Abstract
Purpose:
To report the psychometrics of the Glaucoma Treatment Compliance Assessment Tool (GTCAT), a new questionnaire designed to assess adherence with glaucoma therapy.
Methods:
We developed the questionnaire according to the constructs of the Health Belief Model. We evaluated the questionnaire using data from a cross-sectional study with focus groups (n = 20) and a prospective observational case series (n=58). Principal components analysis provided assessment of construct validity. We repeated the questionnaire after 3 months for test-retest reliability. We evaluated predictive validity using an electronic dosing monitor as an objective measure of adherence.
Results:
Focus group participants provided 931 statements related to adherence, of which 88.7% (826/931) could be categorized into the constructs of the Health Belief Model. Perceived barriers accounted for 31% (288/931) of statements, cues-to-action 14% (131/931), susceptibility 12% (116/931), benefits 12% (115/931), severity 10% (91/931), and self-efficacy 9% (85/931). The principal components analysis explained 77% of the variance with five components representing Health Belief Model constructs. Reliability analyses showed acceptable Cronbach’s alphas (>.70) for four of the seven components (severity, susceptibility, barriers [eye drop administration], and barriers [discomfort]). Predictive validity was high, with several Health Belief Model questions significantly associated (P <.05) with adherence and a correlation coefficient (R2) of .40. Test-retest reliability was 90%.
Conclusion:
The GTCAT shows excellent repeatability, content, construct, and predictive validity for glaucoma adherence. A multisite trial is needed to determine whether the results can be generalized and whether the questionnaire accurately measures the effect of interventions to increase adherence.
INTRODUCTION
Adherence with glaucoma medications is a key component of a successful glaucoma treatment program. C. Everett Koop, the former US Surgeon General, famously remarked, “Drugs don’t work in patients who don’t take them.”1 Researchers define compliance as the extent to which patients take medications as prescribed by their health care provider.1 Some prefer the term adherence, because it suggests a treatment alliance between the patient and provider.1 Regardless of the definition, glaucoma patients attain the full benefits of ocular hypotensive medications only when they use them every day.
The aim of this study is to report the psychometrics of the Glaucoma Treatment Compliance Assessment Tool (GTCAT), a new questionnaire designed to assess adherence with glaucoma therapy. We hypothesize that the questionnaire will show good repeatability, as well as content, construct, and predictive validity, for glaucoma adherence.
THE PROBLEM AND IMPORTANCE OF ADHERENCE TO GLAUCOMA THERAPY
Adherence to ocular hypotensive medications is a critical part of secondary prevention of visual impairment from glaucoma. Ocular hypotensive medications are used by 86% of patients with glaucoma and are the most common treatment for glaucoma.2 Glaucoma patients require long-term treatment over an average of 15 years.3 Finally, medications are very effective—reducing the development or worsening of glaucoma by at least 60%.4–6
However, adherence with prescribed glaucoma treatments is notably poor.7–10 While glaucoma medications require treatment every day, a recent study showed that only 56% of patients used more than 75% of the expected doses.11 Studies using pharmacy records have shown that only 50% of glaucoma patients refill their medication within 6 months of the initial 90-day supply.12–14 Several studies show an average estimate of nonadherence at 40%.8,15,16 Other studies have noted that poor adherence results in greater visual loss and a higher risk of blindness,7,17–19 and nonadherence is thought to be a leading cause of blindness in those with glaucoma.20–22 Overall, glaucoma patients commonly deviate from their prescribed medical regimen; nonadherence represents a significant barrier to successful treatment of glaucoma; and nonadherence is associated with increased risk of visual disability and blindness.
MEASURING ADHERENCE WITH GLAUCOMA THERAPY
Glaucoma adherence is difficult to measure. Patients routinely overestimate their level of adherence with self-report as compared with objective measures.23 Intraocular pressure is a poor surrogate for adherence because patients commonly increase their adherence in the day prior to visiting their eye care provider.10 Observational methods (eg, having a trained observer witness the administration) are impractical and intrusive. Pharmacy records may be valid for measuring adherence of large groups, but can be inaccurate for individual patients24 and are difficult to obtain.
Objective methods, such as electronic dose monitors, are the best method of measuring adherence. However, accurately measuring adherence with glaucoma ocular hypotensive medications presents unique problems when compared to devices to measure oral pill usage, such as tablets for systemic hypertension. The US Food and Drug Administration (FDA) approves an eye drop medication based on the efficacy of the medication, but also on the specific characteristics of the container holding the medication.25 The FDA will not allow researchers to use bottles that have not been approved for a specific medication because of concerns regarding sterility, drop quantity, and drop volume. Therefore, an eye drop dose monitor must utilize the FDA-approved bottle, and these bottles vary widely in their size and shape. Because of these issues, researchers and eye care providers have had difficulty developing a suitable device to measure the drop-taking behavior of glaucoma patients. Another option to objectively measure adherence is the Medication Event Monitoring System (MEMS), where one places an eye drop bottle within the MEMS bottle.26 The MEMS cap records the date and time of unscrewing the cap.27 This “bottle within a bottle” mechanism for eye drop compliance requires multiple extra steps when measuring adherence with an eye drop. This cumbersome process deviates from the usual process of administering eye drops, making it difficult to determine whether one is measuring actual eye drop monitoring behavior.
In the current study, we used an objective eye drop monitor, the Travatan Dosing Aid (TDA; Alcon, Fort Worth, Texas), which is available for travoprost ophthalmic solution.
FACTORS RELATED TO GLAUCOMA ADHERENCE
Previous studies have provided insight into the factors associated with glaucoma adherence. Lacey and associates28 showed adherence to be associated with fear of blindness, forgetfulness, difficulty with drop application, and age. Friedman and associates29 found adherence to be associated with method of communication, patient education, risk of vision loss, cost, traveling, side effects, and demographic factors. Other researchers30,31 have shown that low health literacy is associated with poor adherence. Several studies have shown that glaucoma patients frequently have difficulty with drop instillation by missing the eye and by touching the eye with the eye drop bottle tip.32,33 Overall, these studies suggest that glaucoma adherence is a complex behavior with multiple contributing factors.
HEALTH BEHAVIOR MODELS
One way to help simplify and characterize adherence is to develop a model of the factors related to adherence. For example, eye care providers use an organizing framework to determine the most likely cause of anterior uveitis by assessing the chief complaint, examination findings, review of symptoms, and past medical, family, and social history. Similarly, eye care providers would benefit from an organizing framework to determine the causes for poor adherence with glaucoma therapy.
Health psychologists and researchers have developed many health behavior models to explain adherence, and four are well known: (1) Transtheoretical Model (also known as the Stages of Change); (2) Theory of Reasoned Action/Planned Behavior; (3) Social Cognitive Theory; and (4) the Health Belief Model. The Transtheoretical Model describes the relationships among stages of change, processes of change, decisional balance, situational confidence, and situational temptation to relapse.34 The Theory of Reasoned Action/Planned Behavior postulates that behavior is influenced by the intention to perform the behavior, which is influenced by three variables: subjective norms, attitudes, and self-efficacy.35 Social Cognitive Theory includes both environmental and social factors to predict health behavior.36 The Health Belief Model predicts that patients value health, consider the disease as a threat with avoidable consequences, and expect positive outcomes from treatment.37 While different in some ways, these theories have overlapping constructs or themes for explaining adherence to medical therapy.
HEALTH BELIEF MODEL
We developed the current project using the constructs of the Health Belief Model as the organizing framework for glaucoma adherence.36 The Health Belief Model is a psychologically driven framework of concepts designed to explain and predict health behaviors by examining individuals’ beliefs and attitudes regarding diseases and their treatments. It predicts preventive, screening, and/or treatment adherence based on value expectancy theory, which examines the value individuals place upon their current state of health and their expectancy that some action will maintain or improve that state.37 For example, under the Health Belief Model theory, a person with glaucoma will be more likely to comply with treatment regimens if he or she places a high value on his or her current level of vision and also believes than an ocular hypotensive medication will prevent further vision loss. Table 1 describes the Health Belief Model constructs, which include the severity of the disease, susceptibility to the disease, the benefits offered by a recommended action, and the barriers to taking said action. The mediating factors of self-efficacy (individual’s perception of his or her ability to perform a recommended action) and cues-to-action (external encouragements to perform a recommended action) have been added to the original four constructs.37
TABLE 1.
