Abstract
Background and Aims
Facultative root hemiparasitic plants generally have a wide host range, but in most cases show an obvious host preference. The reasons for the marked difference in growth performance of hemiparasites when attached to different hosts are not fully understood. In this study, the hypothesis was tested that hemiparasites showing a preference for different hosts have different nutrient requirements.
Methods
Two facultative root hemiparasitic Pedicularis species (P. rex and P. tricolor) with a different host dependency and preference were used to test their responses to inorganic solutes. The effects of nitrogen, phosphorus and potassium on growth of the hemiparasitic plants not attached to a host were determined, using an orthogonal design in pot cultivation under greenhouse conditions. Variables including biomass, shoot nutrient concentration, root:shoot (R:S) ratios and the number of haustoria were measured.
Key Results
As in autotrophic plants, nutrient deficiency reduced dry weight (DW) and nutrient concentrations in the root hemiparasites. Nitrogen and phosphorus significantly influenced growth of both Pedicularis species, while potassium availability influenced only shoot DW of P. rex. Nitrogen had far more effect on growth of P. rex than on P. tricolor, while phosphorus deficiency caused more marked growth depression in P. tricolor than in P. rex. Pedicularis rex grew faster than P. tricolor in a range of nutrient supplies. Different patterns of biomass allocation between the two Pedicularis species were observed. While P. rex invested more into roots (particularly fine rootlets) than P. tricolor, the number of haustoria produced by P. rex was relatively much lower than that produced by P. tricolor, which had a much smaller root system.
Conclusions
The two Pedicularis species differ in nutrient requirements and biomass allocation. Distinct interspecific traits in growth and nutrient requirements can be driving forces for the differential interactions between hemiparasites and their hosts.
Keywords: Inorganic solute, root hemiparasitic plant, Orobanchaceae, Pedicularis, pot cultivation, nitrogen, phosphorus, potassium
INTRODUCTION
Root hemiparasitic plants are green with retained photosynthetic capability, but they form direct connections with the root vasculature of other plants for nutrient acquisition, via parasitic organs known as haustoria (Westwood et al., 2010). The majority of root hemiparasitic plants have poorly developed root systems and are generally non-mycorrhizal (Harley and Harley, 1987; Phoenix and Press, 2005), which puts them at a disadvantage in terms of nutrient uptake from soil. As a consequence, root hemiparasitic plants depend largely on their hosts for acquisition of inorganic nutrients and water (Seel et al., 1993; Matthies, 1996; Cameron et al., 2006; Jiang et al., 2010; Mudrak and Leps, 2010). Despite variations in host dependency and duration of the pre-parasitic phase prior to the development of functional haustoria, all root hemiparasitic plants can grow for a period of time (ranging from a few hours to several months) without attachment to a host (Seel et al., 1993). A few facultative root hemiparasitic plants (e.g. Bartsia trixago) can complete their life cycle in the absence of a host (Press et al., 1993), showing a retained capability for nutrient acquisition through their own root systems.
Investigations of nutrient requirements of root hemiparasitic plants have been scarce, partly due to the fact that the majority grow poorly in the absence of a host. Most attention has been paid to the influence of host plants on growth. According to the existing literature, nearly all root hemiparasitic plants show host preference to some extent (Seel and Press, 1993; Cameron et al., 2006; Ren et al., 2010; Li et al., 2012a). Apart from a differential host defence reaction upon parasitic infection (Cameron et al., 2006; Jiang et al., 2008), the nature and flux of solutes from the host may play a role in determining growth performance of the root hemiparasite (Seel et al., 1993). Because the flow of solutes from a host is often non-selective (Jiang et al., 2004, 2010; Irving and Cameron, 2009), it is difficult if not impossible to determine which nutrients actually limit the growth of the hemiparasites. Controlled nutrient supply to cultivated hemiparasites in the absence of their hosts may provide information about the nutrient requirements. Seel et al. (1993) found that phosphorus (P), but not nitrogen (N) or potassium (K), limited growth of the facultative root hemiparasite Rhinanthus minor in the absence of a host plant. Because they did not include nutrient gradients in their experimental design, optimal nutrient supply for the hemiparasite remained unknown.
Pedicularis (Orobanchaceae) is a large lineage of herbaceous root hemiparasitic plants (approx. 600 species), widely distributed in the temperate zone of the northern hemisphere and best represented (352 species) in south-west China (Yang et al., 1998). It forms interspecific haustoria with xylem connections to host roots as well as intraspecific haustoria parasitizing rootlets of its own species/individual (Li et al., 2012a, b), in some cases even on inorganic objects such as soil debris (Piehl, 1963; Ren et al., 2010), indicating a primitive parasitic status. In a pot cultivation experiment to test host dependency, two sympatric Pedicularis species, P. rex and P. tricolor, were found to be facultative root hemiparasites that grew and developed in the absence of host plants (Li et al., 2012a). The two Pedicularis species showed a different host preference and growth rate. Additionally, P. rex showed weaker host dependence and produced far fewer haustoria than P. tricolor (Li et al., 2012a). These results suggest that they have very different physiological traits and different capability for nutrient acquisition from soil. Furthermore, they probably have different nutrient requirements that may account for their different host preference. However, no effort has been made to test nutrient requirements of Pedicularis species, and little information is available regarding the inorganic nutrients limiting the growth of these root hemiparasites.
