Abstract
Background and Aims
Intraspecific reproductive differentiation into sexual and apomictic cytotypes of differing ploidy is a common phenomenon. However, mechanisms enabling the maintenance of both reproductive modes and integrity of cytotypes in sympatry are as yet poorly understood. This study examined the association of sexual and apomictic seed formation with ploidy as well as gene flow towards sexuals within populations of purely polyploid Potentilla puberula.
Methods
The study is based on 22 populations representing various combinations of five polyploid cytotypes (tetraploid–octoploid) from East Tyrol, Austria. Embryo ploidy and the endosperm/embryo ploidy ratio obtained by a flow cytometric seed screen were used to infer reproductive modes of seed formation and to calculate the male and female genomic contributions to the embryo and endosperm. Self-incompatibility (SI) patterns were assessed and a new indirect approach was used to test for the occurrence of intercytotype matings based on the variation in the male genomic contribution to sexually derived embryos on the level of developed seed.
Key Results
Tetraploids formed seeds almost exclusively via sexual reproduction, whereas penta- to octoploids were preferentially apomictic. Non-random distribution of reproductive modes within maternal plants further revealed a tendency to separate the sexual from the apomictic mode among individuals. Self-incompatibility of sexuals indicated functionality of the gametophytic SI system despite tetraploidy of the nuclear genome. We found no indication for significant cross-fertilization of tetraploids by the high polyploids.
Conclusions
The study revealed a rare example of intraspecific differentiation into sexual and apomictic cytotypes at the polyploid level. The integrity of the sexual tetraploids was maintained due to reproductive isolation from the apomictic higher polyploids. Functionality of the gametophytic SI system suggested that the tetraploids are functional diploids.
Keywords: Apomixis, endosperm, European Alps, FCSS, flow cytometry, pollen, polyploidy, Potentilla puberula, reproductive isolation, Rosaceae, sexual reproduction
INTRODUCTION
Angiosperms show three principal modes of seed formation: regular sexual reproduction involving female meiosis and fertilization of the egg cell, and the asexual modes gametophytic and sporophytic (adventitious embryony) apomixis (Asker and Jerling, 1992; Savidan, 2007). Among the asexual modes, gametophytic apomixis constitutes the prevailing developmental pathway represented in several major plant families including the Asteraceae, Poaceae and Rosaceae (Asker and Jerling, 1992; Carman, 1997). It refers to various pathways involving a female gametophyte (or embryo sac) formed by the modification or loss of meiosis (i.e. apomeiosis), embryo formation usually from an unfertilized egg cell (i.e. parthenogenesis) and the development of the endosperm with (i.e. pseudogamy) or without (i.e. autonomous) fertilization (Nogler, 1984).
Gametophytic apomicts (for convenience we use the term apomicts from hereon) are almost exclusively polyploid (Asker and Jerling, 1992; Carman, 1997) with well-documented exceptions limited to diploid genotypes of Boechera (Böcher, 1951) and Paspalum (Siena et al., 2008). Apomicts are either allopolyploids – as in most cases [e.g. Antennaria (Bayer, 1997); Potentilla (Dobeš et al., 2004; Paule et al., 2011)] – or autopolyploids [e.g. Paspalum (Hojsgaard et al., 2008); Ranunculus (Cosendai et al., 2011); Townsendia (Thompson and Whitton, 2006)], whereas their sexual relatives are usually diploid. Concerning the need for excess copies of apomixis factors, polyploidy was proposed to be a requirement for the expression of apomixis (Mogie, 1988). Polyploidy and hybridization may deregulate and repattern gene expression of the normal sexual pathway resulting in apomixis (e.g. Carman, 1997). In accordance with this hypothesis, the sexual ancestors of apomicts are usually outcrossing diploids (Asker and Jerling, 1992). Reproductive differentiation, however, is not necessarily restricted to diploid–polyploid contrasts because it has been rarely observed among polyploids (e.g. Rotreklová et al., 2002).
Space is a crucial factor for the understanding of the evolutionary significance of reproductive differentiation. Sexual and apomictic cytotypes may be spatially separated from each other (i.e. allopatric distribution; e.g. Mráz et al., 2009) or co-occur at the population level (i.e. in sympatry; e.g. Elzinga et al., 1987; Menken et al., 1995; Talent and Dickinson, 2007). Co-existence of reproductively differentiated cytotypes thereby raises questions about mechanisms maintaining their genomic and genetic integrity. The number of (monoploid) genomes per se might promote the integrity of cytotypes, since heteroploid crosses frequently show reduced offspring vitality and/or fertility (Ramsey and Schemske, 1998; Hardy et al., 2001). Thus, in the absence of pre-zygotic barriers, sexual individuals differing in ploidy from co-occurring pollen donor plants were shown to be excluded via the minority cytotype exclusion principle (Levin, 1975). However, no cross-fertilization is needed for apomictic embryo development. Consequently, apomicts may not necessarily be suppressed in heteroploid crosses if variable paternal genomic contributions to the endosperm are tolerated (e.g. Kao, 2007) or the endosperm develops autonomously. Furthermore, pollen transferred in heteroploid crosses and pollen of poor quality induced selfing in otherwise self-incompatible sexuals in cross-pollinations (i.e. mentor effects), promoting the genetic integrity and the maintenance of sexuals in the presence of apomictic relatives (Hörandl and Temsch, 2009). In the absence of reproductive barriers isolating sexuals from apomicts, apomixis may swamp sexuals and come to fixation (Mogie, 1992; Adolfsson and Bengtsson, 2007).
Flow cytometry has been established as an effective and reliable tool for the estimation of nuclear DNA contents and DNA ploidy levels (Doležel et al., 2007b; Greilhuber et al., 2007). The method is particularly useful for high-density (DNA)ploidy screens of individuals even on fine spatial scales (Suda et al., 2007) as well as for high-throughput reproductive mode seed screening. The latter has been established as a flow cytometric technique called the flow cytometric seed screen (FCSS) (Matzk et al., 2000) and is based on a comparison of DNA content (or DNA ploidy) of the endosperm and embryo. The method differentiates between the meiotic and the apomeiotic formation of the embryo sac, the parthenogenetic and zygotic origin of the embryo, and the autonomous vs. pseudogamous development of the endosperm. Recently, FCSS has been extended to calculate male and female genomic contributions to the embryo and endosperm, respectively, in sexual and pseudogamous apomicts (Dobeš et al., 2013). Calculation of the genomic contribution is based on the assumption that endosperms receive a bi-nucleate female contribution, a prerequisite which first can be proven based on cyto-embryological evidence and (molecular marker-aided) progeny surveys (cf. Dobeš et al., 2013). The mathematical formulae developed by these authors, however, are applicable independently of the origin (meiotic vs. apomeiotic) of the male and female gametophytes and the ploidy of parents, i.e. they allow estimation of gamete ploidy variation caused by meiotic disturbances and intercytotype crosses.
The genus Potentilla (Rosaceae) shows considerable variation in reproductive mode among and within species, particularly in the derived and species-rich core group (Dobeš and Paule, 2010; Dobeš et al., 2013). Apomictic elements (i.e. apomeiosis and parthenogenetic origin of embryos) have been documented in at least 16 species (Gentscheff, 1938; Gustafsson, 1947; Löve, 1954; Asker, 1970a), while sexual reproduction was claimed for five species based on embryological evidence (Rutishauser, 1945; Håkansson, 1946; Czapik, 1961, 1962a). In order to initiate seed formation, in all Potentilla species including apomicts functional pollen is needed to fertilize the endosperm (Asker, 1970b), which usually receives a bi-nucleate female contribution (Dobeš et al., 2013).