DEFINITIONS OF THE HEALTH BELIEF MODEL CONSTRUCTS AS APPLIED TO GLAUCOMA TREATMENT. GLAUCOMA TREATMENT COMPLIANCE ASSESSMENT TOOL STUDY
| CONSTRUCT | DEFINITION |
EXAMPLE FROM FOCUS GROUP TRANSCRIPT |
ALTERNATE EXAMPLES |
|---|---|---|---|
| Perceived severity | One’s opinion of how serious glaucoma and its consequences are | “I could go blind from glaucoma.” | Loss of function |
| Perceived susceptibility | One’s opinions of the chances of developing worsening glaucoma | “My mom went blind from glaucoma, so I wasn’t surprised that I could lose vision.” | Risk factors Family history |
| Perceived benefits | One’s belief in the efficacy of using eye drops to reduce the risk or seriousness of glaucoma | “I think the drops will keep me from going blind.” | Lowers intraocular pressure Preserves current sight |
| Perceived barriers | One’s opinion of the tangible and psychological costs of taking the drops | “The drops are too expensive.” | Side effects Cost |
| Cues-to-action | External strategies or encouragements to take drops | “I keep my drops by my bed so I can use them at night.” | Reminder system Encouragement from family |
| Self-efficacy | Confidence in one’s ability to use the drops correctly and effectively | “I have a hard time getting the drops in my eyes.” | Size of bottle Unable to squeeze bottle |
| Other reasons | Comment not better described by any of the above | “You must have a good rapport with your doctor.” | Education Depression |
The Health Belief Model has been effective in predicting adherence with various preventative and treatment approaches across a variety of medical conditions. It has been correlated with adherence in conditions such as breast self-examinations,38–43 condom use,44,45 psychiatric medications,46,47 coronary heart disease,48 diabetes treatments,49–54 vaccinations,55,56 and exercise programs.57 For example, patients with systemic hypertension who believed their medication prevented stroke and coronary artery disease (perceived benefits) were more likely to be compliant with treatment regimens.58 Overall, these studies demonstrate that the Health Belief Model is able to predict health behavior in a variety of medical conditions.
We considered alternative health behavior models to examine glaucoma adherence. However, glaucoma adherence may not require models, such as the Transtheoretical (Stages of Change) Model, that conceptualize contemplation and action with long-term treatment of a chronic disease such as glaucoma. For example, a health educator may use this model to gauge interest in stopping smoking and encourage a smoker to reach a stage termed contemplative. Only when this person reaches this stage will the health educator recommend an intervention to prevent smoking. This assessment of stage of change for a single action (stopping smoking) may not apply for glaucoma, which requires daily treatment over many years.
Ophthalmology studies have generally not used an organizing framework for determining the factors related to adherence to glaucoma therapy. In this study we report the psychometric results of the GTCAT, a new questionnaire based on the Health Belief Model to assess adherence with glaucoma therapy. We present evidence supporting its use as a research tool to characterize and measure the factors related to adherence with glaucoma therapy.
METHODS
Researchers have created valid, reproducible questionnaires, such as the National Eye Institute 51-item Visual Function Questionnaire (NEI-VFQ-51) and the revised NEI-VFQ-25, using focus groups and field testing.59–61 We followed a similar process. Table 2 describes the study designs and analyses for assessing the psychometrics of the GTCAT. The Institutional Review Board at Legacy Health System approved both studies included in this thesis: (1) cross-sectional study design with focus groups and (2) a prospective observational case series. All participants signed an informed consent. The study was conducted in accordance with the tenets of the Declaration of Helsinki.
TABLE 2.
VALIDATION OF THE GTCAT: SUMMARY OF STUDY DESIGN AND ANALYSIS. GLAUCOMA TREATMENT COMPLIANCE ASSESSMENT TOOL STUDY
|
CONTENT VALIDITY |
CONSTRUCT VALIDITY |
PREDICTIVE VALIDITY |
TEST-RETEST RELIABILITY |
|
|---|---|---|---|---|
| Study design | Cross-sectional focus groups | Prospective, observational case series | Prospective, observational case series | Prospective, observational case series with repeated measures |
| Analysis/outcome measures | Scope: unique responses Frequency of responses Distribution: number of persons with similar responses Categorization of responses into Health Belief Model constructs |
Principal components analysis Internal consistency reliability (Cronbach’s α) Frequency analysis, floor/ceiling effects |
Univariate and multivariate regression | Paired samples testing (paired t tests, nonparametric correlation, Bland- Altman analysis74) |
THE GLAUCOMA TREATMENT COMPLIANCE ASSESSMENT TOOL
We created a questionnaire, the GTCAT, to study glaucoma medication adherence. We developed the questionnaire using expert opinion, previous studies regarding adherence in glaucoma patients,10,62,63 and the Health Belief Model.37 This questionnaire includes items related to the six Health Belief Model constructs listed previously, as well as other relevant information, such as age, medical history, gender, income, education levels, and type of insurance coverage. Most questions include a 10-interval Likert scale response with anchoring definitions (eg, 0=absolutely disagree, 10=absolutely agree). The GTCAT questionnaire contains language at a 6th grade reading level (as shown by a Flesch-Kincaid reading level of 6.2 and reading ease of 69.3 [11- to 12-year-old level]). Research assistants required (mean ± SD) 18.0 ± 4.0 minutes (range, 12–28 minutes) to administer the questionnaire.
FOCUS GROUP STUDY
The purpose of the focus group design is twofold: (1) to determine content validity and (2) to determine whether the GTCAT encompasses the factors relevant to glaucoma. Focus groups provide in-depth insight into a particular issue, capture group interactions and group norms on a broad range of topics, and can be formative in the development of questionnaire instruments.
The eligibility criteria for participants in the focus group study were as follows: (1) had been prescribed one or more ocular hypotensive medications and (2) had received a visual field examination during the previous year. The exclusion criterion was cognitive impairment limiting ability to understand the GTCAT questions. We recruited participants using flyers located in clinical areas of Devers Eye Institute.
A research assistant with a psychology background and previous experience leading focus groups moderated the sessions. The moderator followed a written interviewing guide and used nondirective and open discussion techniques to facilitate participant discussion. Fictitious patient scenarios of those with a diagnosis of glaucoma suspect, early glaucoma, or severe glaucoma were used to stimulate discussion. A second research assistant audio taped the sessions, using a primary and a backup audio recorder, and took detailed notes of the order of speaking.
We used contextual analysis to separate the transcript into statements relevant to the topic of adherence with ocular medications.64,65 Two research assistants independently categorized the statements into the six constructs of the Health Belief Model (Table 1), or as “other reasons.” They also determined whether the same issue was mentioned in an earlier part of the transcript or a previous focus group. If not, they labeled this statement unique. The research assistants adjudicated any disagreements in categorization, and a third expert mediated any unresolved disagreements.
Focus Group Study Data Analysis
We used the adjudicated contextual results and frequency statistics to determine the proportion of focus group responses that included Health Belief Model constructs, proportion of responses within each Health Belief Model construct category, the proportion of participants mentioning a particular Health Belief Model construct (also known as distribution), and the number of unique responses.
Focus groups do not lend themselves to typical statistical power analysis because one is unable to determine standard deviation, detectable difference, beta, and alpha levels. Therefore, we used the recommendation to enroll additional focus groups until the content of additional focus groups becomes largely redundant.65 This stopping rule occurs when subsequent focus group statements include less than 10% unique statements.65
OBSERVATIONAL CASE SERIES
The purpose of the observational case series was to determine (1) construct validity, (2) reliability, (3) predictive validity, and (4) test-retest repeatability. To be eligible to participate in the observational case series, patients had to (1) be 18 years or older; (2) have a diagnosis of open-angle glaucoma in one or both eyes, or ocular hypertension; and (3) use a prostaglandin ocular hypotensive medication in one or both eyes. The protocol excluded patients with uncontrolled intraocular pressure, known contraindications to travoprost, clinically significant systemic disease that would interfere with the study, participation in any other research study within 30 days, or a change in systemic medications that may alter intraocular pressure within 30 days before recruitment.
In addition to the GTCAT, information on the number of ocular medications, baseline intraocular pressure of each eye, and severity of the visual field deviation in each eye was assessed from the current medical record. The GTCAT was administered at the enrollment visit and the 3-month follow-up visit, and the same trained research assistant administered the GTCAT on both dates. At the time of the second visit, interviewers and participants were masked to the baseline GTCAT results.
After administering the GTCAT at the enrollment visit, a research assistant demonstrated proper use of the TDA to the participant. The TDA is a battery-operated device that records the time, date, and number of drops of medication released from the bottle. The device also has a programmable audio and visual reminder system, which we deactivated for this particular study. Alcon designed the device to be used with either the 2.5 mL or 5 mL bottles of travoprost ophthalmic solution (Travatan; Alcon, Fort Worth, Texas). The bottle is inserted into a plastic “basket” within the device. Each time the patient presses the lever to release a drop of solution, the TDA records the time and date of drop release. The TDA stores this information on a computer chip embedded in the base and exports the data via a wired hub and Windows-based software program.