Using pot cultivation and an orthogonal design, we evaluated nutrient (N, P and K in this study) requirements of the two root hemiparasitic Pedicularis species, P. rex and P. tricolor, in the absence of a host plant. The following specific questions were addressed. (1) Does inorganic nutrient supply have any influence on their growth? (2) Do they differ in nutrient requirements? (3) Which individual nutrients can limit their growth? Knowledge obtained will help us better understand the capability for direct nutrient uptake in the root hemiparasites via their own root system and will help explain why the sympatric species have differential pattern of parasite–host interactions. More importantly, new clues may be provided for the underlying reasons for host selection by root hemiparasitic plants.
MATERIALS AND METHODS
Experimental design
Taguchi's orthogonal array L9 (33) was used for the experimental design. Three macronutrients, N, P and K, were included for determination of the optimal nutrient supply to the two Pedicularis species in pot cultivation experiments. Based on the Long Ashton nutrient solution formula (Hewitt, 1966), three concentration levels were used for each macronutrient: (1) the nutrient was absent from the nutrient solution; (2) there was a standard value; and (3) there was a doubled concentration. In total, nine nutrient regimes (with four replications for each) were randomly arranged. Detailed information about the nutrient composition is given in Table 1.
Table 1.
Composition of nutrient solutions used in the orthogonal experiment (L9) for determination of optimal nutrient supply to Pedicularis species
| Treatment no. | Orthogonal array | N concentation |
P concentration, Na2HPO4 | K concentration, K2SO4 | Other nutrients | |
|---|---|---|---|---|---|---|
| (NH4)2SO4 | NaNO3 | |||||
| 1 | N P K | 2 mm | 4 mm | 1·33 mm | 2 mm | 1·5 mm MgSO4·7H2O, 4 mm CaCl·2H2O, 0·1 mm FeEDTA, 2·86 mg L−1 H3BO3, 1·81 mg L−1 MnCl2·4H2O, 0·5 mg L−1 ZnSO4·7H2O, 0·08 mg L−1 CuSO4·5H2O, 0·025 mg L−1 NaMoO4·2H2O |
| 2 | N –P –K | 2 mm | 4 mm | 0 | 0 | |
| 3 | N 2P 2K | 2 mm | 4 mm | 2·66 mm | 4 mm | |
| 4 | –N P –K | 0 | 0 | 1·33 mm | 0 | |
| 5 | –N –P 2K | 0 | 0 | 0 | 4 mm | |
| 6 | –N 2P K | 0 | 0 | 2·66 mm | 2 mm | |
| 7 | 2N P 2K | 4 mm | 8 mm | 1·33 mm | 4 mm | |
| 8 | 2N –P K | 4 mm | 8 mm | 0 | 2 mm | |
| 9 | 2N 2P –K | 4 mm | 8 mm | 2·66 mm | 0 | |
In the orthogonal array, ‘–’ indicates absence of the nutrient in the nutrient solution; ‘2’ indicates doubled concentration of the nutrient in the solution. (NH4)2SO4 and NaNO3 were used in combination instead of either one alone in order to facilitate nutrient uptake, because preference of N forms remains unknown. All solutions were adjusted to a pH value of 6·5 (with 0·1 m NaOH or 0·1 m HCl) before use.
Plant materials
Seeds of P. rex and P. tricolor were collected from Shangri-la, Yunnan Province, China, in September 2008 and stored in paper bags at 4 °C until used, except for transport to Australia. Seed nutrient reserves were on average 112 µg (N), 18 µg (P) and 22 µg (K) per seed for P. rex and 70 µg (N), 13 µg (P) and 15 µg (K) for P. tricolor. To promote germination, seeds were surface-sterilized in 4·5 % commercial sodium hypochlorite for 10 min, rinsed thoroughly with running water purified by reverse osmosis (RO) and soaked in 1 g L−1 gibberellic acid for 2 h, and then stratified at 4 °C for 1 week (Li et al., 2012b). Germination was carried out on moist filter papers at 20 °C in the dark for 6 d.
Planting and growth conditions
Shortly after the cotyledons had emerged, uniform seedlings of Pedicularis were transplanted to white plastic pots containing a 1·4 kg mix of 10 % soil and 90 % fine sand. Soil was collected from the Waite Arboretum, University of Adelaide, Australia. Soil was sieved through a 2 mm sieve, autoclaved at 121 °C (twice on separate days, 1 h each time) and then mixed with autoclaved fine sand. The soil mix had 27·2 mg kg−1 plant-available N (sum of nitrate and ammonium N measured using a Lachat Flow Injection Analyzer) (Searle, 1984), 2·6 mg kg−1 plant-available P by the resin extraction method (McLaughlin et al., 1994) and 141·7 mg kg−1 plant-available K by atomic absorption spectrophotometry after extraction with ammonium acetate (Gong et al., 2011). The pH (in 0·01 m CaCl2 solution) was approx. 6·0.