In Potentilla, reproductive differentiation into sexuals and apomicts is often associated with extensive variation in ploidy also observed at the population level (e.g. Skalinska and Czapik, 1958; Smith, 1971; Dobeš, 1999; Paule et al., 2011). Despite earlier claims of diploid apomixis in Potentilla (Håkansson, 1946; Asker, 1967, 1970c), seed formation by diploids appears to be sexual (Holm and Ghatnekar, 1996b; Holm et al., 1997; Dobeš et al., 2013), leading to sexual diploid–apomictic polyploid contrasts. However, reproductive differentiation at the polyploid level also seems to exist. In Potentilla incana tetraploidy was associated with sexual reproduction (Czapik, 1962a) and hexaploidy with apomixis (Ch. Dobeš, unpubl. res.). Tetraploids were sexual in Potentilla crantzii (Czapik, 1961, 1962b) and P. tabernaemontani (= P. verna; inclusively P. puberula) (Håkansson, 1946), whereas cytotypes of higher ploidy were apomictic in these species (Müntzing, 1931, 1958; Smith, 1963a, b; Asker, 1985). However, this evidence is based on limited sample sizes, and the generality of the ploidy–reproductive mode distinctions remains uncertain.
Potentilla puberula exhibits extensive intra- and interpopulation variability in ploidy (Dobeš, 1999). High frequencies of odd-ploids in natural populations (Dobeš, 1999) and clonal population structure (Paule and Dobeš, 2010) provide indirect evidence for apomixis, whereas the observation of meiotically reduced megaspores suggested sexual reproduction for a tetraploid individual (Håkansson, 1946). However, direct evidence for apomixis and the conclusive proof of sexual reproduction is missing for the species.
Selfing of tetraploid individuals caused a significantly lower seed set (40–100 %) compared with the open-pollinated control in P. puberula (Ch. Dobeš, unpubl. res.), suggesting functionality of a self-incompatibility (SI) system. The homomorphic gametophytic SI system is the common type in the Rosaceae (Barrett, 1988; Weller et al., 1995; Sassa et al., 1996), wherein compatibility of the pollen in a cross is determined by the genotype of the male gametophyte and the (sporophytic) genotype of the pollen recipient. A negative correlation between the effectiveness of SI systems and the ploidy level of a species has been reported from the Rosaceae (Dickinson et al., 2007). The generality of this pattern was proposed by Miller and Venable (2000) who observed a breakdown of incompatibility in 92 % of polyploids associated with diploid self-incompatible plants from families known to have gametophytic SI. The phenomenon is explained by the expression of two pollen S-alleles in a single pollen grain inhibiting all S-RNases in the style of a flower (Stone, 2002).
In the following, we provide a comprehensive characterization of the reproductive system of five cytotypes of P. puberula differing in ploidy, and examine the effects of cytotype mixture on embryo ploidy in sexually derived seed. Specifically, we ask the following questions. (1) Is there an association of reproductive modes with ploidy levels as suggested by the embryological record and the high frequency of odd-ploid cytotypes. (2) Is the gametophytic SI system still functional in sexual polyploids? (3) Do apomictic and particularly odd-ploid individuals expectedly show reduced pollen vitality compared with sexual and/or even-ploids? (4) Are there effective reproductive barriers among cytotypes as suggested by the high frequency of cytologically mixed populations? In particular, are sexual individuals reproductively isolated from apomicts?
MATERIALS AND METHODS
Study system
Potentilla puberula Krašan (= P. pusilla Host, Soják, 2010) belongs to a group of species (Aureae Vernae sensu Wolf, 1908) of mainly European distribution which, according to the latest taxonomic treatment, comprises seven sexual and apomictic species (Kurtto et al., 2004). The species exhibits tetraploids (x = 7; 2n = 28), pentaploids (2n = 35), hexaploids (2n = 42), heptaploids (2n = 49), octoploids (2n = 56) and nonaploids (2n = 63) in the Eastern Alps (Dobeš, 1999). Except for nonaploids, these cytotypes have been observed within the scope of a ploidy screen of about 2000 individuals from sympatric populations within East Tyrol, Austria (Hülber et al., 2013). Although direct evidence for apomixis is missing for P. puberula, embryological studies documented apomixis for its former conspecific P. tabernaemontani (Rutishauser, 1943b; Smith, 1963a). Apomixis was realized in that species as diplospory, i.e. embryo sac mother cells develop from the archespore. Aposporous development of embryo sacs sometimes also occurred side by side with diplospory in the same ovule. The majority of archesporial cells entered a well-defined synapsis condition followed by a complete breakdown of the meiotic process. Egg cells developed parthenogenetically. Mostly two polar nuclei lying close to each other were observed and fused to form the central cell nucleus.
Plant material
A total of 115 individuals of known ploidy (determined by Hülber et al. 2013), covering one to three ploidy levels in each of the 22 sampled populations, were included in the study (Table 1). To uncover reproductive modes of seed formation, field-collected mature seeds sampled from the same plants in 2010 were stored in paper bags until analysed flow cytometrically in spring 2012. Plants were transplanted to the experimental garden of the Department of Pharmacognosy, University of Vienna and grown in pots (14 cm in diameter) using a substrate composed of six parts ground soil, two parts of bark humus and two parts of quartz sand. We used flowers of these plants to determine pollen quality in the following year.
Table 1.
General description of 22 populations of Potentilla puberula in East Tyrol, Austria, including the geographic origin and pathways of seed formation obtained using the flow cytometric seed screen (FCSS) classified by the ploidy of the maternal plant
| Geographic origin |
FCSS analysis |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Population | Latitude | Longitude | m a.s.l | Ploidy | N specimen | N seeds | Reproductive pathway |
N failed | ||||
| Apo | Sex | L | Z | Unknown | |||||||||
| 1 | Gonzach | 46·87578 | 12·66265 | 870 | 5x | 3 | 12 | 9 | 3 | ||||
| 6x | 4 | 20 | 10 | 2 | 1 | 6 | 1 | ||||||
| 2 | Unterleibnig | 46·90337 | 12·63542 | 805 | 5x | 4 | 13 | 12 | 1 | ||||
| 6x | 1 | 5 | 2 | 3 | |||||||||
| 3 | Außer Klaunzer-Berg | 46·97385 | 12·55678 | 1100 | 5x | 3 | 13 | 9 | 1 | 3 | |||
| 7x | 1 | 5 | 3 | 2 | |||||||||
| 4 | Oberpeischlach | 46·93583 | 12·59405 | 1090 | 5x | 2 | 9 | 6 | 2 | 1 | |||
| 7x | 1 | 5 | 3 | 2 | |||||||||
| 5 | Rabenstein | 47·00903 | 12·46575 | 1360 | 5x | 4 | 18 | 14 | 1 | 2 | 1 | ||
| 6 | Obermauern | 47·00472 | 12·43544 | 1320 | 4x | 3 | 13 | 11 | 2 | ||||
| 6x | 1 | 4 | 3 | 3 | |||||||||
| 7 | Hainfels | 46·75068 | 12·43715 | 1190 | 4x | 5 | 20 | 17 | 2 | 1 | |||
| 7x | 4 | 17 | 13 | 3 | 1 | ||||||||
| 8 | Bobojach | 47·01700 | 12·40368 | 1390 | 4x | 5 | 15 | 15 | |||||
| 5x | 1 | 5 | 4 | 1 | |||||||||
| 9 | Raut | 46·78112 | 12·57448 | 1470 | 4x | 4 | 12 | 12 | |||||
| 5x | 1 | 4 | 4 | ||||||||||