Once retrieved, the computer program displays the drop data in either a calendar or log format. The calendar format displays the selected month with the number of drops administered on a particular day either in blue, showing that the time and number of drops administered were consistent with the recommended dose, or in red, showing that the administration of drops falls outside the recommended parameters. Nonadherence could mean that either too few drops were administered or that the drops were administered outside the recommended time frame. The drop log format displays the date, the number of drops administered, and the time they were administered, and asterisks identify days of nonadherence. We downloaded data from the TDA at each visit.
The TDA allows only travoprost bottles. Therefore, if needed, we switched participants using a different prostaglandin analogue (such as latanoprost or bimatoprost) to travoprost. Travoprost bottles, along with the electronic dose monitor, were provided free of charge to subjects. At the enrollment visit, the study coordinators observed the participants administering the drops while using the electronic dose monitor to ensure proper training in device usage66 and understanding of directions. The coordinators asked the participants to administer their travoprost using the electronic dosing monitor according to their normal dosing schedule.
Participants brought their electronic dose monitor to the 1- and 3-month follow-up visits. During these visits, the research assistant downloaded the information from the TDA, changed the battery if necessary, and confirmed that the participant was using the device satisfactorily. A brief series of questions was administered to estimate self-reported adherence and satisfaction with the device.
The TDA has an accuracy of 93%67 but can underreport in some cases.67,68 Therefore, we created our own software to analyze the data. Our computer programmer created a program using C programming language (C++) to analyze the TDA output and increase our flexibility for measuring adherence. For example, the TDA identified days as “nonadherent” when drops were dispensed just after the 3-hour deadline. The C++ program allowed us to directly use the date and time stamp data to allow more flexible classifications. We quantified adherence in three ways: Definition 1 was the proportion of days in which the patient used the appropriate number of drops (eg, 2 drops for bilateral treatment) within 3 hours of the designated time. Definition 2 was the proportion of days in which any drop was taken within 3 hours of the designated time. Definition 3 was the proportion of days in which any drop was taken within 6 hours of the designated time. The C++ program determined the number of days the TDA recorded dosing within each of the three definitions, and divided these days by the number of days in the study cycle. Thus, a participant who missed 9 days out of 90 days was considered 90% compliant. As a patient-derived, subjective measure of adherence, we asked the participants to report the number of days they missed taking their drop at the 3-month visit for the previous period of 3 months.
Observational Case Series Analysis: Construct Validity
All statistical analyses were performed using SPSS (v16.0; SPSS, Inc, Chicago, Illinois). We used the baseline GTCAT results to determine construct validity. Construct validity refers to the degree to which the questions categorized into a construct (such as the Health Belief Model constructs) actually measure the construct they are intended to measure. We used principal components analysis with orthogonal Varimax rotation, which is a multivariate method of identifying trends and redundancies in data, while allowing all loadings to vary freely to find the most efficient data reduction.69 This data reduction procedure begins by finding a linear combination of variables (called a “component”) that explains as much variation as possible in the original variables. It then finds another component that accounts for as much of the remaining variation as possible and is uncorrelated with the previous component, continuing in this way until the program identifies as many components as necessary. The procedure may not load some variables into a component, suggesting that they have little explanatory power. The subset of components usually accounts for a large proportion of the variation of the data, and a researcher can use this subset in place of the full complement of original variables.
Another purpose of the principal component analysis was to determine the relationship between the GTCAT questions and what they represent. This method of structure detection allows one to determine if the GTCAT questions load into a structure consistent with the Health Belief Model; for example, questions investigating susceptibility load together into a component, questions regarding barriers into a second component, and so on. This allows us to confirm construct validity of the GTCAT toward the theoretical structure of the Health Belief Model. We examined both liberal (≥.40)70 and rigorous (>.65)71 cutoffs for factor loadings. We estimated the internal consistency reliability using Cronbach’s α for level of agreement between questions within the same construct, and used a Cronbach’s α greater than .70 as demonstrating internal consistency.72
Observational Case Series Analysis: Predictive Validity
We determined predictive validity using a three-stage linear regression model using standard model building techniques.73 The first stage was to determine the demographic, socioeconomic, and medical factors at the enrollment visit associated with adherence. We used the proportion of days in which any drop was taken within 6 hours of the designated time (Definition 3) as the dependent variable, and used the following as independent variables: age, gender, ethnicity (white vs other), education (some college vs other), amount paid out-of-pocket for prescription medications, baseline intraocular pressure in the right eye, baseline intraocular pressure in the left eye, number of eye drops per day, visual field sensitivity in the better eye, and visual field sensitivity in the worse eye. We first performed a univariate analysis, and then a multivariate linear regression using an automated forward stepwise (Wald) selection procedure using only those variables from the univariate model with P≤.10. We used Definition 3 because it had a larger time window of 6 hours and therefore was more flexible for adherence in comparison to Definition 1 and Definition 2 with 3-hour windows. However, similar results were observed using Definition 1 and Definition 2 (data not shown).
The second stage was to determine the GTCAT questions associated with adherence. Similar to the previously described stage, we used Definition 3 as the dependent variable and used the GTCAT questions as independent variables. We performed a univariate linear regression analysis, followed by a stepwise multivariate linear regression selection procedure using only those GTCAT questions from the univariate model with P≤.10.
Finally, the third stage was to determine a final multivariate model from the combination of independent variables from the multivariate results of the first and second stages of model building. This final model would determine the participant demographic, socioeconomic, medical, and health belief factors most associated with adherence.
Observational Case Series Analysis: Test-Retest Reliability
A research assistant administered the GTCAT at the initial (Time 1) and 3-month (Time 2) visits to 27 participants from the cross-sectional survey to determine the test-retest reliability of the 21 Likert scale questions. We used paired t tests to determine repeatability of individual questions after confirming a normal distribution (P>.05, skewness and kurtosis) of the paired differences between Time 1 and Time 2 scores. To further assess test-retest reliability, we determined the nonparametric correlation (Spearman’s rho) of Time 1 and Time 2 scores. Finally, we created a summary score of Time 1 questions and Time 2 questions by simple addition of the questions and used a Bland-Altman analysis74 to show outliers, fixed bias (difference between Time 1 and Time 2), and proportional bias (increasing variability at higher summary scores).
RESULTS
FOCUS GROUP RESULTS
We conducted five focus groups with three to five participants per group and 20 total participants. Among the 20 focus group participants, 70% (14 of 20) were female, ranging in age from 42 to 93 years (mean age, 71.3 ± 12.0 years). They provided 931 statements that related to adherence with glaucoma medications. The research assistants initially had 75% (698 of 931) agreement in categorizing the statements into a Health Belief Model construct (κ=.70), and 99% after adjudication. The investigators categorized 88.7% (826 of 931) of statements into one of the constructs of the Health Belief Model.
Barriers were the most commonly mentioned topic with regard to adherence (n=288 statements, as shown in Figure 1; 30.9% of all statements), followed by cues-to-action (n=131; 14.1%), susceptibility (n=116; 12.5%), benefits (n=115; 12.4%), other reasons (n=105; 11.3%), severity (n=91; 9.8%), and self-efficacy (n=85; 9.1%). The research assistants examined each focus group transcript for unique statements about glaucoma. Figure 2 shows that the number of unique statements decreased from the first (63%, 96 of 150 unique statements) to the fifth focus group (8%, 25 of 312 unique statements). The fifth focus group had less than 10% of unique statements, suggesting that the results are redundant and no further focus groups are needed.65 We measured distribution, defined as the proportion of participants who mentioned a particular Health Belief Model construct. Distribution of responses was 100% (20 of 20) for barriers (all participants made at least one statement related to the construct), 95% (19 of 20) for cues-to-action, 90% (18 of 20) for self-efficacy, 85% (17 of 20) for benefits, 85% (17 of 20) for severity, 80% (18 of 20) for susceptibility, and 80% (18 of 20) for other reasons.
FIGURE 1.

Number of focus group statements related to glaucoma adherence for each construct of the Health Belief Model. There were a total of 931 statements (n=20 participants). Table 1 describes the operational definitions for the Health Belief Model constructs. Glaucoma Treatment Compliance Assessment Tool Study.
FIGURE 2.
