Abstract
Background and Aims
The hormone auxin and reactive oxygen species (ROS) regulate root elongation, but the interactions between the two pathways are not well understood. The aim of this study was to investigate how auxin interacts with ROS in regulating root elongation in tomato, Solanum lycopersicum.
Methods
Wild-type and auxin-resistant mutant, diageotropica (dgt), of tomato (S. lycopersicum ‘Ailsa Craig’) were characterized in terms of root apical meristem and elongation zone histology, expression of the cell-cycle marker gene Sl-CycB1;1, accumulation of ROS, response to auxin and hydrogen peroxide (H2O2), and expression of ROS-related mRNAs.
Key Results
The dgt mutant exhibited histological defects in the root apical meristem and elongation zone and displayed a constitutively increased level of hydrogen peroxide (H2O2) in the root tip, part of which was detected in the apoplast. Treatments of wild-type with auxin increased the H2O2 concentration in the root tip in a dose-dependent manner. Auxin and H2O2 elicited similar inhibition of cell elongation while bringing forth differential responses in terms of meristem length and number of cells in the elongation zone. Auxin treatments affected the expression of mRNAs of ROS-scavenging enzymes and less significantly mRNAs related to antioxidant level. The dgt mutation resulted in resistance to both auxin and H2O2 and affected profoundly the expression of mRNAs related to antioxidant level.
Conclusions
The results indicate that auxin regulates the level of H2O2 in the root tip, so increasing the auxin level triggers accumulation of H2O2 leading to inhibition of root cell elongation and root growth. The dgt mutation affects this pathway by reducing the auxin responsiveness of tissues and by disrupting the H2O2 homeostasis in the root tip.
Keywords: Auxin, ROS, hydrogen peroxide, root elongation, tomato, Solanum lycopersicum, diageotropica, dgt
INTRODUCTION
Plant root growth depends on production of new cells in the root apical meristem and cell elongation once cells leave the meristem. Cell expansion generally depends on turgor pressure and modulations of the cell-wall extensibility. Plant organ growth and cell length are targets of the hormone auxin, which seems to act differentially depending on concentration, specific tissues and growth conditions. For example, auxin promotes shoot growth and root hair formation and elongation (Pitts et al., 1998; Rahman et al., 2002) but strongly suppresses root growth when applied even at relatively low doses. When applied at nanomolar concentrations (30 nm and 100 nm, respectively), the natural auxin indol-3-acetic acid (IAA) and the synthetic form 1-naphthaleneacetic acid (NAA) have been reported to inhibit root growth in Arabidopsis thaliana by reducing the length of the root elongation zone (Rahman et al., 2007). However, these treatments did not significantly decrease cell division in the root apical meristem, as judged from expression level of the cell-cycle marker construct At-CyclinB1;1:GUS (Rahman et al., 2007). In contrast, when applied at micromolar concentrations (10 µm for 40 h) NAA was shown to cause an almost complete consumption of the tomato root apical meristem and replacement of the meristem by developing lateral root primordia (Ivanchenko et al., 2006). These examples illustrate the ability of auxin to regulate root growth and development in a concentration-dependent manner.
In addition to hormones, the regulation of root growth has been strongly linked to the generation of reactive oxygen species (ROS). The superoxide radical (O2•–), hydrogen peroxide (H2O2) and the highly reactive and unstable hydroxyl radical (•OH) are produced during normal plant metabolism and in response to biotic and abiotic stresses (Neill et al., 2002; Dietz et al., 2006; Gapper and Dolan, 2006). Compared with other ROS, H2O2 is relatively more stable. H2O2 is also able to diffuse freely among cellular compartments and between cells facilitated by movement through specific aquaporin membrane channels (Henzler and Steudle, 2000; Bienert et al., 2007). Along the root, O2•– has been shown to localize predominantly in the elongation zone whereas H2O2 was found predominantly in the differentiation zone and in root hairs (Dunand et al., 2007). Normal ROS levels are maintained by ROS-scavenging enzymes, superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and glutathione transferase (GST) (reviewed by Mittler et al., 2004). In plants, H2O2 is also scavenged non-enzymatically by the antioxidants glutathione and ascorbate (reviewed by Ogawa, 2005). The level of reduced (active) glutathione is regulated by the rate-limiting biosynthetic enzyme gamma-glutamylcysteine synthetase (GCS) and by glutathione reductase (GR), whereas the level of ascorbate is regulated through oxidation by ascorbate oxidase (AO) and by regeneration of oxidized ascorbate. Scavenging of H2O2 by ascorbate generates monodehydroascorbate and ultimately dehydroascorbate, which is regenerated back to ascorbate by dehydroascorbate reductase (DHAR) using glutathione as an electron donor.
The importance of ROS and their spatial distribution along the root for normal root growth has been demonstrated through mutant analysis. In the upbeat 1 (upb1) A. thaliana mutant, the level of O2•– was increased and that of H2O2 decreased in the root tip, resulting in meristem enlargement, increased cell elongation and generally increased root growth (Tsukagoshi et al., 2010). The auxin and cytokinin responses of upb1 proved normal, suggesting that modulations of the ROS level in the upb1 root tip did not dramatically affect the hormonal responses involved in root growth (Tsukagoshi et al., 2010). By contrast, whether hormones regulate ROS level in the root tip and/or the balance between different types of ROS thereby affecting root growth is less understood.
The dgt tomato (Solanum lycopersicum) mutant was discovered by Zobel in the early 1970s (Zobel, 1973a, b) and is frequently studied in this plant species. It has been long known to be auxin resistant and to exhibit abnormal expression of auxin-related genes (Kelly and Bradford, 1986; Mito and Bennett, 1995; Nebenführ et al., 2000). Among other studies, dgt has served as a model for investigating gravitropic response (Madlung et al., 1999; Rice and Lomax, 2000), fruit development (Balbi and Lomax, 2003; Mignolli et al., 2012), root branching (Ivanchenko et al., 2006), adventitious root formation (Vidoz et al., 2010; Lombardi-Crestana et al., 2012) and arbuscular mycorrhiza initiation (Hanlon and Coenen, 2010). Cloning of the DGT gene revealed that DGT encodes a homolog of human cyclophilin-A, a protein known for affinity for the immunosuppressant drug cyclosporine-A but poorly understood at the cellular level due to protein redundancy in many species (Oh et al., 2002, 2006). To date, no dgt-like mutant has been described in A. thaliana, although similar mutants were recently reported in rice (Oryza sativa) (Kang et al., 2013; Zheng et al., 2013) and the moss Physcomitrella patens (Lavy et al., 2012) indicating that DGT-like proteins have conserved function in auxin signalling.
Here we report interactions between auxin and H2O2 in regulating root elongation that were discovered through analyses of the dgt tomato mutant. We found that H2O2 was maintained at a relatively low level in the wild-type tomato root tip but was increased upon auxin treatment and apparently acted as a downstream component of auxin in reducing root cell elongation. The dgt mutant was found to exhibit a constitutively increased level of H2O2 in the root tip that contributed to dgt's inability to respond flexibly to auxin treatments. These findings suggest that auxin regulates the H2O2 level in the root tip in order to control root growth and this pathway is disrupted by the dgt mutation in tomato.