| 10 | Zabernig | 47·00467 | 12·5192 | 1330 | 4x | 3 | 11 | 10 | 1 | ||||
| 5x | 4 | 12 | 11 | 1 | |||||||||
| 11 | Kosten | 47·01883 | 12·33275 | 1450 | 5x | 3 | 9 | 9 | |||||
| 8x | 2 | 10 | 6 | 1 | 3 | ||||||||
| 12 | Hopfgarten | 46·78628 | 12·60243 | 1250 | 5x | 4 | 14 | 12 | 2 | ||||
| 7x | 1 | 3 | 3 | ||||||||||
| 13 | Groder | 46·92607 | 12·52558 | 1530 | 4x | 4 | 14 | 1 | 12 | 1 | |||
| 5x | 3 | 9 | 9 | ||||||||||
| 14 | Erlbach | 46·74653 | 12·36964 | 1290 | 5x | 2 | 6 | 6 | |||||
| 7x | 3 | 11 | 8 | 1 | 2 | ||||||||
| 8x | 7 | 34 | 18 | 1 | 13 | 2 | |||||||
| 15 | Lana | 46·98575 | 12·63190 | 1320 | 5x | 3 | 9 | 9 | |||||
| 6x | 3 | 9 | 9 | ||||||||||
| 8x | 1 | 4 | 3 | 1 | |||||||||
| 16 | St. Veit | 46·92663 | 12·42213 | 1580 | 5x | 3 | 9 | 9 | |||||
| 17 | Stein | 47·02757 | 12·52672 | 1350 | 5x | 3 | 12 | 7 | 1 | 4 | |||
| 7x | 2 | 8 | 4 | 3 | 1 | ||||||||
| 8x | 3 | 12 | 8 | 4 | |||||||||
| 18 | Innervillgraten | 46·81183 | 12·36085 | 1450 | 5x | 1 | 3 | 3 | |||||
| 8x | 1 | 4 | 4 | ||||||||||
| 20 | Dorfmäder | 47·02528 | 12·36367 | 1720 | 4x | 5 | 15 | 15 | |||||
| 21 | Moaalm | 47·03358 | 12·62811 | 1800 | 5x | 1 | 3 | 3 | |||||
| 6x | 1 | 4 | 4 | ||||||||||
| 22 | Katalalm | 47·05761 | 12·48822 | 1720 | 5x | 3 | 9 | 9 | |||||
| 38 | Obergaimberg | 46·84612 | 12·78215 | 930 | 7x | 2 | 7 | 5 | 1 | 1 | |||
Latitude/longitude are provided in WGS84 standard. 4x, 5x, 6x, 7x and 8x refer to tetra-, penta-, hexa, hepta- and octoploids, respectively. ‘N specimen’ and ‘N seeds’ specify the number of individuals and seeds used in the FCSS, respectively. ‘Reproductive pathways’ refers to the apomictic (Apo) and sexual (Sex) origin of seeds. Following Dobeš et al. (2013), ‘L’ indicates fertilization of an unreduced egg cell and ‘Z’ indicates apomixis involving an embryo sac of twice the ploidy of the maternal plant. ‘Unknown’ indicates seeds missing a distinct fluorescence signal for the endosperm. ‘N failed’ is the number of seeds which failed in the FCSS.
Flow cytometric seed screen (FCSS)
The relative fluorescence intensity of embryo and endosperm nuclei was determined by flow cytometric analysis of single seeds following Dobeš et al. (2013). Three to five seeds were analysed per individual, 432 seeds in total. Pisum sativum ‘Kleine Rheinländerin’ and Glycine max ‘Inovec’ (Doležel et al., 2007a) were chopped together with the sample and served as internal standards. 4',6-Diamidino-2-phenylindole (DAPI) served as the DNA-selective stain. Measurements were performed on a CyFlow Ploidy Analyser equipped with a 365 nm light-emitting diode (LED Partec, Germany). The sample/standard fluorescence ratio and the endosperm/embryo fluorescence ratio (i.e. the peak index) were calculated from the means of the corresponding fluorescence histograms. The DNA ploidy of embryos was inferred from comparison of the sample/standard fluorescence ratio of seeds with the sample/standard fluorescence ratio of reference individuals of known chromosome number (Ptl4048, 2n = 4x = 28; Ptl4184, 2n = 5x = 35; Ptl4187, Ptl4188, 2n = 7x = 49: Paule et al., 2012). For convenience, we refer to the measured DNA ploidy (Suda et al., 2006) as ploidy.
Inference of reproductive modes and of the male and female genomic contribution
We distinguish between the sexual and the apomictic origin of the embryo. Peak indices <2 are indicative of a sexual origin, while values >2 in combination with the recovery of the maternal ploidy by the embryo indicate apomixis. The female and male genomic contributions are calculated from the embryo and endosperm ploidies using the mathematical formulae introduced by Dobeš et al. (2013). The female genomic contribution to the embryo and endosperm is once and twice the ploidy of the embryo sac, respectively. The male genomic contribution is the number of male genomes transferred by the two sperm to the embryo and endosperm in seeds with sexually derived embryos and by one or two sperm to the endosperm in seeds with parthenogenetically derived embryos. We use, according to Greilhuber (2005), n (the haplophasic chromosome number) to indicate the number of holoploid genomes (i.e. the whole chromosome complement with chromosome number n) and x (the chromosome number of the monoploid genome) when referring to the number of chromosome sets (i.e. the generative ploidy). The female genomic contribution is provided as n or x. The male genomic contribution is calculated as x only because n of the pollen donor is unknown. Estimates of male and female genomic contributions were used to calculate maternal : paternal genome ratios in the endosperm.
To test whether sex and apomixis were randomly associated with each other in a single maternal plant, we applied a Monte Carlo randomization technique using R (R Development Core Team, 2011). The empirical association of reproductive modes was compared with the distribution of associations of 10 000 replications randomly assigning modes to seeds. As a measure of association, we used the percentage of individuals with at least one sexual seed which also derived at least one seed via apomixis – and vice versa.
The effect of population cytotype diversity on the male genomic contribution to the embryo was tested using linear regressions performed for sexually derived seeds of tetraploids. Diversity and the male genomic contribution were measured as the Shannon diversity index based on the cytological diversity of populations (Supplementary Data Table S1) and as x, respectively. Analyses were performed using R (R Development Core Team, 2011).
Breeding system of sexuals
We combined controlled pollinations and the determination of pollen/ovule (P/O) ratios (Cruden, 1977) to infer the breeding system of sexuals. Pollination experiments were carried out from March to May 2011. Flowers were emasculated and bagged a few days before anthesis. Bridal veil was used for bagging as it has the least effect on the microclimate of the bagged flowers (Wyatt et al., 1992). At stigma maturity, flowers were selfed and outcrossed, respectively, by rubbing mature anthers over the recipient stigmas. Each treatment was applied to two flowers of each of 38 individuals. At seed maturity, the number of viable seeds and empty testae was assessed, enabling the calculation of seed/ovule and P/O ratios of each flower. We performed pairwise reciprocal cross-pollinations with all individuals from two randomly selected populations inhabited by tetraploids (populations 6 and 13). The number of filled seed was compared as a measure of the reproductive success between selfed and outcrossed flowers using a generalized linear model. We assumed the number of seeds to be a Poisson-distributed random variable and, thus, applied a log-link function. To consider potential autocorrelation of values derived from flowers of the same individual, we included treatment as a random effect for each pollen receptor plant. The analysis was performed using the function glmer of the library lme4 (Douglas Bates, Martin Maechler and Ben Bolker, 2012. lme4: Linear mixed-effects models using S4 classes. R package version 0·999999-0. http://CRAN.R-project.org/package=lme4#) in R (R Development Core Team, 2011).