As seedlings of Pedicularis species often grow close together in the wild, two individuals (from the same species) were planted into one pot to create a plant density similar to that in their natural habitats. A fixed distance (approx. 3 cm) was set between the two plants in each pot to reduce distance effects. The surface of the soil–sand mix was covered with autoclaved polyethylene beads (Qenos Pty Ltd, Australia) to retain moisture. The nine modified Long Ashton nutrient solutions were applied weekly (15 mL per pot) to the appropriate pots 1 week after planting. Pots were watered to weight with RO water whenever necessary to maintain the water content at around 10 % oven-dry soil. Pots were fully randomized and re-randomized at each watering to reduce position effects.
The experiment was conducted from early September to early December (Southern Hemisphere Spring) in an environmentally controlled glasshouse at the Waite Campus, University of Adelaide. Night–day temperature range in the glasshouse was 16–31·3 °C. During cloudy days, supplementary lights were turned on to increase irradiance, which was in the range of 237–1000 µmol m−2 s−1.
Harvest and sampling
Survival of both Pedicularis species was recorded each week after planting. Plants were harvested after 13 weeks, when both Pedicularis species flowered. As the two species will not set seeds without pollinators in the glasshouse, we did not test seed productivity in this study. At harvest, shoots were cut at the soil surface and fresh weights (FWs) determined. Shoot dry weight (DW) per pot was determined after oven drying at 85 °C for 48 h. Roots were washed thoroughly and their FW was determined after blotting with paper towels. A weighed sub-sample of root material was taken and stored in 50 % ethanol for later assessment of haustorium formation in the different nutritional treatments. The remainder of each root sample was separated into two categories based on root diameter (fine rootlets <0·5 mm in diameter and the remaining root tissue) and oven-dried at 85 °C for 48 h to determine the DW. The DW of the sub-sample used for checking haustorium formation was obtained from the FW:DW ratio of the remainder and the FW of the sub-sample. Total DW per pot and the root:shoot (R:S) ratio were calculated using the DW of corresponding materials. Fine rootlets:total root DW ratios were also calculated to determine the proportion of biomass allocation into fine rootlets. Because the root systems of the two individuals proved to be impossible to separate from each other, roots from the same pot were treated as one sample. Accordingly, the R:S ratio was calculated per pot rather than per plant. Shoot DW and root DW per plant were calculated by dividing the corresponding DW per pot by two.
In order to determine if different nutrient supplies have any influence on formation of intraspecific haustoria, we recorded the numbers of haustoria in this study. To facilitate examination of internal structures of haustoria, sampled roots were washed free of ethanol, cleared in 10 % KOH and stained in a 5 % ink–vinegar solution (Vierheilig et al., 1998). The number of haustoria (H) in the sub-sample was counted under a bright field microscope at ×40 magnification. The incidence of haustorium formation was recorded as number of H per gram of root DW. Haustoria with distinct xylem bridges were recorded as presumably functional haustoria (PFH; Li and Guan, 2008).
Tissue element analysis
Dried shoot tissue was ground in a pestle and mortar, and then digested in a 5 mL sulfuric–salycilic acid mix using the Kjeldahl technique (Allen et al., 1974). Shoot N concentrations were determined using a Kjeldahl apparatus (BUCHI K360, Switzerland). Shoot P concentrations were determined using the phosphovanado-molybdate method (Hanson, 1950) and a spectrophotometer (UV1601 Shimadzu, Japan). Shoot K concentration was determined using a flame photometer (FP640, China). Shoot calcium (Ca) and magnesium (Mg) were determined by flame atomic absorption spectrometry (AA-6601F Shimadzu, Japan).
Statistical analysis
Univariate analysis of variance (ANOVA) was performed using Statistical Product and Service Solutions (SPSS) software (version 16.0; SPSS China Ltd, Shanghai, China) for most of the data. Data for R:S ratios were arcsine transformed and the number of haustoria and PFH were square-root transformed prior to analysis in order to meet ANOVA assumptions of normality and homogeneity. Duncan's multiple range test was used for comparison of the means. PERMANOVA, a non-parametric method for ANOVA, was used for data that did not fulfil the assumptions of either normality or homogeneity of variances required for a parametric ANOVA. Pair-wise a posteriori comparisons of the means were done wherever necessary according to the User's Guide of this program (Anderson, 2005).
RESULTS
Plant DW and R:S ratios in relation to different nutrient supply
Both Pedicularis species showed obvious growth responses to variations in supply of the three nutrient elements, but in very different patterns. Pedicularis rex grew faster and accumulated higher biomass than P. tricolor in a range of nutrient supplies (Fig. 1). In addition, P. rex had better developed roots than P. tricolor under all nutrient regimes.
Fig. 1.