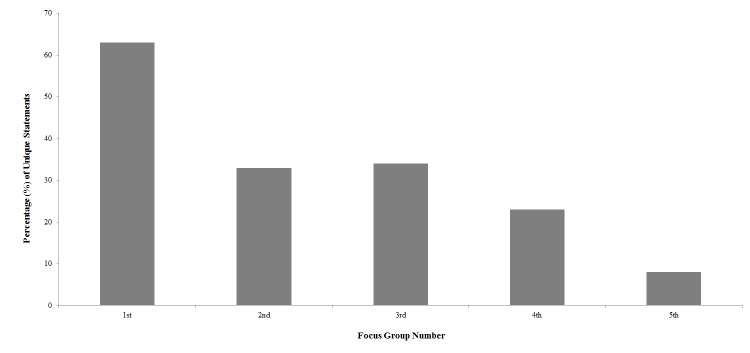
Percentage of unique statements related to glaucoma adherence for each focus group from contextual analysis of focus group transcripts (n=20 participants). The percentage of unique statements dropped to less than 10% of statements during the 5th focus group indicating that the statements became redundant and no further focus groups are needed. Glaucoma Treatment Compliance Assessment Tool Study..
The most common themes with regard to the barriers construct involved cost (“the drops are too expensive”), forgetfulness (“I can’t tell you whether I took my drops”), and side effects (“it puts little cuts in the corner of your eye…very painful”). Statements describing the most common cues-to-action themes dealt with the establishment of routines and reminders to take medications (“the older you get, the more important it is to establish a routine”). Statements about severity revolved around a lack of symptoms (“with no symptoms people don’t realize how severe their disease is”), whereas themes about susceptibility were expressed in statements such as “Young people are at a very active stage of life and tend to say ‘…I don’t need those drops’.” Benefits themes included statements about how well the drops work (“I stayed on them because [the doctors] liked the results”), whereas self-efficacy themes involved bottle size (“if the bottles were a trifle bigger they’d be easier to handle”), and comorbidities that interfere with ability (“my husband has arthritis and sometimes he finds it very hard to squeeze”). Some “other reasons” mentioned by the participants include depression, provider-patient relationships, and continuing education about glaucoma.
OBSERVATIONAL CASE SERIES RESULTS
We enrolled 60 consecutive glaucoma patients into the 3-month observational case series. Two participants dropped out prior to the 1-month visit: one did not tolerate travoprost, and the other could not operate the TDA. Therefore, we included 58 participants in the analysis of construct validity and test-retest reliability. The TDA malfunctioned for one person. We also excluded this subject, resulting in a cohort of 57 subjects for the analysis of adherence and predictive validity. The first column of Table 3 reports the participant characteristics at the enrollment visit.
TABLE 3.
DEMOGRAPHIC, SOCIOECONOMIC, AND MEDICAL CHARACTERISTICS OF ALL PARTICIPANTS, THE ADHERENT GROUP,* AND THE NONADHERENT GROUP AT THE ENROLLMENT VISIT IN THE OBSERVATIONAL CASE SERIES STUDY. GLAUCOMA TREATMENT COMPLIANCE ASSESSMENT TOOL STUDY
| VARIABLE† |
ALL PARTICIPANTS (N=58) |
ADHERENT GROUP (N=28) |
NONADHERENT GROUP‡ (N=29) |
P§ |
|---|---|---|---|---|
| Age, years | 65.2 ± 14.9 | 69.4 ± 13.1 | 61.6 ± 15.6 | .05 |
| Female, % | 56.9 | 57.1 | 55.2 | .88 |
| White primary ethnicity¶, % | 79.3 | 89.3 | 69.0 | .06 |
| Some college or more, % | 75.9 | 78.6 | 72.4 | .59 |
| Medication cost out-of-pocket per year, $ | 601 ± 818 | 741 ± 933 | 488 ± 702 | .30 |
| Intraocular pressure (right eye), mm Hg | 16.1 ± 5.5 | 15.1 ± 4.8 | 17.0 ± 6.0 | .20 |
| Intraocular pressure (left eye), mm Hg | 16.4 ± 4.6 | 15.5 ± 3.1 | 17.2 ± 5.6 | .17 |
| Number of eye drops per day | 3.3 ± 2.1 | 3.2 ± 1.5 | 3.4 ± 2.6 | .77 |
| Visual field mean deviation in better eye, dB | −3.5 ± 6.0 | −4.0 ± 5.8 | −3.6 ± 5.5 | .78 |
| Visual field mean deviation in worse eye, dB | −8.3 ± 8.7 | −8.0 ± 7.1 | −9.7 ± 9.5 | .46 |
Adherent persons were those that had 90% or more days in which any drop was taken within 6 hours of the designated time.
Two (n=2) participants in the adherent group did not know whether they had insurance coverage for medication; 10 participants (5 from the adherent group and 5 from the nonadherent group) did not know their medication cost out-of-pocket; and 1 person in the nonadherent group did not have intraocular pressure evaluated at the enrollment visit.
We excluded one participant from the nonadherent group, and the analysis comparing the nonadherent group to the adherent group, because of a malfunction of the Travatan Dosing Aid (Alcon, Fort Worth, Texas).
Comparison of the adherent and nonadherent groups using an independent-samples t test or chi-square test as applicable.
Ethnicity included white (n=46), African American (n=6), Asian (n=4), Hispanic/Latino (n=1), and other (n=1).
Observational Case Series: Construct Validity Results and Reliability
We excluded 3 GTCAT questions (out of 21) from the principal components analysis because of ceiling effects (≥90% of participants selecting a 10 on the Likert scale). The principal component analysis resulted in the extraction of seven components containing all 18 questions and explaining 77% of the variance when using a less restrictive factor loading value of 0.40. When using a more restrictive minimum factor loading of 0.65, only 14 questions loaded into these same seven components. We performed a reliability analysis for this more restrictive criterion. Components 1 (Cronbach’s α=.83; composed of severity items), 2 (Cronbach’s α=.89; susceptibility items), 3 (Cronbach’s α=.83; barrier items), and 4 (Cronbach’s α=.76; barrier items) showed acceptable internal consistency, whereas components 5 (Cronbach’s α=.57; unidentified), 6 (Cronbach’s α=.63; knowledge items), and 7 (Cronbach’s α=.39; severity items) showed poor reliability. Therefore, we identified three Health Belief Model constructs (severity, susceptibility, and barriers), as well as one component representing knowledge; self-efficacy, cues to action, and benefits constructs were not represented in the seven components extracted. Table 4 shows the results of the principal component analysis and suggests good construct validity for three constructs of the Health Belief Model.
TABLE 4.
FACTOR LOADINGS AND CONSTRUCTS OF THE GTCAT QUESTIONNAIRE. GLAUCOMA TREATMENT COMPLIANCE ASSESSMENT TOOL STUDY
| COMPONENT | QUESTION |
FACTOR LOADING* |
CONSTRUCT |
|---|---|---|---|
| 1 | Q21, How much vision would you say you have lost because of glaucoma? | .86 | Severity |
| Q27, How would you rate the level of your disease? | .87 | ||
| Q42, How likely do you think you are to become blind in 10 years if you do use your eye drops? | .48 | ||
| 2 | Q41, How likely do you think you are to become blind in 10 years if you do not use your eye drops? | .90 | Susceptibility |
| Q40, How likely do you think you are to become blind in 5 years if you do not use your eye drops? | .77 | ||
| Q29, How well do you think eye drops can control the negative progress of glaucoma? | .62 | ||
| Q28, How likely do you think it is that you will develop other potentially blinding eye diseases, such as macular degeneration, retinal detachment, etc.? | .41 | ||
| 3 | Q39, How easy to use are your eye drops? | .95 | Barriers |
| Q30, How much difficulty do you have administering your eye drops? | −.89 | ||
| 4 | Q34, What level of side effects do you experience when using your eye drops? | .86 | Barriers |
| Q32, How much pain/discomfort do your eye drops cause you? | .81 | ||
| 5 | Q33, How likely is it that you think you will always use your eye drops every night? | .83 | Unidentified |
| Q15, How would you rate your overall general health? | .76 | ||
| Q35, How would you rate the cost of your eye drops? | .58 | ||
| 6 | Q17, How would you rate your personal knowledge of the symptoms of glaucoma? | .83 | Knowledge |
| Q16, How would you rate your personal knowledge of the risk factors for glaucoma?† | .74 | ||
| 7 | Q26, How much do you think further vision loss would change the quality of your life (in work, family, and social situations) as it is now? | .82 | Severity |
| Q18, How much of your vision do you think can be lost from glaucoma? | .80 |
Bolded values represent factor loading with a more stringent cutoff of > 0.65. Regular font represents questions loading only with a cutoff of .4.
This question also loaded onto Component 1, but with a lower factor loading of .44. Therefore, we include this question with Component 6.
