967 Aug 28;28(1):45–51. doi: 10.1016/s0022-2836(67)80076-7. [DOI] [PubMed] [Google Scholar]
- Martinez R. J., Gordee E. Z. Formation of bacterial flagella. I. Demonstration of a functional flagellin pool in spirillum serpens and bacillus subtilis. J Bacteriol. 1966 Feb;91(2):870–875. doi: 10.1128/jb.91.2.870-875.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R. J., Ichiki A. T., Lundh N. P., Tronick S. R. A single amino acid substitution responsible for altered flagellar morphology. J Mol Biol. 1968 Jun 28;34(3):559–564. doi: 10.1016/0022-2836(68)90180-0. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. The formation of bacterial flagella. II. The relative stability of messenger RNA for flagellin biosynthesis. J Mol Biol. 1966 May;17(1):10–17. doi: 10.1016/s0022-2836(66)80090-6. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Schaller H. The topology of DNA from the small filamentous bacteriophage fd. J Mol Biol. 1966 Jan;15(1):1–7. doi: 10.1016/s0022-2836(66)80204-8. [DOI] [PubMed] [Google Scholar]
- Marvin D. A. X-ray diffraction and electron microscope studies on the structure of the small filamentous bacteriophage fd. J Mol Biol. 1966 Jan;15(1):8–17. doi: 10.1016/s0022-2836(66)80205-x. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Takemoto K. K., Daniel W. A. Replication of polyoma virus in mouse embryo cells: electron microscopic observations. Virology. 1966 Oct;30(2):242–256. doi: 10.1016/0042-6822(66)90099-7. [DOI] [PubMed] [Google Scholar]
- Matthews R. E. Reconstitution of turnip yellow mosaic virus RNA with TMV protein subunits. Virology. 1966 Sep;30(1):82–96. doi: 10.1016/s0042-6822(66)81012-7. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J., Roberts R. B. The Synthesis of Ribosomes in E. coli: III. Synthesis of Ribosomal RNA. Biophys J. 1962 Jan;2(1):57–82. doi: 10.1016/s0006-3495(62)86841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClare C. W. Bonding between proteins and lipids in the envelopes of Halobacterium halobium. Nature. 1967 Nov 25;216(5117):766–771. doi: 10.1038/216766a0. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W. THE REPLICATION OF DNA IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):671–682. doi: 10.1073/pnas.44.7.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M. R., Hirt B., Weil R. Mouse cellular DNA enclosed in polyoma viral capsids (pseudovirions). Proc Natl Acad Sci U S A. 1967 Oct;58(4):1381–1388. doi: 10.1073/pnas.58.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. I. Structure of the contracted sheath and polysheath. J Mol Biol. 1967 Apr 28;25(2):167–200. doi: 10.1016/0022-2836(67)90136-2. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. II. Rearrangement of the sheath subunits during contraction. J Mol Biol. 1967 Apr 28;25(2):201–208. doi: 10.1016/0022-2836(67)90137-4. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Nomura M., Traub P. Structure and function of Escherichia coli ribosomes. 3. Stoichiometry and rate of the reconstitution of ribosomes from subribosomal particles and split proteins. J Mol Biol. 1968 Jun 28;34(3):609–619. doi: 10.1016/0022-2836(68)90184-8. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Olsen B. R. Electron microscope studies on collagen. V. The structure of segment-long-spacing aggregates consisting of molecules renaturated from the isolated alpha-3-fraction of rat tail tendon collagen. J Ultrastruct Res. 1967 Aug 30;19(5):432–445. doi: 10.1016/s0022-5320(67)80072-8. [DOI] [PubMed] [Google Scholar]