The P/O ratios were estimated for a single flower per individual. Anthers were preserved in Carnoy's fixative (60 % ethanol:30 % chloroform:10 % acetic acid) and stained with a solution of Malachite green, acid fuchsin and Orange G (Peterson et al., 2010) for approx. 12 h. Subsequently one mature undehiscent anther per flower was transferred to a glass slide and covered in a drop of 100 µL of distilled water, finely chopped with a razor blade and the resulting suspension homogenized. A 20 µL aliquot of the suspension was transferred to a Fuchs Rosenthal counting chamber (Hecht Assistent, Altau, Switzerland) and pollen grains were counted using a light microscope (Nikon Eclipse 600, Nikon, Japan). Ovules per flower were counted with the aid of a stereo lens (Nikon SMZ-U, Nikon, Japan).
Estimation of pollen quality
Pollen quality was estimated based on the percentage of physiologically vital and morphologically intact pollen grains of a single anther per individual. Anthers were stained using the vitality stain invented by Peterson et al. (2010), which discriminates aborted from non-aborted pollen based on the stainability of the protoplasm. In addition, the shape of pollen grains was used to discriminate morphologically intact pollen (regularly round to oval) from degraded (i.e. deformed) pollen. Only stained and morphologically regular grains were regarded as viable. Pollen was embedded in a drop of distilled water dispersed between an object and cover slide, and 94–211 pollen grains per individual were screened for their viability using a Nikon Eclipse 600 light microscope and bright-field illumination.
Differences in pollen viability among ploidy levels were tested by means of logistic regressions using the proportion of viable pollen grains as response and ploidy level as a categorical predictor. The number of individuals was used as a weighting factor, because proportions of viable pollen were pooled over individuals for each cytotype within populations. In regression analyses, categorical predictors such as ploidy allow for pairwise comparisons only with a pre-defined baseline level. Thus, it was necessary to re-fit the model using different cytotypes as baseline levels, i.e. each cytotype was compared with the remaining ones in a separate model. An inflation of Type I errors due to multiple comparisons was avoided by applying a Bonferroni correction of resulting P-values. Analyses were performed using R (R Development Core Team, 2011).
RESULTS
Variation in reproductive mode
Clear fluorescence signals for both the embryo and the endosperm were obtained from 354 (81·94 %) seeds using FCSS. The remaining seeds either failed (3·93 %) or showed signals for the embryo only (14·13 %). A total of 102 (28·81 %) of the seeds with embryo and endosperm signals were derived through regular sexual reproduction (i.e. fertilization of the reduced egg cell), while 249 seeds (70·34 %) were of apomictic origin. Two seeds originated from the fertilization of an unreduced egg cell (exemplary measurements graphically representing these modes are provided in Supplementary Data Fig. S3). The ploidy of the maternal plant (2n = 5x, population 5) was doubled in a single apomictically derived embryo (4n = 10x). The association of inferred reproductive pathways and modes of seed formation with the ploidy of maternal plants is shown in Table 2. Tetraploids formed 98·9 % of their seeds via regular sexual reproduction. In contrast, higher polyploids (penta- to octoploids) were preferentially apomictic (88·6–100 % of the analysed seeds depending on cytotype). Seven out of the 115 maternal plants formed seeds via both apomixis and sexual reproduction. This share was significantly lower (Monte Carlo randomization: P < 0·001 in both cases) than expected for a random association of reproductive modes based on the observed frequencies of reproductive modes and, thus, shows some tendency to separate the sexual from the apomictic modes among individuals. In nine out of the 22 populations, sexually as well as apomictically derived seeds were found. In contrast, sexual reproduction and apomixis only were observed in two and 11 populations, respectively. A detailed description of the FCSS results is given in the Supplementary Data Results.
Table 2.
Reproductive modes of seed development observed in five cytotypes of Potentilla puberula (Rosaceae)
| Ploidy of the maternal plant | Sexual | Apomictic | Irregular |
|---|---|---|---|
| Tetraploid | 92 | 1 | 0 |
| Pentaploid | 0 | 145 | 2 |
| Hexaploid | 4 | 25 | 1 |
| Heptaploid | 5 | 39 | 0 |
| Octoploid | 1 | 39 | 0 |
Three to five seeds of each of 115 maternal plants were analysed using flow cytometric seed screen (FCSS).
The male and female genomic contribution
The male genomic contribution was related to the reproductive mode. Endosperms and embryos derived via sexual reproduction received a male genomic contribution of 1·57x to 4·06x (Supplementary Data Fig. S2). The female genomic contribution to sexually derived embryos ranged between 0·88n and 1·20n. In the tetraploids, the male and female genomic contribution to sexually derived embryos varied between 1·57x and 2·24x, and 1·78x and 2·39x, respectively. The contributions were negatively correlated with each other (r2 = 0·755, P < 0·001; not significant for the other cytotypes) and resulted in embryo ploidies of 3·87–4·17x. The ratio of the female (maternal m) to male (paternal p) genomic contribution to the endosperm for sexually derived seeds was 2m:0·7–1·4p. The male genomic contribution to the endosperm in apomictically derived seeds was greatly raised compared with sexual seed and varied between 1·71x and 15·74x (Supplementary Data Fig. S2). The female genomic contribution to apomictically derived embryos equals by definition the ploidy of the embryo (see the Materials and Methods). The ratio of the female to male genomic contribution to the endosperm was 2m:0·3–2·2p.
Linear regressions (F1,5 = 3·96, P = 0·103, R2 = 0·44) revealed no significant relationship of the cytotype diversity of populations (Shannon diversity index) to the male genomic contribution to the embryo (and endosperm) of sexually derived seeds of tetraploids (Supplementary Data Fig. S4).
Breeding system of sexuals
The breeding system was established for tetraploid sexual individuals. The proportion of flowers with at least one viable seed was 9·3 % and 65·5 % for selfed and outcrossed individuals, respectively. A generalized linear model revealed a significantly higher number of viable seeds for outcrossed than for selfed flowers: fixed effect coefficient ± s.e. = 5·83 ± 1·03; z = 5·63; P < 0·001; number of groups (i.e. pollinated plants) = 38; number of observations (i.e. flowers) = 484. The P/O ratio ± s.d was 7288·92 ± 5018·08. Thus, tetraploids can be classified as obligate outcrossers following the classification of Cruden (1977).
Pollen quality
Pollen quality varied greatly among individuals, from (almost) complete failure to very high percentages of viable pollen in all cytotypes (1·0–99·1 %, 0·0–92·9 %, 12·6–96·9 %, 0·0–88·1 % and 0·0–86·5 % for tetra-, penta-, hexa-, hepta- and octoploid individuals, respectively). The proportion of viable pollen pooled within populations (Fig. 1) differed significantly among cytotypes of P. puberula (P < 0·001 for all pairwise comparisons; Supplementary Data Table S2). The highest pollen quality was detected in tetraploids, followed by hepta-, hexa-, octo- and pentaploids.
Fig. 1.