Observational Case Series: Test-Retest Reliability
Figure 3 illustrates the comparison of mean Time 1 (initial visit) and Time 2 (3-month visit) question scores for the 21 Likert scale questions in the GTCAT (n=35 participants). It shows a significant nonparametric correlation for 19 questions, whereas 2 questions (questions 18 and 26) did not have a significant correlation (P>.05). Bland-Altman plots showed large differences in responses between Time 1 and Time 2 (not shown) for both question 18: “How much of your vision do you think can be lost from glaucoma?” and question 26: “How much do you think further vision loss would change the quality of your life?” The analysis with paired t tests between Time 1 and Time 2 scores showed the same results (not shown). Figure 4 shows a Bland-Altman plot for Time 1 and Time 2. It shows a fixed bias defined as the mean (± SD) difference (Time 1 – Time 2) between scores of .41 (± 15.5) and no sign of proportional bias with higher or lower scores. There were no outliers, which are defined as a difference in scores outside the 95% confidence interval (dotted lines). Overall, test-retest reliability was 90.4% (19 of 21 questions) and suggests good test-retest reliability.
FIGURE 3.
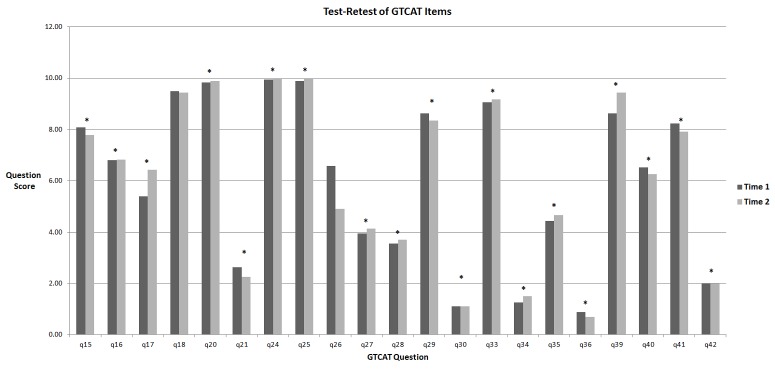
Mean Likert scores for the 21 Likert scale questions at Time 1 (initial visit) and Time 2 (3-month visit) in 35 participants with glaucoma or ocular hypertension. The asterisks(*) indicate that the Spearman’s rho shows statistically significant nonparametric correlation (P≤.05) between Time 1 and Time 2 for all questions except Q18 and Q26. Glaucoma Treatment Compliance Assessment Tool Study.
FIGURE 4.
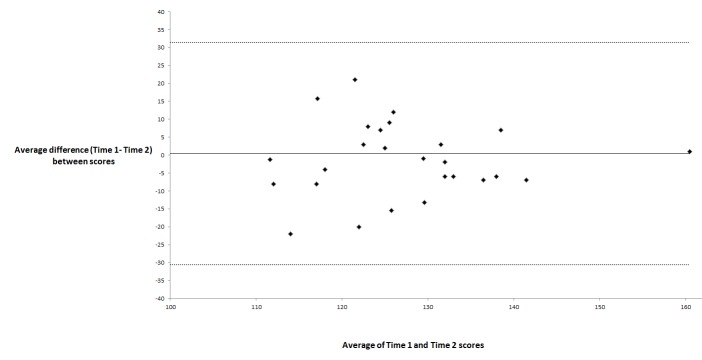
Bland-Altman analysis74 of the total score at Time 1 (initial visit) and Time 2 (3-month visit) in 35 participants with glaucoma or ocular hypertension. We created a total score of the Glaucoma Treatment Compliance Assessment Tool (GTCAT) at each time period by simple addition of the 21 Likert scale questions. It shows a fixed bias defined as the mean (± SD) difference (Time 1 – Time 2) between scores of .41 (±15.5) and no sign of proportional bias with higher or lower scores. No participant was an outlier as defined as a difference in scores outside the 95% confidence interval (dotted lines). Glaucoma Treatment Compliance Assessment Tool Study.
Observational Case Series: Adherence
We defined adherence using self-report of adherence, and three objective definitions using the TDA device (Definition 1, Definition 2, and Definition 3 as described in the “Methods” section). Over the approximate 90-day duration of the study, patients self-reported a mean 2.4 ± 3.3 (range, 0–18) days without medication, with a self-reported adherence of 97.2 ± 3.9% (range, 81%–100%). Using TDA as an objective measure, the proportions of days adherent by Definition 1, Definition 2, and Definition 3 were as follows: mean 71.7% ± 20.3% (range, 18%–100%), 81.3% ± 18.8% (range, 20%–100%), and 87.4% ± 13.6% (range, 36%–100%), respectively. Our analysis subtracted the proportion of days in which any drop was taken within 6 hours of the designated time (Definition 3) from the self-reported adherence to determine the difference between self-report and an objective measure of adherence. The difference between self-report and objective measures was 9.3% ± 11.3% (range, −5.9% to 48%). Overall, this shows overestimation of self-reported adherence as well as poor adherence based on objective measures.
When we define adherence dichotomously as 90% of expected doses,26 91.2%, 21.1%, 42.1%, and 49.1% were adherent for self-report, Definition 1, Definition 2, and Definition 3, respectively. Using Definition 3 with a 90% cut-off for “adherent,” Table 3 shows adherent participants were more likely to be older (P=.05) and have white primary ethnicity (P=.06).
Observational Case Series: Predictive Validity
To determine predictive validity, we used a three-stage model building procedure using univariate and multivariate linear regression. The dependent variable for all three stages was the proportion of days in which any drop was taken within 6 hours of the designated time (Definition 3 as a continuous variable). Table 5 shows the results of the first stage of model building using the demographic, socioeconomic, and medical characteristics of the participants at the enrollment visit. The univariate analysis showed older age, white primary ethnicity vs other ethnicity, and lower intraocular pressure in the left eye to be associated with adherence (all with a P≤.10). The R2 was .27, suggesting that 27% of the variance in adherence could be explained by these three questions. The multivariate analysis showed similar results as Table 3, with older age (P=.002) and white vs other primary ethnicity (P=.01) to be associated with adherence.
TABLE 5.
UNIVARIATE AND MULTIVARIATE ASSOCIATIONS BETWEEN ADHERENCE AND DEMOGRAPHIC, SOCIOECONOMIC, AND MEDICAL FACTORS AS PART OF THE OBSERVATIONAL CASE SERIES. GLAUCOMA TREATMENT ASSESSMENT TOOL STUDY*
| VARIABLE† |
UNIVARIATE ANALYSIS B |
P‡ |
MULTIVARIATE ANALYSIS B |
P§ |
|---|---|---|---|---|
| Age, years older | .37 | .002 | .47 | .002 |
| Female vs male | −2.38 | .52 | … | … |
| White primary ethnicity vs other ethnicity | 10.73 | .01 | 14.22 | .01 |
| Some college or more vs less education | −1.19 | .78 | … | … |
| Cost out-of-pocket per year for prescriptions, per $ higher | .002 | .32 | … | … |
| Intraocular pressure (right eye), mm Hg | −.51 | .13 | … | … |
| Intraocular pressure (left eye), mm Hg | −.66 | .10 | −.031 | .80 |
| Number of eye drops per day | 1.08 | .21 | … | … |
| Visual field mean deviation in better eye, dB | −.16 | .63 | … | … |
| Visual field mean deviation in worse eye, dB | −.12 | .60 | … | … |
The only questions eligible for multivariate analysis were those questions with P≤.10 using univariate analysis. This analysis includes n=57 participants, and excluded one participant because of a Travatan Dosing Aid (Alcon, Fort Worth, Texas) malfunction. Adherence was defined as the proportion of days (from 0 to 100%) in which any drop was taken within 6 hours of the designated time. Data are presented as unstandardized beta coefficients. R2 was .27 for the final multivariate model including age and ethnicity.
Two (n=2) participants in the adherent group did not know whether they had insurance coverage for medication; 10 participants (5 from the adherent group and 5 from the nonadherent group) did not know their medication cost out-of-pocket; and 1 person in the nonadherent group did not have intraocular pressure evaluated at the enrollment visit.
P value for univariate analysis for higher percentage of adherence (linear regression model).
P value for multivariate analysis for higher percentage of adherence (linear regression model with covariates eligible if univariate P ≤ .10).
Table 6 shows the results of the second stage of model building using the Health Belief Model questions as independent variables. A univariate analysis found seven questions (with a P≤.10) to be associated with adherence and an R2 of .42. These seven questions represent the Health Belief Model constructs of barriers (2 questions), self-efficacy (1 question), cues-to-action (2 questions), and benefits (1 question). Knowledge of glaucoma was also represented as a question. The multivariate model showed that three Health Belief Model questions and an R2 of .40 were significantly associated with adherence. These questions were (1) Personal knowledge of risk factors for glaucoma; (2) I am likely to use the drops every night; and (3) What level of side effects do you experience with your drops?
TABLE 6.