- Olsen B. R. Electron microscope studies on collagen. VI. The structure of segment-long-spacing aggregates consisting of molecules renatured from isolated alpha-fractions of codfish skin collagen. J Ultrastruct Res. 1967 Aug 30;19(5):446–473. doi: 10.1016/s0022-5320(67)80073-x. [DOI] [PubMed] [Google Scholar]
- Osawa S. Biosynthesis of ribosomes in bacterial cells. Prog Nucleic Acid Res Mol Biol. 1965;4:161–188. doi: 10.1016/s0079-6603(08)60787-4. [DOI] [PubMed] [Google Scholar]
- Otaka E., Itoh T., Osawa S. Protein components in the 40s ribonucleoprotein particles in Escherichia coli. Science. 1967 Sep 22;157(3795):1452–1454. doi: 10.1126/science.157.3795.1452. [DOI] [PubMed] [Google Scholar]
- Outka D. E., Kluss B. C. The ameba-to-flagellate transformation in Tetramitus rostratus. II. Microtubular morphogenesis. J Cell Biol. 1967 Nov;35(2):323–346. doi: 10.1083/jcb.35.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERUTZ M. F. THE HEMOGLOBIN MOLECULE. Sci Am. 1964 Nov;211:64–76. doi: 10.1038/scientificamerican1164-64. [DOI] [PubMed] [Google Scholar]
- PETRUSKA J. A., HODGE A. J. A SUBUNIT MODEL FOR THE TROPOCOLLAGEN MACROMOLECULE. Proc Natl Acad Sci U S A. 1964 May;51:871–876. doi: 10.1073/pnas.51.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGLAZOV B. F., BORHSENIUS S. N., BELAVTSEVA E. M. RECONSTITUTION AND CRYSTALLIZATION OF TAIL SHEATHS OF PHAGE T2. Virology. 1965 Apr;25:650–658. doi: 10.1016/0042-6822(65)90093-0. [DOI] [PubMed] [Google Scholar]
- Penniston J. T., Harris R. A., Asai J., Green D. E. The conformational basis of energy transformations in membrane systems. I. Conformational changes in mitochondria. Proc Natl Acad Sci U S A. 1968 Feb;59(2):624–631. doi: 10.1073/pnas.59.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poglazov B. F., Mesyanzhinov V. V. Crystallization of the protein of the head of bacteriophage T2 in vitro. Virology. 1967 Mar;31(3):449–452. doi: 10.1016/0042-6822(67)90225-5. [DOI] [PubMed] [Google Scholar]
- REISSIG M., KAPLAN A. S. The morphology of noninfective pseudorabies virus produced by cells treated with 5-fluorouracil. Virology. 1962 Jan;16:1–8. doi: 10.1016/0042-6822(62)90196-4. [DOI] [PubMed] [Google Scholar]
- Razin S., Boschwitz C. The membrane of the Streptobacillus moniliformis L-phase. J Gen Microbiol. 1968 Nov;54(1):21–32. doi: 10.1099/00221287-54-1-21. [DOI] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. The cell membrane of mycoplasma. Ann N Y Acad Sci. 1967 Jul 28;143(1):115–129. doi: 10.1111/j.1749-6632.1967.tb27651.x. [DOI] [PubMed] [Google Scholar]
- Rhodes M. E. Flagellation as a criterion for the classification of bacteria. Bacteriol Rev. 1965 Dec;29(4):442–465. doi: 10.1128/br.29.4.442-465.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo D. L. The arrangement of subunits in flagellar fibers. J Ultrastruct Res. 1967 Feb;17(3):266–277. doi: 10.1016/s0022-5320(67)80048-0. [DOI] [PubMed] [Google Scholar]
- Roberts F. F., Jr, Doetsch R. N. Some singular properties of bacterial flagella, with special reference to monotrichous forms. J Bacteriol. 1966 Jan;91(1):414–421. doi: 10.1128/jb.91.1.414-421.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Steitz J. E. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth L. E. Electron microscopy of mitosis in amebae. 3. Cold and urea treatments: a basis for tests of direct effects of mitotic inhibitors on microtubule formation. J Cell Biol. 1967 Jul;34(1):47–59. doi: 10.1083/jcb.34.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOESTRAND F. S., ELFVIN L. G. THE GRANULAR STRUCTURE OF MITOCHONDRIAL MEMBRANES AND OF CYTOMEMBRANES AS DEMONSTRATED IN FROZEN-DRIED TISSUE. J Ultrastruct Res. 1964 Apr;10:263–292. doi: 10.1016/s0022-5320(64)80009-5. [DOI] [PubMed] [Google Scholar]