MATERIALS AND METHODS
Plant material and growth conditions
Tomato (Solanum lycopersicum) wild-type and dgt1-1 plants in the ‘Ailsa Craig’ background (Ivanchenko et al., 2006) were used in this study. Seeds were surface-sterilized in 20 % commercial bleach for 30 min and rinsed four times for 10 min with sterile water. Sterilized seeds were incubated at 4 °C for 2 d to ensure even germination and then planted on media containing 0·2× Murashige and Skoog basal medium with vitamins (PhytoTechnology; http://www.phytotechlab.com), 1 % sucrose, 10 mm MES buffer pH 5·7 and 0·8 % agar. Plants were grown on vertically orientated 150-mm-diameter Petri dishes in a growth chamber at 21 °C under long day (16 h light, 8 h dark) conditions and light intensity of 26 µE m−2 s−1. For histological analyses, plants were usually grown for 7 d on control medium and then transferred to a new Petri dish containing the same medium (control) or medium supplemented as indicated in the figure legends. IAA and H2O2 (Sigma-Aldrich; http://www.sigmaaldrich.com) were applied for 5 h (unless noted otherwise) after which plates were immediately photographed, then roots were fixed and processed for clearing and subsequent histological measurements. Media contained 0, 5, and 25 nm IAA or 0, 0·25 and 1 mm H2O2. On average, 10–12 seedlings were analysed per sample in at least two independent experiments. For analyses of IAA effects on H2O2 level or gene expression, 7-d-old seedlings were treated with the corresponding IAA concentrations in liquid medium with gentle shaking for 12 h. On average, 50–100 root tips were collected from below the first root hair bulge per sample in three independent experiments.
Analyses of root growth parameters and microscopy
Measurements of root growth increments were performed on images taken from the plates using IMAGE-Pro Plus (Media Cybernetics, http://www.mediacy.com). For meristem, elongation zone and cell morphology analyses, roots were fixed in ethanol/acetic acid (3 : 1) for 24 h, cleared as described (Malamy and Benfey, 1997) and then mounted in saturated chloral hydrate solution containing 10 % glycerol. Root samples were analysed under a Zeiss Axiovert microscope with differential interference contrast (DIC) optics. Measurements of the root zones lengths and cell lengths were performed under a microscope using an ocular micrometer and the 20× or 40× objective, respectively. Meristem length was measured as the distance from the quiescent centre (QC)/central root cap border to the region where cells started to progressively increase in length as the result of rapid elongation and the elongation zone length was measured from this point to the first root hair bulge. Cell length was measured in the second exterior cortex layer by measuring the length of the cell that was just below the first root hair bulge. Digital images were taken using a SPOT CCD camera and software (Diagnostic Instruments; http://www.spotimaging.com/).
Staining for ROS and histological analyses
The superoxide radical (O2•–) was detected based on Nitroblue tetrazolium (NBT)-reducing activity as previously described (Doke, 1983). Eight-day-old seedlings were immersed in a solution of 2·0 mm NBT (Sigma-Aldrich) in 20 mm MES buffer pH 6·1 for 5 min at room temperature after which the reaction was stopped by transferring the seedlings into distilled water. Hydrogen peroxide (H2O2) was detected with 3,3′-diaminobenzidine tetrachloride (DAB) reagent (Sigma-Aldrich) as described (Thordal-Christensen et al., 1997). Seedlings were immersed in 0·05 % DAB in PBS buffer pH 7·4 for 2 h and the reaction was stopped by transferring the seedlings into distilled water. After staining, roots were fixed, cleared and processed for microscopy as described above.
For histological sections, roots were fixed in 1·5 % (v/v) glutaraldehyde and 0·3 % (v/v) paraformaldehyde in 25 mm PIPES (1,4-piperazinediethanesulfonic acid) and embedded in Historesin (Leica Instruments GmbH, Heidelberg, Germany). Sections 2 µm thick were stained using a Periodic acid-Schiff reaction as described (Baum and Rost, 1996).
Imaging of extracellular ROS accumulation
Extracellular release of ROS was determined using the fluorogenic reagent OxyBURST green H2HFF conjugated to BSA (Invitrogen; http://www.invitrogen.com) as described (Monshausen et al., 2007). Wild-type and dgt seedlings were grown on wet filter paper for 6 d and then mounted side by side on agar in an experimental chamber for 5–6 h as previously described (Monshausen et al., 2007). Roots were then cut free of agar and bathed in 100 µL low-salt nutrient medium containing 0·3 mm KNO3, 0·2 mm Ca(NO3)2.4H2O, 0·05 mm MgSO4.7H2O, 0·1 mm (NH4)2PO4 and 1 % sucrose for approximately 2 min before adding 500 µL of 100 µg mL−1 OxyBURST into the low-salt medium. Images were acquired on a Zeiss LSM 510 microscope using a 10 × , 0·3 numerical aperture objective. Fluorescence was excited with the 488-nm line of the argon laser and emission was collected using a 488 dichroic mirror and 505-nm long-pass filter. Images were acquired at 5-s intervals for 30 s prior to OxyBURST addition to determine background fluorescence and for 90 s subsequently.
Quantification of the IAA effect on H2O2 accumulation in the root tip
To prepare root extracts, root tips excised from below the first root hair bulge were ground on ice in 50 mm Na-KPO4 buffer (pH 7·4) and cleared by centrifugation at 10 000g for 5 min at 4 °C. H2O2 accumulation was quantified using Amplex Red hydrogen peroxide/peroxidase assay kit (Invitrogen) following the manufacturer's instructions. Briefly, 50 µL of cleared supernatant was added to 50-μL reaction mixtures containing 100 µm Amplex Red reagent and 0·1 U mL−1 horseradish peroxidase in black-bottom microtitre plates (Corning Inc.; http://www.corning.com/index.aspx). After incubation for 30 min in the dark at room temperature, fluorescence was measured using a Synergy II microtitre plate reader (Biotek; http://www.biotek.com/) with excitation and emission at 530 and 590 nm, respectively. Readings from samples were calibrated against a standard curve prepared with known H2O2 concentrations. In both sample assays and standard curve assays appropriate negative controls (only buffer or no-H2O2) were used. H2O2 content was expressed as μmol g−1 f. wt.
Quantitative real-time PCR (qRT-PCR) for gene expression
Total RNA was extracted using TRIzol reagent (Invitrogen) and purified by phenol/chloroform extraction and LiCl precipitation followed by an RNeasy Mini kit (Qiagen; http://www.qiagen.com) with in-column DNAase treatment step according to the manufacturer's protocol. Synthesis of cDNA was performed using QuantiTect Reverse transcription kit (Qiagen) with a genomic DNA wipeout step when the RNA yields were around 50 ng μL−1 or higher. In samples with lower RNA concentration, an iScript cDNA synthesis kit (Bio-Rad; http://www.bio-rad.com) was used according to the manufacturer's protocol. In both cases, the primers were validated for efficiency using a 10(−1/slope) – 1 method and slopes were obtained between values of –3·23 and –3·18 (92–94 % efficiency). Real-time PCR was performed in triplicate with iTaq SYBR Green Supermix with a Rox reference dye (Bio-Rad) on either an ABI StepOne Plus real-time machine or realplex ep2 Mastercycler (Eppendorf; http://www.eppendorf.com) under default thermal cycling conditions with an added melt curve. Primers were designed using Primer Quest and obtained from Integrated DNA Technologies (https://www.idtdna.com). For primer sequences, see Supplementary Data Table S1. Data were analysed using the 2−ΔΔCT method and values normalized to two housekeeping genes, RPL2 (Balbi and Lomax, 2003) and Ubi3 (Rotenberg et al., 2006), which were found to be expressed stably in roots and not affected by treatments and genotypes.