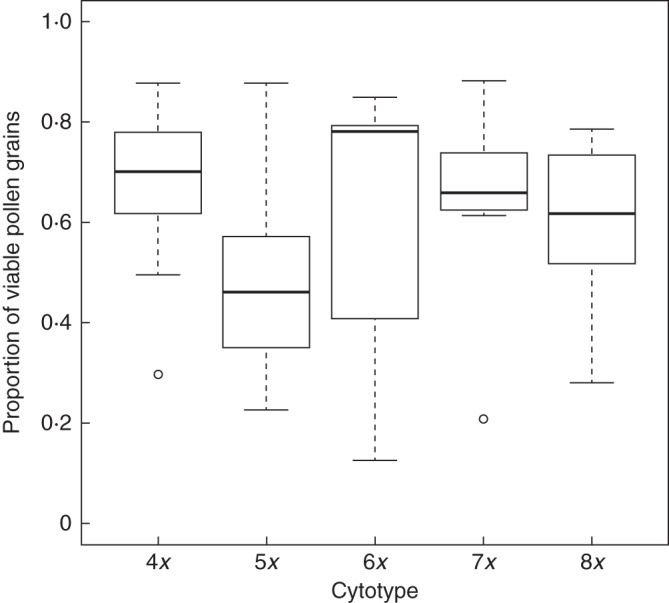
Pollen viability of the five cytotypes of Potentilla puberula. Values represent the proportion of viable grains pooled over individuals of each cytoypes in each population. Tetra- (4x), penta- (5x), hexa- (6x), hepta- (7x) and octoploids (8x) are represented by nine, 22, five, 11, and six populations, respectively.
DISCUSSION
Apomixis and sexual reproduction are non-randomly associated on the level of the individual
Regular sexual reproduction and apomixis were the dominant modes of seed formation in P. puberula (observed for 28·81 and 70·34 % of seeds, respectively). These reproductive modes were non-randomly associated on the level of the individual, as sexually and apomictically derived seeds had a much lower probability of co-occurrence within a single individual than can be expected based on their frequencies (Monte Carlo randomization: P < 0·001). Based on the observed relative frequencies of sex and apomixis and under the hypothetical assumption of random association of modes, the probability of an individual to form its seeds – from three to five according to our sampling design – exclusively via one mode is 0·2–2·4 % (sex) and 17·2–34·8 % (apomixis). Actually, 93·9 % of the individuals formed all analysed seeds via a single reproductive mode. This value is expected (based on our sampling) if seeds have a probability of 98·8–97·9 % to form either via sexuality or apomixis. Thus, our data indicated a tendency of individuals to produce their seeds via either the one or the other mode. Our sampling design, however, precluded a classification of individuals as obligate or facultative apomicts/sexuals. Nevertheless, this finding is concordant with high levels of either apomixis or sexuality found in individuals from various Potentilla species. On the one hand, high degrees of apomixis were observed in numerous embryological studies (e.g. Rutishauser, 1943a; Smith, 1963a; Asker, 1970b), progeny surveys (Müntzing, 1928; Holm and Ghatnekar, 1996a) and an FCSS-based study (Dobeš et al., 2013). On the other hand, sexual reproduction but no or only marginal frequencies of apomixis were detected based on segregation patterns of isozyme markers and FCSS in individuals of P. argentea (Holm et al., 1997) and other Potentilla species (Dobeš et al., 2013), respectively. Furthermore, histoembryological studies substantiated high degrees of sexual reproduction in Potentilla, but available analyses are restricted to few individuals (Håkansson, 1946; Czapik, 1961, 1962a).
Strong reproductive differentiation between tetraploid and high polyploid cytotypes parallels diploid–polyploid systems
Tetraploid P. puberula were mainly sexual, whereas penta-, hexa-, hepta- and octoploids preferentially reproduced via apomixis. As already discussed, for numerous angiosperms, intraspecific reproductive differentiation among ploidy levels has been established. Commonly, diploid cytotypes are sexual while polyploids are apomictic (Yahara, 1990; Savidan et al., 2001; Thompson and Whitton, 2006; Lo et al., 2009; Mráz et al., 2009; Cosendai et al., 2011). In contrast, intraspecific differentiation into sexual and apomictic polyploid cytotypes is a rare situation. In embryological studies, association of tetraploidy and high polyploidy with sexuality and apomixis, respectively, was found for P. crantzii, P. incana and P. tabernaemontani (Müntzing, 1931; Håkansson, 1946; Müntzing, 1958; Czapik, 1961, 1962b; Smith, 1963b; Asker, 1985), suggesting some degree of generality of this pattern in the genus Potentilla. However, to the best of our knowledge, differentiation into sexual and apomictic cytotypes on the polyploid level from outside the genus Potentilla is documented only for two genera: three Paspalum species (Savidan et al., 2001) and Pilosella officinarum (Rotreklová et al., 2002). These examples and our study system show important parallels to those involving diploids. Individuals of the lowest ploidy level (i.e. either tetraploids or diploids) are sexual and self-incompatible (Savidan, 2001; Mráz, 2008; Hörandl, 2010; Prohaska, 2013). Furthermore, an allopolyploid origin was suggested for both P. officinarum (Mráz et al., 2008) and P. puberula (Wolf, 1908; Ehrendorfer, 1970; Soják, 2010). In allotetraploids, inheritance patterns are disomic in most cases because only chromosomes from the same parental species are able to pair in meiosis (Soltis and Soltis, 2009). We have no empirical data on the mode of inheritance or the genetic organization of the tetraploid P. puberula genome. However, cytological and genetic diploidization of the genome may be assumed because only functionally disomic incompatibility loci are likely to survive in a polyploid (Richards, 1997). Hence, functionality of the SI system in P. puberula may indicate that the tetraploids are functional diploids, as suggested by Mráz et al. (2008) for P. officinarum.
Reproductive inter-relationships among cytotypes
Besides reproductively uniform populations (two sexual and 11 apomictic), we found both reproductive modes coexisting in varying proportions in nine populations involving different apomictic cytotypes (Table 1). The presence of heteroploid pollen donors potentially fosters changes in ploidy from the maternal plant to sexually derived embryos due to the possibility for intercytotype pollination. In cases where seeds develop from intercytotype crosses, the variability in endosperm ploidies might be related to the cytological diversity of populations in both sexually and apomictically derived seeds. However, we did not find a correlation between cytological diversity and the male genomic contribution to the embryo and the endosperm in sexual tetraploid P. puberula (Supplementary Data Fig. S4). The result is supported by the ploidy of embryos and of involved gametes: the measured ploidy of sexually derived embryos deviated only slightly from 4x (3·87–4·17x), the maternal ploidy. The relatively higher variation in gamete ploidies (1·57–2·24x and 1·78–2·39x for the male and female genomic contributions, respectively) contributing to the tetraploid embryos is considered a mathematical artefact because the male genomic contribution is calculated as the difference between the ploidy of the embryo and the female genomic contribution to the embryo (Dobeš et al., 2013). The dependence is seen from the strongly negative correlation between the male and female genomic contributions (Supplementary Data Fig. S2). Hence, gamete ploidies – and resultant embryo ploidies – suggested that for the tetraploid cytotype, sexually derived seeds originated from intracytotype crosses or selfing. Thus, we found no indication for extensive intercytotype gene flow towards the tetraploids on the level of seeds, suggesting integrity through generations of this cytotype in cytologically mixed populations.
Understanding reproductive relationships in apomictic P. puberula is complicated by the high variation in the male genomic contribution to the endosperm observed for all high polyploid cytotypes (Supplementary Data Fig. S2), which can be explained by – in addition to intercytotype cross-fertilization of the central cell – irregular meiotic segregation of chromosomes, the methodological error of the inference process and particularly the contribution of either one or two sperm nuclei to the endosperm. Comparable variability in the male genomic contribution (0·35–1·9n) to the endosperm of apomictically derived seeds was observed in the offspring of selfed Potentilla individuals (Dobeš et al., 2013), suggesting that seed formation in apomictic P. puberula likewise may have originated from selfings or intracytotype crosses. However, we cannot distinguish between the effect of intercytotype crosses, i.e. variable sperm ploidy, and that of variation in the number of sperm nuclei on the variability of endosperm ploidy in apomictically derived seeds. Hence, more comprehensive experimental investigations are necessary to disentangle these factors accurately.