- SLAUTTERBACK D. B. CYTOPLASMIC MICROTUBULES. I. HYDRA. J Cell Biol. 1963 Aug;18:367–388. doi: 10.1083/jcb.18.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Netschey A. Physical chemistry of isolated bacterial membranes. Biochim Biophys Acta. 1965 Oct 18;107(3):539–545. doi: 10.1016/0304-4165(65)90198-4. [DOI] [PubMed] [Google Scholar]
- Santer M., Ruebush T. K., Van Brunt J., Oldmixon E., Hess R., Primakoff P., Palade P. Identification of a precursor pool of ribosome protein in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1355–1367. doi: 10.1128/jb.95.4.1355-1367.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalla T. A., Amici A. The distribution of viral antigen in cells infected with tobacco mosaic virus as revealed by electron microscopy. Virology. 1967 Jan;31(1):78–91. doi: 10.1016/0042-6822(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Silverman P. M., Rosenthal S., Mobach H., Valentine R. C. Two new classes of F-pili mutants of Escherichia coli resistant to infection by the male specific bacteriophage F2. Virology. 1968 Sep;36(1):142–146. doi: 10.1016/0042-6822(68)90125-6. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Allosteric regulation of enzyme activity. Adv Enzymol Relat Areas Mol Biol. 1966;28:41–154. doi: 10.1002/9780470122730.ch2. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Identification of the A protein as a structural component of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):923–936. doi: 10.1016/0022-2836(68)90328-8. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Isolation of the A protein from bacteriphage R17. J Mol Biol. 1968 May 14;33(3):937–945. doi: 10.1016/0022-2836(68)90329-x. [DOI] [PubMed] [Google Scholar]
- Stephens R. E. Reassociation of microtubule protein. J Mol Biol. 1968 Apr 28;33(2):517–519. doi: 10.1016/0022-2836(68)90210-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Hebert R. R., Hartman K. A. Ribonucleoprotein complexes formed between bacteriophage MS2 RNA and MS2 protein in vitro. J Mol Biol. 1967 May 14;25(3):455–463. doi: 10.1016/0022-2836(67)90198-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakada D. Translational control of bacteriophage MS2 RNA cistrons by MS2 coat protein: polyacrylamide gel electrophoretic analysis of proteins synthesized in vitro. J Mol Biol. 1968 Feb 14;31(3):431–440. doi: 10.1016/0022-2836(68)90419-1. [DOI] [PubMed] [Google Scholar]
- Sund H., Weber K. The quaternary structure of proteins. Angew Chem Int Ed Engl. 1966 Feb;5(2):231–245. doi: 10.1002/anie.196602311. [DOI] [PubMed] [Google Scholar]
- Takai M. Complex-formation by protein and nucleic acid. I. Evidence for specific interaction between phi-X174 phage protein and phi-X174 phage deoxyribonucleic acid in vitro. Biochim Biophys Acta. 1966 Apr 18;119(1):20–28. [PubMed] [Google Scholar]
- Terry T. M., Engelman D. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. II. Modes of aggregation of solubilized membrane components. Biochim Biophys Acta. 1967 Jul 3;135(3):391–405. doi: 10.1016/0005-2736(67)90029-6. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Porter K. R. Studies on the microtubules in heliozoa. II. The effect of low temperature on these structures in the formation and maintenance of the axopodia. J Cell Biol. 1967 Jul;34(1):327–343. doi: 10.1083/jcb.34.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Hosokawa K., Craven G. R., Nomura M. Structure and function of E. coli ribosomes, IV. Isolation and characterization of functionally active ribosomal proteins. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2430–2436. doi: 10.1073/pnas.58.6.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of Escherichia coli ribosomes. I. Partial fractionation of the functionally active ribosomal proteins and reconstitution of artificial subribosomal particles. J Mol Biol. 1968 Jun 28;34(3):575–593. doi: 10.1016/0022-2836(68)90182-4. [DOI] [PubMed] [Google Scholar]