RESULTS
The dgt mutation of tomato causes quantitative histological defects in the root tip
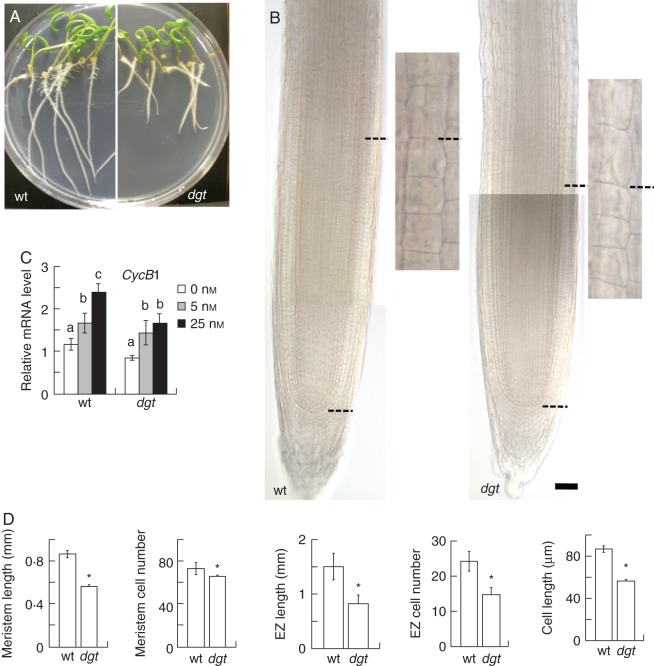
The dgt mutant has been previously reported to have shorter root meristem and elongation zone compared with wild-type (Ivanchenko et al., 2006) but these defects were not analysed in detail. We observed that the dgt root tip was slightly wider, but did not display any severe meristem disorganization (Fig. 1A, B). The wild-type root apical meristem, estimated as the distance from the quiescence center (QC)/root cap border to the site where cells show the first evidence of fast elongation, was on average 0·86 ± 0·03 mm long containing 75 cells whereas the dgt meristem was on average 0·56 ± 0·01 mm long containing 68 cells (Fig. 1B, D). The length of the elongation zone was measured as the distance from the first visible sign of cell elongation in the second exterior cortex layer to the first root hair bulge in the epidermis. This distance was on average 1·4 ± 0·2 mm containing 25 cells in wild-type and 0·7 ± 0·1 mm containing 14 cells in dgt (Fig. 1D). The length of the elongated cortex cells at the site of the first root hair bulge was on average 84 ± 4 µm in wild-type and 59 ± 2 µm in dgt (Fig. 1D). Thus, dgt is unable to maintain normal meristem length, elongation zone length and cell elongation, which correlated with reduced root growth. qRT-PCR analyses of the cell-cycle-related Sl-CyclinB1;1 mRNA in the root tip below the root hair bulge showed that Sl-CyclinB1;1 was upregulated by auxin in both wild-type and dgt but its expression level remained much lower in dgt even after the auxin treatment (Fig. 1C). Thus, the reduction of dgt meristem length correlates with reduced cell division in the root tip.
Fig. 1.
The dgt mutant of tomato displays reduced root growth correlated with reduced meristem length, number of cells in the elongation zone and elongated cell length. (A) Images of 8-d-old wild-type and dgt seedlings. Two partially overlapping images were combined for each genotype by precisely aligning the contours of overlapping cells. (B) Root meristem in wild-type and dgt depicted as the distance between the QC/root cap border and the beginning of cell elongation (dashed lines). Inserts show beginning of cell elongation in the second exterior layer of the cortex. Scale bar = 122 µm. (C) Expression level and IAA response of Sl-CyclinB1;1 cell-cycle gene in wild-type and dgt root tips. Different letters indicate a statistically significant difference when analysed by one-way ANOVA and a multiple comparison using Tukey's test at P ≤ 0·05. Data are combined mean ± s.e. of two experiments performed in triplicate with 50–100 root tips per sample. (D) Quantifications of root meristem length, meristem cell number, elongation zone (EZ) length, elongation zone cell number and elongated root cell length in wild-type and dgt. Quantitative values are combined mean ± s.e. for two independent experiments with 11–12 roots per sample in each experiment. *P ≤ 0·05, Student's t-test.
In addition to cell division, the length of the root apical meristem also depends on the maintenance of a population of initial cells. The QC serves as an organizer of the meristem and a reservoir of initial cells. The tomato QC can be recognized histologically as a ‘hemispherical mass of irregularly-shaped cells’ at the root tip just above the central root cap border (Street et al., 1967). These morphologically distinct cells show low labelling with tritiated thymidine, confirming their low rate of cell division and hence QC nature (Fusconi et al., 1999). Based on the cell-shape criterion, we identified the QC region in wild-type and dgt roots and determined that dgt QC was of normal height (Fig. 2). A normal QC height indicates normal maintenance of initial cells in the meristem (Barlow, 1997). However, the number of QC cells appeared to be increased in the lateral direction, probably resulting from ectopic cell division (Fig. 2). This ectopic cell division could result from auxin imbalance because the auxin transport inhibitor N-naphthylphthalamic acid has been shown to induce QC cell division in maize roots (Ponce et al., 2005). Whether the abnormal mitotic activity of the dgt QC region is somehow related to the reduction of dgt root apical meristem length is at present unclear.
Fig. 2.
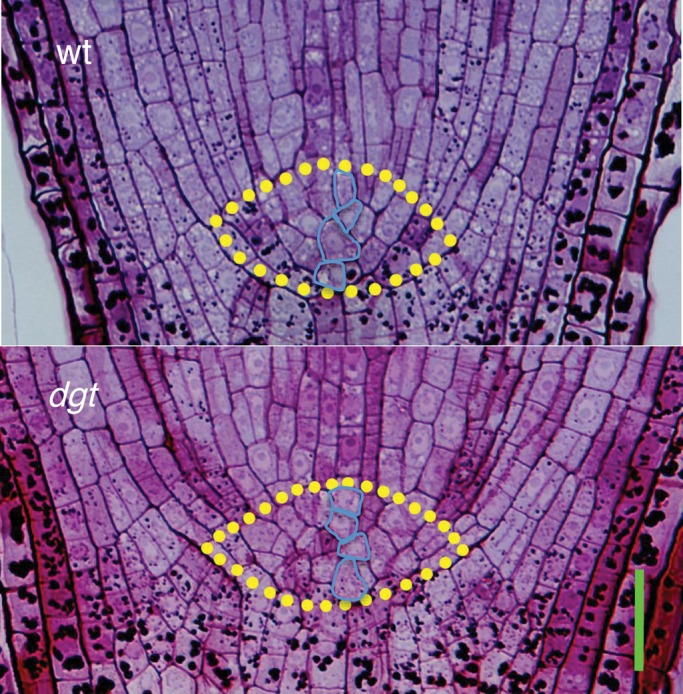
Histological comparison of the distal meristem region from a wild-type and dgt root. The QC cell region is marked. Periodic acid-Schiff stain was used to visualize cells in 8-d-old seedlings. Scale bar = 35 µm.