Taken together, we found no indication for extensive cross-fertilization of tetraploids. Consequently, effective barriers to gene flow must exist in natural populations of P. puberula. On theoretical grounds, barriers might be pre-zygotic (e.g. spatial clustering of cytotypes, ecological differentiation or pollinator preferences, etc.) and/or post-zygotic (e.g. pollen competition, endosperm incompatibilities, etc.). The actual factors maintaining the integrity of cytotypes are not known yet. In addition, the effects of cytotype mixture on seed set and fertility and therefore stability of cytotype mixtures remain to be studied. However, preliminary results from controlled heteroploid crossings show maternal:paternal genome ratios to vary considerably for both sexually (maternal:paternal genome ratios 2:1 to 2:3·4) and apomictically (2:0·6 to 2:4·4) derived seed, indicating a strong relaxation of genomic endosperm balance requirements (Scheffknecht et al., 2013). Furthermore, ecological niche differentiation among tetraploids and high polyploids (Hülber et al., 2013) suggests pre-zygotic isolation of cytotypes to be more important.
Pollen quality is weakly associated with reproductive mode
Each of the five cytotypes displayed a wide range (in common 0·0–96·9 % of viable pollen grains; Fig. 1) of pollen quality. The low pollen viability observed in some tetraploid individuals is in contrast to the results from other sexual Potentilla species showing (close to) 100 % viable pollen (Müntzing, 1928; Czapik, 1961). Lower pollen fertility (53·0–91·5 % viable pollen) was reported from sexual P. arenaria, but the embryology of the studied biotypes exhibited some tendency to apomixis (Czapik, 1962a). A low frequency of apomixis was also observed in tetraploid P. puberula (one out of 93 seeds). Reduced pollen quality in apomicts compared with sexuals was observed for several Potentilla species [e.g. approx. 15–80 % viable pollen in P. tabernaemontani (Müntzing, 1928; Asker, 1985); approx 40–60 % in P. argentea, approx. 0–40 % in P. collina (Müntzing, 1928, 1958); and 64 % in P. intermedia (Asker, 1970a)]. Besides apomixis, hybridity was associated with low pollen quality (Müntzing, 1928; Asker, 1970a). Thus, the low pollen quality in some tetraploid P. puberula individuals might be the combined effects of a tendency to apomixis and the hybrid origin of the species.
In almost all cases documented in Potentilla species, poor pollen quality was linked to disturbances of male meiosis, indicated by irregular chromosome pairing, laggards, sticking chromosome bridges, microcyte formation or degeneration of nuclei (e.g. Müntzing, 1928; Asker, 1970a; Czapik, 1975), resulting in aneuploid offspring (Asker, 1971). Such irregularities can be particularly expected in odd-ploids because of the high chance of unpaired chromosomes disrupting meiosis (Dawe, 1998). However, high pollen quality was found in heptaploid P. puberula, which might be accomplished by the formation of unreduced male gametes (e.g. Voigt et al., 2007). Based on the lower ploidy variation in sperm nuclei and the higher proportion of seeds receiving a 2n male contribution to the endosperm in odd-ploid compared with even-ploid apomictic Potentilla species, Dobeš et al. (2013) hypothesized that pollen tends to be unreduced in odd-ploids. The present results, however, do not support this idea as odd-ploids received, on average, a higher percentage of n male contributions compared with the even-ploid apomicts (based on the assumption of only marginal intercytotype gene flow; Supplementary Data Fig. S2). Alternatively, Müntzing (1928) proposed an increasing number of genomes to alleviate disadvantageous effects of aneuploid chromosome numbers on pollen viability, which probably explains the difference in pollen quality between penta- and heptaploids (Fig. 1). Consequently, pollen quality in the apomictic cytotypes might be governed by the interacting effects of the number of genomes carried by an individual.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to Christof Endl, Department of Pharmacognosy, University of Vienna, for help with the cultivation of the garden plants and with the preparation of the seed samples, and to Katharina Frost, University of Vienna, for proof reading of the manuscript. This work was supported by the Austrian Science Foundation [project AP21661-B17 to C.D.].
LITERATURE CITED
- Adolfsson S, Bengtsson BO. The spread of apomixis and its effect on resident genetic variation. Journal of Evolutionary Biology. 2007;20:1933–1940. doi: 10.1111/j.1420-9101.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- Asker S. Induced sexuality after chromosome doubling in an apomictic Potentilla argentea biotype. Hereditas. 1967;57:339–342. [Google Scholar]
- Asker S. Apomictic biotypes in Potentilla intermedia and P. norvegica. Hereditas. 1970a;66:101–108. [Google Scholar]
- Asker S. Apomixis and sexuality in the Potentilla argentea complex: 1. Crosses within other species. Hereditas. 1970b;66:127–144. [Google Scholar]
- Asker S. Apomixis and sexuality in the Potentilla argentea complex: II. Crosses within the complex. Hereditas. 1970c;66:189–204. [Google Scholar]
- Asker S. Apomixis and sexuality in the Potentilla argentea complex: 3. Euploid and aneuploid derivatives (including trisomics) of some apomictic biotypes. Hereditas. 1971;67:111–142. [Google Scholar]
- Asker S. Polymorphism of Potentilla tabernaemontani and related taxa on Gotland. Hereditas. 1985;102:39–45. [Google Scholar]
- Asker SE, Jerling L. Apomixis in plants. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Barrett SCH. The evolution, maintenance, and loss of self-incompatibiliy systems. In: Lovett Doust J, Lovett Doust L, editors. Plant reproductive ecology. New York: Oxford University Press; 1988. pp. 98–124. [Google Scholar]
- Bayer RJ. Antennaria rosea (Asteraceae) – a model group for the study of the evolution of polyploid agamic complexes. Opera Botanica. 1997;132:53–65. [Google Scholar]
- Böcher TW. Cytological and embryological studies in the amphi-apomictic Arabis holboellii complex. Kongelige Danske Videnskaber Selskabs Biologiske Skrifter. 1951;6:1–59. [Google Scholar]
- Carman JG. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biological Journal of the Linnnean Society. 1997;61:51–94. [Google Scholar]
- Cosendai A-C, Rodewald J, Hörandl E. Origin and distribution of autopolyploids via apomixis in the alpine species Ranunculus kuepferi (Ranunculaceae) Taxon. 2011;60:355–364. [Google Scholar]
- Cruden RW. Pollen-ovule ratios. A conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Czapik R. Embryological studies in the genus Potentilla L. I P. Crantzii. Acta Biologica Cracoviensia, Series Botanica. 1961;4:97–119. [Google Scholar]
- Czapik R. Badania embriologiczne nad rodzajem Potentilla L. II. P. arenaria. Embryological studies in the genus Potentilla L. II. Potentilla arenaria. Acta Biologica Cracoviensia, Series Botanica. 1962a;5:29–42. [Google Scholar]
- Czapik R. Badania embriologiczne nad rodzajem Potentilla L. III. Mieszance miedzy P. Crantzii i P. arenaria. Embryological studies in the genus Potentilla L. III. Hybrids between P. crantzii and Potentilla arenaria. Acta Biologica Cracoviensia, Series Botanica. 1962b;5:43–61. [Google Scholar]
