Abstract
Noise-induced hearing loss (NIHL) is a significant clinical, social, and economic issue. The development of novel therapeutic agents to reduce NIHL will potentially benefit multiple very large noise-exposed populations. Oxidative stress has been identified as a significant contributor to noise-induced sensory cell death and noise-induced hearing loss, and several antioxidant strategies have now been suggested for potential translation to human subjects. One such strategy is a combination of beta-carotene, vitamins C and E, and magnesium, which has shown promise for protection against NIHL in rodent models, and is being evaluated in a series of international human clinical trials using temporary (military gunfire, audio player use) and permanent (stamping factory, military airbase) threshold shift models (NCT00808470). The noise exposures used in the recently completed Swedish military gunfire study described in this report did not, on average, result in measurable changes in auditory function using conventional pure-tone thresholds and distortion product otoacoustic emission (DPOAE) amplitudes as metrics. However, analysis of the plasma samples confirmed significant elevations in the bloodstream 2 hours after oral consumption of active clinical supplies, indicating the dose is realistic. The plasma outcomes are encouraging, but clinical acceptance of any novel therapeutic critically depends on demonstration that the agent reduces noise-induced threshold shift in randomized, placebo-controlled, prospective human clinical trials. Although this noise insult did not induce hearing loss, the trial design and study protocol can be applied to other populations exposed to different noise insults.
Keywords: Noise, hearing, vitamin, magnesium, plasma, temporary threshold shift
Introduction
Recent years have brought a significantly improved understanding of biochemical events occurring in the inner ear during and after noise insult. These biochemical events can be driven by oxidative stress and/or activation of cell death receptors. Identification of oxidative stress with noise and improved knowledge as to how cells die has been particularly significant for the development of novel antioxidant strategies to reduce noise-induced hearing loss (NIHL) [for recent reviews, see 1, 2].
Synergistic protective effects of a combination of β-carotene, ascorbic acid, Trolox® (a water soluble analogue of vitamin E), and Mg were explicitly shown in the inner ear by Le Prell et al. [3], in guinea pigs. Similar (but smaller) levels of protection using a combination of vitamins and Mg was recently reported by Tamir et al. [4], in mice. Data presented in other recent reports include dose-dependent reductions in permanent threshold shift (PTS) in mice maintained on nutrient-enhanced diets [5], and reductions in temporary thresholds shift (TTS) in guinea pigs [6]. Of particular interest, plasma levels achieved during dosing that resulted in effective protection in the guinea pig have been established [6], and these serve as a guide to potential target levels for translation to human use.
Here, we describe the first efforts to evaluate the combination of β-carotene, vitamins C and E, and Mg, for potential protection against NIHL in human subjects; the subject population was Swedish military personnel exposed to impulse noise during weapons training. The Swedish military has had an active hearing conservation program for the past 30 years, and has monitored the rate of hearing loss in 18-year old military conscripts as well as the development of hearing loss during military service for most of that time [7–12].
Materials and Methods
In the current study, we used conventional pure-tone audiometry (the clinical gold standard) and distortion product otoacoustic emission amplitude (DPOAE) measurements, which provide a sensitive and objective measure of outer hair cell (OHC) function [13, 14], to measure noise-induced changes in Swedish military personnel undergoing weapons training. Detailed procedures follow. Psycoacoustic modulation transfer function (PMTF) tests, which reflect the ability of the ear to follow the amplitude modulation [15, 16], were conducted on the first ten subjects tested; these tests are not described further given the small number of subjects tested.
Subjects
All protocols and procedures were approved by the Swedish Ethical Review Committee and the Investigational Review Board (IRB) at the University of Michigan (IRBMED), and all data were collected under the supervision of an NIH-selected Data Safety Monitoring Board. Additional approval was provided by the University of Florida IRB-01, where analysis of the de-identified DPOAE data was performed. Thirty-one individuals served as subjects, including officers in the Swedish military serving at Södra skånska regementet (n=10; age=29.6+/−3.1 years) and Swedish military academy trainees serving at Karlberg (n=21; age=23.3+/− 3.2 years). Demographic information of all subjects is provided in Table 1.
Table 1.
Demographic, Behavioral, and Medical History Data Obtained Before Randomization
| Variable | Södra skånska regementet (n=10 officers) |
Karlberg(n=21 academy trainees) |
N=31 (combined) |
P-value |
|---|---|---|---|---|
| Demographic or Behavioral Variables | ||||
| Age (years) | 29.6 (3.13) | 23.3 (3.2) | 25.4 (4.3) | <0.0001 |
| Sex (% Male) | 90.0% | 85.7% | 87.1% | 1.0 |
| Current Smoker (% Yes) | 10.0% | 9.5% | 9.7% | 1.0 |
| Exposure of loud sounds In Leisure Time (% Yes) |
50.0% | 23.8% | 31.0% | 0.21 |
| Exposure to Loud Music (% Yes) |
37.5% | 66.7% | 58.6% | 0.21 |
| Accidental Exposure to Loud Impulse Noise (% Yes) |
60.0% | 23.8% | 35.5% | 0.10 |
| Medical History Variables | ||||
| History of Loose Stools (% Yes) |
0% | 0% | 0% | NA |
| History of Diarrhea (% Yes) |
0% | 0% | 0% | NA |
| Current Steroid Use (% Yes) |
0% | 0% | 0% | NA |
| History of Tinnitus after Noise exposure (% Yes) |
70.0% | 71.4% | 71.0% | 1.0 |
| Experience of Hearing Loss (% Yes) |
20.0% | 10.0% | 13.0% | 0.30 |
| History of Ear Infections (% Yes) |
44.4% | 33.3% | 37.0% | 0.68 |
Mean (S.D) or Percentage are presented as appropriate. P-value for age is from a two-sample t-test; all other p-values are from Fisher exact tests.
Advertisements posted on the military base invited normally hearing subjects to participate in a study of temporary changes in hearing after military-mandated weapons training exercises. After providing informed consent, all participants completed questionnaires regarding their health and noise exposure histories. Volunteers reporting a history of gastrointestinal disturbances (loose stools, frequent diarrhea, recurrent nausea/vomiting), neurological disturbances (seizures, frequent severe headaches, stroke, fainting spells, disorientation), hematological disorders (problems stopping bleeding), or auditory/vestibular disorders (ear ache, ear drainage, ear infection, dizziness/vertigo) were not eligible to participate in this study. Hearing tests were conducted to confirm eligibility to participate. Eligibility criteria included normal audiologic assessment defined as 1) symmetric hearing with air conduction thresholds no worse than 25 dB HL at tested frequencies from 0.25 – 8 kHz; 2) threshold asymmetry ≤ 15 dB at all test frequencies; 3) ipsilateral reflex intact at 1 kHz at 100 dB HL; and 4) normal Type A tympanograms bilaterally, defined as a range of −140 to +40 daPa based on the 90% range for adults [17]. Two subjects were included by the study site team despite unilateral single-frequency deficits outside the 25 dB HL criteria. These protocol violations were reported to the DSMB and the supervising IRBs.
Treatments
Micronutrient treatment was a combination of β-carotene (18 mg), vitamin C (500 mg ascorbic acid), vitamin E (305 mg α-tocopherol acetate), and Mg (1949 mg magnesium citrate). The placebo pills were inactive tablets identical in appearance to the micronutrient pill. All pills were manufactured by the Chao Center (Purdue University, Lafayette, Indiana) using good manufacturing practices, and active agent concentration and stability were confirmed using analytic techniques performed by the contract manufacturer. The above doses were the full daily dose and 6 pills/day were required to be consumed in order to achieve a full daily dose (delivered as 3 pills twice daily, see overview, above).
Design Overview
Subjects were randomized to one of the two treatment conditions (placebo or nutrients), and assigned a study date for “Arm 1”. Each arm of the study included a single weapons training exercise. After completing all Arm 1 study procedures, there was a washout period (ranging from 1–2 months in duration), and then subjects entered “Arm 2” during which the treatment was reversed but all other procedures were held constant. This was a double-blind study with treatment condition masked for both participants and study team members. The design of the trial was a cross-over design; as illustrated in Figure 1, half of the subjects received placebo first (in Arm 1), and the rest received active agents first (in Arm 1). This design is similar to that used in a recent cross-over study evaluating use of NAC by steel workers in Taiwan [18]. The study procedures repeated within each study arm are listed in order in Table 2; detailed descriptions follow.
Figure 1.