The dgt mutant shows an increased H2O2 level in the root tip and increased level of ROS in the apoplast
We also analysed the level of ROS in the dgt root tip. As a probe for O2•–, we used NBT, a reagent that forms an insoluble purple–blue formazan precipitate upon interaction with O2•– (Bielski et al., 1980). For H2O2, we used 3,3'-diaminobenzidine-HCl (DAB), a chromogen that produces a brown stain in the presence of endogenous H2O2 and peroxidases (Thordal-Christensen et al., 1997). Consistent with earlier observations demonstrating differential distribution of these radicals along the A. thaliana root (Dunand et al., 2007), we observed differential distribution in the wild-type tomato root with O2•– decreasing from root tip toward differentiation zone and H2O2 increasing from root tip toward differentiation zone (Fig. 3A). There was no visually obvious difference between wild-type and dgt root tips in respect to O2•– stain (Fig. 3B). However, the H2O2 stain was increased in the dgt root tip compared with wild-type, especially in the proximal (shootward) portion of the meristem and the elongation zone (Fig. 3C). This demonstrates that H2O2 is maintained at a relatively low level in the growing portion of the wild-type tomato root and this balance is disturbed by the dgt mutation.
Fig. 3.

The dgt mutation increases the H2O2 level in the root tip but has no visible effect on the O2•– level. (A) Images of 8-d-old wild-type roots stained for the superoxide radical (O2•–) using Nitroblue tetrazolium (NBT) and for H2O2 using 3,3′-diaminobenzidine tetrachloride (DAB), respectively. Note that the NBT signal increases toward the root tip whereas the DAB signal increases in the opposite direction (arrows). (B) Comparison of wild-type and dgt root tips stained for O2•–. (C) Comparison of wild-type and dgt root tips stained for H2O2. Scale bars = 122 µm.
The properties of the cell-wall continuum, known as the apoplast, are considered to play an important role during cell elongation; loosening of the wall would allow cells to increase in volume upon increases of turgor pressure whereas wall stiffening would cause an opposite effect (Cosgrove, 1997). To visualize extracellular ROS in dgt with high spatial and temporal resolution, we used an imaging approach based on OxyBURST green H2HFF [(dihydro-2),4,5,6,7,7)-hexafluorofluorescein], a non-fluorescent reagent that becomes fluorescent upon oxidation. OxyBURST was applied as a conjugate with BSA to retain it in the extracellular space. The intensity of OxyBURST fluorescence in this approach does not vary with environmental factors such as changes in pH or ionic strength of the medium (Monshausen et al., 2007). After addition of OxyBURST to the medium bathing wild-type and dgt roots, ROS-dependent fluorescence quickly developed along the surface of the meristem and elongation zone of the dgt root whereas much lower levels of fluorescence were observed along wild-type root tips (Fig. 4A, B).
Fig. 4.

Confocal imaging of extracellular ROS accumulation along wild-type and dgt tomato roots incubated with OxyBURST green H2HFF-BSA. (A) Bright field (left) and fluorescence images (right) of growing wild-type and dgt root tips 1 min after addition of OxyBURST. Regions (1–3) spanning the meristem and the elongation zone used to measure fluorescence are marked. The beginning of root hair initiation is marked in dgt (arrow) and is just above the field of the view for wild-type. Fluorescence quickly developed along the root surface of dgt, indicating higher extracellular ROS accumulation compared with wild-type. Scale bar = 500 µm. (B) Quantification of ROS-dependent OxyBURST fluorescence in region 1 (black), region 2 (red) and region 3 (blue) as denoted in A. Average ROS-dependent fluorescence intensities were analysed by three-way ANOVA followed by Tukey's HSD, which showed a statistically significantly difference between wild-type and dgt (P < 0·05 for regions 1 and 2, P < 0·01 for region 3). Within wild-type there was no significant difference between regions or over time, while in dgt a significant difference was recorded between region 1 and 3 (P < 0·01) and between 2 and 3 (P < 0·05). Data represent means ± s.d. of six independent measurements. a.u., arbitrary units.
OxyBURST is documented to react with only certain forms of ROS showing strongest sensitivity for O2•– and, in the presence of peroxidases, H2O2 (Haugland, 2005). Given that we did not detect any strong increase of O2•– with the NBT stain in the dgt root tip but detected a dramatic increase of H2O2 with the DAB reagent (Fig. 2B), we conclude that the OxyBURST signal probably detected an increased accumulation of H2O2 in the apoplast at the dgt root tip.
IAA treatment increases the level of H2O2 in the wild-type root tip
We next quantified the effect of IAA on H2O2 level in the root tip of wild-type and dgt plants using samples from below the first root hair bulge. When wild-type plants were incubated in medium containing 5 or 25 nm IAA the level of H2O2 in their root tips increased in a dose-dependent manner (Fig. 5A). This clear linear response revealed a relationship between auxin level and H2O2 level in the root tip. In comparison with wild-type, the level of H2O2 in untreated dgt root tips was much increased and an inability to respond to IAA with increasing the H2O2 level was also evident (Fig. 5A). This could be due to dgt's auxin resistance. Alternatively, the auxin treatment did not increase the H2O2 level in dgt because it was already too high in untreated dgt root tips and comparable with that in IAA-treated wild-type root tips (Fig. 5A).
Fig. 5.