- Czapik R. Apomixis in a sterile hybrid species of Potentilla. In: Walters SM, King CJ, editors. European floristic and taxonomic studies. Conference report. Botanical Society of the British Isles. London: E.W. Classey Ltd; 1975. pp. 38–47. [Google Scholar]
- Dawe RK. Meiotic chromosome organization and segregation in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:371–395. doi: 10.1146/annurev.arplant.49.1.371. [DOI] [PubMed] [Google Scholar]
- Dickinson TA, Lo E, Talent N. Polyploidy, reproductive biology, and Rosaceae: understanding evolution and making classifications. Plant Systematics and Evolution. 2007;266:59–78. [Google Scholar]
- Dobeš Ch. Die Karyogeographie des Potentilla verna agg. (Rosaceae) in Österreich – mit ergänzenden Angaben aus Slowenien, Kroatien, der Slowakei und Tschechien. Annalen des Naturhistorischen Museums Wien. 1999;101 B:599–629. [Google Scholar]
- Dobeš Ch, Paule J. A comprehensive chloroplast DNA-based phylogeny of the genus Potentilla (Rosaceae): implications for its geographic origin, phylogeography and generic circumscription. Molecular Phylogenetics and Evolution. 2010;56:156–175. doi: 10.1016/j.ympev.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Dobeš Ch, Lückl A, Hülber K, Paule J. Prospects and limits of flow cytometric seed screen – insights from Potentilla sensu lato (Potentilleae, Rosaceae) New Phytologist. 2013;198(605–616) doi: 10.1111/nph.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobeš Ch, Mitchell-Olds Th, Koch M. Intraspecific diversification in North American Boechera stricta (= Arabis drummondii), Boechera×divaricarpa, and Boechera holboellii (Brassicaceae) inferred from nuclear and chloroplast molecular markers – an integrative approach. American Journal of Botany. 2004;98:2087–2101. doi: 10.3732/ajb.91.12.2087. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols. 2007a;2:2233–2244. doi: 10.1038/nprot.2007.310. [DOI] [PubMed] [Google Scholar]
- Doležel J, Greilhuber J, Suda J. Flow cytometry with plants: an overview. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry in plant cells. Weinheim: Wiley-VCH; 2007b. pp. 41–65. [Google Scholar]
- Ehrendorfer F. Mediterran-mitteleuropäische Florenbeziehungen im Lichte cytotaxonomischer Befunde. Feddes Repertorium. 1970;81:3–32. [Google Scholar]
- Elzinga D, Van Der Kamp J, den Nijs APM, Sterk AA. Cytogeography and ecology of diploids and triploids of Taraxacum section Taraxacum in South Limburg, Netherlands. Proceedings of the Koninklyke Nederlands Akademie van Wetenschenschappen, Series C. 1987;90:431–442. [Google Scholar]
- Gentscheff GJ. Über die pseudogame Fortpflanzung bei Potentilla. Genetica. 1938;20:398–408. [Google Scholar]
- Greilhuber J. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany. 2005;95:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Temsch EM, Loureiro JCM. Nuclear DNA content measurement. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry in plant cells. Weinheim: Wiley-VCH; 2007. pp. 67–101. [Google Scholar]
- Gustafsson Å. Apomixis in higher plants. Part III. Biotype and species formation. Lunds Universitets Arsskrift N.f.Avd.2. 1947;43:181–320. [Google Scholar]
- Håkansson A. Untersuchungen über die Embryologie einiger Potentilla-Formen. Lunds Universitets Arsskrift N.f.Avd.2. 1946;42:1–70. [Google Scholar]
- Hardy OJ, Loose MD, Vekemans X, Meerts P. Allozyme segregation and inter-cytotype reproductive barriers in the polyploid complex Centaurea jacea. Heredity. 2001;87:136–145. doi: 10.1046/j.1365-2540.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- Hojsgaard D, Schegg E, Valis JFM, Martinez EJ, Quarin CL. Sexuality, apomixis, ploidy levels, and genomic ralationships among four Paspalum species of the subsgenus Anachyris (Poaceae) Flora. 2008;203:535–547. [Google Scholar]
- Holm S, Ghatnekar L. Apomixis and sexuality in hexaploid Potentilla argentea. Hereditas. 1996a;125:53–60. [Google Scholar]
- Holm S, Ghatnekar L. Sexuality and no apomixis found in crossing experiments with diploid Potentilla argentea. Hereditas. 1996b;125:77–82. [Google Scholar]
- Holm S, Ghatnekar L, Bengtsson BO. Selfing and outcrossing but no apomixis in two natural populations of diploid Potentilla argentea. Journal of Evolutionary Biology. 1997;10:343–352. [Google Scholar]
- Hörandl E. The evolution of self-fertility in apomictic plants. Sexual Plant Reproduction. 2010;23:73–86. doi: 10.1007/s00497-009-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Temsch EM. Introgression of apomixis into sexual species is inhibited by mentor effects and ploidy barriers in the Ranunculus auricomus complex. Annals of Botany. 2009;104:81–89. doi: 10.1093/aob/mcp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülber K, Scheffknecht S, Dobeš Ch. Partitioning the factors explaining the eco-geography in the amphi-apomictic species Potentilla puberula (Rosaceae) In: Kroh A, Berning B, Haring E, et al., editors. BioSyst.EU 2013. Global systematics! 2nd BioSyst.EU joint meeting. 2013. pp. 18–22. February 2013, Vienna, Austria. Vienna: NOBIS Austria, 98. [Google Scholar]
- Kao RH. Asexuality and the coexistence of cytotypes. New Phytologist. 2007;175:764–772. doi: 10.1111/j.1469-8137.2007.02145.x. [DOI] [PubMed] [Google Scholar]
- Kurtto A, Lampinen R, Junikka L. Helsinki: The Commitee for Mapping the Flora of Europe & Societas Biologica Fennica Vanamo; 2004. Atlas Florae Europaeae 13. Distribution of vascular plants in Europe. Rosaceae (Spiraea to Fragaria, excl. Rubus) [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Lo E, Stefanovic S, Dickinson TA. Population genetic structure of diploid sexual and polyploid apomictic hawthorns (Crataegus; Rosaceae) in the Pacific Northwest. Molecular Ecology. 2009;18:1145–1160. doi: 10.1111/j.1365-294X.2009.04091.x. [DOI] [PubMed] [Google Scholar]
- Löve Á. Cytotaxonomical remarks on some species of circumpolar taxa. Svensk Botanisk Tidskrift. 1954;48:211–233. [Google Scholar]
- Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal. 2000;21:97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Menken SBJ, Smit E, Nijs HJCMd. Genetical population structure in plants: gene flow between diploid sexual and triploid asexual dandelions (Taraxacum section Ruderalia) Evolution. 1995;49:1108–1118. doi: 10.1111/j.1558-5646.1995.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Miller JS, Venable DL. Polyploidy and the evolution of gender dimorphism in plants. Science. 2000;289:2335–2338. doi: 10.1126/science.289.5488.2335. [DOI] [PubMed] [Google Scholar]
- Mogie M. A model for the evolution and control of generative apomixis. Biological Journal of the Linnnean Society. 1988;35:127–153. [Google Scholar]
- Mogie M. The evolution of asexual reproduction in plants. London: Chapman & Hall; 1992. [Google Scholar]
- Mráz P, Singliarova B, Urfus T, Krahulec F. Cytogeography of Pilosella officinarum (Compositae): altitudinal and longitudinal differences in ploidy level distribution in the Czech Republic and Slovakia and the general pattern in Europe. Annals of Botany. 2008;101:59–71. doi: 10.1093/aob/mcm282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mráz P, Chrtek J, Šíngliarová B. Geographical parthenogenesis, genome size variation and pollen production in the arctic–alpine species Hieracium alpinum. Botanica Helvetica. 2009;119:41–51. [Google Scholar]