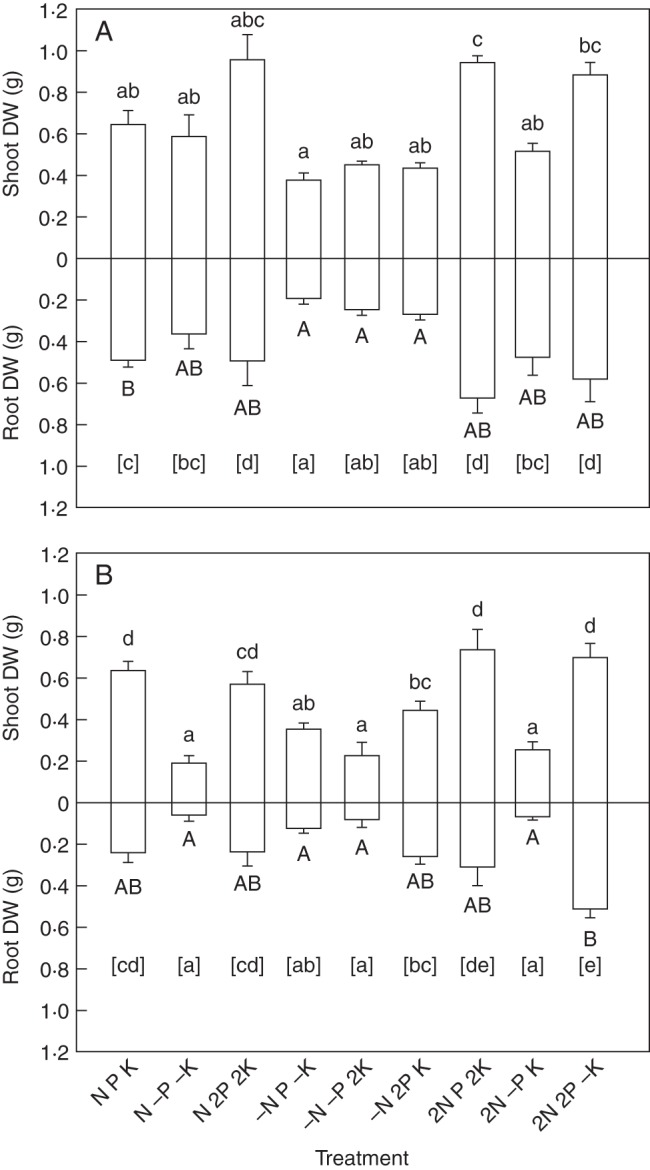
Dry weight (DW) per plant of Pedicularis rex (A) and P. tricolor (B) after 13 weeks when grown with an individual of its own species and supplied with different nutrient solutions. Data are presented as the mean ± s.e. of four replicates. Statistical analyses were performed separately for shoot (above the line), root (below the line) and total DW of each plant. Bars for shoots (lower case), roots (upper case) and total DWs (in square brackets) with different letters indicate statistically significant differences at the P < 0·05 level. Treatments (nutrient solutions based on Long Ashton solution): N, nitrogen; P, phosphorus; K, potassium; ‘–’ indicates absence of the nutrient in the solution; ‘2’ indicates doubled concentration of the nutrient in the solution.
All three nutrient elements tested had significant effects on shoot DW of P. rex (Fig. 1A, Table 2), with N as the most obvious element limiting shoot growth (F = 28·569, P < 0·001). Shoot DWs and root DWs were the lowest in N deficiency treatments, including those with sufficient supply of P and K (Fig. 1A). In contrast, growth depression with deficiency of P or K was not as strong as that shown with N deficiency. Although univariate ANOVA results showed that variations in K supply had a significant influence on R:S ratios (F = 3·643, P = 0·039), no clear pattern could be recognized.
Table 2.
UNIANOVA (general linear model, univariate) results (F-values, followed by P-values in brackets) for the effects of nitrogen (N), phosphorus (P) and potassium (K) on dry weight (DW) per plant, root:shoot (R:S) ratio, number of haustoria (H), number of presumably functional haustoria (PFH) per gram dry root, fine root/total root (FR/TR) ratio and shoot concentrations of N, P K, calcium (Ca) and magnesium (Mg) of Pedicularis rex and P. tricolor
| Nutrient | Pedicularis | Shoot DW | Root DW | R:S ratio | No. of H | No. of PFH | FR/TR ratio | N concentration | P concentration | K concentration | Ca concentration | Mg conc. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P. rex | 28·569 (<0·001) | 17·377 (<0·001) | 2·949 (0·068) | 4·359 (0·022) | 0·389 (0·681) | 0·075 (0·928) | 9·591 (0·001) | 4·061 (0·028) | 11·993 (<0·001) | 0·373 (0·692) | 0·178 (0·838) |
| P. tricolor | 9·245 (0·001) | 5·515 (0·009) | 1·461 (0·249) | 1·142 (0·333) | 0·726 (0·492) | 2·887 (0·072) | 4·869 (0·015) | 1·068 (0·357) | 1·815 (0·181) | 0·872 (0·429) | 1·108 (0·344) | |
| P | P. rex | 10·839 (<0·001) | 1·616 (0·216) | 0·914 (0·412) | 1·260 (0·299) | 0·369 (0·695) | 2·609 (0·091) | 8·270 (0·001) | 20·381 (<0·001) | 7·621 (0·002) | 3·767 (0·035) | 1·321 (0·282) |
| P. tricolor | 31·102 (<0·001) | 17·323 (<0·001) | 8·571 (0·001) | 3·077 (0·061) | 0·489 (0·618) | 33·680 (<0·001) | 46·681 (<0·001) | 36·639 (<0·001) | 8·593 (0·001) | 2·456 (0·103) | 0·059 (0·942) | |
| K | P. rex | 12·681 (<0·001) | 1·319 (0·283) | 3·643 (0·039) | 4·250 (0·024) | 2·390 (0·109) | 0·838 (0·443) | 3·386 (0·048) | 2·412 (0·107) | 3·321 (0·050) | 2·128 (0·137) | 2·459 (0·103) |
| P. tricolor | 1·883 (0·170) | 0·438 (0·649) | 0·862 (0·433) | 0·951 (0·398) | 0·057 (0·945) | 2·379 (0·110) | 0·175 (0·840) | 6·192 (0·006) | 7·552 (0·002) | 4·685 (0·017) | 4·734 (0·017) |
Significant values are highlighted in bold.