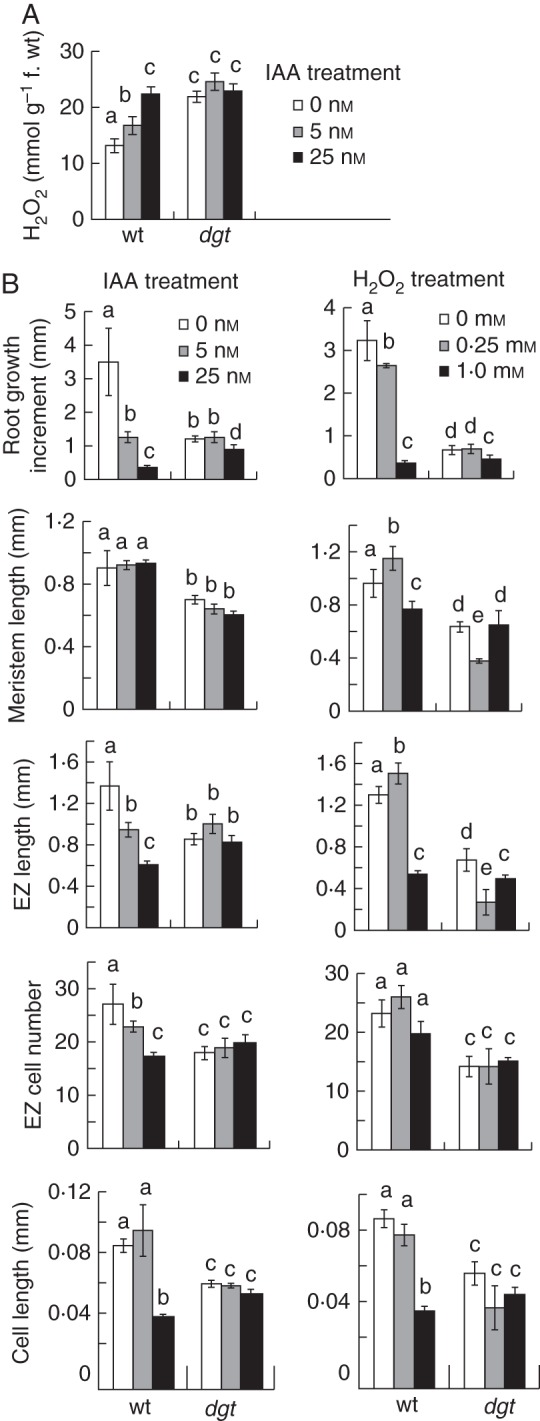
Correlations between IAA level and response and H2O2 level and response in tomato seedlings. (A) Quantifications of the effect of IAA on H2O2 level in root tips of 8-d-old wild-type and dgt seedlings that had received a 12-h IAA treatment. (B) Comparisons of the effects of IAA and H2O2 on root growth, meristem length, elongation zone (EZ) length, elongation-zone cell number, and elongated cell length in 8-d-old wild-type and dgt seedlings after 5 h of treatment. Different letters indicate a statistically significant difference between treatments in each genotype as well as between genotypes at P ≤ 0·05 when analysed by two-way ANOVA followed by Bonferroni's post-test. Data in A represent mean ± s.e.m. from three independent experiments (n = 3) where each sample consists of 50–100 pooled root tips, and data in B represent mean ± s.e.m. of two independent experiments each performed with 10–11 individual roots per group (n = 20–22).
IAA and H2O2 both inhibit cell elongation and root growth in wild-type in a dose-dependent manner
To examine the interaction between IAA and H2O2 more broadly, we compared their histological effects at the cellular level at concentrations that comparably inhibited root growth (Fig. 5B). Treatments of only 5 h were used in these comparisons because the H2O2 effect proved unstable with longer incubation times. In the presence of 5 or 25 nm IAA, the length of the newly formed wild-type root portion was reduced in a concentration-dependent manner, on average from 3·5 ± 1·0 mm in untreated roots to only 0·3 ± 0·1 mm in plants treated with the higher IAA concentration. In the presence of 0·25 and 1·0 mm H2O2, the length of the newly formed root portion was comparably reduced, on average from 3·2 ± 0·5 to 0·3 ± 0·1 mm with the higher H2O2 concentration. Upon analysing the root tip histologically, it was evident that both IAA and H2O2 reduced the elongated cell length (Fig. 5B). However, IAA also reduced the number of cells in the elongation zone and had no inhibitory effect on meristem length whereas H2O2 had little effect in the elongation zone and reduced meristem length significantly (Fig. 5B). Thus, IAA and H2O2 probably interact to inhibit cell elongation, but apparently also have differential effects in the meristem and elongation zone.
In comparison with wild-type, the length of the newly formed dgt root portion was less reduced in the presence of IAA, on average from 1·2 ± 0·1 mm in untreated roots to 0·7 ± 0·2 mm at the higher IAA concentration, consistent with dgt's auxin resistance. In the presence of H2O2, the length of the newly formed dgt root portion was also less reduced compared with the wild-type, on average from 0·7 ± 0·1 to 0·5 ± 0·1 mm with the higher H2O2 concentration. Histological analyses of the root tip revealed that the dgt mutation reduced not only the common effect of IAA and H2O2 on elongated cell length, but also their differential effects on meristem length and number of cells in the elongation zone. Thus, dgt roots grew better than wild-type roots in the presence of inhibitory concentrations of either auxin or H2O2, demonstrating that dgt is resistant to both compounds.
IAA and the dgt mutation affect expression of ROS-related mRNAs in the root tip
To elucidate further the links between IAA, H2O2 and the effect of the dgt mutation, we next analysed how IAA affected the expression of ROS-related mRNAs in the root tip by qRT-PCR. Where information on the subcellular localization of enzymes was available, we focused on cytosolic and apoplastic isoforms as those were more likely to be expressed in the root tip and affect root growth. Root-tip samples were collected from below the first root hair bulge to avoid contamination with high levels of mRNAs related to root hair development. IAA treatments slightly reduced the level of Sl-SOD mRNA and increased those of Sl-CAT1, Sl-APX1, Sl-GPX-like 1 (Sl-GPXL1) and Sl-GST / GPX (Fig. 6). This suggested that simultaneously with increasing the H2O2 level in the root tip the IAA treatment also induced activation of H2O2-detoxifying pathways. The dgt mutant showed an increased level of Sl-CAT1, Sl-APX1 and Sl-GPXL1 mRNAs but demonstrated normal response in terms of IAA effect on the levels of these mRNAs (Fig. 6). Thus, protective feedback upregulation of ROS-scavenging enzymes is probably induced upon IAA treatment to minimize the toxic effect of accumulating H2O2 in the root tip, but this response does not seem to require intact DGT gene function.
Fig. 6.
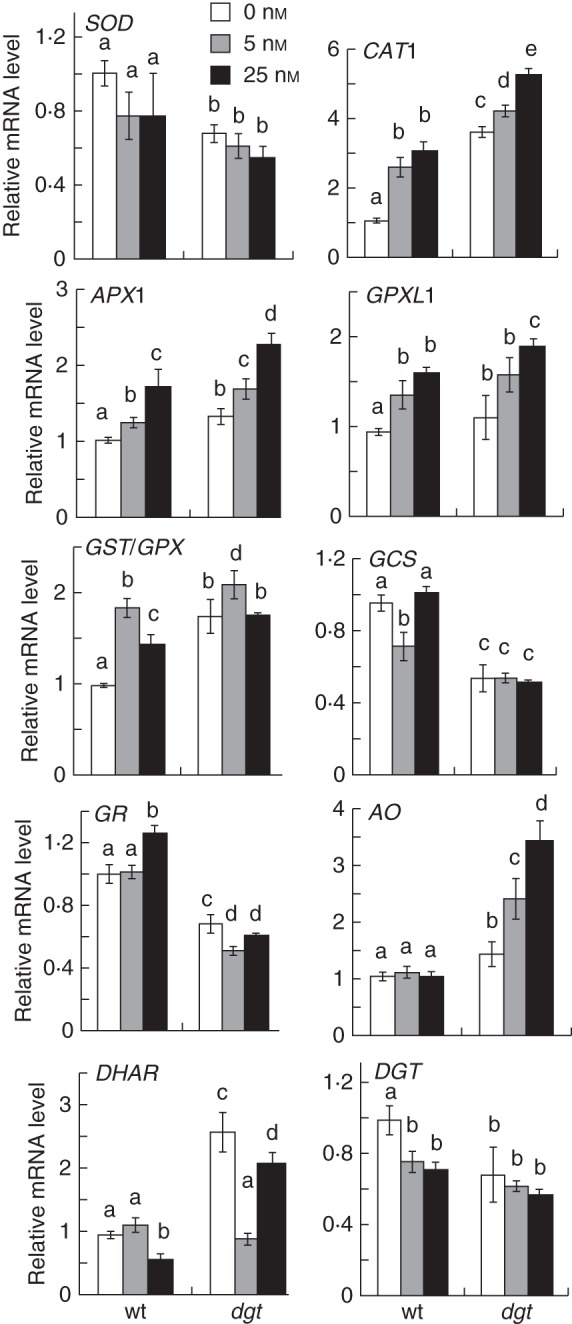
Quantifications of the effect of IAA (as indicated in the key) on expression level of mRNAs of tomato ROS-scavenging enzymes (Sl-SOD accession M37151, Sl-CAT1 accession M93719, Sl-APX1 accession DQ099420, Sl-GPXL accession NM_001247638, Sl-GST/GPX accession NM_001247450), mRNAs related to regulation of antioxidant balance (Sl-GCS accession NM_001247081, Sl-GR accession FJ265823, Sl-AO accession AY971876, Sl-DHAR accession AY971873) and DGT mRNA (accession M55019). Different letters indicate a statistically significant difference at P ≤ 0·05 when analysed by two-way ANOVA followed by Bonferroni's post-test. Data represent combined mean ± s.e. of three biological experiments performed in triplicate (n = 9) with 50–100 pooled root tips per sample.