Subjects were randomly assigned to one of the two study arms, distinguished by the order of the placebo and micronutrient tests. Subjects later crossed into the other arm of the study and participated in the opposite test condition. Treatment condition was masked; neither the participants nor the study team knew which treatment was delivered in each arm.
Table 2.
Study Procedures Repeated Within Each Treatment Condition
| Södra skånska regementet |
Karlberg | ||
|---|---|---|---|
| Screening* *conducted once per subject to determine eligibility; not repeated within each treatment |
Informed Consent | xx | xx |
| Medical Questionnaires | xx | xx | |
| Otoscopy | xx | xx | |
| Tympanometry | xx | xx | |
| Stapedius Reflex | xx | xx | |
| Pure-tone Audiometry | xx | xx | |
| Randomization (Masked) |
xx | xx | |
| One day prior to the shooting exercises; “Pre-1” |
Questionnaires | xx | xx |
| Blood sample | xx | -- | |
| Pure-tone Audiometry | xx | xx | |
| DPOAE tests | xx | xx | |
| PMTF tests | xx | -- | |
| Treatment #1 (1/2 dose) | xx | xx | |
| Treatment #2 (1/2 dose) | xx | xx | |
| Day of the shooting exercises, pre-noise; “Pre-2” |
Treatment #3 (1/2 dose) | xx | xx |
| Questionnaires | xx | xx | |
| Pure-tone Audiometry | xx | xx | |
| DPOAE tests | xx | xx | |
| PMTF tests | xx | -- | |
| Treatment #4 (1/2 dose) | xx | xx | |
| Shooting Exercise | Machine Gun Training | xx | xx |
| Tinnitus Survey | xx | xx | |
| “Post-1” Day of the shooting exercises, starting 15 min post noise |
Pure-tone Audiometry | xx | xx |
| DPOAE tests | xx | xx | |
| Blood sample | xx | -- | |
| “Post-2” Day of the shooting exercises, starting 1 hr 45 min post noise |
Pure-tone Audiometry | xx | xx |
| DPOAE tests | xx | xx | |
| Tinnitus survey | xx | xx | |
| “Post-3” Day of the shooting exercises, starting 3 hr 30 min post noise |
Pure-tone Audiometry | xx | xx |
| DPOAE tests | xx | xx | |
| Tinnitus survey | xx | xx | |
| PMTF tests | xx | -- | |
| “Post-4” One day post shooting |
Pure-tone Audiometry | xx | xx |
| DPOAE tests | xx | xx | |
| Tinnitus survey | xx | xx | |
| PMTF tests | xx | -- |
Assessment Protocols
Questionnaires
The Hearing Survey and the Tinnitus Survey used for screening the current subjects were identical to those recently used in other studies[see Appendices A and B in 19]. These questionnaires were completed after subjects provided informed consent and after the medical history was completed.
Facilities
Military officers were consented and assessed at Södra skånska regementet, P7; military trainees were consented and assessed at Karlberg. Audiometric tests were conducted in a quiet room in a house approximately 100 m from the bunker where the shooting is conducted. Shooting took place during discrete periods of time, and audiometric testing was not conducted during those times. Background noise was monitored with a data-logging noise-dosimeter. Ambient noise levels did not exceed allowable levels (per ANSI S3.1-1999) during testing.
Otoscopy
Visual examination of the ear canal and tympanic membrane was conducted to ensure normal anatomy and no presence of obstructive debris.
Tympanometry
Tympanometric measures were collected using a GSI 38 immittance measurement device that was in compliance with ANSI S3.39 and IEC 601-1 criteria. Normal middle ear function was defined by Type A 226 Hz tympanograms bilaterally with a pressure peak within the region of −140 to +40 daPa based on the 90% range for adults [see 17].
Stapedius Reflex Assessment
Ipsilateral stapedius reflex testing was conducted at 1 kHz at 100 dB HL. All of the subjects had a stapedius reflex under these stimulus conditions.
Pure-Tone Audiometry
Pure-tone threshold measurements were conducted using GSI 61 diagnostic audiometers with 3A insert earphones; audiometers were professionally calibrated according to ANSI 3.6 1996 prior to the start of the trials at both study sites and they were checked biologically each day. Pure-tone air conduction thresholds were obtained using a modified Hughson-Westlake procedure for test frequencies of 0.25-, 0.5-, 1-, 2-, 3-, 4-, 6-, and 8-kHz [20]; left ears were always tested first. Modifications were as follows: 1) initial descent towards threshold was accomplished in 10-dB steps; 2) beginning with the first non-response, levels were increased by 2 dB for each non-response, and decreased by 4 dB after each correct detection response; and 3) threshold was defined as the lowest level at which two responses were obtained out of three presentations on an ascending run. Responses were evaluated for reliability using repeat testing at 2- and 8-kHz in each ear, and the absolute difference between test and retest thresholds was required to be ≤ 5 dB during the baseline tests.
Distortion Product Otoacoustic Emissions (DPOAEs)
DPOAE testing was conducted using Tucker-Davis System III hardware controlled by custom software developed at the Karolinska Institutet, in combination with an Etymotic Research microphone-earphone assembly (ER 10C) (Södra skånska), or using the Mimosa HearID system (Karlberg). In both cases, the closed, calibrated probe assembly was coupled to the subject’s ear by a foam ear tip. All software acquired, averaged, extracted and stored desired DPOAE response amplitude and noise floor data. All instruments were calibrated prior to the onset of the studies. DPOAE responses were acquired from both ears of subjects (left, then right) using two simultaneously-presented ‘primary’ tones, with frequencies f1 and f2 (f2/f1 = 1.2), and levels L1 and L2 (with L2=L1-10 dB). To facilitate comparisons with audiometric thresholds, f2 frequencies (2, 3, 4, 6, and 8 kHz) matched the audiometric test frequencies. To minimize the potential for bias due to measurement-based stopping, a simplified stopping rule was used; all tests were averaged over 10 seconds as in Goldman et al. [21]. There were no effects of noise on the DPOAE responses at any test frequency, at any test time; those data are not presented or discussed.
Noise Exposure
During the shooting session, two rounds of ammunition (20 shots/round) were fired from an automatic machine-gun (Ksp-58) in a bunker over a period lasting less than 1 minute. Two people were in the bunker at the same time for each exercise: the right-handed shooter and a companion on his/her right side, with the weapon situated between them. Standard hearing protectors were worn by all participants during shooting exercises. It was not possible to collect sound level measurements during the shooting exercises at these bases; however, sound levels during firing of these machine gun weapons have been measured previously and peak exposures of 156 dB SPL were reported [12]. Other (unpublished) measurements of free-field sound levels during firing of this weapon were slightly higher (164–166 dB SPL), with measurements of 135–154 dB SPL in the ear canal under the hearing protectors [22].
Blood Sampling and Analysis
For nine of the ten subjects tested at the Södra skånska regementet, P7, site, blood samples were taken by standard venipuncture 1-day prior to the first treatment and 2 hours after the fourth, final, treatment. Because this was a cross-over study, each subject contributed a total of 4 blood samples. The tenth subject did not have readily accessible veins. It was not logistically possible to collect blood samples from subjects tested at the Karlberg site. Blood (8 ml) was collected in a lithium heparin coated Vacutainer® (green top BD# 367964) and after proper mixing (gentle inversion 8–10×), samples were centrifuged (1000–1300 RPM for 10–15 min). Plasma (0.5 ml) was withdrawn and placed in a clean tube (e.g., 1.5 mL Eppendorf centrifuge tube). Plasma was immediately mixed with an equal volume of 10% meta-phosphoric acid and mixed, prior to freezing at −70°C. The 10% meta-phosphoric acid solution was prepared fresh daily by dissolving meta-phosphoric acid (solid, Sigma #04103-250G) crystals in water at a 1:10 ratio. Remaining plasma was transferred to a clean tube, labeled, then wrapped in aluminum foil and frozen at −70°C. The coded samples were shipped by overnight express on dry ice to KAR Bioanalytics (Kalamazoo, MI) where plasma samples were prepared and analyzed as follows.
Vitamin C
Plasma samples were stabilized with metaphosphoric acid and further preserved with additional acetic acid and dithiothreitol (DTT). A calibration curve spiked with vitamin C was prepared so that a linear range of detection of 1.00 to 50.0 µg/mL for a 200 µL sample of stabilized plasma was achieved. Components were separated using an HPLC System (Waters 2695; Waters Corporation; Milford, MA) and analyzed using a Luna 5 µ C18, 250 × 4.6 mm (phenomenex) column. Waters Millennium software was used in conjunction with Watson software to back-calculate concentration of ascorbic acid in extraction solvent from the peak area based on a standard curve using a linear regression equation where y=mx+b (y=peak area, m=slope, x=concentration, b=y intercept).