Variations in N and P supply showed strong effects on both shoot DW and root DW of P. tricolor (Fig. 1B, Table 2), with P (F = 31·102, P < 0·001 for shoot DW; F = 17·323, P < 0·001 for root DW) as a much stronger limiting element than N (F = 9·245, P = 0·001 for shoot DW; F = 5·515, P = 0·009 for root DW). This species suffered extreme growth retardation in P deficiency conditions in comparison with those with normal or enhanced P supply in the nutrient solution. Plant DWs were significantly decreased in P deficiency despite sufficient supply of N and K (Fig. 1B). In contrast, growth depression with deficiency of N or K was not as dramatic as that shown with P deficiency. In addition, P was the only nutrient element tested that significantly influenced the R:S ratios of P. tricolor (F = 8·571, P = 0·001), though with no clear patterns. Potassium had no influence on either DWs or R:S ratios.
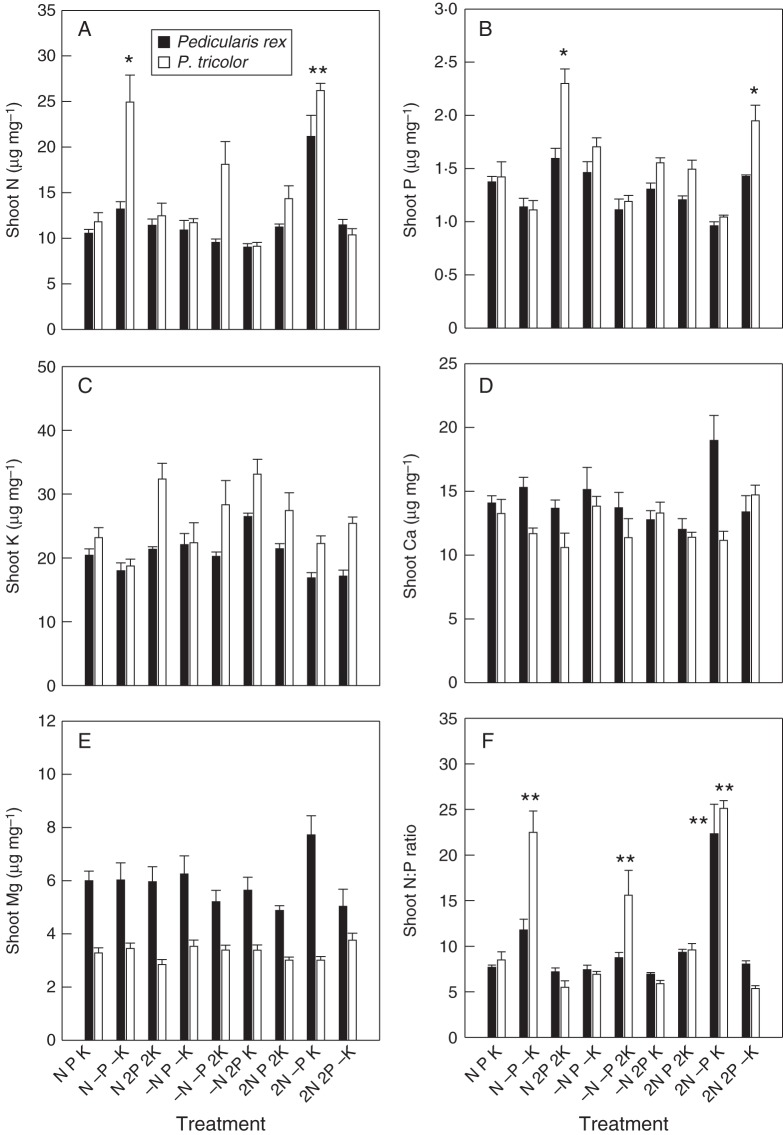
Tissue element concentration
Different patterns of element accumulation were observed in the shoots of the two Pedicularis species, even when supplied with identical nutrient solutions (Fig. 2). The ratios of elements in the nutrient solution showed a stronger influence on tissue element concentration than the amount of nutrient element per se. While P. tricolor had similar or higher concentrations of N, P and K than P. rex in shoots, shoot Mg concentrations in P. rex were much higher than in P. tricolor (Fig. 2E).
Fig. 2.
Element concentrations (μg mg−1) and N:P ratios in shoots of Pedicularis rex and P. tricolor (as indicated in the key). (A) Shoot concentration of nitrogen (N), (B) shoot concentration of phosphorus (P), (C) shoot concentration of potassium (K), (D) shoot concentration of calcium (Ca), (E) shoot concentration of magnesium (Mg) and (F) shoot N:P ratios. Data are presented as the mean ± s.e. of four replicates. Statistical analyses were performed separately for the two Pedicularis species. *Indicates a statistically significant difference at the P < 0·05 level; **indicates a statistically significant difference at the P < 0·01 level. Treatments (nutrient solutions based on Long Ashton solution): N, nitrogen; P, phosphorus; K, potassium; ‘–’ indicates absence of the nutrient in the solution; ‘2’ indicates doubled concentration of the nutrient in the solution.