In contrast to its effect on mRNAs of ROS-scavenging enzymes, the effect of IAA on the expression of mRNAs related to regulation of antioxidant levels was less profound and did not display clear dose dependence, although the effect of the dgt mutation on these mRNAs was dramatic (Fig. 6). The levels of Sl-GCS and Sl-GR were reduced nearly two-fold in the dgt root tip compared with wild-type whereas Sl-DHAR was increased. The level of Sl-AO was also dramatically increased in dgt and in addition showed an unusually strong upregulation by IAA that was not detected in the wild-type root tip. These observations revealed a defect in dgt in regulating the expression of antioxidant-related mRNAs.
We also analysed the expression of the DGT gene by qRT-PCR (Fig. 6). The Sl-DGT mRNA was down-regulated by IAA treatment in the wild-type root tip in a dose-dependent manner but was reduced and unresponsive to IAA in the dgt root tip. Thus, there is apparently a feedback regulation between auxin level and DGT gene expression in the root tip consistent with the participation of DGT in auxin signalling.
DISCUSSION
H2O2 probably acts as a downstream component of auxin in inhibiting root growth
At the concentrations tested, we have observed that IAA increased the H2O2 level in the wild-type root tip in a dose-dependent manner, which demonstrates that H2O2 is under auxin regulation in the growing portion of the root. Furthermore, additions of either IAA or H2O2 to the growth medium clearly elicited similar reductions of cell elongation and root growth, supporting the hypothesis that H2O2 is a component downstream of auxin in inhibiting root elongation. IAA also reduced the number of cells in the elongation zone whereas H2O2 significantly reduced meristem length, indicating that the two compounds have differential effects in specific root zones. Another study reported recently that IAA treatments decreased the H2O2 level in tomato root apices simultaneously with reducing root growth (Tyburski et al., 2009). Potentially, differences in experimental design, e.g. much higher auxin concentrations, longer exposure times and not excluding root hair-containing portions, could lead to results different from these reported here.
In addition to being increased by auxin, we also observed that the spatial distribution of H2O2 along the wild-type tomato root was not random and the H2O2 level increased gradually above the meristem toward the differentiation zone of the root. It is interesting to note that a recent study reported a similar gradient in auxin distribution and response along A. thaliana and S. lycopersicum roots that was dependent on polar auxin transport (Dubrovsky et al., 2011). Potentially, modulations of the auxin gradient in this part of the root through polar auxin transport would affect the H2O2 gradient and lead to modulations in root growth and development.
Our results agree with observations made with the upb1 mutant of A. thaliana, showing that a decrease of the H2O2 level in the root tip is associated with enlargement of the meristem, increase of cell elongation and general increase of root growth (Tsukagoshi et al., 2010). Furthermore, gravistimulated maize seedlings have been reported to display accumulation of H2O2 on the lower non-expanding side of the root, suggesting that H2O2 acts downstream of auxin in gravitropic root bending (Joo et al., 2001). How could IAA reduce root cell elongation by increasing the H2O2 level in the root tip? The mechanisms through which auxin regulates cell elongation remain poorly understood. In shoots, auxin probably promotes growth by increasing the acidification of the cell wall, which should facilitate expansin-induced cell-wall loosening (Cosgrove, 1997). However, auxin may act through a different mechanism in roots. At micromolar concentrations auxin treatment was reported to inhibit root elongation in maize seedlings in a manner that was independent of changes in the external pH, and hence of cell-wall acidification (Büntemeyer et al., 1998). As we observed that increases in the level of H2O2 upon IAA treatment were accompanied by decreases of cell elongation and an increase of apoplastic H2O2 in the tip of the dgt mutant marked with reduced cell elongation, we speculate that accumulation of H2O2 in the root tip might lead to reductions in the cell-wall extensibility of developing root cells.
Simultaneously with increasing the H2O2 level, auxin upregulates transcription of mRNAs of ROS-scavenging enzymes
Major ROS-scavenging enzymes in plants, SOD, CAT, APX, GPX and GST, have been well characterized at the transcriptional level, although their subcellular localizations are still poorly understood (reviewed by Mittler et al., 2004). SOD catalyses the conversion of O2•– to H2O2 whereas peroxidases of different types participate in H2O2 elimination. Because H2O2 diffuses freely among subcellular compartments and among cells (Henzler and Steudle, 2000; Bienert et al., 2007), its increase in the root tip would probably affect root elongation regardless of the subcellular site of its generation.
At least two tomato SODs are expressed in leaves: a potentially cytosolic form (accession M37150) and a form showing similarity to a chloroplastic tobacco SOD (accession M37151) (Perl-Treves, 1988). We could not amplify the M37151 accession in root tips but we were able to analyse M37151. The tomato catalase CAT1 gene (accession M93719) investigated here seems to be involved in H2O2 detoxification as transgenic tomato plants harbouring this sequence in antisense orientation demonstrated a two-fold increase in levels of H2O2 (Kerdnaimongkol and Woodson, 1999). The APX gene family of tomato has been reported to comprise three cytosolic forms (APX1–3) (Najami et al., 2008) of which we analysed APX1 (accession DQ099420) as it was well expressed in roots. Tomato GST/GPX (accession NM_001247450) analyaed here has been reported to enhance resistance to H2O2-induced oxidative stress when overexpressed in yeast (Kampranis et al., 2000). A glutathione peroxidase-like (GPXL1) gene (accession NM_001247638) has been suggested to encode either a plasma membrane-localized or cytosolic enzyme (Depège et al., 1998). In qRT-PCR analyses, we observed that the expression of Sl-SOD was slightly decreased upon auxin treatment whereas those of Sl-CAT1, Sl-APX1, Sl-GST/GPX and Sl-GPXL1 were significantly increased, supporting the hypothesis that these enzymes participate in a feedback response related to detoxification of IAA-induced H2O2 accumulation in the root tip.