Vitamin E and β-carotene
A calibration curve spiked with vitamin E and β-carotene was prepared so that a linear range of detection of 0.800 µg/mL to 40.0 µg/mL for vitamin E, and 0.0250 µg/mL to 10.0 µg/mL for β-carotene, was achieved. This method utilized liquid/liquid extraction with hexane and an internal standard of vitamin E acetate. Plasma was treated with a 10% sodium chloride / 10% ascorbic acid solution and the resulting supernatant was extracted with hexane. The organic phase was evaporated under N2 in a heated water bath and the residue reconstituted in ethanol. Components were separated using an HPLC System (Waters 2695; Waters Corporation; Milford, MA) and Waters 2784 Dual Wavelength UV detector (multiple wavelengths 325, 292, and 450 nm). Millennium software was used in conjunction with Watson LIMS to directly back-calculate concentrations from the peak area ratios based on a calibration curve. A linear fit with 1/x2 weighting was used for the regression analysis.
Magnesium
For initial calibration, serial dilution of a Mg standard was performed in 0.125 N hydrochloric acid with a range of 0.050 µg/mL to 1.30 µg/mL (ppm) with 0.01% lanthanum. Test samples (50 µL) were then diluted into the curve range using 0.125 N HCl and 0.01% lanthanum, then analyzed by atomic absorption spectrometry (Varian SpectraAA 20 Plus with Hollow Cathode Lamp (Mg): Agilent Technologies, Inc.; Wilmington, DE). Data were transcribed into Excel software used in conjunction with Watson LIMS to capture and directly back-calculate concentrations from the absorbance readings based on an eight point calibration curve. A quadratic fit with no weighting was used for the regression analysis.
Statistical Analysis
The primary DSMB-approved study outcome was the greatest audiometric threshold shift at 3, 4, or 6 kHz in either ear. Secondary outcome measures included 1) thresholds at individual frequencies, 2) DPOAE amplitude measurements, 3) PMTF thresholds, and 4) tinnitus measures. Primary and secondary analyses were carried out using repeated measures analyses of variance (ANOVA) models as implemented by PROC MIXED in SAS (SAS/STAT User’s Guide, version 8, p. 2083). Each outcome measure was observed in each subject before and after noise exposure in both the experimental and placebo conditions. The interaction effect involving these two “within subjects” factors (i.e., treatment/control and pre/post) was used to test the null hypothesis that the threshold shifts in the nutrient and placebo conditions are not distinguishable.
Results
Adverse Events
Subjects were asked if they had, “any kind of untoward experience, including an illness or other physical event, in the past 24 hours, whether they think it is study related or not,” during the pre-2 tests (after they had consumed 3 of the 4 half doses) and during the post-4 tests (24 hours after completing the treatments). One subject (1070) reported mild stomach upset during pre2 tests (i.e., after consuming 3 of the 4 doses), in both the placebo arm and the treatment arm of the study. Three additional subjects reported mild stomach upset 24 hours after completing the treatments (post-4) during Arm 1 only. Two of these subjects consumed placebo pills during Arm 1 (1065, 1069) and the third subject consumed nutrients (1068). Taken together, 3 of 31 subjects reported mild stomach upset during or after the placebo treatments, and 2 of 31 subjects reported mild stomach upset during or after the nutrient treatments. One additional subject reported a cough/cold during the placebo condition (1001).
Plasma Levels
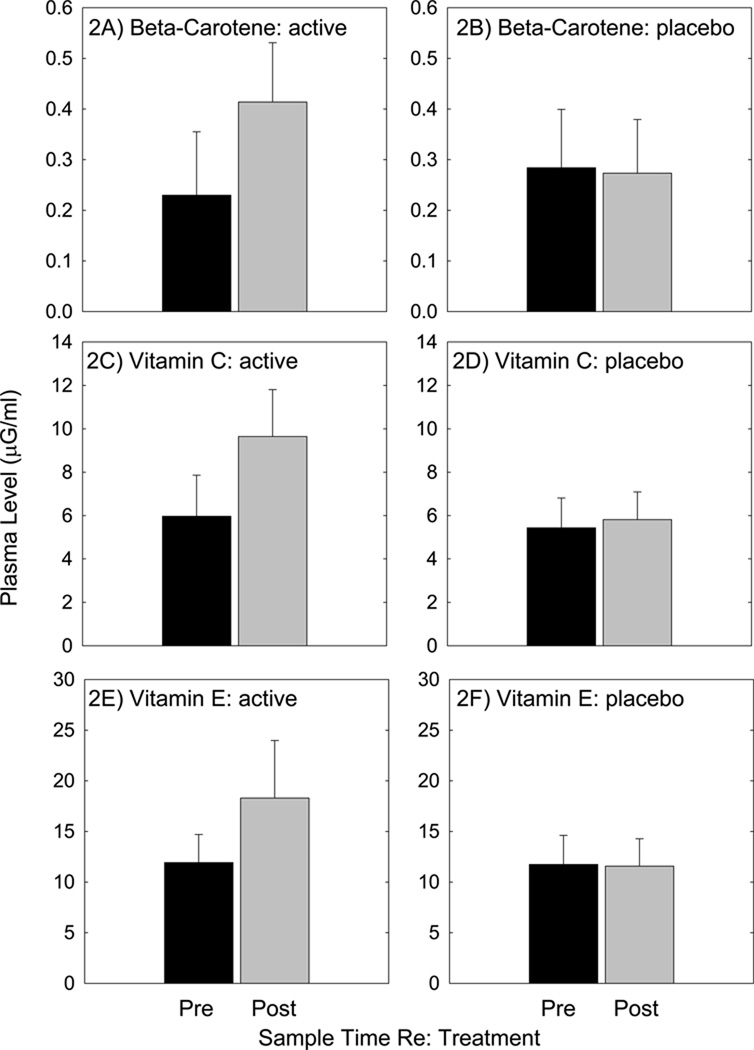
All subjects consumed all four doses of the clinical supplies over each two day study arm period, with two half doses taken twice daily. Sixteen subjects received placebo first, and the other fifteen subjects received active agents first; all subjects participated in both treatment conditions in this cross-over design study. As shown in Figure 2, plasma levels for β-carotene (2A), vitamin C (2C), and vitamin E (2E), were increased two hours after the final treatment when subjects received the active treatments. However, Mg levels were not reliably increased (not shown). There was no reliable change in plasma levels of the vitamins measured after consumption of placebo pills (2B, 2D, 2F); the levels measured after consuming placebo pills approximate the two baseline measures, taken prior to consuming each set of pills. As observed with active pills, there was no increase in plasma Mg (not shown).
Figure 2.
Subjects received blood draws prior to initiating treatment, and 2 hours after consuming the fourth and final treatment. Subjects received active agents (β-carotene, vitamins C and E, and Mg) in one arm of the study, and inactive (placebo) agents in the other arm of the study. Treatment order was randomly assigned, and treatment condition was masked to both the participants and the study team. All plasma analysis was contracted to a professional service provider: KAR Bioanalytics.
Pre-1 Test Outcomes: Age/Experience Effects
Small (≤ 5 dB) but statistically significant differences in threshold sensitivity were observed at a few frequencies when recruits were compared to officers (not shown). For the left ear, statistically reliable group differences were observed at 6 kHz (t=3.11, DF=29, p=0.004), with recruits having better hearing than officers. After adjusting for unequal variances at 4 kHz using Satterthwaite corrections, there was a trend in which recruits also had lower (better) thresholds at 4 kHz as well (t=1.84, DF=11, p=0.09). For the right ear, statistically significant group differences were observed at 0.5 and 1 kHz, with recruits having lower (better) thresholds than officers (0.5 kHz: t=2.30, DF=29, p=0.03; 1 kHz: t=2.98, DF=29, p=0.006). There was a trend in which recruits had lower (better) thresholds at 8 kHz (t=2.00, DF=11.6, p=0.07) after adjusting for unequal variances using Satterthwaite corrections. In contrast, OAE amplitude did not vary for officers and recruits (not shown). Given that group differences were small, and limited to a subset of the pure-tone test frequencies, data from recruits and officers were combined for subsequent analyses.