For P. rex, the shoot element concentrations remained relatively stable when supplied with different nutrient solutions (Fig. 2). However, univariate ANOVA results showed that variations in supply of all three tested nutrients had significant effects on shoot concentrations of N, P and K (Table 2), except that K supply did not show any significant effect on shoot P concentration.
For P. tricolor, P deprivation caused a significant increase in N concentration (Fig. 2A). This species responded positively to additional P supply, as shown by a significant increase in shoot P concentration when supplied with doubled P (Fig. 2B). In addition, shoot K concentrations were higher when P supply was sufficient (Fig. 2C, Table 2). Increased N supply increased the shoot N concentration when at appropriate N:P supply ratios, but not the concentrations of other elements examined. Variations in K supply to P. tricolor significantly influenced shoot P, K, Ca and Mg concentrations (again showing no clear pattern), but had no influence on N concentration (Table 2).
Root morphology and haustorium formation in relation to nutrient supply
Interspecific variations were observed between P. rex and P. tricolor in terms of biomass allocation into roots and the number of haustoria produced when supplied with identical nutrient solutions. While P. rex had larger roots (Fig. 1) and higher biomass allocation into roots (particularly fine rootlets; Fig. 3A, B) than P. tricolor under virtually all nutrient conditions, the number of haustoria produced by P. rex was relatively much lower than that produced by P. tricolor.
Fig. 3.
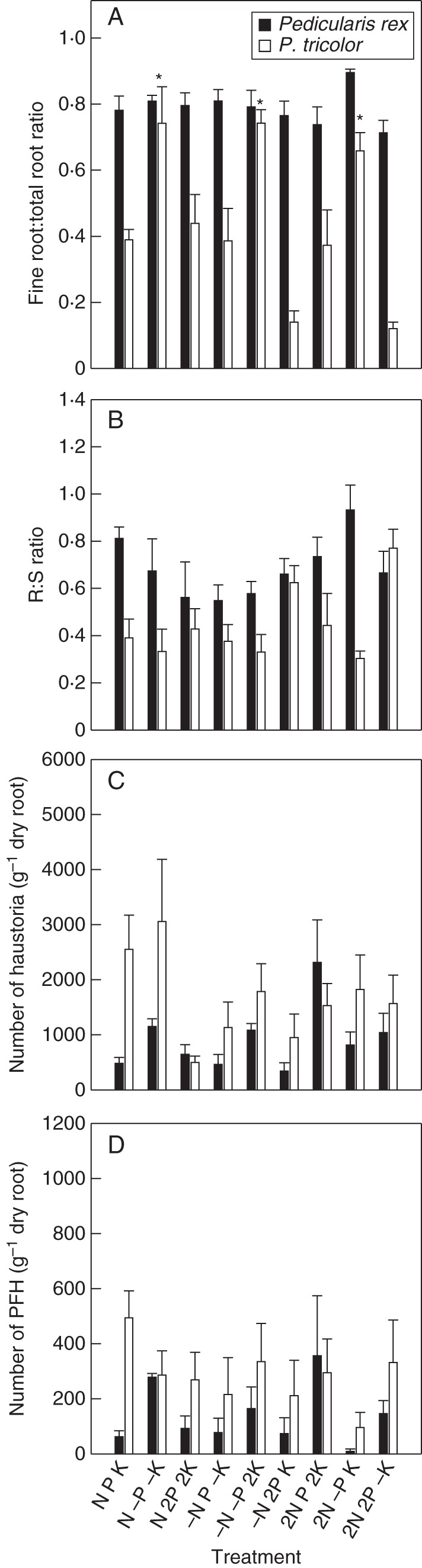
Biomass allocation and haustorium formation in Pedicularis rex and P. tricolor (as indicated in the key). (A) Ratios between the dry weight (DW) of fine rootlets (<0·5 mm in diameter) and total root DW, (B) root:shoot (R:S) ratios, (C) total number of haustoria produced per gram of dry root and (D) number of presumably functional haustoria (PFH) produced per gram of dry root. Data are presented as the mean ± s.e. of four replicates. Statistical analyses were performed separately for the two Pedicularis species. * Indicates a statistically significant difference at the P < 0·05 level. Treatments (nutrient solutions based on Long Ashton solution): N, nitrogen; P, phosphorus; K, potassium; ‘–’ indicates absence of the nutrient in the solution; ‘2’ indicates doubled concentration of the nutrient in the solution.
In general, P. rex allocated much of its root biomass (around 80 % in proportion) to fine rootlets that were <0·5 mm in diameter (Fig. 3A). Variations in nutrient supply showed no obvious effects on the proportion of fine rootlets produced. The availability of N and K, but not P, showed significant effects on haustorium formation in this species (Table 2), but with no clear pattern.
Biomass allocation to fine rootlets in roots of P. tricolor was much lower than in P. rex. The proportions of fine rootlet DWs in total root DWs were in most cases <50 %, except that plants dramatically increased their fine rootlet biomass allocation up to 80 % in all the three P-deficient treatments (Fig. 3A). Overall, an increase in P availability reduced the production of fine rootlets in P. tricolor. Variations in supply of the macronutrients showed no detectable effects on haustorium formation in P. tricolor.