We also analysed Sl-GCS for which a sequence was available (accession NM_001247081), Sl-GR (accession FJ265823) as it has been reported to be induced by abiotic stress in roots (Goupil et al., 2009), as well as Sl-AO (accession AY971876) and Sl-DHAR (accession AY971873) as these sequences have been cloned and partially mapped (Zou et al., 2006). Dose-dependent responses of these mRNAs to auxin treatments were less obvious compared with the responses observed with mRNAs of ROS-scavenging enzymes analysed here.
The dgt mutation increases the H2O2 level in the root tip in part due to misregulation of antioxidant-related mRNAs
The qRT-PCR analyses demonstrated that, in comparison with wild-type, the dgt mutation caused decreased expression of mRNAs related to glutathione synthesis and regeneration, Sl-GCS and Sl-GR, and increased expression of mRNAs related to ascorbate metabolism and regeneration, Sl-AO and Sl-DHAR. The observed decreases in Sl-GCS and Sl-GR expression and the increased expression of Sl-AO are likely to account, at least in part, for the increased level of H2O2 in the dgt root tip as the antioxidants regulated by these genes are involved in non-enzymatic elimination of H2O2 (reviewed by Ogawa, 2005). The IAA treatment did not affect these mRNAs in wild-type root tips. However, both the expression levels and IAA responses of Sl-GCS, Sl-GR and Sl-DHAR were affected profoundly by the dgt mutation, suggesting that antioxidant levels are auxin regulated. In support of this, an earlier study reported an increase of the AO mRNA in cultured maize roots after 2 d of treatment with the synthetic auxin 2,4-D (Kerk and Feldman, 1995).
In addition to response (a term generally defined as the direct effect of auxin on gene expression), a mutant can also be deficient in auxin distribution. The dgt mutant shows an increased accumulation of free (active) auxin and an abnormal auxin gradient in the root as judged from direct auxin measurements (Ivanchenko et al., 2006). This abnormally increased auxin accumulation could contribute to the increase of H2O2 in the dgt root tip that is reported here. Note that plants permanently inactivate IAA by ring oxidation to oxIAA, especially in the presence of an oxidative environment (reviewed by Woodward and Bartel, 2005). A recent study performed in A. thaliana reported that treatment with the phytotoxic atmospheric pollutant ozone, which can increase the production of ROS, caused auxin signalling to be transiently suppressed (Blomster et al., 2011). Thus, we cannot rule out that the high H2O2 level affects the IAA/oxIAA balance in the dgt root tip.
In conclusion, the results reported here suggest that auxin increases the H2O2 level in the root tip of tomato (S. lycopersicum ‘Ailsa Craig’), leading to a decrease of cell elongation and inhibition of root growth. A relatively low level of H2O2 in the proximal portion of the meristem and elongation zone of the wild-type root, regulated by the DGT gene, is essential for flexible auxin-induced increases of H2O2 in control of root growth.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by grants from Agricultural Research Foundation 5723G (M.G.I.); Oregon State University General Research Fund (M.G.I.); National Science Foundation MCB-1121994 (G.B.M.) and EPS-0903787 (N.K.); Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, Universidad Nacional Autónoma de México IN204312 (J.G.D.); Consejo Nacional de Ciencia y Technología (CONACyT) Mexico 127957 (J.G.D.); Mississippi State University MSU#012156-014 (N.K.) and GA USB project No. 062/2011/P., and grant No. P501/10/1215 from the Grant Agency of the Czech Republic (A.B). We thank Dr J. Fowler for providing lab space to M.G.I., M. E. Salas-Ocampo for help with histological sections and K. Hoth for help with editing the manuscript.
LITERATURE CITED
- Balbi V, Lomax TL. Regulation of early tomato fruit development by the diageotropica gene. Plant Physiology. 2003;131:186–197. doi: 10.1104/pp.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P. Stem cells and founder zones in plants, particularly their roots. In: Potten CS, editor. Stem cells. London: Academic Press; 1997. pp. 29–57. [Google Scholar]
- Baum SF, Rost TL. Root apical organization in Arabidopsis thaliana: 1. Root cap and protoderm. Protoplasma. 1996;192:178–188. [Google Scholar]
- Bielski BHJ, Shiue GG, Bajuk S. Reduction of nitro blue tetrazolium by CO2•- and O2•– radicals. Journal of Physiological Chemistry. 1980;84:830–833. [Google Scholar]
- Bienert GP, Møller AL, Kristiansen KA, et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. Journal of Biological Chemistry. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Blomster T, Salojärvi J, Sipari N, et al. Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiology. 2011;157:1866–1883. doi: 10.1104/pp.111.181883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büntemeyer K, Lüthen H, Böttger M. Auxin-induced changes in cell wall extensibility of maize roots. Planta. 1998;204:515–519. [Google Scholar]
- Cosgrove DJ. Cellular mechanisms underlying growth asymmetry during stem gravitropism. Planta. 1997;203(Suppl. 1):S130–S135. doi: 10.1007/pl00008101. [DOI] [PubMed] [Google Scholar]
- Depège N, Drevet J, Boyer N. Molecular cloning and characterization of tomato cDNAs encoding glutathione peroxidase-like proteins. European Journal of Biochemistry. 1998;253:445–451. doi: 10.1046/j.1432-1327.1998.2530445.x. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Jacob S, Oelze ML, et al. The function of peroxiredoxins in plant organelle redox metabolism. Journal of Experimental Botany. 2006;57:1697–1709. doi: 10.1093/jxb/erj160. [DOI] [PubMed] [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiology and Plant Pathology. 1983;23:345–357. [Google Scholar]
- Dubrovsky JG, Napsucialy-Mendivil S, Duclercq J, et al. Auxin minimum defines a developmental window for lateral root initiation. New Phytologist. 2011;191:970–983. doi: 10.1111/j.1469-8137.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- Dunand C, Crèvecoeur M, Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytologist. 2007;174:332–341. doi: 10.1111/j.1469-8137.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- Fusconi A, Gnavi E, Trotta A, Berta G. Apical meristems of tomato roots and their modifications induced by arbuscular mycorrhizal and soilborne pathogenic fungi. New Phytologist. 1999;142:505–516. [Google Scholar]
- Gapper C, Dolan L. Control of plant development by reactive oxygen species. Plant Physiology. 2006;141:341–345. doi: 10.1104/pp.106.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil P, Souguir D, Ferjani E, Faure O, Hitmi A, Ledoigt G. Expression of stress-related genes in tomato plants exposed to arsenic and chromium in nutrient solution. Journal of Plant Physiology. 2009;166:1446–1452. doi: 10.1016/j.jplph.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Hanlon MT, Coenen C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytologist. 2010;189:701–709. doi: 10.1111/j.1469-8137.2010.03567.x. [DOI] [PubMed] [Google Scholar]
- Haugland RP. Handbook of fluorescent probes and research chemicals. 2005 Molecular Probes-Invitrogen, http://www.invitrogen.com . [Google Scholar]