Pre-2 Outcomes: No Effects of Treatment on Baseline Measurements
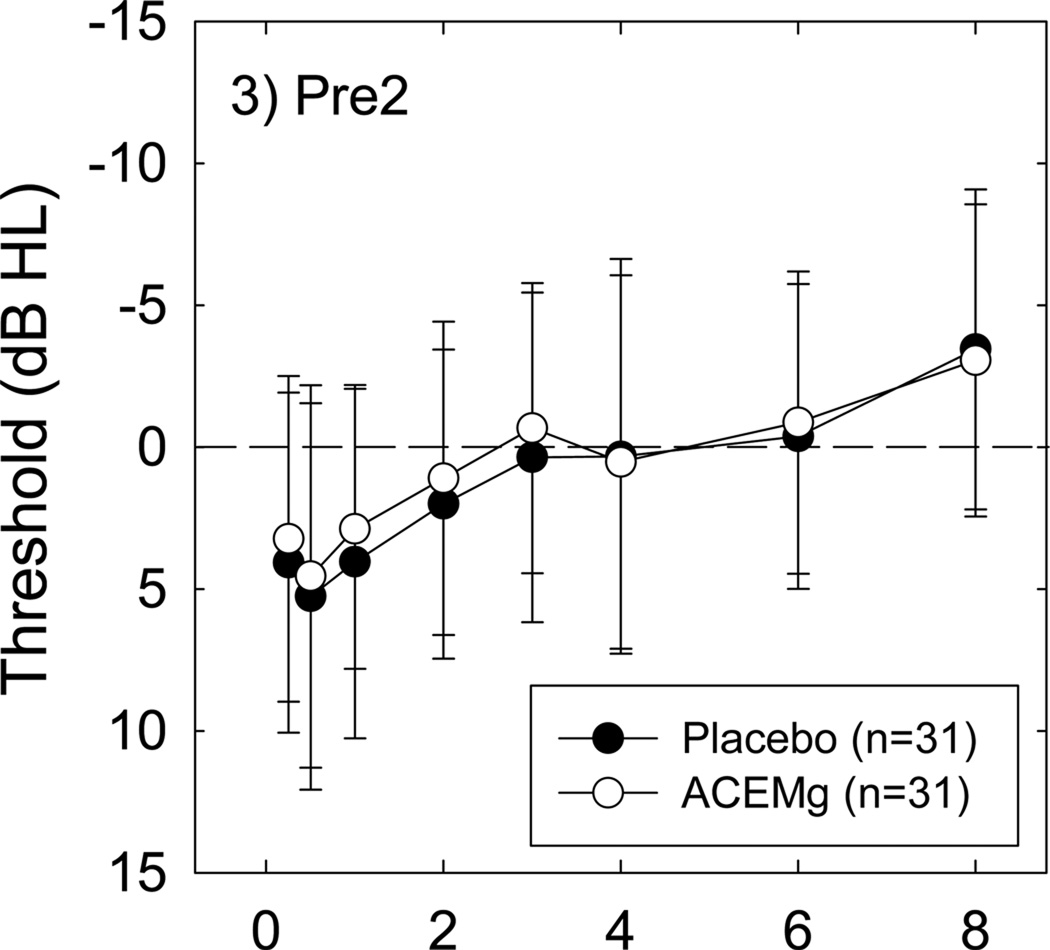
There was no effect of this nutrient regimen on baseline (pre-noise) measures of sensitivity. There were no differences between Pre-1 (pre-treatment, pre-noise) and Pre-2 (pre-noise, after three of four half doses were consumed) pure-tone thresholds regardless of whether comparisons were within the placebo or the nutrient condition (all p’s > 0.05) (see Figure 3). OAE amplitude similarly did not vary as a function of treatment condition (placebo vs nutrients; not shown).
Figure 3.
Pre-2 pre-shooting thresholds, measured after 2 days of treatment with either placebo or nutrients, were not reliably different when placebo-treated condition thresholds were compared to nutrient-treated condition thresholds for all subjects. All data are Mean ± S.D.
Conventional Audiometry: No Effects of Shooting on Baseline Thresholds
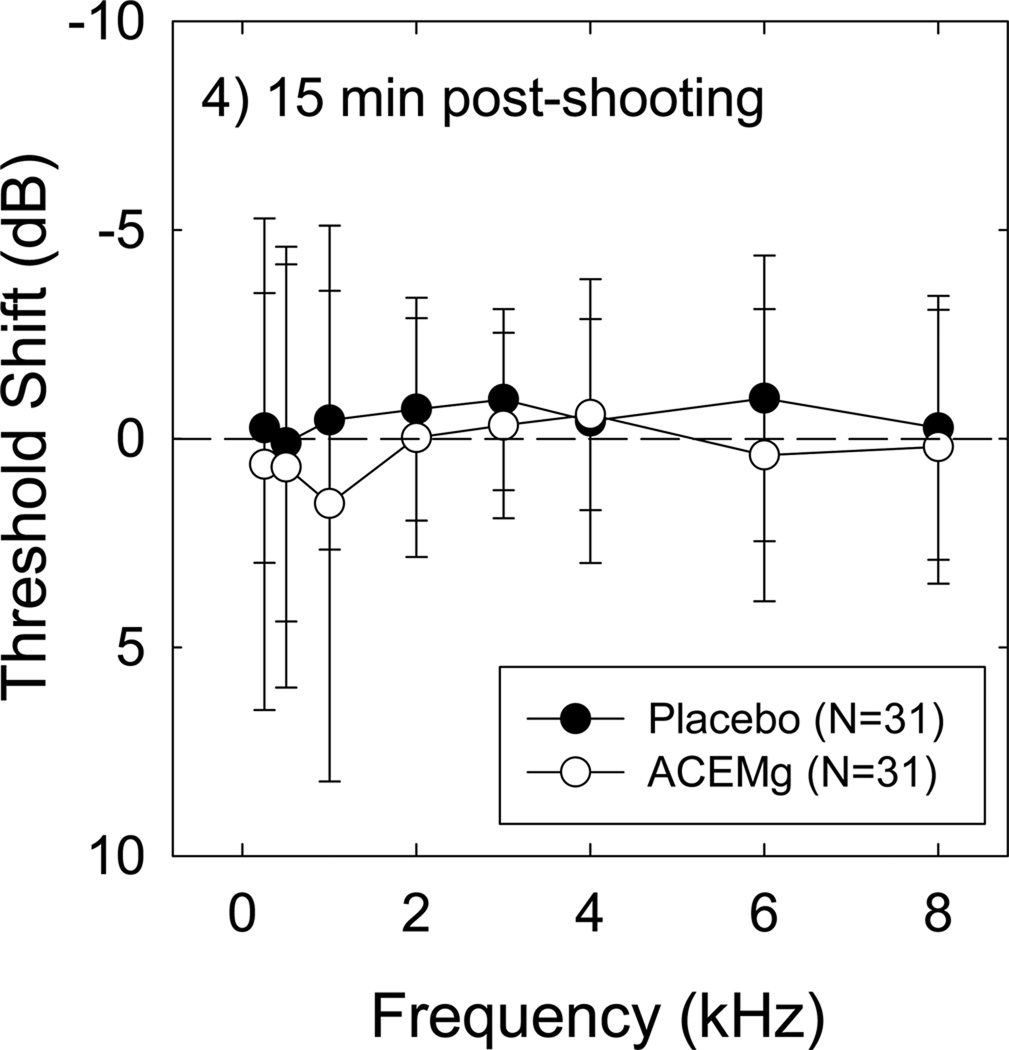
There was no reliable effect of the shooting exercises on hearing thresholds in either the placebo or the nutrient condition at any post-shooting test time, using either the single frequency change data (for average change +/− S.D., see Figure 4), or the maximum change at 3, 4, or 6 kHz in either ear (not shown). Evaluating median change instead of average change similarly failed to produce any evidence for TTS (not shown). In other words, TTS was not reliably induced by this noise insult regardless of treatment condition, regardless of the metric used to define TTS. With no overall effect of noise on threshold sensitivity, there was no opportunity to measure protection against TTS as a function of treatment.
Figure 4.
Threshold shift measured 15 min post-shooting is plotted; the dashed line indicates no change from Pre-2 baseline. There was no reliable effect of noise on threshold sensitivity, and no group difference between placebo- and nutrient- condition. All data are Mean ± S.D. Outcomes were unchanged when Median data were analyzed.