- Müntzing A. Pseudogamie in der Gattung Potentilla. Hereditas. 1928;11:267–283. [Google Scholar]
- Müntzing A. Note on the cytology of some apomictic Potentilla-species. Hereditas. 1931;15:166–178. [Google Scholar]
- Müntzing A. Heteroploidy and polymorphism in some apomictic species of Potentilla. Hereditas. 1958;44:280–330. [Google Scholar]
- Nogler GA. Gametophytic apomixis. In: Johri BM, editor. Embryology of angiosperms. Berlin: Springer; 1984. pp. 475–518. [Google Scholar]
- Paule J, Dobeš Ch. Hybridisation in the genus Potentilla – the case study P. alpicola. In: Kranebitter P, editor. 6. Tagung: zoologische und botanische Forschung in Südtirol. 2010. pp. 2–3. September 2010; Bozen, Italy, 31. [Google Scholar]
- Paule J, Sharbel TF, Dobeš Ch. Apomictic and sexual lineages of the Potentilla argentea L. group (Rosaceae): cytotype and molecular genetic differentiation. Taxon. 2011;60:721–732. [Google Scholar]
- Paule J, Scherbantin A, Dobeš Ch. Implications of hybridisation and cytotypic differentiation in speciation assessed by AFLP and plastid haplotypes – a case study of Potentilla alpicola La Soie. BMC Evolutionary Biology. 2012;12:132. doi: 10.1186/1471-2148-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R, Slovin JP, Chen Ch. A simplified method for differential staining of aborted and non-aborted pollen grains. International Journal of Plant Biology. 2010;1:66–69. [Google Scholar]
- Prohaska D. Charakterisierung des Reproduktionssystems von Potentilla pusilla Host und methodische Etablierung der Kreuzungstechnik. Diploma thesis, Universität Wien. 2013 [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. www.r-project.org . [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Richards AJ. Plant breeding systems. 2nd edn. London: Chapman & Hall; 1997. [Google Scholar]
- Rotreklová O, Krahulcová A, Vanková D, Peckert T, Mráz P. Chromosome numbers and breeding systems in some species of Hieracium subgen. Pilosella from Central Europe. Preslia. 2002;74:27–44. [Google Scholar]
- Rutishauser A. Konstante Art- und Rassenbastarde in der Gattung Potentilla. Mitteilungen der Naturforschenden Gesellschaft Schaffhausen. 1943a;18:111–134. [Google Scholar]
- Rutishauser A. Untersuchungen über die Fortpflanzung und Bastardbildung apomiktischer Potentillen. Berichte der Schweizer Botanischen Gesellschaft. 1943b;53:5–83. [Google Scholar]
- Rutishauser A. Zur Embryologie amphimiktischer Potentillen. Berichte der Schweizer Botanischen Gesellschaft. 1945;55:19–32. [Google Scholar]
- Sassa H, Nishio T, Kowyama Y, Hirano H, Koba T, Ikehashi H. Self-incompatibility (S) alleles of the Rosaceae encode members of a distinct class of the T2/S ribonuclease superfamily. Molecular and General Genetics. 1996;250:547–557. doi: 10.1007/BF02174443. [DOI] [PubMed] [Google Scholar]
- Savidan Y. Apomixis in higher plants. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel TF, editors. Apomixis. Evolution, mechanisms and perspectives (Regnum Vegetabile) Rugell, Liechtenstein: A.R.G. Ganter; 2007. pp. 15–22. [Google Scholar]
- Savidan Y, Carman JG, Dresselhaus T. The flowering of apomixis: from mechanisms to genetic engineering. Mexico: CIMMYT, IRD, European Commission DG VI (FAIR); 2001. [Google Scholar]
- Scheffknecht S, Hülber K, Prohaska D, Milosevic A, Sharbel TF, Dobeš Ch. Reproductive differentiation into sexual and apomictic polyploid races in Potentilla puberula (Potentilleae, Rosaceae) In: Kroh A, Berning B, Haring E, et al., editors. BioSyst.EU 2013. Global systematics! 2nd BioSyst.EU joint meeting. 2013. pp. 18–22. February 2013, Vienna, Austria. Vienna: NOBIS Austria, 183–184. [Google Scholar]
- Siena LA, Sartor ME, Espinoza F, Quarin CL, Ortiz JPA. Genetic and embryological evidences of apomixis at the diploid level in Paspalum rufum support recurrent auto-polyploidization in the species. Sexual Plant Reproduction. 2008;21:205–215. [Google Scholar]
- Skalinska M, Czapik R. Badania cytologiczne nad rodzajem Potentilla L. Studies in the cytology of the genus Potentilla L. Acta Biologica Cracoviensia, Series Botanica. 1958;1:137–149. [Google Scholar]
- Smith GL. Studies in Potentilla L. I. Embryological investigations into the mechanism of agamospermy in British P. tabernaemontani Aschers. New Phytologist. 1963a;62:264–282. [Google Scholar]
- Smith GL. Studies in Potentilla L. II. Cytological aspects of apomixis in P. crantzii (Cr.) Beck ex Fritsch. New Phytologist. 1963b;62:283–300. [Google Scholar]
- Smith GL. Studies in Potentilla L. III. Variation in British P. tabernaemontani Aschers. and P. crantzii (Cr.) Beck ex Fritsch. New Phytologist. 1971;70:607–618. [Google Scholar]
- Soják J. Origin of Potentilla crantzii, P. verna and P. puberula (Rosaceae) with a note on the nomenclature of P. pusilla. Feddes Repertorium. 2010;121:112–116. [Google Scholar]
- Soltis PS, Soltis DE. The role of hybridization in plant speciation. Annual Review of Plant Biology. 2009;60:561–588. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- Stone JL. Molecular mechanisms underlying the breakdown of gametophytic self-incompatibility. Quarterly Review of Biology. 2002;77:17–32. doi: 10.1086/339200. [DOI] [PubMed] [Google Scholar]
- Suda J, Krahulcová A, Trávnícek P, Krahulec F. Ploidy level versus DNA ploidy level: an appeal for consistent terminology. Taxon. 2006;55:447–450. [Google Scholar]
- Suda J, Kron P, Husband BC, Trávnícek P. Flow cytometry and ploidy: applications in plant systematics, ecology and evolutionary biology. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry in plant cells. Weinheim: Wiley-VCH; 2007. pp. 103–130. [Google Scholar]
- Talent N, Dickinson TA. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytologist. 2007;173:231–249. doi: 10.1111/j.1469-8137.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- Thompson SL, Whitton J. Patterns of recurrent evolution and geographic parthenogenesis within apomictic polyploid easter daises (Townsendia hookeri) Molecular Ecology. 2006;15:3389–3400. doi: 10.1111/j.1365-294X.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- Voigt M-L, Melzer M, Rutten T, Mitchell-Olds Th, Sharbel TF. Gametogenesis in the apomictic Boechera holboellii complex: the male perspective. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel TF, editors. Apomixis. Evolution, mechanisms and perspectives (Regnum Vegetabile) Rugell, Liechtenstein: A.R.G. Ganter; 2007. pp. 235–257. [Google Scholar]
- Weller SG, Donoghue MJ, Charlesworth D. The evolution of self-incompatibility in the flowering plants: a phylogenetic approach. In: Hoch PC, Stephenson AG, editors. Experimental and molecular approaches to plant biosystematics. St. Louis, MO: Missouri Botanic Garden; 1995. pp. 355–382. [Google Scholar]
- Wolf Th. Monographie der Gattung Potentilla. Bibliotheca Botanica. 1908;16(71):1–715. [Google Scholar]
- Wyatt R, Boyles SB, Derda GS. Environmental influences on nectar production in milkweeds (Asclepias syriaca and A. exaltata) American Journal of Botany. 1992;79:636–642. [Google Scholar]
- Yahara T. Evolution of agamospermous races in Boehmeria and Eupatorium. Plant Species Biology. 1990;5:183–196. [Google Scholar]