- Henzler T, Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. Journal of Experimental Botany. 2000;51:2053–2066. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, Coffeen WC, Lomax TL, Dubrovsky JG. Mutations in the Diageotropica (Dgt) gene uncouple patterned cell division during lateral root initiation from proliferative cell division in the pericycle. Plant Journal. 2006;46:436–447. doi: 10.1111/j.1365-313X.2006.02702.x. [DOI] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiology. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampranis SC, Damianova R, Atallah M, et al. A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. Journal of Biological Chemistry. 2000;275:29207–29216. doi: 10.1074/jbc.M002359200. [DOI] [PubMed] [Google Scholar]
- Kelly MO, Bradford KJ. Insensitivity of the diageotropica tomato mutant to auxin. Plant Physiology. 1986;82:713–807. doi: 10.1104/pp.82.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdnaimongkol K, Woodson WR. Inhibition of catalase by antisense RNA increases susceptibility to oxidative stress and chilling injury in transgenic tomato plants. Journal of the American Society for Horticultural Science. 1999;124:330–336. [Google Scholar]
- Kang B, Zhang Z, Wang L, et al. OsCYP2, a chaperone involved in AUX/IAA degradation, plays crucial roles in rice lateral root initiation. Plant Journal. 2013;4:86–97. doi: 10.1111/tpj.12106. [DOI] [PubMed] [Google Scholar]
- Kerk NM, Feldman NJ. A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development. 1995;121:2825–2833. [Google Scholar]
- Lavy M, Prigge MJ, Tigyi K, Estelle M. The cyclophilin DIAGEOTROPICA has a conserved role in auxin signaling. Development. 2012;139:1115–1124. doi: 10.1242/dev.074831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi-Crestana S, da Silva Azevedo M, e Silva GF, et al. The tomato (Solanum lycopersicum cv. Micro-Tom) natural genetic variation Rg1 and the DELLA mutant procera control the competence necessary to form adventitious roots and shoots. Journal of Experimental Botany. 2012;63:5689–5703. doi: 10.1093/jxb/ers221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madlung A, Behringer FJ, Lomax TL. Ethylene plays multiple nonprimary roles in modulating the gravitropic response in tomato. Plant Physiology. 1999;120:897–906. doi: 10.1104/pp.120.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Science. 2004;9:490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mignolli F, Mariotti L, Lombardi L, Vidoz ML, Ceccarelli N, Picciarelli P. Tomato fruit development in the auxin-resistant dgt mutant is induced by pollination but not by auxin treatment. Journal of Plant Physiology. 2012;169:1165–1172. doi: 10.1016/j.jplph.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Mito N, Bennett AB. The diageotropica mutation and synthetic auxins differentially affect the expression of auxin-regulated genes in tomato. Plant Physiology. 1995;109:293–297. doi: 10.1104/pp.109.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proceedings of the National Academy of Sciences of the USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najami N, Janda T, Barriah W, et al. Ascorbate peroxidase gene family in tomato: its identification and characterization. Molecular Genetics and Genomics. 2008;279:171–182. doi: 10.1007/s00438-007-0305-2. [DOI] [PubMed] [Google Scholar]
- Nebenführ A, White TJ, Lomax TL. The diageotropica mutation alters auxin induction of a subset of the Aux/IAA gene family in tomato. Plant Molecular Biology. 2000;44:73–84. doi: 10.1023/a:1006437205596. [DOI] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J. Hydrogen peroxide signaling. Current Opinion in Plant Biology. 2002;5:388–395. doi: 10.1016/s1369-5266(02)00282-0. [DOI] [PubMed] [Google Scholar]
- Oh K, Hardeman K, Ivanchenko MG, et al. Fine mapping in tomato using microsynteny with the Arabidopsis genome: the Diageotropica (Dgt) locus. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-9-research0049. research0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K, Ivanchenko MG, White TJ, Lomax TL. The diageotropica gene of tomato encodes a cyclophilin: a novel player in auxin signalling. Planta. 2006;224:133–144. doi: 10.1007/s00425-005-0202-z. [DOI] [PubMed] [Google Scholar]
- Ogawa K. Glutathione-associated regulation of plant growth and stress responses. Antioxidants and Redox Signalling. 2005;7:973–981. doi: 10.1089/ars.2005.7.973. [DOI] [PubMed] [Google Scholar]
- Perl-Treves R, Nacmias B, Aviv D, Zeelon AP, Galun E. Isolation of two cDNA clones from tomato containing two different superoxide dismutase sequences. Plant Molecular Biology. 1988;11:609–623. doi: 10.1007/BF00017461. [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant Journal. 1998;16:553–560. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- Ponce G, Barlow PW, Feldman LJ, Cassab GI. Auxin and ethylene interactions control mitotic activity of the quiescent centre, root cap size, and pattern of cap cell differentiation in maize. Plant Cell and Environment. 2005;28:719–732. doi: 10.1111/j.1365-3040.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiology. 2002;130:1908–1917. doi: 10.1104/pp.010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant Journal. 2007;50:514–528. doi: 10.1111/j.1365-313X.2007.03068.x. [DOI] [PubMed] [Google Scholar]
- Rice MS, Lomax TL. The auxin-resistant diageotropica mutant of tomato responds to gravity via an auxin-mediated pathway. Planta. 2000;210:906–913. doi: 10.1007/s004250050696. [DOI] [PubMed] [Google Scholar]
- Rotenberg D, Thompson TS, German TL, Willis DK. Methods for effective real-time RT-PCR analysis of virus-induced gene silencing. Journal Virological Methods. 2006;138:49–59. doi: 10.1016/j.jviromet.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Street HE, Opik H, James FEL. Fine structure of the main axis meristems of cultured tomato roots. Phytomorphology. 1967;17:391–401. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant Journal. 1997;11:1187–1194. [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143:606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Tyburski J, Dunajska K, Mazurek P, Piotrowska B, Tretyn A. Exogenous auxin regulates H2O2 metabolism in roots of tomato (Lycopersicon esculentum Mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiologiae Plantarum. 2009;31:249–260. [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant Journal. 2010;63:551–562. doi: 10.1111/j.1365-313X.2010.04262.x. [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Annals of Botany. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li S, Ren B, et al. LATERAL ROOTLESS2, a cyclophilin protein, regulates lateral root initiation and auxin signaling pathway in rice. Molecular Plant. 2013 doi: 10.1093/mp/sst052. in press doi:10.1093/mp/sst052. [DOI] [PubMed] [Google Scholar]
- Zou L, Li H, Ouyang BA, Zhang J, Ye Z. Cloning and mapping of genes involved in tomato ascorbic acid biosynthesis and metabolism. Plant Science. 2006;170:120–127. [Google Scholar]
- Zobel RW. Some physiological characteristics of the ethylene-requiring tomato mutant diageotropica. Plant Physiology. 1973a;52:385–389. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel RW. Control of morphogenesis in the ethylene-requiring tomato mutant diageotropica. Canadian Journal of Botany. 1973b;52:735–741. doi: 10.1104/pp.52.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.