Within-Subject Comparisons of the “Most Vulnerable” Subjects
Data from the most vulnerable subjects are encouraging with respect to the potential that group differences may emerge in future studies, given a more robust noise stress that induces a reliable TTS. We compared TTS in the two treatment conditions (placebo vs nutrient) for the subset of the subjects that were most vulnerable to noise, using the power of the within-subject crossover design. Six of 31 subjects had a threshold shift of 8 dB or more for the “primary outcome measure” (maximum change at 3, 4, or 6 kHz 15 min post-exposure in either ear; TTS Max15) in the placebo condition (see Table 2). The two subjects with the greatest vulnerability (>10 dB TTS in the placebo condition) both had 12 dB less TTS in the treated condition compared to the placebo condition (subjects 1002, 1058). When we considered subjects with less robust change (8 dB TTS in the placebo condition), 3 of the 4 subjects (subjects 1007, 1057, 1062) had 2–4 dB smaller changes in the treated condition than in the placebo condition. The other subject with 8 dB TTS (subject 1010) had the same TTS in both the placebo and treated conditions. Thus, in 5 of 6 cases, the TTS was smaller with the nutrients than it was with the placebo, but 3 of the 5 cases showed a difference of 4 dB or less, while the other two showed a difference of 12 dB. In the sixth case, TTS was equivalent in the two treatment conditions.
Tinnitus
Tinnitus was only sporadically reported. There were 5 reports of post-exposure tinnitus from 3 subjects, with 2 subjects reporting tinnitus after each exposure. One subject reported tinnitus in the nutrient condition but not the placebo condition (subject 1051). One subject reported louder (but not more bothersome) tinnitus in the placebo condition compared to the nutrient condition (subject 1065). The third subject reported equivalent tinnitus in the nutrient and placebo conditions (subject 1069). All reports of post-noise tinnitus were submitted by academy recruits; none of the officers reported tinnitus at any test time.
Discussion
This report describes a human clinical trial to evaluate potential protection against temporary changes in hearing induced by military weapons training, using a nutrient therapy. No reliable noise-induced threshold shift was measured in either the placebo condition or the nutrient condition in the subjects tested in this study, and no noise-induced changes were observed in any of the secondary functional test metrics. Thus no conclusions on the potential for protection of the human ear were possible. Nonetheless, the results are of value as a reliable elevation in vitamin plasma levels was measured, and a small subset of noise ‘susceptible’ individuals showed smaller changes during the active agent treatments than were measured in the same subjects in the placebo arm of the study. While encouraging, the demonstration of group differences in future studies is essential for this combination of agents, or, any agent of interest for potential protection of the human inner ear.
Importantly, we present here a detailed double-blind protocol and an IRB-approved experimental design that may be useful to guide future studies, with the important caveat that more robust noise models are needed. The ethical challenges in designing and conducting human trials, without putting research subjects at risk of permanent damage to their own hearing, are clear. The strategy used here was to draw upon a military population that undergoes weapons training that was expected to exceed the limits of conventional hearing protection. Thus, all subjects were guaranteed protection using the same, traditional mechanical devices (ear plugs, ear muffs) that anyone NOT participating in the study would use. Although an earlier (unpublished) study revealed measurable TTS in soldiers participating in similar urban combat training exercises [22], there was no evidence for significant noise-induced changes in function using any of the metrics employed in this study.
It is possible that conventional hearing protection devices, which were required to be used, were used more effectively by subjects participating in the current study given increased attention to hearing outcomes as a consequence of study participation. Such effects are well-known, and are termed the Hawthorne effect; the concept is that subject behavior can be modified simply by the subjects’ knowledge that they are participating in an experiment. It is of course also possible that the hearing protection devices and/or the hearing conservation training program in use now is more effective than the devices and/or training protocols in use at the time of the earlier study. The Swedish military routinely updates its hearing conservation program as new devices are available for adoption.
Given the lack of noise-induced change in each of the measures tested, it was not possible to determine whether the nutrients had beneficial effects with respect to protection against NIHL. However, it is well known that there is significant variation across individuals with respect to vulnerability to noise insult, with data coming from both animal models [see 23 for data from guinea pigs], and humans [see, for example, 24, 25, 26]. Indeed, variability in threshold shift after music exposure was almost 30 dB across subjects [27], and variability in threshold shift after impulse noise was approximately 40 dB across subjects [28]. In those earlier studies with heterogeneous subject populations, it is possible that unidentified individual differences in dietary nutrient intake could have influenced individual vulnerability; such a finding would be consistent with emerging epidemiological evidence that dietary nutrient intake is correlated with hearing outcomes in humans [29–31]. The observation that a subset of the most vulnerable subjects tended to have less TTS in the nutrient condition than in the placebo condition in this study is intriguing, but will need to be substantiated by additional study. Specifically, although there appeared to be a benefit to taking the nutrients in 5 of the 6 cases in which TTS reached or exceeded 8 dB in the placebo condition, the fact that the other 25 cases had small TTS (<8 dB in the placebo condition) and no seeming benefit of the nutrients makes that interpretation suspect. Taken together, the reliability of these effects must be confirmed in other populations with more robust, and more reliable, TTS, given that the majority of the subjects had no change in thresholds in either condition.
Although the study did not provide robust efficacy data, critical new information on changes in vitamin plasma level was obtained in this study. The vitamin dose parameters used here were largely modeled after the Age-Related Eye Disease study (AREDS)[32]. The vitamintreated subjects in that study received 500 mg vitamin C, 400 IU vitamin E, and 15 mg betacarotene. This long-term study (average follow-up: 6.3 years) provides important safety data for long-term use of vitamin agents, and provides data that vitamins (in combination with zinc) have an important protective role for a visual sensory disorder [33]. Plasma levels measured in other studies are reported below using the units specified in the original report, with conversions to a single consistent unit of measurement (µg/mL) following in parentheses to facilitate comparisons across studies.
β-carotene
In the Swedish military officers, baseline levels of plasma β-carotene ranged from 0.23 µg/mL (Fig 2A) to 0.28 µg/mL (Fig 2B), values which are consistent with the baseline β-carotene levels of 25–28 µg/dL (0.25–0.28 µg/mL) measured in the AREDS study [33, 34]. Plasma β-carotene levels roughly doubled with the two day treatments used here; in contrast, the AREDS report describes an average change of 482% increase in median plasma levels after 1-year of daily vitamin use (15 mg/day β-carotene) [33, 34]. Taken together, our data confirm rapid increases in plasma β-carotene levels after 2 days of vitamin dosing, and long-term data from the AREDS study suggests plasma concentrations have the potential for greater increase with longer term treatments. It should be noted that use of high-level β-carotene supplements (20–30 mg/day) is now contra-indicated for those with a significant history of tobacco use [for recent review, see 35]. Otherwise, it is generally regarded as safe. Target plasma levels for protection of the inner ear are unknown [see 6].
Vitamin C
In the Swedish military officers, baseline levels of plasma vitamin C ranged from 5.4 µg/mL (Fig 2D) to 6.0 µg/mL (Fig 2C). These baseline values were a bit lower than those measured in other human studies, which report normal (unsupplemented) vitamin C levels ranging from 0.8 mg/dL (8 µg/mL) [36] to 1.4 mg/dL (14 µg/mL) [37–39]. In the AREDS subjects, baseline vitamin C levels in plasma were 1.1 mg/dL (11 µg/mL) [33, 34]. Vitamin C plasma levels in the current military subject population increased by approximately 60% after the two day treatments used here (to 9.6±2.2 µg/mL, Fig 2C).
Given suggestions that plasma levels in the range of 1.6–2.5 mg/dL (16–25 µg/mL) are one potential target level for translation to human use [6], treatments lasting longer than two-days may ultimately be more likely to provide benefits in humans as plasma levels clearly increase with duration of dosing. Single-subject examples presented in two earlier reports showed stable vitamin C plateaus after 25–35 days of daily dosing [40, 41]. These longer-term vitamin C supplements (400 gram/day or greater) have achieved plasma levels ≥ 2.0 mg/dL (20 µg/mL) in human subjects[42–44]; such levels are within the target range suggested by Le Prell et al. [6]. As noted in that report, lower dose supplements such as those found in many daily multi-vitamins may not be effective for increasing plasma levels into the suggested vitamin C target range (i.e., 16–25 µg/mL). In a long-term (8 year) study examining daily Centrum multi-vitamin use, daily intake of the multi-vitamin containing 60 mg ascorbic acid resulted in vitamin C plasma levels that increased only minimally, from ~45 µMol/L to ~60 µMol/L (e.g., ~0.8 mg/dL to 1.0 mg/dL; or 8 µg/mL to 10 µg/mL) [45]. A second study also using a 60 mg/day ascorbic acid supplement resulted in a similarly small increase in plasma levels, from 1.1 mg/dL to 1.4 mg/dL (11 µg/mL to 14 µg/mL) [42]. It is well known that peak vitamin C plasma levels increase with increasing vitamin C dose in human subjects [40, 41, 46].