RESULTS OF UNIVARIATE AND MULTIVARIATE LINEAR REGRESSION BETWEEN HEALTH BELIEF MODEL QUESTIONS AND ADHERENCE USING DATA FROM THE OBSERVATIONAL CASE SERIES. GLAUCOMA TREATMENT ASSESSMENT TOOL STUDY*
| HEALTH BELIEF QUESTIONS† |
UNIVARIATE ANALYSIS B |
P‡ |
MULTIVARIATE ANALYSIS B |
P§ |
|---|---|---|---|---|
| Question 16: Personal knowledge of risk factors for glaucoma | −1.26 | .06 | −1.15 | .04 |
| Question 29: How well do drops control glaucoma? | 2.56 | .10 | 1.07 | .44 |
| Question 32a: I forget to use the drops | −9.16 | .01 | −4.62 | .18 |
| Question 32c: The drops aren’t with me when it is time to take them. | −12.19 | .007 | −6.26 | .22 |
| Question 32d: How much pain or discomfort do your eye drops cause you? | −2.21 | .02 | 9.59 | .16 |
| Question 33: I am likely to use the drops every night. | 5.66 | .001 | 5.61 | <.001 |
| Question 34: What level of side effects do you experience with your drops? | −2.95 | .001 | −2.53 | .001 |
This table includes only those questions with P ≤ .10 using univariate analysis. This analysis includes n=57 participants and excluded one participant because of a Travatan Dosing Aid malfunction. Adherence was defined as the proportion of days (from 0 to 100%) in which any drop was taken within 6 hours of the designated time. Beta (β) coefficients are presented as unstandardized. R2 was .40 for the multivariate model including only questions 16, 33, and 34 in bold.
We excluded 3 questions because of ceiling effects (≥90% listing “10” on the Likert scale) from analysis. These were: (1) How much do you agree with your doctor’s diagnosis of glaucoma?; (2) How important is it to maintain your current level of eyesight in your better-seeing eye?; and (3) How important is it to maintain your current level of eyesight in your worse-seeing eye?.
P value for univariate analysis for higher percentage of adherence (linear regression model).
P value for multivariate analysis for higher percentage of adherence.
Table 7 shows the third stage of model building and indicates that the three Health Belief Model questions from Table 6 have the strongest association with adherence when included in a multivariate model including age and primary ethnicity. It also shows only a small (although not significant) improvement in the R2, from .40 to .44, when adding age and ethnicity to a model containing these three Health Belief Model questions.
TABLE 7:
FINAL MULTIVARIATE LINEAR REGRESSION MODEL OF THE GTCAT QUESTIONS. GLAUCOMA TREATMENT ASSESSMENT TOOL STUDY*
| GLAUCOMA TREATMENT COMPLIANCE ASSESSMENT TOOL QUESTIONS |
MULTIVARIATE ANALYSIS β |
P† |
|---|---|---|
| Question 16: Personal knowledge of risk factors for glaucoma | −1.16 | .03 |
| Question 33: I am likely to use the drops every night. | 4.08 | .01 |
| Question 34: What level of side effects to you experience with your drops? | −1.96 | .02 |
| Age, years older | .18 | .11 |
| White primary ethnicity vs other ethnicity | 4.89 | .20 |
Table includes only variables that were significant at a P ≤ .05 in the multivariate linear regression model from first stage (demographic, socioeconomic, and medical factors) and second stage (Health Belief Model questions) of model building. This analysis includes n=57 participants, and excluded one participant because of a Travatan Dosing Aid (Alcon, Fort Worth, Texas) malfunction. Adherence was defined as the proportion of days (from 0 to 100%) in which any drop was taken within 6 hours of the designated time. Beta coefficients are presented as unstandardized. R2 was .44 for the full multivariate model including all variables below, and .40 for the reduced model including only those questions significant at P value ≤ .05 (questions 16, 33, and 34 in bold).
P value for multivariate analysis for higher percentage of adherence.
Overall, the staged multivariate model building suggests that the predictive validity of the GTCAT is strong because several Health Belief Model questions and constructs are associated with adherence. The R2 value of .40 suggests that 40% of the variability in adherence can be explained by a subset of GTCAT questions. It also suggests that a shorter questionnaire with similar explanatory power may be feasible in the future.
DISCUSSION
Our study used focus groups and an observational case series to evaluate the psychometrics of the GTCAT. The focus group component demonstrated that the content validity was high, with 89% of responses fitting a construct of the Health Belief Model. The principal component analysis showed an organizing structure consistent with the Health Belief Model. Regression models with adherence as the outcome of interest and GTCAT questions as explanatory variables showed predictive validity. Finally, the repeatability of the GTCAT questionnaire was excellent. Overall, this suggests that the Health Belief Model may represent the organizing structure for glaucoma adherence; that the GTCAT may be a survey tool to determine the factors related to adherence with glaucoma medications in individual glaucoma patients; and that one could use the GTCAT to measure the effect of interventions to improve glaucoma adherence.
Our study found that patients underestimated their adherence when we compared self-report to the objective measures of adherence. This is similar to findings in previous studies.10,68,75 We also show that older participants and those of white ethnicity were more likely to be adherent. The increased adherence in those of white ethnicity as compared to other ethnicities was also found by Okeke and associates.76
Previous studies examining glaucoma adherence have not used the Health Belief Model as organizing framework for understanding and characterizing compliance. However, their results suggest that the Health Belief Model may be applicable to their results. Lacey and associates28 demonstrated adherence to be associated with fear of blindness, forgetfulness, difficulty with drop application, and age; Friedman and associates29 found adherence to be associated with patient education, risk of vision loss, cost, traveling, side effects, and demographic factors. Using the Health Belief Model, one could organize these results into demographic factors and the Health Belief Model constructs of perceived susceptibility, barriers, and self-efficacy. Tsai and coworkers9 reported the first systematic classifications of barriers to adherence with glaucoma medications. They used a structured interview of glaucoma patients, and experts categorized responses based on their similarity. They demonstrated regimen factors, patient factors, provider factors, and situational/environmental factors. When using the Health Belief Model as an organizing framework, the responses of the Tsai study could be explained as the constructs of barriers, self-efficacy, cues-to-action, and benefits. Sleath and coworkers77 developed a questionnaire based on self-efficacy. Overall, the findings of these previous studies are similar to ours and can be reasonably interpreted within the structure of the Health Belief Model.
Health literacy and doctor-patient communication may also influence adherence in glaucoma treatment. Several publications highlight the importance of health literacy to glaucoma adherence.30,31,78 They show that poor educational attainment and poor knowledge of glaucoma decreased adherence with glaucoma medications. Gelb and associates79 found lower adherence with certain physician beliefs (“reactives” and “skeptics”), low patient education, low risk of vision loss, higher costs of medications, longer travel distance, increased side effects, and patient age <50 years or ≥80 years. Friedman and associates29 showed that the doctor-patient communication can be related to adherence. Overall, these studies suggest that educating patients and the style of communication may be important methods to improve adherence.
Our results also suggest that modifications of the current GTCAT may improve its performance and feasibility. A new version of the GTCAT (available from the principal author on request) includes a 5-point Likert scale, rather than a 10-point scale,80 and the same response scale for each item. We also added questions regarding patient-provider relationships, education level, and depression. These topics were mentioned during the focus groups, and previous studies have shown them to be associated with adherence.29,81,82 Hopefully, these modifications will improve the R2 beyond the results of the current study (0.40 with GTCAT questions only). The GTCAT questionnaire required a mean of 18 minutes to administer, which is too long in busy clinical settings. Ceiling effects, principal component analysis, and regression analysis suggest that a shorter questionnaire may be possible. Finally, a future goal will be to compare the results of self-administered vs interviewer-administered GTCAT.
HOW DO YOU ADDRESS ADHERENCE WITH YOUR GLAUCOMA PATIENTS?
Some researchers consider poor adherence as a condition in itself, requiring constant vigilance, reinforcement, and cooperation between the clinician and patient. Studies have shown that multimode interventions are more effective than a single type of intervention for improving adherence for a group of patients.83 While this may be true, eye care providers do not have a valid method of determining the factors related to adherence in their patients. For example, patients who frequently miss their eyes during administration of the drops may become frustrated, both at the waste of a costly medication and with their own ability to perform the task. A clinician who knows this in advance can demonstrate different ways of administering the drops. Future versions of the GTCAT should facilitate this assessment.
Without a questionnaire, the clinician must address adherence on an individual basis. The first step is to have a good rapport with your patient. The second step is to ask about adherence using open questions in a “safe” environment. For example, “It can be hard to use your eye drops. How often do you think you miss them?” These types of statements will give a basic starting point for addressing adherence. As stated previously, patients will overestimate their adherence and may not admit to difficulty.