DISCUSSION
Our data show that appropriate inorganic nutrient supply is important for growth and development of the two Pedicularis species in the absence of their hosts. The root hemiparasites retained the capacity for inorganic solute acquisition and assimilation, since growth was stimulated by the addition of appropriate nutrient levels. Growth responsiveness of both Pedicularis species to different supplies of solutes was rather high. Plant DWs were increased by 2–3 times when supplied with appropriate nutrient solutions, in most cases higher than when attached to poor host plants and even similar to those with moderate host plants (Li et al., 2012a). This is different from the root hemiparasitic Rhinanthus minor which showed very low responsiveness to variations in nutrient supply (Seel et al., 1993).
An interspecific difference was observed between the two Pedicularis species in terms of their nutrient requirements. Growth of both species was significantly affected by complete N, P or K deprivation. Nevertheless, the two species showed very different responses to nutrient deficiency. While P. rex experienced a more marked decrease in biomass production than P. tricolor in N deficiency, P. tricolor showed more dramatic biomass reduction than P. rex in P deficiency. Our findings suggest that both N and P, and probably K, can influence growth and element accumulation in the two Pedicularis species that we tested. In their experiment with R. minor, Seel et al. (1993) found that P was the only limiting element among N, P and K for growth. Because they did not take into account the effects of the ratios of the supplied nutrient element on growth of R. minor, which we found play key roles in many cases for Pedicularis, we cannot exclude the possibility that N and/or K also play a role in growth of R. minor. However, our data and the existing literature all agree that P is at least one of the limiting elements for growth of root hemiparasitic plants.
The two Pedicularis species showed different acquisition capability for different nutrient elements in terms of internal concentrations. While P. tricolor had similar or higher concentrations of N, P and K than P. rex in shoots, shoot Mg concentrations in P. rex were much higher than in P. tricolor in all cases. A higher capability for Mg acquisition in P. rex could result from its relatively better developed root system.
In the absence of a host plant, P. rex tends to allocate more biomass to roots than P. tricolor, as shown by generally higher R:S ratios observed in P. rex. In particular, P. rex invested more in producing fine rootlets than P. tricolor. This explains why P. rex overall produced higher biomass than P. tricolor in all nutrient treatments (Fig. 1), since fine rootlets are more active than thick roots in nutrient uptake. In addition, a difference in root architecture can at least partially explain the different extent of growth depression in P deficiency between P. rex and P. tricolor. For terrestrial plants, species with fine and extensive root systems (P. rex in this case) tend to have low external P requirements for maximum growth, while those with thick and small root systems (P. tricolor in this experiment) generally had high external P requirements (Hill et al., 2006). However, P. tricolor retained the capability to adjust its investment to produce more fine rootlets in P deficiency (Fig. 3C), though the increased fine rootlets:total root DW ratios did not make up for growth depression caused by P deficiency due to the small root systems (as shown in Fig. 1B).
As a whole, P. rex is more like an autotrophic species than P. tricolor, in terms of a higher R:S ratio, stronger growth responses to nutrient supply, and extensive root systems with a higher proportion of fine rootlets. This explains why P. rex shows less host dependency than P. tricolor (Li et al., 2012a). Nitrogen deficiency showed stronger limiting effects on P. rex, while P deficiency had stronger limiting effects on P. tricolor. These results agree with the observations that P. rex preferred legume hosts that are known for high N content but P. tricolor preferred grass hosts which had higher capability for P acquisition (Li et al., 2012a). We suggest that different nutrient requirements among root hemiparasites can play significant roles in the evolution of host preference by the parasites.
In conclusion, the two Pedicularis species with different host preference differ in nutrient requirements and growth traits. The different responses of the root hemiparasites to deficiency of different nutrient elements may account for their host preference. This study provides a new clue regarding the driving forces for evolution of host preference by root hemiparasitic plants. Apart from host defence responses, nutrient requirements of the hemiparaites per se should be taken into account for a better understanding of marked difference in interactions between different root hemiparasite–host pairs.
ACKNOWLEDGEMENTS
This study was part of a project carried out at the University of Adelaide. We thank Ms Rebecca Stonor for her excellent help with setting up and harvesting of the experiments. We are grateful to the anonymous reviewers for their valuable comments and suggestions to improve the paper. The research was supported by the Natural Science Foundation of China (grant no. 30970288), Natural Science Foundation of Yunnan Province (grant no. 2009CD114), Youth Innovation Promotion Association of Chinese Academy of Sciences (CAS), a scholarship of Overseas Training Program from CAS for the first author, and a research fund (NO. P2012-KF03) from the Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, CAS.