- Traub P., Söll D., Nomura M. Structure and function of Escherichia coli ribosomes. II. Translational fidelity and efficiency in protein synthesis of a protein-deficient subribosomal particle. J Mol Biol. 1968 Jun 28;34(3):595–608. doi: 10.1016/0022-2836(68)90183-6. [DOI] [PubMed] [Google Scholar]
- Traut R. R., Moore P. B., Delius H., Noller H., Tissières A. Ribosomal proteins of Escherichia coli. I. Demonstration of different primary structures. Proc Natl Acad Sci U S A. 1967 May;57(5):1294–1301. doi: 10.1073/pnas.57.5.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Dreizen P., Stracher A. Studies on subfragment-I, a biologically active fragment of myosin. Proc Natl Acad Sci U S A. 1968 Oct;61(2):659–666. doi: 10.1073/pnas.61.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BRUGGENE, SCHUITEN V., WIEBENGA E. H., GRUBER M. STRUCTURE AND PROPERTIES OF HEMOCYANINS. III. ELECTRON MICROGRAPHS OF HEMOCYANINS FROM DIFFERENT GASTROPODA AND CRUSTACEA. J Mol Biol. 1963 Sep;7:249–253. doi: 10.1016/s0022-2836(63)80005-4. [DOI] [PubMed] [Google Scholar]
- Vaituzis Z., Doetsch R. N. Flagella of Escherichia coli spheroplasts. J Bacteriol. 1966 May;91(5):2103–2104. doi: 10.1128/jb.91.5.2103-2104.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine R. C., Pereira H. G. Antigens and structure of the adenovirus. J Mol Biol. 1965 Aug;13(1):13–20. doi: 10.1016/s0022-2836(65)80076-6. [DOI] [PubMed] [Google Scholar]
- Wagner G. W., Bancroft J. B. The self-assembly of spherical viruses with mixed coat proteins. Virology. 1968 Apr;34(4):748–756. doi: 10.1016/0042-6822(68)90095-0. [DOI] [PubMed] [Google Scholar]
- Warner J. R. The assembly of ribosomes in HeLa cells. J Mol Biol. 1966 Aug;19(2):383–398. doi: 10.1016/s0022-2836(66)80012-8. [DOI] [PubMed] [Google Scholar]
- Weber K. Amino acid sequence studies on the tryptic peptides of the coat protein of the bacteriophage R17. Biochemistry. 1967 Oct;6(10):3144–3154. doi: 10.1021/bi00862a023. [DOI] [PubMed] [Google Scholar]
- Weier T. E., Engelbreicht A. H., Harrison A., Risley E. B. Subunits in the membranes of chloroplasts of Phaseolus vulgaris, Pisum sativum, and Aspidistra sp. J Ultrastruct Res. 1965 Aug;13(1):92–111. doi: 10.1016/s0022-5320(65)80091-0. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. Studies on head-tail union in bacteriophage lambda. J Mol Biol. 1968 Apr 28;33(2):483–489. doi: 10.1016/0022-2836(68)90204-0. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Sakai H., Mazia D. Old and new protein in the formation of the mitotic apparatus in cleaving sea urchin eggs. J Mol Biol. 1967 Jul 14;27(1):1–7. doi: 10.1016/0022-2836(67)90346-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S., King J., Lielausis I., Henninger M. Bacteriophage assembly. Fed Proc. 1968 Sep-Oct;27(5):1160–1166. [PubMed] [Google Scholar]
- Young M., Blanchard M. H., Brown D. Selective nonenzymic cleavage of the myosin rod: isolation of the coiled-coil alpha-rope section from the c-terminus of the molecule. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1087–1094. doi: 10.1073/pnas.61.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Iterson W., Hoeniger J. F., Nijman van Zanten E. A "microtubule" in a bacterium. J Cell Biol. 1967 Jan;32(1):1–10. doi: 10.1083/jcb.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]