Measuring, educating, reinforcing, and treating adherence with glaucoma medications may create time constraints, especially when declining reimbursements require providers to examine more patients. Perhaps eye care providers would incorporate methods of measuring and improving adherence if third-party payers provided incentives similar to those given to providers to encourage patients to stop smoking or as part of a Physician Quality Reporting Initiative. This is not likely to occur until studies document the short- and long-term success of interventions to improve adherence.
LIMITATIONS
The current report suggests excellent psychometric properties of the GTCAT. However, we recruited participants from a single, tertiary care glaucoma center. Results in other clinic populations may be different. We included only approximately 80 participants as part of the focus and observational case series. However, our results suggest that the sample size was adequate because the focus group contextual analysis became redundant, and the principal component analysis was able to converge into seven components. We used principal component analysis to test whether the underlying structure of responses will support the Health Belief Model, but principal component analysis can be subjective with different results for different cutoffs of factor loading. We used a conservative and more stringent cutoff for factor loadings with similar convergence into seven components. Alternative methods (eg, Rasch analysis) will be applied in the future to development of the instrument and to ensure that the scales are unidimensional. Additionally, a multicenter study including larger sample size would provide further evidence regarding the reliability of the questionnaire with wider, more diverse participants. Finally, we provided medications free of charge to participants, and although our study used an electronic dosing monitor to obtain an objective measure of adherence, our patients knew that their medication use was being monitored. Therefore, it is possible that our adherence results are biased toward higher adherence.
CONCLUSION
In this initial demonstration, the GTCAT showed promise as a tool for researchers. With additional development, the GTCAT may have utility for eye care providers. One future goal is to create an instrument to determine the most likely factors related to adherence in individual patients, which offers the opportunity to create a tailored educational approach. For example, a patient who frequently misses his or her eyes during administration of drops may become frustrated. An eye care provider who knows this in advance can demonstrate different ways of getting the drops into the eye. Likewise, for a patient whose primary reason for failing to use the drops is one of forgetfulness, a clinician can address the development of external reminders. Further studies should examine the ability of the GTCAT to identify and treat factors related to poor adherence.
Acknowledgments
Funding/Support: This work is funded in part by grant EY0155501-01 from the National Eye Institute, the American Glaucoma Society, and the Good Samaritan Foundation.
Financial Disclosures: Dr Mansberger is a consultant/advisor to Allergan, Santen, Glaukos, Genentech, and Alcon; has grant support from the National Eye Institute and the Centers for Disease Control and Prevention (CDC); and receives lecture fees from Merck. Dr Lambert has grant support from the Office of Minority Health, Department of Health and Human Services, and the CDC.
Author Contributions: Conception and design (S.M., W.L., Z.S., I.S.); Analysis and interpretation (S.M., W.L., C.S., C.V.); Writing the article (S.M., C.S., C.V.); Critical revision of article (S.M., W.L., C.S., Z.S., T.M., I.S., C.V.); Final approval of article (S.M., W.L., C.S., Z.S., T.M., I.S., C.V.); Data collection (S.M., Z.S., T.M., I.S.); Provision of materials, patients, or resources (S.M.); Statistical expertise (S.M., W.L., C.S.); Obtaining funding (S.M.); and Literature search (S.M.).
Other Acknowledgments: The authors would like to thank the study participants. Stuart Gardiner, PhD, provided statistical advice.
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, Nordstrom B, Mozaffari E, Quigley HA. Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology. 2005;112(9):1500–1504. doi: 10.1016/j.ophtha.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 3.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49(1):66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. doi: 10.1001/archopht.120.6.701. discussion 829–730. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106(4):653–662. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman TJ, Zalta AH. Facilitating patient compliance in glaucoma therapy. Surv Ophthalmol. 1983;28(Suppl):252–258. doi: 10.1016/0039-6257(83)90142-x. [DOI] [PubMed] [Google Scholar]
- 8.Laster SF, Martin JL, Fleming JB. The effect of a medication alarm device on patient compliance with topical pilocarpine. J Am Optom Assoc. 1996;67(11):654–658. [PubMed] [Google Scholar]
- 9.Tsai JC, McClure CA, Ramos SE, Schlundt DG, Pichert JW. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12(5):393–398. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101(5):515–523. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- 11.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically: the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140(4):598–606. doi: 10.1016/j.ajo.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Lee PP, Walt JG, Chiang TH, Guckian A, Keener J. A gap analysis approach to assess patient persistence with glaucoma medication. Am J Ophthalmol. 2007;144(4):520–524. doi: 10.1016/j.ajo.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Reardon G, Schwartz GF, Mozaffari E. Patient persistency with topical ocular hypotensive therapy in a managed care population. Am J Ophthalmol. 2004;137(1 Suppl):S3–12. doi: 10.1016/j.ajo.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Deokule S, Sadiq S, Shah S. Chronic open angle glaucoma: patient awareness of the nature of the disease, topical medication, compliance and the prevalence of systemic symptoms. Ophthalmic Physiol Opt. 2004;24(1):9–15. doi: 10.1046/j.1475-1313.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 16.Olthoff CM, Schouten JS, van de Borne BW, Webers CA. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology. 2005;112(6):953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Stewart WC, Chorak RP, Hunt HH, Sethuraman G. Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol. 1993;116(2):176–181. doi: 10.1016/s0002-9394(14)71282-6. [DOI] [PubMed] [Google Scholar]
- 18.Rossi GC, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21(4):410–414. doi: 10.5301/EJO.2010.6112. [DOI] [PubMed] [Google Scholar]
- 19.Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. doi: 10.1016/j.ophtha.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashburn FS, Jr, Goldberg I, Kass MA. Compliance with ocular therapy. Surv Ophthalmol. 1980;24(4):237–248. doi: 10.1016/0039-6257(80)90045-4. [DOI] [PubMed] [Google Scholar]
- 21.Spaeth GL. Visual loss in a glaucoma clinic. I. Sociological considerations. Invest Ophthalmol. 1970;9(1):73–82. [PubMed] [Google Scholar]
- 22.Van Buskirk EM. The compliance factor. Am J Ophthalmol. 1986;101(5):609–610. doi: 10.1016/0002-9394(86)90954-2. [DOI] [PubMed] [Google Scholar]
- 23.Kass MA, Gordon M, Meltzer DW. Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy? Am J Ophthalmol. 1986;101(5):524–530. doi: 10.1016/0002-9394(86)90940-2. [DOI] [PubMed] [Google Scholar]
- 24.Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- 25.US Dept of Health and Human Services . Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics. Rockville, MD: May, 1999. [Google Scholar]
- 26.Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. doi: 10.1016/j.ajo.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261(22):3273–3277. [PubMed] [Google Scholar]
- 28.Lacey J, Cate H, Broadway DC. Barriers to adherence with glaucoma medications: a qualitative research study. Eye. 2009;23(4):924–932. doi: 10.1038/eye.2008.103. [DOI] [PubMed] [Google Scholar]
- 29.Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115(8):1320–1327. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Muir KW, Santiago-Turla C, Stinnett SS, et al. Health literacy and adherence to glaucoma therapy. Am J Ophthalmol. 2006;142(2):223–226. doi: 10.1016/j.ajo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Juzych MS, Randhawa S, Shukairy A, Kaushal P, Gupta A, Shalauta N. Functional health literacy in patients with glaucoma in urban settings. Arch Ophthalmol. 2008;126(5):718–724. doi: 10.1001/archopht.126.5.718. [DOI] [PubMed] [Google Scholar]
- 32.Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eye drop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127(6):732–736. doi: 10.1001/archophthalmol.2009.96. [DOI] [PubMed] [Google Scholar]
- 33.Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, Dada T. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21(3):189–192. doi: 10.1097/IJG.0b013e31820bd2e1. [DOI] [PubMed] [Google Scholar]