LITERATURE CITED
- Allen SE, Grimshaw HM, Parkinson JA, Quarmby C. Chemical analysis of ecological materials. London, UK: Blackwell Scientific; 1974. [Google Scholar]
- Anderson MJ. PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. University of Auckland: New Zealand; 2005. Department of Statistics. [Google Scholar]
- Cameron DD, Coats AM, Seel WE. Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Annals of Botany. 2006;98:1289–1299. doi: 10.1093/aob/mcl218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XY, Chen Q, Dittert K, Taube F, Lin S. Nitrogen, phosphorus and potassium nutritional status of semiarid steppe grassland in Inner Mongolia. Plant and Soil. 2011;340:265–278. [Google Scholar]
- Hanson WC. The photometric determination of phosphorus in fertilizers using the phosphovanado–molybdate complex. Journal of the Science of Food and Agriculture. 1950;1:172–173. [Google Scholar]
- Harley JL, Harley EL. A check-list of mycorrhiza in the British flora. New Phytologist. 1987;105:1–102. [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. Farnham Royal, UK: Commonwealth Agricultural Bureaux; 1966. [Google Scholar]
- Hill JO, Simpson RJ, Moore AD, Chapman DF. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant and Soil. 2006;286:7–19. [Google Scholar]
- Irving LJ, Cameron DD. You are what you eat: interactions between root parasitic plants and their hosts. Advances in Botanical Research. 2009;50:87–138. [Google Scholar]
- Jiang F, Jeschke WD, Hartung W. Solute flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite nutrient relations. Functional Plant Biology. 2004;31:633–643. doi: 10.1071/FP03225. [DOI] [PubMed] [Google Scholar]
- Jiang F, Jeschke WD, Hartung W, Cameron DD. Does legume nitrogen fixation underpin host quality for the hemiparasitic plant Rhinanthus minor? Journal of Experimental Botany. 2008;59:917–925. doi: 10.1093/jxb/ern015. [DOI] [PubMed] [Google Scholar]
- Jiang F, Jeschke WD, Hartung W, Cameron DD. Interactions between Rhinanthus minor and its hosts: a review of water, mineral nutrient and hormone flows and exchanges in the hemiparasitic association. Folia Geobotanica. 2010;45:369–385. [Google Scholar]
- Li AR, Guan KY. Arbuscular mycorrhizal fungi may serve as another nutrient strategy for some hemiparasitic species of Pedicularis (Orobanchaceae) Mycorrhiza. 2008;18:429–436. doi: 10.1007/s00572-008-0196-z. [DOI] [PubMed] [Google Scholar]
- Li AR, Smith FA, Smith SE, Guan KY. Two sympatric root hemiparasitic Pedicularis species differ in host dependency and selectivity under phosphorus limitation. Functional Plant Biology. 2012a;39:784–794. doi: 10.1071/FP12159. [DOI] [PubMed] [Google Scholar]
- Li AR, Smith SE, Smith FA, Guan KY. Inoculation with arbuscular mycorrhizal fungi suppresses initiation of haustoria in the root hemiparasite Pedicularis tricolor. Annals of Botany. 2012b;109:1075–1080. doi: 10.1093/aob/mcs028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: heterotrophic benefit and parasite-mediated competition. Oikos. 1996;75:118–124. [Google Scholar]
- McLaughlin MJ, Lancaster PA, Sale PG, Uren NC, Peverill KI. Comparison of cation–anion exchange resin methods for multielement testing of acidic soils. Australian Journal of Soil Research. 1994;32:229–240. [Google Scholar]
- Mudrak O, Leps J. Interactions of the hemiparasitic species Rhinanthus minor with its host plant community at two nutrient levels. Folia Geobotanica. 2010;45:407–424. [Google Scholar]
- Phoenix GK, Press MC. Linking physiological traits to impacts on community structure and function: the role of root hemiparasitic Orobanchaceae (ex-Scrophulariaceae) Journal of Ecology. 2005;93:67–78. [Google Scholar]
- Piehl MA. Mode of attachment, haustorium structure, and hosts of Pedicularis canadiensis. American Journal of Botany. 1963;50:978–985. [Google Scholar]
- Press MC, Parsons AN, Mackay AW, Vincent CA, Cochrane V, Seel WE. Gas-exchange characteristics and nitrogen relations of 2 mediterranean root hemiparasites – Bartsia trixago and Parentucellia viscosa. Oecologia. 1993;95:145–151. doi: 10.1007/BF00649518. [DOI] [PubMed] [Google Scholar]
- Ren YQ, Guan KY, Li AR, Hu XJ, Zhang L. Host dependence and preference of the root hemiparasite, Pedicularis cephalantha Franch. (Orobanchaceae) Folia Geobotanica. 2010;45:443–455. [Google Scholar]
- Searle PL. The berthelot or indophenol reaction and its use in the analytical-chemistry of nitrogen – a review. Analyst. 1984;109:549–568. [Google Scholar]
- Seel WE, Press MC. Influence of the host on 3 sub-arctic annual facultative root hemiparasites.1. growth, mineral accumulation and aboveground dry-matter partitioning. New Phytologist. 1993;125:131–138. doi: 10.1111/j.1469-8137.1993.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Seel WE, Parsons AN, Press MC. Do inorganic solutes limit growth of the facultative hemiparasite Rhinanthus minor L. in the absence of a host. New Phytologist. 1993;124:283–289. doi: 10.1111/j.1469-8137.1993.tb03818.x. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piche Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology. 1998;64:5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood JH, Yoder JI, Timko MP, dePamphilis CW. The evolution of parasitism in plants. Trends in Plant Science. 2010;15:227–235. doi: 10.1016/j.tplants.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Yang H, Holmgren NH, Mill RR. Pedicularis Linnaeus. Flora of China. 1998;18:97–209. [Google Scholar]