Vitamin E
Baseline levels of plasma vitamin E were low in the Swedish military officers, ranging from 11.6 µg/mL (Fig 2F) to 11.9 µg/mL (Fig 2E). Vitamin E plasma levels in the current military subject population increased by approximately 60% over the two day treatments used here (to 18.3±5.7 µg/mL, see Fig 2E). Even with supplements, the plasma levels were low compared to those measured in other human populations. In other supplement studies, serum levels increased from baselines of 8.4 mg/dL (84 µg/mL) to 20.7 mg/dL (207 µg/mL) in subjects taking 200 IU/day vitamin E for 8 weeks, and they increased from baselines of 8.9 mg/dL (89 µg/mL) to 52.8 mg/dL (528 µg/mL) in those taking 2000 IU/day for 8 weeks [47]. Maximum levels appear to be reached approximately 4 weeks after onset of daily treatment [48]. It is not clear why vitamin E levels in plasma were low in the military personnel relative to other similarly healthy populations, and it would have been helpful to have additional information on normal dietary nutrient intake. Several Food Frequency Questionnaires (FFQ) have been designed to capture individuals’ usual dietary intake, typically by asking the respondent to report their frequency of consumption of a list of foods over a specific period of time [49]. In future investigations, it will be important to assess not only dietary nutrient intake using FFQs, but also perhaps patterns of activity, including routine exercise. Exercise has the potential to influence the absorption of vitamins and other nutrients; for example, exercise mobilizes vitamin E and increases circulating plasma levels [50].
Magnesium
Mg levels did not change in the Swedish military officer samples, which could perhaps be a consequence of the technical challenges associated with Mg measurement. There is little consensus on the optimal strategy for Mg measurement, as plasma Mg is not sensitive to subtle change and may not reflect whole body Mg stores [51–53]. Erythrocyte or monocyte levels may have been more sensitive metrics [see 54 for discussion of challenges associated with each assay]; these measures should be considered as part of future investigations. Mg supplements have a variety of health benefits in humans, including reductions in NIHL [55–57]. In those studies, Mg doses were 167 mg Mg aspartate (delivered orally). The dose used here was 315 mg/day. At much higher levels, Mg can act as a laxative. Over-the-counter (OTC) Mg-based laxatives typically include 2400–4800 mg oral magnesium hydroxide (MgOH2,). Doses that do not exceed the UL should not cause adverse GI outcomes in most healthy adult populations. In this study, 3 of 31 subjects reported mild stomach upset during or after the placebo treatments, and 2 of 31 subjects reported mild stomach upset during or after the nutrient treatments; thus adverse GI outcomes were not detected at a higher rate with nutrients than with placebo.
Summary
There were no consistent changes in any measure of sensitivity during shooting exercises conducted during two different treatment conditions (placebo, nutrients). It was therefore not possible to determine whether the nutrient treatment was beneficial with respect to preserving auditory function. The current data do, however, provide important new evidence documenting nutrient plasma level changes achieved with two-day dosing in human subjects. These plasma levels provide evidence that short-term (two-day) dosing with a nutrient combination elevates vitamin levels in the bloodstream. Mg levels in the plasma did not increase, which may reflect technical hurdles previously identified by others. Taken together, this study demonstrates significant plasma level changes for the vitamins, provides a highly powered within-subjects crossover design, and it highlights the sometimes uncontrollable factors encountered in translational research studies, including, in this case, a noise trauma that was inadequate to induce robust TTS in the military subject population. Similar issues have been encountered in other studies, with one report indicating that only 30% of subjects in another military study experienced measurable hearing loss after shooting exercises [see 58; as of the time this article was prepared, those data have not appeared in the peer-reviewed literature].
It is critically important that trial designs and outcomes be published regardless of the success of the agent in providing protection, such that those study designs that are not successful are not repeated by others. With respect to TTS studies, laboratory-based studies using music to induce TTS have been proposed [59] and preliminary data on the reliability of TTS across subjects has been presented at several scientific meetings [60, 61]. Selection of sound levels will be critically important; not all investigator-selected exposure paradigms have induced TTS [62–64]. Equivalent laboratory-based test paradigms using gunshot-like digital noise signals presented during electronic game play are being developed in ongoing laboratory-based studies. Indeed, the use of antioxidants to reduce or prevent NIHL is of broad interest, with multiple groups soon to be initiating, or already conducting, clinical trials on prevention of NIHL [i.e., 65, 66, 67, 68, see also NCT00552786, NCT00802425]. The ongoing translation of these agents from animal models to human trials provides a compelling rationale for continued development of controlled paradigms for investigations into the use of these and other antioxidant agents.
Table 3.
Primary Outcome for Placebo and Nutrient Conditions 15 min after shooting: Most Vulnerable Subjects
| Subject | TTS Max15; Placebo Arm |
TTS Max15, Nutrient Arm |
|---|---|---|
| 1002 | 10 | −2 |
| 1007 | 8 | 4 |
| 1010 | 8 | 8 |
| 1057 | 8 | 6 |
| 1058 | 14 | 2 |
| 1062 | 8 | 6 |
Acknowledgements
The project was supported by U01 DC 008423 from the National Institute On Deafness And Other Communication Disorders, National Institutes of Health, awarded to the University of Michigan (JMM), with subcontracts awarded to Karolinska Institutet (MU), and the University of Florida (CGL). Plasma analysis was funded by OtoMedicine, Inc. Additional support was provided by the Swedish Council for Working Life and Social Research FAS Center programme (2006-1526). The trial is registered at clinicaltrials.gov; see NCT00808470. This manuscript is dedicated to the memory of Per-Anders Hellström, Ph.D., our colleague and collaborator from the Swedish Armed Forces. Colonel Anders Emanuelsson and Lieutenant-Commander Fredrik Blomqvist were also instrumental. We thank the members of the Data Safety Monitoring Board, including Robert Dobie, Joseph Hall, Rick Mowery, Catherine Ross, Darby Thompson, as well as Gordon Hughes at the NIH, for helpful feedback and suggestions during their oversight of these studies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute On Deafness And Other Communication Disorders or the National Institutes of Health. We thank Sharon Kujawa for assistance developing the DPOAE test paradigm and for helpful comments on an earlier version of this manuscript. We thank Peter Boxer and Clark Bennett for their assistance with plasma analysis, Bjorn Hagerman and Åke Olofssonfor technical assistance during data collection, Ulf Rosenhall for medical supervision in Sweden, and Sebastian de la Calle, Kari Morgenstein, and Danielle Rosier Youngstrom, for technical assistance with DPOAE data entry and summaries. Finally, we thank Susan DeRemer for her assistance with IRB applications at the University of Michigan.
Drs. Le Prell and Miller are co-inventors on US Patent Application 20090155390, which is assigned to the University of Michigan [77]. The micronutrient treatment was licensed to OtoMedicine, Inc., at the time this study was conducted, and is now licensed to Hearing Health Sciences, Inc. Dr. Miller was a founding member of OtoMedicine and is a scientific advisor to Hearing Health Science. Dr. Le Prell previously worked as a paid consultant to OtoMedicine and is a scientific advisor to Hearing Health Science.
Footnotes
Disclosure
These relationships have been disclosed to the Conflict of Interest Board at the University of Michigan (Miller) and the University of Florida (Le Prell).