- 34.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Fishbein M. A theory of reasoned action: some applications and implications. Nebr Symp Motiv. 1980;27:65–116. [PubMed] [Google Scholar]
- 36.Glanz K, Rimer BK, Lewis FM. Health behavior and health education: theory, research, and practice. 3rd ed. San Francisco: Jossey-Bass; 2002. [Google Scholar]
- 37.Strecher VJ, Rosenstock IM. The Health Belief Model. In: Glanz K, Lewis FM, Rimer BK, editors. Health behavior and health education: theory, research, and practice. 2nd ed. San Francisco: Jossey-Bass; 1997. pp. 41–58. [Google Scholar]
- 38.Norman P, Brain K. An application of an extended health belief model to the prediction of breast self-examination among women with a family history of breast cancer. Br J Health Psychol. 2005;10(Pt 1):1–16. doi: 10.1348/135910704X24752. [DOI] [PubMed] [Google Scholar]
- 39.Benedict S, Goon G, Hoomani J, Holder P. Breast cancer detection by daughters of women with breast cancer. Cancer Pract. 1997;5(4):213–219. [PubMed] [Google Scholar]
- 40.Erwin DO, Spatz TS, Stotts RC, Hollenberg JA, Deloney LA. Increasing mammography and breast self-examination in African American women using the Witness Project model. J Cancer Educ. 1996;11(4):210–215. doi: 10.1080/08858199609528430. [DOI] [PubMed] [Google Scholar]
- 41.Murray M, McMillan C. Health beliefs, locus of control, emotional control and women’s cancer screening behaviour. Br J Clin Psychol. 1993;32(Pt 1):87–100. doi: 10.1111/j.2044-8260.1993.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 42.Umeh K, Rogan-Gibson J. Perceptions of threat, benefits, and barriers in breast self-examination amongst young asymptomatic women. Br J Health Psychol. 2001;6(Part 4):361–372. doi: 10.1348/135910701169269. [DOI] [PubMed] [Google Scholar]
- 43.Wyper MA. Breast self-examination and the health belief model: variations on a theme. Res Nurs Health. 1990;13(6):421–428. doi: 10.1002/nur.4770130610. [DOI] [PubMed] [Google Scholar]
- 44.Maes CA, Louis M. Knowledge of AIDS, perceived risk of AIDS, and at-risk sexual behaviors among older adults. J Am Acad Nurse Pract. 2003;15(11):509–516. doi: 10.1111/j.1745-7599.2003.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 45.Zak-Place J, Stern M. Health belief factors and dispositional optimism as predictors of STD and HIV preventive behavior. J Am Coll Health. 2004;52(5):229–236. doi: 10.3200/JACH.52.5.229-236. [DOI] [PubMed] [Google Scholar]
- 46.Perkins DO. Adherence to antipsychotic medications. J Clin Psychiatry. 1999;60(Suppl 21):25–30. [PubMed] [Google Scholar]
- 47.Adams J, Scott J. Predicting medication adherence in severe mental disorders. Acta Psychiatr Scand. 2000;101(2):119–124. doi: 10.1034/j.1600-0447.2000.90061.x. [DOI] [PubMed] [Google Scholar]
- 48.Mirotznik J, Feldman L, Stein R. The health belief model and adherence with a community center–based, supervised coronary heart disease exercise program. J Community Health. 1995;20(3):233–247. doi: 10.1007/BF02260407. [DOI] [PubMed] [Google Scholar]
- 49.Alogna M. Perception of severity of disease and health locus of control in compliant and noncompliant diabetic patients. Diabetes Care. 1980;3(4):533–534. doi: 10.2337/diacare.3.4.533. [DOI] [PubMed] [Google Scholar]
- 50.Koch J. The role of exercise in the African-American woman with type 2 diabetes mellitus: application of the health belief model. J Am Acad Nurse Pract. 2002;14(3):126–129. doi: 10.1111/j.1745-7599.2002.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 51.Pham DT, Fortin F, Thibaudeau MF. The role of the Health Belief Model in amputees’ self-evaluation of adherence to diabetes self-care behaviors. Diabetes Educ. 1996;22(2):126–132. doi: 10.1177/014572179602200205. [DOI] [PubMed] [Google Scholar]
- 52.Polly RK. Diabetes health beliefs, self-care behaviors, and glycemic control among older adults with non-insulin-dependent diabetes mellitus. Diabetes Educ. 1992;18(4):321–327. doi: 10.1177/014572179201800411. [DOI] [PubMed] [Google Scholar]
- 53.Schatz PE. An evaluation of the components of compliance in patients with diabetes. J Am Diet Assoc. 1988;88(6):708–712. [PubMed] [Google Scholar]
- 54.Swift CS, Armstrong JE, Beerman KA, Campbell RK, Pond-Smith D. Attitudes and beliefs about exercise among persons with non-insulin-dependent diabetes. Diabetes Educ. 1995;21(6):533–540. doi: 10.1177/014572179502100607. [DOI] [PubMed] [Google Scholar]
- 55.Larson EB, Olsen E, Cole W, Shortell S. The relationship of health beliefs and a postcard reminder to influenza vaccination. J Fam Pract. 1979;8(6):1207–1211. [PubMed] [Google Scholar]
- 56.Rundall TG, Wheeler JR. Factors associated with utilization of the swine flu vaccination program among senior citizens in Tompkins County. Med Care. 1979;17(2):191–200. doi: 10.1097/00005650-197902000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Chen CY, Neufeld PS, Feely CA, Skinner CS. Factors influencing compliance with home exercise programs among patients with upper-extremity impairment. Am J Occup Ther. 1999;53(2):171–180. doi: 10.5014/ajot.53.2.171. [DOI] [PubMed] [Google Scholar]
- 58.Ross S, Walker A, MacLeod MJ. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens. 2004;18(9):607–613. doi: 10.1038/sj.jhh.1001721. [DOI] [PubMed] [Google Scholar]
- 59.Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116(2):227–233. doi: 10.1001/archopht.116.2.227. [DOI] [PubMed] [Google Scholar]
- 60.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 61.Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116(11):1496–1504. doi: 10.1001/archopht.116.11.1496. [DOI] [PubMed] [Google Scholar]
- 62.Granstrom PA. Glaucoma patients not compliant with their drug therapy: clinical and behavioural aspects. Br J Ophthalmol. 1982;66(7):464–470. doi: 10.1136/bjo.66.7.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74(8):477–480. doi: 10.1136/bjo.74.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kidd PS, Parshall MB. Getting the focus and the group: enhancing analytical rigor in focus group research. Qual Health Res. 2000;10(3):293–308. doi: 10.1177/104973200129118453. [DOI] [PubMed] [Google Scholar]
- 65.Morgan DL, Krueger RA, King JA. The focus group kit. 1–6. Thousand Oaks, CA: Sage Publications, Inc; 1998. [Google Scholar]
- 66.Cronin TH, Kahook MY, Lathrop KL, Noecker RJ. Accuracy and performance of a commercially available Dosing Aid. Br J Ophthalmol. 2007;91(4):497–499. doi: 10.1136/bjo.2006.103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman DS, Jampel HD, Congdon NG, Miller R, Quigley HA. The TRAVATAN Dosing Aid accurately records when drops are taken. Am J Ophthalmol. 2007;143(4):699–701. doi: 10.1016/j.ajo.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 68.Boden C, Sit A, Weinreb RN. Accuracy of an electronic monitoring and reminder device for use with travoprost eye drops. J Glaucoma. 2006;15(1):30–34. doi: 10.1097/01.ijg.0000196654.77836.61. [DOI] [PubMed] [Google Scholar]
- 69.Jolliffe IT. Principal component analysis. 2nd ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 70.Carmines EG, Zeller RA. Reliability and validity assessment. Thousand Oaks, CA: Sage Publications, Inc; 1979. [Google Scholar]
- 71.DeVellis RF. Scale development: theory and applications. 3rd ed. Thousand Oaks, CA: Sage Publications, Inc; 2011. [Google Scholar]
- 72.Spector PE. Summated rating scale construction: an introduction. Thousand Oaks, CA: Sage Publications, Inc; 1992. [Google Scholar]
- 73.Kutner MH, Nachtsheim C, Neter J. Applied linear regression models. 4th ed. Boston, New York: McGraw-Hill/Irwin; 2004. [Google Scholar]
- 74.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 75.Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21(4):234–240. doi: 10.1097/IJG.0b013e31821dac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmol. 2009;116(12):2286–2293. doi: 10.1016/j.ophtha.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sleath B, Blalock SJ, Stone JL, et al. Validation of a short version of the glaucoma medication self-efficacy questionnaire. Br J Ophthalmol. 2012;96(2):258–262. doi: 10.1136/bjo.2010.199851. [DOI] [PubMed] [Google Scholar]
- 78.Muir KW, Ventura A, Stinnett SS, Enfiedjian A, Allingham RR, Lee PP. The influence of health literacy level on an educational intervention to improve glaucoma medication adherence. Patient Educ Couns. 2012;87(2):160–164. doi: 10.1016/j.pec.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelb L, Friedman DS, Quigley HA, et al. Physician beliefs and behaviors related to glaucoma treatment adherence: the Glaucoma Adherence and Persistency Study. J Glaucoma. 2008;17(8):690–698. doi: 10.1097/IJG.0b013e31816b3001. [DOI] [PubMed] [Google Scholar]
- 80.Lozano LM, Garcia-Cueto E, Muniz J. Effect of the number of response categories on the reliability and validity of rating scales. Methodology (Gott) 2008;4(2):73–79. [Google Scholar]
- 81.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 82.Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11 Suppl):S30–36. doi: 10.1016/j.ophtha.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 83.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]