Literature Cited
- 1.Abi-Hachem RN, Zine A, Van De Water TR. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Patents on CNS Drug Discovery. 2010;5:147–163. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]
- 2.Le Prell CG, Bao J. In: Prevention of noise-induced hearing loss: Potential therapeutic agents, in Noise-induced hearing loss: Scientific advances, springer handbook of auditory research. Le Prell CG, Henderson D, Fay RR, Popper AN, editors. New York: Springer Science+Business Media, LLC; 2011. [Google Scholar]
- 3.Le Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins a, c, and e plus magnesium reduce noise trauma. Free Radic. Biol. Med. 2007;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamir S, Adelman C, Weinberger JM, Sohmer H. Uniform comparison of several drugs which provide protection from noise induced hearing loss. J. Occup Med. Toxicol. 2010;5:26–32. doi: 10.1186/1745-6673-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Prell CG, Gagnon PM, Bennett DC, Ohlemiller KK. Nutrient-enhanced diet reduces noise-induced damage to the inner ear and hearing loss. Transl. Res. 2011;158:38–53. doi: 10.1016/j.trsl.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Prell CG, Dolan DF, Bennett DC, Boxer PA. Nutrient treatment and achieved plasma levels: Reduction of noise-induced hearing loss at multiple post-noise test times. Transl. Res. 2011;158:54–70. doi: 10.1016/j.trsl.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson BO, Svedberg A, Gothe CJ. Longitudinal changes in hearing ability among swedish conscripts. Scand. Audiol. 1993;22:141–143. doi: 10.3109/01050399309046030. [DOI] [PubMed] [Google Scholar]
- 8.Axelsson A, Rosenhall U, Zachau G. Hearing in 18-year-old swedish males. Scand. Audiol. 1994;23:129–134. doi: 10.3109/01050399409047497. [DOI] [PubMed] [Google Scholar]
- 9.Muhr P, Månsson B, Hellström PA. A study of hearing changes among military conscripts in the swedish army. Int. J. Audiol. 2006;45:247–251. doi: 10.1080/14992020500190052. [DOI] [PubMed] [Google Scholar]
- 10.Rosenhall U, Pyykko I, Rasmussen F, Muhr P. Hearing loss in young men: Possible aetiological factors. Noise Health. 2006;8:40–44. doi: 10.4103/1463-1741.32466. [DOI] [PubMed] [Google Scholar]
- 11.Muhr P, Rasmussen F, Rosenhall U. Prevalence of hearing loss among 18-year-old swedish men during the period 1971–1995. Scand. J. Public Health. 2007;35:524–532. doi: 10.1080/14034940701281477. [DOI] [PubMed] [Google Scholar]
- 12.Muhr P, Rosenhall U. Self-assessed auditory symptoms, noise exposure, and measured auditory function among healthy young swedish men. Int. J. Audiol. 2010;49:317–325. doi: 10.3109/14992020903431280. [DOI] [PubMed] [Google Scholar]
- 13.Kujawa SG, Glattke TJ, Fallon M, Bobbin RP. A nicotinic-like receptor mediates suppression of distortion product otoacoustic emissions by contralateral sound. Hear. Res. 1994;74:122–134. doi: 10.1016/0378-5955(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 14.Kemp D. In: Otoacoustic emissions in perspective, in Otoacoustic emissions: Clinical applications. Robinette M, Glattke T, editors. New York: Thieme; 1997. pp. 1–21. [Google Scholar]
- 15.Hagerman B, Olofsson A, Lindblad AC. Relations between speech intelligibility, psychoacoustical modulation transfer function (pmtf) Scand. Audiol. 1987;16:121–128. doi: 10.3109/01050398709042166. [DOI] [PubMed] [Google Scholar]
- 16.Lindblad A-C, Hagerman B. Hearing tests for selection of sonar operators. Acta Acustica. 1999;85:870–876. [Google Scholar]
- 17.Margolis RH, Hunter LL. In: Acoustic immittance measurements, in Audiology diagnosis. Roeser RJ, Valente M, Hosford-Dunn H, editors. New York: Thieme; 2000. pp. 381–342. [Google Scholar]
- 18.Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM, Ma MC, Guo YL. N-acetyl-cysteine against noise-induced temporary threshold shift in male workers. Hear. Res. 2010;269:42–47. doi: 10.1016/j.heares.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Le Prell CG, Hensley BN, Campbell KCM, Hall JWI, Guire K. Hearing outcomes in a “normally-hearing” college-student population: Evidence of hearing loss. Int. J. Audiol. 2011;50:S21–S31. doi: 10.3109/14992027.2010.540722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughson W, Westlake HD. Manual for program outline for rehabilitation of aural casualties both military and civilian. Trans. Amer. Acad. Opthamology Otolaryngol. 1944;48(Suppl):1–15. [Google Scholar]
- 21.Goldman B, Sheppard L, Kujawa SG, Seixas NS. Modeling distortion product otoacoustic emission input/output functions using segmented regression. J. Acoust. Soc. Am. 2006;120:2764–2776. doi: 10.1121/1.2258871. [DOI] [PubMed] [Google Scholar]
- 22.Lindblad A-C, Olofsson A. Temporary hearing changes in urban combat conditions: Karolinska institutet technical and clinical audiology report ta135. 2003 [Google Scholar]
- 23.Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J. Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindgren F, Axelsson A. In: Human noise experiments using a temporary threshold shift model, in Basic and applied aspects of noise-induced hearing loss. Salvi RJ, Henderson D, Hamernik RP, Colleti V, editors. New York: Plenum Publishing Corporation; 1986. pp. 313–321. [Google Scholar]
- 25.Mills JH, Schulte BA, Boettcher FA, Dubno JR. In: A comparison of age-related hearing loss and noise-induced hearing loss, in Noise induced hearing loss: Basic mechanisms, prevention and control. Henderson D, Prasher D, Kopke R, Salvi RJ, Hamernik R, editors. London: 2001. pp. 497–511. [Google Scholar]
- 26.Strasser H, Irle H, Legler R. Temporary hearing threshold shifts and restitution after energy-equivalent exposures to industrial noise and classical music. Noise Health. 2003;5:75–84. [PubMed] [Google Scholar]
- 27.Müller J, Dietrich S, Janssen T. Impact of three hours of discotheque music on Pure-tone thresholds and distortion product otoacoustic emissions. J. Acoust. Soc. Am. 2010;128:1853–1869. doi: 10.1121/1.3479535. [DOI] [PubMed] [Google Scholar]
- 28.Pfander F, Bongartz H, Brinkmann H, Kietz H. Danger of auditory impairment from impulse noise: A comparative study of the chaba damage-risk, criteria and those of the federal republic of germany. J. Acoust. Soc. Am. 1980;67:628–633. doi: 10.1121/1.383886. [DOI] [PubMed] [Google Scholar]
- 29.Michikawa T, Nishiwaki Y, Kikuchi Y, Hosoda K, Mizutari K, Saito H, Asakura K, Milojevic A, Iwasawa S, Nakano M, Takebayashi T. Serum levels of retinol, and other antioxidants for hearing impairment among japanese older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2009;64:910–915. doi: 10.1093/gerona/glp038. [DOI] [PubMed] [Google Scholar]
- 30.Gopinath B, Flood VM, McMahon CM, Burlutsky G, Spankovich C, Hood LJ, Mitchell P. Dietary antioxidant intake is associated with the prevalence but not incidence of age-related hearing loss. J. Nutr. Health Aging. 2011 doi: 10.1007/s12603-011-0119-0. in press. [DOI] [PubMed] [Google Scholar]
- 31.Spankovich C, Hood L, Silver H, Lambert W, Flood V, Mitchell P. Associations between diet, and both high and low pure tone averages, and transient evoked otoacoustic emissions in an older adult population-based study. J. Am. Acad. Audiol. 2011;22:49–58. doi: 10.3766/jaaa.22.1.6. [DOI] [PubMed] [Google Scholar]
- 32.Age-Related Eye Disease Study Research Group. The age-related eye disease study (areds): Design implications. Areds report no. 1 Control. Clin. Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins c, e, beta carotene, and zinc for age-related macular degeneration, and vision loss. Areds report no. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins c, e, and beta carotene for age-related cataract and vision loss. Areds report no. 9. Arch. Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: A systematic review metaanalysis of randomized controlled trials. Int. J. Cancer. 2010;127:172–184. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- 36.Barbosa E, Faintuch J, Machado Moreira EA, Goncalves da Silva VR, Lopes Pereima MJ, Martins Fagundes RL, Filho DW. Supplementation of vitamin e, vitamin c and zinc attenuates oxidative stress in burned children: A randomized, double-blind, placebo-controlled pilot study. J. Burn Care Res. 2009;30:859–866. doi: 10.1097/BCR.0b013e3181b487a8. [DOI] [PubMed] [Google Scholar]
- 37.Pollard J, Wild CP, White KL, Greenwood DC, Cade JE, Kirk SF. Comparison of plasma biomarkers with dietary assessment methods for fruit, and vegetable intake. Eur. J. Clin. Nutr. 2003;57:988–998. doi: 10.1038/sj.ejcn.1601634. [DOI] [PubMed] [Google Scholar]
- 38.Aydin S, Aral I, Kilic N, Bakan I, Erman F. The level of antioxidant enzymes, plasma vitamins c and e in cement plant workers. Clin. Chim. Acta. 2004;341:193–198. doi: 10.1016/j.cccn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Karajibani M, Hashemi M, Montazerifar F, Bolouri A, Dikshit M. The status of glutathione peroxidase, superoxide dismutase vitamins a c e and malondialdehyde in patients with cardiovascular disease in zahedan, southeast iran. J. Nutr. Sci. Vitaminol. (Tokyo) 2009;55:309–316. doi: 10.3177/jnsv.55.309. [DOI] [PubMed] [Google Scholar]
- 40.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin c pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U. S. A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin c for healthy young women. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson D. Factors that contribute to biomarker responses in humans including a study in individuals taking vitamin c supplementation. Mutat. Res. 2001;480–481:337–347. doi: 10.1016/s0027-5107(01)00193-2. [DOI] [PubMed] [Google Scholar]
- 43.Pancorbo D, Vazquez C, Fletcher MA. Vitamin c-lipid metabolites: Uptake and retention effect on plasma c-reactive protein, and oxidized ldl levels in healthy volunteers. Med. Sci. Monit. 2008;14:CR547–CR551. [PubMed] [Google Scholar]
- 44.Washio K, Inagaki M, Tsuji M, Morio Y, Akiyama S, Gotoh H, Gotoh T, Gotoh Y, Oguchi K. Oral vitamin c supplementation in hemodialysis patients and its effect on the plasma level of oxidized ascorbic acid and cu/zn superoxide dismutase an oxidative stress marker. Nephron Clin. Pract. 2008;109:c49–c54. doi: 10.1159/000137628. [DOI] [PubMed] [Google Scholar]
- 45.Maraini G, Williams SL, Sperduto RD, Ferris FL, Milton RC, Clemons TE, Rosmini F, Ferrigno L. Effects of multivitamin/mineral supplementation on plasma levels of nutrients. Report no. 4 of the italian-american clinical trial of nutritional supplements and age-related cataract. Ann. Ist. Super. Sanita. 2009;45:119–127. [PubMed] [Google Scholar]
- 46.Polidori MC, Mecocci P, Levine M, Frei B. Short-term long-term vitamin c supplementation in humans dose-dependently increases the resistance of plasma to ex vivo lipid peroxidation. Arch. Biochem. Biophys. 2004;423:109–115. doi: 10.1016/j.abb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 47.Meagher EA, Barry OP, Lawson JA, Rokach J, FitzGerald GA. Effects of vitamin e on lipid peroxidation in healthy persons. JAMA. 2001;285:1178–1182. doi: 10.1001/jama.285.9.1178. [DOI] [PubMed] [Google Scholar]
- 48.Kappus H, Diplock AT. Tolerance and safety of vitamin e: A toxicological position report. Free. Radic. Biol. Med. 1992;13:55–74. doi: 10.1016/0891-5849(92)90166-e. [DOI] [PubMed] [Google Scholar]
- 49.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the block, willett, and national cancer institute food frequency questionnaires : The eating at america's table study. Am. J. Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 50.Margaritis I, Rousseau AS. Does physical exercise modify antioxidant requirements? Nutr Res Rev. 2008;21:3–12. doi: 10.1017/S0954422408018076. [DOI] [PubMed] [Google Scholar]
- 51.Wary C, Brillault-Salvat C, Bloch G, Leroy-Willig A, Roumenov D, Grognet JM, Leclerc JH, Carlier PG. Effect of chronic magnesium supplementation on magnesium distribution in healthy volunteers evaluated by 31p-nmrs and ion selective electrodes. Br. J. Clin. Pharmacol. 1999;48:655–662. doi: 10.1046/j.1365-2125.1999.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas J, Millot JM, Sebille S, Delabroise AM, Thomas E, Manfait M, Arnaud MJ. Free and total magnesium in lymphocytes of migraine patients - effect of magnesium-rich mineral water intake. Clin. Chim. Acta. 2000;295:63–75. doi: 10.1016/s0009-8981(00)00186-8. [DOI] [PubMed] [Google Scholar]
- 53.Feillet-Coudray C, Coudray C, Wolf FI, Henrotte JG, Rayssiguier Y, Mazur A. Magnesium metabolism in mice selected for high and low erythrocyte magnesium levels. Metabolism: Clinical and Experimental. 2004;53:660–665. doi: 10.1016/j.metabol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Ranade VV, Somberg JC. Bioavailability pharmacokinetics of magnesium after administration of magnesium salts to humans. Am. J. Ther. 2001;8:345–357. doi: 10.1097/00045391-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Joachims Z, Netzer A, Ising H, Rebentisch E, Attias J, Weisz G, Gunther T. Oral magnesium supplementation as prophylaxis for noise-induced hearing loss: Results of a double blind field study. Schriftenr. Ver Wasser Boden. Lufthyg. 1993;88:503–516. [PubMed] [Google Scholar]
- 56.Attias J, Weisz G, Almog S, Shahar A, Wiener M, Joachims Z, Netzer A, Ising H, Rebentisch E, Guenther T. Oral magnesium intake reduces permanent hearing loss induced by noise exposure. Am. J. Otolaryngol. 1994;15:26–32. doi: 10.1016/0196-0709(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 57.Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin. Otolaryngol. 2004;29:635–641. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 58.Powell K. Like an earplug in a pill. article in the los angeles times Los Angeles Times 2007, July 2 [cited; Available from: http://articles.latimes.com/2007/jul/02/health/he-lab2.
- 59.Le Prell CG, Yang Q, Harris J. Modification of digital music files for use in human temporary threshold shift studies. J. Acoust. Soc. Am. Ex. Lett. 2011 doi: 10.1121/1.3630017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Prell CG, Hall JWI, Sakowicz B, Campbell KCM, Kujawa SG, Antonelli PA, Green GE, Miller JM, Holmes AE, Guire K. Temporary threshold shift subsequent to music player use: Comparison with hearing screenings in populations of adolescents and young adults; 35th Annual National Hearing Conservation Conference-Explore the World of Hearing Loss Prevention; NHCA Spectrum; 2010. p. 28. [Google Scholar]
- 61.Le Prell CG, Kujawa SG, Dell S, Hensley BN, Hall JWI, Campbell KCM, Antonelli PA, Green GE, Miller JM, Guire K. Temporary threshold shifts and otoacoustic emission amplitude reductions subsequent to music player use by young adults; 36th Annual National Hearing Conservation Conference-Innovation and Technology; NHCA Spectrum; 2011. pp. 36–37. [Google Scholar]
- 62.Krishnamurti S, Grandjean PW. Effects of simultaneous exercise and loud music on hearing acuity and auditory function. J Strength Cond Res. 2003;17:307–313. doi: 10.1519/1533-4287(2003)017<0307:eoseal>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Bhagat SP, Davis AM. Modification of otoacoustic emissions following ear-level exposure to mp3 player music. Int. J. Audiol. 2008;47:751–760. doi: 10.1080/14992020802310879. [DOI] [PubMed] [Google Scholar]
- 64.Keppler H, Dhooge I, Maes L, D'Haenens W, Bockstael A, Philips B, Swinnen F, Vinck B. Short-term auditory effects of listening to an mp3 player. Arch. Otolaryngol. Head Neck Surg. 2010;136:538–548. doi: 10.1001/archoto.2010.84. [DOI] [PubMed] [Google Scholar]
- 65.Toppila E, Starck J, Pyykko I, Miller JM. Protection against acute noise with antioxidants; presented at Nordic Noise: An International Symposium on Noise and Health, in Nobel Forum, Karolinska Institutet; Stockholm, Sweden. 2002. [Google Scholar]
- 66.Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke RD, Scranton S, O'Leary M. Efficacy of the antioxidant n-acetylcysteine (nac) in protecting ears exposed to loud music. J. Am. Acad. Audiol. 2006;17:265–278. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]
- 67.Kopke RD, Jackson RL, Coleman JKM, Liu J, Bielefeld EC, Balough BJ. Nac for noise: From the bench top to the. clinic. Hear. Res. 2007;226:114–125. doi: 10.1016/j.heares.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Lynch ED, Kil J. Development of ebselen, a glutathione peroxidase mimic, for the prevention and treatment of noise-induced hearing loss. Semin. Hearing. 2009;30:47–55. [Google Scholar]